Introduction
Atherosclerosis (AS) is a type of chronic
inflammatory disease involving large and medium arteries.
Atherosclerotic plaques composed of lipids, cholesterol, calcium
and abnormal surrounding cells deposited on the vascular wall can
lead to the narrowing of the corresponding blood vessels. At the
same time, the plaques can also rupture to form a local thrombus,
thus causing a series of ischemic diseases (1) AS is the main pathological basis of
cardiovascular and cerebrovascular diseases, which are
characterized by high morbidity, disability and mortality and
seriously threaten human life and health (2,3).
Currently, it is generally accepted that chronic inflammation and
oxidative stress are key factors in AS (4). Therefore, by taking vascular
endothelial cells and their secreted inflammatory factors as the
main research topic in AS, an in-depth study of the molecular
mechanism of the occurrence and development of AS has far-reaching
clinical significance for early detection, diagnosis and treatment
of this disease, and decrease of the associated mortality.
Dual specificity phosphatase (DUSP) is a family of
tyrosine-specific phosphatase proteins discovered in the last
decade. These proteins are mainly responsible for signal
transduction through selective dephosphorylation of MAPK proteins,
thus participating in a variety of biological functions including
protein ubiquitination, proteasome degradation, oxidation,
phosphorylation and methylation (5). DUSP12 is a member of the DUSP family.
A previous study showed that DUSP12 has a regulatory role in the
process of cellular inflammation and oxidative stress. DUSP12 was
shown to prevent hepatic steatosis and inflammation in L02 cells
after palmitic/oleic acid treatment. Overexpression of DUSP12 in
hepatocytes could reduce high-fat diet-induced hepatic steatosis,
insulin resistance and inflammation (6). DUSP12 alleviates oxidative stress
damage and apoptosis in diabetic cardiomyopathy through the
MAP3K5-JNK/p38 signaling pathway (7). The same study also showed that DUSP12
expression was significantly decreased in ischemia-reperfusion (IR)
following major liver surgery and DUSP12 negatively regulated the
pro-inflammatory and pro-apoptotic pathways MAP3K5/JNK-p38 and MAPK
signaling pathways during liver IR, which could be used as a
potential therapeutic target for liver IR (8). However, to the best of the authors'
knowledge, the regulatory effects of DUSP12 on inflammation and
oxidative stress injury of vascular endothelial cells in AS remain
to be elucidated.
The current study found that Forkhead box P1 (FOXP1)
could bind to the DUSP12 promoter through bioinformatics binding
site prediction. The transcriptional regulatory factor FOXP1 is
distributed in different tissues and cell types of the
cardiovascular system and plays an important role in regulating
cardiovascular system homeostasis (9). Gene deletion of FOXP1 could lead to
severe congenital heart defects and embryonic death, pathological
myocardial fibrosis and myocardial hypertrophy, exacerbation of
atherosclerotic lesions and prolonged elimination of thrombosis
(10). It has been shown that
FOXP1 transcription activated SESN1 to reduce oxidized low-density
lipoprotein (ox-LDL)-induced macrophage inflammation and lipid
accumulation (11). However, to
the best of the authors' knowledge, the regulatory effects of
FOXP1-induced DUSP12 on inflammation and oxidative stress injury of
vascular endothelial cells in AS and its mechanisms have not been
reported.
The present study aimed to investigate and discuss
the regulatory effects and mechanism of action of DUSP12 on
ox-LDL-induced inflammation and oxidative stress injury of vascular
endothelial cells in AS. Its results provided a theoretical basis
for DUSP12 as a potential therapeutic target for AS-related
diseases.
Materials and methods
Database
Prediction of promoter binding sites of
transcription factors FOXP1 and DUSP12 were obtained from the
JASPAR database (https://jaspar.genereg.net/) (12).
Cell culture
Human umbilical vein endothelial cells (HUVECs; cat.
no. QC-0122) were purchased from Shanghai Qincheng Biotechnology
Co., Ltd. The cells were cultured in DMEM supplemented with 10% FBS
and 1% penicillin-streptomycin and maintained in a humidified 5%
CO2 atmosphere in a cell incubator at 37˚C. For the
establishment of the AS in vitro model, HUVECs were treated
with 100 µg/ml ox-LDL added to the cells for 24 h at 37˚C (13).
Cell transfection
DUSP12 plasmid (Oe-DUSP12), FOXP1 plasmid (Oe-FOXP1)
and the empty vector plasmid (Oe-NC), small interfering (si)RNAs
targeting FOXP1 (si-FOXP1#1 or si-FOXP1#2) and the scrambled siRNA
negative control (si-NC) were synthesized by Genepharm, Inc. HUVECs
were seeded on 6-well plates (2x105 cells/well) and
cultured until the cell confluence reached 80%. A total of 100 nM
plasmids were transfected into HUVECs using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 48 h. After 48 h of transfection,
reverse transcription-quantitative (RT-q) PCR and western blotting
were used to measure cell transfection efficiency. All
transfections in the present study were transient. The sequences of
two human siRNA-FOXP1 and the siRNA-NC were as follows:
siRNA-FOXP#1 sense: 5'-UCAAAAGGUCACGUCUUACCC-3', and siRNA-FOXP#1
antisense: 5'-GUAAGACGUGACCUUUUGAGG-3'; siRNA-FOXP#2 sense:
5'-UUAUAGUCACCUCAAAAGGUC-3', and siRNA-FOXP#2 antisense:
5'-CCUUUUGAGGUGACUAUAACU-3'; siRNA-NC-sense:
5'-UUCUCCGAACGUGUCACGUTT-3', and siRNA-NC-antisense:
5'-ACGUGACACGUUCGGAGAATT-3'.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from HUVECs (1x104 cells) was
extracted using TRIzol® (Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. The total RNA
was then reverse transcribed into cDNA using a cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Subsequently, a qPCR assay was performed to measure
the mRNA levels of DUSP12 and FOXP1 using SYBR Green Master Mix
(Takara Bio, Inc.) according to recommended instructions. The
following thermocycling conditions were used for qPCR:
Pre-denaturation at 95˚C for 1 min, followed by 40 cycles of
denaturation at 95˚C for 15 sec, annealing at 60˚C for 40 sec and
extension at 72˚C for 15 sec. Relative expression changes were
calculated using the 2-ΔΔCq method (14). Sequences of the primers were:
DUSP12 forward, 5'-TGTCATGCAGGAGTCAGTCG-3' and reverse,
5'-CCTCCCTGTGGTAAGCATGG-3'; FOXP1 forward,
5'-AGGCTTCCCTCTGTGTGTTG-3' and reverse, 5'-ACTCCTAGAGGGCTGATGGT-3'
and GAPDH forward, 5'-CATGAGAAGTATGACAACAGCCT-3' and reverse,
5'-AGTCCTTCCACGATACCAAAGT-3'.
Western blotting
Total protein was extracted from HUVECs using RIPA
buffer (Cell Signaling Technology, Inc.) and quantified using a BCA
kit (Beyotime Institute of Biotechnology). Total protein (30
µg/lane) was separated by SDS-PAGE on 10% gel and transferred to
PVDF membranes. The membranes were blocked with 5% BSA (Beyotime
Institute of Biotechnology) for 1 h at 37˚C and incubated with
primary antibodies (Abcam) overnight at 4˚C. The primary antibodies
were DUSP12 (1:1,000; cat. no. ab228987; Abcam), Bcl-2 (1:1,000;
cat. no. ab32124; Abcam), Bax (1:1,000; cat. no. 182733; Abcam),
Cleaved PARP (1:1,000; cat. no. ab32064; Abcam), PARP (1:1,000;
cat. no. ab191217; Abcam), VCAM-1 (1:1,000; cat. no. ab134047;
Abcam), ICAM-1 (1:1,000; cat. no. ab282575; Abcam), p-eNOS
(1:1,000; cat. no. ab215717; Abcam), eNOS (1:1,000; cat. no.
ab300071; Abcam), FOXP1 (1:1,000; cat. no. ab134055; Abcam), MAP3K5
(1:1,000; cat. no. ab45178; Abcam), p-MAP3K5 (1:1,000; cat. no.
ab278547; Abcam) or GAPDH (1:1,000; cat. no. ab9485; Abcam). The
membranes were then treated with HRP-linked secondary antibodies
(goat anti-rabbit; 1:5,000; cat. no. ab6721; Abcam) for 2 h at 37˚C
and were visualized using an ECL kit (MilliporeSigma) and
semi-quantified using ImageJ software (version 1.42; National
Institutes of Health).
Cell Counting Kit-8 assay
For cell viability assay, HUVECs were plated in
triplicate at an initial density of 2,000 cells/well into 96-well
plates. DMEM medium containing 10 µl CCK-8 was then added for 4 h.
Cell viability was determined at 24 h using the Cell Counting Kit-8
assay (Dojindo Laboratories, Inc.) at 450 nm using a microplate
reader (Bio-Rad Laboratories, Inc.), according to the
manufacturer's instructions.
TUNEL staining
Cell apoptosis in HUVECs was measured using the
TUNEL staining assay (Beyotime Institute of Biotechnology).
Briefly, cells were permeabilized using proteinase K solution (20
µg/ml) at 37˚C for 30 min. Subsequently, the terminal
deoxynucleotidyl transferase and fluorescein were added to the
cells and incubated in a humidified box at 37˚C for 1 h. A
fluorescence microscope (ECLIPSE C1; Nikon Corporation) was used to
capture images of the TUNEL-positive cells.
ELISA
The levels of IL-6 (cat. no. H007-1-2), IL-1β (cat.
no. H002-1-1) and TNF-α (cat. no. H052-1-2) were measured in cell
lysates of HUVECs using ELISA kits (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
instructions. The levels of 8-hydroxy-2-deoxyguanosine (8-OHdG;
cat. no. H165-1-2) and nitric oxide (NO; cat. no. A013-2-1) were
measured in cell lysates of HUVECs with the corresponding kits
(Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's instructions.
Oxidative stress index detection
The reactive oxygen species (ROS) level was measured
with a 2',7'-dichlorofluorescein diacetate (DCFH-DA; cat. no. E004,
Jiancheng Bioengineering, Nanjing, China) staining assay, according
to the manufacturer's instructions. Commercial kits (Nanjing
Jiancheng Bioengineering Institute) were used to measure superoxide
dismutase (SOD; cat. no. A001-3-2), malondialdehyde (MDA; cat. no.
A003-1-2) and catalase (CAT; cat. no. A007-1-1) activity.
Dual-luciferase reporter assay
The mutant (MUT) or wild-type (WT) 3'-untranslated
region (UTR) sequence of DUSP12 was cloned into the dual luciferase
reporter vectors (DUSP12-WT or DUSP12-MUT). Subsequently, DUSP12-WT
or DUSP12-MUT 3'UTR plasmids containing Oe-FOXP1 or Oe-NC that were
constructed by GenePharma were co-transfected into HUVECs using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h after transfection, the luciferase
activities were determined through a dual-luciferase reporter assay
kit (Promega Corporation).
Chromatin immunoprecipitation
(ChIP)-seq analysis
A ChIP assay kit (Beyotime Institute of
Biotechnology) was used to perform the ChIP-seq analysis, according
to the manufacturer's instructions. Briefly, HUVECs were fixed with
1% formaldehyde for 10 min at room temperature. Next,
1x106 HUVECs were collected via centrifugation at 300 x
g for 3 min at 25˚C and washed twice with phosphate-buffered
saline. Subsequently, HUVECs were lysed RIPA buffer (Cell Signaling
Technology, Inc.) and sonicated for 30 min. The sonicated cell
lysates (2 µl) were immuno-precipitated with 4 µg antibodies
against FOXP1 (1:800; cat. no. ab134055; Abcam) or IgG (negative
control, 1:800; cat. no. ab109489; Abcam) at 4 ˚C overnight. The
next day, the samples were conjugated with Protein A agarose
(Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h at 4˚C.
Finally, the immunoprecipitated DNAs were purified with a ChIP DNA
purification kit (Beyotime Institute of Biotechnology) and
amplified by virtue of qPCR as aforementioned. RT-qPCR was applied
to analyze the enriched DNA. The primers used for the ChIP assay
were the same as those used in RT-qPCR.
Statistical analysis
All experiments were replicated for three times.
Statistical analyses were performed using SPSS software (version
no. 22.0; IBM Corp. USA). Data are presented as the mean ± standard
deviation. Data comparisons were made with one-way ANOVA followed
by Tukey's post hoc test. P#x003C;05 was considered to indicate a
statistically significant difference.
Results
Overexpression of DUSP12 inhibits
apoptosis in ox-LDL-induced HUVECs
DUSP12 was overexpressed in HUVECs and the
transfection efficiency was measured by RT-qPCR and western
blotting. The results showed that compared with that in the Oe-NC
group, the expression of DUSP12 was significantly increased in the
Oe-DUSP12 group, indicating successful transfection (Fig. 1A). The cells were then divided into
control, ox-LDL, ox-LDL + Oe-NC and ox-LDL + Oe-DUSP12 groups. The
results of RT-qPCR and western blotting showed that DUSP12
expression in the ox-LDL group was significantly decreased compared
with that in the control group. Compared with that in the ox-LDL +
Oe-NC group, DUSP12 expression was decreased in the ox-LDL +
Oe-DUSP12 group (Fig. 1B). CCK-8
results showed that ox-LDL significantly inhibited cell viability.
After further overexpression of DUSP12, cell viability was
recovered (Fig. 1C). TUNEL and
western blot assays found that ox-LDL significantly promoted
apoptosis, decreased the expression of Bcl-2 and increased
expression of Bax and cleaved PARP. Compared with that in the
ox-LDL + Oe-NC group, apoptosis was significantly decreased in
ox-LDL + Oe-DUSP12 group (Fig. 1D
and E).
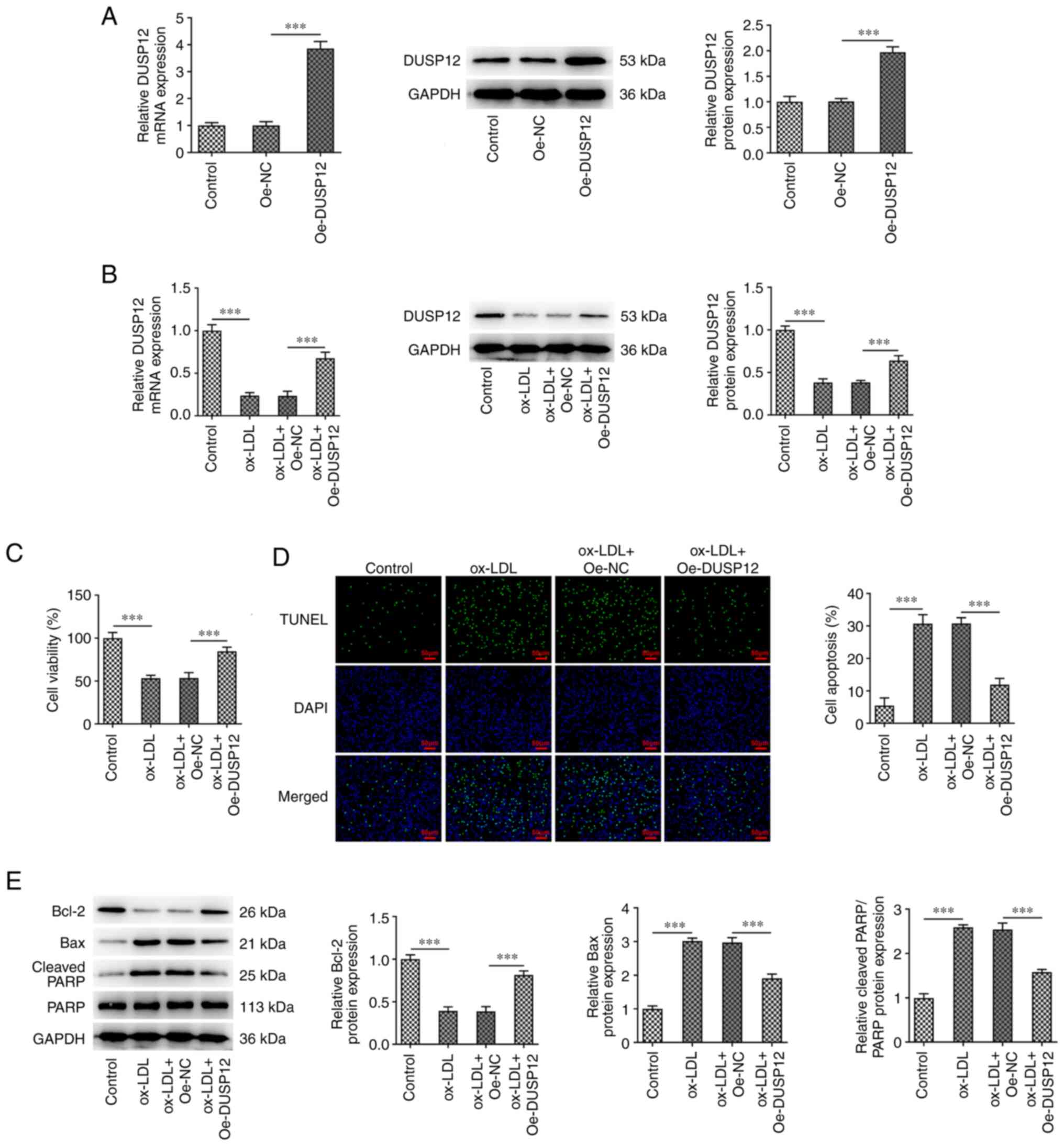 | Figure 1Overexpression of DUSP12 inhibits
apoptosis in ox-LDL-induced HUVECs. (A) DUSP12 was overexpressed in
HUVEC, and the transfection efficiency was measured using RT-qPCR
and western blotting. (B) Cells were then divided into control,
ox-LDL, ox-LDL + Oe-NC and ox-LDL + Oe-DUSP12 groups and the
expression of DUSP12 was measured using RT-qPCR and western
blotting. (C) Cell Counting Kit-8 assay was used to measure cell
viability. (D) TUNEL assay was used to measure cell apoptosis. (E)
Apoptosis-related proteins Bcl-2, Bax and cleaved PARP were
measured by western blotting. ***P#x003C;0.001. DUSP12,
dual specificity phosphatase 12; ox-LDL, oxidized low-density
lipoprotein; HUVECs, human umbilical vein endothelial cells;
RT-qPCR, reverse transcription-quantitative PCR; Oe,
overexpression; NC, negative control; PARP, poly (ADP-ribose)
polymerase. |
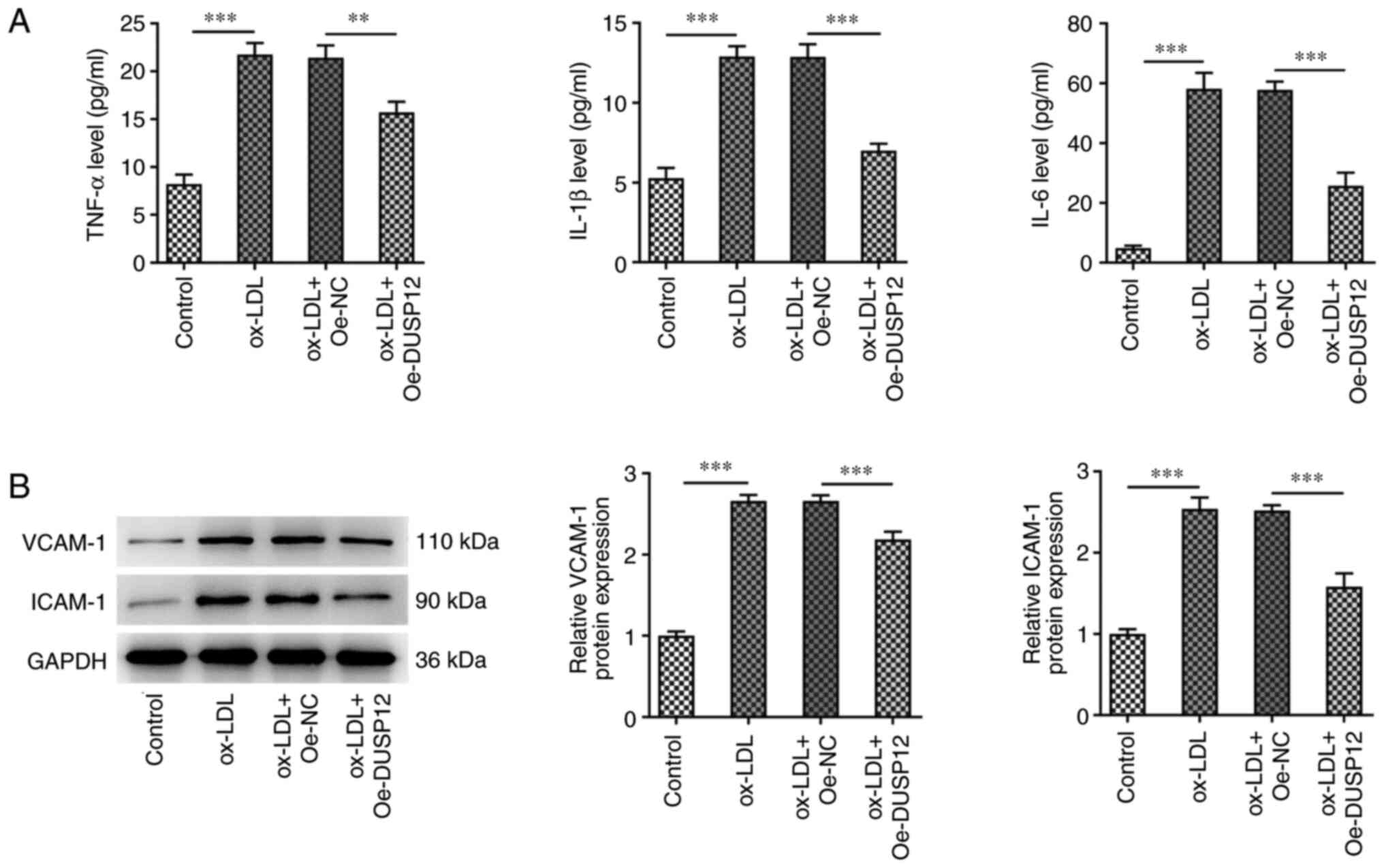
Overexpression of DUSP12 inhibits
inflammation and oxidative stress damage in ox-LDL-induced
HUVECs
The effect of DUSP12 expression on ox-LDL-induced
cellular inflammation was investigated and ELISA results showed
that the levels of TNF-α, IL-1β and IL-6 were significantly
increased in the ox-LDL group compared with those in the control
group. Compared with those in the ox-LDL + Oe-NC group, the levels
of IL-6, IL-1β and TNF-α were inhibited in the ox-LDL + Oe-DUSP12
group (Fig. 2A). Western blotting
of the inflammatory proteins vascular cell adhesion molecule 1
(VCAM-1) and intracellular cell adhesion molecule 1 (ICAM-1) showed
that the expression levels of these two proteins were significantly
increased after ox-LDL induction. The effect on the expression of
VCAM-1 and ICAM-1 was reversed after overexpression of DUSP12
(Fig. 2B). DCFH-DA fluorescent
probe was used to measure ROS levels in HUVECs. The results showed
that the ROS levels in the ox-LDL group were significantly higher
than those in the control group. The levels of ROS in the ox-LDL +
Oe-DUSP12 group were significantly decreased compared with those in
the ox-LDL + Oe-NC group (Fig.
3A). Subsequently, the levels of oxidative stress markers MDA,
SOD and CAT were measured using commercial kits. The results showed
that the activities of SOD and CAT were significantly decreased
after ox-LDL induction, while the levels of MDA were significantly
increased. Compared with those in the ox-LDL + Oe-NC group, SOD and
CAT activities in the ox-LDL + Oe-DUSP12 group were significantly
increased, while the MDA level was significantly decreased
(Fig. 3B). The 8-OHdG level was
measured using a specific commercial the kit and the results showed
that ox-LDL induced the increase of 8-OhdG in cells, while the
level of 8-OHdG in HUVECs was decreased after overexpression of
DUSP12 (Fig. 3C).
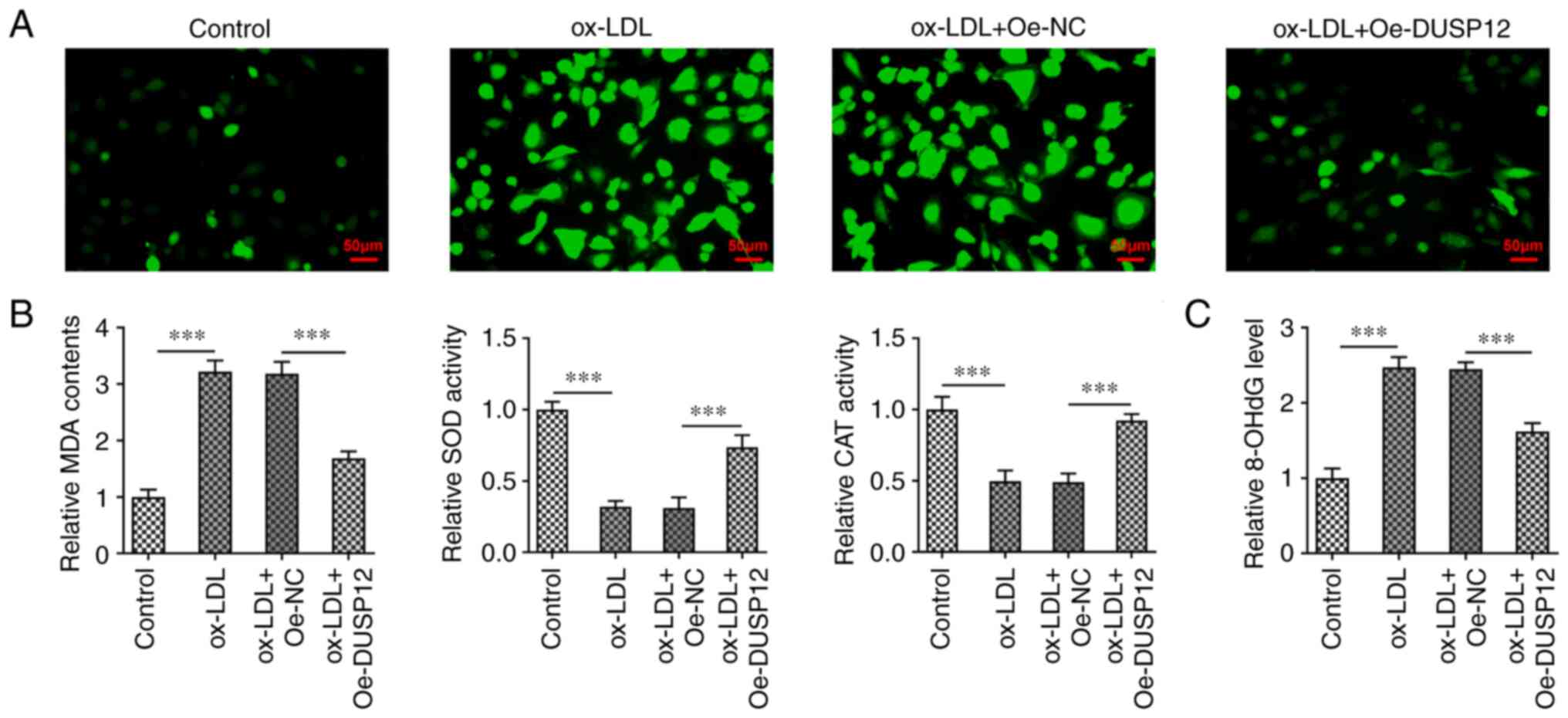 | Figure 3Overexpression of DUSP12 inhibits
oxidative stress damage in oxidized low-density lipoprotein-induced
HUVECs. (A) The DCFH-DA fluorescent probe was used to measure the
levels of reactive oxygen species in cells. (B) Levels of oxidative
stress markers MDA, SOD and CAT. (C) Levels of 8-OHdG.
***P#x003C;0.001. DUSP12, dual specificity phosphatase
12; HUVECs, human umbilical vein endothelial cells; ox-LDL,
oxidized low-density lipoprotein; SOD, superoxide dismutase; CAT,
catalase; MDA, malondialdehyde; DCFH-DA, 2',7'-dichlorofluorescein
diacetate; 8-OHdG, 8-hydroxy-2-deoxyguanosine. |
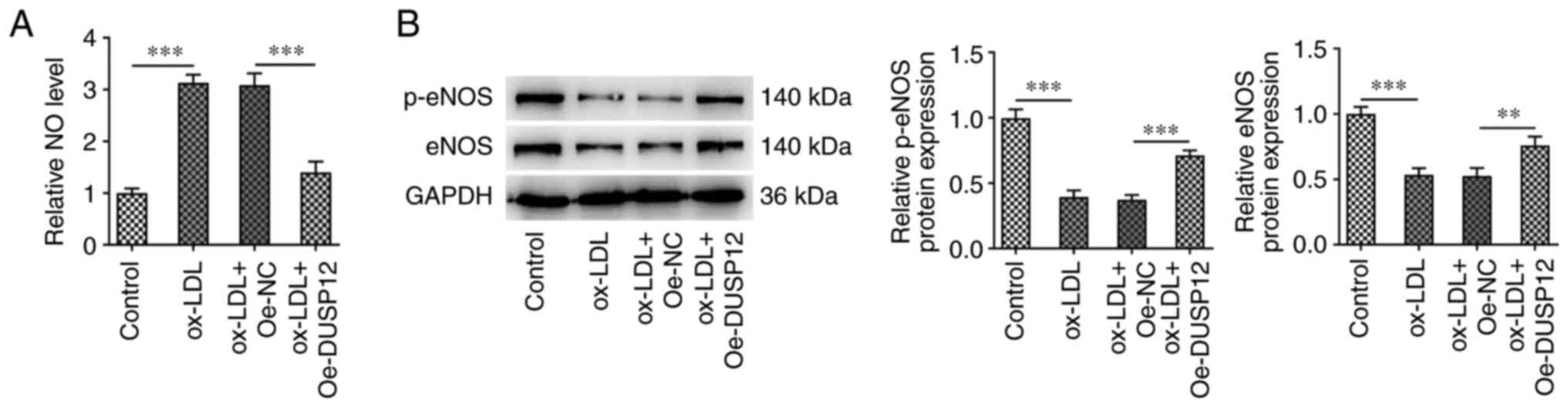
Overexpression of DUSP12 alleviates
endothelial dysfunction in ox-LDL-induced HUVECs
The level of NO in HUVECs was significantly
increased following ox-LDL induction. After further overexpression
of DUSP12, the level of NO in the cells was decreased (Fig. 4A). Western blotting showed that,
compared with those in the control group, the expression levels of
endothelial NO synthase (eNOS) and phosphorylated (p)-eNOS and eNOS
decreased following ox-LDL induction. Compared with those in the
ox-LDL + Oe-NC group, p-eNOS and eNOS protein levels were
significantly increased in the ox-LDL + Oe-DUSP12 group (Fig. 4B).
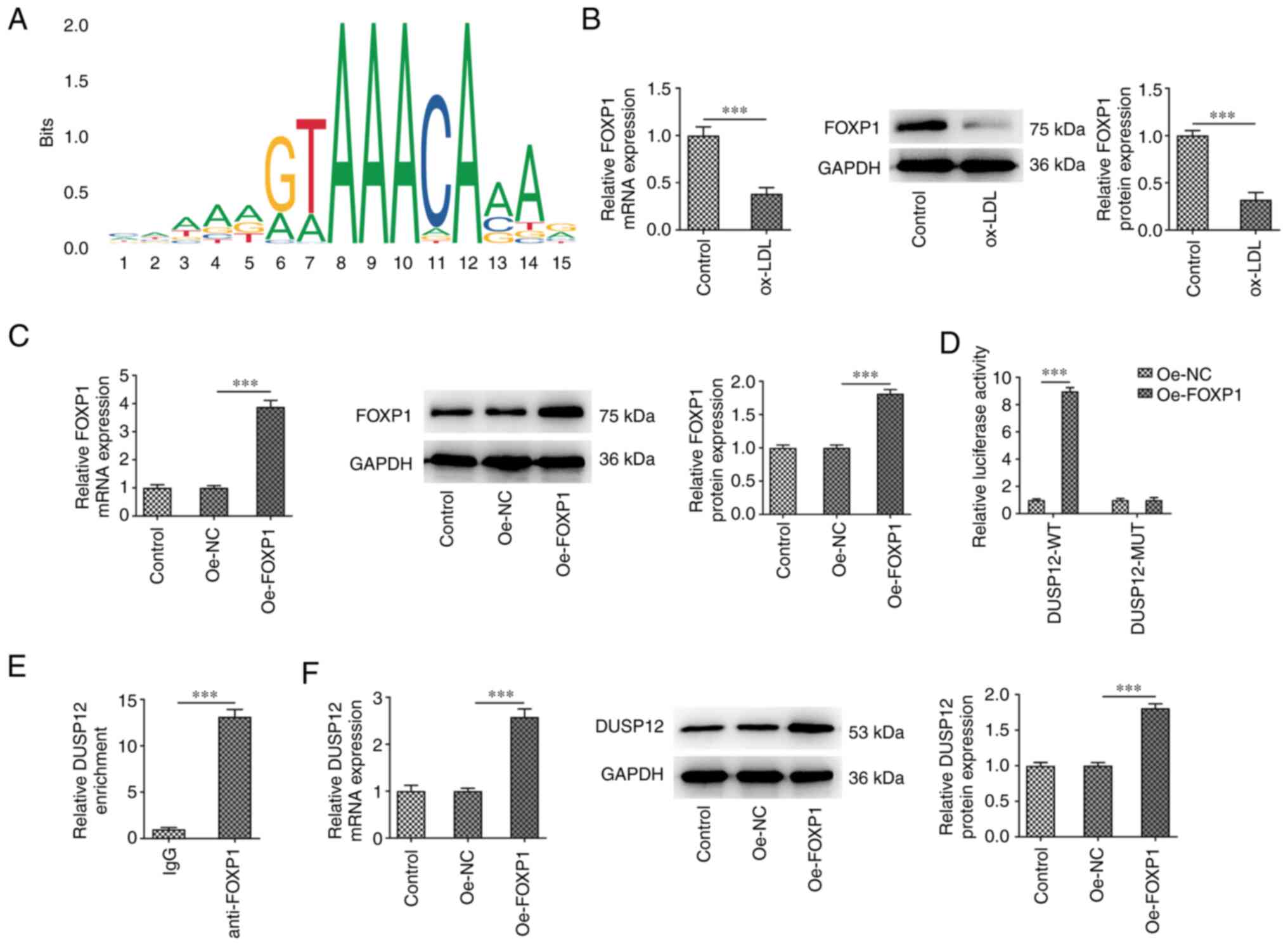
FOXP1 promotes the transcription of
DUSP12
The JASPAR database predicted that the promoters of
DUSP12 and transcription factors FOXP1 had binding sites (Fig. 5A). Therefore, RT-qPCR and western
blotting were used to measure FOXP1 mRNA and protein expression in
HUVECs. The results showed that FOXP1 expression in cells was
significantly decreased after ox-LDL induction (Fig. 5B). Subsequently, FOXP1 was
overexpressed in HUVECs and the transfection efficiency was
measured (Fig. 5C). The binding
between DUSP12 and FOXP1 was verified using duel-luciferase
reporter and ChIP assays (Fig. 5D
and E). In addition, the
expression of DUSP12 in cells was also significantly increased
after overexpressing FOXP1 (Fig.
5F).
FOXP1 alleviates ox-LDL-induced
apoptosis in HUVECs by regulating the expression of DUSP12
The expression of FOXP1 in cells was inhibited
through siRNA transfection and the transfection efficiency was
measured using RT-qPCR and western blotting (Fig. 6A). Since si-FOXP1#2 has a more
significant inhibition efficiency, this was chosen for follow-up
experiments. HUVECs were divided into control, ox-LDL, ox-LDL +
Oe-DUSP12, ox-LDL + Oe-DUSP12 + si-NC and ox-LDL + Oe-DUSP12 +
si-FOXP1 groups. CCK-8 assay showed that cell viability in the
ox-LDL + Oe-DUSP12 + si-FOXP1 group was significantly decreased
compared with that in the ox-LDL + Oe-DUSP12 + si-NC group
(Fig. 6B). TUNEL assay and western
blotting showed that, compared with that in the ox-LDL + Oe-DUSP12
+ si-NC group, apoptosis was significantly increased in the ox-LDL
+ Oe-DUSP12 + si-FOXP1 group, along with decreased levels of Bcl-2
and Bax and increased levels of cleaved PARP (Fig. 6C and D).
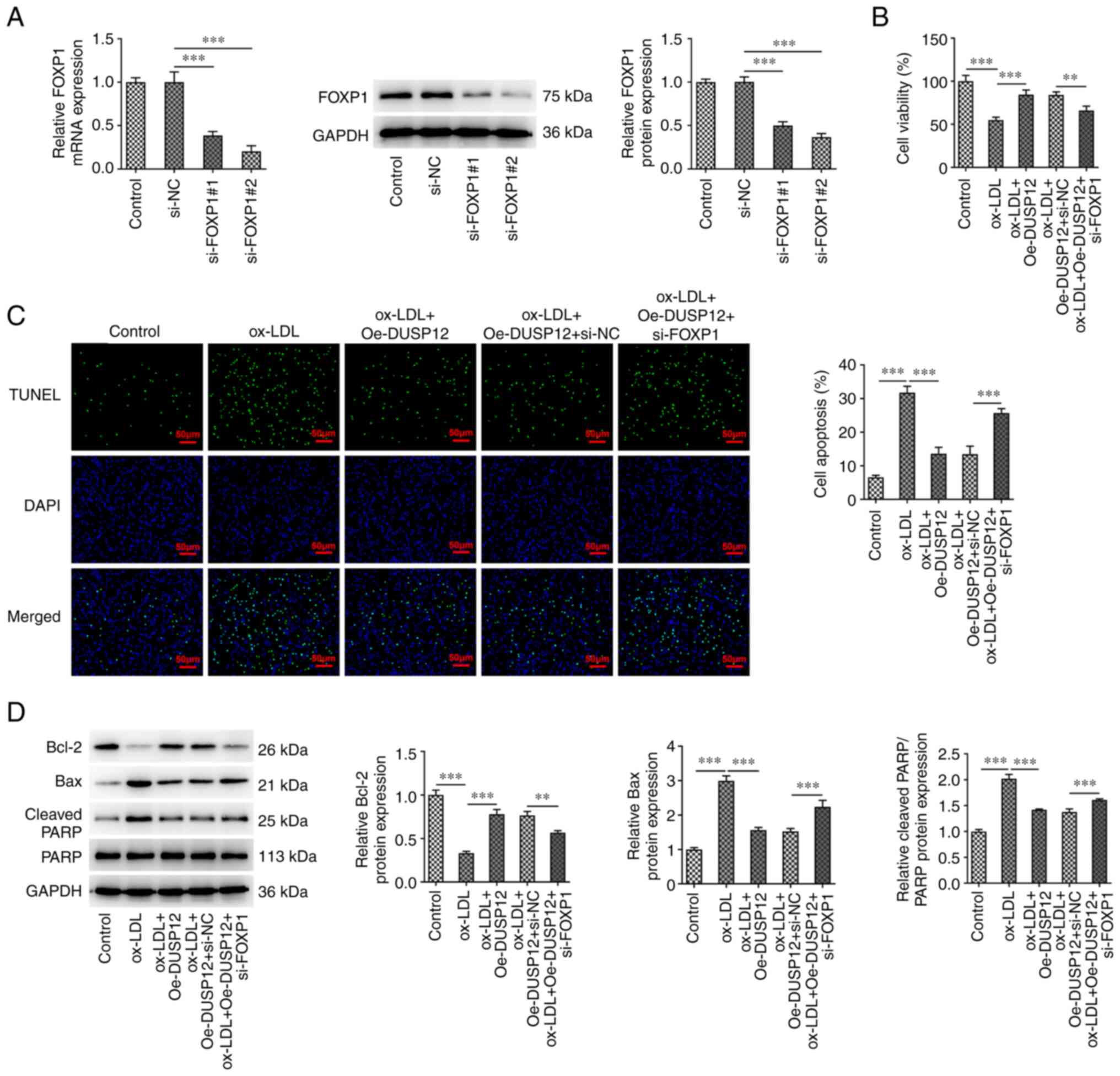 | Figure 6FOXP1 alleviates ox-LDL-induced
apoptosis in HUVECs by regulating the expression of DUSP12. (A) The
expression of FOXP1 in cells was inhibited using siRNA transfection
and the transfection efficiency was measured using RT-qPCR and
western blotting. (B) The cells were then divided into control,
ox-LDL, ox-LDL + Oe-DUSP12, ox-LDL + Oe-DUSP12 + si-NC and ox-LDL +
Oe-DUSP12 + si-FOXP1 groups and Cell Counting Kit-8 was used to
measure the cell viability. (C) TUNEL assay was used to measure
cell apoptosis. (D) The apoptosis-related proteins Bcl-2, Bax and
cleaved PARP were measured by western blotting.
**P#x003C;0.01 and ***P#x003C;0.001. FOXP1,
Forkhead box P1; ox-LDL, oxidized low-density lipoprotein; HUVECs,
human umbilical vein endothelial cells; DUSP12, dual specificity
phosphatase 12; si, short interfering; RT-qPCR, reverse
transcription-quantitative PCR; Oe, overexpression; NC, negative
control. |
FOXP1 reduces ox-LDL-induced
inflammation and oxidative stress injury in HUVECs by regulating
the expression of DUSP12
Compared with those in the ox-LDL + Oe-DUSP12 +
si-NC group, the levels of TNF-α, IL-1β and IL-6 were significantly
increased (Fig. 7A), VCAM-1 and
ICAM-1 protein levels were increased (Fig. 7B) in the ox-LDL + Oe-DUSP12 +
si-FOXP1 group. Compared with those in the ox-LDL + Oe-DUSP12 +
si-NC, ROS and MDA levels were increased in the ox-LDL + Oe-DUSP12
+ si-FOXP1 group, while SOD and CAT activities were decreased
(Fig. 7C and D). In addition, compared with that in the
ox-LDL + Oe-DUSP12 + si-NC, the expression of 8-OhdG was
significantly increased in the ox-LDL + Oe-DUSP12 + si-FOXP1 group
(Fig. 7E). The analysis of
endothelial dysfunction related indicators showed that the level of
NO in the ox-LDL + Oe-DUSP12 + si-FOXP1 group was significantly
higher than that in the ox-LDL + Oe-DUSP12 + si-NC group. However,
the levels of p-eNOS and eNOS in the ox-LDL + Oe-DUSP12 + si-FOXP1
group decreased (Fig. 7F and
G).
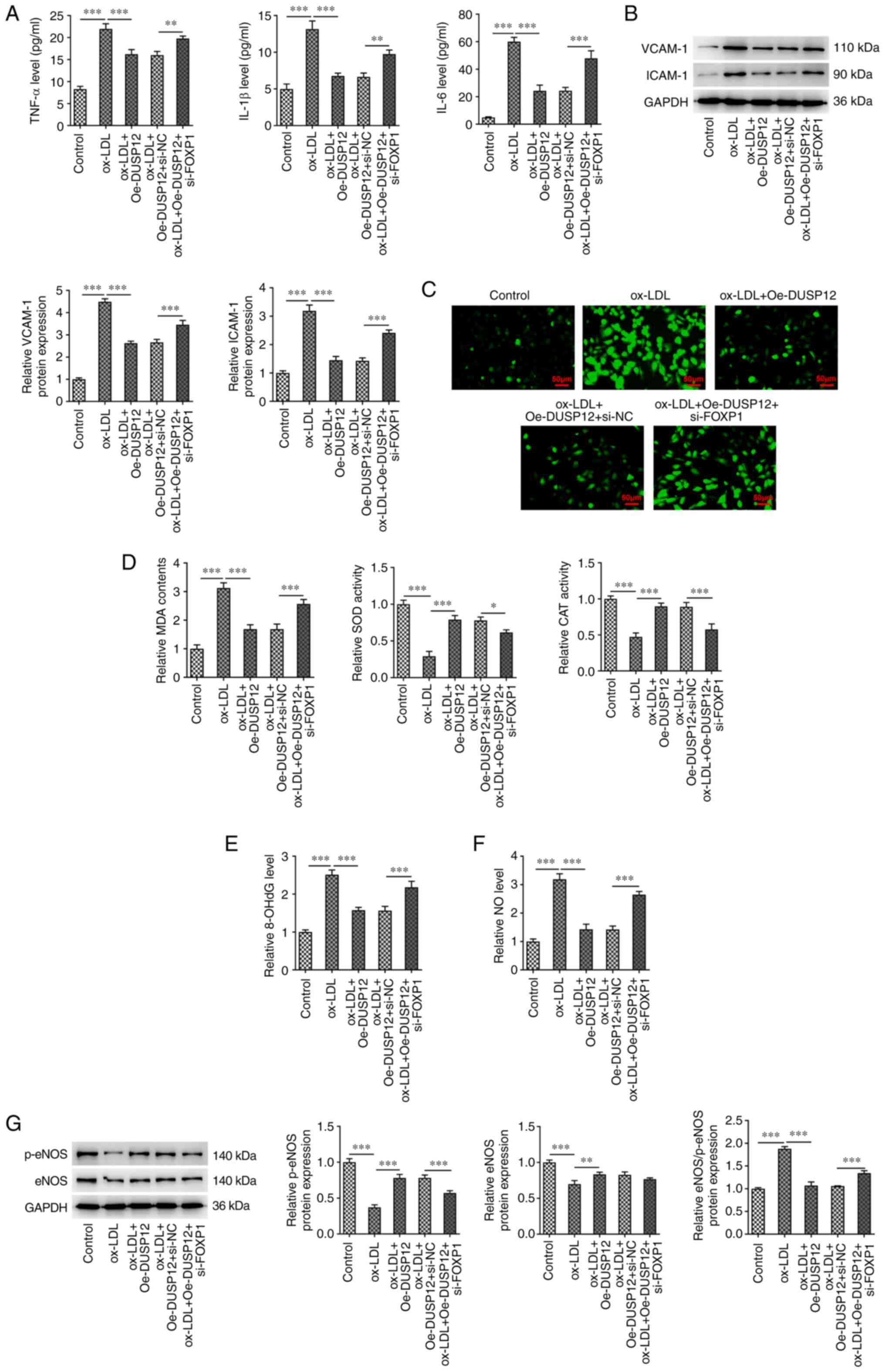 | Figure 7FOXP1 reduces ox-LDL-induced
inflammation and oxidative stress injury in HUVECs by regulating
the expression of DUSP12. (A) ELISA was used to measure the
inflammation-related indicators IL-6, IL-1β and TNF-α to examine
the effect of DUSP12 expression on ox-LDL-induced cellular
inflammation. (B) Western blotting was used to measure the levels
of inflammatory proteins vascular cell adhesion molecule 1 and
intracellular cell adhesion molecule 1. (C) DCFH-DA fluorescent
probe was used to measure the levels of reactive oxygen species
levels. (D) Activities of MDA, SOD and CAT. (E) Levels of OHdG. (F)
Levels of NO. (G) Western blotting analysis of the expression of
eNOS and p-eNOS. *P#x003C;0.05,
**P#x003C;0.01 and ***P#x003C;0.001. FOXP1,
Forkhead box P1; ox-LDL, oxidized low-density lipoprotein; HUVECs,
human umbilical vein endothelial cells; DUSP12, dual specificity
phosphatase 12; SOD, superoxide dismutase; CAT, catalase; MDA,
malondialdehyde; DCFH-DA, 2',7'-dichlorofluorescein diacetate;
8-OHdG, 8-hydroxy-2-deoxyguanosine; NO, nitric oxide; eNOS,
endothelial NO synthase; p, phosphorylated. |
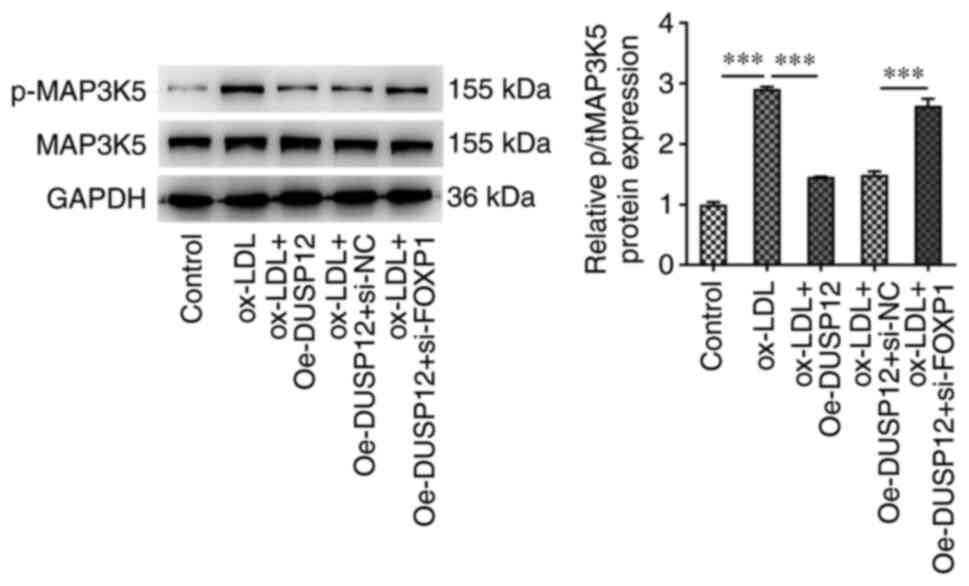
FOXP1 inhibits the MAP3K5 pathway by
regulating the expression of DUSP12, thereby reducing
ox-LDL-induced inflammation and oxidative stress injury in
HUVECs
To further explore the regulatory mechanism, western
blotting was performed to measure the expression of proteins
related to the MAP3K5 signaling pathway downstream to DUSP12. The
results showed that the level of p-MAP3K5 was significantly
increased after ox-LDL induction compared with that in the control
group. After further overexpression of DUSP12, the level of
p-MAP3K5 in HUVECs was significantly inhibited. Compared with that
in the ox-LDL + Oe-DUSP12 + si-NC group, the level of p-MAP3K5 was
significantly increased in the ox-LDL + Oe-DUSP12 + si-FOXP1 group
(Fig. 8).
Discussion
LDL and its modifier ox-LDL are the main
lipoproteins deposited in atherosclerotic plaques in vascular walls
and the level of ox-LDL in patients with AS is significantly higher
compared with that of normal people; therefore, the level of ox-LDL
in plasma can be used as an early screening indicator for AS
(15). In vitro experiments
show that ox-LDL has a damaging effect on vascular endothelial
cells, which can be used as an in vitro model to study the
mechanism of AS (16,17). In the present study, ox-LDL was
used to induce HUVECs to form an in vitro endothelial cell
injury model and the apoptosis of HUVEC was detected by CCK8 and
Tunel assay. Apoptosis, inflammation, oxidative stress related
indicators were tested to determine whether the HUVEC damage model
had been successfully established.
Inflammation, oxidative stress and apoptosis of
vascular endothelial cells are important processes in the
development of AS. Once endothelial cells produce a large number of
inflammatory cytokines and eventually undergo apoptosis, the
vascular endothelium may lose its ability to regulate the
equilibrium of the liposome and the regulation of immunity and
inflammation, resulting in the deposition and retention of
lipoproteins under the endodermis, forming an environment that
promotes the formation of arterial plaque (18). Therefore, regulation of vascular
endothelial cell inflammation and oxidative stress injury is a
potential preventive and therapeutic approach for AS. Therefore,
the current study was of theoretical value and practical
significance for the prevention and treatment of AS by seeking the
causes of inflammation and oxidative stress of vascular endothelial
cells and exploring their pathways.
A previous study showed that DUSP12 expression level
is decreased in LPS-induced endothelial cells and mice
overexpressing DUSP12 could reduce the inflammation and injury of
vascular endothelial cells induced by LPS (19). However, to the best of the authors'
knowledge, the role of DUSP12 in ox-LDL-induced HUVECs injury has
yet to be reported. The present study showed that DUSP12 expression
was significantly decreased in ox-LDL-induced HUVECs.
Overexpression of DUSP12 significantly inhibited apoptosis,
inflammation and oxidative stress in ox-LDL-induced HUVECs. In a
recent study, overexpression of DUSP12 reduced the expression of
lactate dehydrogenase, reduced the size of heart infarction in rats
and inhibited the apoptosis of myocardial tissue and oxidative
stress (20). Overexpression of
DUSP12 inhibited the production of pro-inflammatory cytokines and
chemokines that activate TLR4, stimulated heat-inactivated
Mycobacterium tuberculosis and infected intracellular
bacteria including Listeria monomonas and Mycobacterium
bovis BCG by specifically inhibiting p38 and JNK (21). The aforementioned studies were
consistent with the present results.
The binding between FOXP1 and the DUSP12 promoter
was predicted using the JASPAR database. FOXP1 and the DUSP12
promoter had transcriptional regulatory effects in ox-LDL-induced
HUVECs and FOXP1 promoted the transcription of DUSP12. FOXP1 is
involved in cardiovascular disease (22). A previous study showed that
endothelial FOXP1 could inhibit AS by regulating the activation of
NLRP3 inflammasome (23). FOXP1
inhibits ox-LDL-induced inflammation and lipid accumulation in
macrophages (11). In the current
study, FOXP1 expression was significantly decreased in
ox-LDL-induced HUVECs. Inhibition of FOXP1 expression in cells
significantly reversed the inhibitory effect of overexpressed
DUSP12 on ox-LDL-induced apoptosis and oxidative stress damage in
HUVECs. These results suggested that, in AS disease, FOXP1 promoted
transcription of DUSP12, thereby reducing ox-LDL-induced
inflammation and oxidative stress injury in HUVECs.
The present study further explored the mechanism.
Through literature review, it was found that DUSP12 reduced lung
vascular endothelial cell injury in mouse models of LPS-induced
acute lung injury through the MAP3K5-JNK pathway (19). DUSP12 could act as a novel
endogenous protective signal against hepatic ischemia-reperfusion
injury through the inhibition of MAP3K5(8). In addition, inhibition of macrophage
MAP3K5-JNK signaling may be a useful strategy for antagonizing AS
diseases (24). Ox-LDL induced
endoplasmic reticulum stress and endothelial cell injury through
inflammasome activation mediated by the MAP3K5/NLRP3 signaling
pathway (25). Therefore, it was
hypothesized that DUSP12 was involved in ox-LDL-induced cellular
inflammation and oxidative stress injury by regulating the
downstream MAP3K5 signaling pathway. The current study demonstrated
that FOXP1-induced DUSP12 alleviated vascular endothelial cell
inflammation and oxidative stress injury induced by ox LDL via the
MAP3K5 signaling pathway. The present findings provided a solid
theoretical basis for the clinical treatment of AS disease.
The present study has some limitations. First, it
employed cell experiments, but further verification is needed in
animal experiments. Secondly, in terms of experimental grouping
design, there was no comparison between the si-FOXP1 + Oe-DUSP12
group and the si-FOXP1 alone group, to confirm whether si-FOXP1
alone could have any effect in the absence of DUSP12
overexpression. This will also be improved in future
experiments.
Therefore, the results indicated that FOXP1
inhibited the MAP3K5 pathway by regulating the expression of
DUSP12, thereby reducing ox-LDL-induced inflammation and oxidative
stress injury in HUVECs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ conceived the study. YL and LG performed the
experiments and wrote the manuscript. JZ, CH and WZ processed the
experimental data and analyzed the data. YL and WZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan J and Watanabe T: Atherosclerosis:
Known and unknown. Pathol Int. 72:151–160. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fuster JJ, MacLauchlan S, Zuriaga MA,
Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S,
Muralidharan S, Rius C, et al: Clonal hematopoiesis associated with
TET2 deficiency accelerates atherosclerosis development in mice.
Science. 355:842–847. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lv YC, Yang J, Yao F, Xie W, Tang YY,
Ouyang XP, He PP, Tan YL, Li L, Zhang M, et al: Diosgenin inhibits
atherosclerosis via suppressing the MiR-19b-induced downregulation
of ATP-binding cassette transporter A1. Atherosclerosis. 240:80–89.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen HF, Chuang HC and Tan TH: Regulation
of dual-specificity phosphatase (DUSP) ubiquitination and protein
stability. Int J Mol Sci. 20(2668)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang Z, Wu LM, Zhang JL, Sabri A, Wang
SJ, Qin GJ, Guo CQ, Wen HT, Du BB, Zhang DH, et al: Dual
specificity phosphatase 12 regulates hepatic lipid metabolism
through inhibition of the lipogenesis and apoptosis
signal-regulating kinase 1 pathways. Hepatology. 70:1099–1118.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li H, Yang Q, Huang Z, Liang C, Zhang DH,
Shi HT, Du JQ, Du BB and Zhang YZ: Dual-specificity phosphatase 12
attenuates oxidative stress injury and apoptosis in diabetic
cardiomyopathy via the ASK1-JNK/p38 signaling pathway. Free Radic
Biol Med. 192:13–24. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boldorini R, Clemente N, Alchera E and
Carini R: DUSP12 acts as a novel endogenous protective signal
against hepatic ischemia-reperfusion damage by inhibiting ASK1
pathway. Clin Sci (Lond). 135:161–166. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu XM, Du SL, Miao R, Wang LF and Zhong
JC: Targeting the forkhead box protein P1 pathway as a novel
therapeutic approach for cardiovascular diseases. Heart Fail Rev.
27:345–355. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mulla W, Hajaj B, Elyagon S, Mor M, Gillis
R, Murninkas M, Klapper-Goldstein H, Plaschkes I, Chalifa-Caspi V,
Etzion S and Etzion Y: Rapid atrial pacing promotes atrial
fibrillation substrate in unanesthetized instrumented rats. Front
Physiol. 10(1218)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao F, Zhao Y, Zhang B, Xiao C, Sun Z, Gao
Y and Dou X: Forkhead box protein 1 transcriptionally activates
sestrin1 to alleviate oxidized low-density lipoprotein-induced
inflammation and lipid accumulation in macrophages. Bioengineered.
13:2917–2926. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fornes O, Castro-Mondragon JA, Khan A, van
der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M,
Baranašić D, et al: JASPAR 2020: update of the open-access database
of transcription factor binding profiles. Nucleic Acids Res. 48
(D1):D87–D92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang M, Zhu Y, Zhu J, Xie Y, Wu R, Zhong
J, Qiu Z and Jiang L: circ_0086296 induced atherosclerotic lesions
via the IFIT1/STAT1 feedback loop by sponging miR-576-3p. Cell Mol
Biol Lett. 27(80)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Trpkovic A, Resanovic I, Stanimirovic J,
Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D and Isenovic ER:
Oxidized low-density lipoprotein as a biomarker of cardiovascular
diseases. Crit Rev Clin Lab Sci. 52:70–85. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kattoor AJ, Kanuri SH and Mehta JL: Role
of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 26:1693–1700.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Q, Liu J, Duan H, Li R, Peng W and
Wu C: Activation of Nrf2/HO-1 signaling: An important molecular
mechanism of herbal medicine in the treatment of atherosclerosis
via the protection of vascular endothelial cells from oxidative
stress. J Adv Res. 34:43–63. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Conti P and Shaik-Dasthagirisaeb Y:
Atherosclerosis: A chronic inflammatory disease mediated by mast
cells. Cent Eur J Immunol. 40:380–386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hui Z, Jie H and Fan GH: Expression of
DUSP12 reduces lung vascular endothelial cell damage in a murine
model of lipopolysaccharide-induced acute lung injury via the
apoptosis signal-regulating kinase 1 (ASK1)-Jun N-terminal kinase
activation (JNK) pathway. Med Sci Monit. 27(e930429)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cheng J, Ji M, Jing H and Lin H: DUSP12
ameliorates myocardial ischemia-reperfusion injury through
HSPB8-induced mitophagy. J Biochem Mol Toxicol.
37(e23310)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cho SSL, Han J, James SJ, Png CW,
Weerasooriya M, Alonso S and Zhang Y: Dual-specificity phosphatase
12 targets p38 MAP kinase to regulate macrophage response to
intracellular bacterial infection. Front Immunol.
8(1259)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, Zheng Y, Wang S, Fan Y, Ye Y,
Jing Y, Liu Z, Yang S, Xiong M, Yang K, et al: Single-nucleus
transcriptomics reveals a gatekeeper role for FOXP1 in primate
cardiac aging. Protein Cell. 14:279–293. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhuang T, Liu J, Chen X, Zhang L, Pi J,
Sun H, Li L, Bauer R, Wang H, Yu Z, et al: Endothelial Foxp1
suppresses atherosclerosis via modulation of Nlrp3 inflammasome
activation. Circ Res. 125:590–605. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu Q, Pan J, Bao L, Xu C, Qi Y, Jiang B,
Wang D, Zhu X, Li X, Zhang H, et al: Major vault protein prevents
atherosclerotic plaque destabilization by suppressing macrophage
ASK1-JNK signaling. Arterioscler Thromb Vasc Biol. 42:580–596.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hang L, Peng Y, Xiang R, Li X and Li Z:
Ox-LDL causes endothelial cell injury through ASK1/NLRP3-mediated
inflammasome activation via endoplasmic reticulum stress. Drug Des
Devel Ther. 14:731–744. 2020.PubMed/NCBI View Article : Google Scholar
|