Introduction
Gestational diabetes mellitus (GDM) is a significant
pregnancy complication that is defined as glucose intolerance
occurring in the second and third trimester of pregnancy with no
preexisting diabetes diagnosis (1), which poses a great risk to the mother
and fetus. Although most of these patients have normal glucose
metabolism after childbirth, they are at higher risk for type 2
diabetes mellitus (T2DM) (2). The
pathogenesis of GDM includes genetic factors and pancreatic β cell
failure due to placental factors and common risk factors for GDM
include metabolic inflammation, increased adipose tissue mass, a
family history of diabetes mellitus, advanced maternal age, a lack
of nutritional intake, a lack of physical activity, polycystic
ovary syndrome and oxidative stress (3-5).
Nevertheless, the molecular pathways of GDM are not clearly
understood.
Growth differentiation factor-15 (GDF-15), which is
also known as macrophage-inhibiting cytokine-1, is a member of the
TGF-β superfamily and is present in a wide variety of cells and
tissues (6,7). The level of GDF-15 in the circulation
is closely related to the progression of a number of diseases
(8). GDF-15 expression is
increased in various acute and chronic inflammatory states,
including tissue injury, cancer, cardiovascular disease and
diabetes (9-12).
Various animal and human studies have shown the association of
GDF-15 and glycometabolic disorders (13,14).
High plasma glucose levels and hyperinsulinemia significantly
increase GDF-15 levels in humans (15). Furthermore, the expression level of
serum GDF-15 increases with older age and higher body mass index
(BMI) (16-18).
Studies have shown that GDF-15 levels are higher in patients
diagnosed with GDM at 24-28 weeks of gestation than in nondiabetic
pregnant women (19,20). However, there are few relevant
studies regarding the expression level of GDF-15 before 24 weeks of
gestation.
The present study measured serum GDF-15 levels in
pregnant women with GDM and healthy pregnant women at 20-24 weeks
of gestation, as well as their relationship with various
traditional indicators of diabetes mellitus and explored the risk
factors for microalbuminuria in patients with GDM.
Materials and methods
Ethical considerations
The present study was approved by the Medical Ethics
Committee of the Nantong Haimen People's Hospital (approval no.
C20131102) and carried out in accordance with the Declaration of
Helsinki.
Participant population
The present study recruited 237 pregnant women at
20-24 weeks gestation at Nantong Haimen People's Hospital between
January 2014 and December 2020. The criteria for inclusion in the
study were an uncomplicated pregnancy and good health, judged from
the medical history. At entry, all subjects were nonsmokers and did
not consume alcohol. The exclusion criteria were a history of
anemia, hypertension, diabetes mellitus, or chronic kidney disease
before pregnancy. When participants were enrolled in this study, an
oral glucose tolerance test (OGTT) was performed according to a
previous study (21). The
classification of GDM was based on the World Health Organization
guidelines published in 2013(22),
which was consistent with International Association of the Diabetes
and Pregnancy Study Groups (IADPSG) diagnostic criteria: A fasting
plasma glucose (FPG) level ≥5.1 mmol/l, 1-h plasma glucose (1-h PG)
level ≥10.0 mmol/l, or 2-h plasma glucose (2-h PG) level ≥8.5
mmol/l (23). All 237 women were
classified into either the GDM group (n=167) or normal glucose
tolerance group (n=70) (non-GDM) according to plasma glucose
levels. A urinary microalbumin level >30 mg/24 h and <300
mg/24 h was defined as positive for microalbuminuria based on the
description in a previous study (24).
Clinical and serum GDF-15 measurement
methods
Fasting venous blood was collected. Urine was
collected for 24 h and sent to the clinical laboratory centre of
Nantong Haimen People's Hospital. FPG, 1-h PG and 2-h PG levels
were measured using the glucose oxidase method and glycated
hemoglobin and urinary microalbumin levels were measured using the
immunoturbidimetric method. Fasting insulin was measured by the
chemiluminescence method. Homeostasis model assessment-insulin
resistance (HOMA-IR) indexes were calculated as HOMA-IR=FPG x
fasting insulin/22.5.
The levels of serum GDF-15 were determined by ELISA
kit (Abcam; cat. no. ab155432) according to the manufacturer's
instructions. Briefly, 100 µl of standard and sample were added
into appropriate wells and incubated for 2.5 h at room temperature.
After discarding the solution and washing three times with
phosphate buffer solution, 100 µl of prepared biotinylated antibody
was added to each well before incubation for 1 h at room
temperature with gentle shaking. After the plate was washed three
times, 100 µl of Streptavidin solution was added to each well.
Following incubation for 45 min at room temperature, the plate was
again washed three times with phosphate buffer solution. Then, 100
µl of TMB One-Step Substrate Reagent was added to each well,
followed by the addition of stop solution. The absorbance at 450 nm
was measured using an ELISA plate reader (Bio-Rad Laboratories,
Inc.).
Statistical analysis
The Shapiro-Wilk test was used to check the
normality of the distribution of continuous variables. The averages
of the variables are expressed as the mean ± standard deviation
(normally distributed data) or the median (interquartile range)
(nonnormally distributed data). An independent-sample t test was
used to compare differences between the two groups for the normally
distributed parameters, whereas the Mann-Whitney U test was used
for the nonnormally distributed parameters. Comparisons between
proportions were performed with the chi-square test. The strength
and direction of the relationships between continuous variables
were analyzed by Pearson's analysis as applicable. Logistic
regression was used to identify risks associated with elevated
urinary microalbumin levels. Statistical analyses were performed
using SPSS 20.0 software (IBM Corp.). GraphPad Prism 8.0 software
(Dotmatics) was used for plotting.
Results
Basic clinical characteristics of the
normal pregnancy and GDM groups
A total of 237 pregnant women participated in the
present study. The two groups were matched by age. There was no
significant difference in gestational age between the GDM and
normal pregnancy groups (22.04±1.35 vs. 21.98±1.27; P=0.767). The
BMI of the GDM group was higher than that of the normal pregnancy
group (24.72±1.86 vs. 22.82±0.74; P<0.001). FPG (7.77±1.45 vs.
5.11±0.31; P<0.001), 1-h PG (9.81±1.53 vs. 5.79±0.52;
P<0.001), 2-h PG (12.33±1.49 vs. 7.41±1.17; P<0.001), fasting
insulin (8.45±1.05 vs. 6.08±0.50; P<0.001), HOMA-IR (1.38±0.21
vs. 2.94±0.77; P<0.001) and glycated hemoglobin (7.63±1.20 vs.
5.61±0.40; P<0.001) levels were significantly higher in the GDM
group than in the normal pregnancy group. Microalbuminuria was
significantly more common in the GDM group than in the normal
pregnancy group (36.52% vs. 0%; P<0.001; Table I).
 | Table IClinical characteristics and
biochemical parameters of the participants. |
Table I
Clinical characteristics and
biochemical parameters of the participants.
| Characteristic or
parameter | Normal pregnancy
group (n=70) | Gestational diabetes
mellitus group (n=167) |
t/χ2/Z | P-value |
|---|
| Age, median
yeara (lower, upper quartile) | 28 (27, 29) | 28 (24, 29) | -1.788 | 0.074 |
| BMI,
kg/m2 | 22.82±0.74 | 24.72±1.86 | -11.202 | 0.000 |
| Gestational age,
weeks | 21.98±1.27 | 22.04±1.35 | -0.297 | 0.767 |
| Fasting plasma
glucose, mmol/l | 5.11±0.31 | 7.77±1.45 | -22.311 | <0.001 |
| 1-h plasma glucose,
mmol/l | 5.79±0.52 | 9.81±1.53 | -30.008 | <0.001 |
| 2-h plasma glucose,
mmol/l | 7.41±1.17 | 12.33±1.49 | -115.27 | <0.001 |
| Fasting insulin,
IU/ml | 6.08±0.50 | 8.45±1.05 | -23.272 | <0.001 |
| Homeostasis model
assessment-insulin resistance | 1.38±0.21 | 2.94±0.77 | 16.653 | <0.001 |
| Glycosylated
haemoglobin, % | 5.61±0.40 | 7.63±1.20 | -90.194 | <0.001 |
| positive urinary
microalbumin | 0 (0%) | 61 (36.52%) | 34.431 | <0.001 |
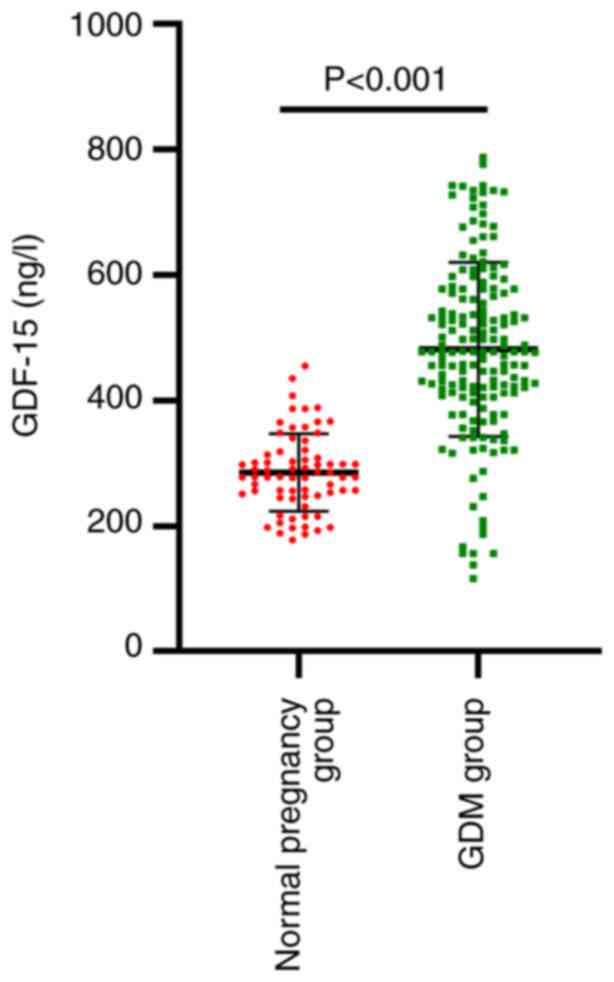
Comparison of serum GDF-15 levels
between the normal pregnancy and GDM groups
Based on the World Health Organization guidelines
published in 2013, participants were divided into a GDM group and a
normal pregnancy group. Fig. 1
shows the concentration of serum GDF-15 in the two groups. Compared
with the levels in the normal pregnancy group, the GDF-15 levels in
the GDM group were significantly elevated (P<0.001; Fig. 1).
Correlations between serum GDF-15
levels and clinical characteristics and indicators in the GDM
group
Through a Pearson correlation analysis, the
relationship between serum GDF-15 levels and glucose
metabolism-related indicators in pregnant women was analyzed.
GDF-15 levels were positively correlated with BMI (r=0.223;
P=0.004), FPG (r=0.401, P<0.001), glycosylated hemoglobin
(r=0.339; P<0.001), HOMA-IR (r=0.474; P<0.001) and fasting
insulin levels (r=0.375; P=0.000; Fig.
2).
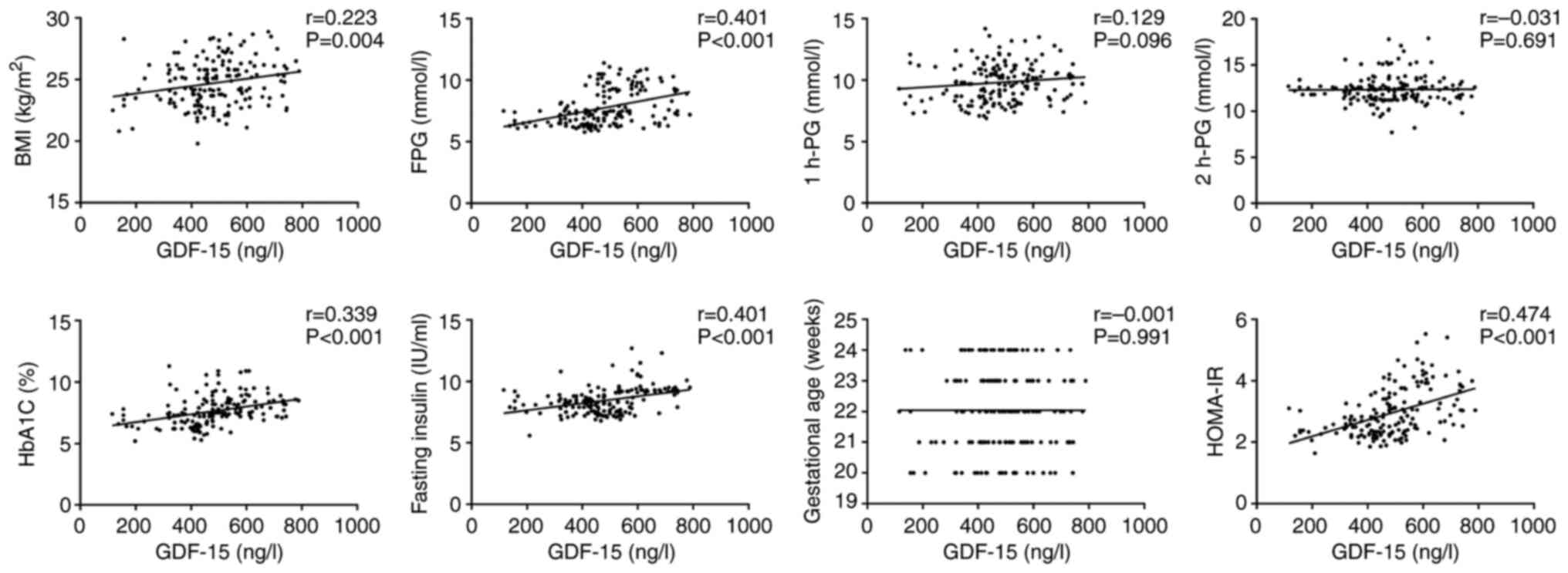 | Figure 2Correlations of GDF-15 levels with
BMI, gestational age, FPG levels, 1-h PG levels, 2-h PG levels,
glycosylated haemoglobin levels, HOMA-IR levels and fasting insulin
levels in the GDM group. GDF-15, growth differentiation factor-15;
BMI, body mass index; FPG, fasting plasma glucose; PG, plasma
glucose; HOMA-IR, homeostasis model assessment-insulin resistance;
GDM, gestational diabetes mellitus. |
Analysis of risk factors for
microalbuminuria in the GDM group
As a result of the univariate logistic regression
analysis, older age [odds ratio (OR)=1.101, 95% confidence interval
(CI) 1.001-1.211] and FPG (OR=6.564, 95% CI 3.840-1.222), 1-h PG
(OR=1.451, 95% CI 1.162-1.812), fasting insulin (OR=2.237, 95% CI
1.534-3.262), glycosylated hemoglobin (OR=1.521, 95% CI
1.100-2.103) and GDF-15 levels (OR=1.011, 95% CI 1.007-1.015) were
related to elevated urinary microalbumin levels. Factors such as
FPG, 1-h PG, fasting insulin, glycosylated hemoglobin and GDF-15
were included in the multivariable logistic regression analysis. In
the multivariate logistic regression analysis, elevated GDF-15 (OR
1.013, 1.006-1.020) and FPG levels (OR 6.069, 3.130-11.874) were
independent risk factors for microalbuminuria (Table II).
 | Table IIRisk factors significantly correlated
with urinary microalbumin in gestational diabetes mellitus
group. |
Table II
Risk factors significantly correlated
with urinary microalbumin in gestational diabetes mellitus
group.
| Risk factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age | 1.101 | 1.001-1.211 | 0.048 | 1.197 | 0.983-1.457 | 0.074 |
| Fasting plasma
glucose | 6.564 | 3.840-1.222 | 0.000 | 6.069 | 3.130-11.874 | <0.001 |
| 1-h plasma
glucose | 1.451 | 1.162-1.812 | 0.013 | 1.371 | 0.835-2.249 | 0.212 |
| 2-h plasma
glucose | 0.854 | 0.674-1.081 | 0.189 | - | - | - |
| Fasting
insulin | 2.237 | 1.534-3.262 | 0.000 | 0.777 | 0.361-0.975 | 0.518 |
| Glycosylated
haemoglobin | 1.521 | 1.100-2.103 | 0.011 | 0.566 | 0.235-1.361 | 0.204 |
| growth
differentiation factor-15 | 1.011 | 1.007-1.015 | 0.000 | 1.013 | 1.006-1.020 | <0.001 |
Discussion
GDM is defined as diabetes diagnosed in the second
or third trimester of pregnancy without overt diabetes prior to
gestation (25). GDM increases the
risk of adverse pregnancy outcomes and causes adverse effects on
both the mother and fetus. GDM often leads to diabetic nephropathy,
retinopathy, fetal macrosomia and unexplained fetal death and
increases the risk of neonatal hypoglycemia, hypocalcemia,
jaundice, acute respiratory distress syndrome and cardiomyopathy
(26). Women with GDM are also
prone to postpartum obesity, metabolic syndrome and T2DM (27,28).
In addition, the risk of long-term cardiovascular disease is
significantly increased in women with GDM (29). The incidence of GDM varies among
different races and populations, with incidence rates of 2-6% in
European countries, 1-14% in the United States, 9.6-13.9% in the
developing countries of South Asia and 14.8% in China (30).
The pathogenesis of GDM is not fully known. In
pregnancy, the levels of estrogen, progesterone, placental
lactogen, human placental growth hormone and cortisol in the
placenta significantly increase, which promotes the aggravation of
insulin resistance. With the gradual decrease in insulin
sensitivity, insulin secretion gradually increases to keep blood
glucose at a normal level. Therefore, pregnancy itself is a state
of hyperinsulinemia (31). If
pancreatic islet β-cells cannot secrete enough insulin to
compensate for pregnancy-related insulin resistance, GDM may occur.
Most women with GDM are overweight or obese, with the clinical
features of metabolic syndrome (31). Yarsilikal Guleroglu et al
(32) reported that FPG and HbA1c
levels in women with GDM and obesity were higher than those in
normal pregnant women. The present study found that the incidence
of obesity in the GDM group was significantly higher than that in
the normal pregnancy group and the mean BMI was also significantly
higher. In addition, FPG, 1-h PG, 2-h PG, fasting insulin and
glycated hemoglobin levels were significantly higher in the GDM
group than in the normal pregnancy group. Furthermore, the positive
rate of microalbuminuria in the GDM group was significantly higher
than that in the normal pregnancy group.
GDF-15 widely expressed in various cells and tissues
and plays an important role in inflammatory response regulation and
cell growth and differentiation (33). Biomechanical stress, local
ischemia, hypoxia and inflammatory cytokine stimulation can lead to
increased GDF-15 expression (33).
Hyperglycemia or obesity can promote GDF-15 expression through the
reactive oxygen species-p53 pathway (33). In the adipose tissue of patients
with GDM, GDF-15 expression is significantly elevated, which is the
main source of abnormally elevated GDF-15 in the maternal
circulatory system (34). Banerjee
et al (20) found that the
mean serum GDF-15 level was significantly higher in subjects with
GDM at 24-28 weeks of gestation than in nondiabetic pregnant women.
Li et al (19) reported
that GDF-15 was significantly elevated in the GDM group compared
with that in the group with normal glucose tolerance at 24-28 weeks
of gestation. Yakut et al (35) found that serum GDF-15 levels were
higher in patients with GDM than in non-GDM pregnant women at 24-28
weeks of gestation. Lu et al (36) reported that the expression levels
of GDF-15 mRNA and GDF-15 protein in late pregnancy were
significantly higher in GDM patients than in non-GDM pregnant
women. Andersson-Hall et al (37) demonstrated that GDF-15 levels
significantly increased in each trimester in pregnant women with
normal weight and obesity. Notably, they also found that GDF-15
levels were increased in cerebrospinal fluid during pregnancy
compared with those after pregnancy (38). Banerjee et al (20) reported that serum GDF-15 levels
were higher in GDM patients in comparison to age-matched pregnant
subjects without GDM in the early third trimester of pregnancy in
Indian women. Moreover, in the third trimester, GDF-15 levels
increased with increases in plasma glucose and insulin resistance.
However, there are few reports on serum GDF-15 levels in GDM
patients before 24 weeks of gestation. Berkowitz et al
reported that 28.8% of cases of GDM were diagnosed before 24 weeks
of gestation in 354 GDM patients (39). Therefore, it is important to
explore the expression of GDF-15 in GDM patients before 24 weeks of
gestation. In the present study, the serum GDF-15 level in the GDM
group was significantly higher than that in the normal pregnancy
group at 20-24 weeks of gestation.
In patients with T2DM, GDF-15 is positively
correlated with BMI, body fat, FPG, glycated hemoglobin, insulin
resistance, arterial blood pressure, triglycerides and the
incidence of diabetic nephropathy and is negatively correlated with
the degree of anemia (40).
Elevated GDF-15 levels can be used as a predictor of diabetic
cardiomyopathy in patients with T2DM (31). GDF-15 elevation has good predictive
value for all-cause mortality and cardiovascular death in patients
with diabetes mellitus, cardiovascular disease, kidney disease, or
rheumatic disease (23). Shin
et al (40) reported that
serum GDF-15 levels were independently correlated with
cardiovascular risk scores in patients with new-onset T2DM.
Yarsilikal Guleroglu et al (32) found that the serum GDF-15 level was
higher in GDM patients with obesity than in healthy pregnant women,
indicating that GDF-15 is not only associated with gestational
hypertension but may also be associated with obesity. Banerjee
et al (20) showed that
GDF-15 had a significant positive correlation with fasting serum
insulin, HOMA-IR and FPG at 24-28 weeks of gestation in subjects
with GDM. However, the relationship between GDF-15 and fasting
serum insulin, HOMA-IR and FPG in GDM patients at 20-24 weeks of
gestation has not been reported. The present study found that serum
GDF-15 was positively correlated with BMI, FPG, glycosylated
hemoglobin, fasting insulin and HOMA-IR at 20-24 weeks of gestation
in subjects with GDM. Compared with traditional indicators, GDF-15
has a superior predictive value for the transition from
microalbuminuria to macroalbuminuria in patients with T2DM
(40). In type 1 diabetes mellitus
patients with macroalbuminuria, elevated GDF-15 levels were closely
associated with decreased renal function and increased risk of
progression to end-stage renal disease (28,41).
In the present study, multivariate logistic regression analysis
revealed that GDF-15 was an independent risk factor for
microalbuminuria in women with gestational diabetes at 20-24 weeks
of gestation.
There were a number of limitations in this study.
First, this was a cross-sectional study; therefore, any change in
GDF-15 levels over time could not be documented. Second, this was a
single-centre study and the sample size was relatively small.
Third, because some patients did not deliver in Nantong Haimen
People's Hospital, pregnancy outcomes were not fully recorded.
Further research is needed to determine the exact role and
mechanisms of GDF-15 in the process of the disease.
In conclusion, serum GDF-15 levels in GDM patients
have good correlations with traditional indicators of diabetes
mellitus, such as FPG, glycosylated hemoglobin and fasting insulin
levels. Serum GDF-15 levels are also an independent risk factor for
microalbuminuria. The results suggested that GDF-15 could serve as
a biomarker of GDM at 20-24 weeks of gestation. Nevertheless,
whether GDF-15 plays a causal role in the pathogenesis of GDM or is
just a bystander requires further investigation.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Maternal and Child
Health alliance scientific research project of Nantong (grant no.
TFM202302).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG conceived and designed the present study. JS and
YG performed data collection. JL and LL analyzed the data. LL and
JS confirm the authenticity of all the raw data. YG wrote the
original draft, which LL reviewed and edited. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Nantong Haimen People's Hospital (approval no.
C20131102) and carried out in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2019. Diabetes Care. 42 (Suppl 1):S13–S28.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Skajaa GO, Fuglsang J, Knorr S, Møller N,
Ovesen P and Kampmann U: Changes in insulin sensitivity and insulin
secretion during pregnancy and post partum in women with
gestational diabetes. BMJ Open Diabetes Res Care.
8(e001728)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Prentki M and Nolan CJ: Islet beta cell
failure in type 2 diabetes. J Clin Invest. 116:1802–1812.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Catalano PM: Trying to understand
gestational diabetes. Diabet Med. 31:273–281. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khambule L and George JA: The role of
inflammation in the development of GDM and the use of markers of
inflammation in GDM screening. Adv Exp Med Biol. 1134:217–242.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bootcov MR, Bauskin AR, Valenzuela SM,
Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor
K, et al: MIC-1, a novel macrophage inhibitory cytokine, is a
divergent member of the TGF-beta superfamily. Proc Natl Acad Sci
USA. 94:11514–11519. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ding Q, Mracek T, Gonzalez-Muniesa P, Kos
K, Wilding J, Trayhurn P and Bing C: Identification of macrophage
inhibitory cytokine-1 in adipose tissue and its secretion as an
adipokine by human adipocytes. Endocrinology. 150:1688–1696.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brown DA, Breit SN, Buring J, Fairlie WD,
Bauskin AR, Liu T and Ridker PM: Concentration in plasma of
macrophage inhibitory cytokine-1 and risk of cardiovascular events
in women: A nested case-control study. Lancet. 359:2159–2163.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Marjono AB, Brown DA, Horton KE, Wallace
EM, Breit SN and Manuelpillai U: Macrophage inhibitory cytokine-1
in gestational tissues and maternal serum in normal and
pre-eclamptic pregnancy. Placenta. 24:100–106. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu J, Kimball TR, Lorenz JN, Brown DA,
Bauskin AR, Klevitsky R, Hewett TE, Breit SN and Molkentin JD:
GDF15/MIC-1 functions as a protective and antihypertrophic factor
released from the myocardium in association with SMAD protein
activation. Circ Res. 98:342–350. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ho JE, Mahajan A, Chen MH, Larson MG,
McCabe EL, Ghorbani A, Cheng S, Johnson AD, Lindgren CM, Kempf T,
et al: Clinical and genetic correlates of growth differentiation
factor 15 in the community. Clin Chem. 58:1582–1591.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mehta RS, Song M, Bezawada N, Wu K,
Garcia-Albeniz X, Morikawa T, Fuchs CS, Ogino S, Giovannucci EL and
Chan AT: A prospective study of macrophage inhibitory cytokine-1
(MIC-1/GDF15) and risk of colorectal cancer. J Natl Cancer Inst.
106(dju016)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiao QA, He Q, Zeng J and Xia X: GDF-15, a
future therapeutic target of glucolipid metabolic disorders and
cardiovascular disease. Biomed Pharmacother.
146(112582)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yalcin MM, Altinova AE, Akturk M, Gulbahar
O, Arslan E, Ors Sendogan D, Yetkin I and Toruner FB: GDF-15 and
hepcidin levels in nonanemic patients with impaired glucose
tolerance. J Diabetes Res. 2016(1240843)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schernthaner-Reiter MH, Kasses D,
Tugendsam C, Riedl M, Peric S, Prager G, Krebs M,
Promintzer-Schifferl M, Clodi M, Luger A and Vila G: Growth
differentiation factor 15 increases following oral glucose
ingestion: Effect of meal composition and obesity. Eur J
Endocrinol. 175:623–631. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Simm A, Nass N, Bartling B, Hofmann B,
Silber RE and Navarrete Santos A: Potential biomarkers of ageing.
Biol Chem. 389:257–265. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vila G, Riedl M, Anderwald C, Resl M,
Handisurya A, Clodi M, Prager G, Ludvik B, Krebs M and Luger A: The
relationship between insulin resistance and the cardiovascular
biomarker growth differentiation factor-15 in obese patients. Clin
Chem. 57:309–316. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hong JH, Chung HK, Park HY, Joung KH, Lee
JH, Jung JG, Kim KS, Kim HJ, Ku BJ and Shong M: GDF15 is a novel
biomarker for impaired fasting glucose. Diabetes Metab J.
38:472–479. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li E, Chen P, Lu J, Dai J, Yi J, Zhang S,
Jin H, Guo M, Wang H and Yu X: Serum growth differentiation factor
15 is closely associated with metabolic abnormalities in Chinese
pregnant women. J Diabetes Investig. 12:1501–1507. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Banerjee S, Bhattacharjee R, Sur A,
Adhikary P and Chowdhury S: A study of serum growth differentiation
factor 15 in Indian women with and without gestational diabetes
mellitus in the third trimester of pregnancy and its association
with pro-inflammatory markers and glucose metabolism. Diabetol Int.
12:254–259. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Immanuel J and Simmons D: Screening and
treatment for early-onset gestational diabetes mellitus: A
systematic review and meta-analysis. Curr Diab Rep.
17(115)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
No authors listed. Diagnostic criteria and
classification of hyperglycaemia first detected in pregnancy: A
World Health Organization guideline. Diabetes Res Clin Pract.
103:341–363. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chi C, Loy SL, Chan SY, Choong C, Cai S,
Soh SE, Tan KH, Yap F, Gluckman PD, Godfrey KM, et al: Impact of
adopting the 2013 World Health Organization criteria for diagnosis
of gestational diabetes in a multi-ethnic Asian cohort: A
prospective study. BMC Pregnancy Childbirth. 18(69)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
de Jong PE, Gansevoort RT and Bakker SJL:
Macroalbuminuria and microalbuminuria: Do both predict renal and
cardiovascular events with similar strength? J Nephrol. 20:375–380.
2007.PubMed/NCBI
|
|
25
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2018. Diabetes Care. 41 (Suppl 1):S13–S27.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Görkem Ü, Küçükler FK, Toğrul C and Güngör
T: Are adipokines associated with gestational diabetes mellitus? J
Turk Ger Gynecol Assoc. 17:186–190. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reece EA, Leguizamón G and Wiznitzer A:
Gestational diabetes: The need for a common ground. Lancet.
373:1789–1797. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bellamy L, Casas JP, Hingorani AD and
Williams D: Type 2 diabetes mellitus after gestational diabetes: A
systematic review and meta-analysis. Lancet. 373:1773–1779.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carr DB, Utzschneider KM, Hull RL, Tong J,
Wallace TM, Kodama K, Shofer JB, Heckbert SR, Boyko EJ, Fujimoto WY
and Kahn SE: Gestational diabetes mellitus increases the risk of
cardiovascular disease in women with a family history of type 2
diabetes. Diabetes Care. 29:2078–2083. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gao C, Sun X, Lu L, Liu F and Yuan J:
Prevalence of gestational diabetes mellitus in mainland China: A
systematic review and meta-analysis. J Diabetes Investig.
10:154–162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Burlina S, Dalfrà MG, Chilelli NC and
Lapolla A: Gestational diabetes mellitus and future cardiovascular
risk: An update. Int J Endocrinol. 2016(2070926)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yarsilikal Guleroglu F, Selvi E, Turan
Bakirci I, Bafalı O, Argun Atalmis H, Yasti Dayan M, Balkan Ozmen
A, Yurtcu N, Seker Atas B, Ozdemir Anayurt E and Cetin A: Clinical
value of serum BMP-4, BMP-2, GDF-15, MMP-9, GP39 levels in pregnant
women with obesity and the related comorbidities diabetes mellitus
and gestational hypertension. Z Geburtshilfe Neonatol. 227:42–50.
2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Berezin AE: Diabetes mellitus related
biomarker: The predictive role of growth-differentiation factor-15.
Diabetes Metab Syndr. 10 (1 Suppl 1):S154–S157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sugulle M, Dechend R, Herse F,
Weedon-Fekjaer MS, Johnsen GM, Brosnihan KB, Anton L, Luft FC,
Wollert KC, Kempf T and Staff AC: Circulating and placental
growth-differentiation factor 15 in preeclampsia and in pregnancy
complicated by diabetes mellitus. Hypertension. 54:106–112.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yakut K, Öcal DF, Öztürk FH, Öztürk M,
Oğuz Y, Sınacı S and Çağlar T: Is GDF-15 level associated with
gestational diabetes mellitus and adverse perinatal outcomes?
Taiwan J Obstet Gynecol. 60:221–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lu YC, Liu SL, Zhang YS, Liang F, Zhu XY,
Xiao Y, Wang J, Ding C, Banerjee S, Yin JY and Ma QP: Association
between growth differentiation factor 15 levels and gestational
diabetes mellitus: A combined analysis. Front Endocrinol
(Lausanne). 14(1084896)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Andersson-Hall U, Joelsson L, Svedin P,
Mallard C and Holmäng A: Growth-differentiation-factor 15 levels in
obese and healthy pregnancies: Relation to insulin resistance and
insulin secretory function. Clin Endocrinol (Oxf). 95:92–100.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Andersson-Hall U, Svedin P, Mallard C,
Blennow K, Zetterberg H and Holmäng A: Growth differentiation
factor 15 increases in both cerebrospinal fluid and serum during
pregnancy. PLoS One. 16(e0248980)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Berkowitz GS, Roman SH, Lapinski RH and
Alvarez M: Maternal characteristics, neonatal outcome, and the time
of diagnosis of gestational diabetes. Am J Obstet Gynecol.
167:976–982. 1992.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shin MY, Kim JM, Kang YE, Kim MK, Joung
KH, Lee JH, Kim KS, Kim HJ, Ku BJ and Shong M: Association between
growth differentiation factor 15 (GDF15) and cardiovascular risk in
patients with newly diagnosed type 2 diabetes mellitus. J Korean
Med Sci. 31:1413–1418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hellemons ME, Mazagova M, Gansevoort RT,
Henning RH, de Zeeuw D, Bakker SJ, Lambers-Heerspink HJ and Deelman
LE: Growth-differentiation factor 15 predicts worsening of
albuminuria in patients with type 2 diabetes. Diabetes Care.
35:2340–2346. 2012.PubMed/NCBI View Article : Google Scholar
|