Introduction
Subacute combined degeneration (SCD) is a
neurological disease caused by reduced intake, insufficient
absorption or abnormal metabolism of vitamin B12, which mainly
involves the posterior and lateral cords of the spinal cord and
peripheral nerves (1,2). Its clinical manifestations mainly
include numbness of the lower limbs, deep sensory abnormalities,
ataxia and spastic paralysis, and the white matter of the brain and
optic nerves may also be involved in severe cases (3). The age of onset is mainly during
middle age (45-64 years), with no significant differences between
men and women, and most cases are subacute or chronic in onset,
with slow progression (4,5). SCD has certain characteristic
manifestations on MRI images. The typical manifestation of MRI is
long T2 signals in the posterior and lateral cord of the spinal
cord, and ‘stripe like’ or ‘patchy’ lesion signals can be seen in
the sagittal position of the posterior spinal cord; the
characteristic ‘anti rabbit ear sign’, ‘inverted V-shaped sign’,
and ‘figure-eight sign’ can be found in the axial position of the
posterior part of the spinal cord (3). The neurological damage of SCD is
reversible in the early stage and the key to its cure is early
diagnosis and timely treatment; if left untreated, it causes
irreversible damage to the nervous system and disability (6). To the best of our knowledge, there
are no previous reports of brain lesions caused by SCD. However, in
the present case, symmetrical demyelinating changes were observed
in the patient's brain. The aim of the present study was to report
on the imaging manifestations of SCD brain lesions and to improve
the understanding of SCD, thereby reducing the likelihood of
misdiagnosis and incorrect treatment.
Case report
A 36-year-old female patient was admitted to the
Affiliated Hospital of Gansu University of Chinese Medicine
(Lanzhou, China) in December 2021. The main complaints were
numbness and weakness in both lower limbs for >2 months, and
reported aggravated walking difficulties for 1 week prior to
presentation. In addition, the patient appeared sluggish and had
significant memory loss. The patient reported no other specific
illnesses but had consumed a vegetarian diet for 7 years.
Furthermore, there was no personal or family history of other
diseases.
Physical examination
The patient exhibited a clear mind and indifferent
expression. Her thinking ability, comprehension, memory and
calculation skills were decreased, in addition to difficulties with
orientation. There was increased limb muscle tone, bilateral
Hoffmann's, Babinski's and Chaddock's signs were positive. The
patient did not cooperate during the depth of sensory examination
of the limbs, bilateral finger to nose and alternate motion. Both
the bilateral heel-knee-tibia test and Romberg's sign test were
negative.
Electromyography did not elicit any sensory nerve
conduction velocity of the right superficial peroneal nerve and
double posterior tibial nerves, meaning a possible lesion in the
peripheral nerve. The sensory conduction velocity of the left
superficial peroneal nerve and the potential amplitude were
decreased compared with expected values. The motor nerve
transmission velocities of the double common peroneal and tibial
nerves were normal, while the amplitude of the evoked action
potential was decreased compared with expected values. Prolonged
incubation period of the H-reflex of the left tibial nerve was
noted.
Laboratory examinations
The results of blood tests revealed the following
abnormal findings: Homocysteine (Hcy), 92.9 µmol/l (normal range:
0-15 µmol/l); and serum vitamin B12, 92.41 pg/ml (normal range:
197-771 pg/ml). Other blood test results were within the expected
ranges. Gastroscopy revealed the presence of chronic gastritis.
Imaging examinations
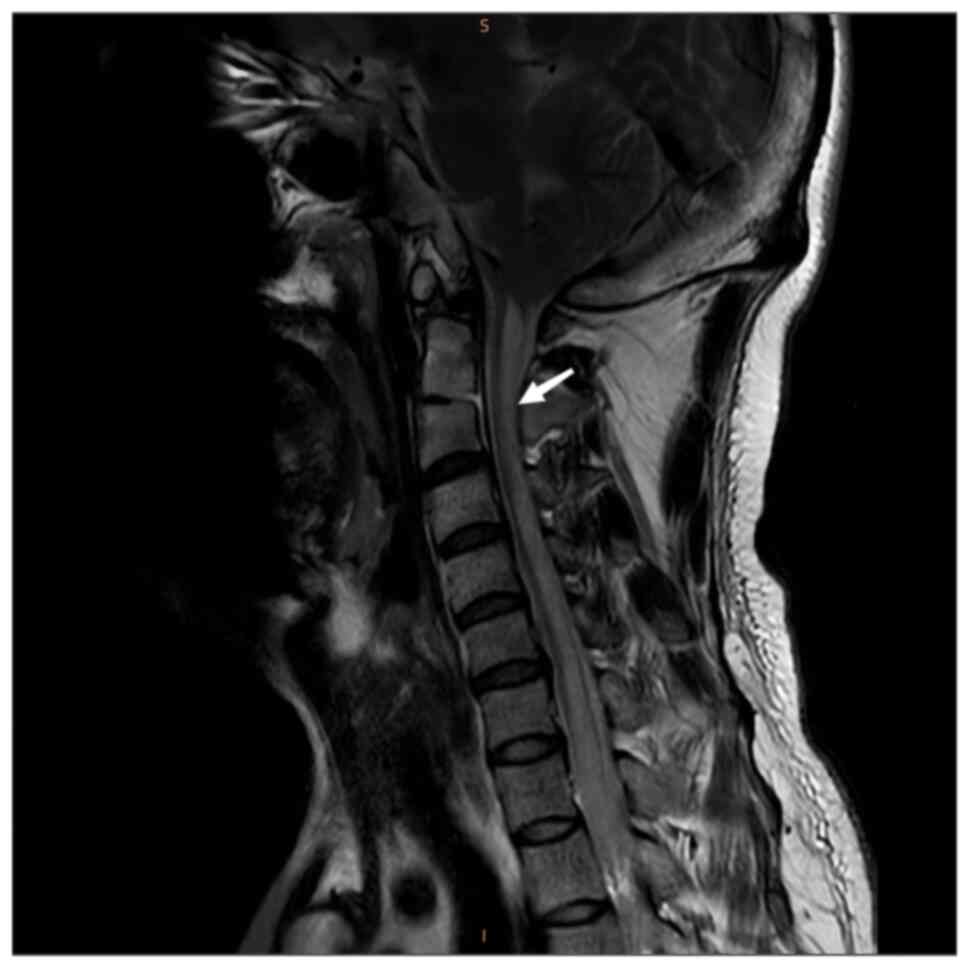
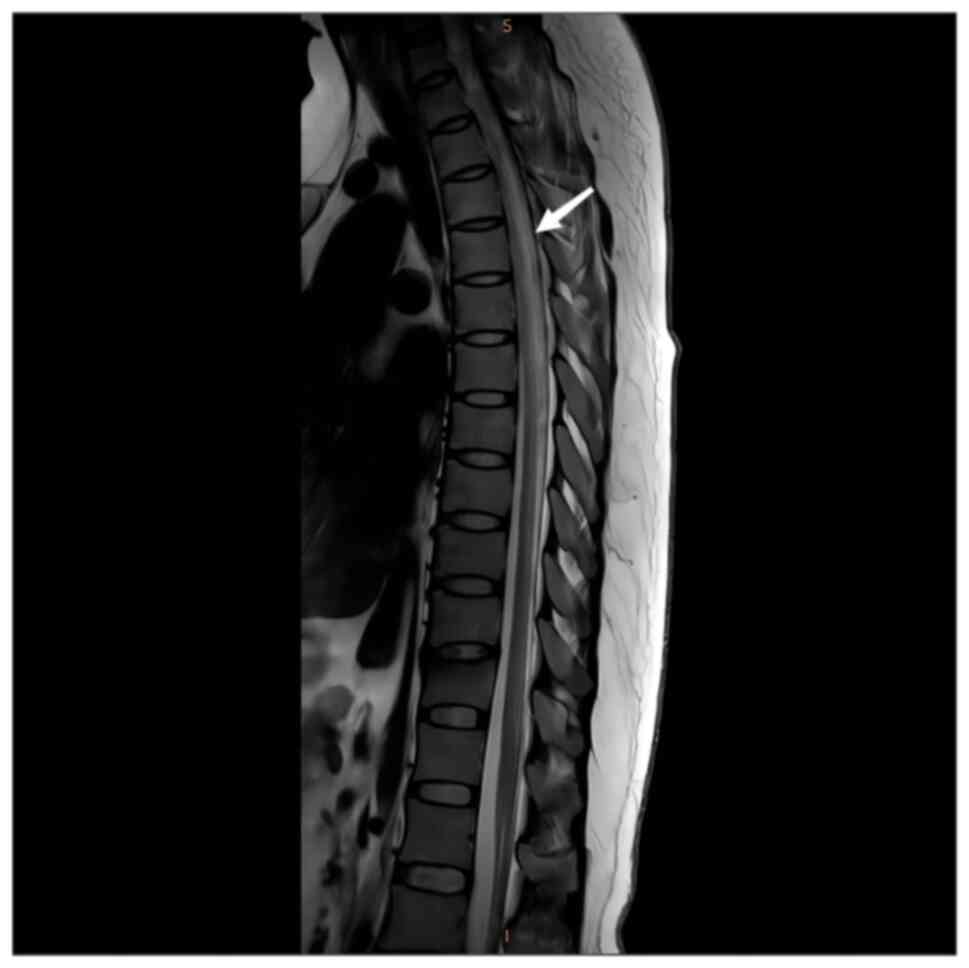
Magnetic resonance imaging (MRI) of the cervical and
thoracic spinal cord indicated an abnormal T2-weighted
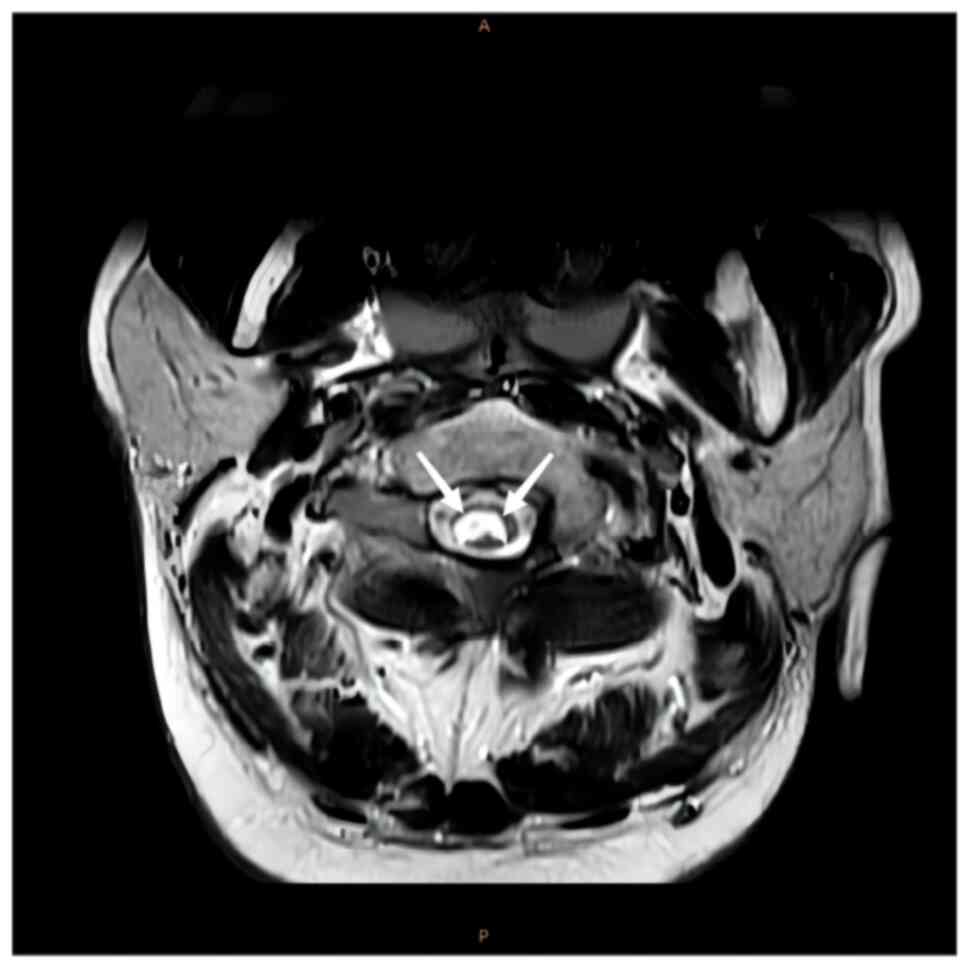
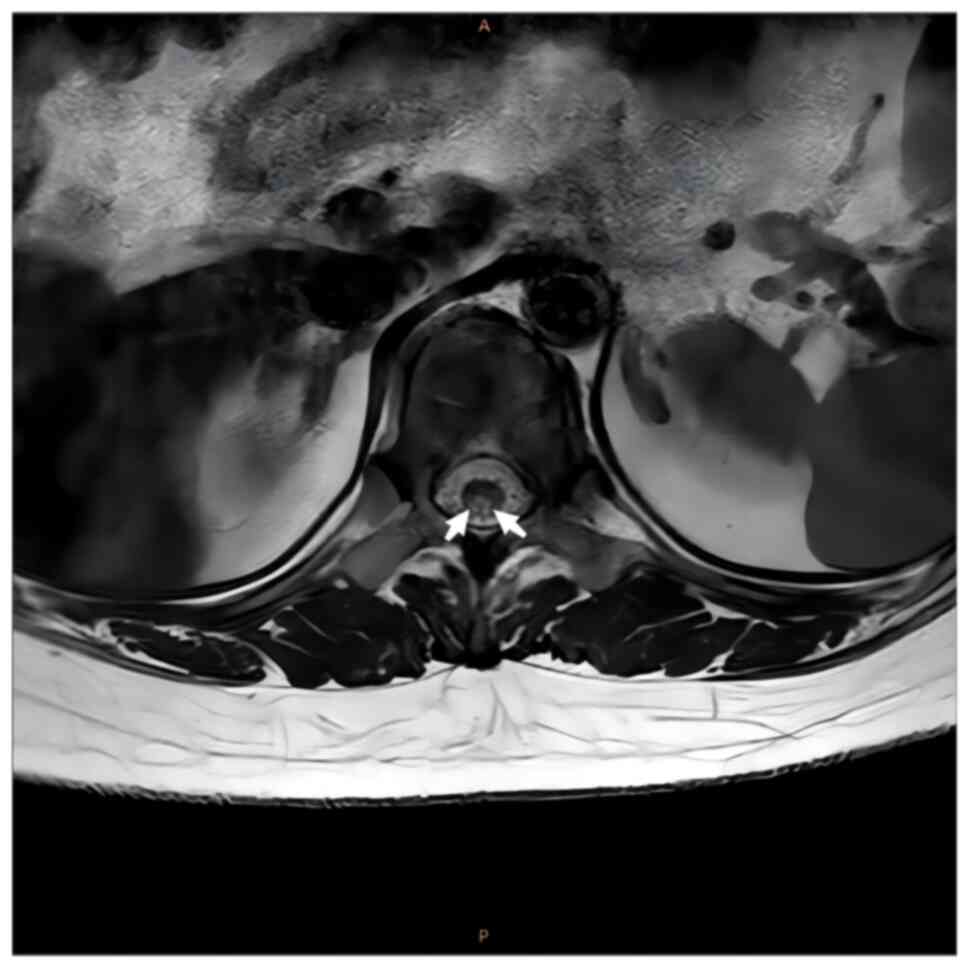
intramedullary signal with a high signal (Figs. 1 and 2). Axial images indicated that the
lesions mainly occupied the posterior portion of the spinal cord in
a ‘figure-eight sign’ (Figs. 3 and
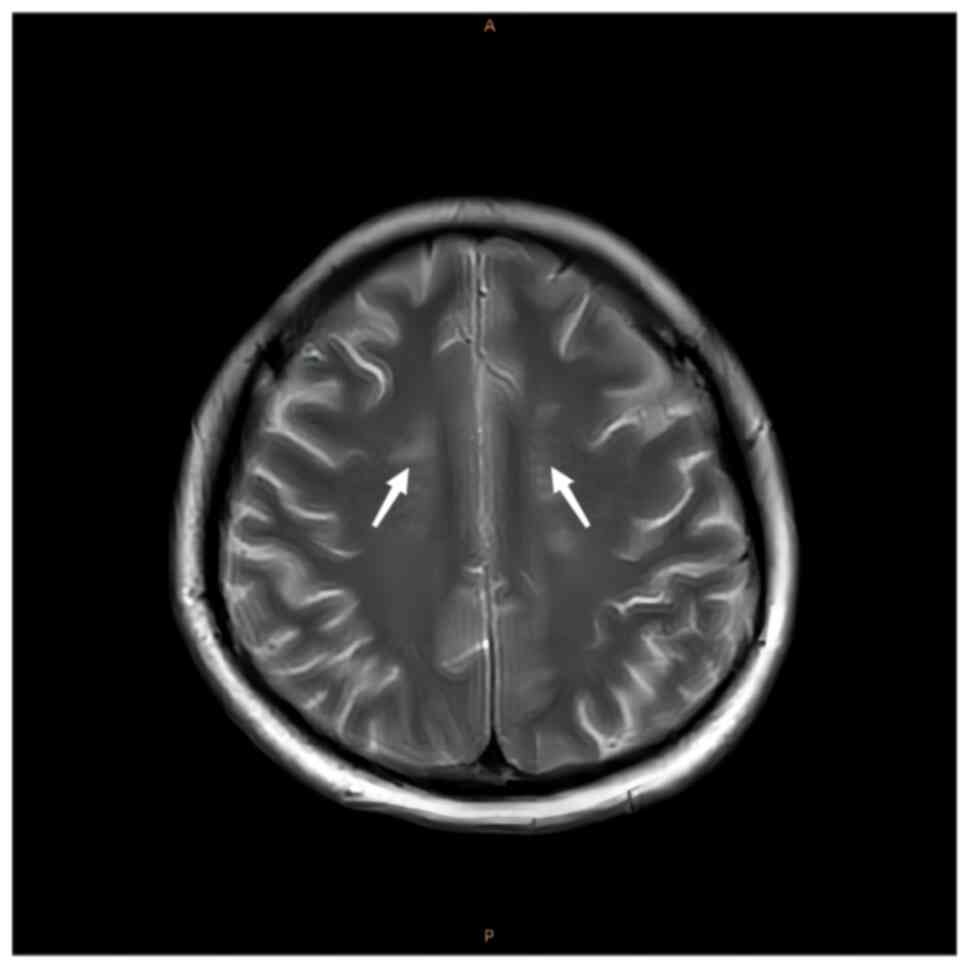
4). MRI of the brain demonstrated
spot-like and short-stripe abnormal signals that were distributed
symmetrically in the centrum semiovale, with equal or a slightly
low signal intensity on T1W1 and a slightly high signal intensity
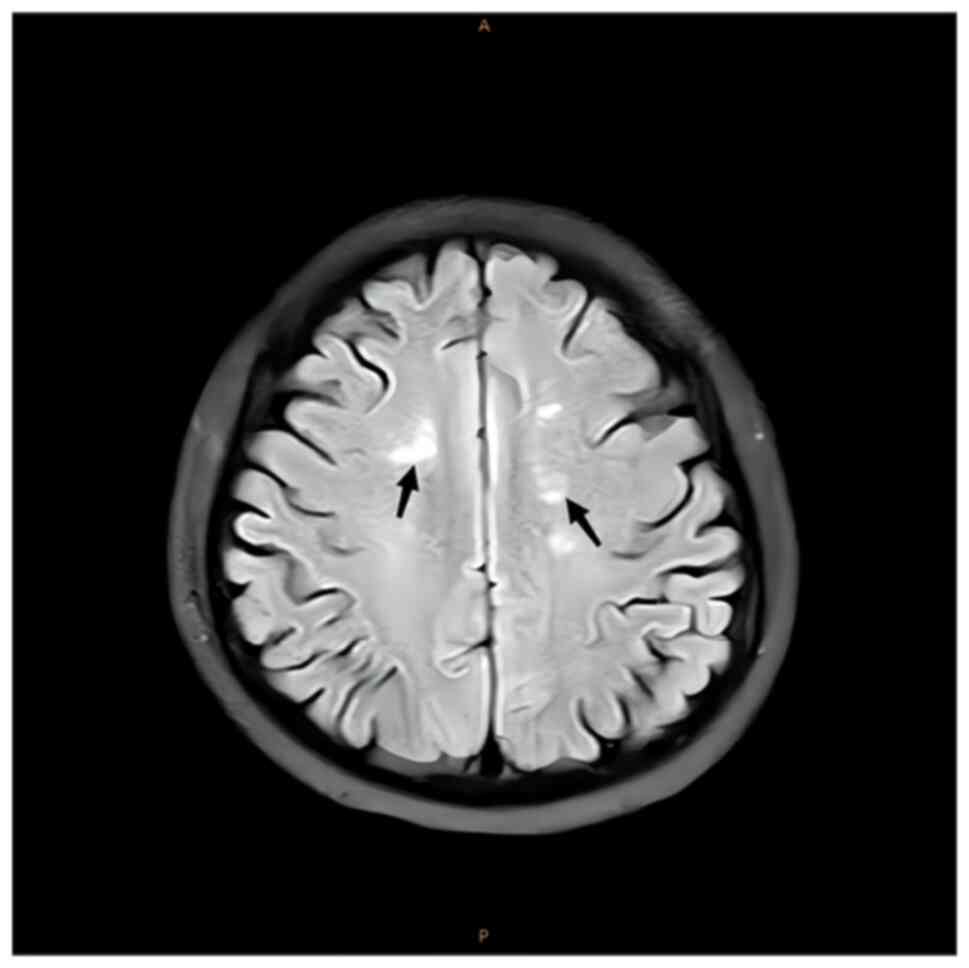
on T2W1 (Fig. 5) and
hyperintensity on fluid-attenuated inversion recovery (FLAIR;
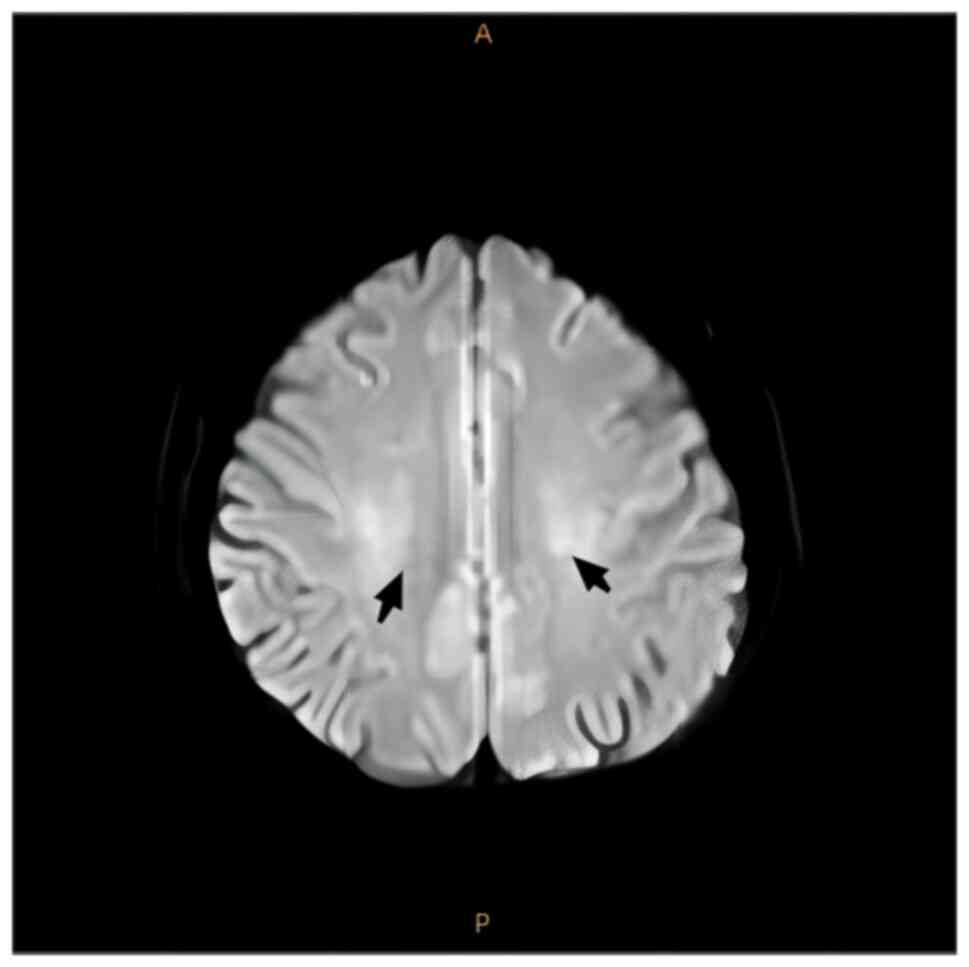
Fig. 6). Diffusion-weighted
imaging revealed low signal intensity (Fig. 7) and the long axis of the lesion
was perpendicular to the lateral ventricles. The patient was
finally diagnosed with SCD.
Treatment
The patient was treated with the following: Vitamin
B1 injection [100 mg, intramuscular injection, every day (QD)], and
intravenous mecobalamin [1 mg, intravenous injection (IV), QD] to
improve nerve function and increase vitamin B12 levels; Intravenous
sodium chloride (100 ml, IV, QD), and oral folic acid tablets (5
mg, per os, QD) cooperating with mecobalamin, to improve
symptoms.
Outcome and follow-up
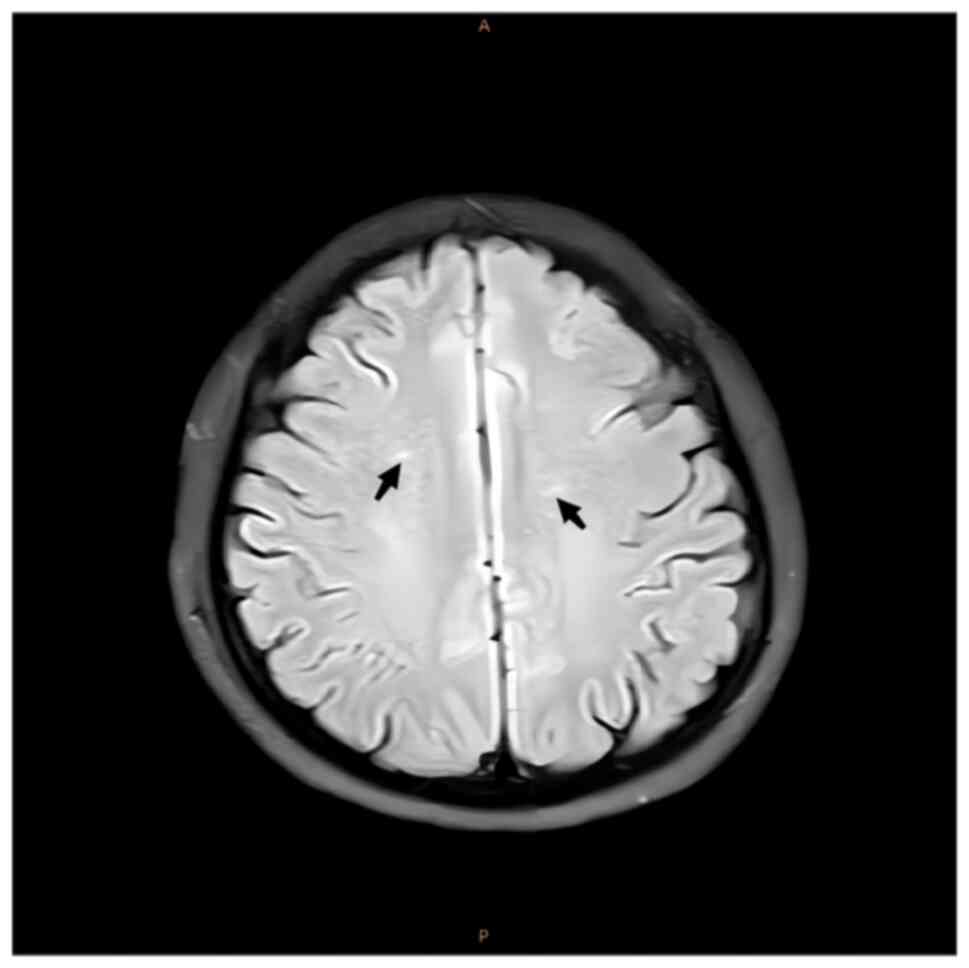
After 6 months of follow-up treatment, cranial MRI
images demonstrated that the symmetrical spot-like and short-stripe
abnormal signals in the centrum semiovale were reduced compared to
previous scans (Fig. 8). The Hcy
and serum vitamin B12 levels of the patient were also within the
normal range.
Discussion
The main cause of SCD, a neurological condition
characterised by demyelination in the posterior and lateral columns
of the cervical spine and spinal cord, is vitamin B12 deficiency
(7). The clinical signs of SCD of
the spinal cord are determined by the degree of damage to the
peripheral nerve, posterior cord and pyramidal tracts (8,9). If
the peripheral nerves are predominantly affected, SCD manifests as
decreased limb muscle tension, decreased tendon reflexes and a
flaccid paralysis. If the pyramidal tracts are predominantly
affected, SCD manifests as limb rigidity, increased muscle tension,
tendon hyperreflexia and positive pathological signs. Sphincter
dysfunction may also be present in advanced stages of the disease.
In the present study, an increase in muscle tension in the limbs
was detected and bilateral Hoffmann's, Babinski's and Chaddock's
signs were positive. SCD typically occurs in the cervical spinal
cord and the lesions usually cause a ‘figure-eight’ change in the
posterior cord.
While SCD rarely causes changes in the brain, in the
present study, the patient with SCD demonstrated abnormal changes
in the white matter of the brain. SCD of the spinal cord is a
neurological disease caused by vitamin B12 deficiency; of note,
vitamin B12 is essential for development of the central nervous
system, formation of the initial myelin sheath and maintenance of
normal neurological function (4,10).
Therefore, vitamin B12 deficiency leads to demyelination of the
external and dorsal spinal columns, which can result in progressive
weakness, sensory ataxia and paraesthesia (11). In the present study, the patient's
lesions were mainly in the bilateral centrum semiovale as there was
a high signal in this region on T2 MRI and FLAIR. In the present
study, the lesions were symmetrical and perpendicular to the long
axis of the bilateral lateral ventricles, which is a characteristic
manifestation of SCD. These lesions are either directly or
indirectly related to the vitamin B12 deficiency observed in the
patient.
Vitamin B12 deficiency mainly affects glial cells,
the myelin sheath and the interstitium, and causes an increase in
the number of positive keratinocyte acidic proteins in astrocytes
and microkeratinocytes (12-14).
Vitamin B12 is converted by absorption and transport into two
active components, methylcobalamin and adenosylcobalamin.
Methylcobalamin is a coenzyme of methionine synthase and is
involved in methionine and homocysteine metabolism. In the
methionine cycle, methylcobalamin catalyzes the conversion of
homocysteine to methionine as a coenzyme of
N5-methyltetrahydrofolate transmethylase, and when tissues are
deficient in vitamin B12, the methionine cycle fails to proceed
normally, resulting in an increase in serum homocysteine
concentration. Adenosylcobalamin deficiency leads to mitochondrial
dysfunction, reduced myelin synthesis and incorporation of abnormal
fats into neuronal lipids (15).
The typical pathological changes of SCD are multifocal
demyelination, vacuolation and axonal degeneration. The
hyperhomocysteinemia exhibited in the patient of the present study
was due to a dysfunction in methionine metabolism caused by a
vitamin B12 deficiency, which manifested as an increase in Hcy,
resulting in hyperhomocysteinemia (16). Previous studies have reported that
Hcy is highly sensitive to vitamin B12 deficiency, lack of vitamin
B12 in plasma may cause an increase in Hcy, which can serve as an
indirect diagnostic basis for vitamin B12 deficiency, and
hyperhomocysteinemia is a risk factor for SCD, and therefore may be
associated with the degree of disability caused by this disease
(17,18).
The main causes of vitamin B12 deficiency include
pernicious anaemia, vitamin B12 malabsorption [congenital or
acquired (e.g. senile gastric acid deficiency, stomach or ileal
resection, chronic pancreatic insufficiency and Crohn's disease)],
medications (e.g. colchicine and neomycin), increased demand (e.g.
hyperthyroidism, α-thalassaemia) or inadequate intake (e.g.
vegetarian diet) (19). SCD
typically develops after middle age, occurs equally between the
sexes, and can have a subacute or chronic onset and gradually
worsens (4,5).
It can be misdiagnosed as other types of
neurological demyelinating diseases, such as Guillain-Barre
syndrome, multiple sclerosis or alcoholic encephalopathy (20). The clinical treatment for SCD of
the spinal cord is vitamin B12 and folic acid supplementation,
nutritional support therapy and aggressive treatment of the primary
cause if a clear primary disease is identified (21).
SCD can result from a vegetarian diet (12,22,23).
Although, to the best of our knowledge, there have been no reports
to date regarding alterations in the brain of patients with SCD,
when patients with SCD have neurological symptoms, clinicians
should consider that this condition may be accompanied by
inflammatory demyelination of the brain. In conclusion, because of
the rarity and non-specific imaging characteristics of SCD, the
brain damage SCD causes can be difficult to diagnose. Thus, the
different symptoms, clinical course of the disease, laboratory test
results and imaging findings should be considered in order to
comprehensively diagnose SCD. A summary of the clinical and imaging
features of the present study may aid the future diagnosis of
SCD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL designed the study. XX collected relevant
clinical data of the patient. XX and HL confirm the authenticity of
all the raw data. XX and HL compared images of the patient before
and after follow-up and queried relevant clinical data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this report and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao J, Su ZY, Xu SB and Liu CC: Subacute
combined degeneration: A retrospective study of 68 cases with
short-term follow-up. Eur Neurol. 79:247–255. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu J and Jia J: Neurology. 3rd edition.
People's Health Publishing House, Beijing, pp159-163, 2018.
|
|
3
|
Zhang YF, Liu YJ, Gan FZ and Zhang Q:
Research progress on subacute combined degeneration of the spinal
cord. J Ningxia Med Col. 41:206–210. 2019.(In Chinese).
|
|
4
|
Lee WJ, Hsu HY and Wang PY: Reversible
myelopathy on magnetic resonance imaging due to cobalamin
deficiency. J Chin Med Assoc. 71:368–372. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miscusi M, Testaverde L, Rago A, Raco A
and Colonnese C: Subacute combined degeneration without nutritional
anemia. J Clin Neurosci. 19:1744–1745. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang X, Liu XL and Liu Y: Diagnostic
value of serum vitamin B12 and homocysteine detection in subacute
combined spinal cord degeneration. J Mod Lab Med. 32(4)2017.(In
Chinese).
|
|
7
|
Van Berkel B, Vandevenne J, Vangheluwe R
and Van Cauter S: Subacute combined degeneration of the cervical
and dorsal spinal cord in a 40-year-old male patient: A case
report. Radiol Case Rep. 16:13–17. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Turner MR and Talbot K: Functional vitamin
B12 deficiency. Pract Neurol. 9:37–41. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Puri V, Chaudhry N and Gulati P:
Syringomyelia-like manifestation of subacute combined degeneration.
J Clin Neurosci. 11:672–675. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen Y, Chen MY, Wu MQ, et al: Clinical
characteristics analysis of subacute combined degeneration of the
spinal cord. China Health Standards Management, (013-011),
2022.
|
|
11
|
Sun W, Li G, Lai Z, Lu Z, Lin Y, Peng J,
Huang J and Hu K: Subacute combined degeneration of the spinal cord
and hydrocephalus associated with vitamin B12 deficiency. World
Neurosurg. 128:277–283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Briani C, Dalla Torre C, Citton V, Manara
R, Pompanin S, Binotto G and Adami F: Cobalamin deficiency:
Clinical picture and radiological findings. Nutrients. 5:4521–4539.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kitamura T, Gotoh S, Takaki H, Kiyuna F,
Yoshimura S and Fujii K: A case of vitamin B12 deficiency with
involuntary movements and bilateral basal ganglia lesions. Rinsho
Shinkeigaku. 56:499–503. 2016.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
14
|
Chen H, Li H, Li Y, Jing J, Raza HK, Zhang
Z, Dong L, Ye X, Hua F and Cui G: Clinical and imaging
characteristics of subacute combined degeneration complicated with
white matter lesions in the brain: A report of five cases.
Somatosens Mot Res. 35:119–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Feng XT: The relationship between vitamin
B12 deficiency and related diseases. Chin J Pract Neurol. 17:96–99.
2014.(In Chinese).
|
|
16
|
Kaptan K and Beyan C: Vitamin B12
deficiency as a cause of hyperhomocysteinaemia. Aliment Pharmacol
Ther. 19(703)2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Green R: Indicators for assessing folate
and vitamin B-12 status and for monitoring the efficacy of
intervention strategies. Am J Clin Nutr. 94:666S–672S.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ma C, Wang Ll, Wang L, Zhao D, Xiaodan XD,
Wei ZH, Qin N, Xia F, Wang JC, Yang F, et al: The association
between serum total homocysteine and subacute combined degeneration
of spinal cord. Zhonghua Yu Fang Yi Xue Za Zhi. 55:1442–1448.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
19
|
Wang L and Song GJ: Report of a case of
subacute joint degeneration of the spinal cord. Capital City Food
Med. 29:2022.
|
|
20
|
Li HY, Chen H, Xu K, et al: Clinical
characteristics of 107 patients with subacute combined degeneration
of the spinal cord., 20, 20, Injury Funct Reconstruction 13:
456-569+66, 2018.
|
|
21
|
Wang YD, Zhang MJ and Li YX: MRI diagnosis
of subacute combined degeneration of the spinal cord. Chin J Med
Imaging. 135–366. 2002.
|
|
22
|
Hathout L and El-Saden S: Nitrous
oxide-induced B12 deficiency myelopathy: Perspectives on
the clinical biochemistry of vitamin B12. J Neurol Sci.
301:1–8. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lavoie MR, Cohen NC, Gregory TA and Weber
PV: Subacute combined degeneration: A case of pernicious anaemia
without haematological manifestations. BMJ Case Rep.
13(e234276)2020.PubMed/NCBI View Article : Google Scholar
|