Introduction
Podocytes, the visceral epithelial cells of the
renal capsule, attach to the outside of the glomerular basement
membrane, and together with vascular endothelial cells and the
glomerular basement membrane, constitute the glomerular
hemofiltration barrier. The foot processes between podocytes are
bridged by the slit diaphragm. The morphological and structural
integrity of podocytes is crucial for maintaining the normal
function of glomerular basement membrane cells (1). Glomerular podocyte dysfunction, due
to factors such as oxidative stress, genetic mutations and
inflammation, can lead to protein leakage into the urine, resulting
in proteinuria. Podocyte injury is a common cause of glomerular
disease and a hallmark of disease progression (2).
DJ-1 is a member of the peptidase C56 protein family
encoded by the PARK7 gene, which is mainly present in the heart,
liver, kidney, skeletal muscle, gonads and other organs. It is a
critical functional protein in the apoptotic signal transduction
pathway and is involved in cell metabolism and energy conversion
(3). Mitophagy is a physiological
process that selectively eliminates damaged mitochondria to promote
mitochondrial renewal and maintain a regular supply of
intracellular energy (4). PTEN is
an upstream regulatory protein of signaling pathways, such as
PTEN-induced putative kinase 1 (PINK1)/Parkin and PI3K/Akt/mTOR,
which specifically regulate mitophagy and participate in
mitochondrial homeostasis (5).
Mitochondria are significant producers of reactive oxygen species
(ROS) in cells. Several studies have suggested that DJ-1 is an
upstream regulator of PTEN and that DJ-1 acts as a sensitive
oxidative stress sensor in vivo (6,7).
When DJ-1 reacts with intracellular ROS, its molecular conformation
significantly changes. This change can increase DJ-1 protein
levels; the binding of DJ-1 and ROS regulates PTEN function, and
affects mitophagy and cellular energy supply (8). The kidney possesses one of the
highest rates of energy metabolism out of all the organs in the
body and mitochondria are essential for the protection of kidney
function. With the progression of molecular experimental methods,
such as PCR and western blotting, mitophagy has gradually become a
new focus of research in elucidating the pathogenesis of kidney
disease (9).
Few previous studies have investigated how DJ-1
regulates the physiological function of glomerular podocytes
(10,11). It has been suggested that autophagy
activation and the inhibition of apoptosis have protective effects
in high glucose-induced podocyte injury models, and that this
protective effect is associated with DJ-1(12). Our preliminary study indicated that
podocyte injury and DJ-1 expression are positively associated
(10). However, the specific
mechanism underlying the regulatory effects of DJ-1 on podocyte
function remains to be elucidated; therefore, the present study
aimed to clarify this. In the present study, mouse glomerular
podocyte DJ-1 gene overexpression and silencing models were
constructed to further explore the role and significance of DJ-1 in
maintaining podocyte morphological structure and mitophagy.
Materials and methods
Podocyte culture
MPC5 mouse glomerular podocytes were purchased from
BeNa Culture Collection; Beijing Beina Chunglian Institute of
Biotechnology and were stored in liquid nitrogen. Before the
experiment, podocytes were recovered, collected, and cultured in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing
antibiotics (1% penicillin and streptomycin) and 10% FBS (Gibco;
Thermo Fisher Scientific, Inc. at 33˚C in a humidified 5%
CO2/95% air atmosphere. Recombinant mouse γ-interferon
(10 U/ml, BINDER GmbH) was used to induce podocyte proliferation.
After podocyte confluence reached 80-90%, 0.25% trypsin/0.02% EDTA
(Gibco; Thermo Fisher Scientific, Inc.) was added. Subsequently,
the podocytes were moved to a 37˚C environment for differentiation
and maturation for 10-14 days. The differentiated and matured
podocytes were then seeded into six-well plates (Corning), and
after the cells reached 60-70% confluence, RPMI 1640 alone was used
to starve the podocytes for 12 h to synchronize the proliferation
of the cells, before the experimental grouping was performed.
Experimental grouping
Podocytes were randomly divided into the following
four groups: i) Control group, cells were cultured in RPMI 1640;
ii) DJ-1 overexpression (OE) group (OE-DJ-1), cells were
transfected with the OE-DJ-1 plasmid; iii) empty vector group,
cells were transfected with an empty vector as a negative control;
iv) DJ-1 silencing group (Si-DJ-1), cells were transfected with
small interfering RNA (siRNA)-DJ-1 for gene silencing.
Model establishment.
Overexpression
Cells were passaged 1 day before transfection, and
transfection was conducted using Lipofectamine® 2000
transfection reagent (cat. no. 11668019l; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
When cell confluence reached 70-80%, 3 µg OE-DJ-1 plasmid
[pcDNA3.1(+), Invitrogen; Thermo Fisher Scientific, Inc.] or empty
vector and AB mixture were added to each well of a 6-well plate
where cells were seeded; solution A refers to 100 µl Opti-MEM cell
culture medium (cat. no. 31985070; Gibco; Thermo Fisher Scientific,
Inc.) and solution B refers to 3 µl Lipofectamine 2000 solution
dissolved in Opti-MEM medium. The cells were transfected at 37˚C in
a 5% CO2 atmosphere for 5 h. Subsequently, the cells
were cultured in complete medium at 37˚C and DJ-1 expression was
detected after 48 h.
Silencing
Podocytes in the logarithmic growth phase were
selected, the cell suspension was adjusted to a density of
1x105/ml following trypsinization and the cells were
inoculated into six-well plates. Subsequently, 20 µm siRNA storage
solution was added to Opti-MEM medium to reach final concentrations
of 100, 50 and 25 nM, which was designated as solution A. Then, 10
µl Lipofectamine 2000 transfection reagent was added to 100 µl
Opti-MEM medium as solution B. Solutions A and B were mixed and
incubated at room temperature for 15-20 min to prepare the
transfection complex. The transfection complex was then added to
complete medium, which was added to a 6-well plate with a final
volume of 2 ml (100 nM) per well. After 5 h of incubation of cells
at 37˚C, the medium was replaced with cell culture medium. After 24
h of transfection, the culture medium was discarded, and washed
twice with PBS.
Detection of transfection
efficiency
To verify the transfection efficiency of OE-DJ-1 and
Si-DJ-1, reverse transcription-quantitative PCR (RT-qPCR) was used
to detect the mRNA expression levels of DJ-1 in cells compared with
those in cells transfected with the empty vector or si-NC (cat. no.
siN0000001-1-5; Guangzhou RiboBio Co., Ltd.).
Podocyte morphology
Podocyte morphology was observed using an inverted
phase-contrast microscope (Axio Vert 25; Carl Zeiss AG) at a
magnification of x200.
Flow cytometry-based apoptosis
assay
After rinsing MPC-5 cells with pre-cooled PBS,
EDTA-free trypsin was added to digest the cells and the suspended
cells were collected. The cells were centrifuged at room
temperature for 5 min (400 x g), the supernatant was removed,
rinsed twice with pre-cooled PBS and the cells were collected.
Cells (1x106) were added to 400 µl binding buffer
(BioVision, Inc.) to resuspend the cells, after which, 10 µl
annexin V-FITC (BioVision, Inc.) and 5 µl PI (BioVision, Inc.) were
added, and allowed to stand at room temperature for 10 min in the
dark. Subsequently, flow cytometry (FACSCanto; BD Biosciences) was
conducted and data were analyzed using FlowJo-V10 software (FlowJo
LLC).
RT-qPCR
Total RNA was extracted according to the
instructions of the RNAgents® Total RNA Isolation System
(Promega Corporation) (13). Then,
the purity and integrity of the total RNA was detected. Briefly,
the purity of the RNA was detected by measuring the OD 260/OD 280
ratio with a nucleic acid protein analyzer (BioPhotometer Plus;
Shanghai Musen Biotechnology Co., Ltd.). The integrity of the total
RNA was assessed by 1% agarose gel electrophoresis, which was
performed at 80 V for 20 min, and the total RNA bands were observed
with a gel imaging system (Zhuhai Hema Medical Instrument Co.,
Ltd.). Thereafter, the extracted RNA was transcribed into cDNA
using PrimeScript™ RT Master Mix Kit (Takara Bio, Inc.) according
to the manufacturer's protocol. SYBR Premix Ex Taq™ II (Takara Bio,
Inc.) was used for qPCR and reactions were performed using a
LightCycler480 Real-Time PCR System (Roche Diagnostics) with the
following protocol: 95˚C for 5 min, followed by 40 cycles at 95˚C
for 15 sec, 60˚C for 15 sec and 72˚C for 30 sec. The primer
sequences were as follows: DJ-1 forward, 5' AACACACCCACTGGCTAAGG
3', reverse, 5' GGCTAGTGCAAACTCAAAGC 3'; PTEN forward,
5'-TGAGTTCCCTCAGCCATTGCCT-3', reverse,
5'-GAGGTTTCCTCTGGTCCTGGTA-3'; and β-actin forward, 5'
ACATCCGTAAAGACCTCTATGCC 3', reverse, 5' TACTCCTGCTTGCTGATCCAC 3'.
Primers were designed and synthesized by Shanghai Sangon
Bioengineering Co., Ltd. Data were calculated using the
2-ΔΔCq method (14).
Western blot analysis
The MPC-5 cells in each group were rinsed twice with
PBS, and the PBS was aspirated prior to the addition of RIPA cell
lysis solution (Thermo Fisher Scientific, Inc.). The cells were
then shaken on ice for 30 min and lysed by pipetting. Total protein
concentration was measured using the BCA method and the proteins
were denatured at 100˚C for 10 min. Total proteins (30 µg) were
separated by SDS-PAGE on 12% gels and were transferred to PVDF
membranes. Blocking was performed with 5% nonfat milk powder at
25˚C on a shaker for 2 h, after which, dropwise rabbit anti-DJ-1
(1:1,000; cat. no. ab76008; Abcam), rabbit anti-PTEN (1:1,000; cat.
no. ab137337; Abcam), rabbit anti-LC3B (1:1,000; cat. no. ab192890;
Abcam), rabbit anti-Bad (1:1,000; cat. no. ab32445; Abcam) or
rabbit anti-GAPDH (1:1,000; cat. no. ab9485; Abcam) antibodies were
added, and incubated overnight at 4˚C on a shaker. The horseradish
peroxidase-conjugated secondary antibody (1:1,000; cat. no. ab6721;
Abcam) was then added for 1 h at room temperature on a shaker.
Subsequently, 1 ml western blot ECL reagent (MilliporeSigma) was
added to the blots, which were exposed, and images were captured.
The exposed image was scanned using ImageJ (V1.8.0; National
Institutes of Health) and the gray value of the protein band was
analyzed.
Immunofluorescence staining and
confocal laser scanning microscopy
MPC-5 cells were cultured and separated into groups.
Subsequently, the cells were fixed with 4% paraformaldehyde at 4˚C
for 8 min, ruptured with Triton-100 at room temperature for 5 min
and blocked with 5% BSA (Gibco; Thermo Fisher Scientific, Inc.) at
37˚C for 1 h. Subsequently, DJ-1 (1:50; cat. no. ab76008; Abcam) or
PTEN (1:50; cat. no. ab137337; Abcam) primary antibodies were added
dropwise to the cell slides, and the cells were incubated in a
humidified atmosphere at 4˚C overnight. After washing three times
with PBS, an Alexa Fluor™ 568 goat anti-rabbit secondary antibody
(1:200; cat. no. A11011; Invitrogen; Thermo Fisher Scientific,
Inc.) was added and incubated at 37˚C for 1 h, and nuclear staining
was performed with 0.1% DAPI at room temperature for 10 min. Excess
DAPI was washed three times with PBS-0.1% Tween, the slides were
sealed with a mounting solution containing anti-fluorescence
quencher, and the cells were observed under a fluorescence
microscope. To semi-quantify DJ-1 and PTEN levels in the cells,
three typical images from each group were analyzed by ImageJ based
on a reported method (15).
Transmission electron microscopy
Podocytes from each group were collected, fixed with
glutaraldehyde and 1% osmium tetroxide for 15-30 min at 4˚C, and
dehydrated at room temperature with gradient concentrations of
acetone. The dehydrated samples were embedded in pure
acetone-EPON812 embedding medium for 2 h at room temperature, baked
to solidify, sectioned (1 µm), stained dropwise with sodium acetate
for 30 min at room temperature and washed with PBS. Samples were
imaged using a transmission electron microscope (Libra 120
microscope; Carl Zeiss AG) to observe autophagosomes.
Statistical analyses
All experiments were repeated at least three times.
All data were statistically processed using SPSS17.0 statistical
software (SPSS, Inc.). The measurement data are expressed as the
mean ± standard deviation. Unpaired Student's t-test was used for
comparisons between two groups, and one-way ANOVA followed by
Tukey's post hoc test was used for comparisons among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphological changes of
podocytes
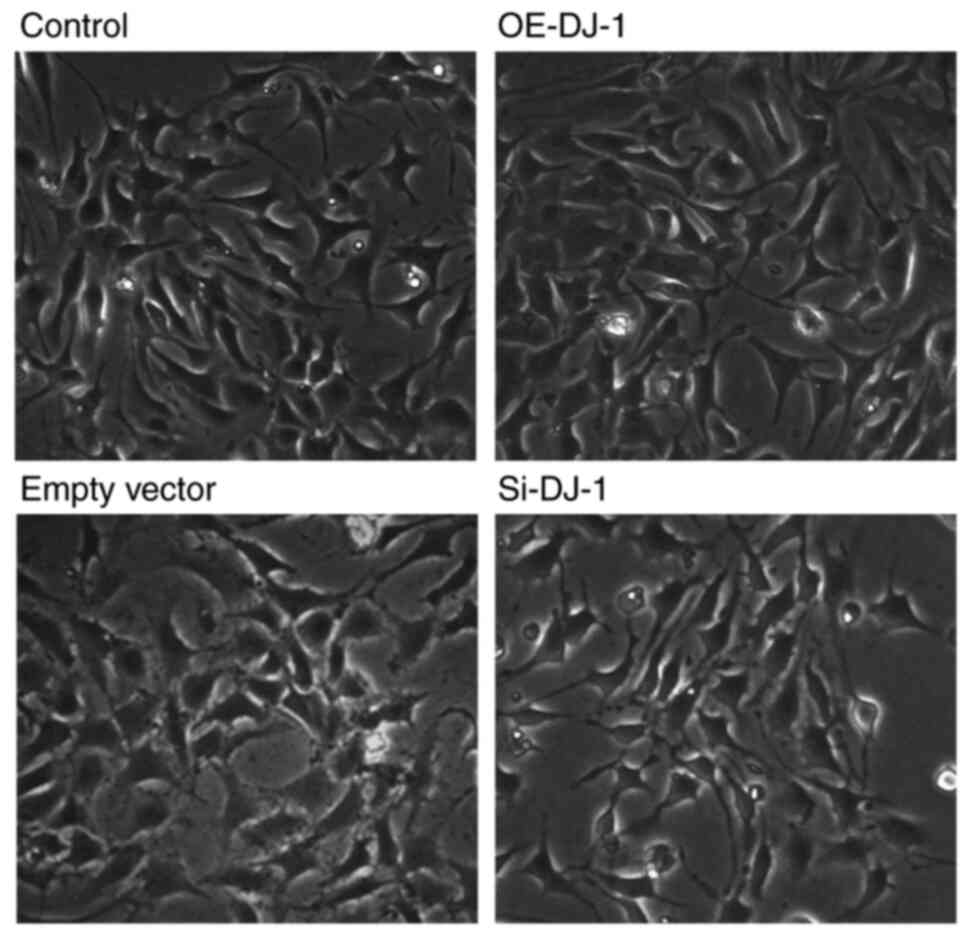
As shown in Fig. 1,
there were differences in cell morphology among the different
treatment groups. There was no marked difference in cell morphology
between the empty vector and control groups. Compared with the
control group, the podocytes in the OE-DJ-1 group exhibited a
larger volume, more obvious foot processes, more regularly shaped
nuclei, and tight junctions between adjacent cells, whereas the
podocytes in the Si-DJ-1 group exhibited a decrease in volume and
the foot processes contracted inward. Neighboring cells were
loosened and some dead cells were observed. The morphology of
podocytes observed in the present study is in line with findings
from previous studies (16,17).
Podocyte transfection efficiency
After assessing three different siRNA transfection
concentrations, the optimal concentration required was determined.
Agarose gel electrophoresis (Fig.
2A) showed that the RNA extracted had high purity and contained
no DNA contamination or degradation. Podocytes were treated with
different DJ-1 siRNA transfection concentrations (25, 50 and 100
nM). Compared with in the si-NC group, the expression levels of
DJ-1 were significantly reduced after transfection with Si-DJ-1
(P<0.05; Fig. 2B), and there
were no obvious differences among the different transfection
concentrations and siRNAs used. Therefore, 25 nM si3 was selected
as the experimental working concentration for subsequent silencing.
Furthermore, the expression levels of DJ-1 in the OE-DJ-1 group
were significantly increased compared with those in the empty
vector group (Fig. 2C).
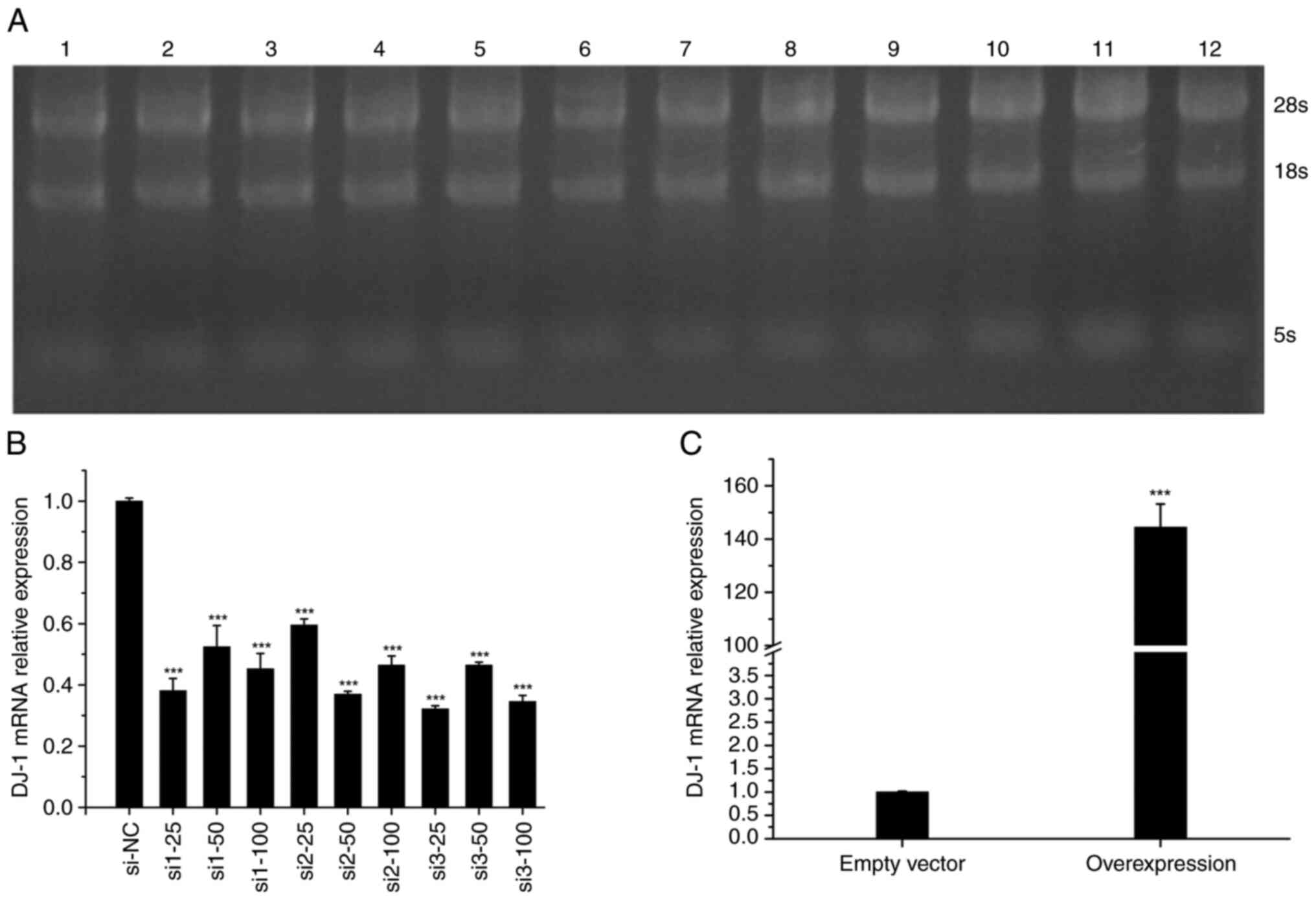 | Figure 2(A) RNA electrophoresis of 12
samples. Lane 1, si1-25; lane 2, si1-50; lane 3, si1-100; lane 4,
si2-25; lane 5, si2-50; lane 6, si2-100; lane 7, si3-25; lane 8,
si3-50; lane 9, si3-100; lane 10, si-NC; lane 11, OE-DJ-1; lane 12,
empty vector. (B) DJ-1 mRNA expression in 10 silencing samples.
***P<0.05 vs. si-NC. (C) DJ-1 mRNA expression in the
overexpression group and the empty vector group.
***P<0.05 vs. empty vector. OE, overexpression; NC,
negative control; si, small interfering. |
Changes in podocyte apoptosis
rate
The apoptotic rate of podocytes (Fig. 3A; quadrants 2 and 3) in the control
group and empty vector group was lower than that in the Si-DJ-1
group ((P<0.05; Fig. 3A), and
there was no significant difference between the control and empty
vector groups; however, the apoptotic rate of the control group and
the empty group was significantly higher than that in the OE-DJ-1
group (P<0.05; Fig. 3A). In
addition, compared with those in the control group and empty vector
group, the expression levels of the proapoptotic protein Bad were
significantly reduced in the OE-DJ-1 group, whereas they were
significantly increased in the Si-DJ-1 group (P<0.05; Fig. 3B).
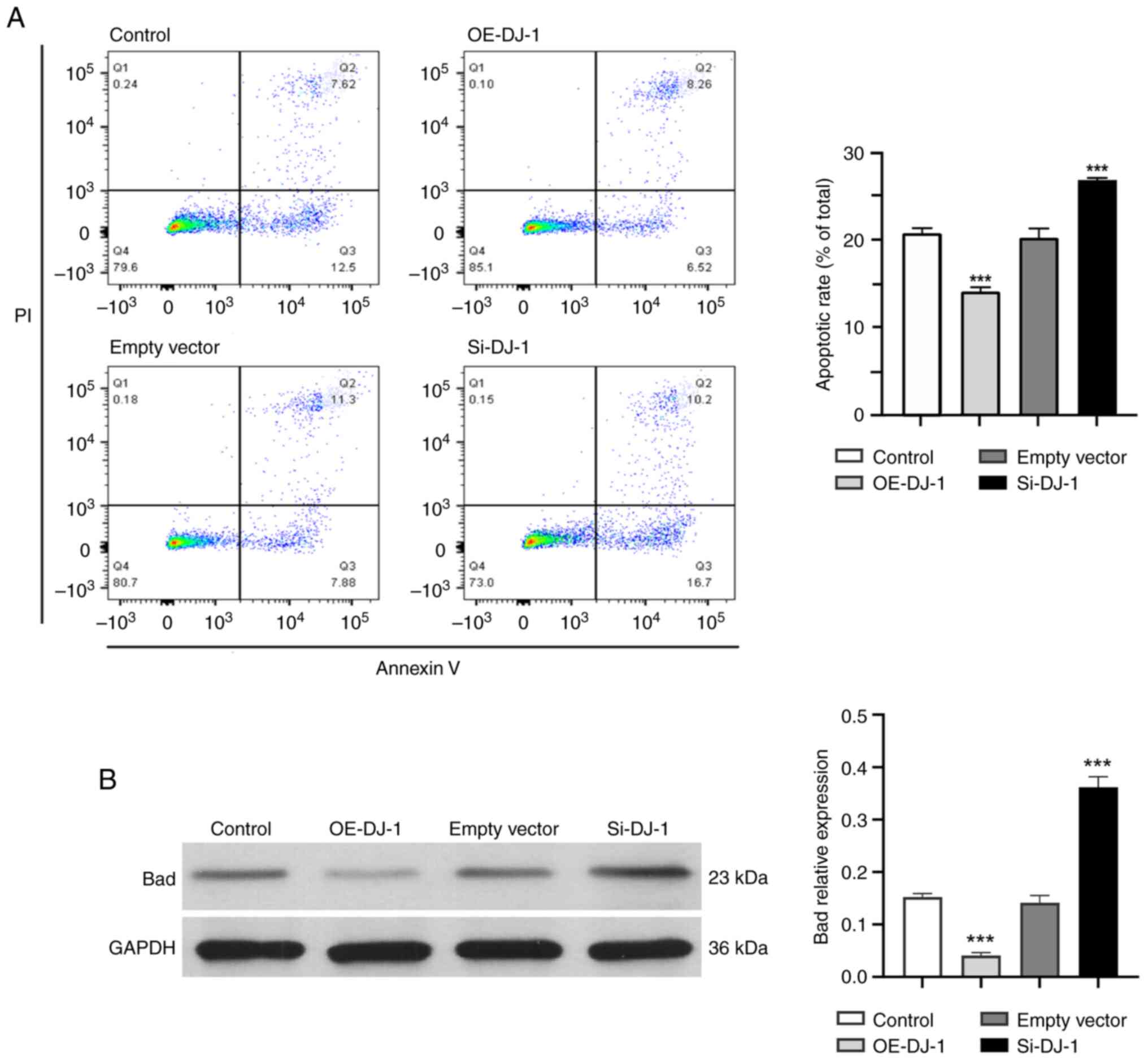 | Figure 3(A) Apoptotic rate of podocytes was
detected by flow cytometry. Q1, PI (+) and annexin V (-),
necrotic/dead cells; Q2, PI (+) and annexin V (+), late apoptotic
or dead cells; Q3, PI (-) and annexin V (+), early apoptotic cells;
Q4, annexin V (-) and PI (-), normal living cells. (B) Expression
of the proapoptotic protein Bad in each group.
***P<0.05 vs. control group. OE, overexpression; Si,
silencing. |
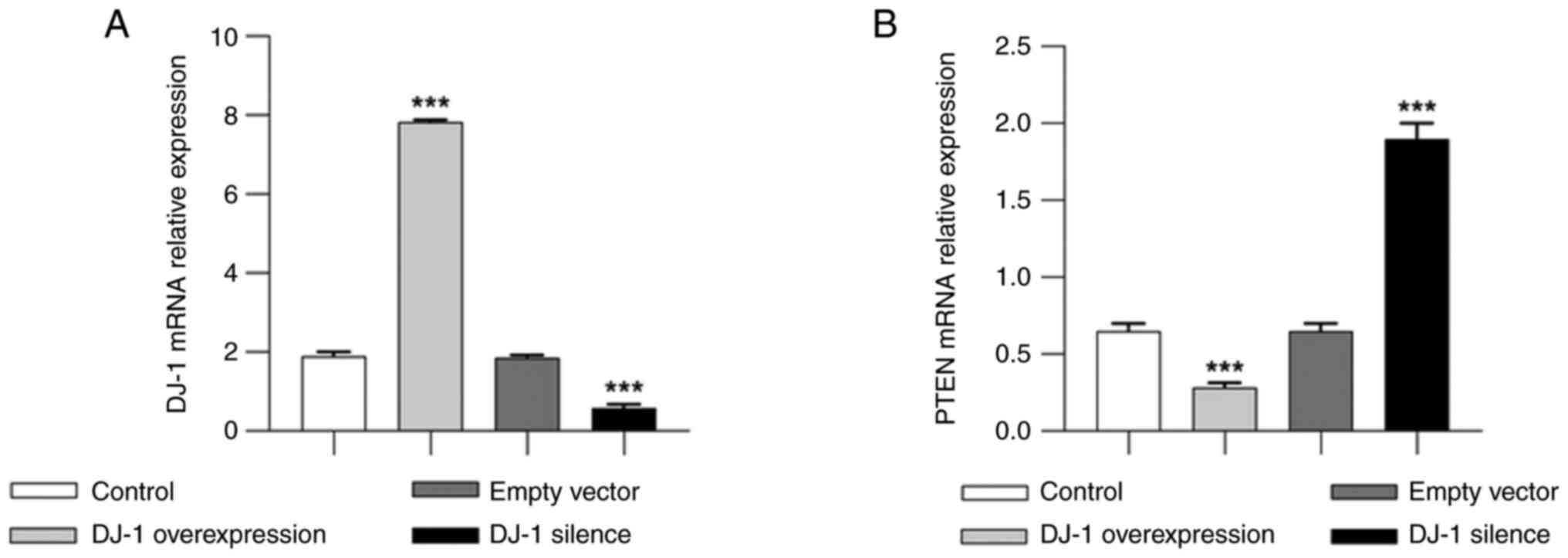
mRNA expression levels of DJ-1 and
PTEN in podocytes
The results of qPCR showed that the relative mRNA
expression levels of DJ-1 in the control and empty vector groups
were lower than those in the OE-DJ-1 group. Moreover, DJ-1
expression was significantly higher in the OE-DJ-1 group than that
in the control group (P<0.05), whereas it was significantly
lower in the Si-DJ-1 group than that in the control group
(P<0.05) (Fig. 4A). The
relative mRNA expression levels of PTEN were significantly lower in
the OE-DJ-1 group than those in the control group (P<0.05) and
were significantly higher in the Si-DJ-1 group (P<0.05; Fig. 4B).
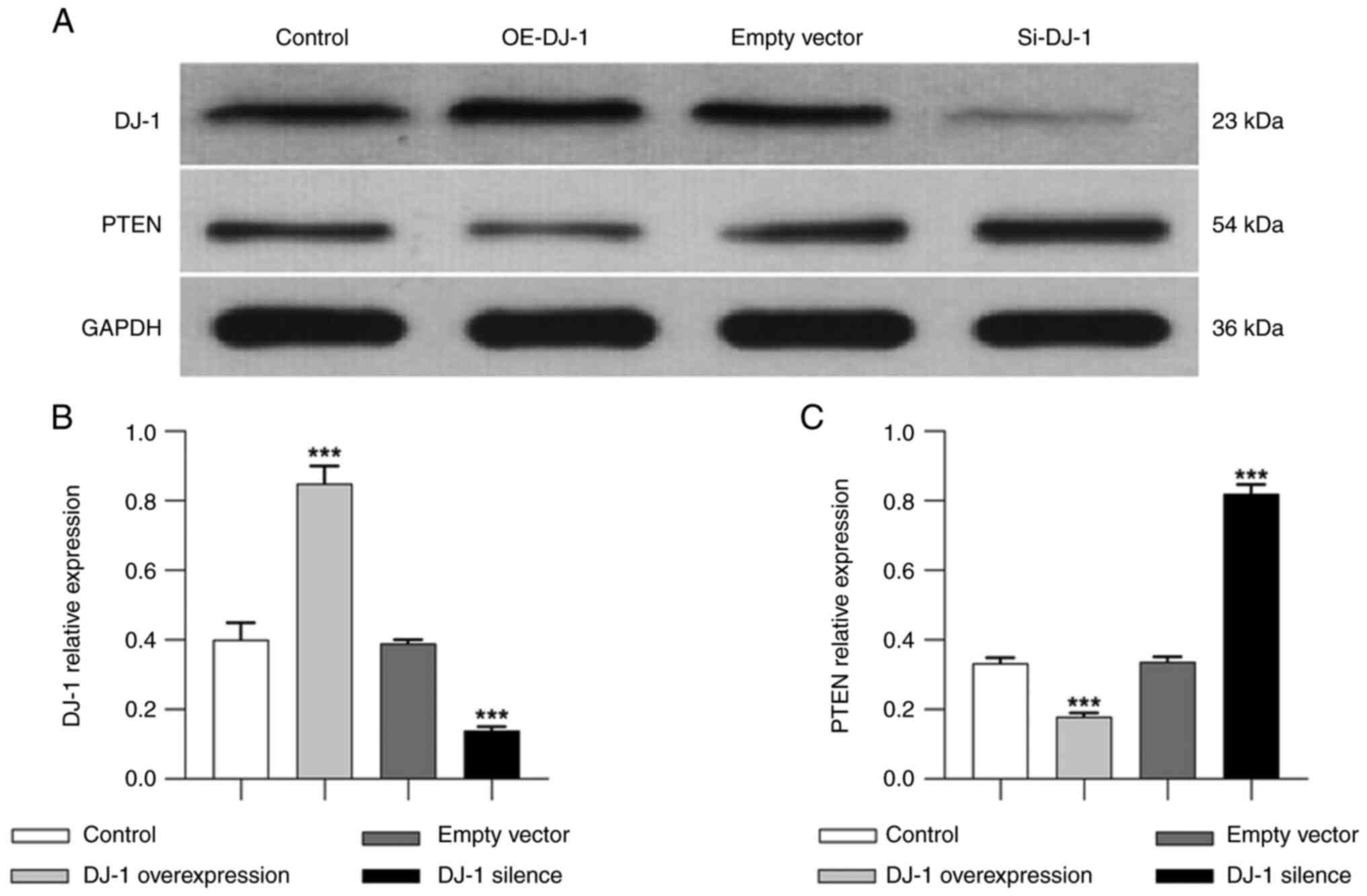
Protein expression levels of DJ-1 and
PTEN in podocytes
Western blotting results showed no significant
difference in the protein expression levels of DJ-1 between the
control and empty vector groups. The protein expression levels of
DJ-1 were significantly higher in the OE-DJ-1 group than those in
the control group (P<0.05). By contrast, the protein expression
levels of DJ-1 were significantly lower in the Si-DJ-1 group than
those in the control group (P<0.05; Fig. 5A). The protein expression levels of
PTEN were relatively low in both the control and empty vector
groups. The protein expression levels of PTEN were significantly
lower in the OE-DJ-1 group than those in the control group
(P<0.05). By contrast, the protein expression levels of PTEN
were significantly increased after DJ-1 gene silencing compared
with those in the control group (P<0.05; Fig. 5B).
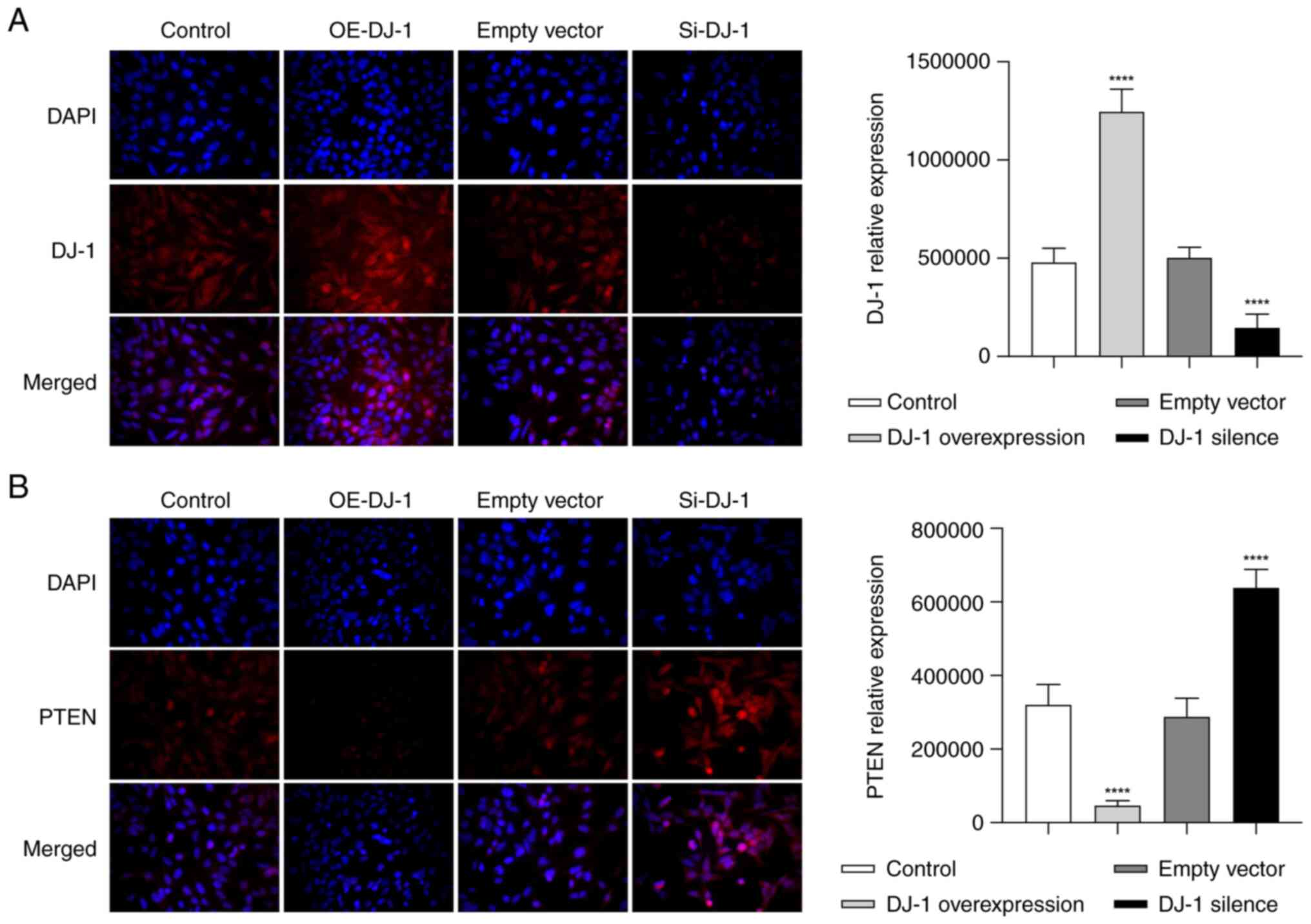
Distribution of DJ-1 and PTEN
proteins
The DJ-1 protein was evenly distributed throughout
the cell membrane in the control and empty vector groups, and there
was no significant difference between the two groups. In the
OE-DJ-1 group, the expression of DJ-1 was significantly higher than
that in the control group (P<0.05; Fig. 6A). In the Si-DJ-1 group, the
expression of DJ-1 was significantly reduced, compared with that in
the control group (P<0.05; Fig.
6A).
In the control and empty vector groups, PTEN was
mainly distributed discontinuously on the surface of the cell
membrane, a small amount of PTEN was evenly distributed in the
cytoplasm, and granular distribution was also observed around the
nucleus. When DJ-1 was overexpressed, the distribution of PTEN in
the cell membrane was markedly reduced, and a granular uneven
distribution was observed in the cytoplasm. The difference in PTEN
expressions levels was statistically significant compared with that
in the control group (P<0.05; Fig.
6B). In addition, the distribution of PTEN in the cell membrane
and cytoplasm of the cells in the Si-DJ-1 group was notably
increased, and evenly and densely distributed in clumps, with a
statistically significant difference in PTEN expression levels
compared with those in the control group (P<0.05; Fig. 6B).
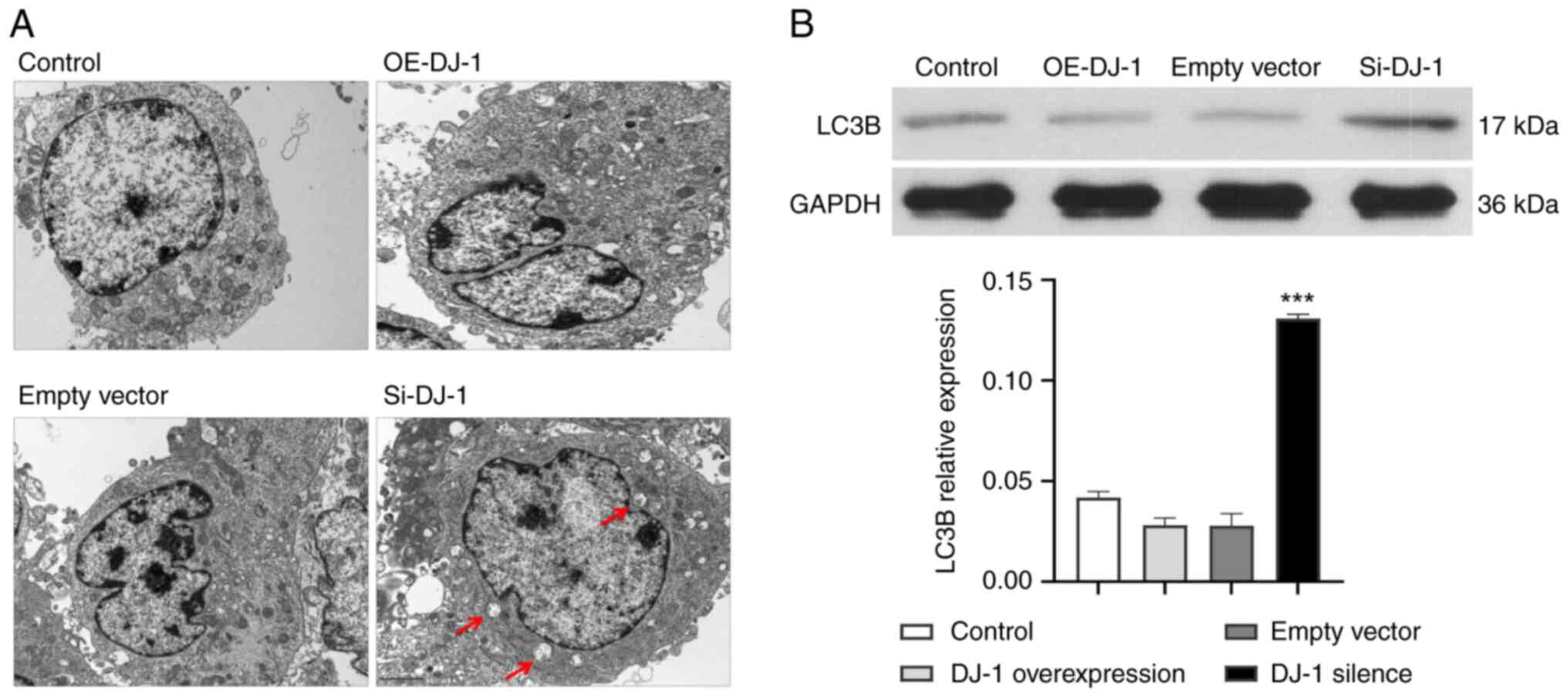
Mitochondrial morphology and
autophagosomes
In the control and empty vector groups, the
structure of most mitochondria was clear and the mitochondrial
cristae were complete. The structure of the mitochondria in the
OE-DJ-1 group was relatively clear and the mitochondrial cristae
were complete, similar to the control group. In the Si-DJ-1 group,
the mitochondrial structure was swollen, mitochondrial cristae were
scattered, vacuoles appeared and the number of autophagosomes was
markedly increased (Fig. 7A).
Compared with the control group, the expression levels of the
autophagy marker LC3B were significantly increased in the Si-DJ-1
group (P<0.05; Fig. 7B).
Discussion
The DJ-1 gene, also known as PARK7, is located on
chromosome 1p36.23. It is widely expressed in various tissues in
eukaryotic and prokaryotic organisms, it encodes the DJ-1 protein
and belongs to the C56 peptidase family. DJ-1 is a nucleic acid
disaccharidase with a molecular weight of ~20 kDa and contains 189
amino acids, existing as a homodimer (18). Oxidative stress promotes the
dissociation of cytoplasmic DJ-1 dimers into monomers that are
translocated to the nucleus. DJ-1 acts as a co-activator of various
signaling pathways and regulates cell death in the nucleus
(19). The crystal structure of
human DJ-1 contains three cysteine residues at amino acids 46, 53
and 106 (Cys46, Cys53 and Cys106), which is its main functional
domain. Notably, DJ-1 is a sensitive oxidative stress sensor in
vivo and can determine the intracellular redox level according
to its different oxidation states [non-oxidation, SO2-
(free radical), SO3- (free radical)] (20). DJ-1 has powerful anti-oxidative,
immunoregulatory, anti-apoptotic and molecular chaperone functions
(21). Its adaptive regulation of
cells under oxidative stress is attributed to its regulation of
multiple signal transduction pathways, especially its
neuroprotective effect (22).
Previous studies have shown that DJ-1 can improve cell survival and
induce proliferation by activating ERK1/2 and PI3K/Akt signaling
pathways, and nuclear factor erythroid 2-related factor 2
pathway-mediated antioxidant responses, and can attenuate cell
death by inhibiting apoptotic signal-regulated kinase 1 and
P53-related apoptotic pathways (23,24).
Autophagy is a catabolic process involving the
formation and targeted degradation of autophagosomes in cells,
which aims to support the recycling and energy regeneration of
intracellular nutrients. In addition to non-selective degradation,
mitophagy is a type of selective autophagy that removes damaged
mitochondria and maintains a healthy mitochondrial network
(25). It is used to maintain
homeostasis in the intracellular environment and is significantly
related to the level of oxidative stress in vivo (4). PTEN is one of the main downstream
molecules involved in the regulation of the DJ-1 protein (26). PINK1 is a serine/threonine kinase
that recruits Parkin through phosphorylation; Parkin is an
intracellular E3 ubiquitin-protein ligase that targets
ubiquitinated outer mitochondrial membrane and induces
mitochondrial autophagy (27).
Oxidized DJ-1 can closely bind to PTEN and inhibit its function,
thereby increasing the phosphorylation activity of PI3K/Akt
(28), which is another mechanism
by which DJ-1/PTEN participates in autophagy regulation.
DJ-1 was first discovered in a study of the
pathogenesis of Parkinson's disease (29); therefore, it is also called
Parkinson's protein. Previous studies on DJ-1 function have mainly
focused on neurodegenerative diseases, such as Parkinson's disease
(30,31). However, its pathophysiological role
in the kidneys remains largely unknown. Few studies have focused on
systemic diseases involving glomeruli and renal tubules, mainly in
in vitro cell and animal experiments, such as diabetic
nephropathy (32), hypertensive
nephropathy (33), infectious
renal failure (34,35) and ischemia/reperfusion renal injury
(36). Therefore, the present
study aimed to explore the role of DJ-1 in the pathogenesis of
primary kidney disease.
The results of the present study demonstrated that
the morphology and structure of podocytes in the Si-DJ-1 group were
worse than those in the control group, and the number was markedly
reduced, whereas the morphology and number of podocytes in the
OE-DJ-1 group were not noticeably different from those in the
control group. The apoptotic rate of podocytes in the Si-DJ-1 group
was markedly increased compared with that in the control group,
whereas that in the OE-DJ-1 group was lower. It could be
hypothesized that DJ-1 may be an effective protective protein in
glomerular podocytes, and could serve an important role in
maintaining their normal morphology and structure. Silencing the
DJ-1 gene may affect the normal proliferation and metabolism of
podocytes, leading to an increase in their apoptotic rate. Under a
transmission electron microscope, the morphology and structure of
mitochondria in the OE-DJ-1 group were shown to be intact, and
there was no marked difference compared with the control group. In
the Si-DJ-1 group, the mitochondrial structure swelled, cristae
dispersed, vacuoles appeared and the number of autophagosomes was
increased. It is suggested that increasing the expression of DJ-1
may reduce the level of mitochondrial autophagy in podocytes to
some extent. PTEN is an important protein phosphatase in the human
body that regulates mitochondrial autophagy by increasing protein
phosphorylation in signal transduction pathways, such as PI3K/Akt
and PINK/Parkin. The present study confirmed that DJ-1 can
negatively regulate the expression of PTEN. Combined with all of
the present experimental results, it was deduced that PTEN may be
one of the main downstream molecules in the DJ-1-regulated signal
transduction pathway. DJ-1 may regulate mitochondrial autophagy and
participate in podocyte protection via PTEN, which is consistent
with previous reports. Overexpression of the DJ-1 gene and
reduction of PTEN expression can reduce the mitochondrial autophagy
in podocytes, maintain healthy and complete morphological structure
of mitochondria, stabilize the effective energy supply of
mitochondria, and protect the normal function of podocytes. By
contrast, silencing the DJ-1 gene can increase the expression of
PTEN, leading to excessive mitochondrial autophagy, which cannot
ensure normal energy supply, thus leading to podocyte damage.
It is well known that podocyte injury leads to
glomerular filtration membrane dysfunction, one of the main causes
of proteinuria. Few studies have examined the relationship between
mitochondrial autophagy and glomerular podocyte injury. Our
research group has explained this relationship in detail in
previous studies. Yu and Yu (37)
suggested that autophagy is involved in the damage of glomerular
podocytes caused by puromycin, which is closely related to the
damage and repair of podocytes. Xie and Yu (38) reported that the mitochondrial
autophagy process mediated by the PINK1/Parkin signaling pathway is
affected by a number of other proteins and related signaling
pathways at the same time, so as to protect podocytes from external
damage. Yang et al (39)
revealed that by increasing the expression of autophagy-related
protein LC3 and enhancing the autophagy activity of glomerular
podocytes, they can significantly reduce the damage to glomerular
podocytes in mice induced by puromycin, reduce the production of
proteinuria and delay glomerular sclerosis. These findings suggest
that increasing autophagy may be an effective protective mechanism
of podocytes under the condition of injury. DJ-1 is a sensitive
oxidative stress receptor. It participates in the development of
various diseases by regulating mitochondrial autophagy. As an
essential organ with active energy metabolism in the body,
oxidative damage to the kidneys is a common factor in the
occurrence of glomerular diseases. Podocytes are rich in
mitochondria. Autophagy is an important process that affects
growth, metabolism, apoptosis and function. Therefore, we
hypothesized that the DJ-1 protein could protect podocytes and
reduce the occurrence of proteinuria by regulating mitophagy.
Combined with previous research on the DJ-1/PTEN pathway and the
results of the present study, it may be suggested that DJ-1
participates in regulating mitochondrial autophagy through the
DJ-1/PTEN pathway. On the one hand, DJ-1 may directly combine with
excessive ROS in podocytes through its own unique structure to
serve an antioxidative stress function; on the other hand,
mitochondrial damage may be reduced by reducing excessive autophagy
and ineffective autophagy of podocyte mitochondria. As a result,
the normal energy supply of podocytes can be ensured, morphological
and functional damage of glomerular podocytes can be avoided, and
the formation of urinary protein can be reduced.
Subsequently, we aim to establish a podocyte injury
model and conduct quantitative analysis of DJ-1 in the experimental
group to assess the protective function of DJ-1 on podocytes under
the condition of kidney disease. There are other limitations in the
present study. Without a three-dimensional internal experiment, it
is not possible to accurately evaluate the function of DJ-1 in
vivo, and this will be improved on in subsequent
experiments.
In conclusion, under the condition of overexpression
or silencing of the DJ-1 gene, the present study assessed its
effects on mitochondrial autophagy, podocyte morphology and
apoptosis. When the DJ-1 gene was silenced, the level of mitophagy
in glomerular podocytes was increased significantly, the expression
of the autophagy marker protein LC3B was increased, and podocytes
exhibited excessive mitophagy and an increased apoptosis rate.
These findings indirectly indicated that DJ-1 may protect podocyte
function by regulating mitophagy. Therefore, DJ-1 may be considered
a new target for the treatment of kidney diseases caused by
mitochondrial autophagy-mediated podocyte injury.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Science Foundation
of Guangzhou First People's Hospital (grant no. M2019020), the
National Natural Science Foundation of China (grant nos. 81273205
and 81670652), the Guangdong Planned Project of Science and
Technology (grant no 2016A020215010), the Youth Cultivation Fund of
Guangdong Medical University (grant no. GDMUQ2022008) and the
Project of Guangdong Medical Science and Technology Research Fund
(grant no. A02022128).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX was involved in data analysis and writing. GL and
LY performed conceptualization, validation, supervision, and
reviewed and edited the manuscript. JT and SY were involved in
collecting experimental data and software application. JX and JT
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mukherjee K, Gu C, Collins A, Mettlen M,
Samelko B, Altintas MM, Sudhini YR, Wang X, Bouley R, Brown D, et
al: Simultaneous stabilization of actin cytoskeleton in multiple
nephron-specific cells protects the kidney from diverse injury. Nat
Commun. 13(2422)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gourlay CW, Carpp LN, Timpson P, Winder SJ
and Ayscough KR: A role for the actin cytoskeleton in cell death
and aging in yeast. J Cell Biol. 164:803–809. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oh SE and Mouradian MM: Regulation of
signal transduction by DJ-1. Adv Exp Med Biol. 1037:97–131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Palikaras K, Lionaki E and Tavernarakis N:
Mechanisms of mitophagy in cellular homeostasis, physiology, and
pathology. Nat Cell Biol. 20:1013–1022. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li G, Yang J, Yang C, Zhu M, Jin Y, McNutt
MA and Yin Y: PTEN α regulates mitophagy and maintains
mitochondrial quality control. Autophagy. 14:1742–1760.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Qiu L, Ma Z, Li X, Deng Y, Duan G, Zhao
LE, Xu X, Xiao L, Liu H, Zhu Z and Chen H: DJ-1 is involved in the
multidrug resistance of SGC7901 gastric cancer cells through
PTEN/PI3K/Akt/Nrf2 pathway. Acta Biochim Biophys Sin (Shanghai).
52:1202–1214. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li H, Hu X, Ma B and Zhang H: Effect of
Parkinson's disease-relevant protein DJ-1 on cell proliferation,
apoptosis, invasion and migration in human osteosarcoma cells.
Zhong Nan Da Xue Xue Bao Yi Xue Bao. 43:1054–1060. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
8
|
Ma Z, Yang J, Yang Y, Wang X, Chen G, Shi
A, Lu Y, Jia S, Kang X and Lu L: Rosmarinic acid exerts an
anticancer effect on osteosarcoma cells by inhibiting DJ-1 via
regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine.
68(153186)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang L, Lu G and Shen HM: The long and the
short of PTEN in the regulation of mitophagy. Front Cell Dev Biol.
8(299)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tan JJ, Yu SY, Zhang Y, Hao ZH and Yu L:
Effect of tacrolimus on the expression of Park7 in glomerular
podocytes injured by puromycin aminonucleoside. Zhongguo Dang Dai
Er Ke Za Zhi. 23:951–958. 2021.PubMed/NCBI View Article : Google Scholar : (In Chinese,
English).
|
|
11
|
Yin L, Li H, Liu Z, Wu W, Cai J, Tang C
and Dong Z: PARK7 protects against chronic kidney injury and renal
fibrosis by inducing SOD2 to reduce oxidative stress. Front
Immunol. 12(690697)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu F, Li S, Zhang N, Huang W, Li X, Wang
M, Bai D and Han B: Hispidulin alleviates high-glucose-induced
podocyte injury by regulating protective autophagy. Biomed
Pharmacother. 104:307–314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bo YY, Liang LD, Hua YJ, Zhao Z, Yao MS,
Shan LB and Liang CZ: High-purity DNA extraction from animal tissue
using picking in the TRIzol-based method. Biotechniques.
70:186–190. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao M, Altankov G, Grabiec U, Bennett M,
Salmeron-Sanchez M, Dehghani F and Groth T: Molecular composition
of GAG-collagen I multilayers affects remodeling of terminal layers
and osteogenic differentiation of adipose-derived stem cells. Acta
Biomater. 41:86–99. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang X, Gan H and Du X: High glucose
induces podocyte apoptosis and constitutive photomorphogenic 1
expression changes. CRTER. 15:4461–4464. 2011.
|
|
17
|
Ge M, Molina J, Kim JJ, Mallela SK, Ahmad
A, Varona Santos J, Al-Ali H, Mitrofanova A, Sharma K, Fontanesi F,
et al: Empagliflozin reduces podocyte lipotoxicity in experimental
Alport syndrome. Elife. 12(e83353)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng Y, Marion TN, Cao X, Wang W and Cao
Y: Park 7: A novel therapeutic target for macrophages in
sepsis-induced immunosuppression. Front Immunol.
9(2632)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bahmed K, Boukhenouna S, Karim L, Andrews
T, Lin J, Powers R, Wilson MA, Lin CR, Messier E, Reisdorph N, et
al: The effect of cysteine oxidation on DJ-1 cytoprotective
function in human alveolar type II cells. Cell Death Dis.
10(638)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song IK, Kim MS, Ferrell JE Jr, Shin DH
and Lee KJ: Stepwise oxidations play key roles in the structural
and functional regulations of DJ-1. Biochem J. 478:3505–3525.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Wang J, Wang J, Yang B, He Q and
Weng Q: Role of DJ-1 in immune and inflammatory diseases. Front
Immunol. 11(994)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Neves M, Grãos M, Anjo SI and Manadas B:
Modulation of signaling pathways by DJ-1: An updated overview.
Redox Biol. 51(102283)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang XL, Wang ZZ, Shao QH, Zhang Z, Li L,
Guo ZY, Sun HM, Zhang Y and Chen NH: RNAi-mediated knockdown of
DJ-1 leads to mitochondrial dysfunction via Akt/GSK-3ß and JNK
signaling pathways in dopaminergic neuron-like cells. Brain Res
Bull. 146:228–236. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Niki T, Endo J, Takahashi-Niki K, Yasuda
T, Okamoto A, Saito Y, Ariga H and Iguchi-Ariga SMM: DJ-1-binding
compound B enhances Nrf2 activity through the PI3-kinase-Akt
pathway by DJ-1-dependent inactivation of PTEN. Brain Res.
1729(146641)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ali T, Hussain F, Kayani HUR, Naeem M and
Anjum F: The role of mitochondria and mitophagy in cell senescence.
Adv Protein Chem Struct Biol. 136:93–115. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Oswald MC, Brooks PS, Zwart MF, Mukherjee
A, West RJ, Giachello CN, Morarach K, Baines RA, Sweeney ST and
Landgraf M: Reactive oxygen species regulate activity-dependent
neuronal plasticity in Drosophila. Elife. 7(e39393)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li W, Du M, Wang Q, Ma X, Wu L, Guo F, Ji
H, Huang F and Qin G: FoxO1 promotes mitophagy in the podocytes of
diabetic male mice via the PINK1/Parkin pathway. Endocrinology.
158:2155–2167. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yu S and Yu L: The mechanism of PI3K/Akt
signaling pathway in the apoptosis of glomerular podocytes. J
Pediatr Pharm. 25:1–5. 2019.(In Chinese).
|
|
29
|
Dolgacheva LP, Berezhnov AV, Fedotova EI,
Zinchenko VP and Abramov AY: Role of DJ-1 in the mechanism of
pathogenesis of Parkinson's disease. J Bioenerg Biomembr.
51:175–188. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Heremans IP, Caligiore F, Gerin I, Bury M,
Lutz M, Graff J, Stroobant V, Vertommen D, Teleman AA, Van
Schaftingen E and Bommer GT: Parkinson's disease protein PARK7
prevents metabolite and protein damage caused by a glycolytic
metabolite. Proc Natl Acad Sci USA. 119(e2111338119)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Imberechts D, Kinnart I, Wauters F,
Terbeek J, Manders L, Wierda K, Eggermont K, Madeiro RF, Sue C,
Verfaillie C and Vandenberghe W: DJ-1 is an essential downstream
mediator in PINK1/parkin-dependent mitophagy. Brain. 145:4368–4384.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Das F, Dey N, Venkatesan B, Kasinath BS,
Ghosh-Choudhury N and Choudhury GG: High glucose upregulation of
early-onset Parkinson's disease protein DJ-1 integrates the
PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal.
23:1311–1319. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cuevas S, Yang Y, Konkalmatt P, Asico LD,
Feranil J, Jones J, Villar VA, Armando I and Jose PA: Role of
nuclear factor erythroid 2-related factor 2 in the oxidative
stress-dependent hypertension associated with the depletion of
DJ-1. Hypertension. 65:1251–1257. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Leeds J, Scindia Y, Loi V, Wlazlo E, Ghias
E, Cechova S, Portilla D, Ledesma J and Swaminathan S: Protective
role of DJ-1 in endotoxin-induced acute kidney injury. Am J Physiol
Renal Physiol. 319:F654–F663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yao Y, Wei H, Liu L, Liu L, Bai S, Li C,
Luo Y, Zeng R, Han M, Ge S and Xu G: Upregulated DJ-1 promotes
renal tubular EMT by suppressing cytoplasmic PTEN expression and
Akt activation. J Huazhong Univ Sci Technolog Med Sci.
31(469)2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yin J, Xu R, Wei J and Zhang S: The
protective effect of glutaredoxin 1/DJ-1/HSP70 signaling in renal
tubular epithelial cells injury induced by ischemia. Life Sci.
223:88–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu-Shengyou and Li Y: Dexamethasone
inhibits podocyte apoptosis by stabilizing the PI3K/Akt signal
pathway. Biomed Res Int. 2013(326986)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xie Q and Yu L: The role of Pink1/Parking
signaling pathway in Pink1 gene silencing-mediated mitochondrial
dysfunction in glomerular podocytes. Chin J Pract Pediatr.
33:347–352. 2018.(In Chinese).
|
|
39
|
Yang XQ, Yu SY, Yu L, Ge L, Zhang Y, Hao
ZG and Liu GS: Effects of tacrolimus on autophagy protein LC3 in
puromycin-damaged mouse podocytes. J Int Med Res.
48(300060520971422)2020.PubMed/NCBI View Article : Google Scholar
|