Introduction
Contact dermatitis (CD) is one of the most prevalent
inflammatory dermatological disorders and is caused by exposure to
immune response-triggering exogenous substances and induces skin
inflammation (1,2). It is classified as irritant CD (ICD)
or allergic CD (ACD), with ICD accounting for 80% of all CD cases.
ICD typically affects the hands, its occurrence is irrespective of
age or sex and is one of the most common occupational diseases
worldwide. In Europe, ICD contributes to >30% of all reported
occupational diseases and constitutes 70 and >80% of
occupational skin diseases in Australia and Asia, respectively
(3-5).
Individuals at high risk for ICD include workers in the healthcare,
food industry and agricultural sectors, and hairdressers, who are
exposed to various irritants (4).
In addition to occupational exposure, ICD incidence has recently
increased with the frequent use of soap and alcohol-based hand
sanitisers and increased hand washing owing to the coronavirus
disease 2019 (COVID-19) pandemic (3).
ICD is an inflammatory response of the skin to a
variety of irritant products, including soap, water, cosmetics,
dust, foods and solvents (6). In
the case of water, if the skin is repeatedly exposed to water, it
can easily become dry and humid, weakening the skin barrier and
causing ICD (7). The clinical
manifestation of ICD varies from mild dryness and redness to severe
reactions with oedema, inflammation and vesiculation (8). It occurs as a result of acute and
direct skin injury that activates innate immunity without preceding
sensitization (9). Exposure to
irritants that are toxic to epidermal keratinocytes disrupts the
skin barrier and triggers an innate immune response with the
release of various pro-inflammatory cytokines, such as IL-1β, IL-6
and TNF-α (10,11). Subsequently, these cytokines
activate neighbouring cells, which release chemokines that induce
the migration of neutrophils and macrophages to the damage site
(12). Among recruited immune
cells, macrophages are key regulators and inducers of immune
responses in ICD lesions (13).
Uncontrolled inflammation in acute ICD leads to psoriasis, a
chronic inflammatory skin disease (14,15).
Although steroidal and non-steroidal anti-inflammatory drugs are
used to treat ICDs, their long-term use has adverse effects
(14,16). Therefore, developing a novel
therapeutic agent with safe and efficient anti-inflammatory effects
is necessary.
Humulus japonicus (HJ), also known as
Japanese hop, is a perennial herb in the Cannabaceae family widely
distributed in Asian countries, including Korea, Japan and China.
The anti-inflammatory, anti-atherogenic, antioxidative and
anti-ageing effects of HJ extract were previously reported
(17-19).
Moreover, a clinical study demonstrated the protective effect of HJ
extract in patients with mild atopic dermatitis (20). HJ contains various active
compounds, of which luteolin was identified as the major component
(21). Luteolin inhibits
pro-inflammatory mediators and regulates inflammation-related
signalling pathways, such as NF-κB p65. In addition, luteolin was
shown to modulate several inflammatory responses in the skin
(22). Although the
anti-inflammatory effects of HJ were already demonstrated in
several inflammatory diseases, its effects on ICD remain unclear.
Therefore, the present study investigated the anti-dermatitis
effects of HJ and its possible mechanisms using
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced dermatitis mice
models and murine macrophage cell lines.
Materials and methods
Preparation of HJ extract
HJ was purchased from Gangwon Yakcho in July 2014.
Professor Won Keun Oh identified the voucher specimen (deposit no.
SNU-2014-0004), which was then deposited at the College of Pharmacy
of Seoul National University (Seoul, Korea). The HJ extract was
prepared and supplied by the Korea Bioactive Natural Material Bank
(Seoul, Korea). Briefly, the dried aerial parts of HJ were soaked
in 70% ethanol in an extraction container for 2 days at room
temperature. The ethanol-soluble extract was filtered through
cheesecloth, exhaustively concentrated and dried to produce an
ethanolic extract under reduced pressure. The extract of HJ was
stored at room temperature (25±2˚C) until further use.
Animal studies
C57BL/6J mice (11-week-old; Korea Research of
Bioscience and Biotechnology, Ochang, Korea) were acclimatized to a
12 h light/dark cycle at 22±2˚C for 1 week with unlimited food and
water in a specific pathogen-free facility. A total of 58 mice were
used for experiments. Mice were randomly divided into four groups:
i) Vehicle group treated with 1% DMSO in PBS; ii) HJ300 group
treated with 300 mg/kg of HJ; iii) HJ500 group treated with 500
mg/kg of HJ; and iv) Luteolin group treated with 30 mg/kg of
luteolin (cat. no. L9283; Sigma-Aldrich; Merck KGaA). A total of
two experimental sets were used. In the first set, the experiment
was conducted in four groups (Vehicle, HJ300, HJ500 and luteolin),
with 7 animals in each group. In the second set, the experiment was
conducted in three groups (Vehicle, HJ500 and luteolin), with 10
animals in each group. HJ and luteolin were orally administered
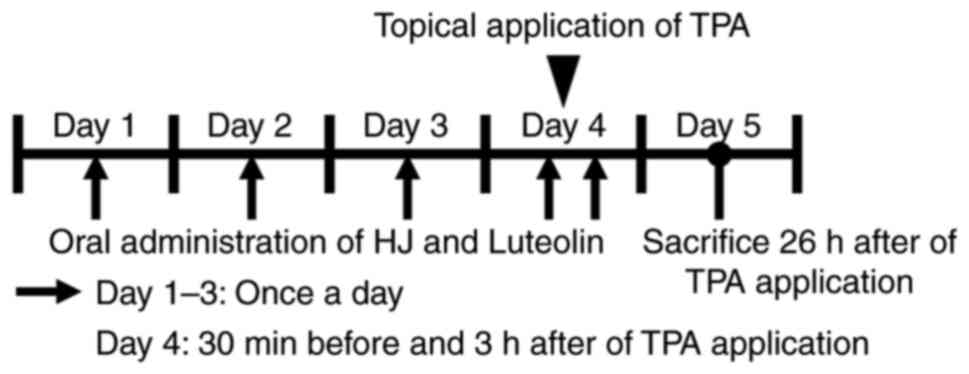
daily for 3 days before topical application of TPA. Subsequently,
on the day of TPA application, HJ and luteolin administration was
performed 30 min before and 3 h after TPA treatment (Fig. 1). TPA (3 µg in 30 µl in 1% DSMO/99%
acetone; cat. no. P8139; Sigma-Aldrich; Merck KGaA) was applied
topically on both internal and external side of each mouse right
ear. Similarly, the left ear was treated with 30 µl 1% DMSO/99%
acetone. DMSO 1% was used to improve the solubility of HJ and
luteolin, which are not completely soluble in PBS, and acetone was
used as a solvent to dissolve the TPA powder. The ear thickness was
assessed as the index of ear skin inflammation at 0, 8 and 26 h
after TPA application using a Digital Thickness Gauge (Mitutoyo
Corporation). As another indicator of inflammation, the ears of
each mouse were cut to the same size and weighed 26 h after TPA
application. All mice were humanely euthanized via CO2
inhalation with a CO2 displacement rate of 30-70% of the
chamber volume per minute. Death was confirmed by the absence of
heart rate, breathing and reflexes. During the experiment, the
temperature, humidity, feed, etc., of the breeding room were
properly managed, and no animals died or were euthanized before the
end of the experiment.
Cell culture
Murine macrophage cell line RAW 264.7 cells were
purchased from the American Type Cell Culture. The cells were
cultured in DMEM (Welgene, Inc.) supplemented with 10% FBS, 100
unit/ml penicillin and 100 µg/ml streptomycin in a humidified
environment (5% CO2 and 95% air) at 37 C. The cells were
pretreated with 800 µg/ml HJ and 25 µM luteolin for 1 h and were
subsequently stimulated with 1 µg/ml lipopolysaccharide (LPS; cat.
no. 916974; Sigma-Aldrich; Merck KGaA) or PBS as a vehicle for 3
and 24 h, at 37 C.
Histopathology and
immunohistochemistry
Ear samples were fixed in 10% neutral buffered
formalin for at least 2 days at room temperature (RT), embedded in
paraffin, cut into 4-µm thick sections and H&E stained
(hematoxylin; 1 min, eosin; 1 min) at RT. To detect neutrophils and
macrophages infiltration, ear sections were incubated with blocking
reagent (1% normal serum in PBS; VECTASTAIN Elite ABC Kits, Vector
Laboratories, Inc.) for 30 min at RT, and then stained with
NIMP-R14 anti-neutrophil antibody (1:200; cat. no. ab2557; Abcam)
and anti-F4/80 antibody (1:500; cat. no. 70076S; Cell Signaling
Technology, Inc.) overnight at 4 C. After repeating the washing
steps with PBS, the sections were incubated with biotinylated
antibody (VECTASTAIN Elite ABC Kits) for 1 h at RT. After further
washing, the sections were incubated with ABC reagent (VECTASTAIN
Elite ABC Kits) for 30 min and visualized with
3,3'-diaminobenzidine (cat. no. SK4100; Vector Laboratories, Inc.).
Hydrogen peroxide (0.3%) was used to block endogenous
peroxidase/phosphatase activity. Epidermal and dermal thickness was
measured as the index of ear oedema and epidermal hypoplasia by
using an image analysis software program (ImageInside version 2.32;
Ehwa Optical Co.). Epidermis and dermis thickness was analyzed
using fifty different parts of each mouse ear tissue section. The
number of neutrophils and F4/80 positive macrophages were counted
in 10 randomly-selected fields of view for each ear section using
ImageJ software version 1.43u (National Institutes of Health).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from mouse ears and RAW 264.7
cells using TRIzol™ reagent (Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA using the UltraScript 2.0 cDNA
Synthesis kit (cat. no. PB30.31-10; PCR Biosystems, Ltd.). The
reaction conditions for cDNA synthesis were as follows: Incubation
at 50˚C for 30 min, followed by denaturing reverse transcriptase at
95˚C for 10 min. Subsequently, qPCR was performed using
AccuPower® 2X Greenstar™ qPCR MasterMix (Bioneer
Corporation) and the StepOne™ Real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling
conditions were as follows: Preheating at 95˚C for 10 min, followed
by 40 cycles at 95˚C for 10 sec and 60˚C for 30 sec. RNA pooling
was conducted by mixing the same volume of each cDNA sample at a
fixed concentration in a single tube followed by a 5-fold dilution.
Relative gene expression levels were analyzed using the
2-∆∆Cq method (23) and
normalized to 18S rRNA expression level. The sequences of the
primer pairs used for qPCR are listed in Table I.
 | Table IPCR primer sequences in the present
study. |
Table I
PCR primer sequences in the present
study.
| | Primer
sequence |
|---|
| Gene | Gene bank accession
no. | Forward | Reverse |
|---|
| Ccl3 | NM_011337.2 |
5'-TCTTCTCAGCGCCATATGGA-3' |
5'-GCAAAGGCTGCTGGTTTCAA-3' |
| Cxcl2 | NM_009140.2 |
5'-GGCTGTTGTGGCCAGTGAA-3' |
5'-CGCCCTTGAGAGTGGCTATG-3' |
| Il-1β | NM_008361.4 |
5'-CTACAGGCTCCGAGATGAACAAC-3' |
5'-TCCATTGAGGTGGAGAGCTTTC-3' |
| Il-6 | NM_031168.2 |
5'-TTCCATCCAGTTGCCTTCTTG-3' |
5'-GGGAGTGGTATCCTCTGTGAAGTC-3' |
| Tnf-α | NM_001278601.1 |
5'-CCCTCACACTCAGATCATCTTCT-3' |
5'-GCTACGACGTGGGCTACAG-3' |
| Cox2 | NM_011198.4 |
5'-GGGTGTCCCTTCACTTCTTTCA-3' |
5'-GAGTGGGAGGCACTTGCATT-3' |
| iNos | NM_001313921.1 |
5'-GTTCTCAGCCCAACAATACAAGA-3' |
5'-GTGGACGGGTCGATGTCAC-3' |
| 18S rRNA | NR_003278.3 |
5'-GACACGGACAGGATTGACAGATTGATAG-3' |
5'-GTTAGCATGCCAGAGTCTCGTTCGTT-3' |
Western blot analysis
RAW264.7 cells (2.5x105 cells) were
homogenized in ice-cold RIPA buffer (pH 7.4, 0.1 mmol/l sodium
vanadate, 1 mmol/l phenylmethanesulfonyl fluoride, 25 mmol/l NaF,
50 mmol/l Tris-HCl, 40 mmol/l β glycol phosphate, 120 mmol/l NaCl,
1% NP40 and 0.5% Triton X-100) containing complete protease
inhibitor (cat. no. 11836170001; Roche Diagnostics), and
phosphatase inhibitor (cat. no. p3200-010; GenDEPOT, LLC). The cell
lysate was centrifuged at 16,000 x g for 15 min at 4 C and the
protein concentration was measured using a Bradford assay (Bio-Rad
Laboratories, Inc.). Protein samples were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis on 10% gels and
transferred onto a polyvinylidene difluoride membrane (Millipore).
Membranes were stained with anti-COX2 (1:1,000; cat. no. ab15191;
Abcam), anti-inducible nitric oxide synthase (iNOS; 1:1,000; cat.
no. ab49999; Abcam), anti-phospho NF-κB p65 (Ser536; 1:1,000, cat.
no. 3033S; Cell Signaling Technology, Inc.), anti-NF-κB p65
(1:1,000, cat. no. 8242S; Cell Signaling Technology, Inc.) and
anti-GAPDH (1:1,000; cat. no. 2118S; Cell Signaling Technology,
Inc.) overnight at 4 C. HRP-conjugated secondary antibodies
(1:1,000; cat. no. 115-035-003; cat. no. 111-035-003; Jackson
ImmunoResearch Laboratories) were then incubated for 1 h at RT.
Protein bands were quantified via densitometric analysis using
ImageJ software version 1.43u (National Institutes of Health).
Statistical Analyses
Numerical data are presented as the mean ± standard
error of the mean. Comparisons of multiple groups were performed
using one-way ANOVA followed by Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Oral administration of HJ alleviates
TPA-induced ICD
TPA-induced acute skin inflammation mice models were
used to examine the anti-inflammatory effects of HJ on ICD. Mice
were treated with HJ and luteolin orally for 3 days before TPA
application on the right ear on day 4. HJ300-, HJ500- and
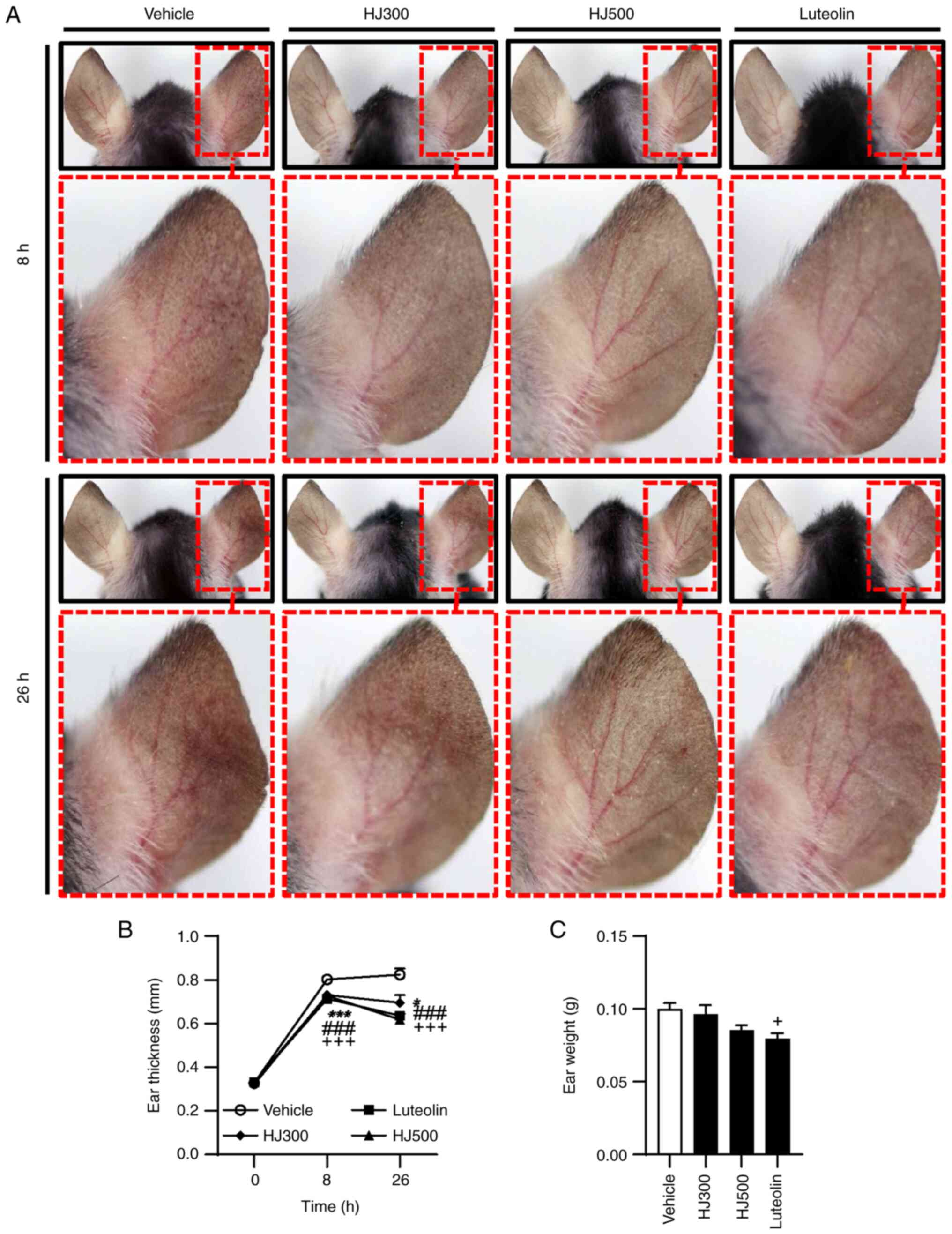
luteolin-treated mice had less ear redness than the vehicle-treated
mice. Particularly, in the HJ500 group, blood vessels were more
apparent than in the other groups, owing to a significant reduction
in ear oedema (Fig. 2A). Ear
oedema was evaluated by measuring ear thickness and weight after
TPA treatment. Compared with that in the vehicle group, the average
ear thickness at 8 h after TPA application was reduced in the HJ
and luteolin groups (0.802±0.011 mm for vehicle vs. 0.731±0.014,
0.727±0.013 and 0.713±0.015 mm for HJ300, HJ500 and luteolin,
respectively). After 26 h of TPA administration, HJ significantly
reduced the mean ear thickness in a concentration-dependent manner
and luteolin exerted the same effect (0.824±0.028 mm for vehicle
vs. 0.695±0.036, 0.617±0.029 and 0.636±0.015 mm for HJ300, HJ500
and luteolin, respectively) (Fig.
2B). The average ear weight at 26 h after TPA application was
considerably lower in the HJ500 and luteolin-treated groups
(0.100±0.004 g for vehicle vs. 0.086±0.003 and 0.080±0.004 g for
HJ500 and luteolin, respectively). However, a significant
difference was not observed in the HJ300 group (Fig. 2C). These results indicate that HJ
ameliorated ICD by reducing ear redness and oedema.
HJ attenuates epidermis and dermis
thickening in TPA-induced ear oedema
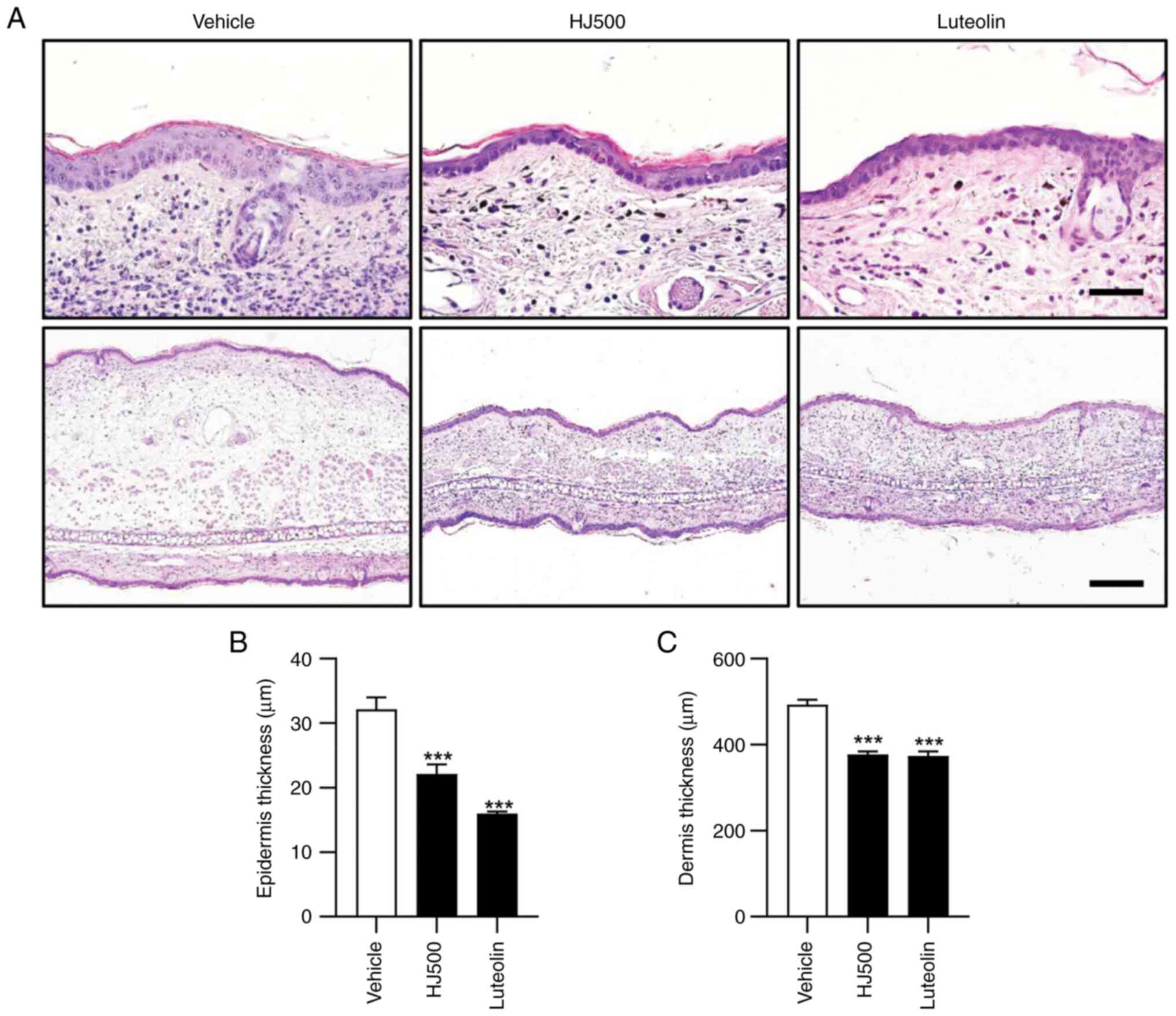
The effect of HJ on epidermis and dermis thickening
in mouse ears after TPA application was investigated. Given that
the HJ300 group did not exhibit obvious effects compared with the
other groups, only the HJ500 and luteolin groups were used for
further analysis. In H&E-stained histological sections of
TPA-treated mouse ears, HJ and luteolin administration decreased
epidermal (32.13±1.87 µm for vehicle vs. 22.10±1.53 and 15.96±0.33
µm for HJ500 and luteolin, respectively) and dermal thickness
(493.13±10.96 µm for vehicle vs. 377.07±7.32 and 373.65±10.47 µm
for HJ500 and luteolin, respectively) (Fig. 3A-C). Additionally, HJ and luteolin
inhibited the infiltration of immune cells into the dermis in the
TPA-treated right ears (Fig. 3A).
These results suggest that HJ may prevent TPA-induced ear oedema by
downregulating epidermal and dermal thickening and inflammatory
cell migration.
HJ reduces neutrophil and macrophage
recruitment in TPA-induced mouse ear inflammation
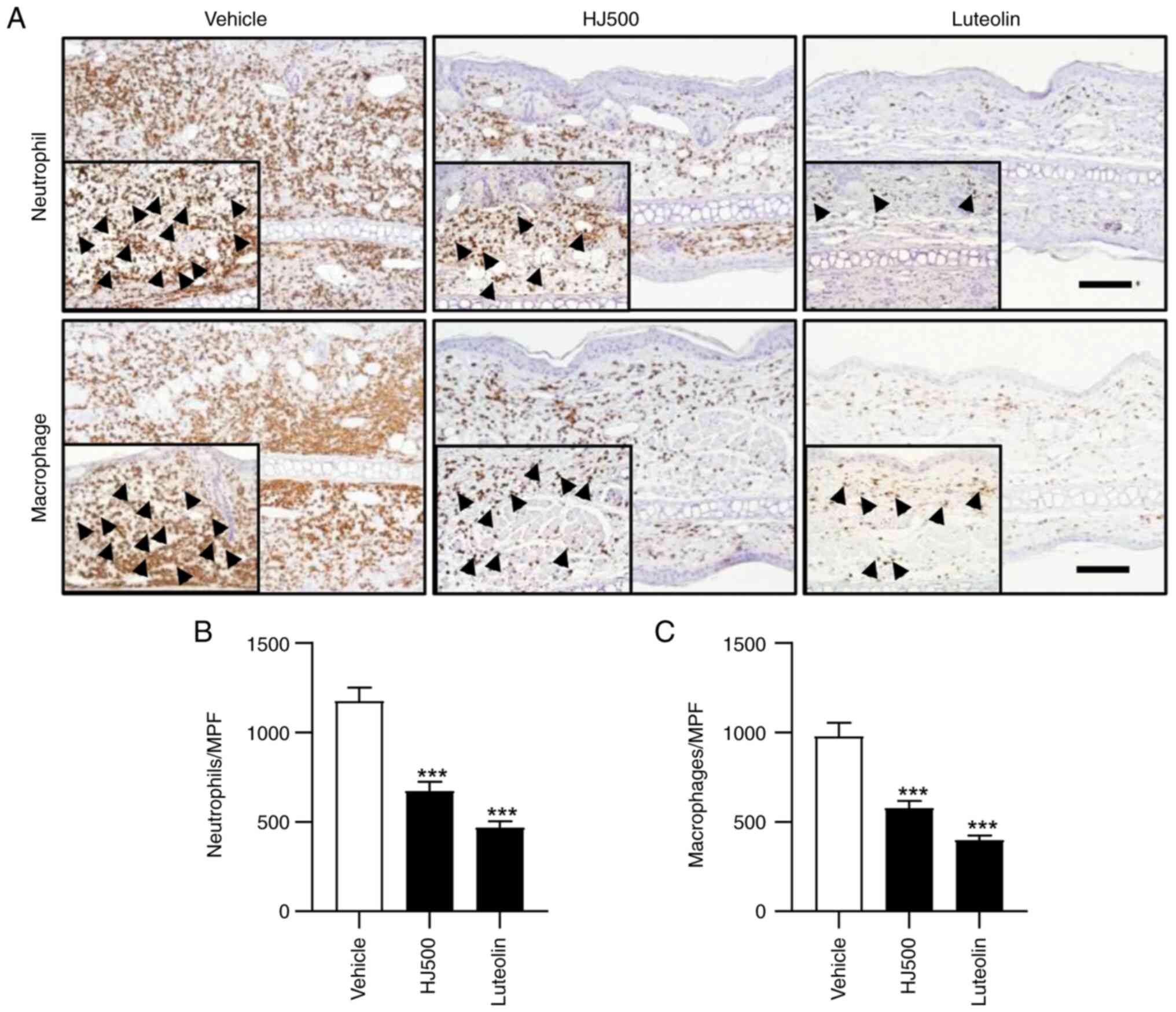
HJ and luteolin suppressed inflammatory cell
infiltration in TPA-treated mouse ears (Fig. 3A). According to a previous study on
ICD, exposure to an irritant activates innate immunity and cellular
recruitment to the damage site, and infiltrating cells, including
neutrophils and macrophages, further promote an inflammatory
cascade (24). Thus, the possible
inhibitory effect of HJ on neutrophil and macrophage recruitment in
mouse ears after TPA application was investigated. To measure the
degree of neutrophil and macrophage infiltration, TPA-treated mouse
ear tissue sections were stained with anti-neutrophil and
anti-F4/80 antibodies (Fig. 4A).
The number of neutrophils was significantly decreased in the HJ and
luteolin groups compared with that in the vehicle group
(1,174.34±76.03 cells for vehicle vs. 673.40±50.77 and 468.19±36.02
cells for HJ500 and luteolin, respectively) (Fig. 4B). HJ and luteolin also reduced
macrophages (977.53±75.33 cells for vehicle vs. 578.10±38.51 and
398.41±25.27 cells for HJ500 and luteolin, respectively) (Fig. 4C). These findings suggest that HJ
mitigated TPA-induced dermatitis by inhibiting the neutrophil and
macrophage infiltration in inflamed mouse ears.
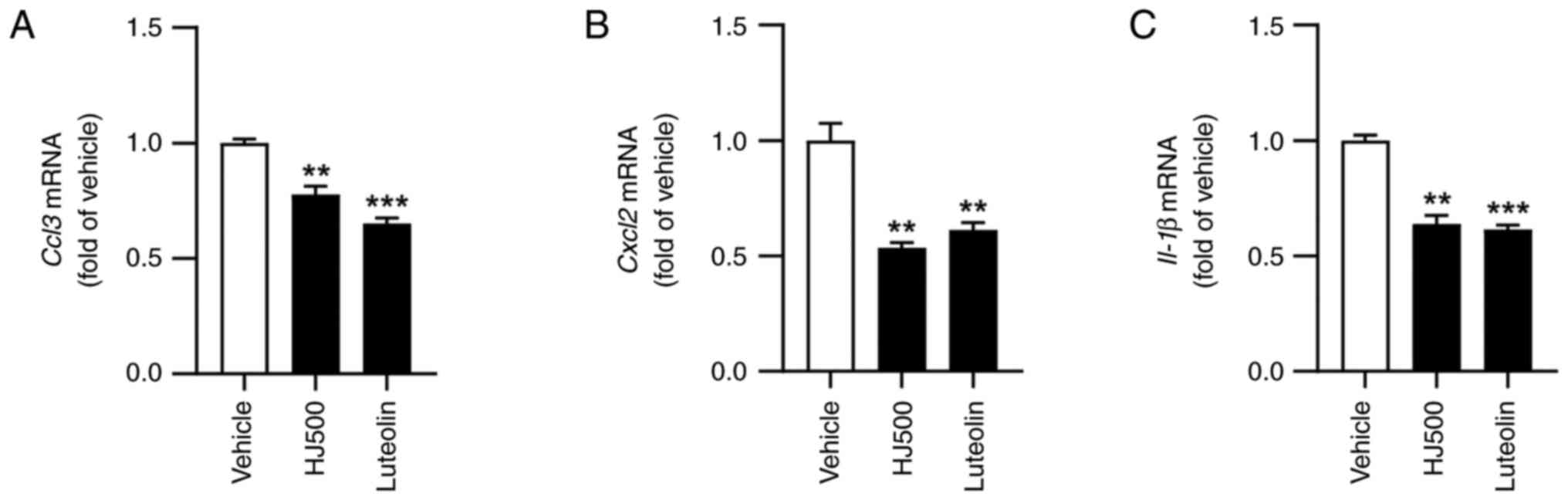
HJ decreases gene expression of
pro-inflammatory cytokine and chemokines associated with neutrophil
and macrophage migration in TPA-induced acute skin
inflammation
Based on the observation that HJ and luteolin affect
neutrophil and macrophage recruitment, the gene expression levels
of chemokines related to the migration of these cells (25,26)
were analysed. The gene expression of chemokine ligand 3 (CCL3) and
chemokine (C-X-C motif) ligand 2 (CXCL2), which recruit neutrophils
and macrophages in several inflammatory diseases and conditions
(27) was measured using RT-qPCR
analysis. In the TPA-induced dermatitis mice models, HJ and
luteolin significantly reduced CCL3 and CXCL2 gene expression
levels (Fig. 5A and B). Additionally, the gene expression of
IL-1β, a pro-inflammatory cytokine that mediates the acute phase of
inflammation (28), was examined;
HJ and luteolin also reduced IL-1β gene expression (Fig. 5C). Collectively, HJ suppressed
inflammation in TPA-induced dermatitis by reducing the expression
of chemokines that promote neutrophil and macrophage migration and
pro-inflammatory cytokine.
HJ inhibits the expression of
inflammatory mediators by modulating NF-κB p65 pathway signalling
in LPS-stimulated RAW264.7 cells
Various inflammatory cells, including macrophages,
are present in inflamed tissue lesions in contact dermatitis
(24). Particularly, macrophages
accumulate in acutely irritated skin and release inflammatory
mediators, such as chemokines, pro-inflammatory cytokines and
pro-inflammatory enzymes (29). It
is well known that macrophages are key immune cells which regulate
ICD progression. RAW264.7 cells are a mouse cell line extensively
used to study macrophage functions, mechanisms and signalling
pathways. Furthermore, in several previous studies on the
TPA-induced ICD mice model, the anti-inflammatory mechanisms of
candidate drugs were identified using LPS-stimulated RAW264.7 cells
(30-32).
Therefore, LPS-stimulated RAW264.7 cells could be a relevant cell
model for ICD. The present study investigated the inhibitory effect
of HJ on the gene expression of several inflammatory mediators in
LPS-stimulated RAW264.7 cells. Chemokines, CCL3 and
CXCL2, whose gene expression levels were decreased by HJ and
luteolin in TPA-treated mouse ears, were also reduced in
LPS-stimulated RAW264.7 cells after HJ and luteolin treatment
(Fig. 6A and B). Furthermore, the gene expression
levels of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α
were reduced in HJ- and luteolin-treated cells compared with those
in LPS-treated cells (Fig. 6C-E).
In addition, the decreased gene and protein expression levels of
pro-inflammatory enzymes, such as COX2 and iNOS, after HJ and
luteolin treatment (Fig. 6F-H)
were confirmed. These inflammatory mediators are commonly regulated
by the transcription factor NF-κB p65; phosphorylation of a
specific residue of NF-κB p65 regulates its transcriptional
activity and is involved in the severity of inflammation (33-35).
Thus, the protein expression of NF-κB p65 and phosphorylated NF-κB
p65 (p-NF-κB p65) in LPS-stimulated RAW264.7 cells was evaluated.
HJ and luteolin downregulated the protein expression of NF-κB p65
and p-NF-κB p65 (Fig. 6I).
Collectively, these results indicate that HJ inhibited the
expression of inflammatory mediators by regulating NF-κB p65 in
LPS-stimulated RAW264.7 cells.
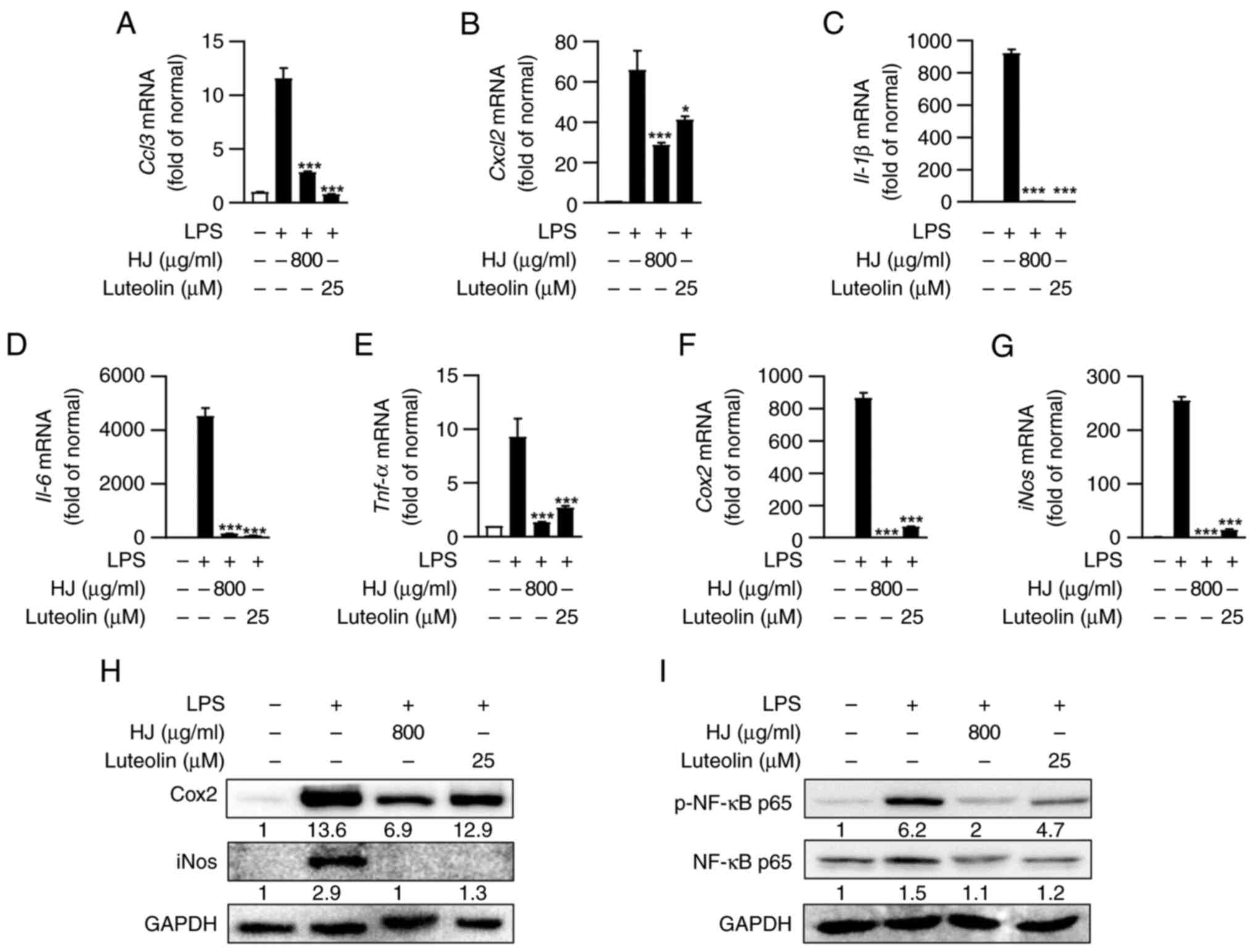 | Figure 6HJ suppresses the expression of
inflammatory mediators by regulating the NF-κB p65 signalling
pathway in LPS-stimulated RAW264.7 cells. (A-H) RAW 264.7 cells
were stimulated using LPS (1 µg/ml) for 24 h after pretreatment
with HJ (800 µg/ml) and luteolin (25 µM) for 1 h. Gene expression
levels of (A) Chemokine ligand 3, (B) Chemokine (C-X-C motif)
ligand 2, (C) IL-1β, (D) IL-6, (E) TNF-α, (F) COX2 and (G) iNOS
were analyzed using RT-qPCR. Gene expression levels were normalized
to 18S rRNA and shown as the fold change relative to the normal
control. (H) Protein levels of COX2 and iNOS were detected using
western blot analysis. Protein bands were quantified and normalized
using GAPDH as loading control and expressed as fold change
relative to the normal control. (I) RAW 264.7 cells were stimulated
using LPS (1 µg/ml) for 3 h after pretreatment with HJ (800 µg/ml)
and luteolin (25 µM) for 1 h. Protein levels of NF-κB p65 and
phosphorylated NF-κB p65 were detected using western blot analysis.
The protein level of NF-κB p65 was quantified and normalized using
GAPDH as the loading control. p-NF-κB p65 was quantified and
normalized using the total NF-κB p65 level. Data are expressed as
mean ± standard error of the mean and analyzed using one-way ANOVA
followed by Tukey's test. *P<0.05 and
***P<0.001 vs. LPS-treated group. HJ, Humulus
japonicus; CCL3, chemokine ligand 3; CXCL2, chemokine (C-X-C
motif) ligand 2; COX2, cyclooxygenase-2; iNOS, inducible nitric
oxide synthase; p-, phsopho-; LPS, lipopolysaccharide. |
Discussion
The present study examined the anti-dermatitis
actions of HJ ethanol extract using TPA-induced ICD mice models and
LPS-stimulated macrophages. Although the anti-inflammatory role of
HJ was already reported (17), its
effect and action mechanism in ICD remains unclear. Furthermore,
luteolin, the main compound in HJ, was reported to exert
anti-inflammatory effects by inhibiting various inflammatory
mediators and regulating NF-κB p65 in skin inflammation (22,36).
Thus, we hypothesized that HJ could alleviate inflammation in ICD
with luteolin as the main active compound.
The present authors previously reported that HJ
could suppress inflammation by regulating Th1 and Th2 cell-mediated
immune responses in chronic inflammatory conditions such as
arthritis (17). In the current
study, HJ regulated inflammation by inhibiting neutrophil and
macrophage migration to the damage site in the TPA-induced acute
ICD mice models. In acute inflammatory diseases, including ICD, the
inflammatory response is controlled by the innate immune system,
which is initiated and triggered in the presence of foreign
particles, known as damage-associated molecular patterns (DAMPs) or
pathogen-associated molecular patterns (PAMPs) (37). DAMPs are generated upon cellular
stress or tissue injury resulting from skin exposure to irritants
and induce potent inflammatory responses by activating the innate
immune system during non-infectious inflammation (38). A crucial role of the innate immune
system is the rapid recruitment of inflammatory cells to the
damaged tissues and the regulation of immune responses by producing
cytokines and chemokines (39).
When innate immune responses fail to eliminate DAMPs or PAMPs, the
adaptive immune system, mediated by antigen-specific T and B cells,
is activated, inducing the development of chronic inflammatory
disease (40). Therefore, although
HJ regulates the T cell-mediated immune responses in chronic
inflammatory states of arthritis, it is also suggested to mitigate
inflammation by modulating innate immune cells, such as neutrophils
and macrophages, in TPA-induced acute ICD.
TPA is an irritant commonly used to establish ICD
mice models, causing redness, oedema, epidermal hyperplasia, skin
barrier disruption and skin inflammation by triggering innate
immunity (41,42). In response to TPA-induced skin
irritation, epidermal keratinocytes release pro-inflammatory
cytokines and stimulate neighbouring cells to promote the release
of chemokine, which promote the recruitment of immune cells, such
as neutrophils and macrophages, to the injury site, and these cells
accelerate inflammatory responses (43,44).
Among the chemokine-recruited immune cells, neutrophils are one of
the first circulating inflammatory cells to infiltrate and are
essential for inflammatory responses in the skin (45). Macrophages also migrate during the
initiation phase of inflammation and contribute to the development
of skin diseases, including psoriasis (46). In the current study, TPA-stimulated
mouse ears showed an increased distribution of neutrophils and
macrophages; however, HJ and luteolin inhibited the recruitment of
these cells. Subsequently, to determine which factors reduce immune
cell migration in inflamed mouse ears, the mRNA levels of
chemokines associated with neutrophils and macrophages were
assessed. CCL3 and CXCL2 mediate the attraction of neutrophils and
macrophages during the initiation stage of ICD (26,47).
CCL3, which belongs to the CC chemokine family, and CXCL2, which
belongs to the CXC chemokine family, are secreted by epidermal
cells exposed to irritants during the acute phase of ICD. These
chemokines stimulate the infiltration and activation of neutrophils
and macrophages in inflamed lesions (48). Furthermore, CCL3 and CXCL2 are
released from recruited neutrophils and macrophages and affect each
other or migrate to other immune cells (49,50).
HJ downregulated the gene expression levels of both chemokines and
luteolin exerted a similar effect. According to the inhibitory
action of HJ on inflammatory cell infiltration, the mRNA expression
level of IL-1β secreted from various immune cells, including
neutrophils and macrophages (51),
was also decreased. These results suggest that HJ has a protective
effect against acute skin inflammation, which reduces the migration
of neutrophils and macrophages into the damage site by inhibiting
chemokine expression.
Macrophages play a pivotal role in the skin's immune
system (13). Infiltrated
macrophages are major producers of inflammatory mediators such as
CCL3, CXCL2 (chemokines), IL-1β, IL-6, TNF-α (pro-inflammatory
cytokines), COX2, and iNOS (pro-inflammatory enzymes) (52). Therefore, the anti-inflammatory
effect of HJ in RAW264.7 cells, a murine macrophage cell line, was
investigated. The present in vitro study revealed that HJ
and luteolin significantly reduced the gene expression of CCL3 and
CXCL2 in LPS-stimulated RAW264.7 cells. Moreover, IL-1β, IL-6,
TNF-α, COX2 and iNOS expression was considerably downregulated by
HJ and luteolin. These inflammatory mediators are generally
regulated by the NF-κB p65 signalling pathway in macrophages
(53). NF-κB p65 is an inducible
transcription factor that controls various genes involved in
diverse immunological and inflammatory responses (54). In macrophages, LPS stimulation
enhances NF-κB p65 transactivation via phosphorylation of the
serine residue (Ser536) (55).
Subsequently, NF-κB p65 phosphorylated at Ser536 is translocated to
the nucleus where it regulates the transcription of various
inflammation-related genes in macrophages (56). In the present study, HJ suppressed
the total NF-κB p65 and p-NF-κB p65 levels, and luteolin exerted a
similar effect. Collectively, these results indicate that HJ
inhibits NF-κB in activated macrophages, consequently reducing
immune cell migration and the expression of inflammatory
mediators.
If acute ICD does not improve, it can develop into
psoriasis, a chronic skin disease. Thus, ICD unavoidably shares
mechanical, clinical and histopathological similarities with
psoriasis (57). Accordingly,
various indicators identified in the present study, such as ear
thickness, ear weight, innate immune cells, CCL3, CXCL2 and IL-1β,
could also be relevant in psoriasis. Furthermore, these markers
were generally adopted and confirmed in several previous studies on
ICD (58-60).
Therefore, the current findings would be relevant to support the
protective effects of HJ on ICD.
In summary, the present findings demonstrate that HJ
ameliorates ICD by inhibiting immune cell infiltration and the
activation of macrophages, which are important inflammatory
modulators in dermatitis. To the best of our knowledge, this study
is the first to identify the effect of HJ in ICD. Furthermore, this
study provides evidence to support the use of HJ extract as a safe
therapeutic strategy for preventing ICD. However, since it is still
in the preclinical stage of efficacy verification, additional
investigation is needed before a direct application to patients
with ICD.
Acknowledgements
The authors would like to thank Mr. Dong-Hee Choi,
Mr. In Bok Lee, Mr. Young-Keun Choi, Mrs. Yun Jeong Seo and Mrs.
Jung Hyeon Choi (Laboratory Animal Resource Center, Korea Research
Institute of Bioscience and Biotechnology, Daejeon, Republic of
Korea) for their technical assistance.
Funding
Funding: This study was supported by the Korea Research
Institute of Bioscience and Biotechnology Research Initiative
Program (grant no. KGS1042322) and the Development of Measurement
Standards and Technology for Biomaterials and Medical Convergence
funded by Korea Research Institute of Standards and Science (grant
no. KRISS-2023-GP2023-0007).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YHK and CHL designed the study. YBK and EJK
performed the experiments and collected the data. WKO provided the
HJ extract and analyzed its active components. YHK, EJK, JRN, JPA,
JTP, WKO, YHK and CHL analysed and interpreted the data. YBK, YHK
and CHL wrote, revised and reviewed the manuscript. YBK, EJK, YHK
and CHL confirm the authenticity of all raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology (approval nos.
KRIBB-AEC-16210 and KRIBB-AEC-23089; Daejeon, Republic of
Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scheinman PL, Vocanson M, Thyssen JP,
Johansen JD, Nixon RL, Dear K, Botto NC, Morot J and Goldminz AM:
Contact dermatitis. Nat Rev Dis Primers. 7(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alinaghi F, Bennike NH, Egeberg A, Thyssen
JP and Johansen JD: Prevalence of contact allergy in the general
population: A systematic review and meta-analysis. Contact
Dermatitis. 80:77–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Patel K and Nixon R: Irritant contact
dermatitis-a review. Curr Dermatol Rep. 11:41–51. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jakasa I, Thyssen JP and Kezic S: The role
of skin barrier in occupational contact dermatitis. Exp Dermatol.
27:909–914. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bhatia R and Sharma VK: Occupational
dermatoses: An Asian perspective. Indian J Dermatol Venereol
Leprol. 83:525–535. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bains SN, Nash P and Fonacier L: Irritant
contact dermatitis. Clin Rev Allergy Immunol. 56:99–109.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsai TF and Maibach HI: How irritant is
water? An overview. Contact Dermatitis. 41:311–314. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rietschel RL: Mechanisms in irritant
contact dermatitis. Clin Dermatol. 15:557–559. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee HY, Stieger M, Yawalkar N and Kakeda
M: Cytokines and chemokines in irritant contact dermatitis.
Mediators Inflamm. 2013(916497)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fartasch M, Schnetz E and Diepgen TL:
Characterization of detergent-induced barrier alterations-effect of
barrier cream on irritation. J Investig Dermatol Symp Proc.
3:121–127. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Smith HR, Basketter DA and McFadden JP:
Irritant dermatitis, irritancy and its role in allergic contact
dermatitis. Clin Exp Dermatol. 27:138–146. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
McKenzie RC and Sauder DN: The role of
keratinocyte cytokines in inflammation and immunity. J Invest
Dermatol. 95 (Suppl 6):105S–107S. 1990.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tang H, Schlapbach C, Hassan AS, Simon D
and Yawalkar N: Characterization of dendritic cells and macrophages
in irritant contact dermatitis. J Dermatol Sci. 57:216–218.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pasparakis M, Haase I and Nestle FO:
Mechanisms regulating skin immunity and inflammation. Nat Rev
Immunol. 14:289–301. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Madsen M, Hansen PR, Nielsen LB,
Hartvigsen K, Pedersen AE, Christensen JP, Aarup A and Pedersen TX:
Effect of 12-O-tetradecanoylphorbol-13-acetate-induced
psoriasis-like skin lesions on systemic inflammation and
atherosclerosis in hypercholesterolaemic apolipoprotein E deficient
mice. BMC Dermatol. 16(9)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
de Groot AC: Systemic allergic dermatitis
(systemic contact dermatitis) from pharmaceutical drugs: A review.
Contact Dermatitis. 86:145–164. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kang EJ, Kim HJ, Choi JH, Noh JR, Kim JH,
Lee IB, Choi YK, Choi DH, An J, Oh WK, et al: Humulus japonicus
extract ameliorates collagen-induced arthritis in mice through
regulation of overall articular inflammation. Int J Mol Med.
45:417–428. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lim H, Noh JR, Kim YH, Hwang JH, Kim KS,
Choi DH, Go MJ, Han SS, Oh WK and Lee CH: Anti-atherogenic effect
of Humulus japonicus in apolipoprotein E-deficient mice. Int J Mol
Med. 38:1101–1110. 2016.
|
|
19
|
Sung B, Chung JW, Bae HR, Choi JS, Kim CM
and Kim ND: Humulus japonicus extract exhibits antioxidative and
anti-aging effects via modulation of the AMPK-SIRT1 pathway. Exp
Ther Med. 9:1819–1826. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Park HS, Kim YM and Kim HT: A clinical
study for the efficacy and safety of functional cosmetics
containing humulus japonicus extract in patients with dry skin due
to mild atopic dermatitis. J Korean Med Ophthalmol Otolaryngol
Dermatol. 32:24–58. 2019.
|
|
21
|
Lee HJ, Dhodary B, Lee JY, An JP, Ryu YK,
Kim KS, Lee CH and Oh WK: Dereplication of components coupled with
HPLC-qTOF-MS in the active fraction of humulus japonicus and it's
protective effects against Parkinson's disease mouse model.
Molecules. 24(1435)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gendrisch F, Esser PR, Schempp CM and
Wolfle U: Luteolin as a modulator of skin aging and inflammation.
Biofactors. 47:170–180. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dhingra N, Gulati N and Guttman-Yassky E:
Mechanisms of contact sensitization offer insights into the role of
barrier defects vs. intrinsic immune abnormalities as drivers of
atopic dermatitis. J Invest Dermatol. 133:2311–2314.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vilgelm AE and Richmond A: Chemokines
modulate immune surveillance in tumorigenesis, metastasis, and
response to immunotherapy. Front Immunol. 10(333)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ridiandries A, Tan JTM and Bursill CA: The
role of chemokines in wound healing. Int J Mol Sci.
19(3217)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mavrogianni D, Tsaftaridis P, Terpos E,
Symeonidis A, Galanopoulus A, Papadaki EA, Zoumbos N, Meletis J,
Pangalis GA and Viniou N: Macrophage Inflammatory Protein-1 alpha
(MIP-1alpha) is over-expressed in a cohort of patients with
myelodysplastic syndromes. Eur J Haematol. 75:85–86.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abramovits W, Rivas Bejarano JJ and
Valdecantos WC: Role of interleukin 1 in atopic dermatitis.
Dermatol Clin. 31:437–444. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
O'Neill LA: Toll-like receptor signal
transduction and the tailoring of innate immunity: A role for Mal?
Trends Immunol. 23:296–300. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim NY, Cheong SH, Lee KJ, Sok DE and Kim
MR: Anti-Inflammatory effects of ribes diacanthum pall mediated via
regulation of Nrf2/HO-1 and NF-kappaB signaling pathways in
LPS-Stimulated RAW 264.7 macrophages and a TPA-Induced dermatitis
Animal Model. Antioxidants (Basel). 9(622)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu J, Huang H, Huang Z, Ma Z, Zhang L, He
Y, Li D, Liu W, Goodin S, Zhang K and Zheng X: Eriocitrin in
combination with resveratrol ameliorates LPS-induced inflammation
in RAW264. 7 cells and relieves TPA-induced mouse ear edema. J
Funct Foods. 56:321–332. 2019.
|
|
32
|
Xu X, Huang D, Liu W, Sheng Z, Liang K, Li
D, Zhao D, Ma Y, Zhang K, Hayat T, et al: Evaluation of the
anti-inflammatory properties of telmesteine on
inflammation-associated skin diseases. RSC Adv. 7:34699–34704.
2017.
|
|
33
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866.
1999.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Christian F, Smith EL and Carmody RJ: The
regulation of NF-κB subunits by phosphorylation. Cells.
5(12)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hoffmann A, Natoli G and Ghosh G:
Transcriptional regulation via the NF-kappaB signaling module.
Oncogene. 25:6706–6716. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aziz N, Kim MY and Cho JY:
Anti-inflammatory effects of luteolin: A review of in vitro, in
vivo, and in silico studies. J Ethnopharmacol. 225:342–358.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Turvey SE and Broide DH: Innate immunity.
J Allergy Clin Immunol. 125 (2 Suppl 2):S24–S32. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Roh JS and Sohn DH: Damage-Associated
molecular patterns in inflammatory diseases. Immune Netw.
18(e27)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Murphy K and Weaver C: Janeway's
Immunobiology. Garland Science, New York, NY, 2016.
|
|
40
|
Janeway CA Jr, Travers P, Walport M and
Shlomchik MJ: Principles of innate and adaptive immunity. In:
Immunobiology: The Immune System in Health and Disease. 5th
edition. Garland Science, New York, NY, 2001.
|
|
41
|
Hvid H, Teige I, Kvist PH, Svensson L and
Kemp K: TPA induction leads to a Th17-like response in transgenic
K14/VEGF mice: A novel in vivo screening model of psoriasis. Int
Immunol. 20:1097–1106. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zielinska M, Ben Haddou T, Cami-Kobeci G,
Sałaga M, Jarmuż A, Padysz M, Kordek R, Spetea M, Husbands SM and
Fichna J: Anti-inflammatory effect of dual nociceptin and opioid
receptor agonist, BU08070, in experimental colitis in mice. Eur J
Pharmacol. 765:582–590. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wei WC, Lin SY, Chen YJ, Wen CC, Huang CY,
Palanisamy A, Yang NS and Sheu JH: Topical application of marine
briarane-type diterpenes effectively inhibits
12-O-tetradecanoylphorbol-13-acetate-induced inflammation and
dermatitis in murine skin. J Biomed Sci. 18(94)2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Slodownik D, Lee A and Nixon R: Irritant
contact dermatitis: A review. Australas J Dermatol. 49:1–9; quiz
10-11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Calhoun KN, Luckett-Chastain LR, Frempah B
and Gallucci RM: Associations between immune phenotype and
inflammation in murine models of irritant contact dermatitis.
Toxicol Sci. 168:179–189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kamata M and Tada Y: Dendritic cells and
macrophages in the pathogenesis of psoriasis. Front Immunol.
13(941071)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yan BX, Chen XY, Wang ZY, Cui YZ, Landeck
L, Fu NC, Yang XY, Xu F, Zhou Y, Chen JQ and Man XY: Mupirocin
blocks imiquimod-induced psoriasis-like skin lesion by inhibiting
epidermal isoleucyl-tRNA synthetase. Cell Commun Signal.
20(185)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fyhrquist-Vanni N, Alenius H and Lauerma
A: Contact dermatitis. Dermatol Clin. 25:613–623, x.
2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sherry B, Tekamp-Olson P, Gallegos C,
Bauer D, Davatelis G, Wolpe SD, Masiarz F, Coit D and Cerami A:
Resolution of the two components of macrophage inflammatory protein
1, and cloning and characterization of one of those components,
macrophage inflammatory protein 1 beta. J Exp Med. 168:2251–2259.
1988.PubMed/NCBI View Article : Google Scholar
|
|
50
|
O'Donovan N, Galvin M and Morgan JG:
Physical mapping of the CXC chemokine locus on human chromosome 4.
Cytogenet Cell Genet. 84:39–42. 1999.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lopez-Castejon G and Brough D:
Understanding the mechanism of IL-1β secretion. Cytokine Growth
Factor Rev. 22:189–195. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Viola A, Munari F, Sanchez-Rodriguez R,
Scolaro T and Castegna A: The metabolic signature of macrophage
responses. Front Immunol. 10(1462)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang N, Liang H and Zen K: Molecular
mechanisms that influence the macrophage m1-m2 polarization
balance. Front Immunol. 5(614)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023-)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sharif O, Bolshakov VN, Raines S, Newham P
and Perkins ND: Transcriptional profiling of the LPS induced
NF-kappaB response in macrophages. BMC Immunol. 8(1)2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hobbs S, Reynoso M, Geddis AV, Mitrophanov
AY and Matheny RW Jr: LPS-stimulated NF-κB p65 dynamic response
marks the initiation of TNF expression and transition to IL-10
expression in RAW 264.7 macrophages. Physiol Rep.
6(e13914)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kamsteeg M, Jansen PA, van Vlijmen-Willems
IM, van Erp PE, Rodijk-Olthuis D, van der Valk PG, Feuth T, Zeeuwen
PL and Schalkwijk J: Molecular diagnostics of psoriasis, atopic
dermatitis, allergic contact dermatitis and irritant contact
dermatitis. Br J Dermatol. 162:568–578. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lee DY, Choi G, Yoon T, Cheon MS, Choo BK
and Kim HK: Anti-inflammatory activity of Chrysanthemum indicum
extract in acute and chronic cutaneous inflammation. J
Ethnopharmacol. 123:149–154. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Frempah B, Luckett-Chastain LR, Calhoun KN
and Gallucci RM: Keratinocyte-specific deletion of the IL-6RΑ
exacerbates the inflammatory response during irritant contact
dermatitis. Toxicology. 423:123–131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Liu W, Huang S, Li Y, Zheng X and Zhang K:
Synergistic effect of tolfenamic acid and glycyrrhizic acid on
TPA-induced skin inflammation in mice. Medchemcomm. 10:1819–1827.
2019.PubMed/NCBI View Article : Google Scholar
|