Introduction
Colorectal cancer (CRC) is the third most common
malignancy worldwide, after lung and breast cancer, and is one of
the leading causes of cancer death, accounting for 9.4% of global
cancer deaths (1,2). Although targeted therapies and
immunotherapies have improved the treatment outcomes of advanced
CRC, the therapeutic efficacy is still limited, especially because
the development of drug resistance affects the further improvement
of therapeutic efficacy (3,4).
Therefore, there is an urgent need to identify and develop safer
and more effective therapeutic agents with anticancer activity.
Natural compounds and their derivatives may be
promising sources of therapeutic agents. Paclitaxel, for example,
which has been used in clinical cancer treatment, is a natural
substance derived primarily from the natural plant yew that
frequently triggers cancer cell apoptosis (5). Cucurbitacins are a class of natural
tetracyclic triterpenoids extracted from plants, which exhibit a
variety of pharmacological activities, such as anti-inflammatory,
anticancer and hepatoprotective activities (6). Previous studies have reported that
cucurbitacins have a broad anticancer spectrum and can inhibit
various types of cancer, such as breast, lung and laryngeal cancer
(7-9).
Cucurbitacin B (CuB) has received attention in cancer research as
one of the most abundant and potent members of this family
(10). It has been reported that
CuB can target the Notch signalling pathway to induce apoptosis in
CRC HCT116 and SW480 cells (11).
Promkan et al (12) also
reported that CuB induced apoptosis in colon cancer Caco-2 and
SW620 cells while triggering autophagy. In addition, CuB may
regulate the polarization of tumour-associated M2-type macrophages
to inhibit the metastasis and invasion of colon cancer cells
through suppression of JAK2/STAT3 signalling (13).
The endoplasmic reticulum (ER) is an intracellular
organelle with important physiological functions, which is involved
mainly in protein synthesis, folding and calcium storage (14). Aggregation of misfolded or unfolded
proteins in the ER caused by various factors triggers ER stress
(ERS) and the unfolded protein response (UPR), with the aim of
maintaining ER homeostasis (15,16).
Reactive oxygen species (ROS) is a general term describing
molecular oxygen derivatives, including the superoxide anion,
hydrogen peroxide and the hydroxyl radical, which are produced
during cellular respiration. These ROS act as agents of cellular
damage, reacting with lipids, proteins and DNA to cause injury
(17-19).
ERS is further initiated when the intracellular environment is
stimulated by ROS. When ERS is prolonged or intensified, apoptosis
is induced (20-22).
In cancer research, ROS have been reported to be able to regulate
ERS to induce cancer cell apoptosis or death (23,24).
Therefore, ERS may be a target for the treatment of cancer.
Notably, it remains to be elucidated whether CuB can
induce apoptosis in CRC cells through ROS-activated ERS; therefore,
the present study investigated the role of ROS and ERS in
CuB-mediated induction of apoptosis in CRC cells.
Materials and methods
Cell treatment
CuB (Dalian Meilun Biology Technology Co., Ltd.) was
dissolved in DMSO, and the stock solution (20 mM) was prepared and
stored at -80˚C. In all experiments, the final concentration of
DMSO was <0.1%, and different concentrations of CuB working
solution were prepared. The HT-29 and SW620 cells (Procell Life
Science & Technology Co., Ltd.) used in the experiment were
cultured in DMEM (Biological Industries) containing 10% FBS
(Biological Industries) and 1% penicillin (Biological Industries)
in a 5% CO2 incubator at 37˚C. In the experiments, HT-29
and SW620 cells were incubated with the CuB (5 µM) for 48 h at
37˚C, and the concentration of DMSO in the control group was 0.1%.
The human CRC HT-29 cell line used in the present study was
verified by STR profiling.
Cell Counting Kit 8 (CCK8) assay
HT-29 and SW620 cells were seeded in a 96-well plate
with complete culture medium (5x103 cells/well). After
the cells attached to the plate, they were treated with different
concentrations of CuB (0-100 µM) working solution and cultured in a
37˚C incubator for 48 h. Subsequently, the drug-containing culture
medium was removed, CCK8 reagent (100 µl; 1:100; Biosharp) was
added to each well, and the 96-well plate was incubated at 37˚C for
2 h. The absorbance was measured at 450 nm using a microplate
reader. Calculate each group's cell proliferation rate was
calculated using the formula follows: Cell viability=1-[(control
group OD value-experimental group OD value)/(control group OD
value-blank group OD value) x100].
Colony formation assay
HT-29 and SW620 cells were evenly seeded in 6-well
cell culture plates (300 cells/well). After the cells were treated
with different concentrations of CuB (0, 0.5, 1.0, 5.0 µM) at 37˚C
for 48 h, and the cell culture media was changed. The cells were
cultivated for 14 days in a 5% CO2 incubator at 37˚C,
with the media changed every three days. Subsequently, the original
medium was removed, and 4% paraformaldehyde was added to fix the
cells for 30 min and the cells were stained with 0.5% crystal
violet at room temperature (25˚C) for 10 min. The total number of
colonies (>50 cells) formed was calculated by ImageJ 1.53t
(National Institutes of Health)
Dichlorodihydrofluorescein diacetate
(DCFH-DA) fluorescence probe ROS detection kit
HT-29 and SW620 cells were seeded in 12-well cell
culture plates (1x104 cells/well), cultured for 24 h and
then treated with CuB (5 µM) in a 37˚C cell incubator for 48 h. The
original culture medium was aspirated and DCFH-DA working solution
(MedChemExpress) was added at a final concentration of 10 µM in a
37˚C incubator was 30 min, and the cells were observed and imaged
under a fluorescence microscope. The average fluorescence intensity
of each image was determined using ImageJ 1.53t software.
Flow cytometric apoptosis assay
HT-29 and SW620 cells were seeded into 6-well cell
culture plates (5x105 cells/well), incubated for 24 h,
and then treated with CuB (5 µM) alone or in combination with
4-phenylbutyric acid (4-PBA; 800 µM; MedChemExpress) or
N-acetylcysteine (NAC; 5 mM; MedChemExpress) in a 37˚C incubator
for 48 h. The cells were collected and resuspended with PBS. Cells
were stained using Annexin V-PE and 7-AAD (for nucleic acid
staining) from a PE Annexin V Apoptosis Detection Kit (BD
Pharmagen), both 5 µl. After gentle shaking, the solutions were
incubated at room temperature (25˚C) in dark for 15 mins. The cells
were subjected to flow cytometry (CytoFLEX S; Beckman Coulter) and
then the data were analysed (FlowJo; version 9.0.4; BD Pharmagen)
with total apoptosis calculated as follow: Total apoptosis
rate=early apoptosis rate + late apoptosis rate.
Establishment of stable cells
CHOP was knocked down in SW620 cells using a short
hairpin RNA (shRNA). For the experiment, OBiO Technology (Shanghai)
Co., Ltd. produced the lentivirus plasmid pCLenti-U6-shRNA
(CHOP)-CMV Uro WPRE. 293T (American Type Culture Collection) cells
that were transfected grew well. The transfected virus vector
plasmid was 32 µg (skeleton plasmid: shuttle plasmid, 1:1) in the
case of a 100 mm disk, packing the virus. Cells were cultured in a
cell culture compartment and 48 h after transfection, the virus was
collected and purified. Then, SW620 cells in logarithmic growth
phase were seeded in a 6-well plate (5x104 cells/well)
and cultured in an incubator at 37˚C. When the cell confluence
reached ~30%, viral particles (multiplicity of infection, 10) were
added for infection for 12 h, and medium containing puromycin
(Beijing Solarbio Science & Technology Co., Ltd.; 2 µg/ml) was
added to screen for positive cells 12 h later. The screened
positive cells were subcultured, and the purinomycin concentration
remained constant at 2 µg/ml. Following each successful infection,
SW620 cells from the first to third generations were subjected to
subsequent experimentation. The cells with CHOP knockdown were
denoted as the shRNA-CHOP group and the cells transduced with the
negative control (NC) vector were denoted the shRNA-NC group. The
shRNA targeting sequences were as follows: shRNA-NC,
5'-TTCTCCGAACGTGTCACGT-3'; shRNA-CHOP,
5'-TGAACGGCTCAAGCAGGAAAT-3'.
Protein isolation and western
blotting
CRC cell lines (HT-29 and SW620) were detached,
suspended and seeded into a 100-mm Petri dish. After adhered cells
were 70% confluent, CuB working solution (5 µM) combined with the
ROS scavengers NAC (5 mM, 1 h pretreatment) and 4-PBA (800 µM, 2 h
pretreatment) were used to treat the cells. The cells were
incubated in a suitable cell incubator at 37˚C for 48 h and the
cells were collected immediately after incubation. RIPA lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.), which
was precooled in advance and mixed with protease and phosphatase
inhibitors, was used to fully lyse the cells. Subsequently, the
protein concentration in each group was determined using a BCA
assay. All protein samples were separated by SDS-PAGE (30 µg/lane)
on 12.5 (proteins >30 kDa) and 15% (proteins <30 kDa) gels
with a molecular weight marker (cat. no. WJ102; Ipsen
Biopharmaceuticals, Inc.), and the separated proteins were
transferred to PVDF membranes with a constant current
electrophoretic transfer system. PVDF membranes were placed in 1X
rapid blocking solution (New Cell & Molecular Biotech Co.,
Ltd.) for 30 min on a shaker at room temperature. Primary
antibodies against the following proteins were used: GAPDH
(1:10,000; 36 KDa; cat. no. 10494-1-AP; Wuhan Sanying
Biotechnology); Bax (1:5,000; 21 kDa; cat. no. GR3275117-D; Abcam)
and Bcl2 (1:2,000; 26 kDa; cat. no. GR3325129-9; Abcam); glucose
regulated protein 78 (GRP78; 1:1,000; 78kDa; cat. no. 11587-1-AP;
Wuhan Sanying Biotechnology), CHOP (27 kDa; cat. no. 44266;
1:1,000; GeneTex, Inc.), activating transcription factor 4 (ATF4;
42 kDa; cat. no. 39568; 1:1,000; GeneTex, Inc.) and X-box binding
protein 1 splicing form (XBP1-s; 55 kDa; cat. no. 44286; 1:1,000;
GeneTex, Inc.); IRE1 (1:1,000; 130 kDa; cat. no. 3294S; Cell
Signaling Technology, Inc.); phosphorylated (p)-protein kinase
R-like ER kinase (PERK; 1:1,000; 125 kDa; cat. no. 6N04; SAB
Biotherapeutics, Inc.); PERK (1;1,000; 115 kDa; cat. no. 5683S;
Cell Signaling Technology, Inc.); p-eukaryotic translation
initiation factor 2α (eIF2α; 1:1,000; 32 kDa; cat. no. AP0692;
ABclonal Biotech Co., Ltd.) and eIF2α (1:1,000; 38 kDa; cat. no.
42774; GeneTex, Inc.). After incubation overnight at 4˚C, the
membranes were incubated with a fluorescent secondary antibody
(anti-rabbit IgG; 1:10,000; cat. no. 5151P Cell; Signaling
Technology, Inc.) at room temperature for 2 h. The LI-COR Odyssey
Infrared Imaging System (LI-COR Biosciences) was used to scan and
visualize protein bands. Following that, the relative protein
expression was determined by evaluating the band densities with
ImageJ software.
Statistical analysis
SPSS 25.0 statistical software (IBM Corp.) was used
for analysis and GraphPad Prism 5.0 software (Dotmatics) was used
for graph generation. The results of at least three independent
experiments were used for statistical analysis in all experiments,
and the experimental data are expressed as the mean ± SD. The data
were analysed using independent samples t-test, or one-way ANOVA
and Tukey post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
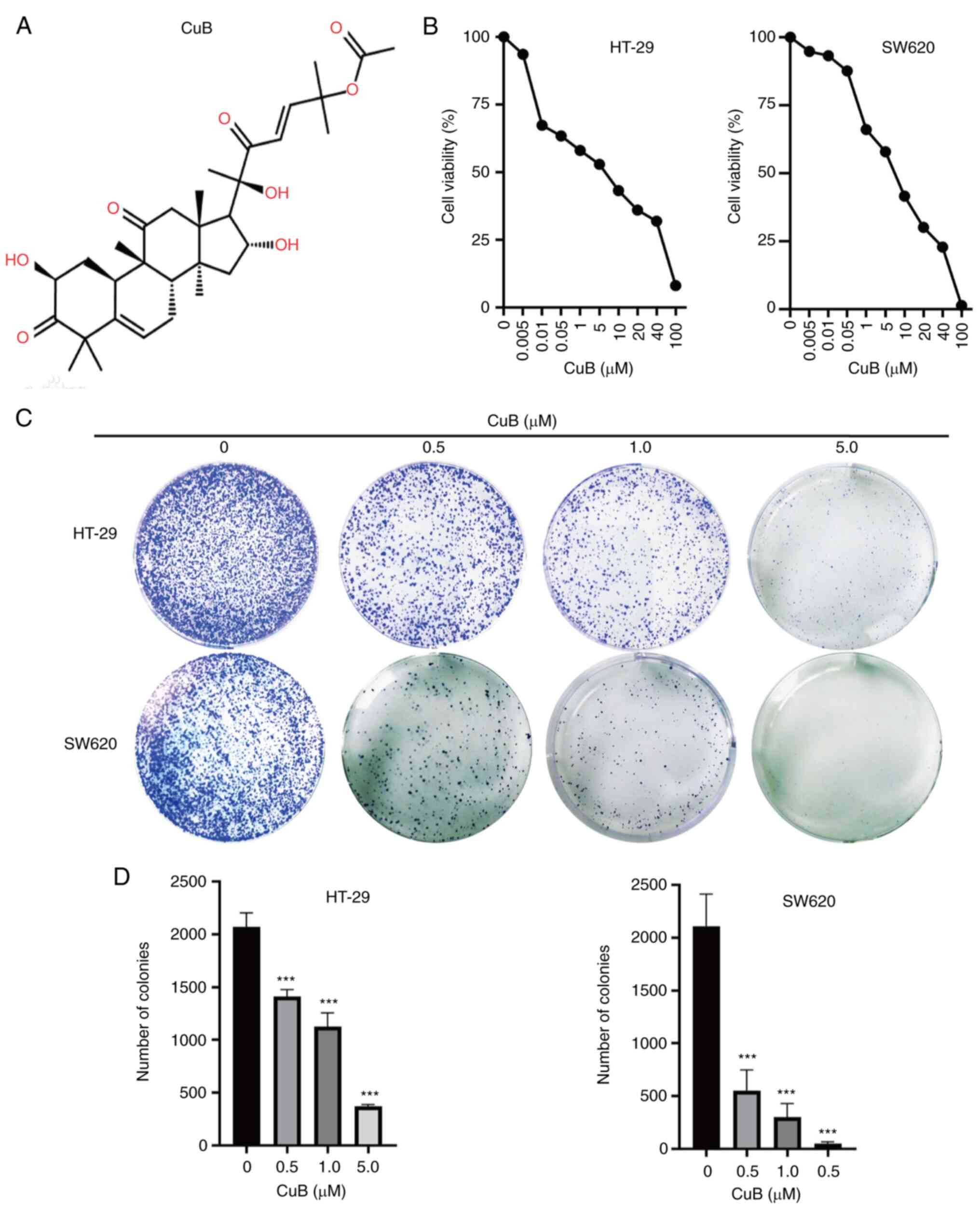
CuB inhibits CRC cell viability and
proliferation
The present study initially assessed the viability
of human CRC cell lines (HT-29 and SW620) using a CCK8 assay. A
series of concentrations of CuB (Fig.
1A) were prepared, which were then used to treat cells for 48
h. In the first experiment, the appropriate concentration of CuB
was selected for subsequent experiments. The results of the CCK8
assay showed that 0.005 µM CuB inhibited the proliferation of CRC
cells, and the inhibitory effect became more obvious with
increasing concentrations (Fig.
1B). At 5 µM, the inhibition rate of CuB-treated HT-29 and
SW620 cells was close to 50%. These results indicated that CuB may
have a marked inhibitory effect on CRC cell viability. In addition,
to study the effect of CuB on the proliferation of CRC cells, a
cell plate colony formation assay was performed, and the results
confirmed that the colony formation ability of cancer cells was
significantly inhibited by treatment with 0.5-5 µM CuB compared
with that in the control group (P<0.05; Fig. 1C and D). These results indicated that CuB may
have strong antitumour activity against CRC cells and 5 µM was
selected as the concentration for the follow-up experiments.
CuB induces ERS in CRC cells
Western blot analysis was performed to determine
whether CuB (5 µM) could activate ERS. The present study detected
the ER stress markers GRP78 and CHOP, and their corresponding
activated UPR signalling pathways were assessed by measuring the
levels of proteins related to the PERK/eIF2α/ATF4 and IRE1/XBP1
signal transduction pathways. Cells exposed to CuB for 48 h
exhibited an upregulation of ER stress markers, and increased
expression levels of the UPR-related proteins p-PERK, p-eIF2α,
ATF4, IRE1α and XBP1, compared with those in the control group
(P<0.05; Fig. 2).
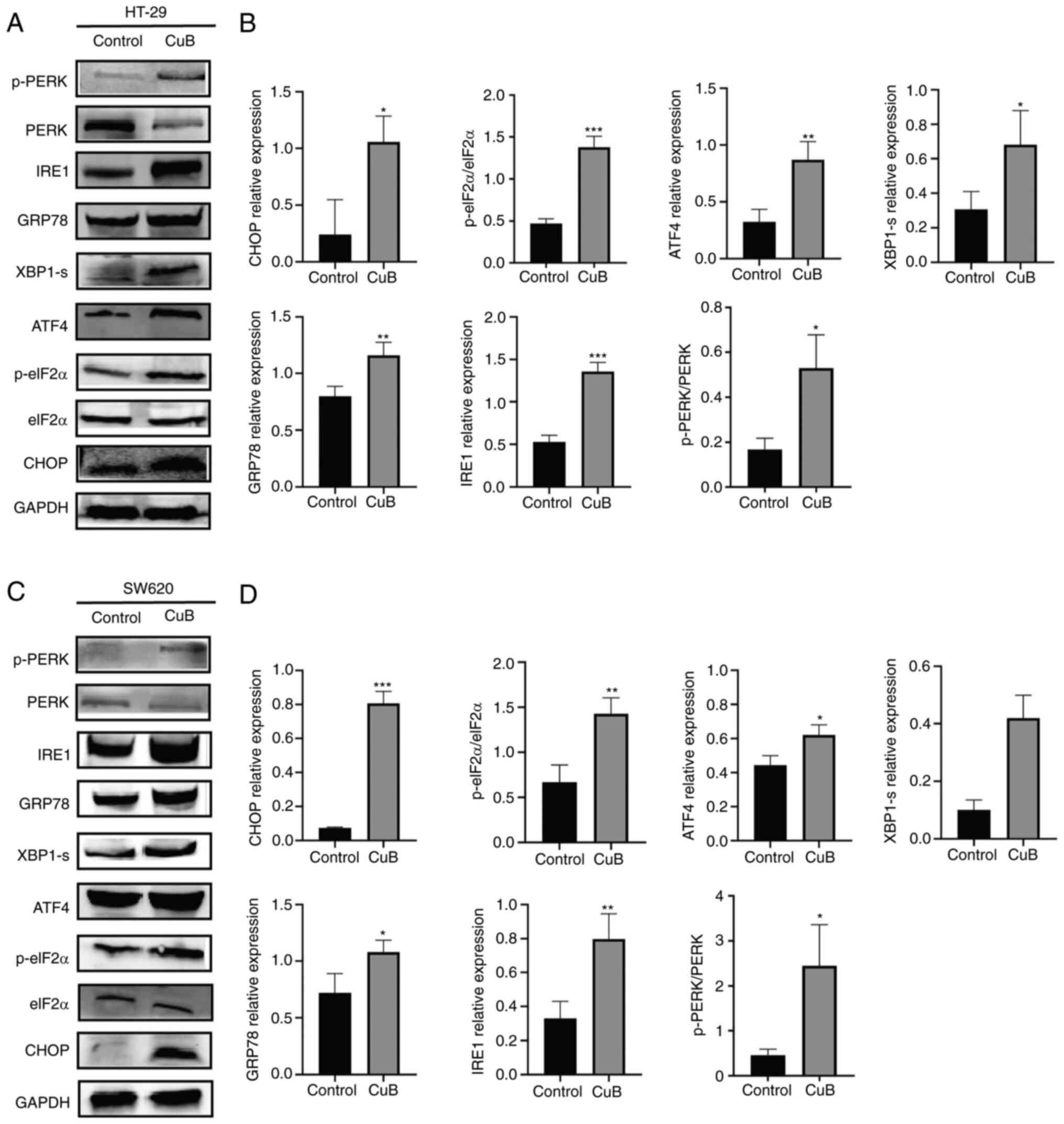 | Figure 2CuB induces ER stress in CRC cells.
After CRC cells were treated with CuB (5 µM) for 48 h, the
expression levels of ER stress-related proteins was evaluated by
western blotting. (A and C) The expression of ERS-related proteins
in different groups of HT-29 and SW620 cells. (B and D)
Quantification of expression levels of ERS-related proteins. Data
are presented as the mean ± SD of three independent experiments.
*P<0.05, **P<0.01,
***P<0.001 vs. control. ATF4, activating
transcription factor 4; CRC, colorectal cancer; CuB, cucurbitacin
B; eIF2α, eukaryotic translation initiation factor 2α; GRP78,
glucose regulated protein 78; p-, phosphorylated; PERK, protein
kinase R-like ER kinase; XBP1-s, X-box binding protein 1. |
CuB mediates ROS production in CRC
cells and induces ERS
To determine whether CuB can promote the production
of ROS in CRC cells, the DCFH-DA fluorescent probe was used to
detect the production of ROS in CRC cells after continuous
treatment with CuB. Compared with those in the untreated group,
following exposure to CuB-mediated toxicity, the amounts of ROS
produced in the two CRC cell lines were significantly increased
with increasing CuB concentration (P<0.05; Fig. 3A and B). These results confirmed that CuB can
promote the production of ROS in CRC cell lines. Furthermore, the
ROS inhibitor NAC (5 mM) was used to examine the relationship
between CuB-induced ROS production and ERS. The results showed that
NAC pretreatment significantly inhibited the CuB-induced increases
in the protein expression levels of GRP78, CHOP, p-PERK, p-eIF2α,
ATF4, XBP1 and IRE1 (P<0.05; Fig.
3C-F). These findings suggested that ROS produced in CRC cells
mediated by CuB treatment may induce ERS.
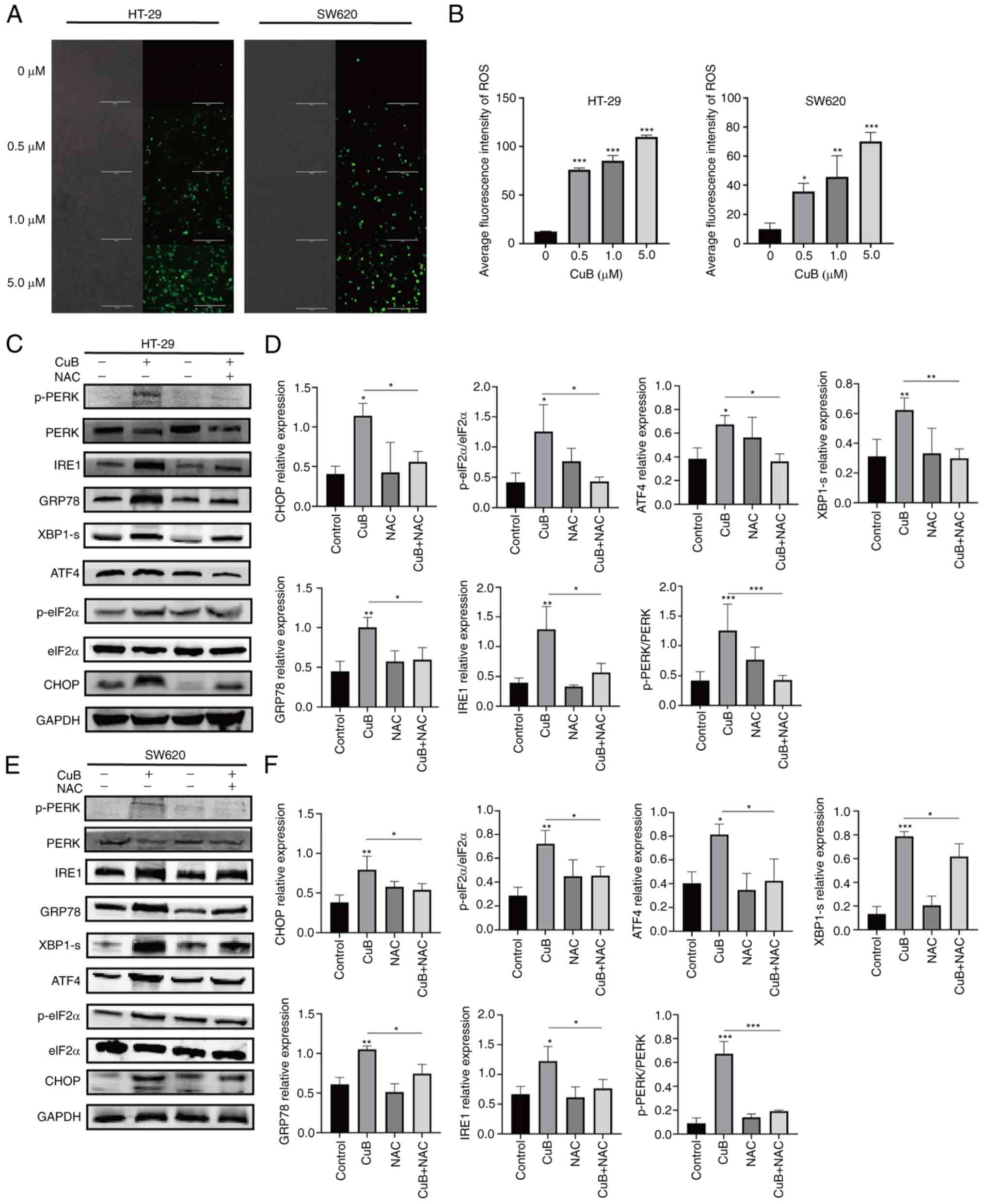 | Figure 3CuB mediates ROS production in CRC
cells and induces ERS. (A and B) After CRC cells were treated with
CuB (0-5 µM) for 48 h, the dichlorodihydrofluorescein diacetate
fluorescent probe was used to evaluate the amount of ROS produced.
Scale bars, 200 µm. CRC cells were pretreated with NAC (5 mM) for 1
h before adding CuB (final concentration of 5 µM). After 48 h of
treatment, the expression of ERS-related proteins was evaluated by
western blotting. (C and E) The expression of ERS-related proteins
in different groups of HT-29 and SW620 cells. (D and F)
Quantification of expression levels of ERS-related proteins. Data
are presented as the mean ± SD of three independent experiments.
*P<0.05, **P<0.01,
***P<0.001 vs. control or as indicated. CRC,
colorectal cancer; CuB, cucurbitacin B; eIF2α, eukaryotic
translation initiation factor 2α; ERS, endoplasmic reticulum
stress; GRP78, glucose regulatory protein 78; NAC,
N-acetylcysteine; p-, phosphorylated; PERK, protein kinase R-like
ER kinase; ROS, reactive oxygen species; XBP1-s, X-box binding
protein 1. |
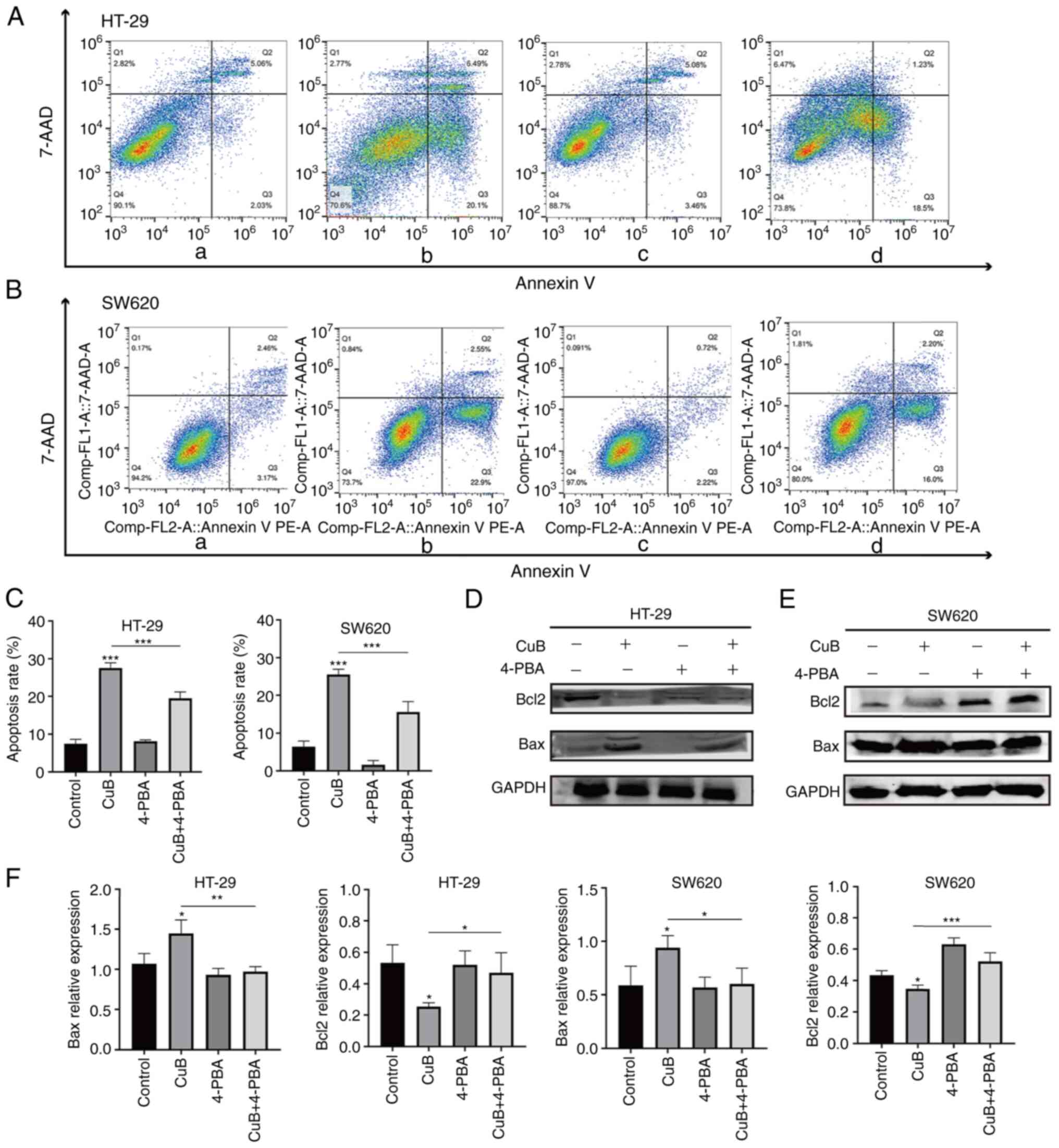
CuB can induce apoptosis in CRC
cells
Following treatment with CuB (5 µM), apoptosis in
HT-29 and SW620 cells was significantly increased compared with
that in the control groups (P<0.05; Fig. 4A-C). By contrast, when HT-29 and
SW620 cells were pretreated with the ERS inhibitor 4-PBA (800 µM)
for 2 h, CuB-induced apoptosis was significantly inhibited
(P<0.05) compared with that in the groups treated with CuB
alone, as determined by flow cytometry (Fig. 4A-C). In addition, CuB induced
significant upregulation of the proapoptotic protein Bax and
significant downregulation of the antiapoptotic protein Bcl2
compared with that in the control group. Furthermore, when compared
to the CuB alone group, the protein expression levels of Bax in the
CuB combined 4-PBA group were reduced, while those of Bcl2 were
increased (P<0.05; Fig.
4D-F).
CuB induces apoptosis via ERS and its
signal transduction pathways
To further explore the effect of CuB-induced
activation of ERS on apoptosis, a model of CHOP knockdown was
established in SW620 cells. By comparing the CHOP knockdown group
with the NC group, the successful establishment of cells with CHOP
knockdown was confirmed (P<0.05; Fig. 5A and B). When CuB (5 µM) working solution was
added to the shRNA CHOP group, it was revealed that CuB treatment
could still promote the expression of CHOP; however, the expression
of CHOP was significantly decreased compared with that in the group
treated with CuB alone (P<0.05; Fig. 5A and B). Subsequently, the effects of CHOP
knockdown were assessed on the inhibition of cell viability and
apoptosis. The results revealed that when CuB was activated, the
CHOP knockdown group increased cell viability relative to the NC
group; by contrast, the apoptosis rate in the shRNA CHOP group
exposed to CuB was significantly lower than that in the group
treated with CuB alone (P<0.05; Fig. 5C-E). The aforementioned data
suggested that CuB may induce apoptosis in CRC cells by mediating
ROS-induced activation of the PERK and IRE1 ERS signalling
pathways.
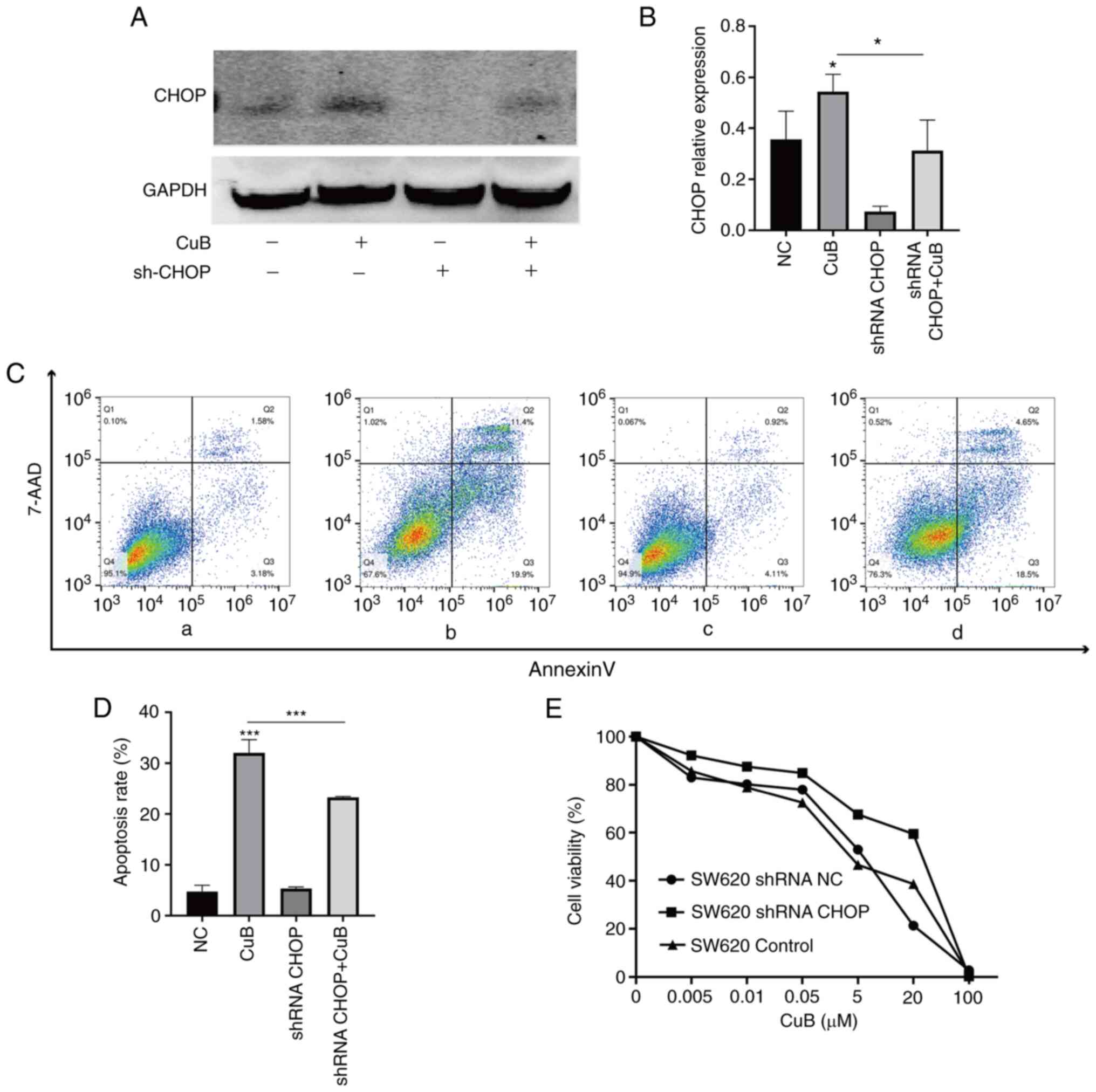 | Figure 5CuB induces apoptosis via endoplasmic
reticulum stress and its signal transduction pathways.
Lentivirus-mediated knockdown of CHOP was performed in SW620 cells,
and the expression of CHOP was measured by western blotting after
cells were treated with CuB (5 µM) for 48 h. (A) The expression of
CHOP protein. (B) Quantification of CHOP protein expression. (C)
After cells were treated with CuB (5 µM) for 48 h, the apoptosis
rates in the (Ca) NC group, (Cb) CuB-treated group, (Cc) shRNA CHOP
group and (Cd) CuB-treated shRNA CHOP group were determined by flow
cytometry. (D) Quantification of apoptosis rates. (E) SW620 cells
were treated with CuB (0-100 µM) for 48 h, and the cell viability
inhibition rates in the shRNA CHOP group, shRNA NC group and
control group were determined using a Cell Counting Kit 8 assay.
Data are presented as the mean ± SD of three independent
experiments. *P<0.05, ***P<0.001 vs.
control or as indicated. CuB, cucurbitacin B; NC, negative control;
shRNA, short hairpin RNA. |
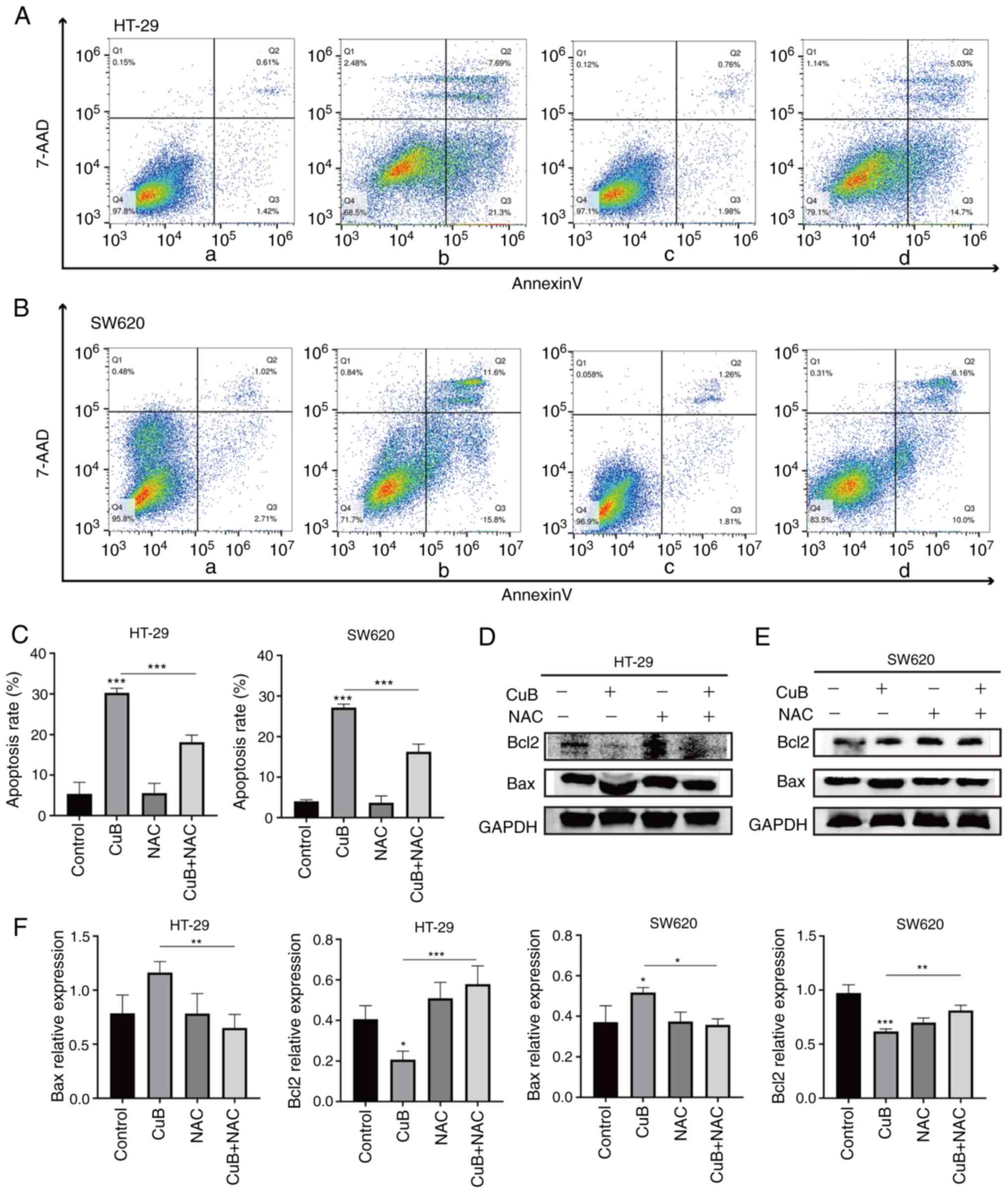
CuB mediates ROS production and
induces apoptosis in CRC cells
It has been shown that ROS overload can induce
apoptosis (25). Therefore, the
present study hypothesized that the production of ROS mediated by
CuB (5 µM) may also promote apoptosis. Notably, pretreatment with
the ROS inhibitor NAC (5 mM) for 1 h significantly inhibited
apoptosis in cancer cells compared with that in cells treated with
CuB alone (P<0.05; Fig. 6A-C).
Moreover, the upregulation of Bax and the downregulation of Bcl2
induced by CuB were reversed by NAC pretreatment (P<0.05;
Fig. 6D-F). These findings
indicated that CuB may also induce apoptosis in cancer cells
through ROS.
Discussion
To date, a number of natural compounds have been
shown to have significant anticancer activity and act on a variety
of targets. Polydatin, for example, inhibits liver cancer cell
migration and invasion by targeting c-MYC and Shikonin stimulates
the MAPK pathway and promotes death in melanoma cells (26,27).
As aforementioned, CuB exhibits significant anticancer activity in
various types of cancer; however, the underlying mechanisms of its
significant anticancer effects have not been fully elucidated. In
the present study on CuB in HT-29 and SW620 CRC cells, it was
revealed that CuB was able to inhibit the proliferation of these
cells in a concentration-dependent manner and could increase their
apoptosis. The main mechanism of action of these effects was
related to the CuB-mediated increase in ROS production and ERS
pathway activation.
Apoptosis is a stable and conservatively controlled
process of autonomous and orderly cell death, which serves an
important role in maintaining the stability of the internal
environment of an organism (28).
From the perspective of tumour therapy, evasion of apoptotic
signalling confers a survival advantage on tumour cells, creating
conditions suitable for angiogenesis, invasive metastasis and even
drug resistance (29). Previously,
it has been reported that CuB may have a role in proliferation
inhibition and apoptosis in Burkitt lymphoma Ramos cells by
inhibiting the phosphorylation of STAT3, thereby downregulating
Bcl-2 and upregulating Bax (30).
The present study also revealed that CuB was effective in
increasing the apoptosis rates of HT-29 and SW620 cells, increasing
the expression levels of Bax and decreasing those of Bcl-2 in CRC
cells.
GRP78 is a molecular chaperone in the lumen of the
ER, which has an important role in the correct folding of proteins
and the maintenance of luminal homeostasis. Elevated GRP78
expression indicates the presence of ERS (31). It has been demonstrated that the
IRE1/XBP1 and PERK/eIF2α/ATF4/CHOP signalling pathways, in the
presence of long-term sustained ERS activation, induce apoptosis
(32,33). In the present study, treatment of
HT-29 and SW620 cells with CuB resulted in increased GRP78
expression, and activation of the IRE1/XBP1 and
PERK/eIF2α/ATF4/CHOP signalling pathways in the UPR, a result that
demonstrates the ability of CuB to induce ERS in CRC cells.
It has been reported that ROS at low and appropriate
concentrations regulate and maintain normal physiological
functions, whereas intracellular ROS overload has destructive
effects (34). In cancer research,
Lai et al (35) reported
that crassolide, a diterpenoid derived from soft coral, could
trigger ERS to induce apoptosis and autophagy through the
production of ROS in lung cancer cells. The present experimental
results demonstrated that ROS production in HT-29 and SW620 cells
was gradually increased with increasing CuB concentration. This is
consistent with the findings of increased ROS production induced by
CuB in a related study in hepatocellular carcinoma (36). Furthermore, the present study
revealed that after treatment with the ROS inhibitor NAC, the
expression of ERS-related proteins was suppressed, suggesting that
CuB may mediate the regulation of both IRE1 and PERK ERS via ROS
production in HT-29 and SW620 cells. In addition, when HT-29 and
SW620 cells were pretreated with the ERS inhibitor 4-PBA,
CuB-induced apoptosis, Bcl2 downregulation and Bax upregulation
were reversed, emphasizing that CuB-induced apoptosis in CRC cells
may be associated with ERS.
CHOP is a marker of ERS-induced apoptosis and
ultimately induces apoptosis through signalling cascades (37-39).
Therefore, the present study also assessed the effects of blocking
the main target of ERS-induced apoptosis by downregulating CHOP
expression in SW620 cells. The results revealed that knockdown of
CHOP reduced CuB-induced apoptosis in SW620 cells compared with
that in control cells. Thus, these results suggested that CuB
induces apoptosis in CRC cells through ROS-mediated activation of
the IRE1 and PERK ERS signalling pathways. When the viability of
cells was examined using a CCK8 assay, knockdown of CHOP was found
to increase the viability of SW620 cells, further demonstrating
that CuB inhibits the viability of CRC through ROS-mediated
activation of ERS.
According to a previous study, ROS can cause
apoptosis (40). The present
results showed that the CuB-induced apoptosis of CRC cells was
decreased after pretreatment with NAC, and that CuB-induced Bcl2
downregulation and Bax upregulation were also reversed, indicating
that CuB can induce apoptosis through ROS. These findings
demonstrated that CuB can induce apoptosis in CRC cells through ROS
and ERS; furthermore, CuB may induce apoptosis and inhibit cancer
cell proliferation through ROS-mediated activation of ERS.
Although the present results confirmed the mechanism
underlying the effects of CuB on the apoptosis of HT-29 and SW620
CRC cells, the present study still has limitations. First, the
present study successfully verified the role of ERS in the
CuB-induced apoptosis of CRC cells in the SW620 cell line, in which
CHOP was knocked down, but did not validate the results in the
HT-29 cell line. Second, to the best of our knowledge, just one
in vivo investigation of CRC has validated the antitumour
effectiveness of CuB (11). As a
result, whether CuB has a similar curative effect in animal models,
and whether it is associated with ERS and ROS should be
investigated in further animal studies.
In conclusion, the results of the present study
demonstrated that ROS and ERS have important roles in CuB-induced
apoptosis in CRC cells, and specifically demonstrated that CuB can
induce apoptosis in CRC cells through ROS-mediated activation of
ERS. These findings may provide new ideas for the clinical
development of novel drugs for the treatment of CRC.
Acknowledgements
Not applicable.
Funding
Funding: This research was financially supported by the Guangxi
Medical and Health Key Discipline Construction Project (grant no.
2022049) and the Special Fund for Scientific Research of the Second
Affiliated Hospital of Guangxi Medical University (grant no.
EFYKY2021013).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH was mainly responsible for experiments, data
analysis and draft writing. LL visualized and analysed the data. PX
performed experiments and data visualization. WS was responsible
for data collation and analysis. LW was responsible for analysing
the data. ZC was responsible for project conceptualization and
design, management, validation, and review and editing of the
manuscript. JH and ZC confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang Y, Yuan H, Li Z, Ji X, Shen Q, Tuo
J, Bi J, Li H and Xiang Y: Global pattern and trends of colorectal
cancer survival: A systematic review of population-based
registration data. Cancer Biol Med. 19:175–186. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Araghi M, Soerjomataram I, Jenkins M,
Brierley J, Morris E, Bray F and Arnold M: Global trends in
colorectal cancer mortality: Projections to the year 2035. Int J
Cancer. 144:2992–3000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Han N, Li J and Li X: Natural marine
products: Anti-Colorectal cancer in vitro and in vivo. Mar Drugs.
20(349)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin
R, He WL, Cai SR, He YL and Ye JN: Anti-EGFR therapy in metastatic
colorectal cancer: Mechanisms and potential regimens of drug
resistance. Gastroenterol Rep (Oxf). 8:179–191. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10(1021)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chai Y, Xiang K, Wu Y, Zhang T, Liu Y, Liu
X, Zhen W and Si Y: Cucurbitacin B Inhibits the Hippo-YAP signaling
pathway and exerts anticancer activity in colorectal cancer
cells-pubmed. Med Sci Monit. 19:9251–9258. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sinha S, Khan S, Shukla S, Lakra AD, Kumar
S, Das D, Maurya R and Meeran SM: Cucurbitacin B inhibits breast
cancer metastasis and angiogenesis through VEGF-mediated
suppression of FAK/MMP-9 signaling axis. Int J Biochem Cell Biol.
77(Pt A):41–56. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu JH, Li C, Cao L, Zhang CH and Zhang
ZH: Cucurbitacin B regulates lung cancer cell proliferation and
apoptosis via inhibiting the IL-6/STAT3 pathway through the lncRNA
XIST/miR-let-7c axis. Pharm Biol. 60:154–162. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu T, Zhang M, Zhang H, Sun C and Deng Y:
Inhibitory effects of cucurbitacin B on laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 265:1225–1232.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wakimoto N, Yin D, O'Kelly J, Haritunians
T, Karlan B, Said J, Xing H and Koeffler HP: Cucurbitacin B has a
potent antiproliferative effect on breast cancer cells in vitro and
in vivo. Cancer Sci. 99:1793–1797. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dandawate P, Subramaniam D, Panovich P,
Standing D, Krishnamachary B, Kaushik G, Thomas SM, Dhar A, Weir
SJ, Jensen RA and Anant S: Cucurbitacin B and I inhibits colon
cancer growth by targeting the Notch signaling pathway. Sci Rep.
10(1290)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Promkan M, Dakeng S, Suebsakwong P,
Suksamrarn A and Patmasiriwat P: Alterations of cellular
proliferation, apoptosis and autophagy by cucurbitacin B treatment
in colon cancer cells. Ann Oncol. 26 (Suppl 9):S151–S152. 2015.
|
|
13
|
Zhang H, Zhao B, Wei H, Zeng H, Sheng D
and Zhang Y: Cucurbitacin B controls M2 macrophage polarization to
suppresses metastasis via targeting JAK-2/STAT3 signalling pathway
in colorectal cancer. J Ethnopharmacol. 287(114915)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Voeltz GK, Rolls MM and Rapoport TA:
Structural organization of the endoplasmic reticulum. EMBO Rep.
3:944–950. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schwarz DS and Blower MD: The endoplasmic
reticulum: Structure, function and response to cellular signaling.
Cell Mol Life Sci. 73:79–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim HS, Kim TJ and Yoo YM: Melatonin
combined with endoplasmic reticulum stress induces cell death via
the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. PLoS One.
9(e92627)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cross CE, Halliwell B, Borish ET, Pryor
WA, Ames BN, Saul RL, McCord JM and Harman D: Oxygen radicals and
human disease. Ann Intern Med. 107:526–545. 1987.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rhee SG, Woo HA, Kil IS and Bae SH:
Peroxiredoxin functions as a peroxidase and a regulator and sensor
of local peroxides. J Biol Chem. 287:4403–4410. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brandes RP, Weissmann N and Schröder K:
Nox family NADPH oxidases: Molecular mechanisms of activation. Free
Radic Biol Med. 76:208–226. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Y, Lu L, Zhang G, Ji G and Xu H: The
role and therapeutic implication of endoplasmic reticulum stress in
inflammatory cancer transformation. Am J Cancer Res. 12:2277–2292.
2022.PubMed/NCBI
|
|
21
|
Zhou Z, Wang Q and Michalak M: Inositol
Requiring Enzyme (IRE), a multiplayer in sensing endoplasmic
reticulum stress. Anim Cells Syst (Seoul). 25:347–357.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Daverkausen-Fischer L and Pröls F: The
function of the co-chaperone ERdj4 in diverse (patho-)physiological
conditions. Cell Mol Life Sci. 79(9)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu SN, Kim SH, Kim KY, Ji JH, Seo YK, Yu
HS and Ahn SC: Salinomycin induces endoplasmic reticulum
stress-mediated autophagy and apoptosis through generation of
reactive oxygen species in human glioma U87MG cells. Oncol Rep.
37:3321–3328. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chok KC, Koh RY, Ng MG, Ng PY and Chye SM:
Melatonin induces autophagy via reactive oxygen species-mediated
endoplasmic reticulum stress pathway in colorectal cancer cells.
Molecules. 26(5038)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Perrone GG, Tan SX and Dawes IW: Reactive
oxygen species and yeast apoptosis. Biochim Biophys Acta.
1783:1354–1368. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bai L, Ma Y, Wang X, Feng Q, Zhang Z, Wang
S, Zhang H, Lu X, Xu Y, Zhao E and Cui H: Polydatin inhibits cell
viability, migration, and invasion through suppressing the c-Myc
expression in human cervical cancer. Front Cell Dev Biol.
9(587218)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee JH, Han SH, Kim YM, Kim SH, Yoo ES,
Woo JS, Jung GH, Jung SH, Kim BS and Jung JY: Shikonin inhibits
proliferation of melanoma cells by MAPK pathway-mediated induction
of apoptosis. Biosci Rep. 41(BSR20203834)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Karagöz GE, Acosta-Alvear D, Nguyen HT,
Lee CP, Chu F and Walter P: An unfolded protein-induced
conformational switch activates mammalian IRE1. Elife.
6(e30700)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Neophytou CM, Trougakos IP, Erin N and
Papageorgis P: Apoptosis deregulation and the development of cancer
multi-drug resistance. Cancers (Basel). 13(4363)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Klungsaeng S, Kukongviriyapan V, Prawan A,
Kongpetch S and Senggunprai L: Cucurbitacin B induces
mitochondrial-mediated apoptosis pathway in cholangiocarcinoma
cells via suppressing focal adhesion kinase signaling. Naunyn
Schmiedebergs Arch Pharmacol. 392:271–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xia S, Duan W, Liu W, Zhang X and Wang Q:
GRP78 in lung cancer. J Transl Med. 19(118)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li J, Zhuo JY, Zhou W, Hong JW, Chen RG,
Xie HY, Zhou L, Zheng SS and Jiang DH: Endoplasmic reticulum stress
triggers delanzomib-induced apoptosis in HCC cells through the
PERK/eIF2α/ATF4/CHOP pathway. Am J Transl Res. 12:2875–2889.
2020.PubMed/NCBI
|
|
33
|
Zhou T, Lv X, Guo X, Ruan B, Liu D, Ding
R, Gao Y, Ding J, Dou KF and Chen Y: RACK1 modulates apoptosis
induced by sorafenib in HCC cells by interfering with the IRE1/XBP1
axis. Oncol Rep. 33:3006–3014. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lai KM, Wang JH, Lin SC, Wen Y, Wu CL, Su
JH, Chen CC and Lin CC: Crassolide Induces G2/M cell cycle arrest,
apoptosis, and autophagy in human lung cancer cells via
ROS-Mediated ER stress pathways. Int J Mol Sci.
23(5624)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Niu Y, Sun W, Lu JJ, Ma DL, Leung CH, Pei
L and Chen X: PTEN activation by DNA damage induces protective
autophagy in response to cucurbitacin B in hepatocellular carcinoma
cells. Oxid Med Cell Longev. 2016(4313204)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 46:629–640. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pytel D, Majsterek I and Diehl JA: Tumor
progression and the different faces of the PERK kinase. Oncogene.
35:1207–1215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Moradzadeh M, Sadeghnia HR, Mousavi SH,
Mahmoodi M and Hosseini A: Ferula gummosa gum induces apoptosis via
ROS mechanism in human leukemic cells. Cell Mol Biol
(Noisy-le-grand). 63:17–22. 2017.PubMed/NCBI View Article : Google Scholar
|