Introduction
Diabetic kidney disease (DKD) is a microvascular complication resulting from diabetes mellitus and represents the most prevalent cause of kidney failure, and ~30% of DKD cases will develop into renal failure (1). In recent years, the increasing incidence of diabetes has led to a higher prevalence of DKD. In Western countries, DKD accounts for 44% of patients with end-stage renal disease, with >80% of these cases originating from type 2 diabetes (2). DKD arises from various consequences associated with aberrant glucose metabolism, hyperglycemia and altered renal hemodynamics, which can activate numerous growth factors and cytokines, ultimately contributing to the development of DKD (3,4). Currently, DKD diagnosis primarily relies on a patient's medical history of diabetes mellitus and laboratory tests, including urine glucose qualitative testing, blood glucose measurement and urine protein measurement. However, urinary albumin abnormalities are not observed in all patients with DKD at stages I and II, making it challenging to confirm the presence of any irreversible kidney damage in these cases.
Hongqi, the dried rhizome of the leguminous plant Hedysarum polybotrys Hand.-Mazz, contains numerous active components, including polysaccharides, flavonoids, trace elements, amino acids and saponins (5). Hedysarum polybotrys polysaccharide (HPS), a complex alkaloid extracted from the plant Hedysarum polybotrys, is the primary component responsible for its reported medicinal effects, such as strengthening the spleen, promoting diuresis, reducing cell swelling and purging pus. A recent study demonstrated that HPS could enhance immunity, regulate endocrine function whilst also exhibiting antioxidant and anti-aging effects, as well as lowering blood sugar and antibacterial anti-inflammatory properties (6). Astragalus and Hongqi both come from the leguminous plant Astragalus, but their species and genera are different, belonging to the same family and different genera of traditional Chinese medicinal plants. Although HPS is not currently used as a standalone treatment, Astragalus, which has a similar composition and efficacy, is a commonly used clinical drug (7). Modern pharmacological research on Astragalus identified astragaloside IV as its main active component, which exhibits anti-oxidative stress, anti-inflammatory, endothelial cell function improvement and insulin resistance amelioration properties. Astragaloside IV has been reported to effectively attenuate glomerular fibrosis and renal hypertrophy in type 2 diabetes rats induced by high-fat diet combined with low-dose streptozotocin (8). In addition, astragaloside IV has been demonstrated to improve the ultrastructure of the distal tubule and collecting duct principal cells in septic mice with cecal ligation and puncture, facilitating humoral regulation and discharge of excess interstitial fluid, ultimately providing renal protection (9). Another previous study also demonstrated that Astragalus polysaccharide could reduce blood glucose in streptozotocin-induced diabetic mice, decrease creatinine clearance in collecting duct principal cells, improve renal ultrastructure, alleviate water and sodium retention in the kidney and delay diabetic kidney disease development (10). Astragalus has been observed to slow down the progression of renal interstitial fibrosis in DKD mice by downregulating the expression of TNF-α and IL-1β in the renal interstitium (11).
Considering the anti-inflammatory effects of Astragalus, it was hypothesized that HPS may be equally protective against inflammatory injury in diabetic nephropathy. Therefore, in the present study, DKD mice were treated with HPS at different doses to observe the changes in body weight, renal function and renal histomorphology to further explore the potential therapeutic effects and possible mechanisms of HPS in DKD.
Materials and methods
Animals
A total of six db/m and 24 db/db C57BL/6J mice (leptin receptor gene-deficient mice, 4-week-old, 37±2 g, half male and half female) were acquired from the Wuhan Medical Laboratory Animal Center. After a 1-week acclimation period, the mice were divided into the following five groups: i) Control group (db/m mice; oral administration of normal saline); ii) db/db group (oral administration of normal saline); iii) HPS (Bixiang Biotechnology Co., Ltd) 30 mg/kg group (db/db mice; oral administration of HPS dissolved in normal saline); iv) HPS 60 mg/kg group; v) and glyburide (Lingrui Pharmaceutical Co., Ltd) group (db/db mice; oral administration of glyburide dissolved in 0.5% sodium carboxymethyl cellulose, 7.2 mg/kg, as positive control). All mice were housed in a specific pathogen-free environment under a 12-h light/dark cycle with free access to food and water. On day 42, mice were anesthetized by 5% isoflurane inhalation and kidney tissues were collected. Subsequently, the mice were euthanized by cervical dislocation. The present study was approved by the Animal Ethics Committee of the Shaanxi University of Chinese Medicine (approval no. SUCMDL20211101001).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from tissue samples using the TRIzol™ reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse-transcribed into single-stranded cDNA using the PrimeScript™ Reagent Kit (cat. no. RR047A; Takara Biotechnology Co., Ltd.) under the following conditions: 42˚C for 50 min, and then 95˚C for 5 min and 0˚C for 2 min. RT-qPCR was performed using the SYBR Premix Ex Taq™ Kit (cat. no. DRR420A; Takara Biotechnology Co., Ltd.) under the following conditions: 95˚C for 1 min, and then 95˚C for 20 sec, 56˚C for 10 sec and 72˚C for 15 sec for 35 cycles. Primers used in this study were designed and synthesized by Sangon Biotech Co., Ltd. The primer pair sequences were as follows: IL-6 forward, 5'-ACAACCACGGCCTTCCCTACTT-3' and reverse, 5'-CACGATTTCCCAGAGAACATGTG-3'; TNF-α forward, 5'-GCCACCACGCTCTTCTG-3' and reverse, 5'-GGTGTGGGTGAGGAGCA-3'; IL-1β forward, 5'-TCGTGCTGTCGGACCCATAT-3' and reverse, 5'-GTCGTTGCTTGGTTCTCCTTGT-3'; and GAPDH forward, 5'-AGAACATCATCCCTGCATCC-3' and reverse, 5'-GGTCCTCAGTGTAGCCCAAG-3'. The mRNA expression levels were quantified using the 2-ΔΔCt method and normalized to the internal reference gene GAPDH (12).
Western blot analysis
Total proteins were extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology). The protein content of each sample was determined by using the BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Then, equal amounts of proteins (15 µg/lane) were separated on a 12% SDS-PAGE and transferred to PVDF membranes (Bio-Rad Laboratories, Inc.). The membranes were then blocked in 5% (w/v) nonfat dry milk for 2 h at room temperature and incubated with primary antibodies overnight at 4˚C (all from Abcam) for 12 h. The primary antibodies used were as follows: GAPDH (1:3,000 dilution; cat. no. ab8245); fibronectin (1:1,500; cat. no. ab2413); α-smooth muscle actin (α-SMA; 1:1,000; cat. no. ab5694), TGF-β1 (1:1,000; cat. no. ab215715), high mobility group box 1 (HMGB1; 1:1,000; cat. no. ab18256), receptor for advanced glycation end-products (RAGE; 1:1,500; cat. no. ab37647), toll-like receptor 4 (TLR4; 1:1,000; ab22048), NF-κB (1:1,500; cat. no. ab32360) and phosphorylated (p-) NF-κB1 (phospho S337, 1:1,000; cat. no. ab194729). Subsequently, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG (1:15,000; cat. no. ab205718; Abcam) or goat anti-mouse IgG (1:5,000; cat. no. ab6789; Abcam) for 1 h at room temperature. The bands were visualized by using an ECL Plus Chemiluminescence Reagent Kit (Pierce; Thermo Fisher Scientific, Inc.) and were photographed by a chemiluminescence imaging system. Image J software (v1.8.0.112; National Institutes of Health) was used to quantify the band densities.
ELISA
The kidney tissue was collected under sterile conditions, cut into small pieces and ground it thoroughly in a mortar to obtain tissue homogenate. The secretion levels of IL-6 (cat. no. SEKM-0007), TNF-α (cat. no. SEKM-0034) and IL-1β (cat. no. SEKM0002) were measured using ELISA kits (Beijing Solarbio Science & Technology Co., Ltd.) following the manufacturer's instructions.
TUNEL staining
Kidney tissue paraffin sections (30 µm) were initially fixed in 4% paraformaldehyde for 30 min at room temperature, followed by a 30 min incubation in a 3% methanol solution at room temperature, and then dehydration was performed according to standard procedures. The sections were then treated with a TUNEL reaction mixture (Beyotime Institute of Biotechnology) for 1 h at 37˚C. Afterwards, the sections underwent 3,3'-diaminobenzidine (DAB; Beyotime Institute of Biotechnology, 25˚C for 10 min) and hematoxylin staining (30 sec at room temperature) before being observed and photographed under a light microscope (Olympus Corporation). Nuclei stained with hematoxylin appeared blue, whilst pyroptotic nuclei stained with DAB were brownish-yellow. TUNEL-positive cells, characterized by their brown staining, were counted at 400x magnification. The apoptosis rate was calculated as the percentage of TUNEL-positive cells with respect to the total number of renal tubular cells.
H&E staining
Kidney tissue sections were fixed in a 4% paraformaldehyde solution for 48 h at 4˚C, followed by decalcification using a 20% buffered EDTA solution. The dehydration was performed according to standard procedures. Subsequently, the tissues were embedded in paraffin and a 30-µm slice was prepared, which was stained with H&E (Sigma-Aldrich; Merck KGaA) and photographed under a microscope (Carl Zeiss AG).
Measurement of biochemical parameters and kidney index
The collected blood samples were centrifuged at 1,500 x g for 20 min at 4˚C, before the serum was isolated to measure the blood glucose levels. According to the instructions provided with the assay kits (Nanjing Jiancheng Bioengineering Institute), serum creatinine (Scr; cat. no. C011-2-1; blood samples), urinary albumin excretion rate (UAER; cat. no. aE038-1-1; 24-h urine sample) and β2-microglobulin (β2-MG; cat. no. H128-1-1; 24-h urine sample) levels were measured.
Statistical analysis
All statistical analyses were performed using SPSS software (version 21.0; IBM Corp). Quantitative data derived from three independent experiments were expressed as mean ± standard deviation. In animal experiments, there were 6 mice in each group. The Shapiro-Wilk test was utilized to verify if the data conformed to the normal distribution, whilst Levene's test was utilized to assess the homogeneity of the variances. Comparisons between multiple groups were performed using one-way ANOVA with Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of HPS on renal function in diabetic mice
The body weight in the DKD model group was found to be lower compared with those in the control group. However, the HPS groups exhibited heavier mice compared with those in the db/db group, with those in the high-dose HPS group showing weights more closely resembling those in the positive control drug group of glyburides (Fig. 1A). Furthermore, blood glucose concentration (Fig. 1B), serum creatinine levels (Fig. 1C), urinary albumin excretion rate (Fig. 1D) and urinary β2-MG levels (Fig. 1E) were all increased in diabetic mice compared with those in the normal control mice. However, HPS treatment significantly reversed the aforementioned increases in renal function indicators, in a dose-dependent manner (Fig. 1B-E).
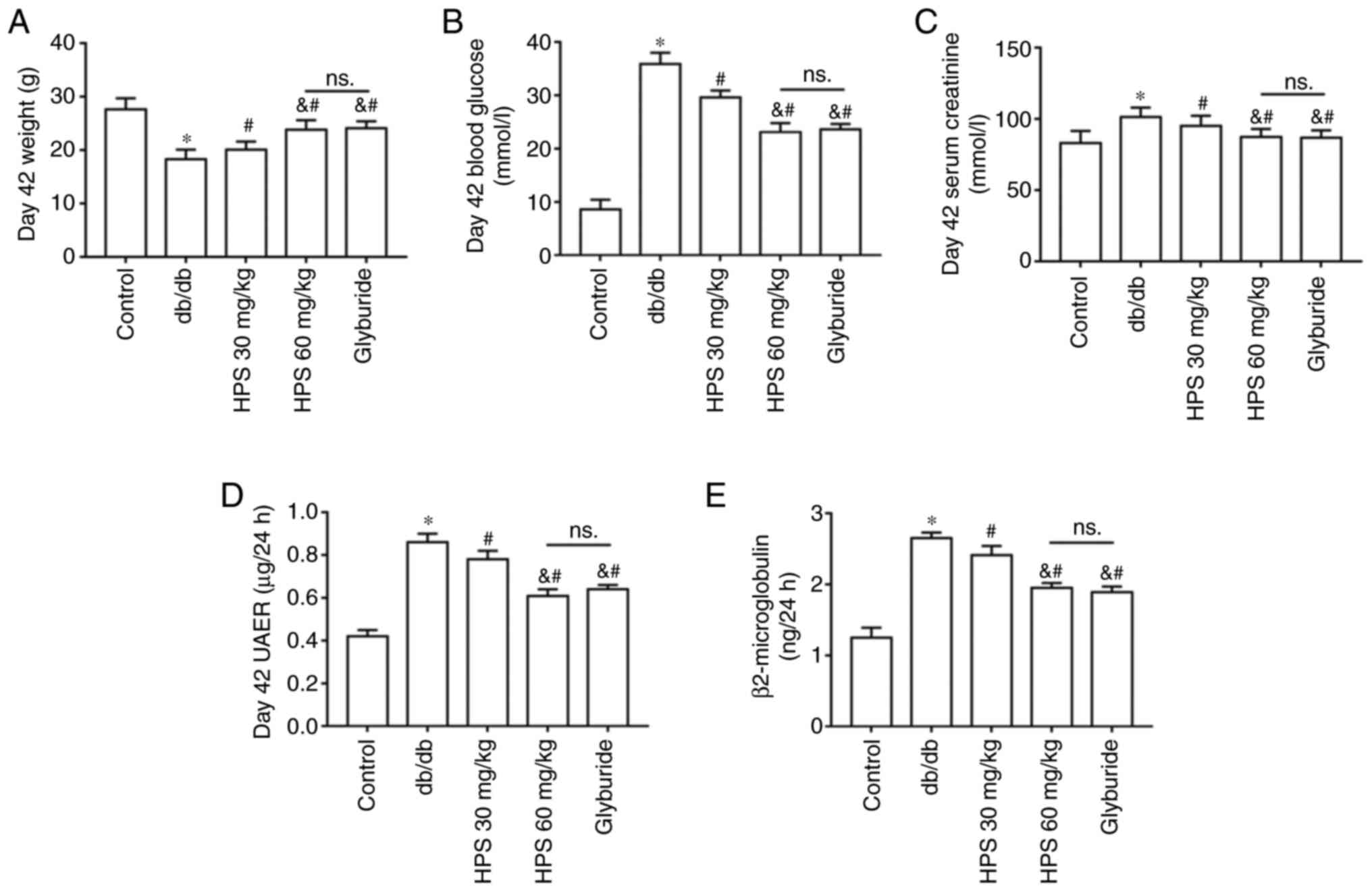 |
Figure 1
Effects of HPS on renal function in diabetic mice. Db/db mice were treated with 30 and 60 mg/kg HPS, respectively. Glyburide was used as a positive control drug. (A) Body weight measured at day 42. (B) Blood glucose concentration was measured on day 42. (C) Serum creatinine level on day 42. (D) UAER on day 42. (E) Urinary β2-microglobulin levels on day 42. *P<0.01 vs. Control. #P<0.01 vs. db/db. &P<0.01 vs. HPS 30 mg/kg. ns., not significant; HPS, Hedysarum polybotrys polysacchcaide; UAER, urinary albumin excretion rate.
|
Effects of HPS on renal apoptosis in diabetic mice
Compared with that in the control group, db/db mice exhibited increased apoptosis in the kidney tissue. HPS treatment reduced the extent of apoptosis in the kidney tissue of db/db mice. The therapeutic effect of 60 mg/kg HPS was found to be superior to that of 30 mg/kg HPS, similar to glyburide (Fig. 2).
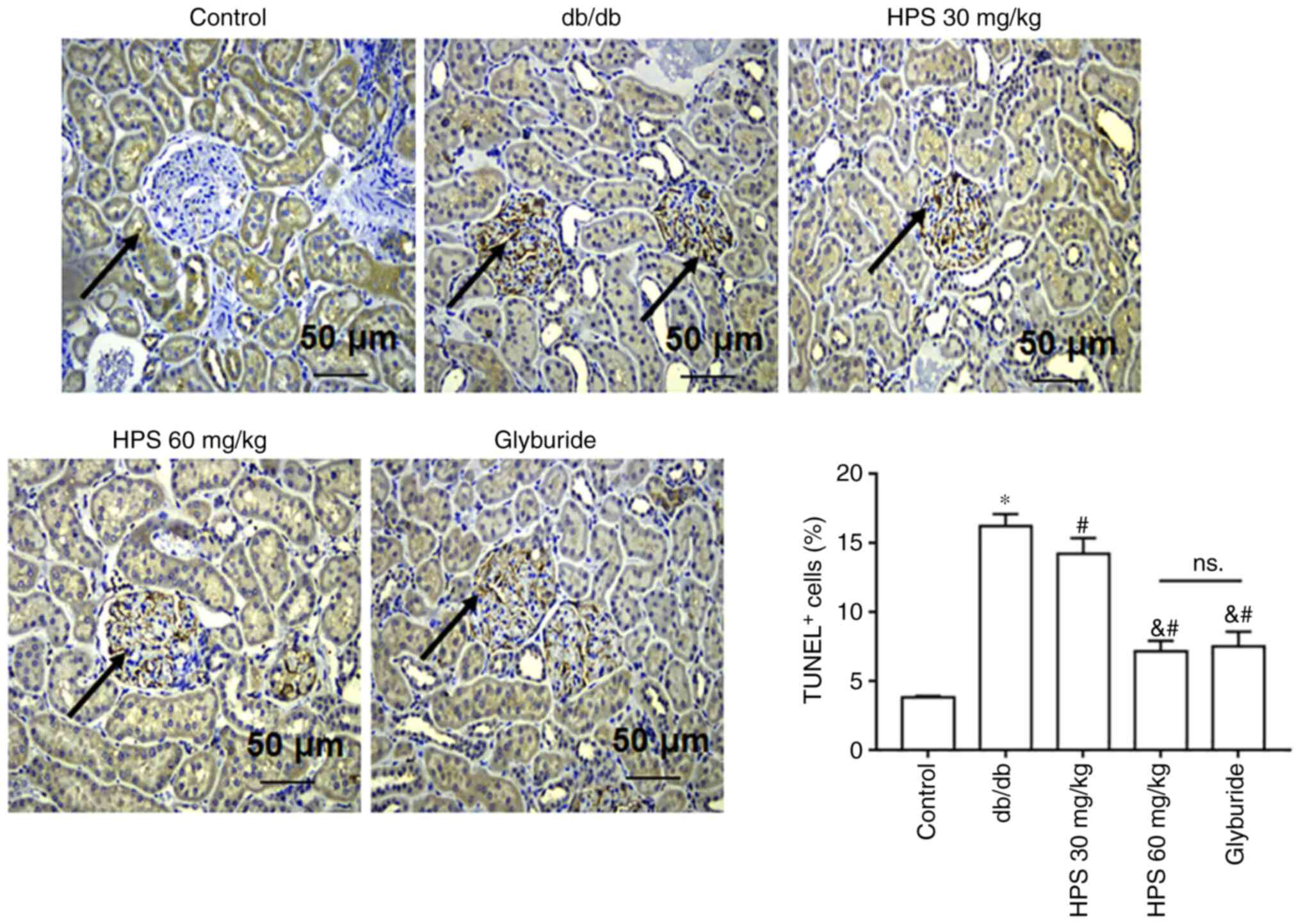 |
Figure 2
Effects of HPS on renal apoptosis in diabetic mice. Db/db mice were treated with various doses of HPS or glyburide as positive control, before apoptosis was observed after the TUNEL staining of kidney tissue sections. *P<0.01 vs. Control. #P<0.01 vs. db/db. &P<0.01 vs. HPS 30 mg/kg. Scale bar, 50 µm. Black arrows represent brown-yellow stained positive cells (apoptotic cells). ns., not significant; HPS, Hedysarum polybotrys polysaccharide; TUNEL+, TUNEL positive cells.
|
Effects of HPS on the renal pathology in diabetic mice
H&E staining results did not indicate abnormal changes in the kidney tissues of the control group, which displayed regular glomerular morphology, clear structure, thin and uniform basement membrane and no abnormal thickening. In the db/db group, the kidneys exhibited abnormal changes characterized by a thickened glomerular basement membrane, abnormally increased extracellular matrix and atrophic or hypertrophic glomeruli with indistinct borders. In the HPS (30 and 60 mg/kg) and glyburide groups, it was indicated that the corresponding treatments ameliorated these pathological changes to varying degrees, resulting in the thinning of the glomerular basement membrane and reduced mesangial matrix proliferation (Fig. 3).
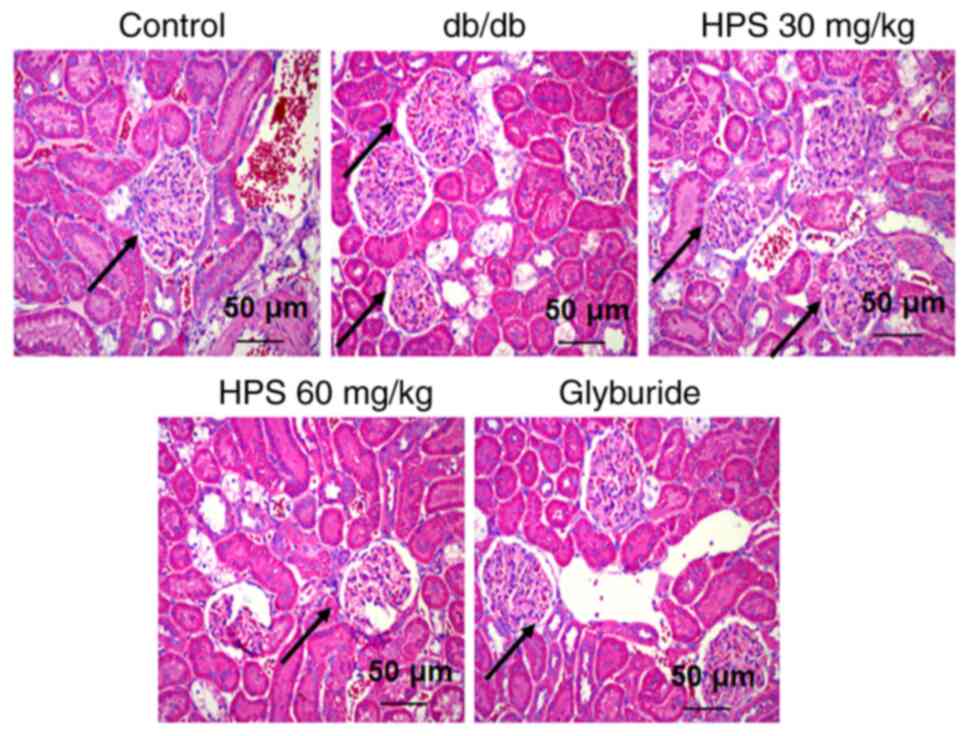 |
Figure 3
Effects of HPS on renal pathology in diabetic mice. The db/db mice were treated with various doses of HPS, with glyburide used as a positive therapeutic agent and the pathological changes in the kidneys of the mice were observed by H&E staining. Scale. bar, 50 µm. Black arrows point to glomeruli. HPS, Hedysarum polybotrys polysaccharide.
|
Effects of HPS on renal fibrosis in diabetic mice
Compared with that in the control group, the expression of fibronectin (Fig. 4A and B), α-SMA (Fig. 4A and C) and TGF-β1 (Fig. 4A and D) proteins was increased in the kidney tissues of db/db mice. HPS treatment inhibited the expression of these aforementioned fibrosis-related proteins in the kidney tissues of mice compared with those in the db/db group, in a dose-dependent manner.
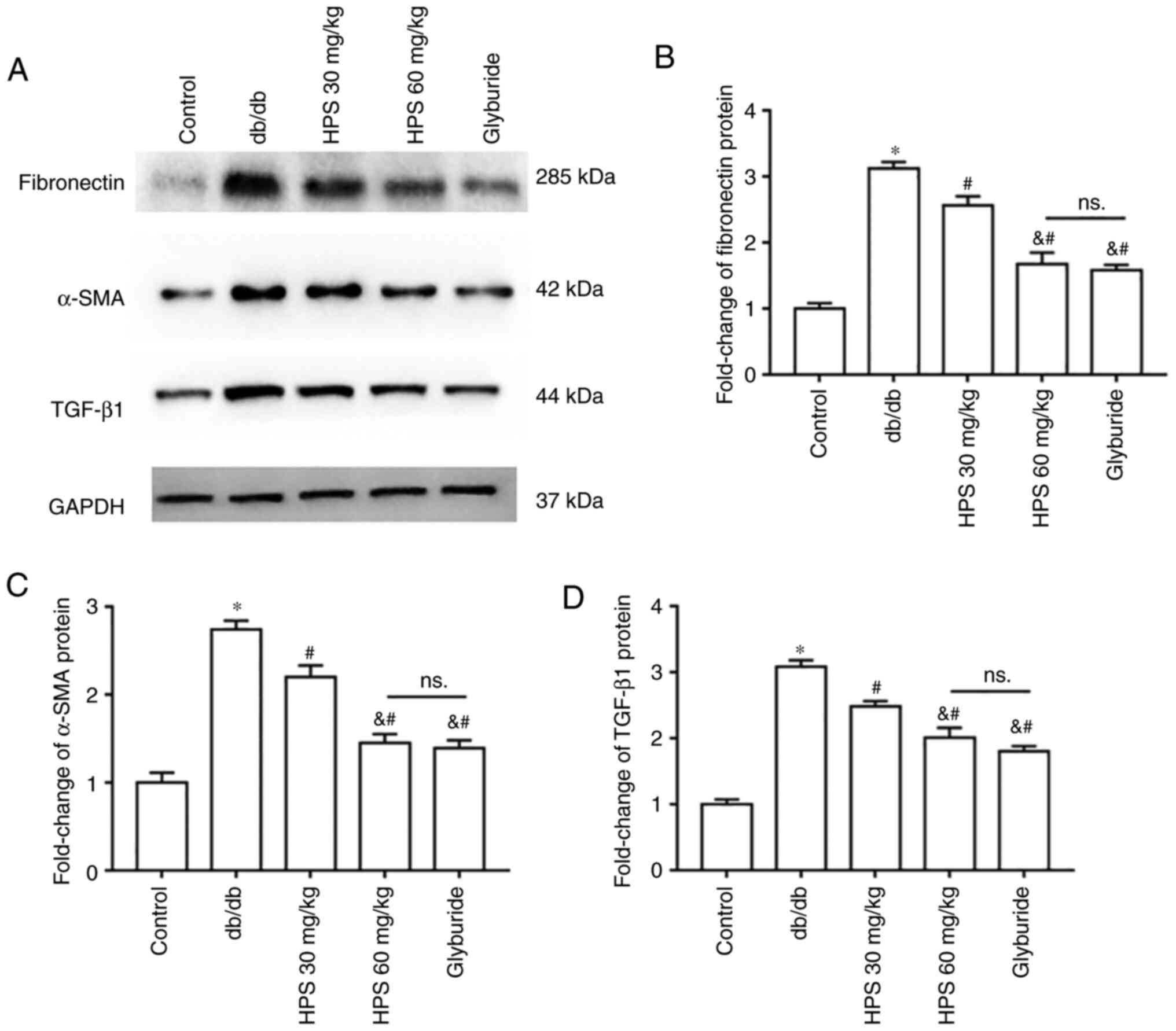 |
Figure 4
Effects of HPS on renal fibrosis in diabetic mice. (A) Representative western blots and protein level quantification of (B) Fibronectin, (C) α-smooth muscle actin and (D) TGF-β1 in kidney tissues. *P<0.01 vs. Control. #P<0.01 vs. db/db. &P<0.01 vs. HPS (30 mg/kg). NS, not significant; α-SMA, α-smooth muscle actin; HPS, Hedysarum polybotrys polysaccharide.
|
Effects of HPS on inflammatory infiltration in the kidneys of diabetic mice
The secretion and mRNA expression of inflammatory factors (Fig. 5A-F) were significantly increased in the kidney tissues of db/db mice compared with those in the control mice. However, the inflammatory factor secretion levels in the kidney tissues were decreased following HPS treatment compared with those in the db/db group, with the treatment effect of HPS 60 mg/kg proving superior to HPS 30 mg/kg.
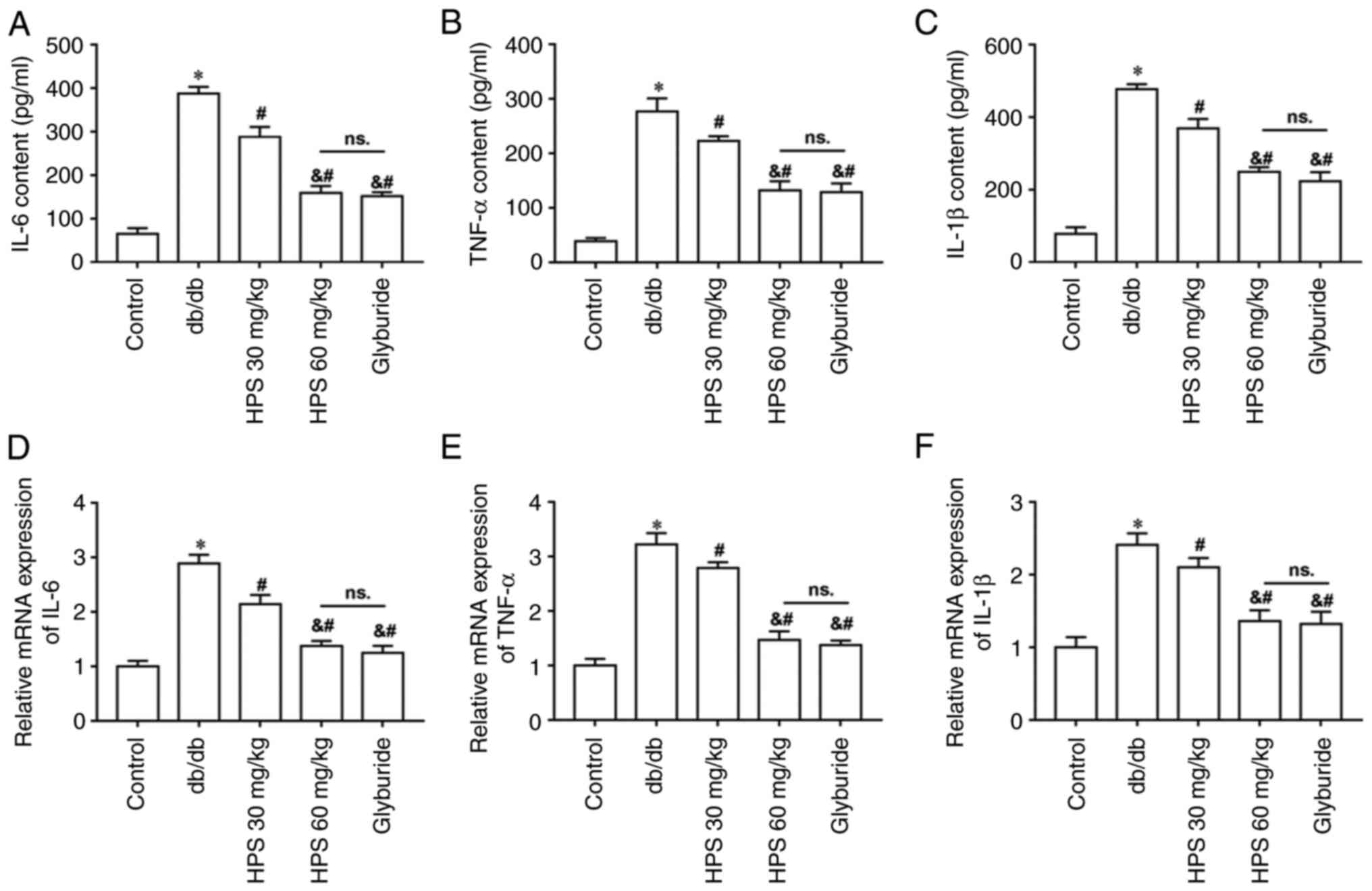 |
Figure 5
Effects of HPS on inflammatory infiltration in the kidneys of diabetic mice. Secretion levels (lower panels) of inflammatory factors (A) IL-6, (B) TNF-α and (C) IL-1β. mRNA expression levels of (D) IL-6, (E) TNF-α and (F) IL-1β. *P<0.01 vs. Control. #P<0.01 vs. db/db. &P<0.01 vs. HPS 30 mg/kg. ns., not significant; HPS, Hedysarum polybotrys polysaccharide.
|
Effects of HPS on the HMGB1/RAGE/TLR4 pathway
Western blotting results demonstrated increased protein expression of HMGB1 (Fig. 6A and B), RAGE (Fig. 6A and C) and TLR4 (Fig. 6A and D) in the kidney tissues of db/db mice, in addition to upregulated phosphorylation of NF-κB (Fig. 6A and E). HPS treatment decreased the protein expression levels of HMGB1, RAGE and TLR4, in addition to the phosphorylation of NF-κB protein, compared with those in db/db group. Treatment with the high-dose HPS appeared to be more effective.
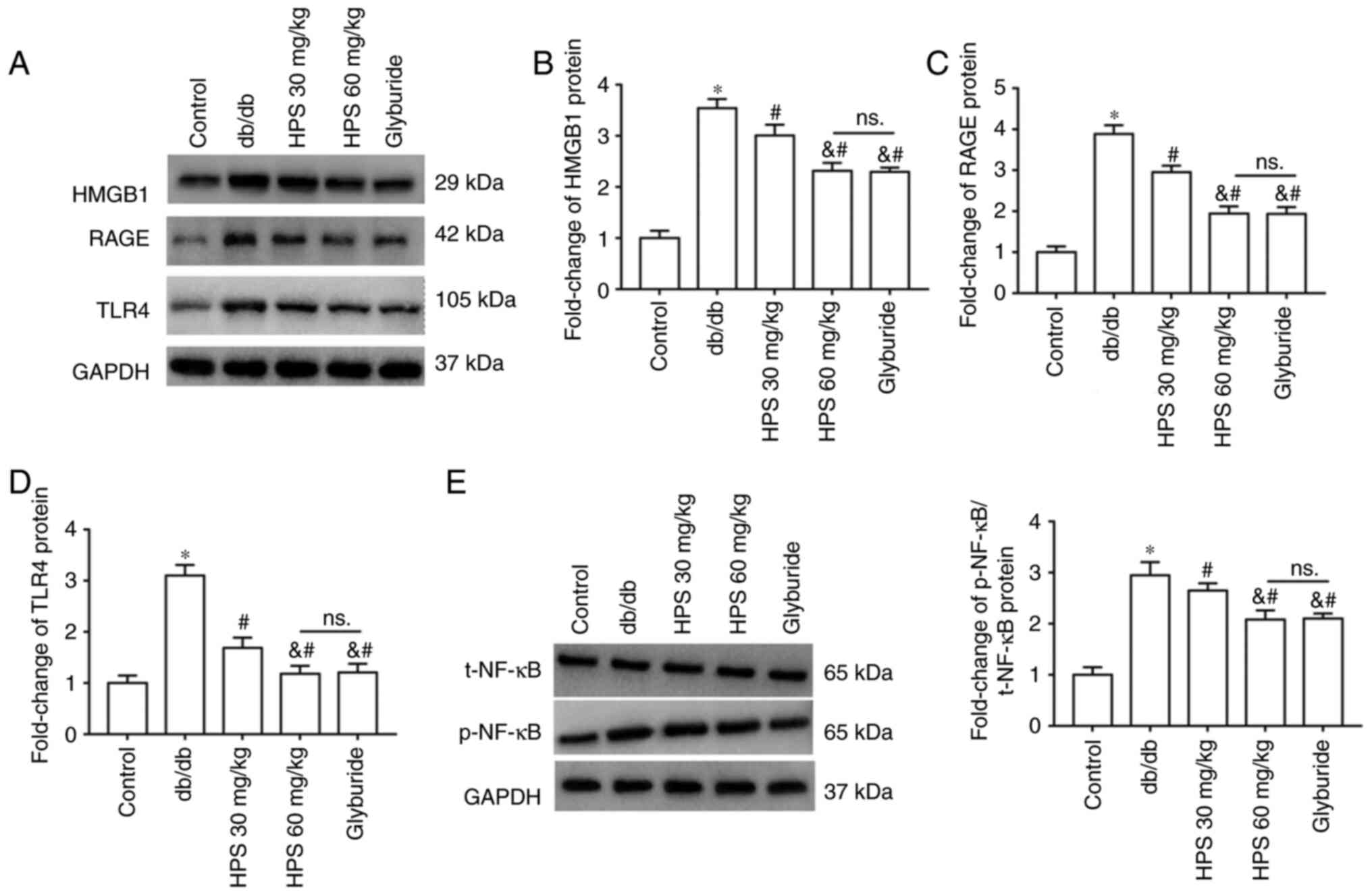 |
Figure 6
Effects of HPS on the HMGB1/RAGE/TLR4 pathway. (A and B) HMGB1, (A and C) RAGE and (A and D) TLR4 protein expression was measured by western blotting. (E) Phosphorylation of NF-κB protein. *P<0.01 vs. Control group. #P<0.01 vs. db/db. &P<0.01 vs. HPS 30 mg/kg. NS, not significant; HPS, Hedysarum polybotrys polysaccharide; HMGB1, high mobility group box 1; RAGE, receptor for advanced glycation end-products; TLR4, toll-like receptor 4.
|
Discussion
DKD is a chronic kidney disorder that is mainly caused by diabetes mellitus. Clinically, it manifests as persistently increased albuminuria excretion or a decrease in the glomerular filtration rate, ultimately progressing to end-stage renal disease (13). Previous evidence suggests that HPS can partially alleviate hyperglycemia and hyperlipidemia associated with type 2 diabetes, by promoting insulin secretion and inhibiting lipid peroxidation (14). Therefore, the present study hypothesized that HPS may also exert protective effects against diabetic nephropathy. Whilst HPS is not currently utilized as a standalone treatment in clinical settings, agents with similar composition and efficacy, such as Astragalus, are commonly used. Previous studies indicated that the active components of Astragalus can alleviate fibrosis and inflammation. A recent study demonstrated that astragaloside IV improved renal function and mitigated podocyte injury to delay the progression of diabetic nephropathy in mice by blocking NLRP3 inflammasome-mediated inflammation (15). Wang et al (16) previously found that astragaloside IV possesses anti-oxidative stress, anti-inflammatory properties and anti-epithelial-mesenchymal transition capabilities. It can inhibit the Wnt/β-catenin signaling pathway, ultimately ameliorating high glucose-induced renal injury. The present study discovered that HPS could attenuate renal inflammatory infiltration and fibrosis in diabetic mice, suggesting that HPS may have a similar effect as Astragalus in reducing DKD.
Under hyperglycemic conditions, cellular metabolism undergoes a series of changes, including increased reactive oxygen species generation and the formation of angiotensin-converting enzymes (AGEs). AGEs binding to RAGE can induce a cascade of metabolic responses, resulting in cytokine production and secretion, including the transfer of HMGB1 to the cytoplasm and extracellular space (17,18). Extracellular HMGB1, acting as a damage-associated molecular pattern, can activate the immune system by binding tightly to its high-affinity receptor RAGE to initiate MAPK and NF-κB signaling downstream (19,20). Activated NF-κB then enters the nucleus and stimulates the transcription of adhesion molecules, chemokines, inflammatory cytokines and other molecules associated with inflammation and proliferation to promote the production of inflammatory factors (IL-6, TNF-α and IL-1β), leading to the development of DKD (21).
Previous studies showed that extracellular HMGB1, as a key proinflammatory factor, can bind to RAGE/TLR on the surface of monocytes to promote the synthesis and release of proinflammatory factors, such as TNF-α, IL-1 and IL-6, with NF-κB potentially serving a direct role in this regulatory process induced by HMGB1 (22,23). In addition, HMGB1 can bind to pattern receptors TLR2 and TLR4 on the cell membrane surface to activate the NF-κB signal transduction pathway hubs, which upregulates various inflammatory factors and promotes inflammatory cascades, further accelerating the progression of DKD (24,25). Zhang (26) previously reported that HMGB1 pathway activation mediated the inflammatory response and epithelial-mesenchymal transition in HK-2 cells, hastening renal fibrosis progression in diabetic nephropathy. The present study reaffirmed that inhibiting the HMGB1 pathway was beneficial in attenuating renal inflammation and fibrosis in mice with diabetic nephropathy.
IL and TNF-α are among the most extensively studied cytokines implicated in the molecular mechanisms of inflammation in DKD. IL is a significant inflammatory factor secreted by various cells and has broad cellular effects (27). IL-1, IL-6 and IL-18 can amplify extracellular matrix deposition, alter cell permeability and stimulate the production of other inflammatory factors, serving a pivotal regulatory role in the inflammatory response of DKD (28,29). In the kidney tissue of DKD mice, the expression level of IL-1 was shown to be elevated, which could trigger the release of prostaglandin E2 and increase the permeability of glomerular endothelial cells, leading to the increase of urinary albumin, which was mediated by inducing the expression of adhesion molecules and chemokines, including cyclooxygenase-2 and prostaglandin receptor 4, which results in abnormal intraglomerular microcirculation (30). IL-1 was also shown to induce the expression of TGF-β, thereby promoting the proliferation of mesangial cells and fibroblasts, which accelerated the onset of renal fibrosis (31).
The present study showed that HPS treatment improved renal function by mitigating renal apoptosis, alleviating pathological damage to glomeruli and tubules, whilst inhibiting fibrosis and inflammatory factor secretion in renal tissue of DKD mice. These effects may be mediated through regulation of the HMGB1/RAGE/TLR4 pathway. As a limitation of the study, background interference of the TUNEL staining was present, which may have been caused by staining reagents or staining conditions, and the protocol may require optimizing to reduce background interference.
The present findings suggest that HPS could be a new alternative treatment method for DKD. Glyburide was employed as a positive control drug and the highest dose of HPS used in this study was 60 mg/kg. Nonetheless, it remains unclear whether a higher dose of HPS treatment could be surpassed by glyburide treatment, necessitating further investigation. In addition, whilst renal tissue inflammatory infiltration and fibrosis in diabetic mice were found to be alleviated by HPS through the modulation of the HMGB1/RAGE/TLR4 pathway, no pathway activators were utilized for further validation in this study, which represents a potential target for future research.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CX: Methodology, investigation, data analysis, original drafting. YC: Data analysis, original drafting. ZL: Manuscript writing, review and editing, and conception and design of the study. XF: Study conception and supervision, manuscript review and editing. CX and YC confirm the authenticity of all the raw data. All the authors have contributed to the completion of this paper, and have read and approved the final manuscript.
Ethics approval and consent to participate
All animal care and experimental procedures were approved by the Ethics Committee of The Second Affiliated Hospital of Shaanxi University of Chinese Medicine (approval no. SUCMDL20211101001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Rousseau M, Denhez B, Spino C, Lizotte F, Guay A, Côté AM, Burger D and Geraldes P: Reduction of DUSP4 contributes to podocytes oxidative stress, insulin resistance and diabetic nephropathy. Biochem Biophys Res Commun. 624:127–133. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Thipsawat S: Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. Diab Vasc Dis Res. 18(14791641211058856)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Giralt-López A, Molina-Van den Bosch M, Vergara A, García-Carro C, Seron D, Jacobs-Cachá C and Soler MJ: Revisiting experimental models of diabetic nephropathy. Int J Mol Sci. 21(3587)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tang PM, Zhang YY, Hung JS, Chung JY, Huang XR, To KF and Lan HY: DPP4/CD32b/NF-κB Circuit: A novel druggable target for inhibiting CRP-Driven diabetic nephropathy. Mol Ther. 29:365–375. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Z, Zhang L and Xu H: Effect of Astragalus polysaccharide in treatment of diabetes mellitus: A narrative review. J Tradit Chin Med. 39:133–138. 2019.PubMed/NCBI
|
|
6
|
Yang S, Wang L, Xie Z, Zeng Y, Xiong Q, Pei T, Wei D and Cheng W: The combination of salidroside and hedysari radix polysaccharide inhibits mitochondrial damage and apoptosis via the PKC/ERK Pathway. Evid Based Complement Alternat Med. 2022(9475703)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tang JL, Xin M and Zhang LC: Protective effect of Astragalus membranaceus and Astragaloside IV in sepsis-induced acute kidney injury. Aging. 14:5855–5877, 35859295. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meng X, Wei M, Wang D, Qu X, Zhang K, Zhang N and Li X: Astragalus polysaccharides protect renal function and affect the TGF-β/Smad signaling pathway in streptozotocin-induced diabetic rats. J Int Med Res. 48(300060520903612)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang JL, Xin M and Zhang LC: Protective effect of Astragalus membranaceus and Astragaloside IV in sepsis-induced acute kidney injury. Aging (Albany NY). 14:5855–5877. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Y, Tao C, Xuan C, Jiang J and Cao W: Transcriptomic analysis reveals the protection of astragaloside IV against diabetic nephropathy by modulating inflammation. Oxid Med Cell Longev. 2020(9542165)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang YY, Tan RZ, Zhang XQ, Yu Y and Yu C: Calycosin ameliorates diabetes-induced renal inflammation via the NF-κB pathway in vitro and in vivo. Med Sci Monit. 25:1671–1678. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jung CY and Yoo TH: Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab J. 46:181–197. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu F, Li X, Zhao L, Feng S and Wang C: Antidiabetic properties of purified polysaccharide from Hedysarum polybotrys. Can J Physiol Pharmacol. 88:64–72. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Feng H, Zhu X, Tang Y, Fu S, Kong B and Liu X: Astragaloside IV ameliorates diabetic nephropathy in db/db mice by inhibiting NLRP3 inflammasome-mediated inflammation. Int J Mol Med. 48(164)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang E, Wang L, Ding R, Zhai M, Ge R, Zhou P, Wang T, Fang H, Wang J and Huang J: Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharmacol Res. 157(104831)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Behl T, Sharma E, Sehgal A, Kaur I, Kumar A, Arora R, Pal G, Kakkar M, Kumar R and Bungau S: Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs. Mol Biol Rep. 48:1869–1881. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang J, Zeng T, Tian Y, Wu Y, Yu J, Pei Z and Tan L: Clinical significance of high-mobility group box-1 (HMGB1) in subjects with type 2 diabetes mellitus (T2DM) combined with chronic obstructive pulmonary disease (COPD). J Clin Lab Anal. 33(e22910)2010.
|
|
19
|
Zhou Y, Liu SX, Zhou YN, Wang J and Ji R: Research on the relationship between RAGE and its ligand HMGB1, and prognosis and pathogenesis of gastric cancer with diabetes mellitus. Eur Rev Med Pharmacol Sci. 25:1339–1350. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Santangelo C, Filardi T, Perrone G, Mariani M, Mari E, Scazzocchio B, Masella R, Brunelli R, Lenzi A, Zicari A and Morano S: Cross-talk between fetal membranes and visceral adipose tissue involves HMGB1-RAGE and VIP-VPAC2 pathways in human gestational diabetes mellitus. Acta Diabetol. 56:681–689. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang L, Zhou L, Wang X, Wang W and Wang J: Inhibition of HMGB1 involved in the protective of salidroside on liver injury in diabetes mice. Int Immunopharmacol. 89(Pt A)(106987)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang J, Wang L, Zhou J, Qin A and Chen Z: The protective effect of formononetin on cognitive impairment in streptozotocin (STZ)-induced diabetic mice. Biomed Pharmacother. 106:1250–1257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
El-Azab MF, Al-Karmalawy AA, Antar SA, Hanna PA, Tawfik KM and Hazem RM: A novel role of Nano selenium and sildenafil on streptozotocin-induced diabetic nephropathy in rats by modulation of inflammatory, oxidative, and apoptotic pathways. Life Sci. 303(120691)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen X, Ma J, Kwan T, Stribos EGD, Messchendorp AL, Loh YW, Wang X, Paul M, Cunningham EC, Habib M, et al: Blockade of HMGB1 attenuates diabetic nephropathy in mice. Sci Rep. 8(8319)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou B, Li Q, Wang J, Chen P and Jiang S: Ellagic acid attenuates streptozocin induced diabetic nephropathy via the regulation of oxidative stress and inflammatory signaling. Food Chem Toxicol. 123:16–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y: MiR-92d-3p suppresses the progression of diabetic nephropathy renal fibrosis by inhibiting the C3/HMGB1/TGF-β1 pathway. Biosci Rep. 41(BSR20203131)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C and Navarro-González JF: Inflammation in Diabetic Kidney Disease. Nephron. 143:12–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang S, Dong J and Huang L: Cytokine polymorphisms and predisposition to diabetic nephropathy: A meta-analysis. Int Arch Allergy Immunol. 182:158–165. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Araújo LS, Torquato BGS, da Silva CA, Dos Reis Monteiro MLG, Dos Santos Martins ALM, da Silva MV, Dos Reis MA and Machado JR: Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrol. 21(308)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guan Y, Davis L, Breyer MD and Hao CM: Cyclooxygenase-2 contributes to diabetic nephropathy through glomerular EP4 receptor. Prostaglandins Other Lipid Mediat. 159(106621)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang L, Wang HL, Liu TT and Lan HY: TGF-Beta as a master regulator of diabetic nephropathy. Int J Mol Sci. 22(7881)2021.PubMed/NCBI View Article : Google Scholar
|




















