Introduction
As an acute necrosis inflammatory bowel disease,
neonatal necrotizing enterocolitis (NNEC) primarily affects preterm
infants. An epidemiology study shows that it is the most common
gastrointestinal emergency in the neonatal intensive care unit
(1) and is characterized as a
worldwide epidemic disease that accounts for significant neonatal
mortality and morbidity (2,3). It
has been estimated that the incidence of necrotizing enterocolitis
(NEC) is 1-3 patients per 1,000 live births and ~90% of all cases
occur in preterm infants. Despite advances in perinatal and
neonatal care have been achieved in past decades, NEC remains a
leading cause of morbidity and mortality in preterm infants, with
mortality rates as high as 30% (4,5).
To the best of the authors' knowledge, several
important factors including prematurity, intestinal immaturity,
hypoxia-ischemia, colonization with pathogenic bacteria and formula
feeding participate in bowel injury and intestinal inflammation.
All the aforementioned factors can result in epithelial injury and
intestinal tissue damage, including necrosis and apoptosis of
intestinal tissue (6). However, a
number of details regarding the pathogenesis of NEC remain to be
elucidated.
Previous studies revealed that TIPE2 might promote
tumor growth and progression. More specifically, Zhu et al
(7) found that the expression of
TIPE2 was reduced in gastric cancer while Liu et al
(8) demonstrated that TIPE2 is
minimally expressed in human glioma tissues and cell lines. In
inflammatory and autoimmune diseases, Wang et al (9) found that Pseudomonas
aeruginosa infection could reduce expression of TIPE2 in mouse
corneas. Zhou et al (10)
demonstrated that the expression of TIPE2 was downregulated in a
collagen-induced arthritis model, which was accompanied by the
occurrence of arthritis. However, Lou et al (11) showed that TIPE2 deficiency reduced
inflammatory responses in a murine acute colitis model by enhancing
immune responses to commensal bacteria. Therefore conflicting
results have been reported regarding the expression of TIPE2 in
different diseases.
The phosphoinositide 3-kinases (PI3Ks) are a
conserved family of signal transduction enzymes (12,13).
PI3Ks and its downstream serine/threonine kinase AKT (also known as
protein kinase B) serve important roles in regulating cell
activation, inflammatory responses, chemotaxis and apoptosis
(13). It has been proved that
modulation of certain PI3K isoforms may elicit beneficial effects,
while activating other PI3K isoforms may result in pathology
changes (14).
In the present study, the expression of TIPE2 and
PI3K/AKT pathway proteins were examined by using a rat model of
NEC. Further mechanism study was conducted using wild type neonatal
SD rats that were infected with recombinant adenovirus
Ad-TIPE2.
Materials and methods
Animals
Neonatal Sprague Dawley (SD) rats (3 days old: 45
rats, 20 of which were used in the follow-up experiments; and 14
days old, 17 rats, 10 of which were used in the follow-up
experiments) were obtained from the Animal Experiment Center of the
Second Hospital of Shandong University. The ambient temperature was
22±3˚C, and the humidity was 40-80%. The 3-days-old rats were
adapted in cages with a 12-h light/dark cycle for 2 days, The
14-days-old SD rats were randomly divided into two groups (OE group
and NC group). After 3 days of normal feeding, rats in the OE group
were transfected with TIPE2-overexpressing adenovirus, while rats
in the NC group were transfected with NC virus. After 7 days of
feeding under the same conditions, the rats were sacrificed for
further experiments. The present study was approved by the Animal
Experiment Center of the Second Hospital of Shandong University
(Shandong, China; approval no. KYLL-2022LW103). All animal-related
procedures complied with the Guide for the Care and Use of
Laboratory Animals published by the National Institutes of Health
(no. 85-23, revised 1996).
Main reagents and antibodies
Polyclonal antibodies against TIPE2 (cat. no.
15940-1-AP) and β-actin (dilution, 1:500; cat. no. Biotin-60008)
were purchased from ProteinTech Group, Inc. Pan-AKT rabbit pAb
(dilution, 1:1,000; cat. no. A18120) and phosphorylated
(p-)AKT1-S473+AKT2-S474+AKT3-S472 rabbit mAbs (dilution, 1:1,000;
cat. no. AP1208) were obtained from ABclonal Biotech Co., Ltd.
Polyclonal antibodies against PI3-kinase p85α/γ (dilution, 1:1,000;
cat. no. AP71896) and PI3-kinase p85α(p-Tyr607) (dilution, 1:1,000;
cat. no. AP67697) were provided by Abcepta. HRP-conjugated
secondary antibody (H+L) (cat. no. BL003A) and bovine serum albumin
V (cat. no. BS114-100g) were obtained from Biosharp Life Sciences.
RIPA lysate (tissue/cell, cat. no. R0020) [including
phenylmethylsulfonyl fluoride (PMSF)] was purchased from Beijing
Solarbio Science & Technology Co., Ltd. Phosphorylase inhibitor
100X (cat. no. SW105-02) and protease inhibitor cocktail 100X (cat.
no. SW107-02) were obtained from Seven Biotech [Saiwen Innovation
(Beijing) Biotechnology Co., Ltd.]. Tissue RNA Purification Kit
Plus (cat. no. EN-RN002plus) was supplied by Yeasen Biotechnology
(Shanghai) Co., Ltd. Evo M-MLV RT Kit with gDNA Clean for qPCR II
(cat. no. AG11711) was provided by Accurate Biology. 2X Universal
SYBR Green Fast qPCR Mix (cat. no. RK21203) was purchased from
ABclonal Biotech Co., Ltd.
Experiment design and model
establishment
The 3-day-old rats (weight 6-7 g) were randomly
divided into two groups: i) Control group (control, n=10) without
any intervention and ii) NEC group (NEC, n=10). The NEC model was
established as described previously (15). Briefly, the NEC model was
established by artificial feeding, hypoxia-cold stimulation and
intraperitoneal injection of lipopolysaccharides (LPS). All animals
were kept in specified facility with 28-30˚C and 45-65% humidity.
Rats in the experimental group were artificially fed with the
formula substitute of rat milk (16) [4.60 g infant formula, 8 g protein
powder and 50 ml lipid emulsion (C14-24) every 100 ml] and 0.2 ml
of the formula was given to each rat at every 4 h interval (q4h)
within the first 24 h and 0.3 ml was given to each rat at q4h
during 24-48 h while 0.4 ml was given to each rat at q4h for the
last 24 h before experiment. NEC rats were placed in a box with
pure nitrogen at 5 l/min for 7 min when the oxygen concentration in
the oxygen concentration control box was 0-0.5% and then the NEC
rats were quickly transferred into a refrigerator at 4˚C for 7 min
for cold stimulation. After cold stimulation, 2 mg/kg LPS (2 mg/ml
dissolved in normal saline) was intraperitoneally injected once a
day for 3 consecutive days. Subsequently, suckling rats were
returned to the incubator for rewarming and artificial feeding was
continued. Hypoxia-cold stimulation was carried out every 12 h at
10 a.m. and 10 p.m., respectively, for 3 consecutive days. Rats in
the control group were placed in the same cage, being suckled by
the mother. All control rats were intraperitoneally injected with
an equal amount of saline, once a day for 3 consecutive days. After
72 h, all rats were fasted for 12 h before being sacrificed with
carbon dioxide euthanasia, and the volume displacement rate of
CO2 used for euthanasia ranged from 30-70% of the
chamber volume per minute.
Adenovirus injection detection
Adenovirus was purchased from OBiO Technology
(Shanghai) Corp., Ltd. Grouping of experimental animals and virus
infection: The 14-day-old rats (weight 30-40 g) were randomly
divided into two groups: i) control group (control, infected with
NC virus group, NC group, n=5) and ii) intervention group (infected
with TIPE2 over-expression virus, OE group, n=5). After being
anesthetized with isoflurane (the induction dose used for
anesthesia was 3-4% and the maintenance dose was 2.0-2.5%), SD rats
of the two groups were injected with NC virus and OE virus at
multiple points in the ileum, respectively. Then the abdominal were
closed and the rats were kept on normal feeding for 7 days. After 7
days, the rats were sacrificed with carbon dioxide euthanasia and
the volume displacement rate of CO2 used for euthanasia
ranged from 30-70% of the chamber volume per minute. Then the ileum
tissues were removed for further experiments. After the rats were
injected with the virus, seven rats showed decreased activity,
shortness of breath and finally succumbed due to respiratory
arrest. It was suspected that the cause of their mortality may be
viral intolerance.
Sample collection and processing
Midline laparotomies were performed on rats in a
sterile environment. The intestinal tube between the lower end of
the duodenum and the ileocecal was collected after isolating the
mesentery and blood vessel. Subsequently, a 3-cm longitudinal
section was made at the terminal ileum which was further divided
into upper and lower segments. The upper segment was stored in 10%
neutral formalin and the lower segment was snap-froze in liquid
nitrogen and stored in -80˚C before experiment.
General observation
The general status of rats were observed and
recorded on a daily basis. Detailed documentations of the changes
were as previously described (17). During the modeling process, it was
found that the general conditions of the rats in the model group
were: No weight gain or loss, little activity or crouching
immobility, low response to stimulation, feeding difficulties,
diarrhea, black stool, and some rats exhibited bloody stool. The
general morphology of intestinal tissues were evaluated after
laparotomy and the presence of enteric cavity pneumatosis,
necrosis, or hemorrhage was visually checked.
Hematoxylin and eosin (H&E)
staining
The intestinal tissues were fixed in 10% neutral
buffered formalin and embedded in a paraffin block (room
temperature) before sectioning at 3 µm. After being dewaxing in
xylene twice (at room temperature, 5-10 min each time), the
sections were rehydrated at room temperature with an ethanol series
(100, 95, 85 and 75%), for 3 min per gradient, and then soaked in
distilled water for 2 min. The sections were stained with
hematoxylin dye solution at room temperature for 20 min. The
sections were differentiated with differentiation solution for 1
min at room temperature. The sections were then dehydrated, sealed
with neutral gum and observed with an OLYMPUS BX53 light microscope
(original magnification, x200).
Immunohistochemical (IHC)
staining
Briefly, 3 µm sections were prepared for IHC
staining. After deparaffinization and rehydration, the sections
were put into a pressure cooker containing EDTA (pH 9.0) for
antigen retrieval. Next, 3% hydrogen peroxide was added to
inactivate endogenous peroxidase. Subsequently, the sections were
successively incubated with the aforementioned primary and
secondary antibodies and rinsed with PBS repeatedly. The primary
antibody was added at 4˚C overnight, used at 1:500 dilution, and
the horseradish peroxidase (HRP)-labeled secondary antibody
(dilution, 1:500) was added at room temperature for 30 min.
Finally, the nuclei were restained with hematoxylin for 1-2 min and
then the tissue sections were sealed with neutral gum. Images of
five non-overlapping fields per section were randomly taken using
an OLYMPUS BX53 light microscope (original maginification, x200).
The IHC staining and image analysis were carried out blindly.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples (<50
mg) using the Tissue RNA Purification Kit Plus according to the
manufacturer's instructions. Purified RNA was reversely transcribed
into cDNA using the Reverse Transcription System (Evo M-MLV Reverse
transcription kit with gDNA Clean for qPCR II; Hunan Aikerui
Biological Engineering Co., Ltd.) and the RT-qPCR was performed
using the 2X Universal SYBR Green Fast qPCR Mix. Briefly, after an
initial denaturation step at 95˚C for 3 min, the amplifications
were carried out with 40 cycles at a melting temperature of 95˚C
for 5 sec and an annealing temperature of 60˚C for 30 sec, followed
by a melting curve analysis at 95˚C for 15 sec, 60˚C for 1 min and
95˚C for 1 sec. Data were collected by CFX Manager Software
(Bio-Rad Laboratories, Inc.) and expressed as quantification cycle
(Cq) values. The samples for RT-qPCR analysis were evaluated using
a single predominant peak as quality control. The relative
expressions of the target genes were calculated using the
2-∆∆Cq method (18) and
GAPDH and β-actin were adopted as the housekeeping gene. RNA
extraction, cDNA synthesis and qPCR were performed according to the
manufacturer's protocols, and all the experiments replicated three
times. The primer sequences were as follows: TIPE2: Forward primer
5'-TCCAAGGCACAACGGGTGA-3' and reverse primer
5'-GGCGAAATCGTGTAGCCAGAG-3'; PI3K: Forward primer
5'-CAATCCAGAAACGCCTCACT-3'and reverse primer
5'-GCAGCCTCTATGGCAATCA-3'; AKT: Forward primer
5'-TACCTGAAGCTACTGGGCAAGGG-3' and reverse primer
5'-CGGTCGTGGGTCTGGAATGAG-3'; GAPDH (for the front experiments):
Forward primer 5'-GCACCGTCAAGGCTGAGAAC-3' and reverse primer
5'-TGGTGAAGACGCCAGTGGA-3'; β-actin (for the later experiments):
Forward primer 5'-CACCCGCGAGTACAACCTTC-3' and reverse primer
5'-CCCATACCCACCATCACACC-3'.
Western blotting analysis
The aforementioned TIPE2 was used at 1:1,000
dilution, and aforementioned HRP-conjugated secondary antibody
(H+L) was used at 1:10,000 dilution. Total protein was extracted
using lysis buffer containing 20 mmol/l Tris-HCl, pH 7.4, 150
mmol/l NaCl, 1% TX-100, 1 mmol/l EDTA, pH 8.0 and 1 mmol/l
phenylmethylsulfonyl fluoride (PMSF). Protein concentration was
determined using a Micro BCA protein kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of proteins (30 µg) were loaded on
10% gels to perform sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) before transferring onto PVDF membranes.
Membranes were blocked with 5% defatted milk in Tris-buffered
saline (TBS) containing 0.1% Tween-20 at room temperature for 1 h
and then incubated with primary antibodies including anti-TIPE2,
anti-p-Akt, anti-Akt and anti-β-actin (ABclonal Biotech Co., Ltd.)
and anti-PI3K and anti-p-PI3K (Abcepta) overnight at 4˚C.
Subsequently, the blots were rinsed and incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (room temperature,
being rocked on a shaker for 1 h slowly). The immunoreactive bands
were visualized using chemiluminescence ECL detection reagents
(cat. no. BL520A; Biosharp Life Sciences) and actin was used as the
loading control. ImageJ 1.51j8 software (National Institutes of
Health) was used for analysis.
Statistical analysis
Data were shown as mean ± standard deviation (SD).
The distribution was assessed with the Shapiro-Wilk test.
Comparisons between two groups were performed using the unpaired
t-test and a P-value <0.05 was considered statistically
significant. Pearson's correlation analysis and linear regression
analysis were performed with two variables (the index of TIPE2 and
p-PI3K) in the NEC group, where TIPE2 was using as the independent
variable and p-PI3K as the dependent variable. All statistical
analyses were performed using the GraphPad Prism 8.0
(Dotmatics).
Results
Establishment of NEC rat model
At the beginning of modeling, the rats showed
varying degrees of excretion of yellow-green mucus with diarrhea,
dark belly, abdominal distension and weight loss, followed by
vomiting, disappearance of group reaction, decreasing activity and
sluggish reactions. These symptoms gradually aggravated. Table I showed the weight changes of the
rats in the NEC group. The average body weight was significantly
decreased in the NEC group compared to the control group
(P<0.05).
 | Table IWeight changes of the two groups in 3
days. |
Table I
Weight changes of the two groups in 3
days.
| | Weight, g | |
|---|
| Group | Number of rats | Day 1 | Day 2 | Day 3a | Weight change, g |
|---|
| Control | 10 | 6.9±0.1 | 7.0±0.1 | 7.1±0.2 | +0.2 |
| NEC | 10 | 6.6±0.6 | 6.4±0.6 | 6.2±0.6 | -0.4 |
Expression of TIPE2 was decreased in
NEC rats
First, H&E staining was used to assess the
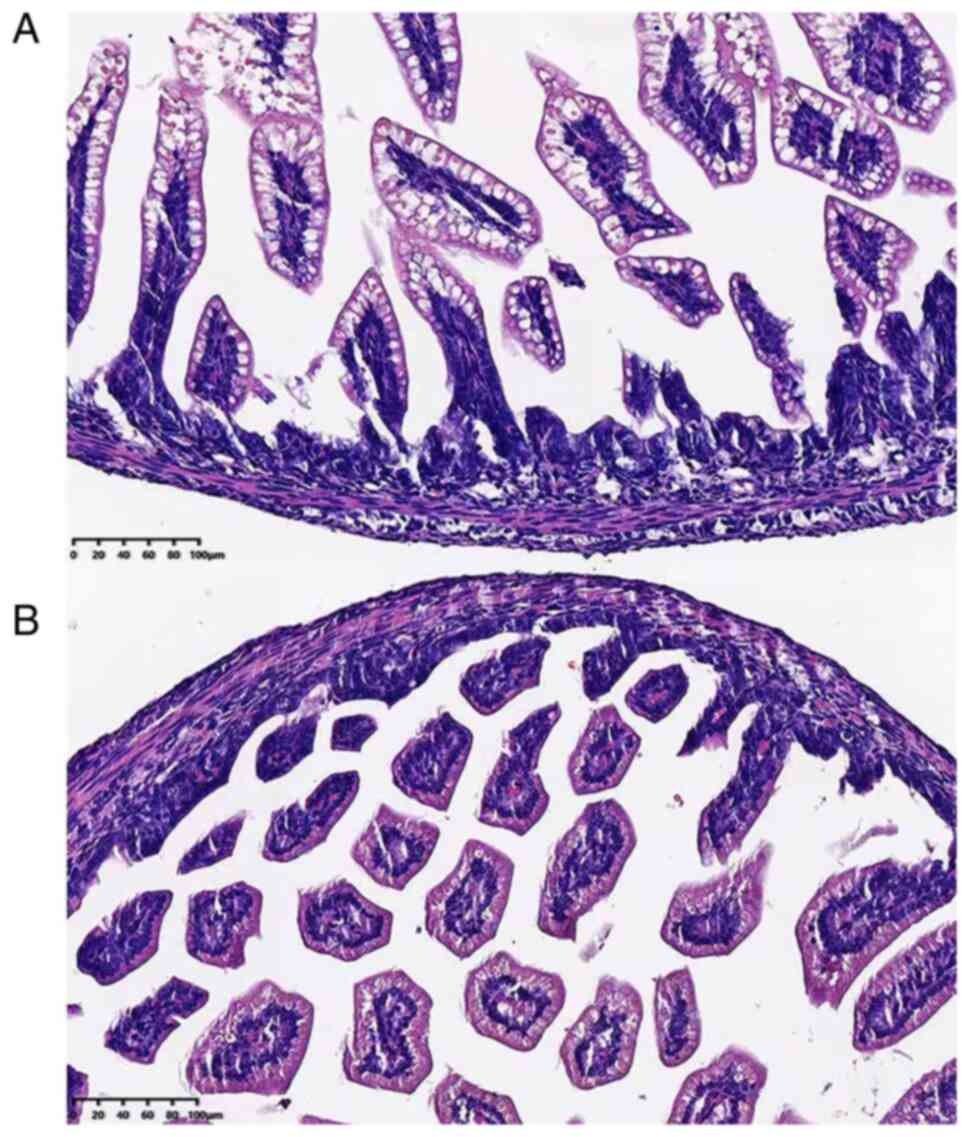
ileocecal intestinal tissue damage. As shown in Fig. 1, rats in the NEC group presented
with surface mucosal ulceration with necrosis. In addition,
moderate or severe separations of submucosal and lamina propria
commonly existed in the tissue, accompanied by moderate
inflammatory cell infiltration and villi edema. Next, the
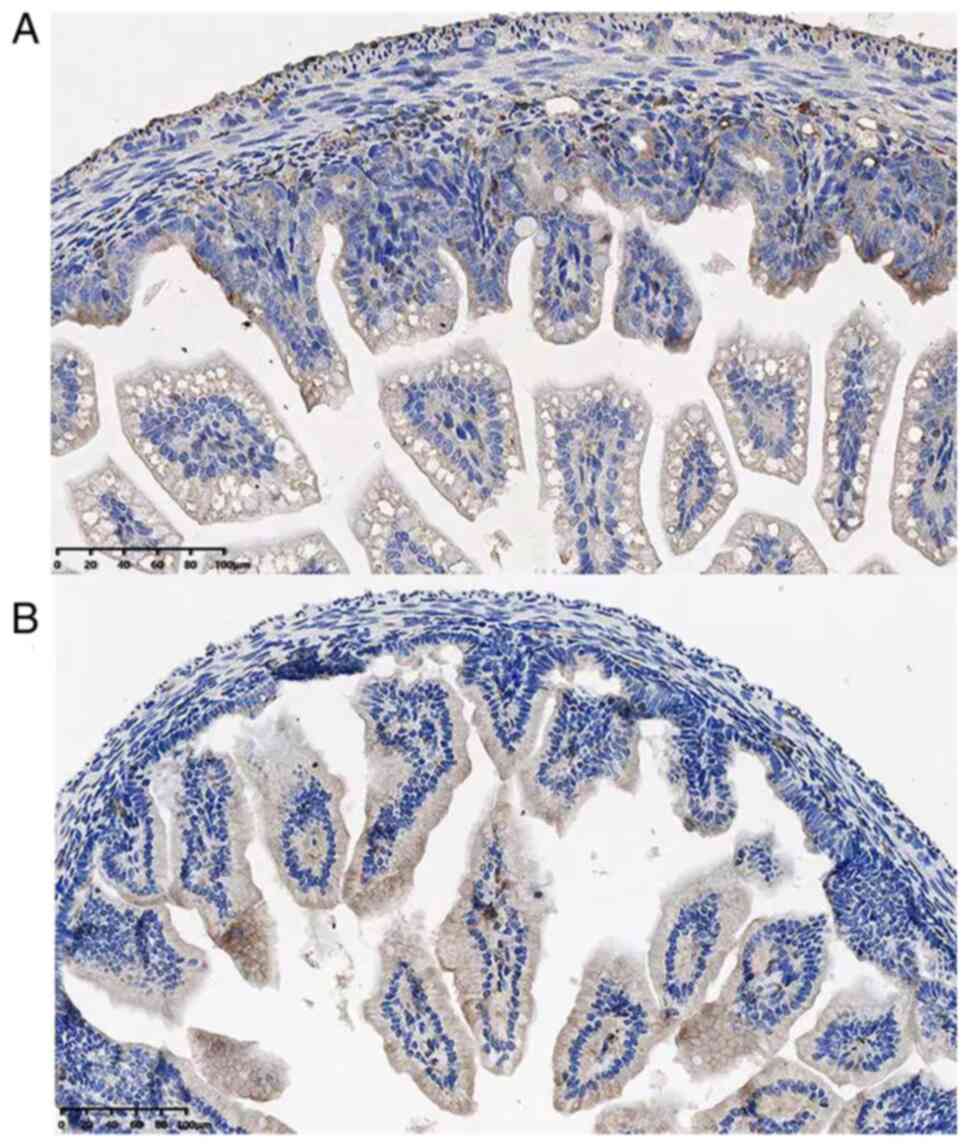
expression of TIPE2 was examined by IHC staining. The
immunoreactivity of TIPE2 was predominantly identified in
infiltrating mononuclear cells in lamina propria and in epithelial
cells to a lesser level. Fig. 2
showed the number of TIPE2-positive mononuclear cells in both
groups.
Expressions of TIPE2, PI3K and AKT at
mRNA level
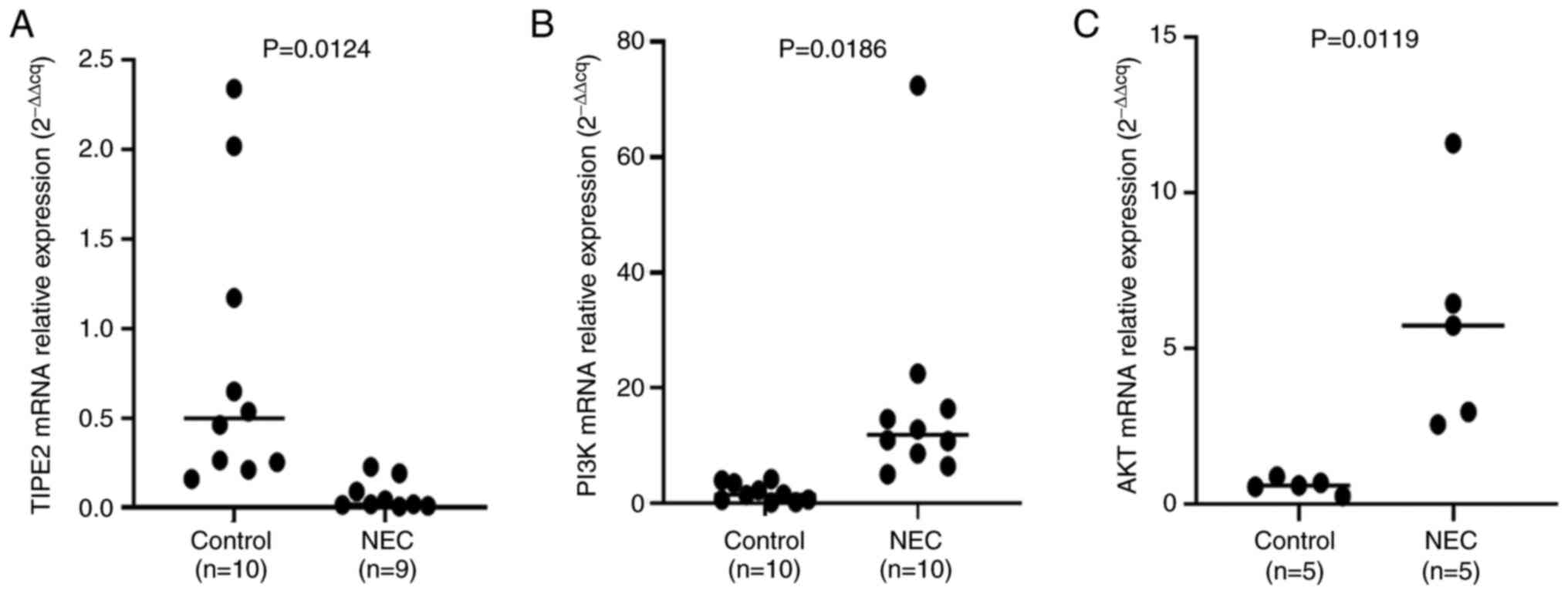
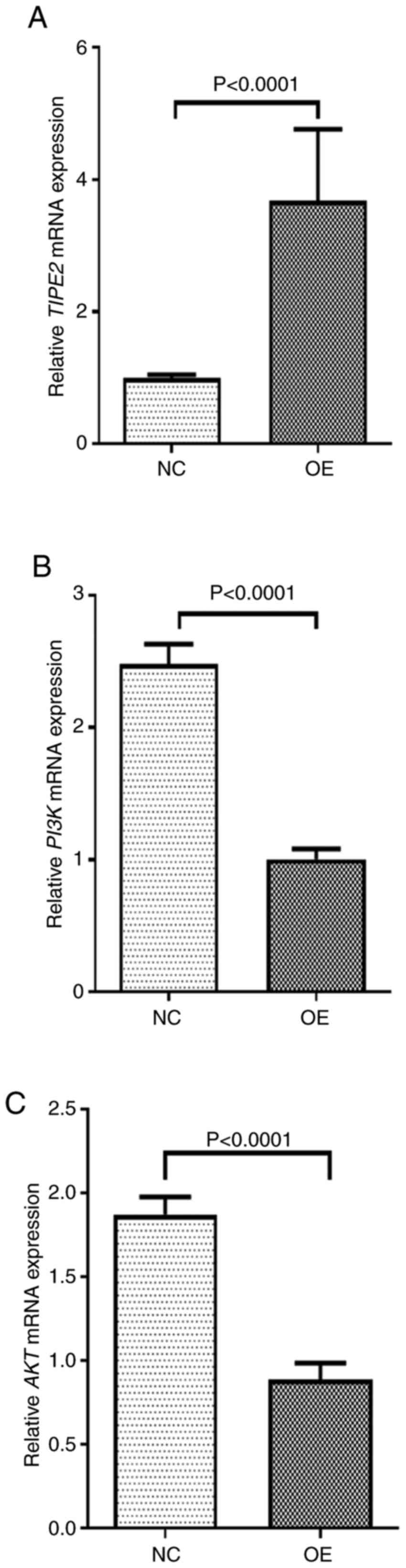
RT-qPCR showed that the expression of TIPE2 mRNA was
significantly reduced while the expressions of PI3K and AKT were
significantly increased in the NEC group compared to the control
group (P<0.05). Fig. 3 showed
the relative expression levels of TIPE2, PI3K and AKT.
Protein expressions of TIPE2,
pan-PI3K, pan-AKT, p-PI3K and p-AKT
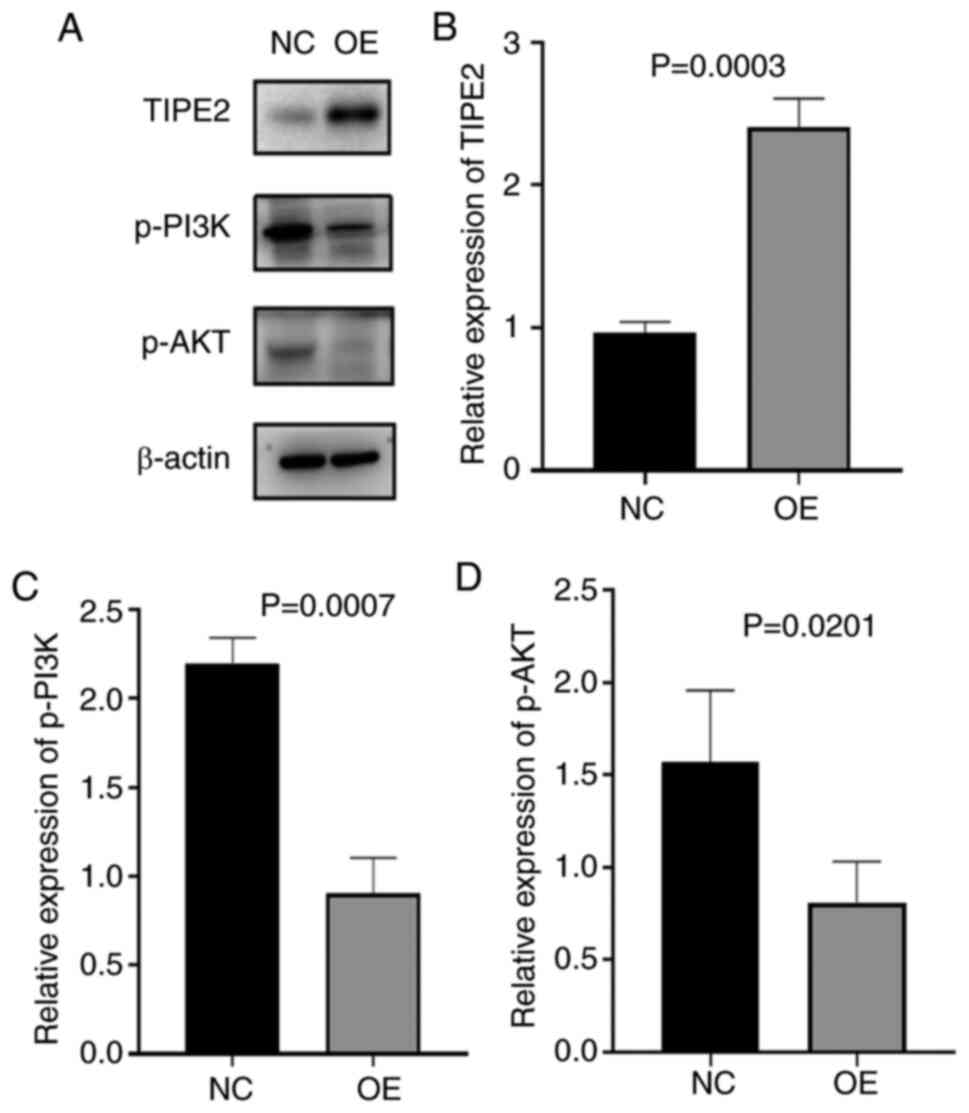
Western blotting analysis showed that the relative
protein expression of TIPE2 in the NEC group was significantly
decreased compared to the control group, while the relative
expressions of p-PI3K, p-Akt, pan-PI3K and pan-Akt were
significantly increased in the experimental group compared to the
control group, as demonstrated by Fig.
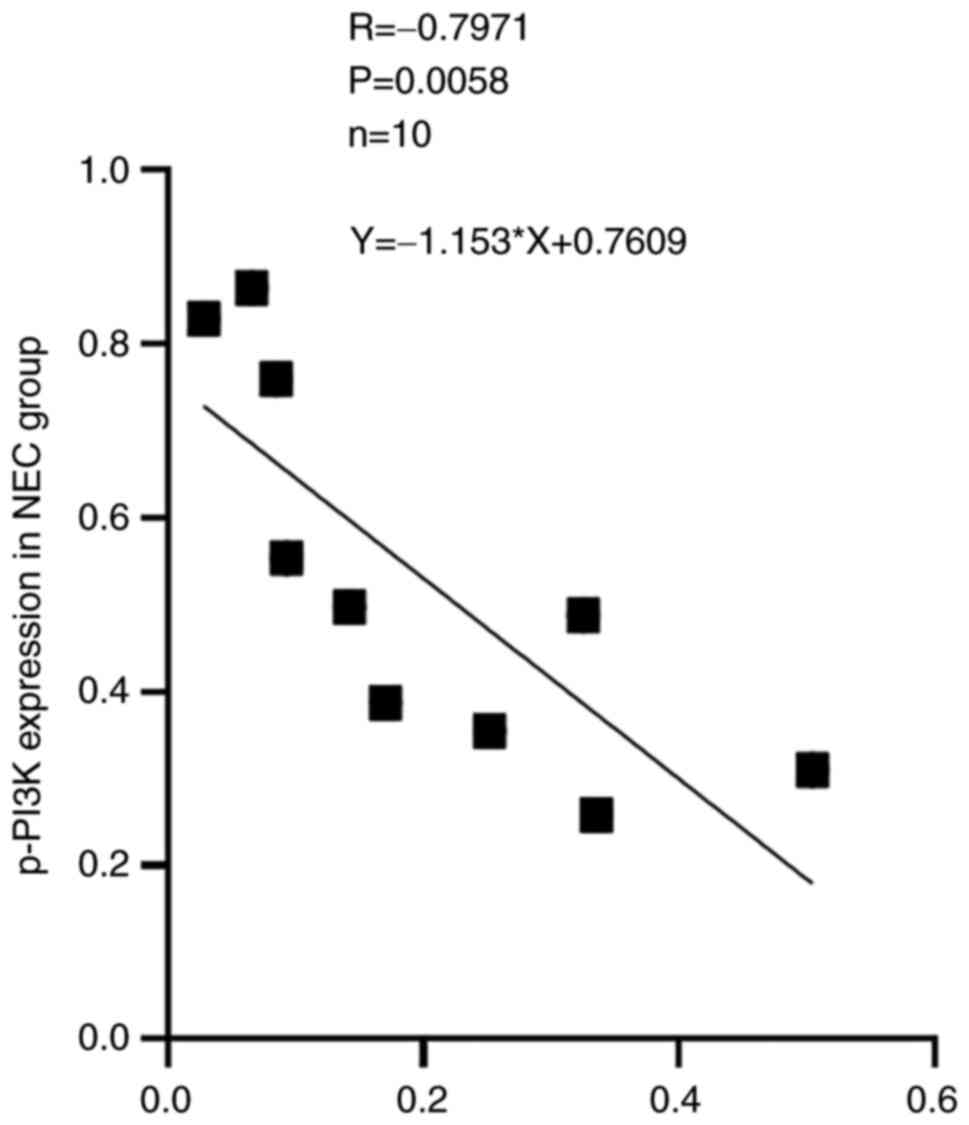
4. Correlation analysis indicated that the protein expression
of TIPE2 was negatively correlated with the expressions of p-PI3K
with a coefficient factor of -0.797 (P=0.0058). Linear regression
analysis of these two parameters showed that the regression
coefficient factor was -1.153 (Fig.
5). This finding suggested that the TIPE2 might participate in
the pathogenesis of NEC through regulating the PI3K/AKT signaling
pathway.
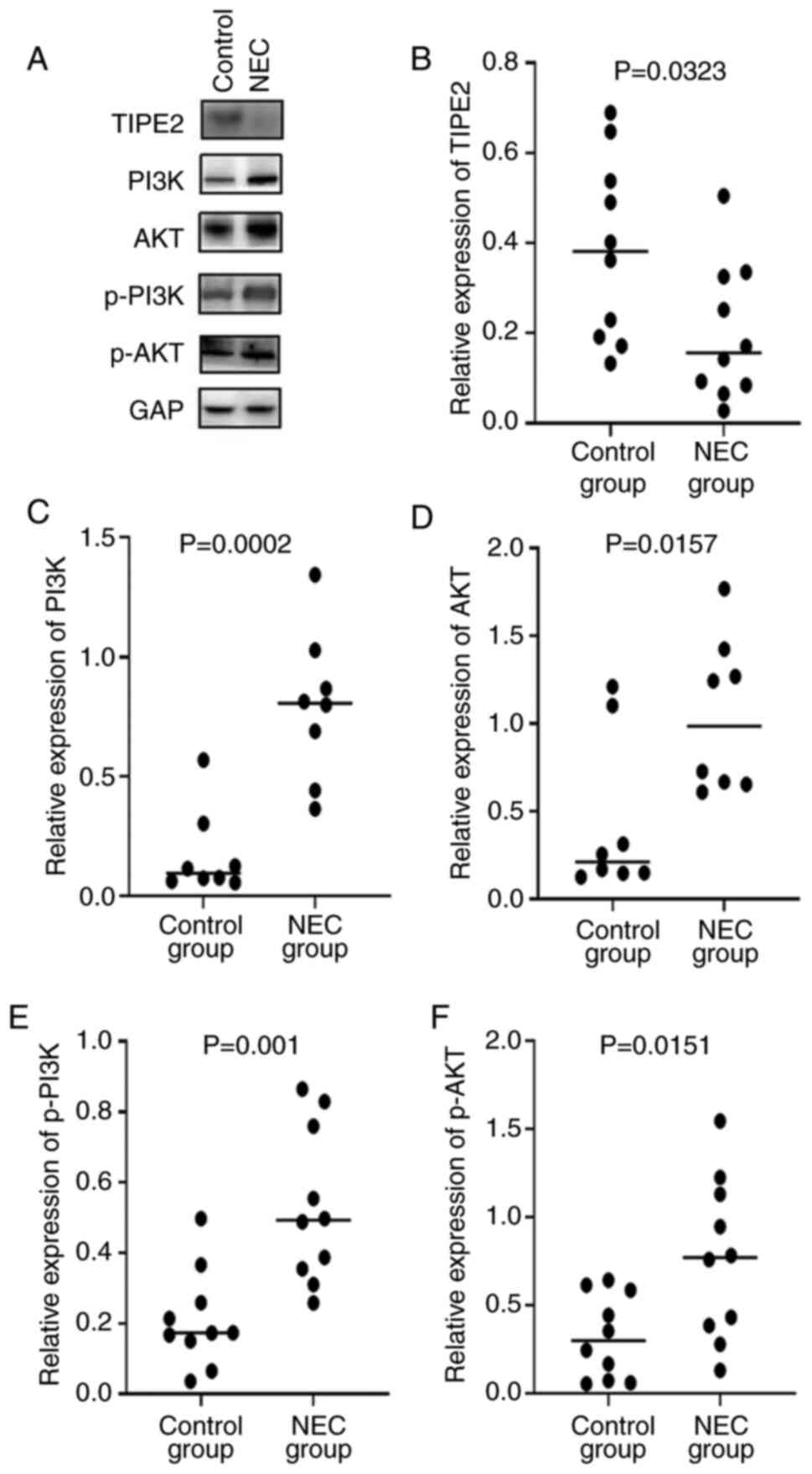 | Figure 4Expressions of TIPE2, pan-PI3K,
pan-AKT, p-PI3K and P-AKT at the protein level. (A) Representative
western blots of TIPE2, pan-PI3K, pan-AKT, p-PI3K and P-AKT
expression in NEC rats and control groups. (B,C,D,E,F) The
comparison of relative expression of (B) TIPE2, (C) pan-PI3K, (D)
pan-AKT, (E) p-PI3K and (F) p-AKT in NEC rats and control groups.
TIPE2, TNF-α-induced protein 8-like 2; PI3K,
phosphatidylinositol-3-kinase; p-, phosphorylated; NEC, necrotizing
enterocolitis. |
Expression of the mRNA and major
proteins of PI3K/AKT pathway in Ad-TIPE2-infected ileum
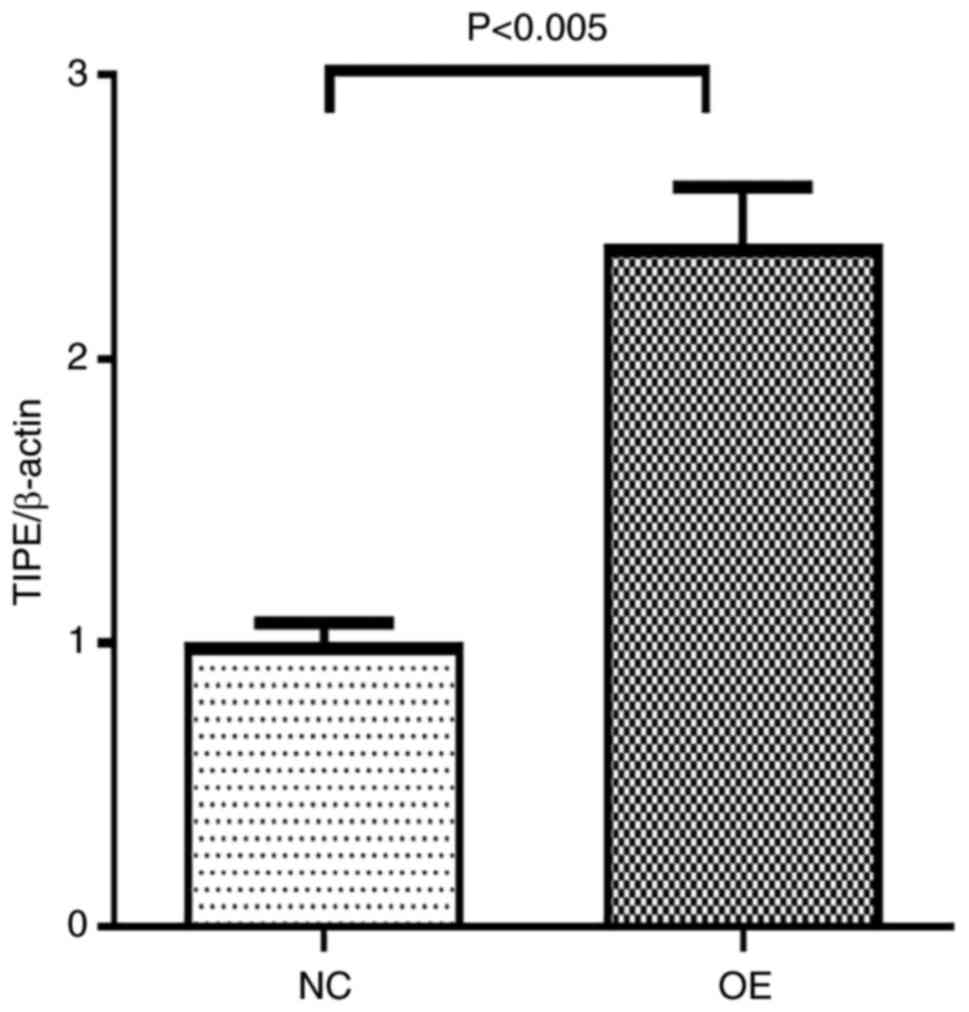
After adenovirus infection, TIPE2 expression in
Ad-TIPE2-infected ileum was significantly increased, indicating
successful infection Fig. 6. Then
the follow-up experiments were conducted. Both RT-qPCR and western
blot assay demonstrated that the expression of TIPE2 was
significantly increased in Ad-TIPE2-infected ileum compared with
Ad-V-infected ileum, while the expressions of PI3K and AKT at the
mRNA and protein level in Ad-TIPE2-infected ileum were
significantly decreased. And the differences of each index between
the two groups were statistically significant (Fig. 7, Fig.
8 and Fig. 9).
Discussion
To the best of the authors' knowledge, this was the
first study that showed that the expression of TIPE2 at both mRNA
and protein levels in a NEC rat model was decreased compared with a
control group. Meanwhile, the expressions of PI3K and AKT at the
mRNA level in the NEC group were significantly increased and the
protein expressions of pan-PI3K, pan-Akt, p-PI3K and p-Akt in NEC
rats were also significantly increased, compared to the controls.
According to the correlation analysis, our data showed that the
expression of TIPE2 was negatively correlated with the expression
of p-PI3K(r =-0.797).
TIPE2 belongs to the TNF-α-induced protein 8
(TNFAIP8) family, which also includes members such as TNFAIP8,
TIPE1 and TIPE3(19). As
aforementioned, TIPE2 has been identified as a tumor suppressor in
multiple malignancies and its expression is significantly
downregulated in a number of inflammatory and autoimmune diseases.
TIPE2 over-expression can inhibit proliferation,
epithelial-mesenchymal transition and migration. The depletion of
TIPE2 can cause severe inflammatory and autoimmune diseases, such
as hepatitis B, systemic lupus erythematosus, asthma,
collagen-induced arthritis and experimental stroke (20-23).
However, a previous study showed that TIPE2 deficiency could reduce
inflammatory responses in a murine acute colitis model (11). Consistently, the present study
found that the expression of TIPE2 was minimally expressed in NEC
rat model. In other words, when NEC occurs in newborn rats, the
body may reduce the expression of TIPE2 to prevent further
activation of immune system to contain the inflammation.
PI3K and its downstream AKT both serve essential
roles in regulating cell proliferation and survival as well as cell
homeostasis (14). PI3K is an
enzyme complex composed of 4 known isoforms (α,β,γ and ζ) and a
catalytic p110 subunit. PI3K catalyzes the conversion of
phosphatidylinositol 4,5 biphosphate PI(4,5)P3 to
PI(3,4,5)P3. A
number of proteins, such as phosphoinositide-dependent kinase-1
(PDK1), PDK2 and AKT, can interact with PI(3,4,5)P3.
It has been known that PDK activates AKT by phosphorylation of
Ser308 and Thr473(13). Studies
have reported that the PI3K/AKT signaling pathway serves a
protective role in myocardial ischemic injury, rotavirus-induced
diarrhea and fulminating polymicrobial sepsis (14,24,25).
However, Camps et al (26)
reported that blocking PI3Kγcould reduce inflammation and joint
damage in murine models of arthritis. In addition, Oudit et
al (27) found that PI3Kγ
participates in the pathogenesis of maladaptive cardiac
hypertrophy, whereas PI3Kα and βserve important roles in regulating
physiologic stimuli that result in adaptive hypertrophy. Thus,
different PI3K isoforms seem to serve significantly different roles
under both physiological and pathological conditions. In the
current study, mRNA expressions of PI3K and AKT as well as
phosphorylation of PI3K and AKT were significantly increased in NEC
rats. These results indicated that the PI3K/AKT pathway might be
activated in NEC to reduce the inflammation and increase the
susceptibility of rats to sepsis.
To further confirm the association between TIPE2 and
PI3K/AKT pathway, wild type rats were infected with recombinant
adenovirus Ad-V and Ad-TIPE2 respectively. The results showed that
the expression of TIPE2 was significantly increased in
Ad-TIPE2-infected rats, compared to controls. However, the
phosphorylation of PI3K and AKT were significantly decreased in
Ad-TIPE2-infected rats and the differences of each index between
the two groups were statistically significant. All the data
suggested that TIPE2 might be involved in the pathogenesis of NEC
by activating the PI3K/AKT signaling pathway.
The data from the present study showed that TIPE2
was involved in the pathogenesis of NEC by activating the PI3K/Akt
signaling pathway and its expression was downregulated in NEC rats.
These findings not only provide an improved understanding of the
pathogenesis of NEC but also shed light upon identifying novel
therapeutic targets.
Acknowledgements
The authors would like to thank Dr Shun Wang
(Department of Clinical Chemistry, The Second Hospital of Shandong
University, Jinan, China), Dr Zhiyang Xiao (Editorial Department of
Urology, The Second Hospital of Shandong University), Dr Lei Liu
(Department of Urology, The Second Hospital of Shandong
University), Dr Qian Xin (Department of Experimental Center, The
Second Hospital of Shandong University) and Dr Qinghong Ji
(Obstetrical Department, The Second Hospital of Shandong
University), for their help with the submission and the
experiments.
Funding
Funding: The present study was supported by the Clinical Science
and Technology Innovation Program of Ji Nan science and Technology
Bureau funding (grant no. 201907088).
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL conceived and designed the present study,
performed the experiments, collated the data and drafted the
manuscript. RW made substantial contributions to conception and
design, and reviewing and revising the manuscript critically for
important intellectual content. SY was involved in drafting the
manuscript, in data analysis and interpretation, and in revising it
critically for important intellectual content. JX and XS
contributed substantially to the experiments. JL and JX confirm the
authenticity of all the raw data. All authors reviewed the
manuscript for important intellectual content, agreed to be held
accountable for all aspects of the work and read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experiment Center of the Second Hospital of Shandong University
(Shandong, China; approval no. KYLL-2022LW103).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang L, Fan J, Chen YX and Wang JH:
Inhibition of A2B adenosine receptor attenuates
intestinal injury in a rat model of necrotizing enterocolitis.
Mediat Inflamm. 2020(1562973)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jung K, Koh I, Kim JH, Cheong HS, Park T,
Nam SH, Jung SM, Sio CA, Kim SY, Jung E, et al: RNA-seq for gene
expression profiling of human necrotizing enterocolitis: A pilot
study. J Korean Med Sci. 32:817–824. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Prencipe G, Auriti C, Inglese R, Gallusi
G, Dotta A and De Benedetti F: The macrophage migration inhibitory
factor-173g/c polymorphism is not significantly associated with
necrotizing enterocolitis in preterm infants. J Pediatr Surg.
48:1499–1502. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aceti A, Beghetti I, Martini S, Faldella G
and Corvaglia L: Oxidative stress and necrotizing enterocolitis:
Pathogenetic mechanisms, opportunities for intervention, and role
of human milk. Oxid Med Cell Longev. 2018(7397659)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fitzgibbons SC, Ching Y, Yu D, Carpenter
J, Kenny M, Weldon C, Lillehei C, Valim C, Horbar JD and Jaksic T:
Mortality of necrotizing enterocolitis expressed by birth weight
categories. J Pediatr Surg. 44:1072–1075; discussion 1075-1076.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Martin CR and Walker WA: Probiotics: Role
in pathophysiology and prevention in necrotizing enterocolitis.
Semin Perinatol. 32:127–137. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu Y, Tao M, Wu J, Meng Y, Xu C, Tian Y,
Zhou X, Xiang J, Zhang H and Xie Y: Adenovirus-directed expression
of TIPE2 suppresses gastric cancer growth via induction of
apoptosis and inhibition of AKT and ERK1/2 signaling. Cancer Gene
Ther. 23:98–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu ZJ, Liu HL, Zhou HC and Wang GC: TIPE2
inhibits hypoxia-induced Wnt/β-catenin pathway activation and EMT
in glioma cells. Oncol Res. 24:255–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Q, Ma L, Liu T, Ge C, Zhou Q, Wei C
and Shi W: TIPE2 suppresses pseudomonas aeruginosa keratitis by
inhibiting NF-κB signaling and the infiltration of inflammatory
cells. J Infect Dis. 220:1008–1018. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou J, Chen P, Li Z and Zuo Q: Gene
delivery of TIPE2 attenuates collagen-induced arthritis by
modulating inflammation. Int Immunopharmacol.
79(106044)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lou Y, Sun H, Morrissey S, Porturas T, Liu
S, Hua X and Chen YH: Critical roles of TIPE2 protein in murine
experimental colitis. J Immunol. 193:1064–1070. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fruman DA and Cantley LC: Phosphoinositide
3-kinase in immunological systems. Semin Immunol. 14:7–18.
2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Williams DL, Ozment-Skelton T and Li C:
Modulation of the phosphoinositide 3-kinase signaling pathway
alters host response to sepsis, inflammation, and
ischemia/reperfusion injury. Shock. 25:432–439. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hou Y, Lu X and Zhang Y: Irak inhibitor
protects the intestinal tract of necrotizing enterocolitis by
inhibiting the toll-like receptor (TLR) inflammatory signaling
pathway in rats. Med Sci Monit. 24:3366–3373. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Auestad N, Korsak RA, Bergstrom JD and
Edmond J: Milk-substitutes comparable to rat's milk; their
preparation, composition and impact on development and metabolism
in the artificially reared rat. Br J Nutr. 61:495–518.
1989.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yin Y, Liu F, Li Y, Tang R and Wang J:
mRNA expression of TLR4, TLR9 and NF-κB in a neonatal murine model
of necrotizing enterocolitis. Mol Med Rep. 14:1953–1956.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity, and cancer. Mol Immunol. 49:4–7.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y,
Qu Z, Cui J, Zhang G, Liang X, et al: Roles of TIPE2 in hepatitis B
virus-induced hepatic inflammation in humans and mice. Mol Immunol.
48:1203–1208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li F, Zhu X, Yang Y, Huang L and Xu J:
Tipe2 alleviates systemic lupus erythematosus through regulating
macrophage polarization. Cell Physiol Biochem. 38:330–339.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ding J, Su J, Zhang L and Ma J: Crocetin
activates Foxp3 through TIPE2 in asthma-associated treg cells. Cell
Physiol Biochem. 37:2425–2433. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Y, Wei X, Liu L, Liu S, Wang Z,
Zhang B, Fan B, Yang F, Huang S, Jiang F, et al: TIPE2, a novel
regulator of immunity, protects against experimental stroke. J Biol
Chem. 287:32546–32555. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li HM, Li KY, Xing Y, Tang XX, Yang DM,
Dai XM, Lu DX and Wang HD: Phenylephrine attenuated sepsis-induced
cardiac inflammation and mitochondrial injury through an effect on
the PI3K/Akt signaling pathway. J Cardiovasc Pharmacol. 73:186–194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang B, Wang Y, Jiang C, Wu C, Guo G,
Chen X and Qiu S: Valeriana jatamansi Jones inhibits
rotavirus-induced diarrhea via Phosphatidylinositol
3-Kinase/Protein Kinase B signaling pathway. J Microbiol
Biotechnol. 31:1115–1122. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Camps M, Rückle T, Ji H, Ardissone V,
Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Françon B,
et al: Blockade of PI3Kgamma suppresses joint inflammation and
damage in mouse models of rheumatoid arthritis. Nature Med.
11:936–943. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Oudit GY, Sun H, Kerfant BG, Crackower MA,
Penninger JM and Backx PH: The role of phosphoinositide-3 kinase
and PTEN in cardiovascular physiology and disease. J Mol Cell
Cardiol. 37:449–471. 2004.PubMed/NCBI View Article : Google Scholar
|