Introduction
Thyroid disorders have a wide range of presentations
and can affect multiple organ systems. The thyroid gland is the
most commonly affected endocrine gland. Such disorders can be
incidentally detected through autopsy as endocrine-related
mortality can be anatomically subtle and may require histological
examination to identify and document the underlying pathologies. It
is important to understand the functioning of the thyroid gland and
its effects on vital organs when determining the cause of mortality
and the contributing factors. Therefore, a thorough examination of
the thyroid gland is recommended, particularly in cases where there
is no apparent anatomical cause of mortality, which can often
reveal the occurrence of occult pathologies (1).
The microscopic morphometric analysis of thyroid
lesions is considered to be an important factor in the diagnosis of
thyroid tumors. Computer-aided image analysis and processing
applications aim to design, validate and implement additional or
complementary tools that can automatically distinguish and classify
malignant thyroid nodules. However, the existing literature on the
applications of computerized morphometry for the histological study
of thyroid lesions is relatively limited compared with the
publications focused on microscopic thyroid cytological morphology.
This could be due to the complexity of the computerized
investigation approach, which requires the availability of
specialized software technology based on artificial intelligence
(2-5).
The anatomopathological evaluation of thyroid gland
investigations typically begins with a cytological examination,
which serves as a preoperative elective method for establishing a
definitive diagnosis of benignity or malignancy (6).
Fine needle aspiration is a widely used, an
efficient, reliable and cost-efficient method that is considered
the gold standard of thyroid investigations. Huang et al
(7) report a sensitivity of 83%
and specificity of 92%, although diagnostic discrepancies can occur
in 1-21% of cases. However, this technique has limitations in
differentiating malignant cells found in lesions that contain a
significant number of apparently benign cells, as is the case with
the cytodiagnosis of follicular neoplasms. Therefore, these smears
require further, more complex cytochemical and computerized
morphometric investigations (7-12).
Additionally, the differential histopathological
diagnosis of thyroid lesions with follicular architecture can be
challenging to interpret, particularly in the case of minimally
invasive follicular carcinomas, which can be easily confused with
follicular adenomas. Capsular or vascular invasion also represent
controversial topics when establishing a definitive diagnosis
(13).
Since it is difficult to diagnose malignancy based
on fine needle aspiration cytology in indeterminate thyroid
nodules, surgery is often recommended for all of these cases.
However, the cancer rate upon the final histology examination is
<30%. To increase the accuracy of diagnosis in such cases,
several other test methods have been proposed, including
Galectin-3-ICC, BRAF mutation analysis, Gene Expression Classifier
(GEC) alone and GEC+BRAF, mutation/fusion (M/F) panel alone, M/F
panel + miRNA GEC and M/F panel by next-generation sequencing,
FDG-PET/CT, MIBI-Scan and TSHR mRNA blood assay (14).
The computer image analysis system has shown
considerable potential for diagnostic application in diverse
histological situations. Microscopic morphometric analysis of
thyroid lesions is considered to be an adjunct in the diagnosis of
thyroid tumors.
Computerized nuclear morphometry can be an effective
and cost-efficient tool for assessing histological features. By
analyzing the size and shape of nuclei, important parameters, such
as nuclear area and nuclear perimeter, can be evaluated, which play
a crucial role in facilitating the diagnosis of various neoplasms.
Despite the potential benefits, there are only a limited number of
research studies on morphometric analyses in thyroid pathologies
and they are not yet extensively used in routine histopathological
diagnosis. However, these studies have shown that nuclear
morphometric parameters, such as perimeter, nuclear area and
coefficients of variability, can differentiate between benign and
malignant thyroid lesions (2,3,15).
The present study applied a semi-automatic system
that can be easily reproduced in any pathology laboratory and aimed
to draw attention to the use of nuclear morphometry in diagnosing
difficult thyroid lesions. Specifically, the goal was to study the
correlations of morphometry in the differential diagnosis of benign
and malignant thyroid lesions, with a focus on forms with highly
aggressive potential.
Materials and methods
Study cases
The present study presented a comprehensive
morphometric analysis of histological specimens from the thyroid
gland, obtained from autopsies conducted within the Brăila Forensic
Medicine Department (Brăila, Romania) and thyroidectomy specimens
analyzed within the Brăila Pathological Anatomy Department (Brăila,
Romania). The present study was conducted in accordance with the
Declaration of Helsinki and approved by the Institutional Review
Board (or Ethics Committee) of Braila Emergency County Hospital
(approval no. 37948/08.10.2020).
The study sample consisted of 36 cases with various
thyroid lesions diagnosed within the Pathology Department of the
Brăila County Hospital (Brăila, Romania) over a period of five
years (2017-2021). Based on the diagnostic reports, the cases were
classified as either benign (follicular adenomas; 10 cases) or
malignant lesions, including follicular carcinomas (10 cases), the
follicular variant of papillary thyroid carcinomas (10 cases), the
diffuse sclerosing variant of papillary thyroid carcinomas (4
cases) and undifferentiated carcinomas (2 cases).
The sections were reviewed by at least two
pathologists to ensure the accuracy of the diagnosis and classified
according to the WHO criteria (16).
The control group employed in the present study
comprised normal thyroid cells that were located in the immediate
vicinity of the lesion tissue, thereby minimizing the possibility
of processing artifacts and increasing the accuracy of the
measurements. To ensure the accuracy of the measurements conducted
in this study, normal thyroid cells identified on the same slide as
the thyroid lesions were utilized.
Oncocytic (Hürthle cell) cases with nuclear
abnormalities were excluded from the cytological study in order to
avoid significant errors in statistical analysis.
Each case underwent a detailed analysis of its
morphological characteristics, including the presence of
cellularity in the smear, the colloid, architectural arrangement,
as well as its nuclear features. The morphometric parameters that
characterized benign and malignant lesions were compared in this
analysis.
Histopathological technique
The 36 histological slide preparations were
processed using standard techniques in the pathological anatomy
laboratory, including fixation, paraffin embedding, sectioning and
staining with hematoxylin and eosin. The chosen tissue samples were
immersed in a 10% neutral-buffered formalin solution with a pH of
7.0 for a duration of 24 to 48 h to achieve optimal fixation. The
processing of the specimens was performed using the Myr STP 120
Carousel Tissue Processor (Especilidades Medicas MYR, S.L),
utilizing 100, 96 and 70˚ ethyl alcohol baths as dehydration
agents, while toluene was used as the clearing agent. The
processing period lasted for 17 h. Paraffin embedding was performed
using the EC 500 Paraffin Embedding Station (Especilidades Medicas
MYR, S.L),.
The embedded tissue blocks were then sectioned at a
thickness of 5 µm to enable detailed examination. These sections
were subjected to the conventional hematoxylin and eosin staining
method (at room temperature, for 70 min), a widely used technique
in histopathology, to facilitate microscopic evaluation and
visualization of cellular structures.
To standardize the morphometry procedure, two new
sections of the paraffin blocks were made by the same technician
using the same microtome. Morphometric tests were performed blindly
without knowledge of the final diagnosis. Images were captured
using a Nikon Eclipse Ci trinocular microscope system (Nikon
Corporation) equipped with an Mshot MS60 digital camera (Guangzhou
Micro-shot Technology Co., Ltd.) and a personal Lenovo computer
with a 2.2 GHz i5 CPU, 8 GB RAM and 1 TB SSD.
The Mshot Image Analysis System 1.0 software
(Guangzhou Micro-shot Technology Co., Ltd.) was used for image
capture.
Data collection
Data collection was achieved by using a manual
method for selecting representative cells and morphometric features
of interest. The morphometric analysis was performed without
knowledge of the final diagnosis, eliminating any potential bias.
Microscopic fields were digitized using a X40 high-power field
(HPF) objective, providing an appropriate degree of image
resolution for micrometric-scale measurements. The X40 HPF lens was
chosen to avoid errors in the measurement process that could have
resulted from using a lower powered lens. The measurements were
calibrated using a Mshot micrometer slide with a calibrated scale
of 0.1 mm.
A mean of 5-10 microscopic fields were analyzed on
each slide, in the selected cases, with a mean of 50
well-visualized nuclei measured on each microscopic field. Nuclei
in areas with fragmented or overlapping nuclei, as well as stromal
cell nuclei, were excluded. Ten nuclear morphometric parameters
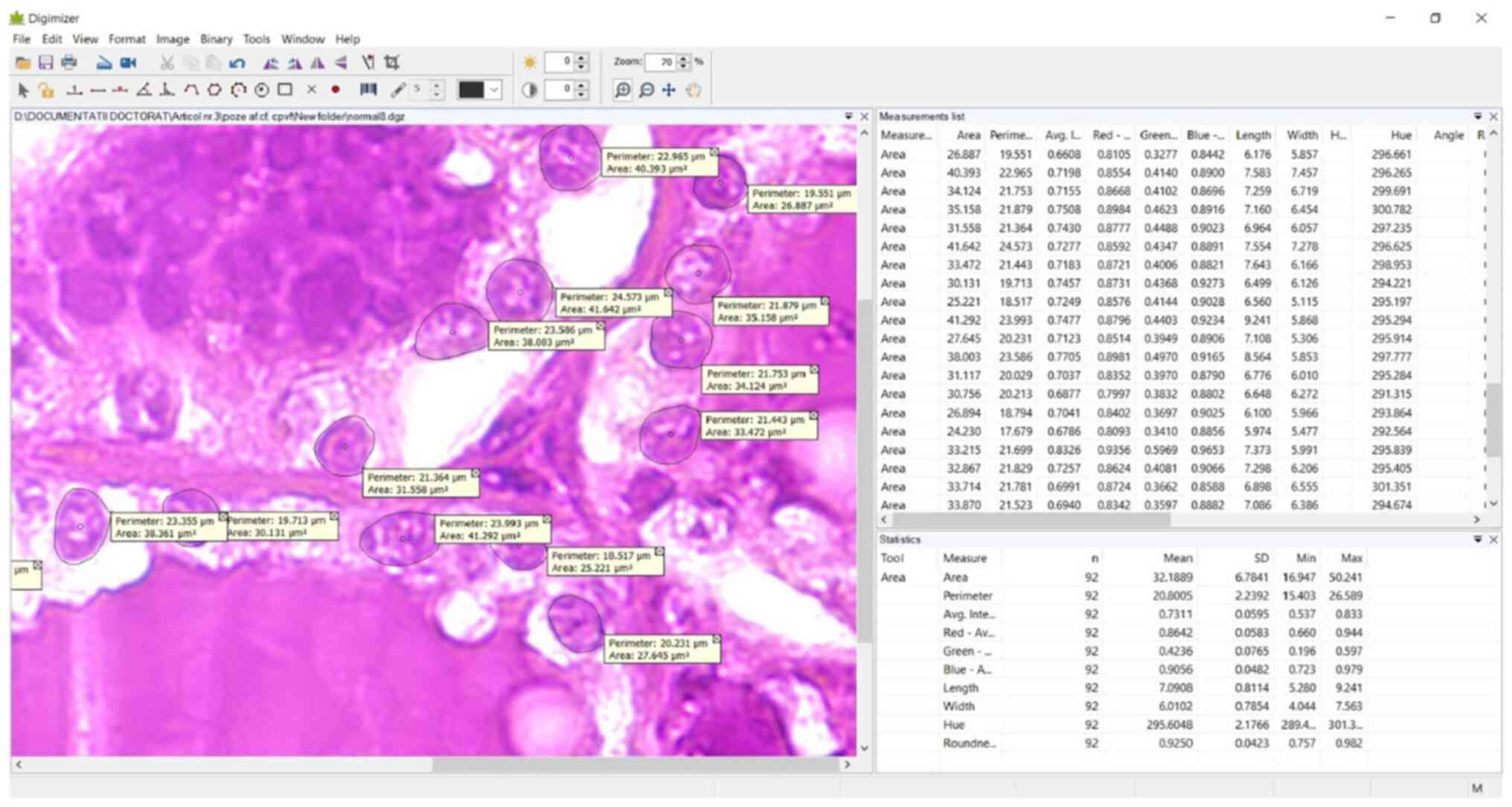
were measured using the drawing tools of the Digimizer v.5 software
(MedCalc Software Ltd.), followed by the processing of the obtained
data (Fig. 1).
Statistical analysis
The collected data were processed using Minitab v.19
(Minitab LLC). The measured parameters included the area,
perimeter, average intensity, red average intensity, green average
intensity, blue average intensity, length, width, hue and
roundness. P<0.05 was considered to indicate a statistically
significant difference. A two-way ANOVA and Bonferroni's post hoc
test were utilized to assess the mean differences among the normal
groups and the groups with follicular adenomas, follicular
carcinomas, follicular variant papillary carcinomas and
undifferentiated carcinomas Comparisons between the nuclear area
and perimeter of normal cells and between benign and malignant
cells were conducted using the paired t-test.
Results
Case group composition
The analyzed group comprised 36 selected cases,
including 10 cases of follicular adenomas, 10 cases of follicular
carcinomas, 14 cases of papillary carcinomas (10, the follicular
variant of papillary thyroid carcinomas and 4, the diffuse
sclerosing variant of papillary thyroid carcinomas), as well as two
cases of undifferentiated carcinomas. It was found that two of the
cases of papillary carcinomas were associated with autoimmune
thyroiditis.
Statistical analysis results
Aggressive papillary carcinomas showed significantly
higher nuclear parameter values than the normal thyrocyte group and
thyroid adenomas.
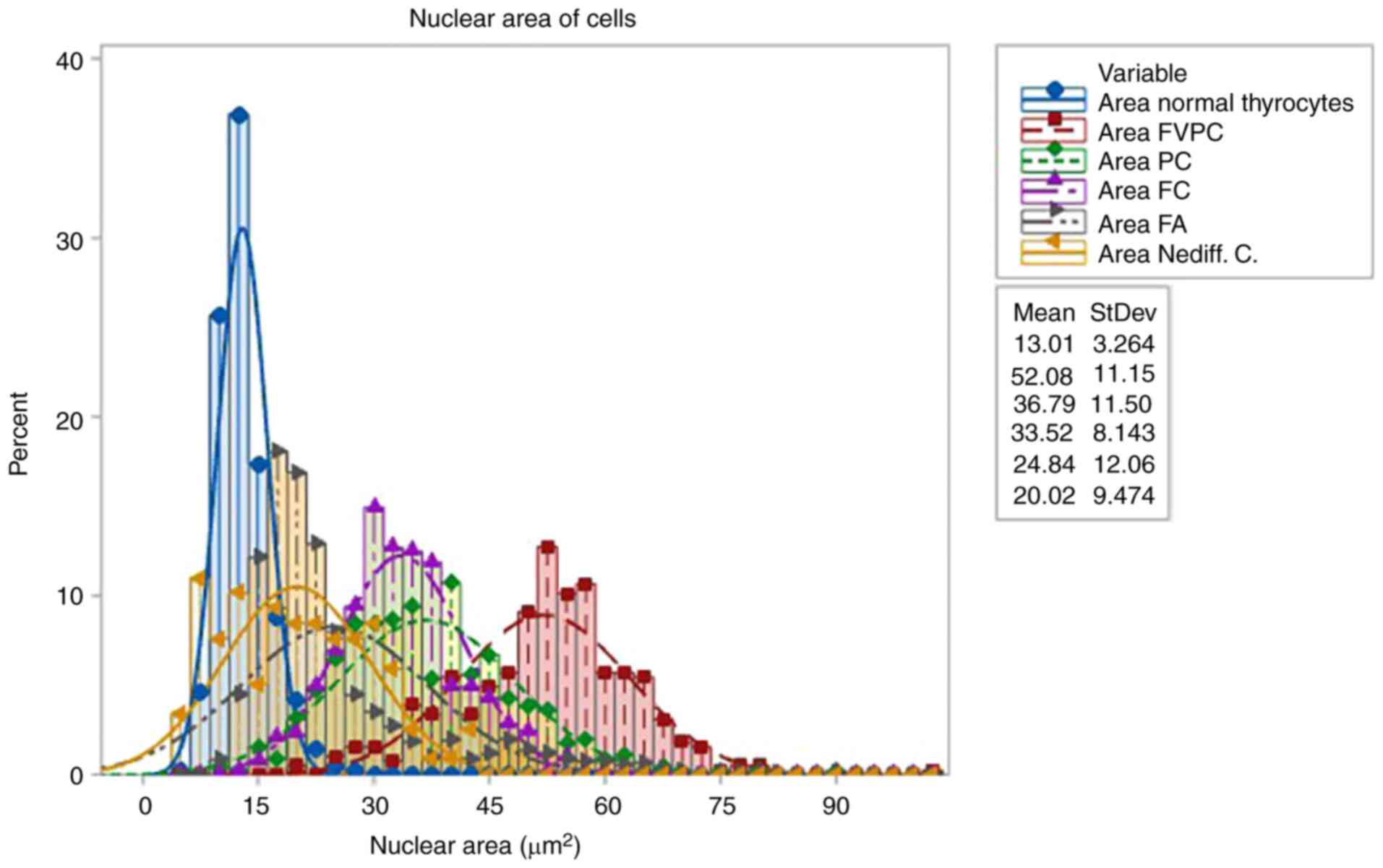
The nuclear areas of the analyzed follicular
carcinomas were larger than in the control group and larger than
the follicular adenomas. Table I
presents a synoptic presentation of the morphological and
morphometric characteristics of the cases analyzed.
 | Table IComparison of nuclear morphometric
parameters between normal cell groups and neoplastic cell groups.
Different superscript letters indicate statistical
significance. |
Table I
Comparison of nuclear morphometric
parameters between normal cell groups and neoplastic cell groups.
Different superscript letters indicate statistical
significance.
| | Normal cell
group/follicular adenoma cell group | Normal cell
group/follicular carcinoma cell group | Normal cell
group/Follicular variant papillary carcinoma cell group | Normal cell
group/undifferentiated carcinoma cell group |
|---|
| Variable, mean ±
SD | Normal
(n=2,754) | Follicular adenoma
(n=2,754) | Normal
(n=2,488) | Follicular
carcinoma n=2488 | Normal
(n=2,384) | Follicular variant
papillary carcinoma (n=2,384) | Normal (n=472) | Undifferentiated
carcinoma (n=472) |
|---|
| Area |
12.908e±3.279 |
24.841c±2.061 |
13.035e±3.688 |
33.524b±8.143 |
13.250e±3.342 |
52.081a±11.147 |
12.963e±3.153 |
20.018d±9.474 |
| Perimeter |
13.440e±1.693 | 18.097c±
3.981 |
13.560e±1.873 |
21.68b±2.616 |
13.562e±1.695 |
26.665a±2.92 |
13.425e±1.635 |
17.177d±4.338 |
| Roundness |
0.887b±0.062 |
0.911a±0.054 |
0.877b±0.073 |
0.885b±0.066 | 0.894b
±0.060 |
0.914a±0.085 |
0.893b±0.059 |
0.815c±0.131 |
| Mean intensity |
0.420d±0.048 |
0.560c±0.081 |
0.421d±0.050 |
0.633b±0.058 |
0.423d±0.048 |
0.691a±0.072 |
0.421d±0.048 |
0.387e±0.062 |
| Meanintensity,
red |
0.375d±0.050 |
0.650c±0.096 |
0.375d±0.053 |
0.746b±0.054 |
0.378d±0.051 |
0.816a±0.072 |
0.376d±0.050 |
0.373d±0.069 |
| Mean intensity,
green |
0.366b±0.058 |
0.336c±0.081 |
0.366b±0.060 |
0.394a±0.071 |
0.369b±0.058 |
0.872a±0.059 |
0.367b±0.059 |
0.349c±0.064 |
| Mean intensity,
blue |
0.520d±0.044 |
0.694c±0.090 |
0.521d±0.044 |
0.757b±0.058 |
0.522d±0.043 |
0.872a±0.059 |
0.521d±0.043 |
0.438e±0.062 |
| Hue |
242.17d±7.98 |
293.150b±5.070 |
242.54d±7.810 |
298.50a±2.74 |
242.71d±8.09 |
293.83b±2.45 |
242.39d±8.39 |
258.09c±20.12 |
The two-way ANOVA test was applied to compare means
between the normal groups and follicular adenomas group, normal
groups and follicular carcinomas group, normal groups and
follicular variant papillary carcinomas group and normal groups and
undifferentiated carcinomas group, as shown in Table I.
The nuclear morphometry parameters used in the
statistical calculations were area, perimeter, roundness, average
intensity red, average intensity green, average intensity blue and
average intensity and hue.
Table I provides
the results obtained from the post hoc analysis, specifically
Bonferroni, concerning the nuclear morphometry parameters. Notably,
means distinguished by different letters signify statistically
significant differences between the groups at a significance level
of P<0.05. The utilization of distinct letters following the
means indicates that they are statistically different from one
another.
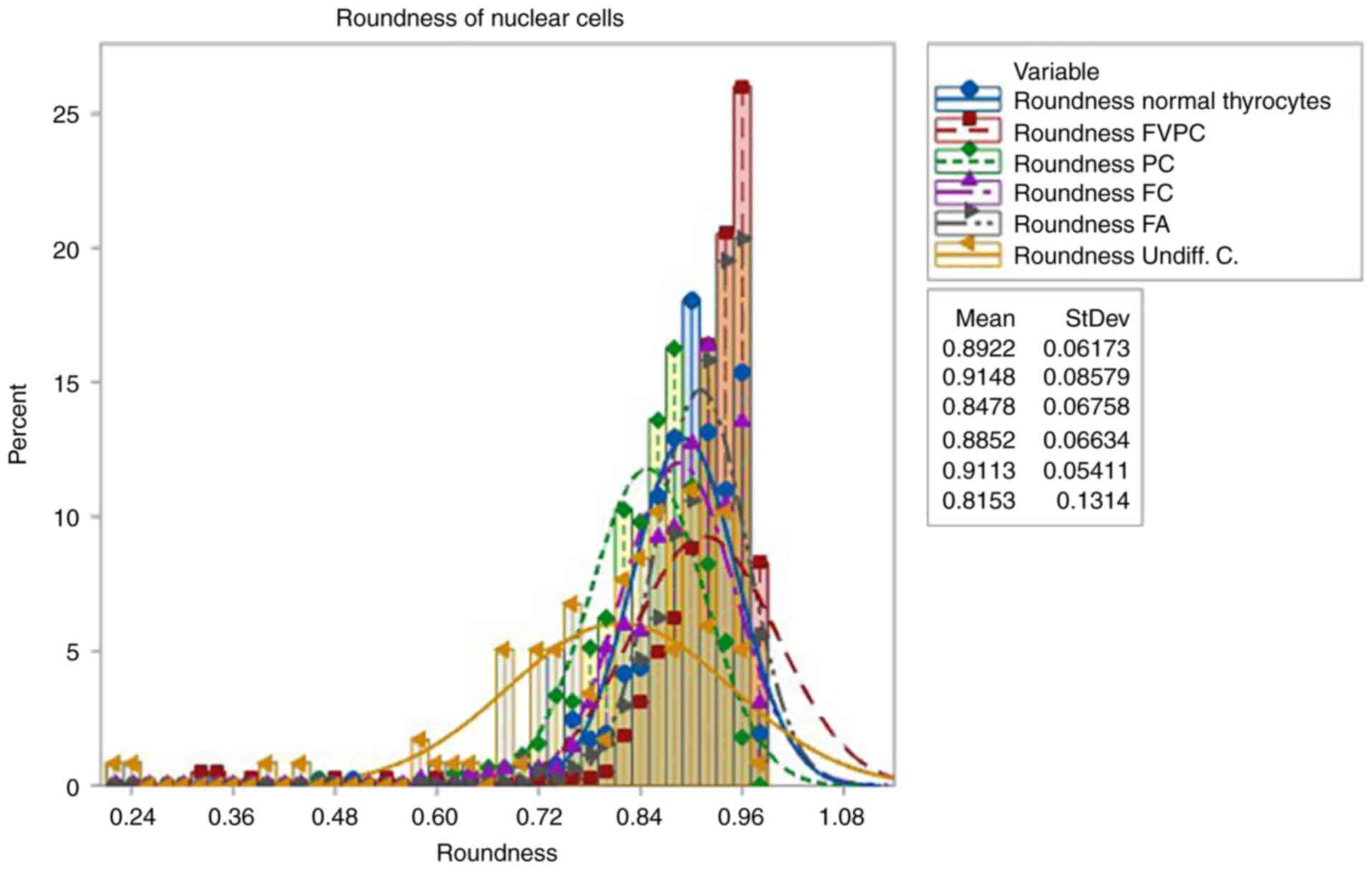
In the case of undifferentiated carcinomas, the
nuclear size morphological parameters were slightly increased as
compared with normal. For these cases, the roundness parameter was
also worth taking into account, with a significantly increased
standard deviation value of 0.131 as compared with the values of
the other cases. This parameter shows the increased degree of
irregularity of the nuclear membrane observed in undifferentiated
carcinomas. Microscope images illustrating morphometric analyses
can be found in Figs. 2, 3 and 4.
In the Digimizer software, the roundness parameter
is defined as a value between 0 and 1, where values closer to 1
indicate a more circular shape. For instance, a perfect circle has
a roundness value of 1.
Furthermore, the Digimizer software can calculate
the average intensity of an area, with an all-white area having an
average intensity of 1 and an all-black area having an average
intensity of 0.
In terms of color, the hue attribute in the
Digimizer software refers to the property that allows a color to be
categorized as a distinct color in the visible spectrum, such as
red, green, yellow, or blue. This attribute is determined by the
frequency of the corresponding wavelength and is expressed as a
number between 0 and 360, as depicted in Fig. 5.
The present study performed a statistical analysis
of the areas and perimeters within the normal group compared with
the benign group and subsequently compared the normal group with
the malignant group using paired t-tests.
Table II presents
the results of paired t-tests for the nuclear morphometry
parameters comparing the normal group with the benign group and
also for the comparison within the normal group.
 | Table IIPaired t-tests for morphometric
parameters (area and perimeter) between normal cell group and
benign cell group, and normal cell group and malignant cell
groups. |
Table II
Paired t-tests for morphometric
parameters (area and perimeter) between normal cell group and
benign cell group, and normal cell group and malignant cell
groups.
| | Normal cell
group/benign cell group | Normal cell
group/malignant cell group |
|---|
| Variable, Mean ±
SD | Normal
(n=2,754) | Benign
(n=2,754) | P-value t-test | Normal
(n=5,344) | Malignant
(n=5,344) | P-value
(t-test) |
|---|
| Area | 12.908±3.279 | 24.841±12.061 | <0.01 | 13.082±3.394 | 39.112±14.683 | <0.01 |
| Perimeter | 13.440±1.693 | 18.097±3.981 | <0.01 | 13.515±1.734 | 23.077±4.360 | <0.01 |
Statistically significant differences between groups
were identified based on the means (P<0.05).
The control group consisted of nuclei from normal
thyrocytes found in the tissue adjacent to the lesion. These nuclei
exhibited a nuclear area with a mean of 13,010 µm2.
The group with neoplastic lesions, specifically that
with follicular adenomas, showed a nuclear area with a mean of
24.841 µm2. These values were significantly higher
compared with those in the control group.
The group with follicular carcinomas had nuclear
areas with a mean of 33.626 µm2.
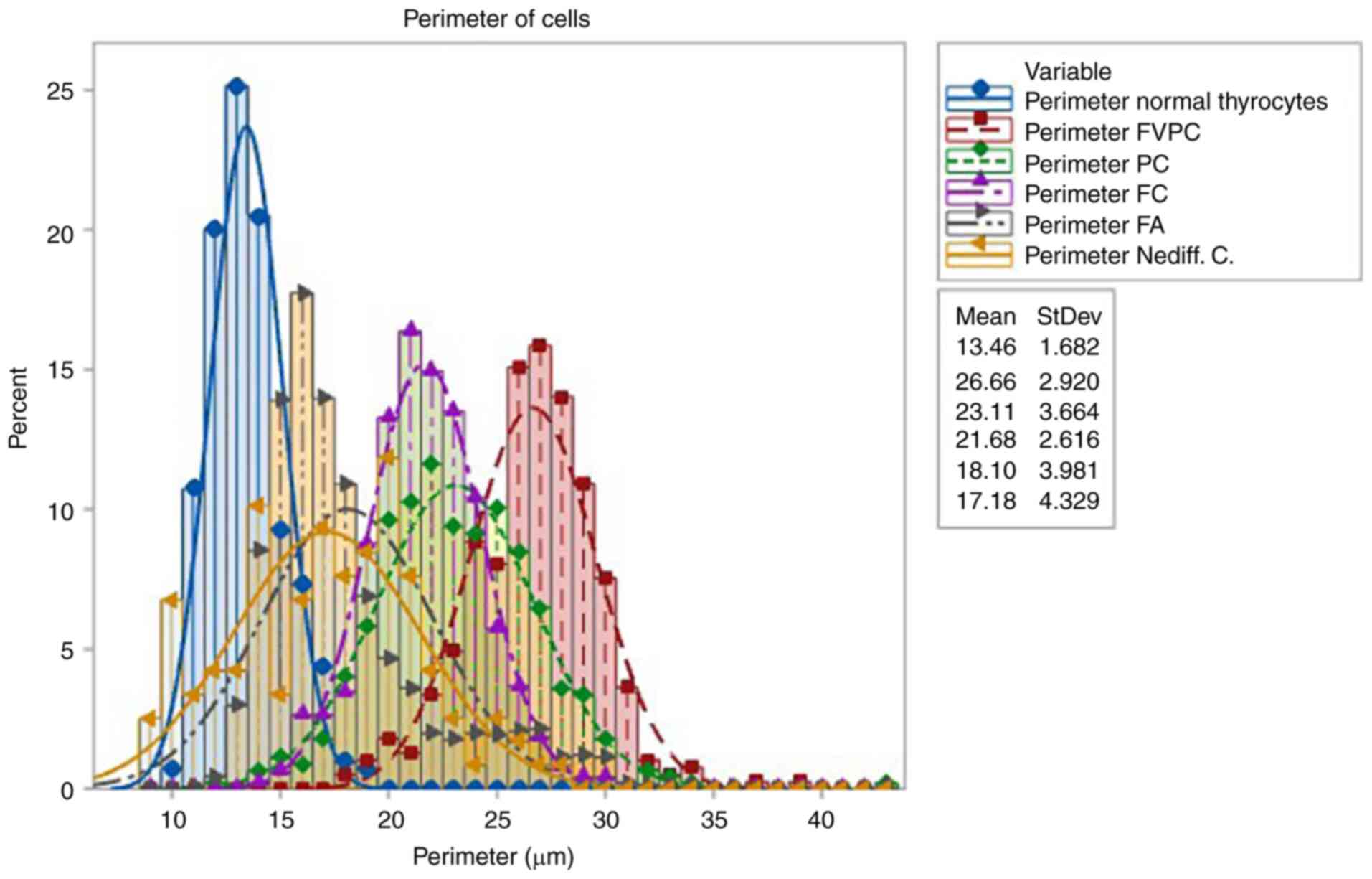
The control group consisted of nuclei from normal
thyrocytes found in the tissue adjacent to the lesion. These nuclei
exhibited a nuclear perimeter with a mean of 13,457 µm.
The group with neoplastic lesions, specifically that
with follicular adenomas, showed a nuclear area with a mean of
18.254 µm. These values were significantly higher compared with
those in the control group.
The group with follicular carcinomas had nuclear
areas with a mean of 39.112 µm.
Patient characteristics
The characteristics of the patients, such as age,
sex, tumor location (unilateral or bilateral) and tumor size, are
presented in Table III.
 | Table IIICharacteristics of patients. |
Table III
Characteristics of patients.
| Case | Histopathological
diagnosis | Age, years | Sex | Tumor location | Tumor diameter,
cm |
|---|
| 1 | Follicular
adenoma | 54 | F | Right thyroid
lobe | 1.5 |
| 2 | Follicular
adenoma | 78 | F | Right thyroid
lobe | 1.8 |
| 3 | Follicular
adenoma | 85 | M | Left thyroid
lobe | 2.0 |
| 4 | Follicular
adenoma | 57 | F | Right thyroid
lobe | 1.5 |
| 5 | Follicular
adenoma | 62 | M | Right thyroid
lobe | 2.5 |
| 6. | Follicular
adenoma | 52 | F | Left thyroid
lobe | 1.5 |
| 7 | Follicular
adenoma | 57 | F | Right thyroid
lobe | 1.5 |
| 8 | Follicular
adenoma | 87 | F | Left thyroid
lobe | 2.0 |
| 9 | Follicular
adenoma | 54 | F | Right thyroid
lobe | 1.8 |
| 10 | Follicular
adenoma | 68 | M | Left thyroid
lobe | 1.5 |
| 11 | Follicular
carcinoma | 57 | F | Right thyroid
lobe | 3.0 |
| 12 | Follicular
carcinoma | 54 | F | Left thyroid
lobe | 2.8 |
| 13 | Follicular
carcinoma | 60 | M | Left thyroid
lobe | 2.5 |
| 14 | Follicular
carcinoma | 52 | F | Left thyroid
lobe | 3.0 |
| 15 | Follicular
carcinoma | 56 | M | Left thyroid
lobe | 2.5 |
| 16 | Follicular
carcinoma | 76 | F | Left thyroid
lobe | 2.5 |
| 17 | Follicular
carcinoma | 79 | F | Right thyroid
lobe | 2.8 |
| 18 | Follicular
carcinoma | 55 | M | Right thyroid
lobe | 3.0 |
| 19 | Follicular
carcinoma | 57 | F | Right thyroid
lobe | 2.8 |
| 20 | Follicular
carcinoma | 54 | F | Right thyroid
lobe | 3.0 |
| 21 | FVPC | 42 | F | Right thyroid
lobe | 3.5 |
| 22 | FVPC | 57 | F | Left thyroid
lobe | 2.8 |
| 23. | FVPC | 38 | F | Left thyroid
lobe | 2.5 |
| 24 | FVPC | 35 | M | Left thyroid
lobe | 1.5 |
| 25 | FVPC | 54 | F | Left thyroid
lobe | 2.5 |
| 26 | FVPC | 60 | M | Right thyroid
lobe | 2.8 |
| 27 | FVPC | 39 | F | Right thyroid
lobe | 2.5 |
| 28. | FVPC | 65 | F | Right thyroid
lobe | 3.5 |
| 29 | FVPC | 67 | F | Left thyroid
lobe | 2.5 |
| 30 | FVPC | 78 | M | Left thyroid
lobe | 3.5 |
| 31 | DSV | 43 | M | Both thyroid
lobes | 5.0 |
| 32 | DSV | 32 | M | Both thyroid
lobes | 4.0 |
| 33 | DSV | 28 | F | Both thyroid
lobes | 5.8 |
| 34 | DSV | 35 | M | Both thyroid
lobes | 3.5 |
| 35 | Undiff. C. | 76 | F | Both thyroid
lobes | 6.5 |
| 36 | Undiff. C. | 83 | F | Both thyroid
lobes | 8.2 |
During the sampling process, no account was taken of
any medications used for different types of thyroid diseases. The
mean age of benign cases was of 65.4 years (range, 52-87 years),
whereas for malignant cases it was of 55.08 years (range, 28-93
years). The majority of benign cases were encountered in women,
with a male-to-female ratio of 3:7, most of the malignant cases
also being encountered in women, with a male-to-female ratio of
9:17.
The tumor size for benign cases ranged between 1.5
and 2.5 cm with a mean ± SD of 1.76±0.33, while for malignant
cases, it ranged between 2.8 and 8.2 cm with a mean ± SD of
3.4±1.45.
The present study found that the most effective
sensitivity and specificity parameters were area, perimeter and
roundness. However, the parameters average intensity, red average
intensity, green average intensity, blue average intensity, length,
width and hue could not be used because their P-value was
>0.05.
Discussion
Complementary immunohistochemistry techniques do not
provide any tested markers with a significant role in
discriminating malignant lesions from benign ones. The CK19 or
HBME-1 immunohistochemical markers are considered good predictors
of malignancy, but cannot be taken for granted as markers of
absolute certainty (17).
Additionally, Galectin3, being negative in the case of these types
of lesions, cannot provide any benefits in elucidating the
diagnosis (4,18,19).
The most common histological subtype of thyroid
papillary carcinoma is the follicular variant of papillary thyroid
carcinoma, which is found in >24% of cases (20). Diagnosis is easily established
based on the specific features of the nucleus, which have the
appearance of optically bare or frosted glass nuclei, the degree of
invasiveness and the presence of lymph node metastases. In one
third of cases, they do not show extrathyroidal infiltration, being
completely encapsulated. In some situations, these cases may
present specific nuclear changes only focally, which is a common
feature in benign lesions, or processing artifacts can be
visualized by using a hyperconcentrated formaldehyde solution
(4,21).
The histopathological diagnosis of non-encapsulated
carcinomas remains one of the most controversial surgical thyroid
pathologies. A correct diagnosis in these cases is crucial because
these tumors may have the most controversial lymphatic and distant
thyroid metastatic potential. Also, the interpretation of a
follicular adenoma as a follicular carcinoma may expose the patient
to unnecessary aggressive surgery (22).
In practice, many confusing situations arise in
which follicular lesions are difficult to assess. Subjective
interpretation of atypical cytological features is not a reliable
criterion for the malignancy of thyroid lesions because these
changes may be present in benign lesions, such as adenomatous
hyperplasia and follicular adenoma. Often, overdiagnosis of these
lesions can lead to overtreatment, which may be too aggressive
(surgical or radioactive iodine) in the case of non-recurrent
lesions that are considered malignant. Therefore, an objective
morphological analysis would be of great value in differentiating
benign from malignant lesions (4,18,23).
Computer-assisted image analysis can vary from
simple software programs, which require human intervention, to
complex applications involving artificial intelligence. The current
state of software development allows for the integration and
processing of histopathological images with automatic measurements.
This acts as an expert system that allows for the automatic
querying of a complex database, facilitating the activity of
morphopathology (2,3,7,11,13,14,17,24).
However, specialized literature rarely provides
concrete data on how authors created software applications for
automated morphometric measurements.
The aim of the computerized morphometric study of
thyroid histological smears was to identify and validate efficient
morphometric parameters that distinguish the differences between
benign and malignant epithelial cells. The most reliable parameters
among them were area, perimeter, length, width, the degree of
roundness and irregularity of the nuclear membrane. The present
study predominantly focused on follicular architecture cases as
follicular tumors cause the greatest diagnostic problems in thyroid
pathology.
The present study found that cases in the group with
malignant thyroid lesions (follicular carcinomas, papillary
carcinomas, follicular versions, papillary carcinomas and the
diffuse sclerosing variant, as well as undifferentiated carcinomas)
had significantly higher values of nuclear morphometric parameters
when compared with benign groups. The highest values were found in
the follicular variant of papillary thyroid carcinomas.
Undifferentiated carcinomas also had a higher value of the
roundness parameter, which measures the nuclear membrane
irregularity degree. It was concluded that the morphometric
parameters that characterize benign and malignant lesions in the
smears that were evaluated in the present study can be considered
within the normal data presented in specialized literature
(11).
Computerized morphometry is a useful and
cost-effective tool that can be implemented in the
anatomopathological management of thyroid gland investigations due
to its simplicity of execution and safety (5). Nuclear morphological changes define
the characteristics of a malignant tumor. The most important
characteristics are enlarged nuclei, hyperchromasia, irregular,
pulverized chromatin and prominent nucleoli.
In some cases, these characteristics may be
interpreted subjectively. Computerized morphometry can be useful in
these cases. Additionally, computerized morphometry appears to be
an inexpensive and reproducible tool for evaluating the
histological characteristics of various lesions (25,26).
Morphometric nuclear parameters such as the nuclear
perimeter and the nuclear area can be useful for differentiating
between different thyroid lesions (27,28).
Although the use of morphometric analyses in thyroid
cytology is not a common practice, it appears to only be used in
studies conducted in the case of scientific research, not for
clinical use (4).
Tseleni-Balafouta et al (16) showed in their study that the
nuclear area is larger in follicular carcinoma compared with the
nuclear area found in adenomatous lesions and in thyroid
hyperplastic nodules. They found significantly higher morphometric
parameters in thyroid papillary carcinomas as compared with thyroid
follicular carcinomas, similar to the measurements found in the
present study.
Wright et al (29) discovered altered morphological
parameters in Giemsa-stained preparations when measuring papillary
and follicular carcinomas as compared with benign thyroid
lesions.
Khatri et al (3) found significant differences in
nuclear morphological parameters between benign and malignant
thyroid lesions, concluding that cytomorphological features of
thyroid lesions can be quantitatively estimated by performing
nuclear measurements.
Atypical lesions of undetermined significance,
including heterogeneous entities, are classified into subgroups
based on cytologic and architectural atypia.
Those with cytological atypia are associated with an
increased risk of malignancies (30).
Mathur et al (30) suggest that in cases with lesions of
undetermined significance, the encountered atypical nuclear
characteristics should be thoroughly evaluated to distinguish
between benign and malignant lesions, as the follow-up and
treatment procedures are different.
Eliminating the category of undetermined
significance lesions leads to a significant decrease in sensitivity
regarding the detection of thyroid lesions.
Sensitivity to detecting papillary carcinoma
decreases from 100 to 7% when undetermined significance cases are
not investigated (31).
Failing to investigate undetermined significance
cases can result in an increase in false-negative and
false-positive diagnoses, with ≤53% of neoplastic thyroid lesions
and 37% of thyroid papillary carcinomas being misdiagnosed as
benign. On the other hand, 38% of benign lesions may be
misdiagnosed as follicular neoplasms or suspected follicular
neoplasia. A thorough examination of the unknown significance
lesion category is necessary in order to avoid misdiagnosis.
Nuclear morphometry can be used to quantify a wide range of
parameters that characterize nuclear size and shape and can
facilitate a more precise diagnosis of thyroid pathology. Studies
have suggested that nuclear morphometric parameters, such as
nuclear area and perimeter, improve diagnosis, treatment and
outcomes in a variety of neoplasms, including mammary gland
carcinoma, bladder carcinoma, skin lymphomas and soft tissue
sarcomas (32-35).
Morphometric analysis is reproducible and inexpensive and objective
information obtained through quantification of the characteristics
of nuclear morphological parameters can be useful in classifying
different lesions (2,16,36,37).
Shih et al (36) and Aiad et al (4) performed retrospective studies on
thyroid cytological preparations, performing multivariate analyses
of computerized morphometry correlated with clinical data.
Parameters related to the size and shape of nuclei had
significantly increased values in the follicular variant of the
papillary thyroid carcinoma as compared with the follicular
neoplasm. Wright et al (29) found significant differences in the
values obtained in the measurements of nuclear areas and perimeters
between cases with multinodular goiter as compared with follicular
adenomas, as well as follicular and papillary carcinomas. Nuclear
morphometry is useful for distinguishing malignant from benign
lesions. The limitations of the present study are the relatively
small sample of patients and the fact that it was conducted only in
one hospital.
The present study employed a semi-automatic system
of nuclear morphometry, which can be easily replicated in any
laboratory setting. This technique offers substantial advantages in
terms of cost-effectiveness and time efficiency for each case. In
comparison to laborious immunohistochemical methods that require
extended work duration and significantly higher costs, our approach
stands out due to its minimal expenses and reduced diagnostic time.
The present study cannot be considered an absolute diagnostic
criterion as it relies solely on statistical differences. The
utilization of morphometric analysis in thyroid pathology is still
limited in clinical research. Computerized morphometry could
positively effect diagnostic accuracy by allowing better clinical
and imaging correlation.
In conclusion, the use of morphometry in cytological
smears for suspected malignant follicular lesions leads to
increased accuracy in clearly establishing suspicious malignant
diagnoses for follicular lesions.
Parameters such as area, perimeter and intensity
have good sensitivity and specificity for detecting malignancy.
Nuclear morphometry provides an unbiased point of
view that increases diagnosis accuracy and differentiation between
lesions bordering on malignancy and benignity. Computerized
morphometry can positively influence diagnostic accuracy, allowing
a better correlation with clinical and imaging data.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IM and MC were responsible for conceiving the
current study. IM, ZC and MC performed validation of data and
formal analysis. IM and MC confirm the authenticity of all the raw
data. BS, AC and DS made substantial contributions to conception
and design. IM, BS, AC, DS, ZC and MC reviewed and edited the
manuscript and supervised the current study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and approved by the Institutional
Review Board/Ethics Committee of Braila Emergency County Hospital
(approval no. 37948/08.10.2020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beynon ME and Pinneri K: An overview of
the thyroid gland and thyroid- related deaths for the forensic
pathologist. Acad Forensic Pathol. 6:217–236. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nagashima T, Suzuki M, Oshida M, Hashimoto
H, Yagata H, Shishikura T, Koda K and Nakajima N: Morphometry in
the cytologic evaluation of thyroid follicular lesions. Cancer.
84:115–118. 1998.PubMed/NCBI
|
|
3
|
Khatri P, Choudhury M, Jain M and Thomas
S: Role of morphometry in the cytological differentiation of benign
and malignant thyroid lesions. J Cytol. 34:1–4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aiad H, Abdou A, Bashandy M, Said A,
Ezz-Elarab S and Zahran A: . Computerized nuclear morphometry in
the diagnosis of thyroid lesions with predominant follicular
pattern. Ecancermedicalscience. 3(146)2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Murata S, Mochizuki K, Nakazawa T, Kondo
T, Nakamura N, Yamashita H, Urata Y, Ashihara T and Katoh R:
Morphological abstraction of thyroid tumor cell nuclei using
morphometry with factor analysis. Microsc Res Tech. 61:457–462.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Patil SR, Patil KR, Andola SK, Viral L and
Mallikarjun B: Efficacy of fine needle aspiration cytology in
diagnosis of lesions of thyroid and histopathological correlation.
J Pub Health Med Res. 1:18–23. 2013.
|
|
7
|
Huang LY, Lee YL, Chou P, Chiu WY and Chu
D: Thyroid fine-needle aspiration biopsy and thyroid cancer
diagnosis: A nationwide population- based study. PLoS One.
10(e0127354)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ciobanu D, Căruntu ID, Vulpoi C, Florea N
and Giuşcă SE: Morphometric parameters and silver stain used in
diagnosis of thyroid follicular diseases. Rom J Morphol Embryol.
47:323–330. 2006.PubMed/NCBI
|
|
9
|
Ferrer-Roca O, Ballester-Guardia E and
Martin-Rodriguez JA: Morphometric, densitometric and flow
cytometric criteria for the automated classification of thyroid
lesions. Anal Quant Cytol Histol. 12:48–55. 1990.PubMed/NCBI
|
|
10
|
De Micco C, Zoro P, Garcia S, Skoog L,
Tani EM, Carayon P and Henry JF: Thyroid peroxidase immunodetection
as a tool to assist diagnosis of thyroid nodules on fine-need
aspiration biopsy. Eur J Endocrinol. 131:474–479. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gupta S, Ajise O, Dultz L, Wang B, Nonaka
MD, Ogilvie J, Heller KS and Patel KN: Follicular variant of
papillary thyroid cancer: Encapsulated, nonencapsulated, and
diffuse: Distinct biologic and clinical entities. Arch Otolaryngol
Head Neck Surg. 138:227–233. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Solymosi T, Tóth V, Sápi Z, Bodó M, Gál I
and Csanádi L: Diagnostic value of AgNOR method in thyroid
cytopathology: Correlation with morphometric measurements. Diagn
Cytopathol. 14:140–144. 1996.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hamburger JI and Husain M:
Semiquantitative criteria for fine-needle biopsy diagnosis: Reduced
false-negative diagnoses. Diagn Cytopathol. 4:14–17.
1988.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Razavi MA, Wong J, Akkera M, Shalaby M,
Shalaby H, Sholl A, Haddad A, Behl P, Kandil E and Lee GS: Nuclear
morphometry in indeterminate thyroid nodules. Gland Surg.
9:238–244. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sciacchitano S, Lavra L, Ulivieri A, Magi
F, De Francesco GP, Bellotti C, Salehi LB, Trovato M, Drago C and
Bartolazzi A: Comparative analysis of diagnostic performance,
feasibility and cost of different test- methods for thyroid nodules
with indeterminate cytology. Oncotarget. 8:49421–49442.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tseleni-Balafouta S, Kavantzas N,
Paraskevakou H and Davaris P: Computerized morphometric study on
fine needle aspirates of cellular follicular lesions of the
thyroid. Anal Quant Cytol Histol. 22:323–326. 2000.PubMed/NCBI
|
|
17
|
Lloyd RV, Osamura RY, Klöppel G and Rosai
J: WHO classification of tumours of endocrine organs. international
agency for research on cancer, 2017.
|
|
18
|
Baloch ZW and Livolsi VA:
Follicular-patterned lesions of the thyroid: The bane of the
pathologist. Am J Clin Pathol. 117:143–150. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Suster S: Thyroid tumors with a follicular
growth pattern: Problems in differential diagnosis. Arch Pathol Lab
Med. 130:984–988. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Trimboli P, Virili C, Romanelli F,
Crescenzi A and Giovanella L: Galectin-3 performance in histologic
a cytologic assessment of thyroid nodules: A systematic review and
meta-analysis. Int J Mol Sci. 18(1756)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Passler C, Prager G, Scheuba C, Niederle
BE, Kaserer K, Zettinig G and Niederle B: Follicular variant of
papillary thyroid carcinoma: A long-term follow-up. Arch Surg.
138:1362–1366. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Naganuma H, Murayama H, Ohtani N, Takaya
K, Mori Y, Sakai N and Kakudo K: Optically clear nuclei in
papillary carcinoma of the thyroid: Demonstration of one of the
fixation artifacts and its practical usefulness. Pathol Int.
50:113–118. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chan JKC: Strict criteria should be
applied in the diagnosis of encapsulated follicular variant of
papillary thyroid carcinoma. Am J Clin Pathol. 117:16–18.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zidan J, Karen D, Stein M, Rosenblatt E,
Basher W and Kuten A: Pure versus follicular variant of papillary
thyroid carcinoma: Clinical features, prognostic factors,
treatment, and survival. Cancer. 97:1181–1185. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kang Y, Lee YJ, Jung J, Lee Y, Won NH and
Chae YS: Morphometric analysis of thyroid follicular cells with
atypia of undetermined significance. J Pathol Transl Med.
50:287–293. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hamilton PW and Allen DC: Morphometry in
histopathology. J Pathol. 175:369–379. 1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fadda G, Rabitti C, Minimo C, Ieraci A,
Verzì A, Bianchi A, Lancia M, Gullotta G and Capelli A: Morphologic
and planimetric diagnosis of follicular thyroid lesions on fine
needle aspiration cytology. Anal Quant Cytol Histol. 17:247–256.
1995.PubMed/NCBI
|
|
28
|
Artacho-Pérula E, Roldán-Villalobos R,
Blanco-García F and Blanco-Rodríguez A: Objective differential
classification of thyroid lesions by nuclear quantitative
assessment. Histol Histopathol. 12:425–431. 1997.PubMed/NCBI
|
|
29
|
Wright RG, Castles H and Mortimer RH:
Morphometric analysis of thyroid cell aspirates. J Clin Pathol.
40:443–445. 1987.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mathur A, Najafian A, Schneider EB, Zeiger
MA and Olson MT: Malignancy risk and reproducibility associated
with atypia of undetermined significance on thyroid cytology.
Surgery. 156:1471–1476. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shi Y, Ding X, Klein M, Sugrue C, Matano
S, Edelman M and Wasserman P: Thyroid fine-needle aspiration with
atypia of undetermined significance: A necessary or optional
category? Cancer. 117:298–304. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kapur U, Antic T, Venkataraman G,
Durazo-Arvizu R, Quek MM, Flanigan RC and Wojcik EM: Validation of
world health organization/international society of urologic
pathology 2004 classification schema for bladder urothelial
carcinomas using quantitative nuclear morphometry: Identification
of predictive features using bootstrap method. Urology.
70:1028–1033. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lira M, Schenka AA, Magna LA, Cotta AC,
Cintra ML, de Souza EM, Brousset P and Vassallo J: Diagnostic value
of combining immunostaining for CD3 and nuclear morphometry in
mycosis fungoides. J Clin Pathol. 61:209–212. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cui Y, Koop EA, van Diest PJ, Kandel RA
and Rohan TE: Nuclear morphometric features in benign breast tissue
and risk of subsequent breast cancer. Breast Cancer Res Treat.
104:103–107. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kazanowska B, Jelen M, Reich A, Tarnawski
W and Chybicka A: The role of nuclear morphometry in prediction of
prognosis for rhabdomyosarcoma in children. Histopathology.
45:352–359. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shih SR, Chang YC, Li HY, Liau JY, Lee CY,
Chen CM and Chang TC: Preoperative prediction of papillary thyroid
carcinoma prognosis with the assistance of computerized morphometry
of cytology samples obtained by fine-needle aspiration: Preliminary
report. Head Neck. 35:28–34. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang SL, Wu MT, Yang SF, Chan HM and Chai
CY: Computerized nuclear morphometry in thyroid follicular
neoplasms. Pathol Int. 55:703–706. 2005.PubMed/NCBI View Article : Google Scholar
|