Introduction
Helicobacter pylori (H. pylori) is a
spiral-shaped Gram-negative bacterium that was first isolated from
the gastric mucosal biopsy of patients with chronic gastritis by
Australian scholars in 1982(1).
Since then, numerous studies have shown that H. pylori is an
important pathogen associated with the etiology of human chronic
gastritis, gastric ulcers and mucosa-associated lymphoid tissue
lymphoma (2-4).
More recently, it has been designated as a Group 1 carcinogen by
the International Agency for Research on Cancer (5). In addition to gastrointestinal
diseases, the latest data indicate that this microorganism may be
related to certain oral diseases, including halitosis (6), caries (7), recurrent oral ulcers (8), chronic gingivitis (9) and periodontitis (10).
Periodontitis is one of the most common diseases of
the human oral cavity. A total of >50% of the global population
suffers from periodontitis (11).
Dental plaque biofilm is the initiating factor of periodontitis. A
wide variety of bacteria are attached to dental plaque. The
existence and interactions of these bacteria cause the occurrence
and development of periodontitis. In 1989, Krajden et al
(12) isolated and cultured H.
pylori from dental plaque. In 1993, Ferguson et al
(13) obtained viable H.
pylori from saliva. The relationship between H. pylori
infection and periodontitis gradually garnered increased attention.
Studies have since indicated that H. pylori infection is
related to periodontitis, and the detection rate of H.
pylori in patients with periodontitis is higher than that in
periodontally healthy individuals (14,15).
However, by contrast, Salehi et al (16) used PCR to detect H. pylori
in the gingival crevicular fluid of periodontally healthy
individuals and patients with periodontitis, and the results showed
that periodontitis was not associated with H. pylori
infection. Al-Ahmad et al (17) also failed to detect the presence of
H. pylori in the saliva, supragingival plaque, subgingival
plaque and tongue mucosa swabs of 15 patients with periodontitis.
These studies suggested that H. pylori infection was not
associated with periodontitis. Considering the inconsistency of the
previous results, the primary aim of the present study was to
explore the relationship between the presence of H. pylori
and the periodontal condition.
Whether H. pylori can cause disease depends
on the virulence factors of different H. pylori strains, and
the strength of virulence in association with the expression of
virulence genes has been previously established (10). The virulence genes of H.
pylori primarily include vacuolating cytotoxin gene A
(vacA) and cytotoxin-associated gene A (cagA)
(18). VacA encoded by the
vacA gene enters the cell by binding to the receptor on the
target cell, causing damage to the lysosome and endoplasmic
reticulum, resulting in vacuolar degeneration. There is a mosaic
structure of alleles in the vacA gene sequence, which
includes two important variant regions: The signal peptide region
(s region) and the middle region (m region). According to the
combination of different s and m regions, the virulence genes of
vacA can be divided into 4 subtypes: s1m1, s1m2, s2m1 and
s2m2. The differences in the combinations of these signal sequence
regions affects the vacuolar cytotoxic activity of H.
pylori, which is closely related to its pathogenicity. A
previous study showed that the virulence of H. pylori is
s1m1 > s1m2 > s2m1 > s2m2 from strong to weak (19). CagA encoded by the
cagA gene is highly immunogenic; it enters the host cell
through the type IV secretion system and undergoes phosphorylation,
which interferes with the EMT-associated signaling pathway,
miRNA-584, miRNA-1290 and other gene expression levels to causes
inflammation in the host cell; there are no subtypes of cagA
(20).
To date, studies have shown that the expression of
vacA and cagA genotypes is associated with the
severity of gastrointestinal diseases (21,22).
However, the relationship between genotype of H. pylori and
periodontal status remains unclear. Therefore, the second aim of
the present study was to investigate the association between
periodontal conditions and H. pylori genotypes.
Materials and methods
Study population
The present study consisted of a cohort of
individuals who visited the Department of Periodontics,
Stomatological Hospital of China Medical University (Shenyang,
China) between April 2020 and May 2021. A total of 53 participants
were selected. The exclusion criteria were as follows: i) <20
natural teeth, ii) symptoms of dyspepsia, iii) systemic disease,
iv) current history of antibiotic usage or use during the previous
2 months, and v) smokers (23).
The demographic information of the participants was recorded,
including sex, age, height and weight. The present study was
approved by the Ethics Committee of the Affiliated Stomatological
Hospital of China Medical University (approval no. 2018-30). Prior
to the examination, the subjects were informed of the purpose of
the study, and written informed consent was obtained.
Measurement of periodontitis
Periodontal examinations were performed by a
periodontist who was not involved in the study, and κ tests were
performed to ensure consistency (κ>0.75). Probing depth (PD) and
clinical attachment loss (CAL) of all the teeth were recorded from
6 points (mesiobuccal, midbuccal, distobuccal and the corresponding
points lingually). The mean values of PD and CAL in every subject
were calculated and recorded. Periodontitis was diagnosed when the
clinical examination met observed ≥2 non-adjacent teeth with CAL;
or ≥2 teeth with buccal or lingual CAL ≥3 mm and simultaneous PD of
≥3 mm (24). Patients with
periodontitis were graded according to the 2017 AAP/EFP
classification (25).
Subgingival plaque collection
The subgingival plaque was collected. Before
periodontal treatment, the subgingival plaque was obtained from the
index teeth (16/11/26/31/36/46) of each participant (26) and frozen at -80˚C for subsequent
use. PD and CAL of the index teeth were recorded from 6 points. For
each sextant only the highest score was recorded (27). The PD and CAL according to the 2017
new classification were divided into healthy group, stage I/II
periodontitis group and stage III/IV periodontitis group to compare
the difference in H. pylori detection rate in subgingival
plaque at the tooth level (25).
DNA extraction and PCR
amplification
DNA was extracted from subgingival samples using the
Magnetic Bead Micro Genomic DNA Extraction Kit [cat. no. B518749;
Sangon Biotech (Shanghai) Co., Ltd.] according to the
manufacturer's protocol.
To detect the presence of H. pylori, PCR was
performed utilizing the primers for the ureC gene (28). All H. pylori-positive
samples in the same individual were mixed for further genotyping at
the individual level using specific PCR to detect the cagA
gene and vacA alleles (s1, s2, m1 and m2), respectively. The
sequences of the primers used in this study are shown in Table I. PCR was conducted using a Takara
Ex Taq DNA polymerase (Takara Bio, Inc.). The thermocycling
conditions were: Initial denaturation at 95˚C for 3 min, followed
by 30 cycles of denaturation at 98˚C for 10 sec, annealing at 58˚C
for 30 min and extension at 72˚C for 1 min, and then a final
extension step at 72˚C for 10 min. The amplified products were
analyzed by electrophoresis in a 1% (w/v) agarose gel (Sheng gong
Biological Company) and stained with ethidium bromide (0.5 µg/ml).
Stained amplicons were visualized on a UV transilluminator at 260
nm, and imaged (Bio-Rad Laboratories, Inc.). H. pylori
standard strain ATCC 43504 (gifted by the laboratory of Guizhou
University of Traditional Chinese Medicine) was used as a positive
control. A PBS solution was used as a negative control. As long as
one or more samples from each subject were positive for
ureC, the subject was marked as positive for H.
pylori.
 | Table ISequences of primers used in the
present study. |
Table I
Sequences of primers used in the
present study.
| Target gene | Sequence | Product size,
bp | (Refs.) |
|---|
| ureC | | 296 | (28) |
|
Forward |
5'-GGATAAGCTTTTAGGGGTGTTAGGGG-3' | | |
|
Reverse |
5'-GCTTACTTTCTAACACTAACGCGC-3' | | |
| cagA | | 400 | (29) |
|
Forward |
5'-AATACACCAACGCCTCCAAG-3' | | |
|
Reverse |
5'-TTGTTGCCGCTTTTGCTCTC-3' | | |
| vacA s1 | | 258 | (30) |
|
Forward |
5'-ATGGAAATACAACAAACACAC-3' | | |
|
Reverse |
5'-CTGCTTGAATGCGCCAAAC-3' | | |
| vacA s2 | | 199 | (31) |
|
Forward |
5'-GCTAACACGCCAAATGATGC-3' | | |
|
Reverse |
5'-CTGCTTGAATGCGCCAAAC-3' | | |
| vacA m1 | | 570 | (30) |
|
Forward |
5'-CAATCTGTCCAATCAAGCGAG-3' | | |
|
Reverse |
5'-GCGTCTAAATAATTCCAAGG-3' | | |
| vacA m2 | | 352 | (32) |
|
Forward |
5'-GGAGCCCCAGGAAACATTG-3' | | |
|
Reverse |
5'-CATAACTAGCGCCTTGCAC-3' | | |
Statistical analysis
SPSS version 21 (IBM Corp.) was used for statistical
analysis. A one-way ANOVA followed by Tukey's post-hoc test to
compare age, BIM, PD and CAL. A χ2 or Fisher's exact
test was used to analyze the categorical data such as the presence
of H. pylori, as well as the frequency of each genotype in
the different groups. All tests were two-tailed tests, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics of
the patients
Among the 53 subjects, there were 20 individuals
with stage I/II periodontitis (6 men and 14 women), with a mean age
of 32.85±7.74 years (16-48), and 18 individuals with stage III/IV
periodontitis (10 men and 8 women), with a mean age 37.50±8.71
years (28~59). The 15 periodontally healthy individuals (5 men and
10 women) had a mean age of 34.53±7.41 years (27~55). There was no
significant difference in terms of sex (χ2=2.932,
P=0.231), age (F=1.533, P=0.226) or body mass index (F=0.426,
P=0.656) among the groups (Table
II).
 | Table IIClinicopathological characteristics
of participants in the present study. |
Table II
Clinicopathological characteristics
of participants in the present study.
| Group | Male/female
patients, n | Age,
yearsa | BMI,
kg/m²,a | PD, mma | CAL,
mma |
|---|
| Periodontally
healthy (n)=15 | 5/10 | 34.530±7.41 | 23.05±2.98 | 2.30±0.72 | 0.47±0.70 |
| Stage I/II
periodontitis (n=20) | 6/14 | 32.85±7.74 | 22.46±1.18 | 3.49±0.36 | 2.50±1.10 |
| Stage III/IV
periodontitis (n=18) | 10/8 | 37.50±8.71 | 22.64±1.23 | 5.60±0.50 | 5.44±1.07 |
|
χ2/F-value | 2.932 | 1.533 | 0.426 | 155.650 | 98.030 |
| P-value | 0.231b | 0.226c | 0.656c |
<0.0001c | 0.0001c |
H. pylori detection rates in
periodontal states
Among the 53 subjects, 21 individuals were positive
for H. pylori infection, accounting for 39.62% of all
subjects. The number of H. pylori-positive individuals in
the periodontally healthy group, stage I/II periodontitis group and
stage III/IV periodontitis group was 2 (13.33%), 8 (40.00%) and 11
(61.11%), respectively (Table
III). The difference between the three groups was significant
(χ2=8.760, P<0.0001). The electrophoresis detection
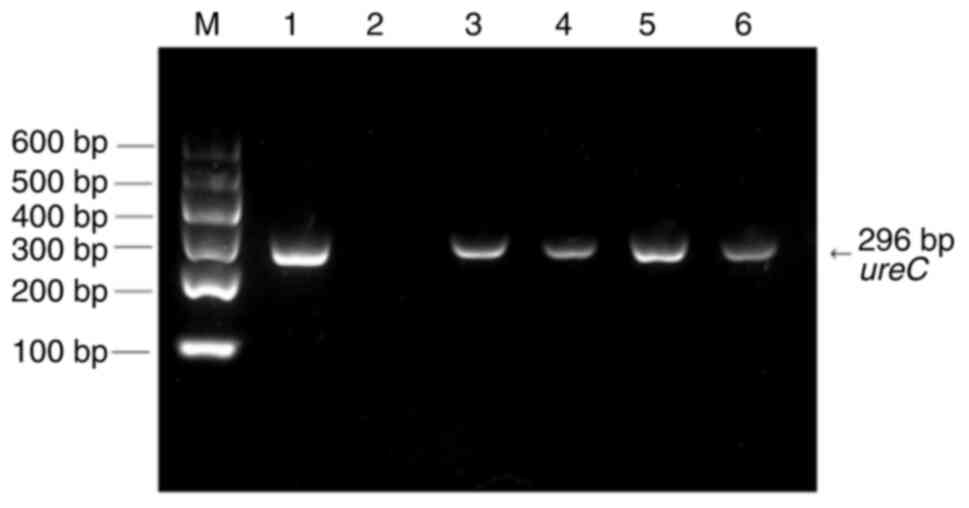
results of the ureC gene are shown in Fig. 1. The results in Table IV show that the detection rate of
H. pylori in subgingival plaque of index teeth increased
with the deepening of PD and CAL. The differences between PD
(χ2=41.909, P<0.0001) and CAL (χ2=41.521,
P<0.0001) among the three groups were significant.
 | Table IIIH. pylori presence in
individuals with different periodontal states. |
Table III
H. pylori presence in
individuals with different periodontal states.
| H. pylori
detection | Periodontally
healthy (n=15) | Stage I/II
periodontitis (n=20) | Stage III/IV
periodontitis (n=18) | Total (n=53) |
χ2-value | P-value |
|---|
| H.
pylori-positive, n | 2 | 8 | 11 | 21 | | |
| H.
pylori-positive, % | 13.33 | 40.00 | 61.11 | 39.62 | 8.76 | <0.0001 |
 | Table IVH. pylori presence in
subgingival plaque with different periodontal conditions. |
Table IV
H. pylori presence in
subgingival plaque with different periodontal conditions.
| Variable | PD ≤3 (n=114) | 3< PD ≤5
(n=120) | PD >5
(n=84) | CAL <1
(n=73) | 1≤ CAL <5
(n=174) | CAL ≥5 (n=71) |
|---|
| H.
pylori-positive, n | 9 | 37 | 41 | 6 | 42 | 39 |
| H.
pylori-positive, % | 7.89 | 30.83 | 48.81a | 8.22 | 24.14 | 54.93a |
| χ2
value | | | 41.91 | | | 41.521 |
| P-value | | | <0.0001 | | | <0.0001 |
Association between the genotype of
H
pylori and periodontal conditions. The genotype of
H. pylori-positive individuals is characterized using PCR by
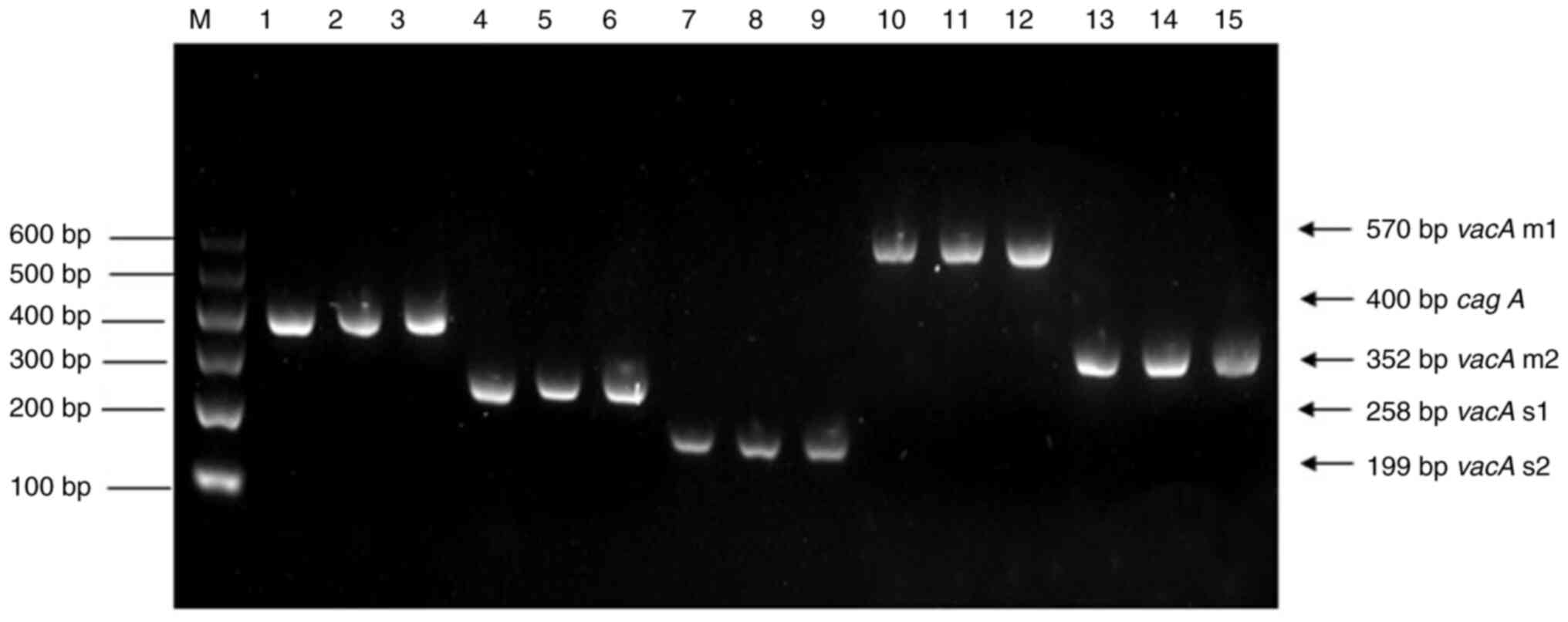
detecting cagA and vacA alleles (Fig. 2). In the periodontally healthy
group, 2 individuals tested positive for H. pylori and both
of these were negative for cagA. The detection rates of
cagA in the stage I/II periodontitis group and the stage
III/IV periodontitis group were 87.50 and 90.91%, respectively
(Table V). The positive rate of
the cagA genotype in the periodontitis group was markedly
higher than that in the healthy group.
 | Table VExpression of each genotype in H.
pylori-positive individuals. |
Table V
Expression of each genotype in H.
pylori-positive individuals.
| H.pylori
genotype | Periodontally
healthy (n=2) | Stage I/II
periodontitis (n=8) | Stage III/IV
Periodontitis (n=11) | Total (n=21) |
|---|
| cagA, n
(%) | | | | |
|
Positive | 0 (0.0) | 7 (87.5) | 10 (90.9) | 17 (81.0) |
|
Negative | 2 (100.0) | 1 (12.5) | 1 (9.09) | 4 (19.0) |
| vacA, n
(%) | | | | |
|
s1a | 0 (0.0) | 4 (50.0) | 7 (63.6) | 11 (52.4) |
|
s2a | 2 (100.0) | 1 (12.5) | 1 (9.1) | 4 (19.0) |
|
s1s2a | 0 (0.0) | 3 (37.5) | 3 (27.3) | 6 (28.6) |
|
m1a | 0 (0.0) | 4 (50.0) | 8 (72.7) | 12 (57.1) |
|
m2a | 2 (100.0) | 3 (37.5) | 3 (27.3) | 8 (38.1) |
|
m
(-)a | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (4.8) |
|
s1m1b | 0 (0.0) | 1 (12.5) | 4 (36.3) | 5 (23.8) |
|
s1m2b | 0 (0.0) | 3 (37.5) | 3 (27.3) | 6 (28.6) |
|
s2m1b | 0 (0.0) | 1 (12.5) | 1 (9.1) | 2 (9.5) |
|
s2m2b | 2 (100.0) | 0 (0.0) | 0 (0.0) | 2 (9.5) |
|
s1s2m1b | 0 (0.0) | 2 (25.0) | 3 (27.3) | 5 (23.8) |
|
s1s2m(-)b | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (4.8) |
|
cagA+/vacA
s1m1c | 0 (0.0) | 1 (12.5) | 4 (36.3) | 5 (23.8) |
|
cagA+/vacA
s1m2c | 0 (0.0) | 3 (37.5) | 3 (27.3) | 6 (28.6) |
|
cagA+/vacA
s2m1c | 0 (0.0) | 1 (12.5) | 1 (9.1) | 2 (9.5) |
|
cag+/vacA
s1s2m1c | 0 (0.0) | 1 (12.5) | 2 (18.2) | 3 (14.3) |
|
cag+/vacA
s1s2m(-)c | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (4.8) |
|
cagA-/vacA
s2m2c | 2 (100.0) | 0 (0.0) | 0 (0.0) | 2 (9.5) |
|
cagA-/vacA
s1s2m1c | 0 (0.0) | 1 (12.5) | 1 (9.1) | 2 (9.5) |
The presence of cagA and vacA
combination genotypes varied with periodontal status. The
cagA-/vacAs2m2 genotype was detected in
100% of H. pylori-positive individuals without
periodontitis. The cagA+/vacAs1m2 (37.5%)
and cagA+/vacAs1m1 (36.3%) were the most
frequently detected allele combinations in the stage I/II
periodontitis and stage III/IV periodontitis groups, respectively
(Table V).
Discussion
Whether H. pylori, colonized in subgingival
plaque, influences the occurrence and development of periodontitis
has been contested for decades. The present study showed that as
the degree of aggravation of periodontal inflammation increased,
the positive rate of H. pylori detection increased
significantly. This suggested that the severity of periodontitis
may be aggravated by the colonization of H. pylori. This is
consistent with the results of studies by Anand et al
(14) and Zheng and Zhou (33). Conversely, successful eradication
of H. pylori can reduce the risk of developing periodontitis
(34). This suggests that in
addition to conventional treatments for periodontitis, a
periodontist should be cognizant of H. pylori infections.
Patients with periodontitis plus H. pylori infection should
be treated with the aim of H. pylori eradication to reduce
the risk of recurrence and aggravation of periodontitis.
Studies have shown that H. pylori in
dyspeptic diseases may enter the mouth through acid reflux and
colonize in the oral cavity (35-37).
This may affect the accuracy of the detection rate of oral H.
pylori. Therefore, participants with symptoms of dyspepsia were
excluded. However, the relationship between subgingival plaque and
gastric H. pylori is still contested. Several studies
reported that there was a close relationship between
gastroesophageal disease and oral status (37,38).
Conversely, other studies found that the occurrence of H.
pylori in the oral cavity was not correlated with an infected
stomach or with the oral dental status of patients (15,17).
Based on the aforementioned contrasting results, it is suggested
that the genotype of H. pylori should be considered when
assessing the differences between subgingival plaque and gastric
H. pylori.
The results of the present study were consistent
with those in the study by Falsafi et al (39), which found that H. pylori
was detectable in the oral cavity of 45% of the population, but
that only a minority of affected individuals demonstrated symptoms
of the disease. It was speculated that this may be attributed to
differences in virulence among different H. pylori strains.
A study by Miehlke et al (40) showed that there was a robust
association between the vacAs1m1 and cagA genotypes
of H. pylori in patients with gastric cancer. It was shown
that the detection of virulence genes may assist in identifying
patients with an increased risk of gastric cancer from the
population. Research by Hu et al (41) found that individuals infected with
the vacAs1m2 strain had a significantly increased risk of
peptic ulcers, while the vacAs1m1 genotype significantly
increased the risk of active gastritis. Based on the aforementioned
findings, we hypothesized that different diseases may be associated
with H. pylori strains with different dominant genes and
different genotypes, and thus different clinical outcomes.
In the H. pylori-positive samples in the
present study, the detection rate of cagA was 80.95%, which
meant that most of the H. pylori strains carried the
cagA gene. This was consistent with the results of the
studies by Link et al (42)
and Falsafi et al (39). In
another study, a biopsy of stomach tissues was performed. The
results showed that the positive rate of H. pylori cagA was
associated with the degree of inflammation of the gastric mucosa
(43). Ferreira et al
(44) found that after infection
of the stomach mucosa with cagA+ H.
pylori, the concentration between IL-8 and neutrophils
increased. Mendoza-Cantú et al (45) assessed 100 samples of supragingival
plaque from patients with chronic gingivitis and showed that the
detection rate of cagA was 16.7 and 80.8% in the healthy and
chronic gingivitis groups, respectively. It was suggested that the
detection rate of the cagA gene was related to gingival
inflammation. The results of the present study indicated that the
detection rate of the cagA gene was related to the extent of
periodontal inflammation. In general, a
cagA-positive strain of H. pylori in the
stomach tissues, gingiva or subgingival plaque is more likely to be
associated with a state of disease.
In the present study, the most frequently detected
genotype was cagA-/vacAs2m2 in the
periodontally healthy group, present in 100% of patients in this
group, whereas the cagA+/vacAs1m2 genotype
was the predominant genotype in stage I/II patients, and the
cagA+/vacAs1m1 was most common in patients
with stage III/IV periodontitis, accounting for 37.5 and 36.3%,
respectively. The aforementioned results illustrated that the
cagA-/vacAs2m2 genotype is the dominant
genotype in the periodontally healthy individuals, while the
cagA+/vacAs1m1 and
cagA+/vacAs1m2 genotypes are dominant in
those with periodontitis. This further confirmed the theory that
different genotypes of H. pylori strains are associated with
different periodontal conditions. Furthermore, a previous study
showed that the majority of H. pylori strains carrying the
cagA+/vacAs1m1 genotype could be isolated
from patients with severe gastric diseases, including duodenal and
gastric ulcers, gastric adenocarcinoma and mucosa-associated
lymphoid tissue lymphoma (46).
Therefore, it is safe to assume that different diseases may be
associated with different dominant genes. Periodontologists should
pay more attention to the cagA+/vacAs1m1
and cagA+/vacAs1m2 genotypes, which were
frequently detected in the periodontitis groups in the present
study. In addition, the results showed that the detection rates of
the cagA+/vacAs1m1 genotype also increased
with the aggravation of periodontal inflammation. However, further
studies are needed to further confirm this speculation to provide a
clinical reference for the treatment of oral H. pylori
infection and periodontitis.
In conclusion, the detection rate and genotypes of
H. pylori in subgingival plaques are associated periodontal
conditions. H. pylori infection with the genotype of
cagA+/vacAs1m1 and
cagA+/vacAs1m2 may be predictive of a
worse periodontal status. However, due to the small sample size in
the present study, a larger scale and high-quality clinical study
is required to confirm the findings.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81970943).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
RL, DZ and YP designed the study. YL, QD and JP
acquired and analyzed the data. YY and YM obtained the clinical
samples and revised the manuscript. RL and JP wrote and revised the
manuscript. All authors have read and approved the final
manuscript. JP and DZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Stomatological Hospital of China
Medical University (Shenyang, China; approval no. 2018-30). Prior
to the examination, the subjects were informed of the purpose of
the study, and written informed consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shokrzadeh L, Baghaei K, Yamaoka Y, Dabiri
H, Jafari F, Sahebekhtiari N, Tahami A, Sugimoto M, Zojaji H and
Zali MR: Analysis of 3'-end variable region of the cagA gene
in Helicobacter pylori isolated from Iranian population. J
Gastroenterol Hepatol. 25:172–177. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yadegar A, Mobarez AM, Alebouyeh M,
Mirzaei T, Kwok T and Zali MR: Clinical relevance of cagL gene and
virulence genotypes with disease outcomes in a Helicobacter
pylori infected population from Iran. World J Microbiol
Biotechnol. 30:2481–2490. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yadegar A, Alebouyeh M and Zali MR:
Analysis of the intactness of Helicobacter pylori cag
pathogenicity island in Iranian strains by a new PCR-based strategy
and its relationship with virulence genotypes and EPIYA motifs.
Infect Genet Evol. 35:19–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abu-Lubad M, Alzoubi H, Jarajreh D,
Sawalqa AA, Bruggemann H, Albataineh E, Aqel A and Al-Zeer M:
Analysis of Helicobacter pylori genotypes amongst Jordanians'
dental plaque samples. Gastroenterology Res. 11:46–51.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Handa O, Naito Y and Yoshikawa T: Redox
biology and gastric carcinogenesis: The role of Helicobacter
pylori. Redox Rep. 16:1–7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zaric S, Bojic B, Popovic B and Milasin J:
Eradication of gastric Helicobacter pylori ameliorates
halitosis and tongue coating. J Contemp Dent Pract. 16:205–209.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
El Batawi HY, Venkatachalam T, Francis A,
Abujabal R and Shehadat SA: Dental caries-A hiding niche for
Helicobacter pylori in Children. J Clin Pediatr Dent.
44:90–94. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ding YJ, Yan TL, Hu XL, Liu JH, Yu CH, Li
YM and Wang QY: Association of salivary Helicobacter pylori
infection with oral diseases: A cross-sectional study in a Chinese
population. Int J Med Sci. 12:742–747. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jalili M, Mahmoodabadi KA and Sayehmiri K:
Relationship between Helicobacter pylori and periodontal
diseases: A metaanalysis study and systematic review. Open Dent J.
14:362–368. 2020.
|
|
10
|
Adachi K, Notsu T, Mishiro T, Yoshikawa H
and Kinoshita Y: Influence of Helicobacter pylori infection
on periodontitis. J Gastroenterol Hepatol. 34:120–123.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Krajden S, Fuksa M, Anderson J, Kempston
J, Boccia A, Petrea C, Babida C, Karmali M and Penner JL:
Examination of human stomach biopsies, saliva, and dental plaque
for Campylobacter pylori. J Clin Microbiol. 27:1397–1398.
1989.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ferguson DA Jr, Li C, Patel NR, Mayberry
WR, Chi DS and Thomas E: Isolation of Helicobacter pylori
from saliva. J Clin Microbiol. 31:2802–2804. 1993.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Anand PS, Kamath KP and Anil S: Role of
dental plaque, saliva and periodontal disease in Helicobacter
pylori infection. World J Gastroenterol. 20:5639–5653.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Silva DG, Stevens RH, Macedo JM, Albano
RM, Falabella ME, Fischer RG, Veerman EC and Tinoco EM: Presence of
Helicobacter pylori in supragingival dental plaque of
individuals with periodontal disease and upper gastric diseases.
Arch Oral Biol. 55:896–901. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Salehi MR, Shah AM, Naghsh N, Hajisadeghi
S and Ajami EA: Comparison in prevalence of Helicobacter
pylori in the gingival crevicular fluid from subjects with
periodontitis and healthy individuals using polymerase Chain
reaction. J Dent Res Dent Clin Dent Prospects. 7:238–243.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Al-Ahmad A, Kürschner A, Weckesser S,
Wittmer A, Rauberger H, Jakob T, Hellwig E, Kist M and Waidner B:
Is Helicobacter pylori resident or transient in the human
oral cavity? J Med Microbiol. 61:1146–1152. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Backert S and Tegtmeyer N: The versatility
of the Helicobacter pylori vacuolating cytotoxin vacA
in signal transduction and molecular crosstalk. Toxins (Basel).
2:69–92. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shiota S, Suzuki R and Yamaoka Y: The
significance of virulence factors in Helicobacter pylori. J
Dig Dis. 14:341–349. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reyes-Leon A, Atherton JC, Argent RH,
Puente JL and Torres J: Heterogeneity in the activity of Mexican
Helicobacter pylori strains in gastric epithelial cells and
its association with diversity in the cagA gene. Infect
Immun. 75:3445–3454. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rudi J, Rudy A, Maiwald M, Kuck D, Sieg A
and Stremmel W: Direct determination of Helicobacter pylori
vacA genotypes and cagA gene in gastric biopsies and
relationship to gastrointestinal diseases. Am J Gastroenterol.
94:1525–1531. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Martínez-Carrillo DN, Garza-González E,
Betancourt-Linares R, Mónico-Manzano T, Antúnez-Rivera C,
Román-Román A, Flores-Alfaro E, Illades-Aguiar B and
Fernández-Tilapa G: Association of IL1B-511C/-31T haplotype and
Helicobacter pylori vacA genotypes with gastric ulcer and
chronic gastritis. BMC Gastroenterol. 10(126)2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wongphutorn P, Chomvarin C, Sripa B,
Namwat W and Faksri K: Detection and genotyping of Helicobacter
pylori in saliva versus stool samples from asymptomatic
individuals in Northeastern Thailand reveals intra-host
tissue-specific H. pylori subtypes. BMC Microbiol.
18(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Papapanou PN, Sanz M, Buduneli N, Dietrich
T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani
F, et al: Periodontitis: Consensus report of workgroup 2 of the
2017 world workshop on the classification of periodontal and
peri-implant diseases and conditions. J Periodontol. 89 (Suppl
1):S173–S182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tonetti MS, Greenwell H and Kornman KS:
Staging and grading of periodontitis: Framework and proposal of a
new classification and case definition. J Periodontol. 89 (Suppl
1):S159–S172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Montero E, Herrera D, Sanz M, Dhir S, Van
Dyke T and Sima C: Development and validation of a predictive model
for periodontitis using NHANES 2011-2012 data. J Clin Periodontol.
46:420–429. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Palmer R and Floyd P (eds): BDJ
clinician's guides. London: Springer Nature Switzerland AG, pp1-15,
2021.
|
|
28
|
Momtaz H, Souod N, Dabiri H and Sarshar M:
Study of Helicobacter pylori genotype status in saliva,
dental plaques, stool and gastric biopsy samples. World J
Gastroenterol. 18:2105–2111. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Valadan Tahbaz S, Yadegar A, Amirmozafari
N, Yaghoobee S, Ehsani Ardakani MJ and Zojaji H: Occurrence of
Helicobacter pylori and its major virulence genotypes in
dental plaque samples of patients with chronic periodontitis in
Iran. Gastroenterol Hepatol Bed Bench. 10 (Suppl 1):S70–S78.
2017.PubMed/NCBI
|
|
30
|
Yamaoka Y, Kodama T, Gutierrez O, Kim JG,
Kashima K and Graham DY: Relationship between Helicobacter
pylori iceA, cagA, and vacA status and clinical
outcome: Studies in four different countries. J Clin Microbiol.
37:2274–2279. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ito Y, Azuma T, Ito S, Miyaji H, Hirai M,
Yamazaki Y, Sato F, Kato T, Kohli Y and Kuriyama M: Analysis and
typing of the vacA gene from cagA positive strains of
Helicobacter pylori isolated in Japan. J Clin Microbiol.
35:1710–1714. 1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
De Gusmão VR, Nogueira Mendes E, De
Magalhães Queiroz DM, Aguiar Rocha G, Camargos Rocha AM, Ramadan
Ashour AA and Teles Carvalho AS: vacA genotypes in
Helicobacter pylori strains isolated from children with and
without duodenal ulcer in Brazil. J Clin Microbiol. 38:2853–2857.
2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zheng P and Zhou W: Relation between
periodontitis and Helicobacter pylori infection. Int J Clin
Exp Med. 8:16741–16744. 2015.PubMed/NCBI
|
|
34
|
Eskandari A, Mahmoudpour A, Abolfazli N
and Lafzi A: Detection of Helicobacter pylori using PCR in
dental plaque of patients with and without gastritis. Med Oral
Patol Oral Cir Bucal. 15:e28–e31. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yee JKC: Are the view of Helicobacter
pylori colonized in the oral cavity an illusion? Exp Mol Med.
49(e397)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bektas M, Soykan I, Altan M, Alkan M and
Ozden A: The effect of Helicobacter pylori eradication on
dyspeptic symptoms, acid reflux and quality of life in patients
with functional dyspepsia. Eur J Intern Med. 20:419–423.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang XM, Yee KC, Hazeki-Taylor N, Li J, Fu
HY, Huang ML and Zhang GY: Oral Helicobacter pylori, its
relationship to successful eradication of gastric H. pylori
and saliva culture confirmation. J Physiol Pharmacol. 65:559–566.
2014.PubMed/NCBI
|
|
38
|
Aksit Bıcak D, Akyuz S, Kıratlı B, Usta M,
Urganci N, Alev B, Yarat A and Sahin F: The investigation of
Helicobacter pylori in the dental biofilm and saliva samples
of children with dyspeptic complaints. BMC Oral Health.
17(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Falsafi T, Khani A, Mahjoub F, Asgarani E
and Sotoudeh N: Analysis of vacA/cagA
genotypes/status in Helicobacter pylori isolates from
Iranian children and their association with clinical outcome. Turk
J Med Sci. 45:170–177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Miehlke S, Kirsch C, Agha-Amiri K, Günther
T, Lehn N, Malfertheiner P, Stolte M, Ehninger G and Bayerdörffer
E: The Helicobacter pylori vacA s1, m1 genotype and
cagA is associated with gastric carcinoma in Germany. Int J
Cancer. 87:322–327. 2000.PubMed/NCBI
|
|
41
|
Hu B, Zhao F, Wang S, Xiang P, Yang C,
Fang Y, Chen F, Yang F, Zhao H and Zhang Y: Correlation of
Helicobacter pylori virulence genotypes and clinical
characteristics. Lab Med. 31:479–485. 2016.
|
|
42
|
Link A, Langner C, Schirrmeister W,
Habendorf W, Weigt J, Venerito M, Tammer I, Schlüter D, Schlaermann
P, Meyer TF, et al: Helicobacter pylori vacA genotype is a
predominant determinant of immune response to Helicobacter
pylori CagA. World J Gastroenterol. 23:4712–4723.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Homan M, Luzar B, Kocjan BJ, Orel R,
Mocilnik T, Shrestha M, Kveder M and Poljak M: Prevalence and
clinical relevance of cagA, vacA, and iceA genotypes
of Helicobacter pylori isolated from Slovenian children. J
Pediatr Gastroenterol Nutr. 49:289–296. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ferreira RM, Pinto-Ribeiro I, Wen X,
Marcos-Pinto R, Dinis-Ribeiro M, Carneiro F and Figueiredo C:
Helicobacter pylori cagA promoter region sequences influence
CagA expression and interleukin 8 secretion. J Infect Dis.
213:669–673. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mendoza-Cantú A, Urrutia-Baca VH,
Urbina-Ríos CS, De la Garza-Ramos MA, García-Martínez ME and
Torre-Martínez HHH: Prevalence of Helicobacter pylori vacA
genotypes and cagA gene in dental plaque of asymptomatic
Mexican children. Biomed Res Int. 2017(4923640)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lukeš P, Pavlík E, Potuznikova B, Nartova
E, Foltynova E, Plzak J, Katra R, Sterzl I, Bartunkova J, Betka J
and Astl J: Detection of Helicobacter pylori in
oropharyngeal lymphatic tissue with real-time PCR and assessment of
its carcinogenic potential. Eur Arch Otorhinolaryngol. 271:399–405.
2014.PubMed/NCBI View Article : Google Scholar
|