Introduction
A desmoid tumor, also termed desmoid-type fibrosis
or aggressive fibromatosis, is a fibroblastic proliferation of
mesenchymal origin, which has no metastasizing potential but is
locally aggressive. It is a rare disease, and in 2013, the
incidence was reported as 5-6 newly diagnosed cases/1 million
individuals/year (1), with the
peak incidence occurring in individuals aged 30-40 years old, with
a slightly higher incidence in women (2,3).
Desmoid tumors are known to occur in women taking oral
contraceptives, women during or after pregnancy, patients with a
history of previous trauma or surgery, and in patients with genetic
conditions such as familial adenomatous polyposis (FAP) and
Gardner's syndrome (4,5). The mortality is rarely reported;
however, in patients with FAP and intra-abdominal desmoid tumors,
the overall mortality has been reported to be ≤76% for stage IV
tumors in a single center cohort study (6). The tumor develops from the deep soft
tissue that is located throughout the body, including the abdominal
wall, intra-abdominal cavity, mesentery, gastrointestinal tract,
extremities, chest wall, breast, and the head and neck (5). Surgical tumor resection has been used
as the primary management for several decades alongside systemic
therapy or radiotherapy (7).
However, previously, treatment has shifted to active surveillance
of the tumor after the initial diagnosis due to its indolent course
and possibility for spontaneous regression (SR) (8,9).
While prompt medical or surgical treatments are reserved for
symptomatic and progressive tumors, intra-abdominal mesenteric
tumors may also require early intervention due to the high
morbidity risk (9). The present
report describes an adolescent male patient with a rapidly growing
38-cm long intra-abdominal desmoid tumor of mesenchymal origin.
Case report
An 18-year-old Asian male patient with no known past
medical history was referred to a tertiary academic medical center
(Severance Hospital, Seoul, Korea) in July 2020 via the outpatient
clinic with an intra-abdominal mass. The patient had been having
upper abdominal discomfort for 2-3 months, which gradually became
worse and was accompanied by abdominal pain. The patient had not
experienced any traumatic events or a familial history of cancer.
As the patient was obese at the first visit (height, 166 cm;
weight, 88 kg; body mass index, 31.9 kg/m2), the mass
was not observed by sight but it was firmly palpable. Abdominal
sonography and abdomino-pelvic computed tomography (APCT) initially
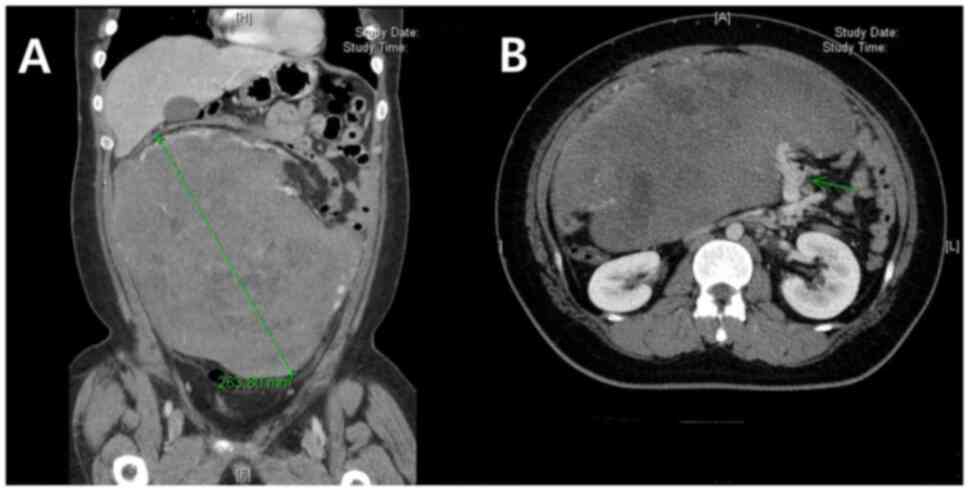
revealed a 26-cm long heterogeneously enhanced, lobulated mass with
necrotic components (Fig. 1). The
mass appeared to originate from the omento-mesentery with feeding
vessels arising from the engorged branches of the superior
mesenteric artery, and although a large portion of the small bowel
was lateralized to the left, there were no definite signs of organ
invasion. Positron emission tomography-CT and whole-body bone scans
indicated no evident distant metastasis. After a transcutaneous
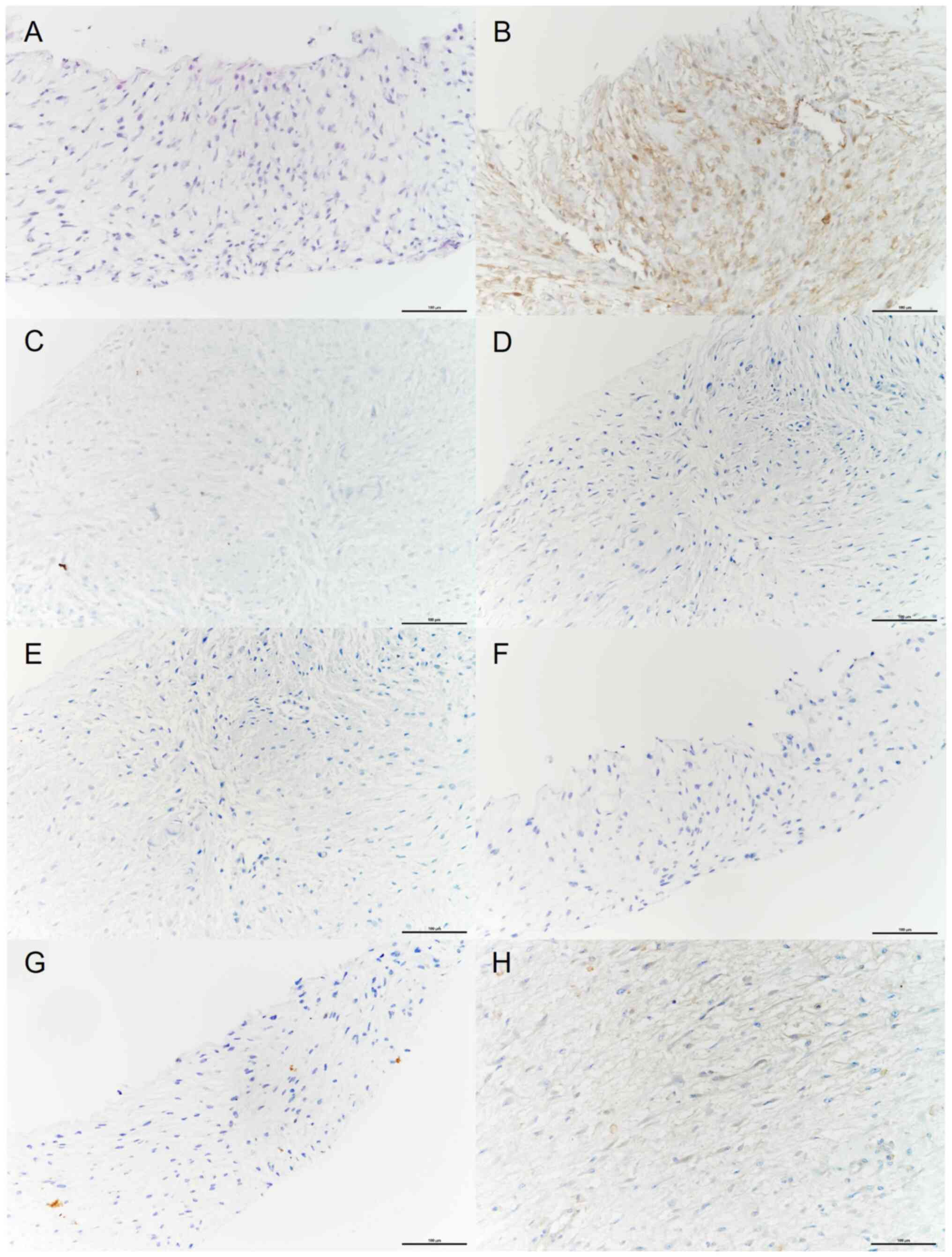
sonography-assisted biopsy, a spindle cell proliferative lesion in
a myxoid background was revealed and immunohistochemical (IHC)
stains were negative for anaplastic lymphoma kinase (ALK), S-100,
mucin 4, CD34, α-smooth muscle actin (α-SMA) and SRY-box
transcription factor 10 (SOX10), and positive for β-catenin, which
indicated that a desmoid-type fibromatosis diagnosis was a high
possibility (Fig. 2). The slide
preparation for IHC and H&E staining was performed as follows:
Formalin fixation was conducted by submerging tissue in 10% neutral
buffered formalin for 24 h at room temperature, followed by
dehydration with ethanol, and clearance with xylene of the tissue.
Paraffin infiltration was then achieved with an automatic tissue
processing machine (HistoCore PELORIS II; Leica Microsystems GmbH),
and the block was cut into 4-µm wide slices. For IHC staining,
deparaffinization, antigen retrieval, primary and secondary
antibody reactions, and counterstaining were further performed with
an automated IHC staining system (Table I). Hematoxylin and eosin staining
was also performed using the automated system (VENTANA HE 600
system; Roche Tissue Diagnostics) as follows: Deparaffinization,
rehydration, hematoxylin staining, differentiation of the nucleus,
bluing, eosin staining, dehydration and clearing (Table II). The light microscope used was
a BX43 microscope (Olympus Corporation). No genetic analysis was
performed for β-catenin (CTNNB1) or adenomatous polyposis
coli (APC) mutations.
 | Table IDetails of procedures for IHC
staining. |
Table I
Details of procedures for IHC
staining.
| Procedures | ALK | S-100 | Mucin 4 | CD34 | SMA | SOX-10 | β-catenin |
|---|
|
Deparaffinization | | | | | | | |
|
Solvent | EZ prep (10x) | EZ prep (10x) | EZ prep (10x) | Clearify Clearing
Agent | Clearify Clearing
Agent | EZ prep (10x) | EZ prep (10x) |
|
Supplier | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Dako; Agilent
Technologies, Inc. | Dako; Agilent
Technologies, Inc. | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics |
|
Temperature,
˚C | 75 | 75 | 75 | 25 | 25 | 75 | 75 |
|
Duration,
min | 4 | 4 | 4 | 1 | 1 | 4 | 4 |
| Antigen
retrieval | | | | | | | |
|
Reagent | Cell conditioning
solution-1 | Cell conditioning
solution-1 | Cell conditioning
solution-1 | EnV FLEX TRS High pH
(50x) | EnV FLEX TRS High pH
(50x) | Cell conditioning
solution-1 | Cell conditioning
solution-1 |
|
Supplier | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Dako; Agilent
Technologies, Inc. | Dako; Agilent
Technologies, Inc. | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics |
|
Temperature,
˚C | 100 | 100 | 100 | 97 | 97 | 100 | 100 |
|
Duration,
min | 64 | 38 | 38 | 30 | 30 | 38 | 38 |
| Primary antibody
reaction | | | | | | | |
|
Dilution | Prediluted | 1:2,000 | 1:1,000 | 1:100 | 1:1,000 | 1:100 | 1:400 |
|
Catalogue
no. | IS641 | Z0311 | MABT395 | M7165 | M0851 | 383R-14 | 224M-15 |
|
Supplier | Dako; Agilent
Technologies, Inc. | Dako; Agilent
Technologies, Inc. | MilliporeSigma | Dako; Agilent
Technologies, Inc. | Dako; Agilent
Technologies, Inc. | MilliporeSigma | MilliporeSigma |
|
Temperature,
˚C | 37 | 37 | 37 | 37 | 32 | 37 | 37 |
|
Duration,
min | 32 | 32 | 32 | 20 | 20 | 32 | 32 |
| Secondary antibody
reactiona | | | | | | | |
|
Catalogue
no. | 760-700 | 05269806001
(760-500) | 05269806001
(760-500) | GV800, GV823,
GV900 | GV800, GV823,
GV900 | 05269806001
(760-500) | 05269806001
(760-500) |
|
Product | Optiview HQ
universal linker | ultraView Universal
DAB | ultraView Universal
DAB | EnVision FLEX
HRP | EnVision FLEX
HRP | ultraView Universal
DAB | ultraView Universal
DAB |
|
Supplier | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Dako; Agilent
Technologies, Inc. | Dako; Agilent
Technologies, Inc. | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics |
|
Temperature,
˚C | 37 | 37 | 37 | 37 | 37 | 37 | 37 |
|
Duration,
min | 8 | 8 | 8 | 20 | 20 | 8 | 8 |
| Counterstain | | | | | | | |
|
Counterstain | Hematoxylin | Hematoxylin | Hematoxylin | Hematoxylin | Hematoxylin | Hematoxylin | Hematoxylin |
|
Temperature,
˚C | 37 | 37 | 37 | 37 | 37 | 37 | 37 |
|
Duration,
min | 8 | 8 | 8 | 4 | 4 | 8 | 8 |
| Automated IHC
staining system | | | | | | | |
|
Instrument | Ventana benchmark
XT | Ventana benchmark
XT | Ventana benchmark
XT | Dako; Agilent
Technologies, Inc. | Dako; Agilent
Technologies, Inc. | Ventana benchmark
XT | Ventana benchmark
XT |
|
Supplier | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics | Dako; Agilent
Technologies, Inc. | Dako; Agilent
Technologies, Inc. | Roche Tissue
Diagnostics | Roche Tissue
Diagnostics |
 | Table IIDetails of procedures for hematoxylin
and eosin staining. |
Table II
Details of procedures for hematoxylin
and eosin staining.
| Procedure | Reagent | Catalogue no. | Supplier | Temperature,
˚C | Duration, sec |
|---|
|
Deparaffinization | Organic
solution | 07095163001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 150 |
| Rehydration | Transfer fluid | 06544380001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 20 |
| Hematoxylin
staining | Hematoxylin | 07024282001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 60 |
| Differentiation of
the nucleus | Differentiation
solution | 06544339001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 120 |
| Bluing | Bluing | 06544347001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 30 |
| Eosin staining | Eosin | 06544304001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 300 |
| Dehydration | Transfer fluid | 06544380001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 20 |
| Clearing | Organic
solution | 07095163001 | Roche Tissue
Diagnostics; Roche Diagnostics, Ltd. | 40±3 | 10 |
The upfront surgical resection seemed challenging
due to the high occupancy of the tumor in the abdominal cavity, so
the patient was first offered treatment with chemotherapy using a
regimen of doxorubicin (20 mg/m2 infusion for 1 h on
days 1-3) and dacarbazine (300 mg/m2 infusion for 1 h on
days 1-3) by a pediatric oncologist. Sperm banking was conducted
prior to the chemotherapy. After two cycles of the regimen with
3-week intervals, the tumor had increased to 32 cm in diameter in 1
month from the first visit, increasing the abdominal discomfort and
pain experienced by the patient. Thus, the chemotherapy regimen was
changed to etoposide (100 mg/m2 infusion for 3 h on days
1-5), carboplatin (200 mg/m2 infusion for 3 h on days 1
and 2) and ifosfamide (1,800 mg/m2 infusion for 3 h on
days 1-5). However, the non-responsiveness of the tumor and the
side effects of the chemotherapy, such as neutropenic fever and
general weakness, led to repeated patient re-admissions and made it
hard to further delay the surgical management. After the decision
for surgical resection of the tumor was made, the patient was
injected with antibiotics (piperacillin-tazobactam; 4.5 g mixed in
100 ml saline; three times a day) for 8 days for the neutropenic
fever, transfused with platelet concentrates (PCs) for
thrombocytopenia and was provided with total parenteral nutrition
for 3 days. Preoperative consultations were conducted with upper
gastrointestinal, hepatobiliary and vascular surgeons for possible
cooperation. On the day before the surgery, percutaneous
angiographic embolization was performed on the feeding vessel of
the tumor, which branched from the superior mesenteric artery, to
prevent intra-operative bleeding. The operation was conducted 2
months after the first outpatient clinic visit.
The surgeon in charge was specialized in colorectal
surgery with >10 years of clinical experience in the tertiary
academic medical center (Severance Hospital, Seoul, Korea). At the
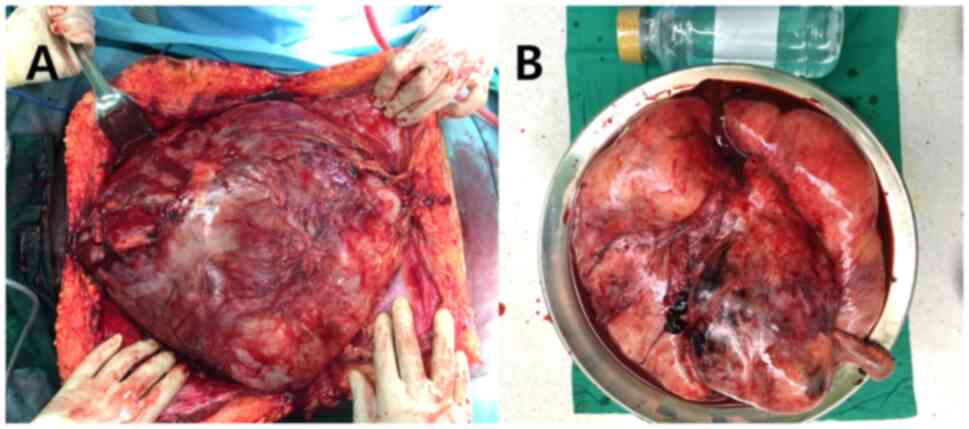
operating field, a long midline incision was insufficient to expose
the mass completely, so a crossed incision with additional
transverse upper midline incisions were made (Fig. 3). Tumor dissection was performed by
peeling off the adjoined bowel, mesentery and retroperitoneum. An
ileocecectomy with ileocolic anastomosis was conducted due to
ileocecal vascular involvement, and the vascular surgical team
engaged in dissecting the superior mesenteric vessel and its
angioplasty. The total operation time was 311 min and estimated
blood loss was 3,000 ml, while intra-operative blood transfusions
of 10 units of packed red blood cells, 6 units of PC and 6 units of
fresh frozen plasma were performed. After the operation, the
patient was admitted to the Surgical Intensive Care Unit, and was
moved to the General Surgical Ward 2 days later. Liquid and soft
diet was started on postoperative day 6 and 9, respectively, and
the patient was discharged at postoperative day 15. The final
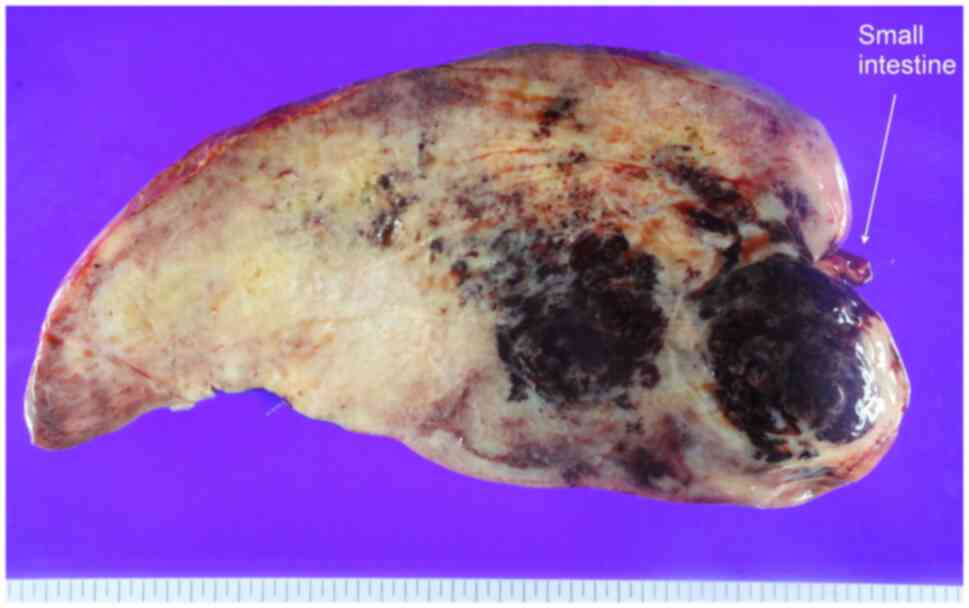
surgical pathology revealed a 38-cm long desmoid-type fibrosis and
a clear resection margin, and the cut surface revealed diffuse
myxoid, hemorrhagic and multilocular cysts with yellowish sebaceous
fluid (Fig. 4). The IHC staining
results were the same as the initial findings: Negative for ALK,
S-100, mucin 4, CD34, α-SMA and SOX10, and positive for
β-catenin.
On the first outpatient clinic visit after discharge
(postoperative month 1), the patient was planned for maintenance
adjuvant chemotherapy (vinblastine 6 mg/m2 infusion for
1 h plus methotrexate 30 mg/m2 injection on days 1, 8,
15 and 23) by the pediatric oncologist, due to the large size of
the tumor despite en bloc resection with an intact tumor
capsule. The chemotherapy was started on postoperative day 40 after
the skin wound had completely healed and ended on postoperative
year 1 (12 cycles total). After the first APCT at 1 month
postoperatively, MRIs were performed every 2 cycles of chemotherapy
(2 months) for response evaluation, to follow up the subcentimeter
T2-hypointense nodular lesion in the upper anterior abdomen, which
appeared to be an uncomplicated postoperative change. Rare and
occasional upper abdominal discomfort that spontaneously subsided
and elevated alanine transaminase (50-80 IU/l) controlled with oral
ursodiol (100 mg three times a day) were noted, and prophylactic
oral sulfamethoxazole/trimethoprim (960 mg per day) was maintained
during chemotherapy.
After completion of chemotherapy, CT and MRI were
alternately performed every 3 months until 2 years after surgery
and every 6 months thereafter. The patient was admitted to hospital
1 year after the surgery due to small bowel obstruction lasting for
5 days. The patient has been followed up for 2.5 years and is
recurrence-free. The patient will be followed up for 5 years, but
the follow-up period may be extended due to the young age. It is
assumed that the tumor will not recur, but if it does, the
multidisciplinary team will discuss the possibility of surgical
resection or enroll the patient in a clinical trial.
Discussion
A desmoid tumor is defined as ‘clonal fibroblastic
proliferations that arise in the deep soft tissues and is
characterized by infiltrative growth and a tendency toward local
recurrence but an inability to metastasize’ by the World Health
Organization (10). While 10-15%
of desmoid tumors derive from germline mutations of the APC
gene and present as FAP, the majority of desmoid tumors are
sporadic and are associated with somatic mutations of the
CTNNB1 gene (7). The
mutation of either gene leads to alterations in the Wnt/β-catenin
signaling pathway and eventually to uncontrolled fibroblastic
proliferations (11). The
APC and CTNNB1 genes are known to be mutually
exclusive, and mutational analysis of the desmoid tumor biopsied
specimen is recommended in the initial diagnostic phase (10). The etiologies of desmoid tumors,
other than hereditary syndromes, include a history of previous
trauma or the hormonal effects of estrogen during pregnancy
(12,13). In the present case study, the
patient did not have any medical or traumatic history, and
mutational analysis was not performed, as relieving the symptomatic
tumor was the treatment priority at the time. In the future, a post
hoc mutational analysis is planned since, although the surgery has
already been performed, detecting the triggering genetic mutation
may be a requirement as the patient gets older.
Despite their high tendency for local recurrence,
desmoid tumors are likely to stay dormant or to spontaneously
regress in 20-30% of patients (14,15).
Penel et al (16)
demonstrated that the 2-year event-free survival rate was not
significantly different between a surgically and non-surgically
treated group in all tumor locations (53 vs. 58%, respectively;
P=0.415) and in locations including the abdominal wall,
intra-abdominal, breast, digestive viscera and lower limb (70 vs.
63%, respectively; P=0.413). Burtenshaw et al (17) demonstrated that among 109 patients
with intra-abdominal and abdominal wall desmoid tumors who were
initially observed, 55 (50.5%) patients underwent medical or
surgical treatments either due to disease progression or symptom
escalation, while 31 (28.4%) and 23 (21.1%) patients exhibited
stable disease (SD) and SR, respectively. Accordingly, the initial
management of desmoid tumors has evolved into observation and
active surveillance with repeated CT or MRI scans.
However, symptomatic and progressive tumors may be
indicated for surgical or medical intervention. In particular,
FAP-associated desmoid tumors are typically managed more actively
due to their more aggressive tumor course (10). Medical management includes
antihormonal therapy, administration of tyrosine kinase inhibitors
(TKI) and chemotherapy. The only TKIs that have been demonstrated
to be effective in randomized trials are sorafenib and pazopanib.
Sorafenib demonstrated a significant higher overall response rate
of SR compared with that of the placebo group (33 vs. 20%,
respectively) and a longer 2-year progression-free survival (PFS)
rate compared with that of the placebo group (81 vs. 36%,
respectively; HR 0.13; P<0.001) (18). Available chemotherapy regimens
include methotrexate plus vinblastine or vinorelbine, oral
vinorelbine, doxorubicin plus dacarbazine, methotrexate with vinca
alkaloids, anthracycline-based regimens and pegylated liposomal
doxorubicin (7,9). Furthermore, the methotrexate with
vinblastine regimen was investigated in two phase II trials;
Azzarelli et al (19)
demonstrated a 67% 5-year PFS and Skapek et al (20) reported that 69% of patients had SD
or SR. In June 2018, a meeting for an evidence-based joint global
consensus on the management of desmoid tumors was held by experts
from the association of European Reference Network on Rare Adult
Solid Cancers, the European Organization for Research and Treatment
of Cancer/Soft Tissue and Bone Sarcoma Group, Sarcoma Patients
EuroNet and The Desmoid Tumor Research Foundation, in Milan, Italy.
The published papers that followed summarized the consensus that,
as mesenteric desmoid tumors are close to vital organs, clinicians
should make an earlier decision towards their active treatment
(8,9,21).
The assessment of the treatment effects and tumor
follow-up examinations are typically implemented with radiological
assessments using CT or MRI. There are currently no standardized
and validated response criteria for desmoid tumors, but the
majority of previous studies used the Response Evaluation Criteria
in Solid Tumors (10).
In the present case study, the tumor grew 20% in
diameter in 6 weeks after applying two cycles of doxorubicin and
dacarbazine. Doxorubicin and dacarbazine have been administered for
fibrous tumors and FAP-related intra-abdominal desmoid tumors, and
in one study (22), implementation
of the regimen caused significant tumor regression in all 7
patients with intra-abdominal mesenteric desmoid tumors. Although
the evidence is weak due to the small number of cases and, unlike
in the present case, the patients in the aforementioned study had
FAP-related tumors, the finding that the tumor grew rapidly in the
present study despite the chemotherapy treatment suggests that it
may have been due to the nature of the tumor rather than the effect
of the chemotherapy.
Adjuvant chemotherapy with a regimen of vinblastine
and methotrexate was performed for the patient in the present
report to reduce recurrence. Postoperative use of this regimen has
rarely been reported but it has been applied to recurrent or
progressive desmoid tumors. Garbay et al (23) reported that among 27 patients who
were treated with vinblastine (6 mg/m2) and methotrexate
(30 mg/m2) on days 1, 8, 15 and 23 for recurrent or
progressive desmoid tumors, 4 (15%), 14 (52%) and 9 (33%) patients
exhibited partial response, SD and progression, respectively. The
number of chemotherapy cycles was not specified in the study. In a
study reporting pediatric patients (24), vinblastine (6 mg/m2) and
methotrexate (30 mg/m2) given for 10 months to a
10-year-old patient with an incompletely resected tumor and for 2
years to a 14-year-old patient with a recurrent tumor resulted in
complete remission and SD, respectively. A phase-II randomized
clinical trial compared pazopanib with vinblastine plus
methotrexate in patients with progressive desmoid tumors and
reported that the 6-month non-progression rate was 83.7 and 45%,
respectively (25). However, the
2-year PFS was 67.2 and 79%, respectively. The present study is one
of the few to describe adjuvant therapy with vinblastine and
methotrexate after complete tumor removal.
Radiation therapy (RTx) before or after the surgery
was not implemented in the present study. Preoperative RTx was not
considered, as it was assumed that the tumor was resectable.
Currently, adjuvant RTx is rarely employed, and when it is, it is
used for retroperitoneal or recurrent tumors (9,21).
Furthermore, as the tumor in the present study was extensively
adjacent to the bowel, it was considered that postoperative RTx may
be complicated by enteritis and that the patient would be followed
up well regardless, as CT and MRI scans are easily available in
South Korea. Additionally, the angiographic embolization that was
performed 1 day before the surgery was conducted to help the
surgeons control the bleeding more easily during the surgery,
rather than to decrease the size of the tumor. Unfortunately, the
procedure did not prevent intra-operative major bleeding, since
various minor vessels caused oozing bleeding.
There are only a small number of case reports
describing patients with large sporadic intra-abdominal
mesentery-derived desmoid tumors ≥19 cm in diameter, with no family
history of cancer and no operative or traumatic history (Table III) (26-31).
All patients in the aforementioned reports were male, with a mean
age of 33 years old. It is unclear whether the reason these tumors
appear to occur more frequently in men is due to a biological cause
or a delay in disease recognition. In addition, the majority of the
tumors reported originated from the ileal mesentery. The largest
sporadic tumor was reported by Williams et al (26), in which the patient exhibited a
45-cm long tumor. The majority of patients underwent upfront
surgery (5 out of 6 patients) and genetic analysis was not
performed in the majority of cases (5 out of 6 patients).
 | Table IIICase reports of patients with a
sporadic intra-abdominal mesentery-derived desmoid tumor ≥19 cm in
diameter. |
Table III
Case reports of patients with a
sporadic intra-abdominal mesentery-derived desmoid tumor ≥19 cm in
diameter.
| First author/s,
year | Country | Age, years | Sex | Duration of
symptoms | Location | Tumor, diameter
cm | Genetic
analysis | Treatment | F/U, months | Recurrence | (Refs.) |
|---|
| Williams et
al, 2016 | USA | 33 | Male | Few months | Ileocolic
mesenterya | 45 | NA | Neoadj
therapyb and
surgery | NA | NA | (26) |
| Mizuta and Tsunemi,
2018 | Japan | 17 | Male | 6 months | Transverse
mesocolon | 30 | NA | Surgery | NA | NA | (27) |
| Sioda et al,
2020 | USA | 24 | Male | 4 weeks | Ileocolic
mesentery | 31 | APC mutation
(-) | Surgery | NA | NA | (28) |
| El-Helou et
al, 2020 | Lebanon | 34 | Male | NA | Ileocolic
mesenterya | 23 | NA | Surgery | 12 | None | (29) |
| Kuwabara et
al, 2021 | Japan | 51 | Male | Acute | Proximal ileal | 19 | NA | Surgery | 36 | None | (30) |
| Elhaddad et
al, 2022 | USA | 38 | Male | 3 months | Ileocolic
mesentery | 40 | NA | Surgery | 5 | None | (31) |
A limitation of the present study was that it lacked
genetic screening of the patient for FAP, Gardner syndrome and
mutation of the CTNNB1 gene. However, to the best of our
knowledge, the present study reported the third largest sporadic
intra-abdominal mesentery-derived desmoid tumor with rapid growth.
Considering the rarity of the disease and the fact that the
treatment for mesentery-derived oversized desmoid tumors has not
been fully established, the present case study may provide
direction for the treatment of similar conditions in the
future.
In conclusion, the present study reported the case
of a patient with a 38-cm intra-abdominal desmoid tumor, who was
treated with chemotherapy followed by surgical resection due to
non-responsiveness and progression of symptoms, then with
maintenance adjuvant chemotherapy to prevent recurrence due to the
large size of the tumor. The patient has been recurrence-free for 2
years, and further follow-up is planned. In the future, prompt
surgical resection may be required for rapidly growing symptomatic
intra-abdominal desmoid tumors of mesenteric origin.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJK contributed to medical record review, patient
data analysis, literature research, and writing. JWH contributed to
conceptualization and review of this paper. TY contributed to
medical record review and obtaining medical images. HC contributed
to specimen collection and interpretation. YDH contributed to
conceptualization, review and supervision of this paper. SJK and
YDH confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and approved by The Ethics Committee
of Severance Hospital of Yonsei University College of Medicine (IRB
no. 4-2023-0487; Seoul, South Korea). Written informed consent was
obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the data and images in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Broekhoven DL, Grünhagen DJ, den
Bakker MA, van Dalen T and Verhoef C: Time trends in the incidence
and treatment of extra-abdominal and abdominal aggressive
fibromatosis: A population-based study. Ann Surg Oncol.
22:2817–2823. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Penel N, Coindre JM, Bonvalot S, Italiano
A, Neuville A, Le Cesne A, Terrier P, Ray-Coquard I, Ranchere-Vince
D, Robin YM, et al: Management of desmoid tumours: A nationwide
survey of labelled reference centre networks in France. Eur J
Cancer. 58:90–96. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kasper B, Ströbel P and Hohenberger P:
Desmoid tumors: Clinical features and treatment options for
advanced disease. Oncologist. 16:682–693. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Riedel RF and Agulnik M: Evolving
strategies for management of desmoid tumor. Cancer. 128:3027–3040.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shinagare AB, Ramaiya NH, Jagannathan JP,
Krajewski KM, Giardino AA, Butrynski JE and Raut CP: A to Z of
desmoid tumors. AJR Am J Roentgenol. 197:W1008–W1014.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Quintini C, Ward G, Shatnawei A, Xhaja X,
Hashimoto K, Steiger E, Hammel J, Uso TD, Burke CA and Church JM:
Mortality of intra-abdominal desmoid tumors in patients with
familial adenomatous polyposis: A single center review of 154
patients. Ann Surg. 255:511–516. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Devata S and Chugh R: Desmoid tumors: A
comprehensive review of the evolving biology, unpredictable
behavior, and myriad of management options. Hematol Oncol Clin
North Am. 27:989–1005. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kasper B, Raut CP and Gronchi A: Desmoid
tumors: To treat or not to treat, That is the question. Cancer.
126:5213–5221. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Desmoid Tumor Working Group. The
management of desmoid tumours: A joint global consensus-based
guideline approach for adult and paediatric patients. Eur J Cancer.
127:96–107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fletcher C, Bridge JA, Hogendoorn PCW and
Mertens F: WHO classification of tumours of soft tissue and bone:
WHO classification of tumours, vol. 5. World Health Organization,
2013.
|
|
11
|
Crago AM, Chmielecki J, Rosenberg M,
O’Connor R, Byrne C, Wilder FG, Thorn K, Agius P, Kuk D, Socci ND,
et al: Near universal detection of alterations in CTNNB1 and Wnt
pathway regulators in desmoid-type fibromatosis by whole-exome
sequencing and genomic analysis. Genes Chromosomes Cancer.
54:606–615. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fiore M, Coppola S, Cannell AJ, Colombo C,
Bertagnolli MM, George S, Le Cesne A, Gladdy RA, Casali PG, Swallow
CJ, et al: Desmoid-type fibromatosis and pregnancy: A
multi-institutional analysis of recurrence and obstetric risk. Ann
Surg. 259:973–978. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schlemmer M: Desmoid tumors and deep
fibromatoses. Hematol Oncol Clin North Am. 19:565–571, vii-viii.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Colombo C, Miceli R, Le Péchoux C,
Palassini E, Honoré C, Stacchiotti S, Mir O, Casali PG, Dômont J,
Fiore M, et al: Sporadic extra abdominal wall desmoid-type
fibromatosis: Surgical resection can be safely limited to a
minority of patients. Eur J Cancer. 51:186–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bonvalot S, Ternès N, Fiore M, Bitsakou G,
Colombo C, Honoré C, Marrari A, Le Cesne A, Perrone F, Dunant A and
Gronchi A: Spontaneous regression of primary abdominal wall desmoid
tumors: More common than previously thought. Ann Surg Oncol.
20:4096–4102. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Penel N, Le Cesne A, Bonvalot S, Giraud A,
Bompas E, Rios M, Salas S, Isambert N, Boudou-Rouquette P, Honore
C, et al: Surgical versus non-surgical approach in primary
desmoid-type fibromatosis patients: A nationwide prospective cohort
from the French sarcoma group. Eur J Cancer. 83:125–131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Burtenshaw SM, Cannell AJ, McAlister ED,
Siddique S, Kandel R, Blackstein ME, Swallow CJ and Gladdy RA:
Toward observation as first-line management in abdominal desmoid
tumors. Ann Surg Oncol. 23:2212–2219. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gounder MM, Mahoney MR, Van Tine BA, Ravi
V, Attia S, Deshpande HA, Gupta AA, Milhem MM, Conry RM, Movva S,
et al: Sorafenib for advanced and refractory desmoid tumors. N Engl
J Med. 379:2417–2428. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Azzarelli A, Gronchi A, Bertulli R, Tesoro
JD, Baratti D, Pennacchioli E, Dileo P, Rasponi A, Ferrari A,
Pilotti S and Casali PG: Low-dose chemotherapy with methotrexate
and vinblastine for patients with advanced aggressive fibromatosis.
Cancer. 92:1259–1264. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Skapek SX, Ferguson WS, Granowetter L,
Devidas M, Perez-Atayde AR, Dehner LP, Hoffer FA, Speights R,
Gebhardt MC, Dahl GV, et al: Vinblastine and methotrexate for
desmoid fibromatosis in children: Results of a pediatric oncology
group phase II trial. J Clin Oncol. 25:501–506. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Casali PG, Abecassis N, Aro HT, Bauer S,
Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee J, Brodowicz
T, et al: Soft tissue and visceral sarcomas: ESMO-EURACAN clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 29:iv51–iv67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gega M, Yanagi H, Yoshikawa R, Noda M,
Ikeuchi H, Tsukamoto K, Oshima T, Fujiwara Y, Gondo N, Tamura K, et
al: Successful chemotherapeutic modality of doxorubicin plus
dacarbazine for the treatment of desmoid tumors in association with
familial adenomatous polyposis. J Clin Oncol. 24:102–105.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Garbay D, Le Cesne A, Penel N, Chevreau C,
Marec-Berard P, Blay JY, Debled M, Isambert N, Thyss A, Bompas E,
et al: Chemotherapy in patients with desmoid tumors: A study from
the French sarcoma group (FSG). Ann Oncol. 23:182–186.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zemer VS, Toledano H, Kornreich L, Freud
E, Atar E, Avigad S, Feinberg-Gorenshtein G, Fichman S, Issakov J,
Dujovny T, et al: Sporadic desmoid tumors in the pediatric
population: A single center experience and review of the
literature. J Pediatr Surg. 52:1637–1641. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Toulmonde M, Pulido M, Ray-Coquard I,
Andre T, Isambert N, Chevreau C, Penel N, Bompas E, Saada E,
Bertucci F, et al: Pazopanib or methotrexate-vinblastine
combination chemotherapy in adult patients with progressive desmoid
tumours (DESMOPAZ): A non-comparative, randomised, open-label,
multicentre, phase 2 study. Lancet Oncol. 20:1263–1272.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Williams AD, Heightchew K and Siripirapu
V: Diagnostic and therapeutic dilemmas in intra-abdominal desmoid
tumors: A case report and literature review. Int J Surg Case Rep.
26:150–153. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mizuta N and Tsunemi K: Giant
intra-abdominal desmoid tumor in a young male without history of
surgery, trauma, or familial adenomatous polyposis. Case Rep Surg.
2018(9825670)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sioda NA, Wakim AA, Wong T, Patel S, Coan
K and Row D: A large sporadic intra-abdominal desmoid-type
fibromatosis in a young male: A case report. Front Surg.
7(60)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
El-Helou E, Alimoradi M, Sabra H, Naccour
J, Zaarour M, Haddad MM and Bitar H: A giant mesenteric
fibromatosis adherent to the appendix and colonic wall, case
report. Int J Surg Case Rep. 77:638–642. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kuwabara H, Katayanagi S, Koganezawa I,
Nakagawa M, Katsumata K, Tsuchida A and Kawachi S: Sporadic
intra-abdominal desmoid tumor with a very unusual onset: Two case
reports. J Med Case Rep. 15(457)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Elhaddad B, Gopireddy D and Liu S: A giant
sporadic intra-abdominal desmoid tumor in a male patient: A case
report. Cureus. 14(e26633)2022.PubMed/NCBI View Article : Google Scholar
|