Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors worldwide, ranking third in morbidity and second
in mortality (1). Approximately
25% of patients with CRC also present with distant metastasis upon
initial diagnosis (2). Without
treatment, the median survival time of patients with advanced CRC
metastasis is 5-6 months (3). With
the in-depth study of the formation, development and treatment of
CRC at the molecular and genetic levels, targeted therapy with
specific selection of binding oncogenic sites has become a research
hot spot (4,5). Therefore, it is necessary to explore
the pathogenesis of CRC.
Neurexophilin 4 (NXPH4) is a synaptic secretory
protein belonging to the NXPH family. Recent studies have revealed
that NXPH4 is highly expressed in bladder cancer (6), liver cancer (7), breast cancer (8) and several other types of cancer. A
previous study showed that NXPH4 knockdown can inhibit cell
proliferation and migration, leading to significant cell cycle
stagnation at the S1 stage in non-small cell lung cancer cells
(9). However, to the best of our
knowledge no studies on NXPH4 in CRC have been reported so far.
Therefore, the aim of the present study was to
preliminarily explore the occurrence and development mechanism of
CRC by studying the mechanism of NXPH4 in CRC. We hope that the
present study can provide a theoretical basis for the clinical
targeted treatment of CRC.
Materials and methods
Databases. The ENCORI database predicted the
expression of NXPH4 in patients with CRC (10). The ENCORI database predicted the
association between high NXPH4 expression and overall survival in
patients with CRC (the median 50% value was used as the cutoff for
high and low) (11). The JASPAR
database predicted the binding sites of transcription factor
forkhead box protein K1 (FOXK1) and NXPH4 promoter (12).
Cell culture. The Caco2, LoVo, SW480 and
HCT116 cells and HIEC normal intestinal epithelial cell lines were
obtained from the American Type Culture Collection and cultured at
37˚C in a 5% CO2 with DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.). RNA concentration was
quantified by spectrophotometry (GeneQuant; GE Healthcare) at 260
nm. Next, cDNA was produced by RT using SuperScript™ IV VILO™
Master Mix (Thermo Fisher Scientific, Inc.) at 37˚C for 60 min and
then at 95˚C for 5 min. The miScript HiSpec Buffer (Qiagen AB) and
ABI 7500 real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) were used for qPCR. The PCR program began with an
initial denaturation at 95˚C for 2 min, followed by 40
amplification reaction cycles (95˚C for 30 sec, 55˚C for 30 sec and
72˚C for 30 sec), with a final extension at 72˚C for 3 min. The
data were processed using the 2-ΔΔCq method (13). GAPDH was used as an endogenous
control. NXPH4 forward, 5'-TGTCGAGTTCGGAGGAGTCT-3' and reverse,
5'-GCTATCCGAAGTAGGGGTGC-3'; FOXK1 forward,
5'-GCGCATTCTTCCTGTTAGCG-3' and reverse, 5'-GGCTACCGGTTCCAAGACAA-3';
and GAPDH forward, 5'-CATGAGAAGTATGACAACAGCCT-3' and reverse,
5'-AGTCCTTCCACGATACCAAAGT-3'.
Western blotting
Cells were lysed with RIPA Lysis Buffer (Thermo
Fisher Scientific, Inc.). A Pierce BCA protein assay kit was used
to measure protein concentration (Thermo Fisher Scientific, Inc.).
A total of 30 µg protein (per lane) was separated using 10%
SDS-PAGE and transferred onto a PVDF membrane (Merck KGaA). After
blocking the membranes with 5% bovine serum albumin (Applygen
Technologies, Inc.) at room temperature for 1.5 h, the protein
bands were incubated overnight at 4˚C with the primary antibodies
NXPH4 (cat. no. ab74999), E-cadherin (cat. no. ab40772), N-cadherin
(cat. no. ab76011), Vimentin (cat. no. ab92547), glucose
transporter 1 (GLUT1; cat. no. ab115730), hexokinase 2 (HK2; cat.
no. ab209847), pyruvate kinase isozymes M1/M2 (PKM2; cat. no.
ab85555), FOXK1 (cat. no. ab309510), and GAPDH (cat. no. ab9485)
(all 1:1,000; Abcam) On the second day, the secondary antibodies
were incubated for 1 h at room temperature and the membranes were
then developed with Clarity Western ECL substrate (Bio-Rad
Laboratories, Inc.), visualized with a ChemiDoc™ MP Imaging System
(Bio-Rad Laboratories, Inc.) and analyzed using Quantity One 4.62
analysis software (Bio-Rad Laboratories, Inc.).
Cell transfection
The expression of NXPH4 was knocked down by short
hairpin (sh)RNAs (sh-NXPH4#1 and sh-NXPH4#2) constructed in
lentiviral plasmids (pCLenti-U6-shRNA-CMV-EGFP-WPRE) using a 2nd
generation system. The Trpv1 pGHH1 plasmid and scrambled control
shRNA (sh-NC; 2 µg) were synthesized by OBiO Technology (Shanghai)
Corp., Ltd. HCT116 cells were transducted with the lentivirus at a
multiplicity of infection of 1 using 50 nM
Lipofectamine® 2000 transfection reagent, according to
the manufacturer's instructions. Cells were transfected with the
pcDNA3.1 vector (GeneChem, Inc.) containing full-length FOXK1
(Oe-FOXK1; 2 µg) and control (Oe-NC; 2 µg) plasmids at 37˚C for 48
h using Lipofectamine 2000 transfection reagent, according to the
manufacturer's instructions. At 24 h after transfection, cells were
selected with puromycin (5 µg/ml) for 2 days and maintained with
0.25 µg/ml puromycin. After 48 h, RT-qPCR and western blotting were
performed to detect the transfection efficiency. The target
sequence of sh-NXPH4#1 is 5'-AGAGTCACGCGCTTTCAATTG-3' and
sh-NXPH4#2 is 5'-AGAAGGTGTGCCCAGACTATA-3'; shRNA-NC,
5'-CAACAAGATGAAGAGCACCAA-3'
Cell Counting Kit (CCK)-8 assay
Transfected cell suspensions were seeded on 96-well
plates at a density of 1,500 cells/well for 24, 48 and 72 h. Next,
10 µl CCK-8 solution (MedChemExpress) was added to each well. After
2 h of incubation, a microplate analyzer was used to read the
absorbance of each well at 450 nm.
5-Ethynyl-2'-deoxyuridine (EdU)
incorporation assay
HCT116 cells were seeded onto 96-well plates at a
density of 5x103 per well. The cells were incubated for
an additional 2 h in medium containing 50 µM EdU (Guangzhou RiboBio
Co., Ltd.). Cells were then fixed and permeabilized with PBS
containing 4% paraformaldehyde for 15 min at room temperature and
0.5% Triton X-100 at room temperature for 10 min, followed by
incubation with 1x Apollo reaction cocktail (100 µl/well) for 30
min at room temperature. DNA was incubated with
4',6-diamidino-2-phenylindole stain (100 µl/well) for 30 min at
room temperature and visualized using an inverted fluorescence
microscope (Leica). Captured images were processed and analyzed
with ImageJ software (version 1.8.0; National Institutes of
Health).
Apoptosis assay
Apoptosis was detected using an annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (Sangon Biotech Co., Ltd.), according to the
manufacturer's instructions. In brief, HCT116 cells were harvested,
incubated with Annexin V-FITC for 15 min at room temperature and
re-stained with PI in the dark for 15 min at room temperature.
Apoptotic cells were measured using a flow cytometer (BD
Biosciences) and analyzed with FlowJo software (version X; FlowJo
LLC).
Cell migration and invasion
assays
HCT116 cells (6x104 cells) were plated in
the upper chambers that had been coated with Matrigel (BD
Biosciences) for 1 h at 37˚C to estimate tumor invasion, and the
upper chambers without Matrigel were used to assess tumor cell
migration, respectively. For cell invasion, the Transwell inserts
(8-µm pore-size; Corning, Inc.) were put into 24-well plates. Cells
(5x104) in serum-free DMEM were seeded into the upper
chambers while the lower chambers were filled with DMEM with 10%
FBS. After 24 h at 37˚C, the upper chambers with the residual cells
were removed and the cells under the surface were stained with 0.5%
crystal violet for 10 min at room temperature and then fixed with
70% ethanol for 30 min at room temperature. Cells were counted with
an inverted light microscope (Olympus) using ImageJ software
(version 1.8.0; National Institutes of Health).
For cell migration, HCT116 cells were seeded in
six-well plates at the density of 5x105 cells/well. When
the cells were grown to 90-100% confluence, artificial wounds were
gently made using a micropipette tip, and confluent monolayers for
wounding were yielded. The original culture medium was replaced
with serum-free DMEM supplemented with 30 µg/ml mitomycin. Cells in
the scratched area were imaged at 0 and 24 h using a light
microscope. The relative migrating distance was the ratio of
migration distance at 24 h and the distance measured at 0 h.
Oxygen consumption rate (OCR) and
extracellular acidification rate (ECAR) detection
Cellular ECAR and OCR were measured with a Seahorse
Bioscience XF96 Extracellular Flux Analyzer. In brief,
2x104 cells were seeded into 96-well cell culture XF
microplates and incubated overnight at 37˚C for further testing,
according to the manufacturer's instructions. The ECAR and OCR
values were calculated after normalization to the total cell
number.
Glucose uptake and lactate product
assay
The relative glucose uptake was assessed by
measuring the glucose concentration in the medium using Glucose
Assay kit (cat. no. CBA086; Merck KGaA), and the lactate production
in the medium was detected using a Lactate Assay kit (cat. no.
BC2235), according to the manufacturer's instructions (Beijing
Solarbio Science & Technology Co., Ltd).
Dual-luciferase reporter assay
Luciferase assays were performed using 96-well
plates. The site of NXPH4 binding of FOXK1 that mutated (MUT-NXPH4)
and the site of wild-type NXPH4 (WT-NXPH4) were amplified by
RT-qPCR and cloned into the pMIR-REPORT™ vector (Ambion; Thermo
Fisher Scientific, Inc.). The constructs Oe-FOXK1 and Oe-NC were
co-transfected into cells with Lipofectamine 2000. Following 48 h
of plasmid co-transfection, the luciferase signals were measured
using 50 µl Stop&Glo Reagent, according to the manufacturer's
instructions [OBiO Technology (Shanghai) Corp., Ltd.]. The
Renilla luciferase levels were normalized to firefly
luciferase levels.
Chromatin immunoprecipitation assay
(ChIP)
ChIP assays were performed with a EZ-Magna ChIP kit
(cat. no. 17-408; Merck KGaA), according to the manufacturer's
instructions. In brief, the cells were lysed in SDS Lysis Buffer in
an ice bath for 10 min. After sonicating the samples (20 kHz; 4
pulses of 12 sec each, followed by 30 sec rest on ice between each
pulse), 1.8 ml ChIP dilution buffer was added. Subsequently, 50 µg
protein-A-agarose was added for 30 min at 4˚C. After centrifugation
at 1,000 x g for 3 min at 4˚C, the supernatant (100 µg) was removed
and incubated with 3 µg indicated antibody FOXK1 (1:200; cat. no.
ab309510; Abcam) and 50 µg protein-A-agarose overnight at 4˚C.
Isotype-matched IgG (1:100; cat. no. ab172730; Abcam) was used as
the negative control. The precipitate was collected after
incubating at 4˚C for 6 h and centrifugated at 1,000 x g at 4˚C for
3 min. Following washing, chromatin from beads was eluted with
gentle vortexing (1,200 rpm) at 65˚C for 30 min. The DNA was then
purified using an RT-qPCR Purification Kit (Qiagen AB).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software, Inc.). All data are expressed as the
mean ± SD (unless otherwise shown). Multiple group comparisons were
performed using ANOVA followed by Tukey's post-hoc test. P<0.05
was considered to indicate a statistically significant difference.
The χ2 test and unpaired Student's t-test were used to
examine the connection between NXPH4 expression and clinical
features. All experiments were repeated independently three
times.
Results
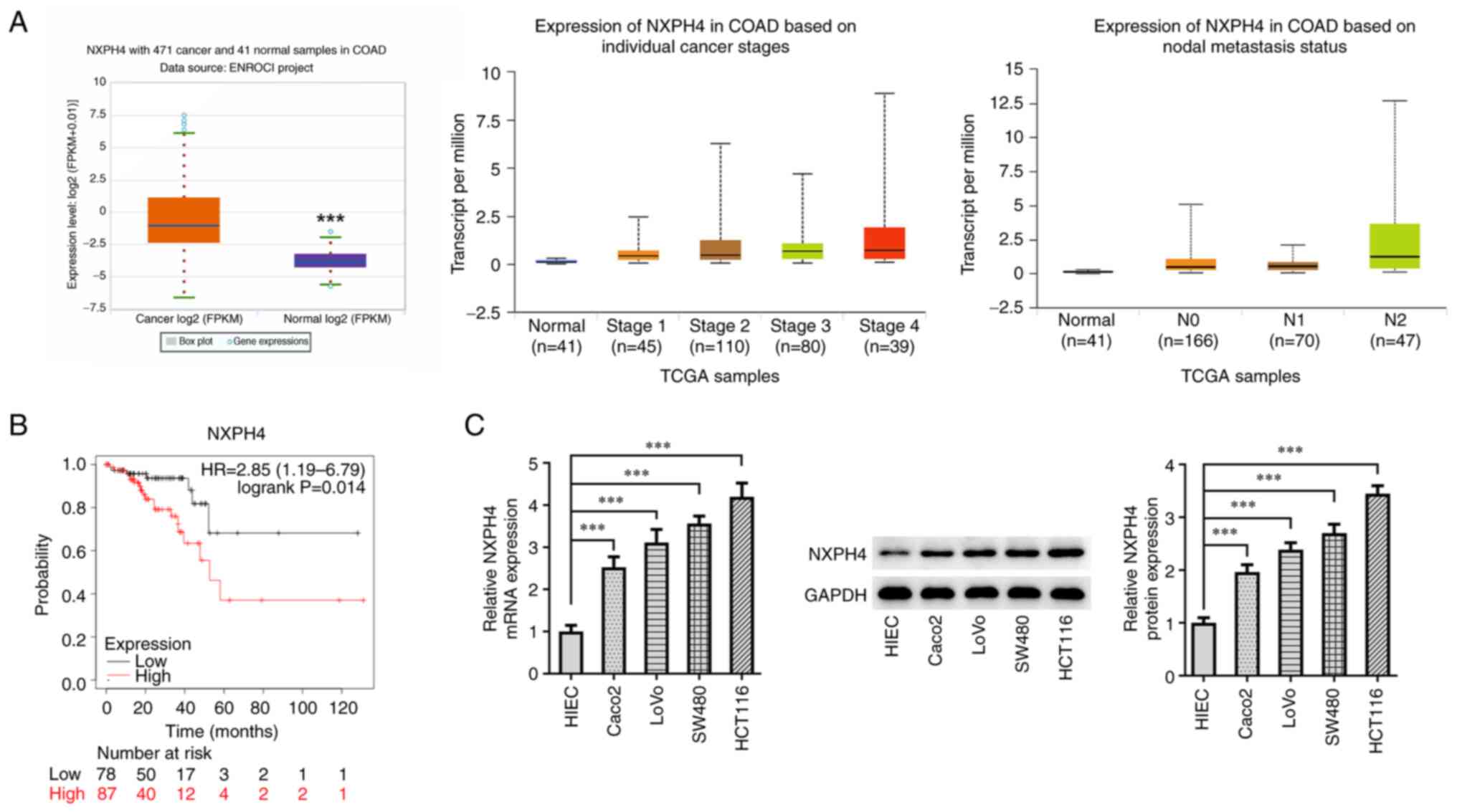
NXPH4 is highly expressed in CRC
The ENCORI database predicted a high expression of
NXPH4 in patients with CRC compared with control patients, and
higher NXPH4 expression was strongly associated with tumor stages
and nodal metastasis status (Fig.
1A). The Kaplan-Meier Plotter database predicted that high
NXPH4 expression was significantly associated with low overall
survival in patients with CRC (Fig.
1B). Clinical data on 590 patients with CRC were gathered from
the TCGA database for the present investigation (Table I). According to the median mRNA
expression levels of NXPH4, patients with CRC were split into low
and high expression groups. The relationship between NXPH4
expression and several clinical features of patients with CRC such
as age and sex were investigated. It was revealed that there was no
association between high NXPH4 mRNA expression and age and sex
(P>0.05). RT-qPCR and western blotting for NXPH4 expression in
CRC cells showed that, as compared with HIEC cells, NXPH4
expression in CRC cell lines was significantly increased (Fig. 1C). Among them, the increase in
HCT116 cells was the most significant, so the HCT116 cell line was
selected for follow-up experiments.
 | Table IAssociation of NXPH4 expression and
clinicopathological parameters in patients with colorectal
cancer. |
Table I
Association of NXPH4 expression and
clinicopathological parameters in patients with colorectal
cancer.
| | NXPH4 expression in
the TCGA database | |
|---|
| Characteristics | Low (n=296) | High (n=294) | P-value |
|---|
| Sex, n (%) | | | 0.218 |
|
Female | 132 (22.4) | 147 (24.9) | |
|
Male | 164 (27.8) | 147 (24.9) | |
| Age, n (%) | | | 0.264 |
|
≤60 | 87 (14.7) | 100 (16.9) | |
|
>60 | 209 (35.4) | 194 (32.9) | |
| Age, median
(IQR) | 67 (59-76) | 66 (56-76) | 0.428 |
NXPH4 interference inhibits CRC cell
proliferation and induces their apoptosis
NXPH4 expression in HCT116 cells was then knocked
down, and transfection efficacy was detected using RT-qPCR and
western blotting. It was discovered that NXPH4 expression was
prominently decreased following transfection of sh-NXPH4#1/2
(Fig. 2A). sh-NXPH4#2 was selected
for follow-up experiments, and cells were divided into the control,
sh-NC and sh-NXPH4 groups. CCK-8 and EdU staining were used to
detect cell viability and proliferation. The results showed that,
as compared with the sh-NC group, cell viability in the sh-NXPH4
group was significantly decreased at 48 and 72 h, as was cell
proliferation (Fig. 2B and
C). Flow cytometry showed that
NXPH4 overexpression significantly increased apoptosis compared
with sh-NC (Fig. 2D).
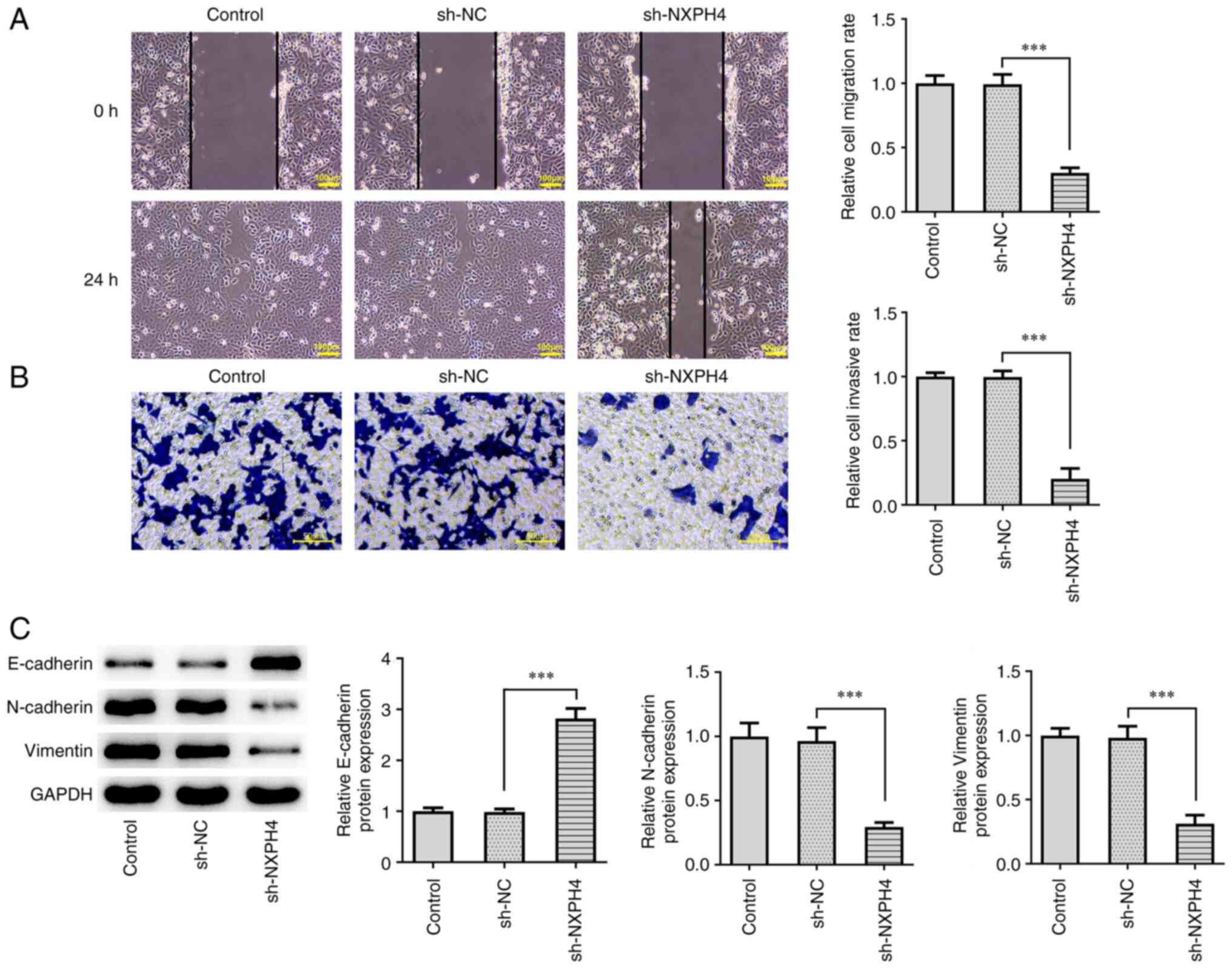
NXPH4 interference inhibits CRC
metastasis and glycolysis
Wound healing and Transwell assays were performed to
evaluate cell migration and invasion. The results showed that,
following NXPH4 interference, the invasion and migration ability of
cells were significantly decreased compared with the sh-NC group
(Fig. 3A and B). Western blotting examined the
expression of epithelial-mesenchymal transition (EMT)-related
proteins and the results indicated that NXPH4 interference elevated
E-cadherin expression, whereas it decreased N-cadherin and Vimentin
expression (Fig. 3C). XF96
extracellular flux analyzer detected ECAR and OCR, and the results
showed that, as compared with sh-NC, ECAR was significantly
decreased and OCR was significantly increased in the sh-NXPH4 group
(Fig. 4A and B). The results of glucose uptake and
lactate product assay revealed a significant decrease in lactic
acid production and glucose consumption in the sh-NXPH4 group, as
compared with the sh-NC group (Fig.
4C and D). Western blotting
for glucose uptake-related protein expression showed that GLUT1,
HK2 and PKM2 expression was significantly decreased following NXPH4
interference (Fig. 4E). The
aforementioned results showed that NXPH4 interference inhibited CRC
metastasis and glycolysis.
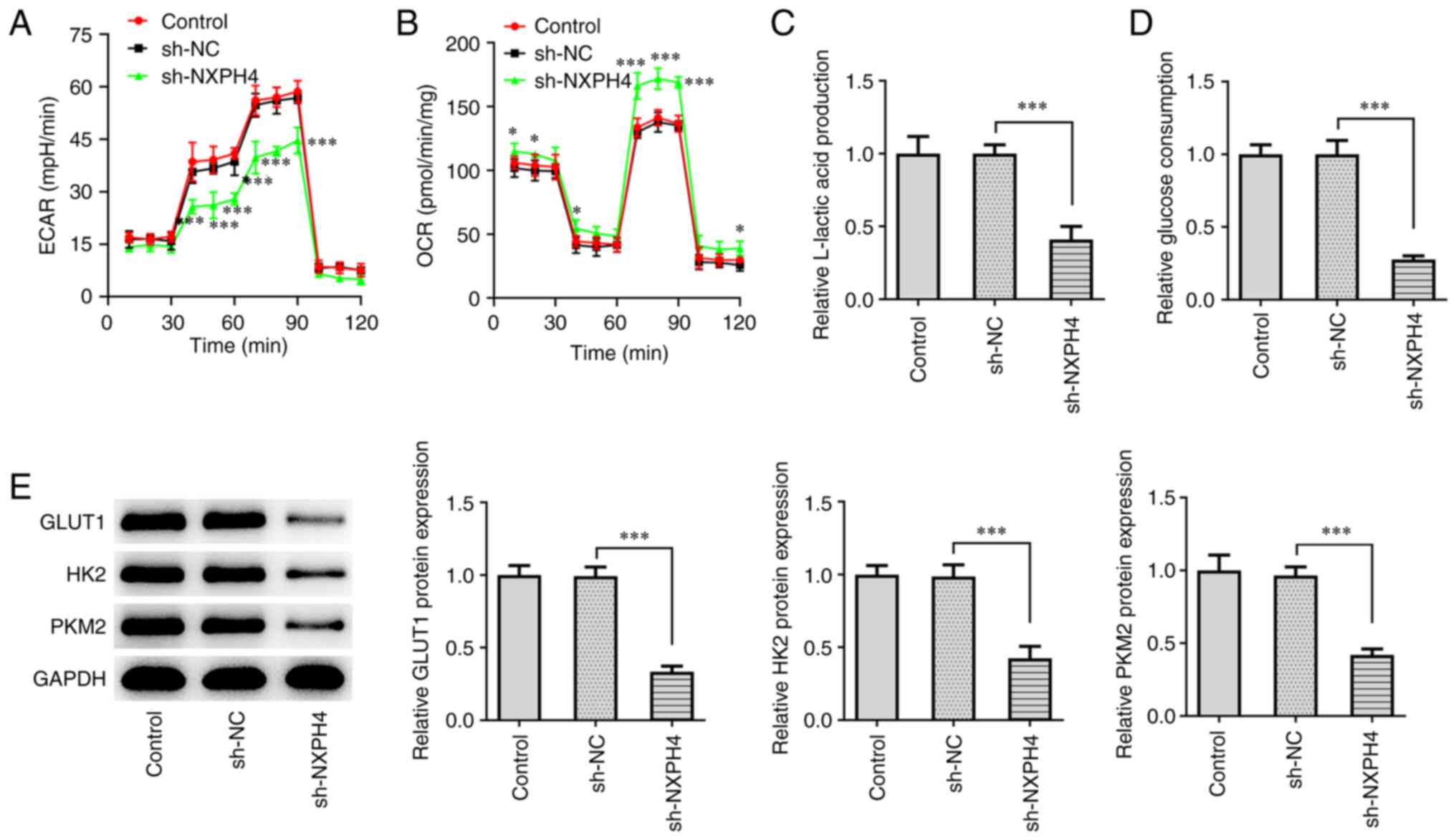 | Figure 4NXPH4 interference inhibits glycolysis
of CRC cells. XF96 extracellular flux analyzer detected (A) ECAR
and (B) OCR. Glucose uptake and lactate product assay were used to
detect (C) lactic acid production and (D) glucose consumption. (E)
Western blotting was used to detect GLUT1, HK2 and PKM2 protein
expression. *P<0.05 and ***P<0.001.
NXPH4, neurexophilin 4; CRC, colorectal cancer; ECAR, extracellular
acidification rate; OCR, oxygen consumption rate; GLUT1, glucose
transporter 1; HK2, hexokinase 2; PKM2, pyruvate kinase isozymes
M1/M2; sh, short hairpin; NC, negative control. |
FOXK1 promotes NXPH4
transcription
The JASPAR database predicted that the transcription
factor FOXK1 and NXPH4 promoter had binding sites (Fig. 5A). RT-qPCR and western blotting
results showed that FOXK1 expression was significantly increased in
CRC cells compared with HIEC cells (Fig. 5B). FOXK1 overexpression plasmid was
constructed, and transfection efficacy was detected by RT-qPCR and
western blotting. FOXK1 expression was increased following
transfection of Oe-FOXK1 (Fig.
5C). Luciferase reporter assay demonstrated that FOXK1
overexpression markedly enhanced the luciferase activity of
NXPH4-WT but not NXPH4-MUT (Fig.
5D). ChIP experiments confirmed that NXPH4 promoter was
abundant in FOXK1 antibody (Fig.
5E), implying the binding relationship between NXPH4 and FOXK1.
Subsequently, following FOXK1 overexpression, RT-qPCR and western
blotting were used to detect the expression of NXPH4 to test its
effect, and NXPH4 expression was discovered to be increasing when
FOXK1 was overexpressed (Fig.
5F).
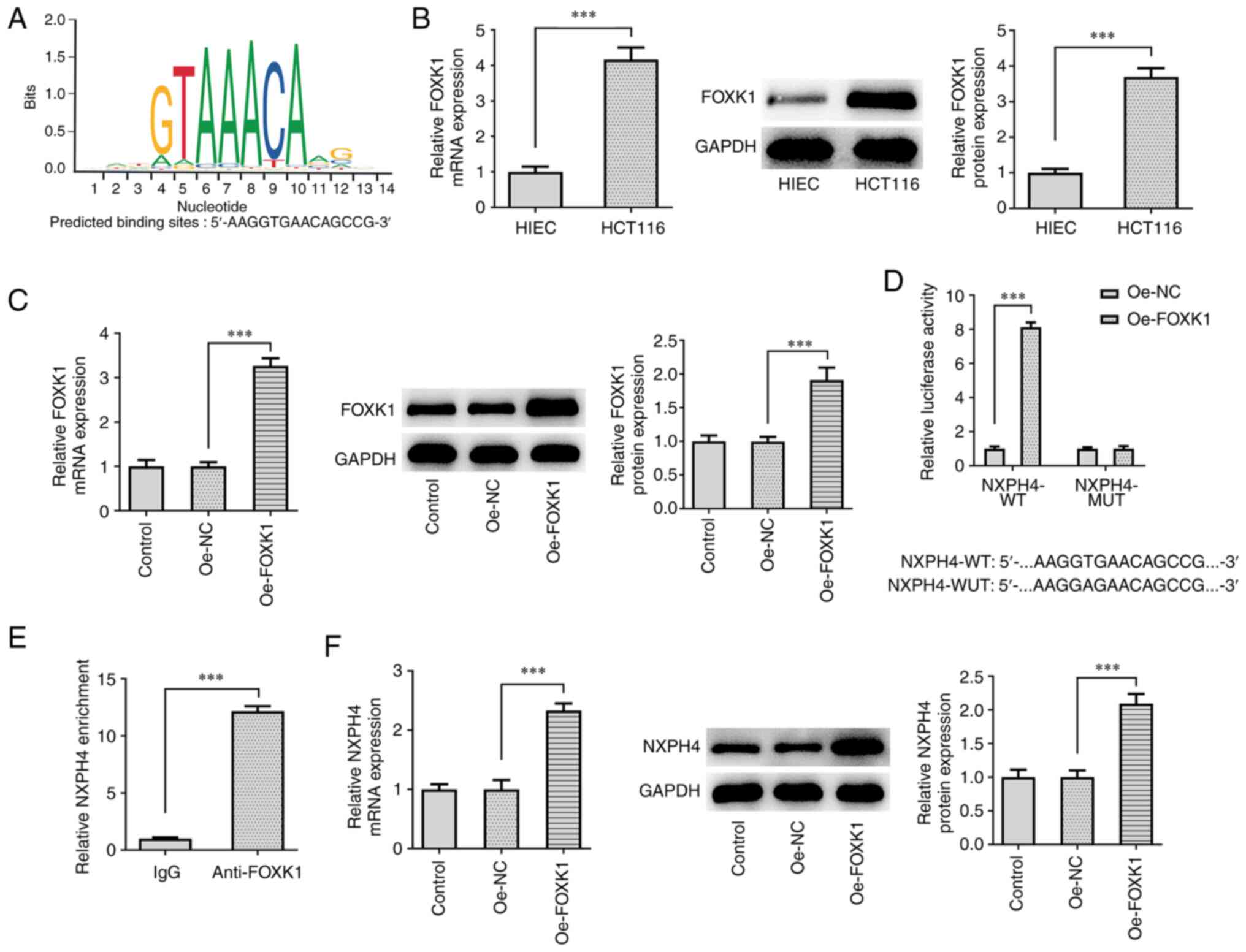 | Figure 5FOXK1 promotes NXPH4 transcription.
(A) JASPAR database predicted that the transcription factor FOXK1
and NXPH4 promoter had binding sites. (B) RT-qPCR and western
blotting were used to detect FOXK1 expression in CRC cells. (C)
FOXK1 overexpression plasmid was constructed, and transfection
efficacy was detected by RT-qPCR and western blotting. (D)
Luciferase and (E) ChIP experiments confirmed the binding
relationship between NXPH4 and FOXK1. (F) Following FOXK1
overexpression, RT-qPCR and western blotting were performed to
detect the expression of NXPH4 to test its effect.
***P<0.001. FOXK1, forkhead box protein K1; NXPH4,
neurexophilin 4; RT-qPCR, reverse transcription-quantitative PCR;
CRC, colorectal cancer; ChIP, chromatin immunoprecipitation assay;
sh, short hairpin; NC, negative control; WT, wild-type; MUT,
mutant. |
Overexpression of FOXK1 reverses the
effect of NXPH4 interference on CRC cells
Cells were grouped into the control, sh-NXPH4,
sh-NXPH4 + Oe-NC and sh-NXPH4 + Oe-FOXK1 groups. The expression of
NXPH4 was detected by RT-qPCR and western blotting, and it was
noted that the depleted NXPH4 expression level due to knockdown of
NXPH4 was markedly increased after overexpression of FOXK1
(Fig. 6). CCK-8 and EdU staining
results showed that cell viability and proliferation were
significantly increased in sh-NXPH4 + Oe-FOXK1, as compared with
sh-NXPH4 + Oe-NC group (Fig. 7A
and B). Flow cytometry showed that
the apoptosis in the sh-NXPH4 + Oe-FOXK1 group was significantly
decreased compared with that in the sh-NXPH4 + Oe-NC group
(Fig. 7C). Cell migration and
apoptosis results showed that cell migration and invasion were
significantly increased in the sh-NXPH4 + Oe-FOXK1 group, as
compared with the sh-NXPH4 + Oe-NC group (Fig. 7D and E). Western blotting results of EMT
markers showed that FOXK1 overexpression could significantly
reverse the effect of NXPH4 interference on EMT-related proteins
(Fig. 7F). Next,
glycolysis-related indicators were detected, and the results showed
that, as compared with the sh-NXPH4 + Oe-NC group, the sh-NXPH4 +
Oe-FOXK1 group exhibited increased ECAR, decreased OCR, increased
lactate level, increased glucose consumption and increased
expression of glycolysis-related proteins GLUT1, HK2 and PKM2
(Fig. 8A-E).
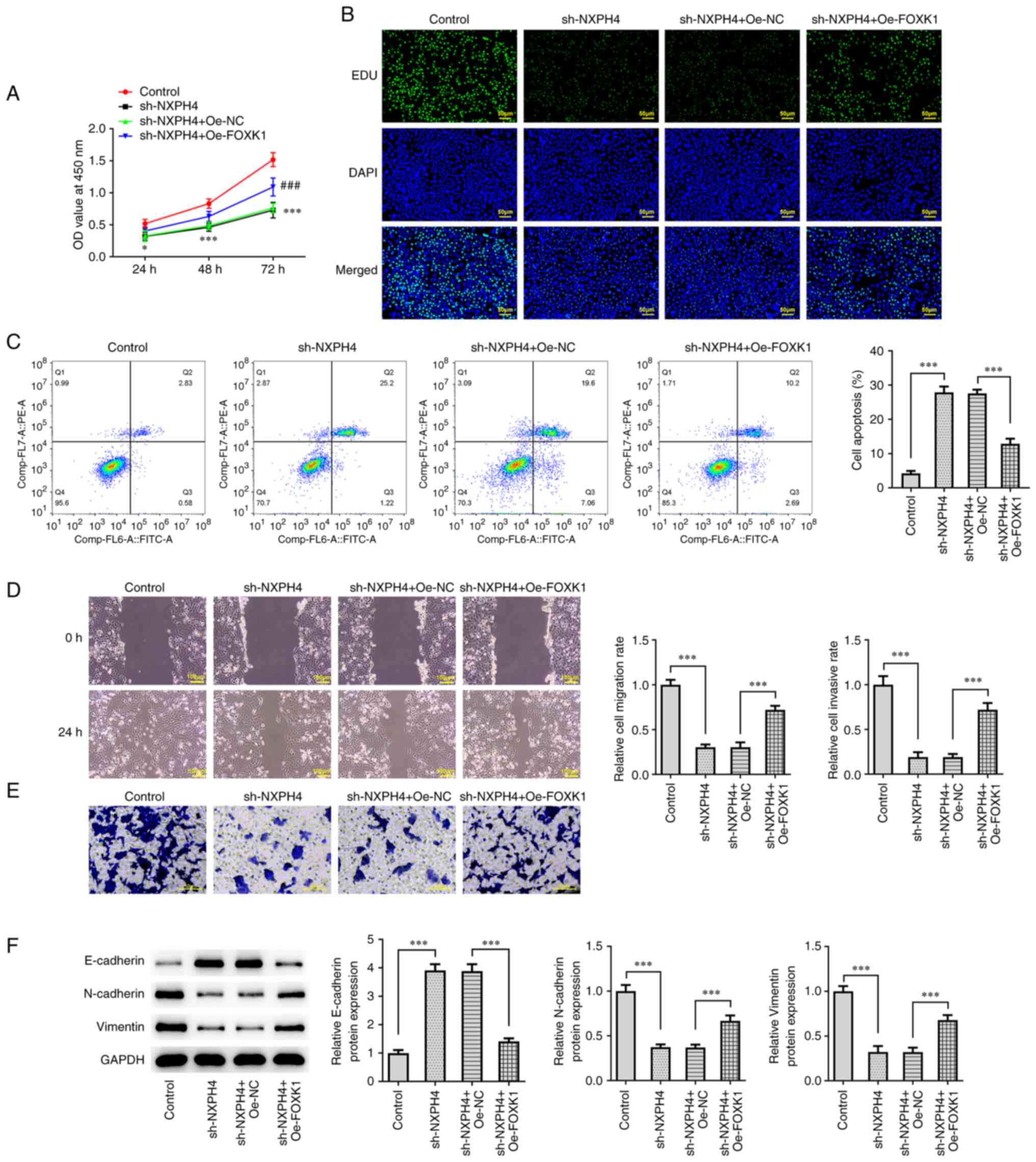 | Figure 7Overexpression of FOXK1 reverses the
effect of NXPH4 interference on CRC cells. (A) Cell-Counting Kit-8
assay and (B) EdU staining were performed to detect cell viability
and proliferation (scale bar, 50 µm). (C) Flow cytometry was
performed to detect apoptosis. (D) Wound healing (scale bar, 100
µm) and (E) Transwell assays (scale bar, 50 µm) were performed for
cell migration and invasion. (F) Western blotting was used to
detect epithelial-mesenchymal transition-related proteins.
*P<0.05, ***P<0.001 and ###P<0.001
vs sh.NXPH4 + Oe-NC. FOXK1, forkhead box protein K1; NXPH4,
neurexophilin 4; CRC, colorectal cancer; EdU,
5-ethynyl-2'-deoxyuridine; sh, short hairpin; NC, negative control;
Oe, overexpression. |
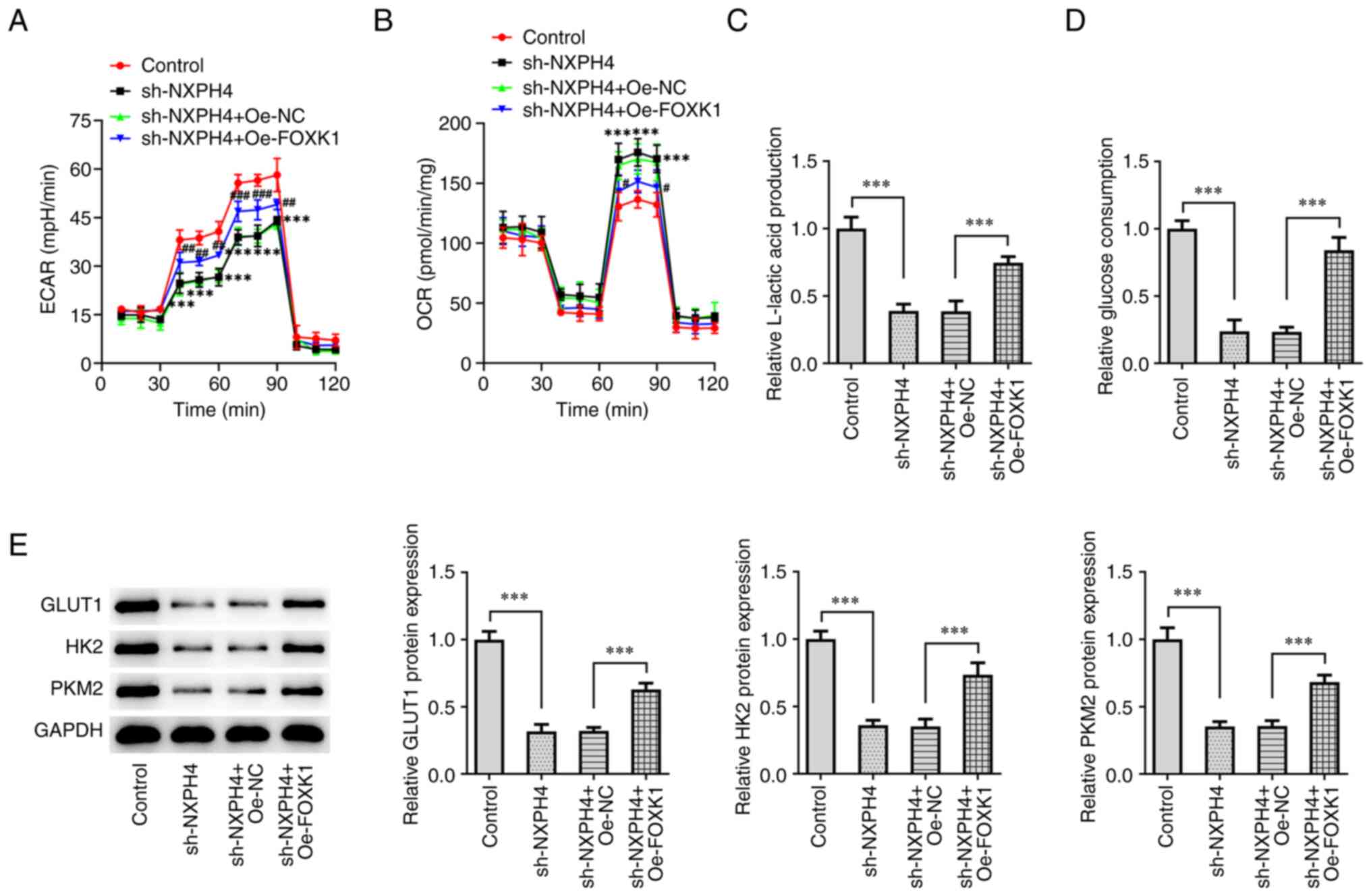 | Figure 8FOXK1 overexpression reverses the
effect of NXPH4 interference on CRC cells. XF96 extracellular flux
analyzer detected (A) ECAR and (B) OCR. ***P<0.001
compared with Control; #P<0.05,
##P<0.01 and ###P<0.001 compared with
sh-NXPH4 + Oe-NC. Glucose uptake and lactate product assay were
performed to detect (C) lactic acid production and (D) glucose
consumption. (E) Western blotting was performed to detect GLUT1,
HK2 and PKM2 proteins. ***P<0.001. FOXK1, forkhead
box protein K1; NXPH4, neurexophilin 4; CRC, colorectal cancer;
ECAR, extracellular acidification rate; OCR, oxygen consumption
rate; GLUT1, glucose transporter 1; HK2, hexokinase 2; PKM2,
pyruvate kinase isozymes M1/M2; sh, short hairpin; NC, negative
control; Oe, overexpression. |
Discussion
CRC can seriously harm human health and has a high
incidence and rate of mortality (14). Traditional treatments for CRC
include surgical therapy and chemoradiotherapy. Currently, the rise
of molecular targeted therapy and immunotherapy has provided new
prospects for the treatment of CRC, but the prognosis of patients
with advanced CRC remains poor. In addition, traditional tumor
markers, such as carcinoembryonic antigen and carbohydrate antigen
199, have poor sensitivity and specificity in predicting the
occurrence of CRC (15). In recent
years, an increasing number of studies have focused on finding new
and more sensitive tumor markers for the diagnosis and treatment of
CRC (16,17).
NXPH4 can be used as a biomarker for the early
diagnosis of hepatocellular carcinoma (18). The upregulation of NXPH4 is
associated with the poor prognosis of hepatocyte carcinoma and
immune cell invasion. NXPH4 knockdown significantly inhibits the
proliferation, migration and invasion of JHH7 and SNU182 cells
(7). In addition, NXPH4 promotes
the proliferation, migration and invasion of bladder cancer cells
by maintaining the stability of NDUFA4 mitochondrial complex
associated-like-2, thereby activating reactive oxygen species and
glycolysis (19). Using relevant
websites, the present study predicted that NXPH4 expression was
significantly increased in patients with CRC, and that high NXPH4
expression was significantly associated with a lower overall
survival in patients with CRC. Therefore, the current study
speculated that NXPH4 also plays a regulatory role in CRC.
Therefore, the regulatory mechanism of NXPH4 was investigated in
colon cancer cells. It was revealed that NXPH4 is also abnormally
expressed in CRC cells, and NXPH4 knockdown can significantly
inhibit CRC cell proliferation, induce apoptosis and inhibit
metastasis and glycolysis.
Next, the regulatory mechanism of NXPH4 in CRC was
discussed. The JASPAR database predicted that the promoters of
transcription factors FOXK1 and NXPH4 had binding sites. Luciferase
and ChIP experiments were also performed to verify the binding
ability between FOXK1 and NXPH4. In addition, the present study
revealed that FOXK1 expression was significantly elevated in CRC
cells. A previous study showed that circAPLP2 interference inhibits
tumor growth in CRC, and inhibits in vitro glycolysis
through the upregulation of microRNA (miR)-485-5p and
downregulation of FOXK1(20).
tRF3008A inhibits the progression and metastasis of CRC by
destabilizing FOXK1 in an AGO-dependent manner (21). The interaction of FOXK1 and four
and a half LIM domains 2 promotes the proliferation, invasion and
metastasis of CRC (22). These
results indicated that FOXK1 plays an important role as a
regulatory factor in the proliferation, metastasis and glycolysis
of CRC. In the present study, it was revealed that the
overexpression of transcription factor FOXK1 reversed the effect of
interfering with NXPH4 on CRC cells.
The present article has some limitations. First of
all, only the expression of NXPH4 was downregulated in the current
paper, and the overexpression of NXPH4 was not investigated. Only
the overexpression of FOXK1 was detected in the current study, and
the knockdown of FOXK1 was not investigated. We will further
supplement relevant content in future experiments. In addition,
another limitation of the present study is that only the HCT116
cell line was used. Due to the amount of data, future experiments
will further verify it in other CRC cell lines.
In conclusion, FOXK1-regulated NXPH4 promotes CRC
cell proliferation, metastasis and glycolysis. The present study
provided a strong theoretical basis for the targeted therapy of CRC
and the search for CRC tumor markers.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS designed the experiments. QF and WH performed the
experiments and wrote the article. QF, WH and YS analyzed the
experimental data. QF, WH and YS confirm the authenticity of all
the raw data. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
ESMO Guidelines Working Group; Van Cutsem
EJD. Advanced colorectal cancer: ESMO clinical recommendations for
diagnosis, treatment and follow-up. Ann Oncol. 18 (Suppl
2):ii25–ii26. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kow AWC: Hepatic metastasis from
colorectal cancer. J Gastrointest Oncol. 10:1274–1298.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Muller MF, Ibrahim AE and Arends MJ:
Molecular pathological classification of colorectal cancer.
Virchows Arch. 469:125–134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Piawah S and Venook AP: Targeted therapy
for colorectal cancer metastases: A review of current methods of
molecularly targeted therapy and the use of tumor biomarkers in the
treatment of metastatic colorectal cancer. Cancer. 125:4139–4147.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gui Z, Ying X and Liu C: NXPH4 Used as a
new prognostic and immunotherapeutic marker for muscle-invasive
bladder cancer. J Oncol. 2022(4271409)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tang Q, Chen YM, Shen MM, Dai W, Liang H,
Liu JN and Gao J: Increased expression of NXPH4 correlates with
immune cell infiltration and unfavorable prognosis in
hepatocellular carcinoma. J Oncol. 2022(5005747)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou L, Rueda M and Alkhateeb A:
Classification of breast cancer Nottingham prognostic index using
high-dimensional embedding and residual neural network. Cancers
(Basel). 14(934)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang Z, Wei B, Qiao A, Yang P, Chen W,
Zhen D and Qiu X: A novel EZH2/NXPH4/CDKN2A axis is involved in
regulating the proliferation and migration of non-small cell lung
cancer cells. Biosci Biotechnol Biochem. 86:340–350.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42 (Database issue):D92–D47. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lanczky A and Gyorffy B: Web-based
survival analysis tool tailored for medical research (KMplot):
Development and implementation. J Med Internet Res.
23(e27633)2021.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Fornes O, Castro-Mondragon JA, Khan A, van
der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M,
Baranašić D, et al: JASPAR 2020: Update of the open-access database
of transcription factor binding profiles. Nucleic Acids Res.
48:D87–D92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stiksma J, Grootendorst DC and van der
Linden PW: CA 19-9 as a marker in addition to CEA to monitor
colorectal cancer. Clin Colorectal Cancer. 13:239–244.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li J, Ma X, Chakravarti D, Shalapour S and
DePinho RA: Genetic and biological hallmarks of colorectal cancer.
Genes Dev. 35:787–820. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lech G, Słotwiński R, Słodkowski M and
Krasnodębski IW: Colorectal cancer tumour markers and biomarkers:
Recent therapeutic advances. World J Gastroenterol. 22:1745–1755.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eun JW, Jang JW, Yang HD, Kim J, Kim SY,
Na MJ, Shin E, Ha JW, Jeon S, Ahn YM, et al: Serum proteins, HMMR,
NXPH4, PITX1 and THBS4; a panel of biomarkers for early diagnosis
of hepatocellular carcinoma. J Clin Med. 11(2128)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang D, Zhang P, Liu Z, Xing' Y and Xiao
Y: NXPH4 promotes gemcitabine resistance in bladder cancer by
enhancing reactive oxygen species and glycolysis activation through
modulating NDUFA4L2. Cancers (Basel). 14(3782)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu J, Zhang J, Wang Z, Xi Y, Bai L and
Zhang Y: Knockdown of circAPLP2 inhibits progression of colorectal
cancer by regulating miR-485-5p/FOXK1 axis. Cancer Biother
Radiopharm. 36:737–752. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Han Y, Peng Y, Liu S, Wang X, Cai C, Guo
C, Chen Y, Gao L, Huang Q, He M, et al: tRF3008A suppresses the
progression and metastasis of colorectal cancer by destabilizing
FOXK1 in an AGO-dependent manner. J Exp Clin Cancer Res.
41(32)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu M, Wang J, Tang W, Zhan X, Li Y, Peng
Y, Huang X, Bai Y, Zhao J, Li A, et al: FOXK1 interaction with FHL2
promotes proliferation, invasion and metastasis in colorectal
cancer. Oncogenesis. 5(e271)2016.PubMed/NCBI View Article : Google Scholar
|