Introduction
Triple-negative breast cancer (TNBC) is an
aggressive disease with a high incidence of recurrence and
metastasis, which often exhibits therapeutic resistance, and is
associated with poor survival outcomes. Tumor recurrence is one of
the primary causes of failure of radiotherapy, and cells that
survive radiotherapy often exhibit rapid proliferative rates
(1-3).
A series of genetic and epigenetic disturbances are reported to be
involved in the radioresistance mechanisms of tumor cells (4,5).
Therefore, research on specific molecular targets and the tumor
immune microenvironment is gradually resulting in advances in
malignant tumor treatment regimens (6,7).
Combination therapy of traditional therapies with targeted drugs or
immune checkpoint inhibitors has seen increased popularity for the
management of malignant tumors.
Receptor tyrosine kinases (RTKs) are the most
widespread type of enzyme-linked receptors in humans and include
the EGFR family and the VEGFR family, which are widely targeted in
clinical practice. By binding to ligands, RTKs induce the
phosphorylation of tyrosine residues of downstream target proteins,
causing downstream intracellular effects, and play an important
role in the occurrence and development of various types of
malignant tumors (8). Axl is a
transmembrane protein belonging to the TAM (TYRO3, AXL, and MERTK)
family of RTKs. Following the binding and activation by
extracellular ligands, the conformation of Axl is altered to form
homodimers or heterodimers. Phosphorylation of tyrosine residues in
the intracellular domain activates the kinase activity and induces
signaling cascades, including the JAK/STAT, PI3K/Akt,
RAS/RAF/MEK/ERK, and NF-κB signaling pathways, which are widely
involved in various biological processes such as tissue generation,
cell proliferation, cellular adhesion and recognition, inhibition
of apoptosis, malignant transformation, and treatment resistance
(9).
In recent years, various studies have shown that Axl
overexpression is closely associated with drug resistance to
targeted therapy, chemotherapy, and immunotherapy. Previous studies
have found that mouse breast cancer tumors with high Axl expression
exhibit resistance to combination therapy consisting of ionizing
radiation and immune checkpoint inhibitors. Inhibition of Axl
expression enhances antigen presentation, affects cytokine
secretion, regulates immune response in tumors, and ultimately
increases the lethality of radiotherapy (10). When drugs target malignant tumors,
the phosphorylation of Axl is also upregulated, followed by the
activation of the downstream cell survival signaling pathways, and
this eventually leads to treatment resistance (11). Inhibition of Axl expression or its
phosphorylation reduces cell proliferation and migration while
maintaining therapeutic drug sensitivity (12-15).
Previous studies have shown that Axl activation
promotes the expression of anti-apoptotic proteins, inhibits the
expression of pro-apoptotic proteins, and ultimately promotes cell
survival by activating the downstream PI3K/Akt signaling pathway
(16,17). It can also induce the expression of
matrix metalloproteins (MMPs), degrade the extracellular matrix,
and promote the invasion and migration of tumor cells (16,18-20).
The activation of the PI3K pathway induces the phosphorylation of
Akt and mTOR, leading to an increase in the proliferation of breast
cancer cells and the occurrence of distant metastasis (21).
Ionizing radiation induces a series of
survival-promoting signaling pathways whilst destroying a tumor.
The activation of the PI3K/Akt signaling pathway following exposure
of malignant tumors to ionizing radiation is often observed
(22). The activation of the
PI3K/Akt signaling pathway usually indicates the resistance of
tumor cells to ionizing radiation. Inhibiting the activation of the
PI3K/Akt signaling pathway increases the radiosensitivity of
various malignant tumor cells, and the potential mechanism may be
associated with the inhibition of DNA damage repair and increased
cell apoptosis (23).
In the present study, the radioresistance of the
TNBC cells that had survived ionizing radiation was investigated,
and the potential value of targeting Axl combined with radiotherapy
in the treatment of TNBC is discussed.
Materials and methods
Tumor tissues and
immunohistochemistry
A total of 45 surgical resection specimens
(including cancer tissue and paracancerous tissue) of primary TNBC
were collected from the biological sample bank of Zhejiang Cancer
Hospital (Zhejiang, China). The median age (range) at diagnosis was
56 years (37 to 69). The paracancerous tissue was at least 2 cm
away from the edge of the tumor tissue. The clinical data of the
specimens were obtained from the medical records of the hospital.
Immunohistochemistry was used to detect the expression of Axl in
TNBC tissues and adjacent paracancerous tissues, as well as the
expression of pAkt in cancerous tissues. The study protocol was
approved by the Ethics Committee of Zhejiang cancer hospital
(approval no, IRB-2020-367). For statistical analysis, the degree
of positive staining was classified as negative (0), weakly
positive (+), moderately positive (++), and strongly positive
(+++), corresponding to the tissues with <10%, 10-25%, 25-75%
and >75% of the tissue stained positive, respectively.
Cell culture and treatment
The mouse TNBC cell line 4T-1 was purchased from the
ATCC cell bank represented by the Beijing Beina Chuanglian
Institute of Biotechnology (cat. no. BNCC273810). Cells were
maintained in RPMI-1640 Medium (HyClone; Cytiva), supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin, and 100 µg/ml streptomycin (Biosharp, Inc.) and
maintained in a humidified incubator at 37˚C supplied with 5%
CO2 air. R428 was purchased from APExBIO Technology and
dissolved in PBS (HyClone; Cytiva).
Establishment of the ionizing
radiation-resistant cell line
The 4T-1 cells resistant to ionizing radiation
(henceforth referred to as 4T-1/IRR) were established using a
gradient irradiation method. 4T-1 cells in the logarithmic growth
phase were cultured in a 25 cm2 culture flask and
irradiated using a 2 Gy X-ray initially. Cells were passaged and
irradiated again with 2 Gy X-ray when the cell density reached
80-90%. The aforementioned 4T-1 cells were irradiated by gradually
increasing doses to 4, 6, 8, and finally 10 Gy, with cells being
irradiated with each dose twice, such that the total irradiation
dose was 60 Gy. After continued culturing and passaging for 15 days
after each irradiation treatment, a few of the cells survived and
proliferated stably. An MTT assay and colony formation assay were
used to identify ionizing radiation-resistant cell lines.
Lentiviral infection
Cell suspension with a density of 3-5x104
cells/ml was prepared by RPMI-1640 complete medium. Then
6-10x104 cells in 2 ml cell suspension were inoculated
into each well of 6-well plates and incubated for 16-24 h to 20-30%
confluency as above. The small interfering RNA (siRNA) sequences
used were as follows: siAxl, TGTCTGCATGAAGGAATTT and NC,
TTCTCCGAACGTGTCACGT. During lentiviral infection, the volume of
serum-free medium in each well of the six-well plate was 1 ml; 40
µl HiTransG A infection reagent (Shanghai GeneChem, Co., Ltd.) and
the appropriate amount of lentivirus (Shanghai GeneChem, Co., Ltd.)
were added to 1 ml serum-free RPMI-1640 medium. The amount of
lentivirus added to each well (µl) was calculated as follows;
multiplicity of infection (MOI) x number of cells/titer (TU/ml)
x1,000, where the MOI value was set to 10. The infection medium was
replaced with fresh supplemented RPMI-1640 medium following
culturing for 12 h. The cells were further cultured for 48 h, after
which the cells were observed under a fluorescence microscope. The
infection efficiency was >95%.
MTT assay
The cells were digested, and a
5x103-1x104 cells/ml cell suspension was
prepared, of which, 100 µl was added to each well of the 96-well
cell culture plate. The seeded cells were cultured for 24 h.
4T-1/IRR cells were treated with different concentrations of R428,
and then the lethal IC50 dose was calculated. To
investigate the role of Axl played in the radioresistance of TNBC
cells, the 4T-1/IRR cells were treated with R428, infected with NC
or Axl siRNA lentivirus, respectively, and then irradiated with 0
or 4 Gy X-rays; 4 Gy X-ray was regarded as the most suitable dose
for the viability test, as it brought about a decrease in cell
viability while minimizing the impact of ionizing radiation damage
on experimental results. The cell viability was measured using MTT
assays after 24 and 48 h. In the dark, 20 µl MTT solution (5 mg/ml)
(Beijing Solarbio Science & Technology Co., Ltd.) was added to
each well, and the cells were incubated for 4 h. The supernatant in
each well was discarded and replaced with 150 µl DMSO (Beijing
Solarbio Science & Technology Co., Ltd.) to dissolve the
formazan. The optical density (OD) values were measured at 492 nm
on a microplate reader. The cell viability was calculated using the
following formula: Cell viability (%)=(OD492 value of experimental
group/OD492 value of control group) x100%.
Colony formation assay
The cells in the logarithmic growth phase were
digested and seeded into six-well plates with gradient densities of
500, 1,000, 2,000, 3,000, and 4,000 cells/well. The detection of
the colony formation ability of 4T-1/IRR cells needs to fit the
dose-survival curve, which was derived from a multi-target
single-hit model as follows: survival fraction
(SF)=1-(1-exp-D/D0)n (24,25).
The numbers of colonies formed after 0, 2, 4, 6, or 8 Gy X-ray
irradiation are usually used to fit the dose-survival curve. This
is a routine operation method for radiobiology tests (24,25).
The plates were irradiated with 2, 4, 6, or 8 Gy X-rays,
respectively, and subsequently incubated for 10-14 days. Following
incubation, the culture supernatant was discarded, and the cells
were fixed at room temperature for 20 min with methanol and stained
at room temperature for 30 min with crystal violet staining
solution. The SF was calculated according to the following formula:
SF=number of colonies/(number of inoculated cells x plating
efficiency). The number of colonies with >50 cells was counted
under a inverted microscope (Olympus Corporation; magnification,
x40). The dose-survival curve was derived from a multi-target
single-hit model as follows:
SF=1-(1-exp-D/D0)n; where D is
the radiation dose (Gy), D0 is the average lethal dose,
Dq is the quasi-domain dose, and N is the extrapolation. The
sensitization enhancing ratio (SER) was calculated as follows:
D0 of the treatment group/D0 of the control
group.
Mouse TNBC 4T-1/IRR cell
xenografts
The female 5-week-old BALB/c mice were purchased
from Shanghai SLAC Laboratory Animal Co., Ltd. The mice were housed
under standard laboratory conditions (25±1˚C with 12-h light-dark
cycle) and given access to sterilized food and water. The mice were
treated in accordance with the ARRIVE guidelines, the UK Animals
(Scientific Procedures) Act, 1986, and associated guidelines
(26). The cells in each group
were inoculated subcutaneously into the right posterior flank of
BALB/c mice. A total of 1x106 cells suspended in 200 µl
PBS were subcutaneously injected into the right posterior flank of
the BALB/c mice. Each group consisted of five mice. The length and
width of the transplanted tumors were measured every 5 days, and
then the tumor volume was calculated using the formula: Volume
(mm3)=length x width2 (mm2)/2. The
maximum diameter of the tumor did not exceed 15 mm. The Small
Animal Radiation Research Platform (SARRP; Xstrahl) was used to
provide a single dose of 6 Gy 220 KV X-ray irradiation.
Intratumoral injection with R428 was used to inhibit the Axl
expression of the transplanted tumors. The volume of the
transplanted tumor was calculated to generate the growth curve. The
mice were sacrificed 20 days after irradiation, and the
transplanted tumor was completely removed. Mice were anesthetized
with 1% sodium pentobarbital (50 mg/kg) by intraperitoneal
injection prior to radiotherapy. The mice were euthanized by
cervical dislocation after anesthetization with 1% sodium
pentobarbital (50 mg/kg) through intraperitoneal injection. The
death of animals was confirmed by a lack of heartbeat, breathing,
dilation of the pupils, and nerve reflexes.
Wound healing assay
Wound healing assays were used to assess the
migratory ability of tumor cells. Cells were seeded into 6-well
plates, treated as described above, and cultured to 80-90%
confluency, typically 24 h. The monolayers were scratched using a
200 µl sterile pipette tip and washed with PBS to discard the
floating and detached cells. Then, fresh serum-free RPMI-1640
medium was added, and the cells were further cultured. The cells
were observed, and images were taken at 0 and 48 h to measure the
wound closure distance. The wound closure rates were calculated as
follows: (Wound distance of cells after 48 h/wound distance of
initial scratch) x100.
Transwell invasion assay
The invasive ability of the cells was assessed using
24-well Matrigel-coated Transwell chambers with 8 µm pore-size
membranes (Corning Inc.). A total of 5x104/ml cells from
each group in serum-free medium were added to the upper chamber.
The inserts were pre-coated with 50 µl Matrigel (BD Biosciences)
diluted with serum-free RPMI-1640 medium at a ratio of 1:8. In the
bottom chamber, 500 µl medium supplemented with 20% FBS was added.
After incubation for 48 h, the Matrigel and cells in the upper
chambers were removed. The cells that had invaded to the lower
chambers were fixed at room temperature for 20 min with 4%
paraformaldehyde, stained at room temperature for 30 min with
crystal violet, and imaged under a light microscope (Olympus
Corporation; magnification, x200).
Immunofluorescence analysis
The rapid phosphorylation of histone H2AX at serine
139 (γH2AX) can be detected by immunofluorescence as DNA
double-stranded breaks (DSBs) induced by ionizing radiation. Cells
cultured and treated on sterile cell culture slides in 24-well
plates, were fixed with 3.7% paraformaldehyde at room temperature
for 20 mins, permeabilized with 0.5% Triton X-100, blocked with 1%
BSA at room temperature for 2 h, and incubated with an anti-γH2AX
antibody (cat. no. ab22551; 1:1,000; Abcam) at 4˚C overnight.
Subsequently, the cells were washed and incubated with a
TRITC-conjugated anti-rabbit secondary antibody (cat. no. ab6786;
1:1,000; Abcam), followed by counterstaining with DAPI (Beyotime
Institute of Biotechnology) at room temperature for 5 mins to mark
the nucleus. The immunofluorescence-stained slices were observed
and imaged using a fluorescence microscope. The mean number of
γH2AX foci per nucleus was quantified in each group.
Western blotting
Cells were lysed in lysis buffer (Thermo Fisher
Scientific, Inc.) containing phosphatase inhibitors (Thermo Fisher
Scientific Inc.) and PMSF (Thermo Fisher Scientific Inc.). The
proteins were loaded on an 10% SDS gel, resolved using SDS-PAGE,
and transferred to PVDF membranes (MilliporeSigma). Subsequently,
the membranes were blocked with 5% skimmed milk, incubated with
primary antibodies against pPI3K (cat. no. ab182651; 1:200; Abcam),
PI3K (cat. no. ab191606; 1:1,000; Abcam), pAxl (cat. no. 96453;
1:1,000; Cell Signaling Technology, Inc.), Axl (cat. no. ab215205;
1:1,000; Abcam), pAkt (cat. no. ab81283; 1:2,000; Abcam), Akt (cat.
no. ab38499; 1:1,000; Abcam), mTOR (cat. no. ab2732; 1:2,000;
Abcam), PTEN (cat. no. ab32199; 1:5,000; Abcam), or GAPDH (cat. no.
ab8245; 1:2,000; Abcam), followed by incubation with the relevant
horseradish peroxidase-conjugated secondary antibody (anti-Rabbit;
cat. no. ab205718; anti-Mouse: cat. no. ab6789; Abcam). GAPDH was
used as the internal control. Signals were visualized using a Laser
Holographic imager (Azure Biosystems).
Statistical analysis
Graph Pad Prism version 5.0 (GraphPad Software,
Inc.) and SPSS version 20.0 (IBM Corp.) were used to analyze the
data. A Mann-Whitney U test was applied to analyze the clinical
data of the TNBC patients. A one-way ANOVA with post hoc
Bonferroni's multiple comparison corrections was used to compare
the differences between groups in vitro or in vivo.
Data are presented as the mean ± standard deviation.
*P<0.05 was considered to indicate a statistically
significant difference. The multi-target single-hit model was used
to fit the dose-survival curves. The ability of a radiation
sensitizer is represented by the SER. SER is the ratio between the
radiation dose that achieves a certain effect in the absence of the
sensitizer and the radiation dose that achieves the same effect in
the presence of the sensitizer. SER >1 was considered to be
indicative of a radiation sensitization effect, whereas an SER
<1 was considered to indicate a radiation-resistant effect.
Results
Axl and pAkt expression in TNBC
tissues
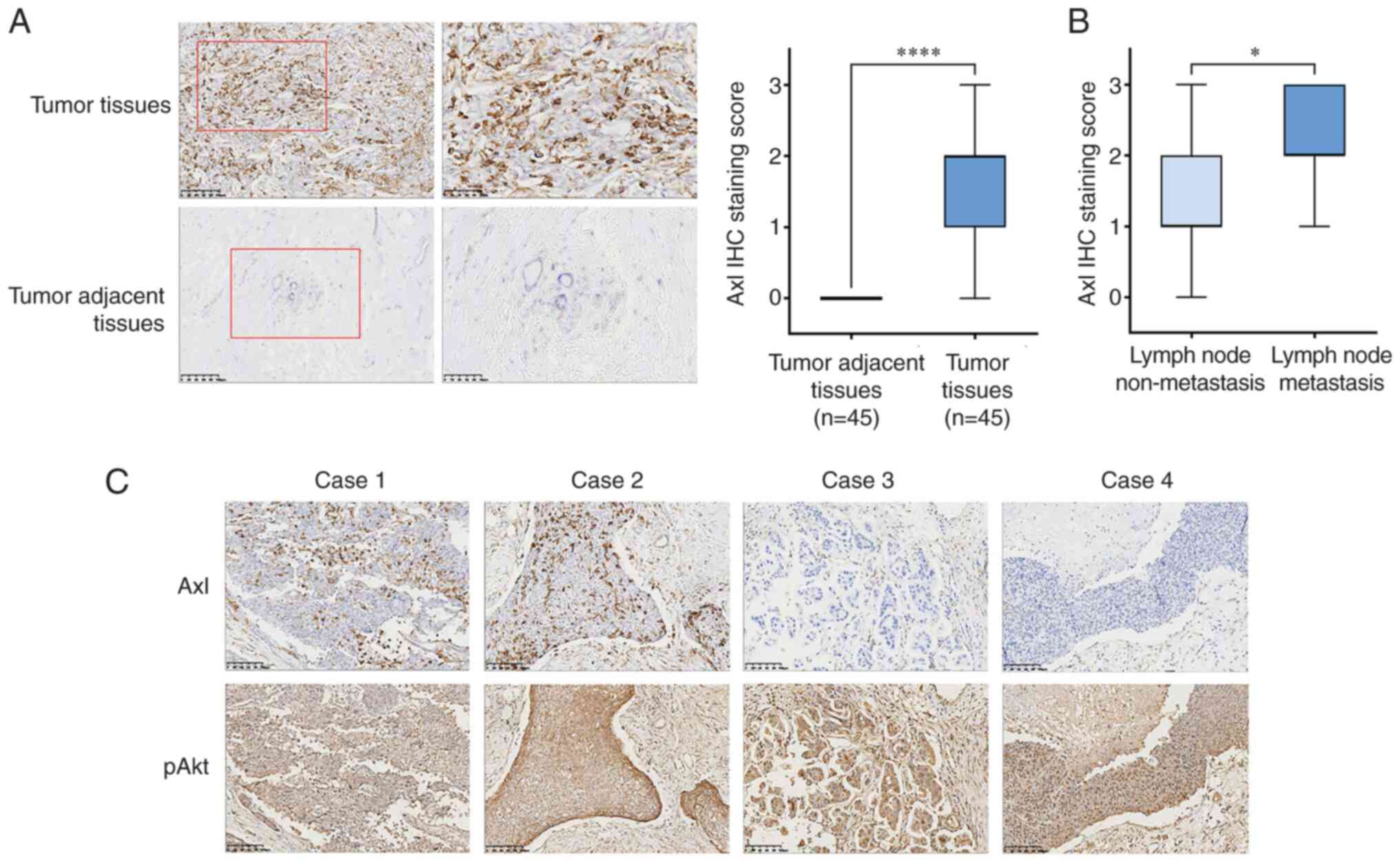
The results of immunohistochemical staining showed
that the Axl protein was primarily expressed in the cytoplasm of
TNBC cells, although some expression was seen in the nucleus
(Fig. 1A). The positive expression
rate of Axl in 45 TNBC cases was 88.9% (40/45), of which 26 cases
were ++ - +++, while that in the adjacent tissues was 0%. It was
thus hypothesized that Axl may play an important role in the
occurrence and development of TNBC.
As shown in Fig.
1B, further analysis of the patients' clinicopathological
characteristics showed that 16 (35.56%) of 45 TNBC patients had
positive axillary lymph node (ALN) metastasis, and Axl expression
in the TNBC cases with ALN metastasis was significantly higher than
that without lymph node metastasis (P<0.05). pAkt expression was
found to be widely distributed in the cytoplasm of tumor cells
(Fig. 1C), and the positive
expression rate was 100% (45/45). Therefore, it was concluded that
the expression of Axl was upregulated in the majority of TNBC
patients, and the PI3K/pAkt signaling pathway was an important
molecular signaling pathway that modulated the biological processes
in TNBC.
Influence of ionizing radiation on the
expression of pAxl and its downstream signaling molecules in 4T-1
cells
The expression levels of pAxl, Axl, and the
downstream signaling pathway molecules including pPI3K, PI3K, pAkt,
Akt, mTOR, and PTEN in 4T-1 cells were detected after 0, 24, 48,and
72 h after 2 Gy X-ray irradiation (Fig. 2A), respectively. The results showed
that the protein expression levels of pAxl, Axl, pPI3K, pAkt, and
mTOR increased gradually as the irradiation time increased. This
suggested that in 4T-1 cells, a series of signaling cascades were
initiated after irradiation to protect against and gradually repair
ionizing radiation damage.
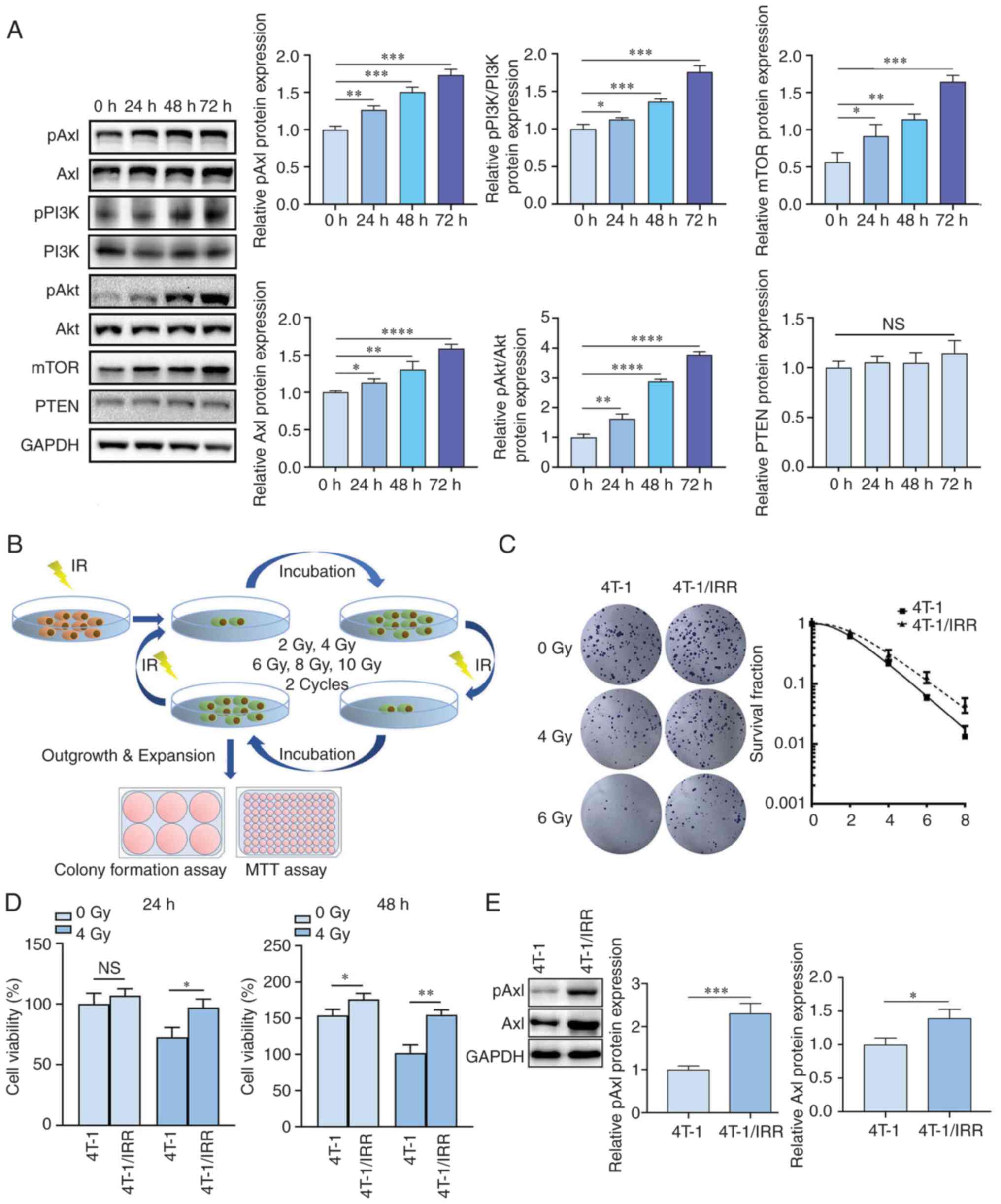 | Figure 2Establishment and identification of
ionizing radiation-resistant mouse TNBC 4T-1/IRR cells. (A) pAxl,
Axl, pPI3K, PI3K, pAkt, Akt, mTOR, and PTEN expression in 4T-1
cells at different times following irradiation. (B) The
construction process of 4T-1/IRR cells using a gradient irradiation
method. The (C) colony formation rate and (D) viability of 4T-1/IRR
cells was increased. (E) pAxl and Axl expression in 4T-1/IRR cells.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. NS, not
significant; IRR, ionizing radiation resistance; TNBC,
triple-negative breast cancer. |
Verification of ionizing
radiation-resistant ability for 4T-1/IRR cells
The method used to establish 4T-1/IRR cells is shown
in Fig. 2B. Following irradiation
with different doses of X-rays, the colony formation rate of
4T-1/IRR cells was notably higher than that of 4T-1 cells
(SER=0.8719; Fig. 2C). As shown in
Fig. 2D, 24 h after 4 Gy X-ray
irradiation, the viability of 4T-1/IRR cells was significantly
higher than that of 4T-1 cells (97.00±7.03% vs. 72.70±8.02%,
P<0.05). After 48 h of cell culture, it was found that in the
non-irradiation group, the viability of 4T-1/IRR cells was higher
than that of 4T-1 cells (175.97±8.28% vs. 154.00±8.19%, P<0.05).
A total of 48 h after X-ray irradiation, the difference between the
two groups became more significant (154.67±6.76% vs. 101.67±11.24%,
P<0.01). The above results confirmed that the colony formation
and proliferative ability of 4T-1/IRR cells were significantly
higher than that of 4T-1 cells, and it was confirmed that 4T-1/IRR
cells had acquired radioresistance. The expression levels of pAxl
and Axl in 4T-1/IRR cells were higher than those in 4T-1 cells,
particularly for pAxl, suggesting that Axl activation likely
participated in the acquisition of radioresistance in TNBC cells
(Fig. 2E).
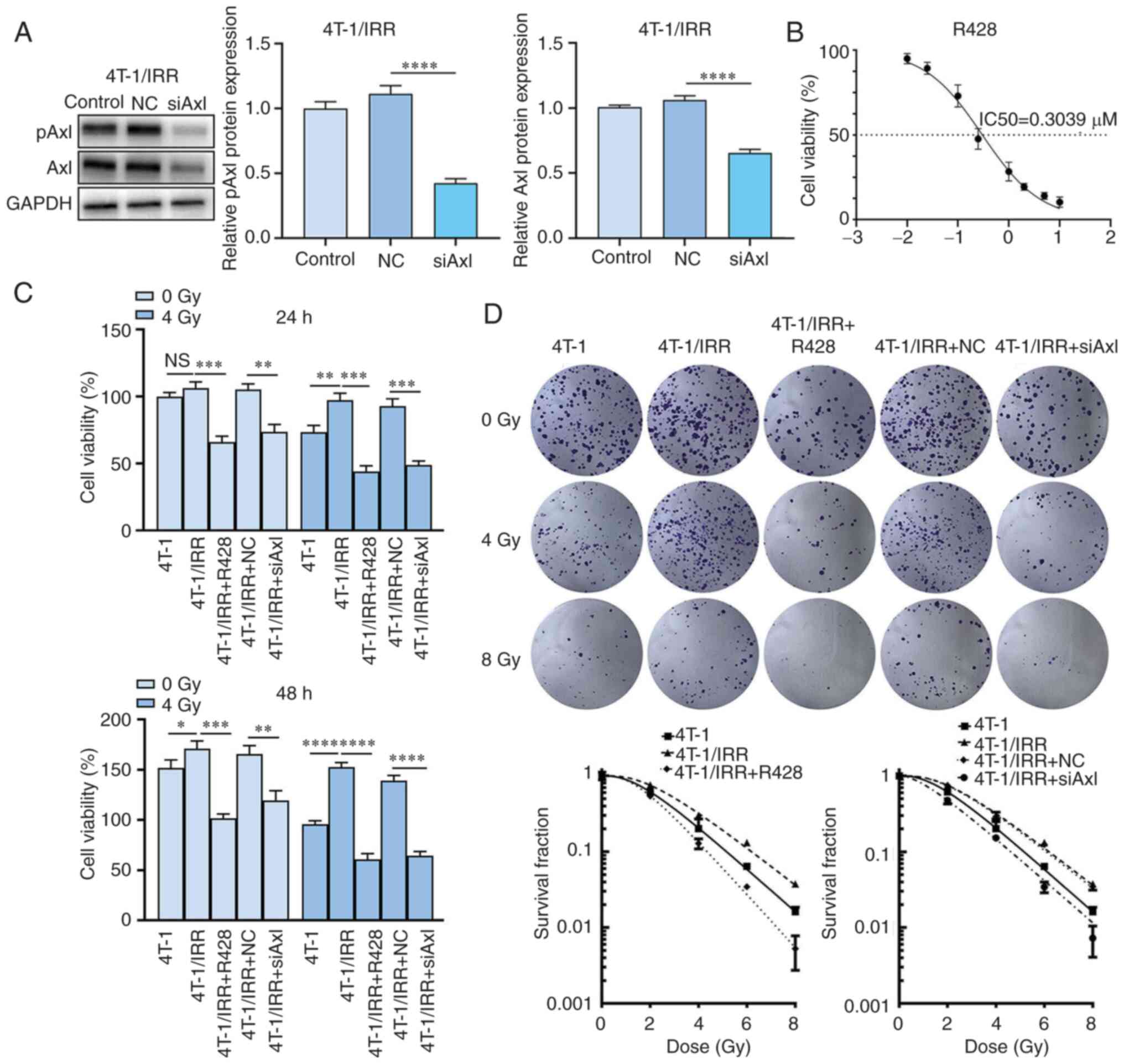
Axl inhibition combined with
irradiation significantly reduces the viability of 4T-1/IRR
cells
As shown in Fig.
3A, the expression of pAxl and Axl was significantly decreased
following Axl siRNA lentiviral infection. In Fig. 3B, the growth curve showed that the
median lethal dose (IC50) of R428 for inhibiting
4T-1/IRR cell growth was 0.3039 µM, thus R428 with a drug
concentration of 0.3 µM was chosen for the subsequent experiments.
As shown in Fig. 3C and Table I, in the unirradiated group, the
viability of R428-treated 4T-1/IRR cells after 24 and 48 h was
significantly reduced to 62.03 and 59.56% of the 4T-1/IRR cells
without R428 treatment. The viability of 4T-1/IRR cells infected
with siAxl lentivirus after 24 and 48 h was also significantly
decreased to 69.96 and 72.11% of the empty vector infected 4T-1/IRR
cells. In the combination treatment groups, the viability of R428
treated 4T-1/IRR cells after 24 and 48 h was significantly reduced
to 53.16 and 35.52% of the 4T-1/IRR cells without any treatment.
The viability of 4T-1/IRR cells infected with siAxl lentivirus
after 24 and 48 h was significantly decreased to 59.86 and 38.98%
of the empty vector infected 4T-1/IRR cells. The above results
suggested that ionizing radiation combined with Axl inhibition
exhibited an enhanced inhibitory role on the proliferation of
radiation-resistant TNBC cells.
 | Table ICell viability of 4T-1/IRR cells
following Axl inhibitione. |
Table I
Cell viability of 4T-1/IRR cells
following Axl inhibitione.
| | 24 h | 48 h |
|---|
| Group | 0 Gy | 4 Gy | 0 Gy | 4 Gy |
|---|
| 4T-1 | 100.00±3.00 | 88.57±5.03 | 151.67±8.08 | 95.67±3.51 |
| 4T-1/IRR | 106.40±4.54 |
102.46±5.23b |
170.80±7.79a |
152.53±4.57b |
| 4T-1/IRR+R428 |
66.00±4.33c |
56.57±4.21c |
101.73±4.28c |
60.67±5.86c |
| 4T-1/IRR+NC | 105.20±4.21 | 99.89±5.41 | 165.67±8.20 | 139.10±5.2 |
| 4T-1/IRR+siAxl |
73.60±5.41d |
62.97±3.02d |
119.47±9.53d |
64.57±4.18d |
Axl inhibition combined with
irradiation reduces the colony formation ability of 4T-1/IRR
cells
As shown in Fig. 3D
and Table II, the colony
formation rate of 4T-1/IRR cells was significantly increased when
compared with the 4T-1 cells (SER=0.8787). R428-treated 4T-1/IRR
cells exhibited a decreased colony formation rate compared with the
4T-1/IRR cells not treated with R428 (SER=1.4104). The colony
formation rate of 4T-1/IRR cells was also reduced by siAxl
lentivirus infection when compared with the NC lentivirus-infected
4T-1/IRR cells (SER=1.1053). The above results showed that the
colony formation ability of 4T-1/IRR cells was promoted when
compared with 4T-1 cells, and it could be significantly reduced by
Axl inhibition, suggesting that Axl played an important role in the
radiation resistance of TNBC cells.
 | Table IIColony formation ability of 4T-1/IRR
cells following Axl inhibitiona. |
Table II
Colony formation ability of 4T-1/IRR
cells following Axl inhibitiona.
| Group | 0 Gy | 2 Gy | 4 Gy | 6 Gy | 8 Gy |
|---|
| 4T-1 | 100.00±7.16 | 61.46±1.85 | 20.15±6.92 | 6.41±0.31 | 1.62±0.23 |
| 4T-1/IRR | 100.00±7.33 | 75.69±1.98 | 30.25±1.74 | 13.19±1.13 | 3.76±0.35 |
| 4T-1/IRR+R428 | 100.00±11.65 | 54.21±1.56 | 12.86±1.94 | 3.45±0.34 | 0.52±0.25 |
| 4T-1/IRR+NC | 100.00±8.10 | 74.54±1.21 | 29.10±4.15 | 12.46±1.03 | 3.49±0.37 |
| 4T-1/IRR+siAxl | 100.00±8.34 | 47.58±4.87 | 15.24±1.11 | 3.44±0.57 | 0.73±0.32 |
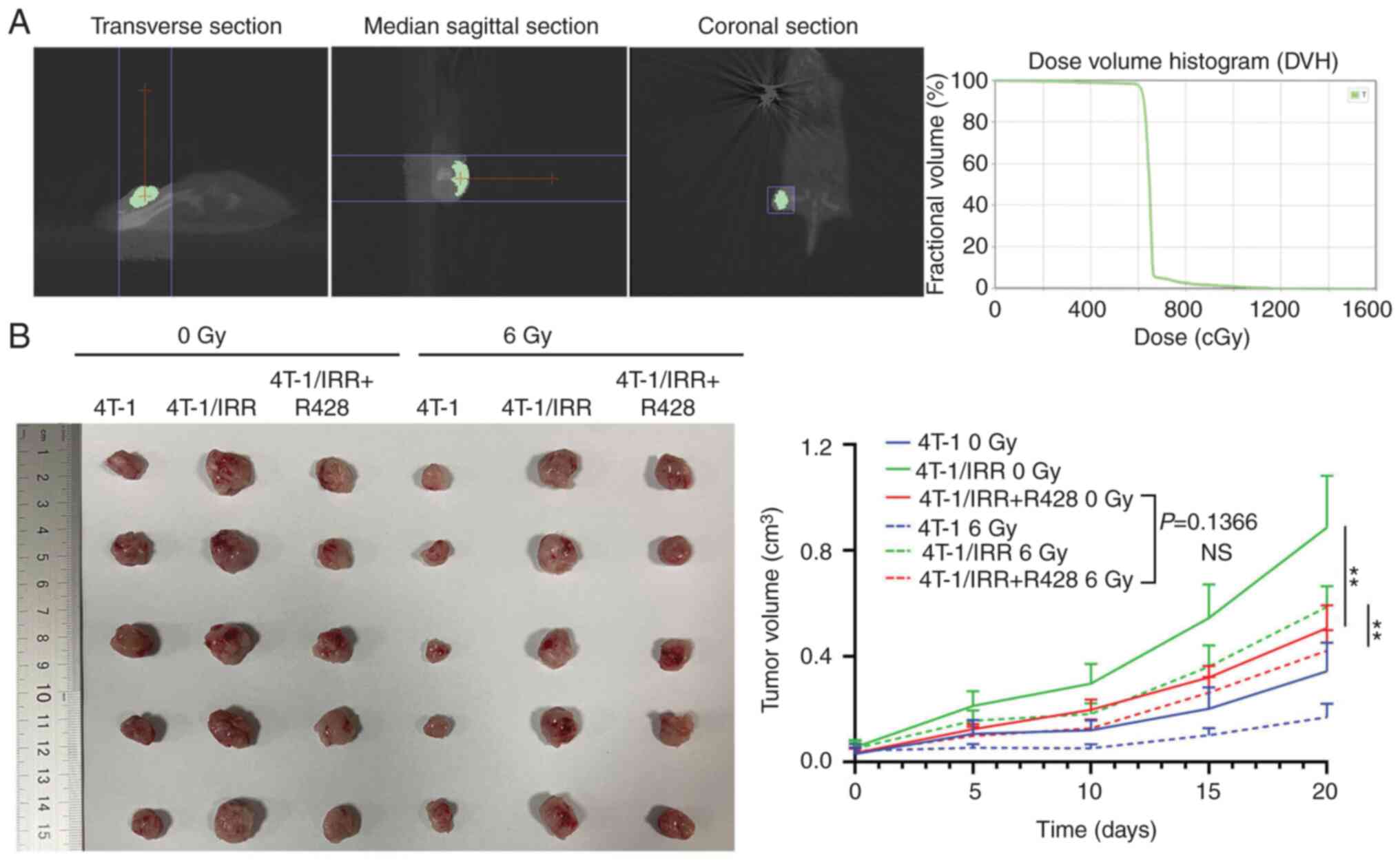
R428 treatment combined with
radiotherapy inhibits the rapid growth of 4T-1/IRR cell
xenografts
As shown in Fig.
4A, the xenografted mice were fixed in the prone position on
the treatment bed, and the analog positioning machine of the SARRP
collected the images in 3D. After the planned target area (PTV) was
outlined, a vertical X-ray irradiation field above the transplanted
tumor on the right hind leg root of the mouse was set up. The
center point of the PTV was located at the center of the
transplanted tumor. The dose-volume histograms were generated from
the treatment planning system of the SARRP, and it was determined
that >95% of xenografts achieved 6 Gy X-ray exposure. The
subcutaneous xenografts of 4T-1/IRR cells that had not been
irradiated grew most rapidly, and this growth was inhibited by R428
treatment (Fig. 4B). In the
ionizing radiation treated groups, xenografts were locally
irradiated with 6 Gy X-rays, the xenografts of the 4T-1/IRR cells
were found to be resistant to ionizing radiation compared with that
of 4T-1 cells. R428 treatment could also interrupt the
radioresistance of 4T-1/IRR cell xenografts (P<0.01). The tumor
volumes of xenografts in the combination treatment group were
slightly smaller than that of the tumors treated with R428 alone,
but the difference was not significant (P=0.1366).
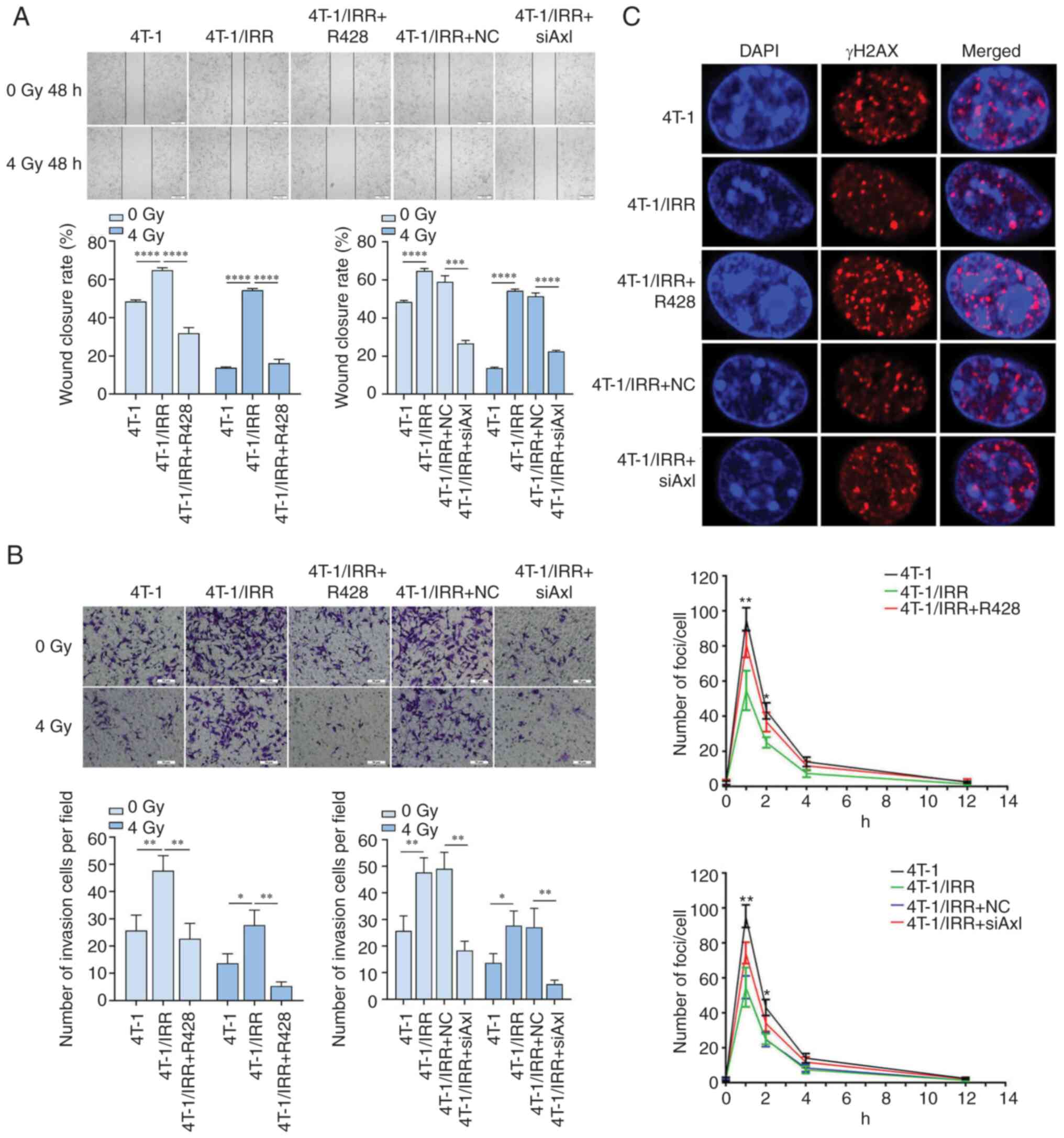
Axl inhibition combined with
irradiation reverses the increase in the migratory capacity of
4T-1/IRR cells
As shown in Fig.
5A, the wound closure rate of 4T-1/IRR cells was 64.77±1.29%,
while that of the 4T-1 cells was 48.44±0.83% (P<0.0001). After
treatment with R428, the wound closure rate of 4T-1/IRR cells
decreased to 31.88±2.91%. The wound closure rate of the 4 Gy X-ray
irradiated 4T-1 cells was 13.78±0.43%, while that of irradiated
4T-1/IRR cells was 54.30±0.92%. The R428 treatment reduced the
wound closure rate of irradiated 4T-1/IRR cells to 16.15±2.09%
(P<0.0001). The wound closure rate was 58.98±3.22% in NC
lentivirus-infected 4T-1/IRR cells, while it was 26.66±1.65% in
siAxl lentivirus-infected 4T-1/IRR cells (P<0.001). After 4 Gy
X-ray irradiation, the wound closure rate of 4T-1/IRR cells
infected with the empty vector lentivirus was 51.41±1.75%, and it
was reduced to 22.45±0.60% after siAxl lentivirus infection
(P<0.0001). The above results indicated that the 4T-1/IRR cells
exhibited increased migratory capacity, and this increase migratory
capacity was dependent on Axl.
Axl inhibition combined with
irradiation reverses the increase in the invasive ability of
4T-1/IRR cells
The number of cells that invaded through Matrigel
and reached the lower chamber of the chamber was counted to
evaluate the invasive ability of tumor cells. As shown in Fig. 5B, the number of invasive 4T-1/IRR
cells per field increased to 1.86x that of the 4T-1 cells, and this
was decreased to 47.55% in 4T-1/IRR cells treated with R428
compared with the untreated 4T-1/IRR cells (P<0.01). After 4 Gy
X-ray irradiation, the invasion of 4T-1/IRR cells was 2.02x that of
the 4T-1 cells, and this was decreased to 19.28% in 4T-1/IRR cells
treated with R428 compared with the untreated 4T-1/IRR cells
(P<0.01). The invasion of siAxl lentivirus-infected 4T-1/IRR
cells was reduced to 37.42% of the NC lentivirus-infected 4T-1/IRR
cells. After 4 Gy X-ray irradiation, the invasive potential of
siAxl lentivirus-infected 4T-1/IRR cells per field was reduced to
20.99% of the NC lentivirus-infected 4T-1/IRR cells (P<0.01).
These results indicated that the ability of 4T-1/IRR cells to
invade and metastasize from the primary site to distant organs and
tissues was also significantly increased, which was associated with
the increased expression of Axl.
Axl inhibition reduces ionizing
radiation-induced DSBs in 4T-1/IRR cells
The number of γH2AX foci in each group of cells was
found to reach a peak 1 h after 2 Gy X-ray irradiation (Fig. 5C), and in 4T-1/IRR cells, the
number of foci was notably reduced compared with the 4T-1 cells
(54.67±11.23 vs. 95.33±6.51, P<0.01), suggesting that DNA damage
in 4T-1/IRR cells was notably reduced. Following treatment of
4T-1/IRR with R428, the degree of ionizing radiation-induced DSBs
increased (81.00±7.55 vs. 54.67±11.23, P<0.01). The number of
γH2AX foci also increased in siAxl lentivirus-infected 4T-1/IRR
cells when compared with that of the NC lentivirus-infected
4T-1/IRR cells (74.33±6.11 vs. 55.0±7.55, P<0.01). The above
results demonstrated that the 4T-1/IRR cells exhibited
significantly less DNA damage than the 4T-1 cells following
irradiation, and the activation of Axl likely protected cells from
ionizing radiation-induced DNA damage. That is, the downregulation
of Axl expression/activity aggravated DNA damage in 4T-1/IRR
cells.
PI3K/pAkt/mTOR pathway activation by
Axl phosphorylation is involved in the radioresistance of 4T-1/IRR
cells
siAxl lentivirus infection of 4T-1/IRR cells
resulted in a significant reduction in pAxl and Axl expression. The
expression levels of the downstream signaling molecules pPI3K,
pAkt, and mTOR were also decreased, although PTEN expression was
not altered (Fig. 6). These
results indicated that pAxl modulated various biological functions
of tumor cells through the activation of the downstream
PI3K/pAkt/mTOR signaling pathway. R428 also suppressed the
expression levels of pAxl, Axl, and the downstream signaling
molecules pPI3K, pAkt, and mTOR in 4T-1/IRR cells (Fig. 7). Ionizing radiation-induced the
upregulation of pAxl expression in 4T-1 cells, and 4T-1/IRR cells
exhibited higher pAxl expression levels. The expression levels of
the downstream signaling molecules pPI3K, pAkt, and mTOR were
accordingly upregulated, which may be a defense mechanism of tumor
cells against ionizing radiation damage. Blocking pAxl activity by
R428 or siRNA lentivirus infection enhanced the radiosensitivity of
4T-1/IRR cells, likely through intercepting the PI3K/pAkt/mTOR
signal cascade.
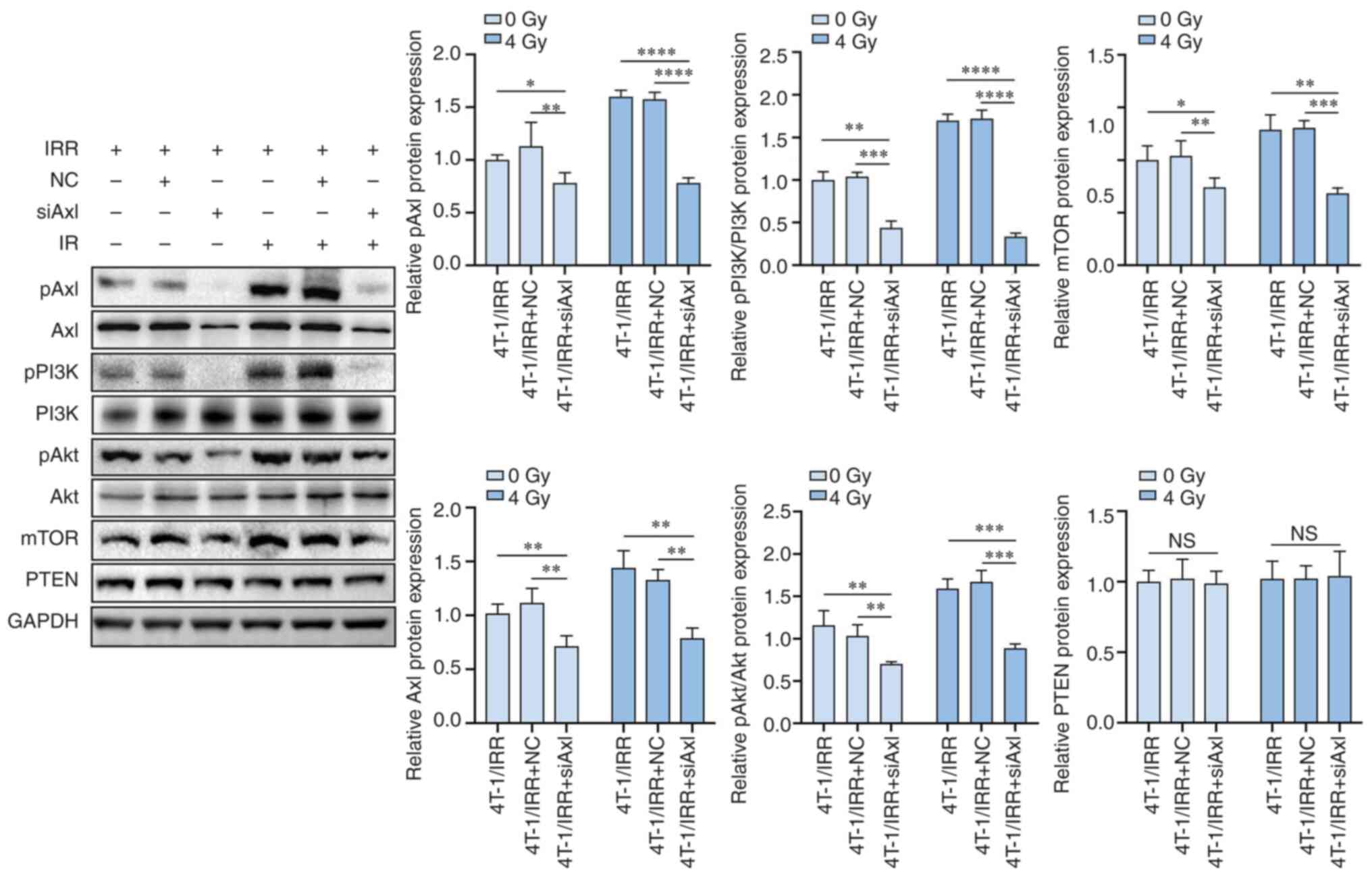 | Figure 6The protein expression levels of
pAxl, Axl, pPI3K, PI3K, pAkt, Akt, mTOR, and PTEN in TNBC cells
with Axl siRNA lentivirus infection and radiotherapy combination
therapy. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. TNBC,
triple-negative breast cancer. |
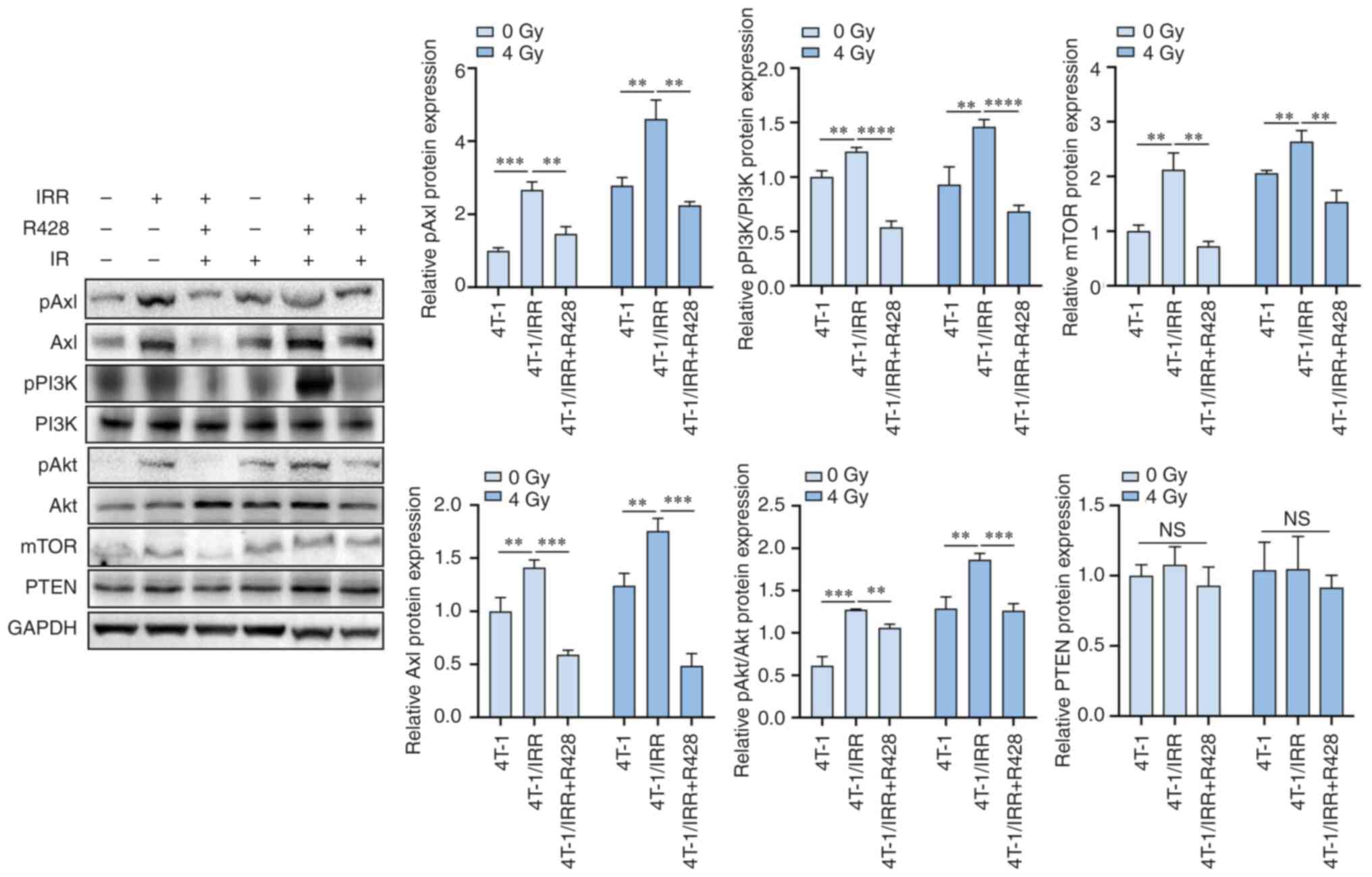 | Figure 7The protein expression levels of
pAxl, Axl, pPI3K, PI3K, pAkt, Akt, mTOR, and PTEN in TNBC cells
following a combination of R428 treatment and radiotherapy.
**P<0.01, ***P<0.001,
****P<0.0001. TNBC, triple-negative breast
cancer. |
Discussion
RTKs are important growth factor receptors present
on the cell surface, and they play critical roles in the survival
and proliferation of breast and other epithelial cells. The
dysregulation of RTKs was found to be closely associated with the
occurrence of breast cancer, and the increased expression of RTKs
was associated with the enhanced invasiveness of breast cancer, as
well as decreased overall survival (OS) and disease-free survival
(DFS) (27,28). Currently, the prognosis of TNBC is
still poor due to the lack of targeted therapeutic drugs; RTK
inhibition may thus have significant potential for the treatment of
TNBC.
Axl is a member of the Tyro-Axl-MER (TAM) family of
RTKs, which regulate a variety of cellular biological processes,
including cell survival, proliferation, autophagy, migration,
angiogenesis, platelet aggregation, and natural killer cell
differentiation. A meta-analysis performed by Zhang et al
(29) demonstrated that high
expression of Axl was associated with a poorer OS in patients with
hepatocellular carcinoma, esophageal cancer, and non-small cell
lung cancer. Tanaka et al (30) reported that the co-expression of
Axl and Vimentin in breast cancer tissues indicated the shortened
OS of patients. Bottai et al (15) found that the expression of Axl was
elevated in the tumor tissues of breast cancer patients with lymph
node metastasis or distant metastasis; however, it was not
associated with tumor size or clinical stage. Gjerdrum et al
(31) reported that Axl
expression, tumor diameter, histological grade, and lymph node
status were negative independent prognostic factors for breast
cancer. Axl expression in the metastatic tissues was significantly
increased when compared with the primary tumor tissues, indicating
that Axl expression was closely associated with distant metastasis
of breast cancer. In the present study, it was found that the
expression levels of Axl in TNBC tissues were significantly higher
than that in adjacent tissues, and Axl expression in the tumor
tissues of patients with lymph node metastasis was significantly
higher than that of patients without lymph node metastases. The
above results indicated that Axl expression may be closely
associated with the occurrence and metastasis of TNBC.
According to previous studies, Axl expression was
upregulated in TNBC cell lines and was associated with a poor
clinical prognosis of breast cancer (32). Downregulation of Axl can
effectively prevent the highly metastatic phenotype breast cancer
cells migrating from the primary tumor to distant organs and
tissues in animal models of breast cancer while improving the OS
rate of the animals (33).
Abdel-Rahman et al (34)
found that the upregulation of Axl in breast cancer cells was
negatively associated with the expression of the epithelial cell
marker E-cadherin. Downregulation of Axl reduced the invasion and
migration ability of breast cancer cells. Another study confirmed
that Axl activation was able to induce the activation of the NF-κB
signaling pathway, promote the expression of MMP-9, and then led to
the enhancement of the invasion and migration of breast cancer
cells (19). A study by Holland
et al (35) demonstrated
that the downregulation of Axl significantly inhibited the growth
of tumors in a nude mouse xenograft model, indicating that Axl also
participated in the proliferation and viability of breast cancer
cells. Therefore, the detection and targeted therapy of Axl may
serve as an important direction for breast cancer treatment.
In the present study, the 4T-1/IRR cells exhibited
significantly enhanced cell viability, colony formation ability,
DNA injury repair capacity, and invasive and migratory ability. Axl
expression was found to be increased in 4T-1/IRR cells, suggesting
the possibility that Axl plays an important role in the resistance
to ionizing radiation. Axl inhibition reversed the radioresistance
of 4T-1/IRR cells, as shown by the attenuated cell viability and
colony formation ability, the increase in DNA damage, and the
reduction of cell invasion and migration. In vivo, it was
observed that the growth rate of the transplanted tumor in the mice
implanted with 4T-1/IRR cells was notably faster than that of the
4T-1 cells. Combination treatment with R428 and radiotherapy
significantly reduced the growth rate of the 4T-1/IRR cell-formed
tumors in the mouse model, consistent with the results in
vitro. The toxicity of combination treatment to animal models
exhibits no difference when compared to other groups. The main
reasons include: i) The small animal radiation research platform
(SARRP) was applied to precisely administer radiotherapy of the
tumor-bearing mice; ii) the tumor was implanted in the right
posterior flank of BALB/c mice; and iii) the intra-tumoral
injection with R428 was the administration pathway. There was
relatively little impact on important tissues and organs of the
animals. There was no significant difference in weight among
groups. The above results showed that Axl played a crucial role in
the radioresistance of TNBC. Targeting Axl combined with
radiotherapy may thus serve as a potential novel direction for TNBC
treatment. However, in the present study, the inhibitory effect of
the combination treatment on tumor xenografts did not differ
significantly when compared to treatment with R428 alone. This may
be explained by the fact the challenges in comparing in vivo
and in vitro experiments; thus, there is a need for further
exploration of radiation doses and segmentation of the xenografts;
and for improvements in R428 administration methods. In animal
experiments and clinical applications, the method of combination
therapy also requires further exploration.
According to previous studies, Axl activation in
breast cancer induces the activation of multiple downstream
signaling transduction pathways, including PI3K/Akt, MAPK, and
NF-κB, and thus accelerates various tumor-promoting processes,
including cell proliferation, survival, invasion, and angiogenesis
(36,37). The PI3K/Akt signaling pathway was
involved in the regulation of tumor cell proliferation, survival,
metastasis, and EMT in various human malignancies, and it was
considered a crucial therapeutic target (38). It has been reported that the
activation of the PI3K/Akt signaling pathway led to the
overexpression and reduced degradation of Snail, which was an
important transcription factor in the EMT process, and ultimately
promoted the radioresistance of tumor cells (39). The PI3K/Akt signaling pathway
activated by miR-410/PTEN led to the occurrence of EMT and
radioresistance of non-small cell lung cancer cells (40). PF-05212384, an inhibitor of
PI3K/mTOR, could effectively suppress the PI3K/mTOR signaling
pathway and lead to increased sensitivity of head and neck squamous
cell carcinoma to ionizing radiation (41). The Akt/mTOR inhibitor everolimus
(RAD001) is currently undergoing clinical trials in combination
with radiotherapy for the treatment of various malignant tumors,
and it may become an effective radiosensitizer (42). The present study showed that Axl
expression was notably increased in the 4T-1/IRR cells. The
expression of the downstream signaling pathway molecules pAkt and
mTOR was simultaneously increased, and significantly downregulated
by R428 treatment or siRNA lentivirus infection, indicating that
the PI3K/Akt/mTOR signaling pathway was a critical molecular
mechanism of TNBC cells resistant to ionizing radiation.
In conclusion, a TNBC cell line resistant to
ionizing radiation was established using a gradient irradiation
method. In vitro, the 4T-1/IRR cells exhibited significantly
enhanced radioresistance, and Axl inhibition resulted in increased
radiosensitivity in these cells; in vivo experiments further
confirmed the above results. The PI3K/Akt/mTOR signaling pathway
activated by Axl phosphorylation was a critical molecular mechanism
of TNBC radioresistance. Axl-targeting therapy combined with
radiotherapy may thus have potential for the management of
TNBC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81602672 and 81902722) and
Medical Health Science and Technology Project of Zhejiang Province
(grant nos. 2020KY061, 2021RC041 and 2023KY594).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ, XL and QG conceived the study. JJ, YD and QG
designed the study. YD and MF searched the literature. JJ and YK
performed the analysis. JJ, YD, MF, QG and XY performed the
experiments. JJ, XL and QG collected the data. JJ wrote the
manuscript. XL and QG reviewed and edited the manuscript. JJ, XL
and QG confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhejiang Cancer Hospital (Zhejiang, China; approval
no, IRB-2020-367). All patients signed informed consent forms prior
to tissue collection for storage in the biological sample bank. The
Ethics Committee of Zhejiang Cancer Hospital waived the need for
informed consent of patients for participation in the present
study. The animal experiments were approved by the Animal Ethics
Committee of Zhejiang Cancer Hospital (Zhejiang, China; approval
no. 2021-11-004).
Patient content for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vagia E, Mahalingam D and Cristofanilli M:
The landscape of targeted therapies in TNBC. Cancers (Basel).
12(916)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiang MJ, Chen YY, Dai JJ, Gu DN, Mei Z,
Liu FR, Huang Q and Tian L: Dying tumor cell-derived exosomal
miR-194-5p potentiates survival and repopulation of tumor
repopulating cells upon radiotherapy in pancreatic cancer. Mol
Cancer. 19(68)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang Y, Zhao M, He S, Luo Y, Zhao Y, Cheng
J, Gong Y, Xie J, Wang Y, Hu B, et al: Necroptosis regulates tumor
repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway.
J Exp Clin Cancer Res. 38(461)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Candas-Green D, Xie B, Huang J, Fan M,
Wang A, Menaa C, Zhang Y, Zhang L, Jing D, Azghadi S, et al: Dual
blockade of CD47 and HER2 eliminates radioresistant breast cancer
cells. Nat Commun. 11(4591)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bai X, Ni J, Beretov J, Wang S, Dong X,
Graham P and Li Y: THOC2 and THOC5 regulate stemness and
radioresistance in triple-negative breast cancer. Adv Sci (Weinh).
8(e2102658)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Colli LM, Machiela MJ, Zhang H, Myers TA,
Jessop L, Delattre O, Yu K and Chanock SJ: Landscape of combination
immunotherapy and targeted therapy to improve cancer management.
Cancer Res. 77:3666–3671. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Seebacher NA, Stacy AE, Porter GM and
Merlot AM: Clinical development of targeted and immune based
anti-cancer therapies. J Exp Clin Cancer Res.
38(156)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trenker R and Jura N: Receptor tyrosine
kinase activation: From the ligand perspective. Curr Opin Cell
Biol. 63:174–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Colavito SA: AXL as a target in breast
cancer therapy. J Oncol. 2020(5291952)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aguilera TA, Rafat M, Castellini L,
Shehade H, Kariolis MS, Hui AB, Stehr H, von Eyben R, Jiang D,
Ellies LG, et al: Reprogramming the immunological microenvironment
through radiation and targeting Axl. Nat Commun.
7(13898)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang Y, Zang H, Wen Q and Fan S: AXL in
cancer: A modulator of drug resistance and therapeutic target. J
Exp Clin Cancer Res. 42(148)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li H, Liu Z, Liu L, Zhang H, Han C, Girard
L, Park H, Zhang A, Dong C, Ye J, et al: AXL targeting restores
PD-1 blockade sensitivity of STK11/LKB1 mutant NSCLC through
expansion of TCF1(+) CD8 T cells. Cell Rep Med.
3(100554)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Noronha A, Nataraj NB, Lee JS, Zhitomirsky
B, Oren Y, Oster S, Lindzen M, Mukherjee S, Will R, Ghosh S, et al:
AXL and error-prone DNA replication confer drug resistance and
offer strategies to treat EGFR-mutant lung cancer. Cancer Discov.
12:2666–2683. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He R, Song Z, Bai Y, He S, Huang J, Wang
Y, Zhou F, Huang W, Guo J, Wang Z, et al: Discovery of AXL
degraders with improved potencies in triple-negative breast cancer
(TNBC) cells. J Med Chem. 66:1873–1891. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bottai G, Raschioni C, Székely B, Di
Tommaso L, Szász AM, Losurdo A, Győrffy B, Ács B, Torrisi R,
Karachaliou N, et al: AXL-associated tumor inflammation as a poor
prognostic signature in chemotherapy-treated triple-negative breast
cancer patients. NPJ Breast Cancer. 2(16033)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han J, Tian R, Yong B, Luo C, Tan P, Shen
J and Peng T: Gas6/Axl mediates tumor cell apoptosis, migration and
invasion and predicts the clinical outcome of osteosarcoma
patients. Biochem Biophys Res Commun. 435:493–500. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Linger RM, Keating AK, Earp HS and Graham
DK: TAM receptor tyrosine kinases: Biologic functions, signaling,
and potential therapeutic targeting in human cancer. Adv Cancer
Res. 100:35–83. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu C, Wei Y and Wei X: AXL receptor
tyrosine kinase as a promising anti-cancer approach: Functions,
molecular mechanisms and clinical applications. Mol Cancer.
18(153)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tai KY, Shieh YS, Lee CS, Shiah SG and Wu
CW: Axl promotes cell invasion by inducing MMP-9 activity through
activation of NF-kappaB and Brg-1. Oncogene. 27:4044–4055.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rankin EB, Fuh KC, Taylor TE, Krieg AJ,
Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA and Giaccia AJ: AXL is
an essential factor and therapeutic target for metastatic ovarian
cancer. Cancer Res. 70:7570–7579. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Miricescu D, Totan A, Stanescu-Spinu II,
Badoiu SC, Stefani C and Greabu M: PI3K/AKT/mTOR signaling pathway
in breast cancer: From molecular landscape to clinical aspects. Int
J Mol Sci. 22(173)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park JH, Kim YH, Shim S, Kim A, Jang H,
Lee SJ, Park S, Seo S, Jang WI, Lee SB and Kim MJ:
Radiation-activated PI3K/AKT pathway promotes the induction of
cancer stem-like cells via the upregulation of SOX2 in colorectal
cancer. Cells. 10(135)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Seol MY, Choi SH and Yoon HI: Combining
radiation with PI3K isoform-selective inhibitor administration
increases radiosensitivity and suppresses tumor growth in non-small
cell lung cancer. J Radiat Res. 63:591–601. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu L, Guo Q, Luo Z, Wang Y, Weng J, Chen
Y, Liang W, Li Y, Zhang Y, Chen K, et al: TXN inhibitor impedes
radioresistance of colorectal cancer cells with decreased ALDH1L2
expression via TXN/NF-κB signaling pathway. Br J Cancer.
127:637–648. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Spring E and Holmberg P: Evaluation of
experimental irradiation fractionation with the single-hit,
multi-target model. Acta Radiol Ther Phys Biol. 7:297–306.
1968.PubMed/NCBI View Article : Google Scholar
|
|
26
|
du Sert NP, Ahluwalia A, Alam S, Avey MT,
Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, et
al: Reporting animal research: Explanation and elaboration for the
ARRIVE guidelines 2.0. PLoS Biol. 18(e3000411)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Butti R, Das S, Gunasekaran VP, Yadav AS,
Kumar D and Kundu GC: Receptor tyrosine kinases (RTKs) in breast
cancer: Signaling, therapeutic implications and challenges. Mol
Cancer. 17(34)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Itoh T, Hatano R, Horimoto Y, Yamada T,
Song D, Otsuka H, Shirakawa Y, Mastuoka S, Iwao N, Aune TM, et al:
IL-26 mediates epidermal growth factor receptor-tyrosine kinase
inhibitor resistance through endoplasmic reticulum stress signaling
pathway in triple-negative breast cancer cells. Cell Death Dis.
12(520)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang S, Xu XS, Yang JX, Guo JH, Chao TF
and Tong Y: The prognostic role of Gas6/Axl axis in solid
malignancies: A meta-analysis and literature review. Onco Targets
Ther. 11:509–519. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tanaka K, Tokunaga E, Inoue Y, Yamashita
N, Saeki H, Okano S, Kitao H, Oki E, Oda Y and Maehara Y: Impact of
expression of vimentin and Axl in breast cancer. Clin Breast
Cancer. 16:520–526. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gjerdrum C, Tiron C, Høiby T, Stefansson
I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT,
et al: Axl is an essential epithelial-to-mesenchymal
transition-induced regulator of breast cancer metastasis and
patient survival. Proc Natl Acad Sci USA. 107:1124–1129.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zajac O, Leclere R, Nicolas A, Meseure D,
Marchiò C, Vincent-Salomon A, Roman-Roman S, Schoumacher M and
Dubois T: AXL controls directed migration of mesenchymal
triple-negative breast cancer cells. Cells. 9(247)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Leconet W, Chentouf M, du Manoir S,
Chevalier C, Sirvent A, Aït-Arsa I, Busson M, Jarlier M,
Radosevic-Robin N, Theillet C, et al: Therapeutic activity of
Anti-AXL antibody against triple-negative breast cancer
patient-derived xenografts and metastasis. Clin Cancer Res.
23:2806–2816. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Abdel-Rahman WM, Al-Khayyal NA, Nair VA,
Aravind SR and Saber-Ayad M: Role of AXL in invasion and drug
resistance of colon and breast cancer cells and its association
with p53 alterations. World J Gastroenterol. 23:3440–3448.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Holland SJ, Powell MJ, Franci C, Chan EW,
Friera AM, Atchison RE, McLaughlin J, Swift SE, Pali ES, Yam G, et
al: Multiple roles for the receptor tyrosine kinase axl in tumor
formation. Cancer Res. 65:9294–9303. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiang C, Cheng Z, Jiang T, Xu Y and Wang
B: MicroRNA-34a inhibits cell invasion and epithelial-mesenchymal
transition via targeting AXL/PI3K/AKT/Snail signaling in
nasopharyngeal carcinoma. Genes Genomics. 42:971–978.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Corno C, Gatti L, Lanzi C, Zaffaroni N,
Colombo D and Perego P: Role of the receptor tyrosine kinase axl
and its targeting in cancer cells. Curr Med Chem. 23:1496–1512.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aoki M and Fujishita T: Oncogenic roles of
the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 407:153–189.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY,
Kim CH, Park HG, Han SI and Kang HS: Induction of metastasis,
cancer stem cell phenotype, and oncogenic metabolism in cancer
cells by ionizing radiation. Mol Cancer. 16(10)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yuan Y, Liao H, Pu Q, Ke X, Hu X, Ma Y,
Luo X, Jiang Q, Gong Y, Wu M, et al: miR-410 induces both
epithelial-mesenchymal transition and radioresistance through
activation of the PI3K/mTOR pathway in non-small cell lung cancer.
Signal Transduct Target Ther. 5(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Leiker AJ, DeGraff W, Choudhuri R, Sowers
AL, Thetford A, Cook JA, Van Waes C and Mitchell JB: Radiation
enhancement of head and neck squamous cell carcinoma by the dual
PI3K/mTOR inhibitor PF-05212384. Clin Cancer Res. 21:2792–2801.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Albert JM, Kim KW, Cao C and Lu B:
Targeting the Akt/mammalian target of rapamycin pathway for
radiosensitization of breast cancer. Mol Cancer Ther. 5:1183–1189.
2006.PubMed/NCBI View Article : Google Scholar
|