Introduction
Cirrhosis is a pathogenic hallmark of advanced liver
injury and fibrosis, and changes resulting from tissue remodeling
in cirrhotic liver are associated with increased intrahepatic
resistance to portal blood flow, leading to portal hypertension
(PH). PH leads to formation of collateral pathways, particularly
esophagogastric varices (1).
Esophagogastric variceal bleeding (EGVB) is a frequent and
dangerous complication associated with PH in patients with
cirrhosis (2). In 1991, a
multicenter study carried out in Boston, New Haven and Barcelona,
determined that the rate of first variceal haemorrhage was 22%
(3). Endoscopic treatment of
esophagogastric varices includes endoscopic variceal ligation (EVL)
and endoscopic injection sclerotherapy (EIS), characterized by
minimal trauma and ease of repeated operation, and is widely used
in clinical practice (4). In 2009,
a study by Cheung et al (5), which involved multiple research
centers in North America/Europe and Asia, found that rebleeding was
still possible following endoscopic treatment with a mortality of
up to 25%. Thus, the Baveno VI consensus (6) recommended early transjugular
intrahepatic portosystemic shunt (TIPS). Early identification of
patients at high risk of rebleeding following endoscopic treatment
improves monitoring and management and timely treatment with
endoscopic secondary prophylaxis, such as EVL every 2-6 weeks, or
early TIPS are important to improve the prognosis of patients
(6). Several non-invasive
assessment models such as albumin (ALB)-bilirubin (ALBI),
ALBI-fibrosis-4 (FIB4), fibrosis index (FI) and platelet
count-spleen diameter ratio are currently used to predict the
occurrence of EGVB and rebleeding (7-9).
ALBI-associated scores have received a lot of
attention and are considered potential alternatives to
Child-Turcotte-Pugh (CTP) and model for end-stage liver disease
(MELD) for prognosis of patients with chronic liver disease, liver
failure and liver cancer (10-12).
However, studies have reported limitations of the ALBI score, which
considered that ALBI has a better prognostic power in patients with
minimal liver dysfunction, and novel scores, such as modified ALBI
(mALBI), platelet-ALBI (PALBI) and ALBI-FIB4, have emerged and are
considered better predictors of liver decompensated events,
especially in stratifying risk for portal hypertension (13-16).
An elevated international normalized ratio (INR) reflects decreased
hepatic reserve capacity and coagulation disorder (17). To the best of our knowledge, the
present study is the first to combine INR with ALBI to predict
rebleeding in patients with EGVB following endoscopic therapy for
liver cirrhosis. Furthermore, ALBI-associated scores and grades
(including mALBI, PALBI and ALBI-FIB4) were evaluated and compared
with the novel scoring system INR-ALBI (IALBI) to analyze their
predictive ability for rebleeding in cirrhotic patients with EGVB
following endoscopic therapy in the short-, medium- and
long-term.
Materials and methods
Patients
The present retrospective study was performed at the
Third Central Hospital of Tianjin (Tianjin, China). A total of
1,348 hospitalized patients with EGVB who underwent endoscopic
treatment following their first bleeding were retrospectively
screened. The entire patient population was divided into training
and validation cohorts based on the date of hospitalization.
Patients hospitalized between January 2015 and January 2020 were
assigned to the training cohort and patients hospitalized between
February 2020 and February 2022 were assigned to the validation
cohort (Fig. 1). The inclusion
criteria were as follows: i) aged ≥18 years; ii) diagnosis of
cirrhosis by liver biopsy or imaging examinations, together with
clinical features and biochemical indices and iii) EGVB caused by
PH due to liver cirrhosis. The exclusion criteria were as follows:
i) Diagnosis of hepatocellular carcinoma (HCC), other malignant
tumors and hematological disease at the time of recruitment or
during follow-up; ii) non-cirrhotic PH; iii) undergoing TIPS,
splenectomy, partial splenic embolization or liver transplantation;
iv) previous history of EGVB, endoscopic treatment of
esophagogastric varices, use of propranolol or other drugs to
reduce PH and v) severe heart and lung disease.
The study was approved by the Ethics Committee of
Tianjin Third Central Hospital (approval no. IRB2021-028-01) and
written informed consent was obtained from all study
participants.
Endoscopic data and treatment
In the present study, endoscopy was performed using
an GIF-Q260J or GIF-H290Z (Olympus Corporation) and all patients
underwent endoscopy within 48 h of admission. Endoscopic
examinations and treatments were performed by expert endoscopists
and uniform standards of treatment and documentation were used.
Bands were applied to each varix in a step ladder pattern up to a
level of 5 cm above the gastroesophageal junction. Endoscopic
injection was performed intravariceally using a therapeutic
endoscope and a transparent T eflon injector. An attempt was made
to obturate the gastric varices completely at one session by
injecting lauromacrogol and tissue adhesive at multiple sites. The
injected the gastric varices was palpated using the hub of the
injector with the needle retracted to look for solidification and
obliteration of the gastric varices. If the gastric varices was not
completely obturated, cyanoacrylate was reinjected till the whole
the gastric varices became solidified (18,19).
Based on Japanese Research Society for Portal
Hypertension classification (20),
the following information on esophageal varices was recorded: i)
Location (locus superior, medialis or inferior varices); ii) form
(F0, lesions lack a varicose appearance; F1, lesions are straight,
small-caliber varices; F2, moderately enlarged lesions, beady
varices and F3, markedly enlarged lesions, nodular or tumor-shaped
varices) and iii) red color signs (red wale markings, cherry red or
hematocytic spots). According to the Sarin classification (21), gastric varices were defined as
gastroesophageal varices (GOV) and isolated gastric varices (IGV).
GOV1 was defined as esophageal varices that extended to the lesser
curvature of the stomach; GOV2 was defined as esophageal varices
that extended to the fundus of the stomach and IGV1 was defined as
localized to the fundus of the stomach. Variceal bleeding was
diagnosed when active bleeding from esophagogastric varices or
signs of recent bleeding, such as ‘white nipples’, were observed
(6). Esophageal varices were
treated with band ligation. Gastric varices were managed with
lauromacrogol and tissue adhesive injections. The number of
injection sites and the dose of lauromacrogol and tissue adhesive
were determined according to the severity of the varices in an
attempt to eradicate the visible varices in one session (19). Lauromacrogol and tissue adhesive
injection combined with band ligation were performed for patients
with gastroesophageal varices.
Clinical and laboratory data
Data from all patients were collected from medical
records, including: i) Age, sex, etiology of liver cirrhosis,
ascites grade and presence of hepatic encephalopathy; ii) white
blood cell (WBC), neutrophil (NEUT) and platelet (PLT) counts,
hemoglobin (HGB), prothrombin time (PT), INR, fibrinogen, ALB,
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
total BIL (TBIL), creatinine (Cr), blood urea nitrogen (BUN),
sodium and blood glucose; iii) imaging indicators, including
abdominal ultrasound and computed tomography (CT) of spleen and
portal vein diameter, presence of portal vein thrombosis (PVT) and
CT portosystemic shunt.
Calculation of non-invasive
markers
CTP, MELD (22) and
ALBI-related scores were calculated as follows:
ALBI=(log10BIL x 0.66) + (ALB x -0.085) [according to
ALBI, scores were graded as follows: Grade 1, ≤-2.60; grade 2,
>-2.60 and ≤-1.39 and grade 3, >-1.39(12). According to mALBI, scores were
graded as follows: Grade 1, ≤-2.60; grade 2a, >-2.60 and ≤-2.27;
grade 2b, >-2.27 and ≤-1.39 and grade 3, >-1.39(13)]; PALBI=2.02 x log10BIL
-0.37 x (log10BIL)2 -0.04 x ALB -3.48 x
log10PLT + 1.01 x (log10PLT)2
[grade 1, ≤-2.53; grade 2, >-2.53 and ≤-2.09 and grade 3,
>-2.09(14)]; ALBI-FIB-4=(ALBI
x1.331) + (FIB-4 x0.165) [high risk, >-1.822; low risk,
≤-1.822(15)] and FIB-4=(age x
AST)/(PLT x ALT1/2) (23). Patients with ALBI and PALBI grade
1, 2 and 3 were assigned a score of 1, 2 or 3, respectively
(12,14). Patients with mALBI grade 1, 2a, 2b
and 3 were assigned a score of 1, 2, 3 or 4, respectively (13). Patients with ALBI-FIB4 score of low
and high risk received a score of 1 or 2, respectively (15).
Definition of combined INR and ALBI
grade
The optimal cut-off value of INR was determined
using Receiver operating characteristic (ROC) curve analysis (SPSS
26.0; IBM Corporation), (24),
based on the most prominent point on the ROC curve for
‘sensitivity’ and ‘1-specificity’. The ideal cut-off value was
computed using the Youden index (sensitivity + specificity-1)
(25). The ideal cut-off value of
INR was 1.205; therefore, low and high INR were defined as INR
values ≤1.205 and >1.205, respectively. Patients with low INR
were allocated a score of 0, whereas those with high INR were
allocated a score of 1. The sum of ALBI (1, 2 or 3) and INR (0 or
1) was defined as the IALBI grade and scored as follows: IALBI
grade 1-2, 1; grade 3, 2 and grade 4, 3 (Table SI).
Study endpoints and follow-up
All patients were treated according to the Baveno VI
criteria (3). To prevent recurrent
hemorrhage, non-selective β-blockers (NSBBs) were used if not
contraindicated and/or endoscopic treatment was performed every 2-6
weeks until varices were eradicated, followed by endoscopy every 3
months after variceal eradication. For patients with high-risk
esophageal varices (large, medium or small varices with red signs),
EVL or EIS were performed. For patients with high-risk
gastroesophageal varices, lauromacrogol and tissue adhesive
injection combined with EVL or EIS were used. All patients were
followed up for 1 year. The endpoint event was EGVB rebleeding,
which was characterized by new hematemesis and/or melena, and the
bleeding lesion was confirmed by esophagogastroduodenoscopy (EGD).
Patients lost to follow-up and patients who did not receive EGD
were excluded during follow-up.
Statistical analysis
SPSS 26.0 (SPSS Inc.; IBM Corporation) and GraphPad
Prism7 (GraphPad Software; Dotmatics) were used for data analysis.
Continuous variables with normal distribution are expressed as the
mean ± standard deviation (SD) and the unpaired t-test was used for
comparison between two groups; non-normally distributed measures
were expressed as the median and interquartile range (IQR) and
Mann-Whitney U-test was used to compare two groups. Categorical
data are shown as frequencies or proportions and analyzed using
χ2 test. The Cox proportional hazards model was used to
identify factors associated with rebleeding. Multicollinearity was
assessed using the variance inflation factor (VIF), (26); VIF>10 was considered to indicate
multicollinearity. Area under ROC curve (AUC) was calculated to
evaluate the discriminatory ability of each non-invasive marker.
Kaplan-Meier method was used to estimate the cumulative risk of
rebleeding and differences were tested using the log-rank test. The
R statistical package ‘pROC’ (version 3.5.2) was used to calculate
time-dependent ROC curves (27). A
two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of the training
cohort
In the training cohort, 437 patients met the
inclusion criteria (Fig. 1).
Patients were divided into a non-rebleeding (n=284) and rebleeding
group (n=153). The rebleeding rates at 1,3 and 12 months were 7.1
(31/437), 11.4 (50/437) and 35.0 (153/437)%, respectively. There
were nine individuals who died during the follow-up period; causes
of death were gastrointestinal bleeding (n=4), liver failure (n=3)
and abdominal infection and pneumonia (both n=1). The mortality
rate was 0.3, 0.7 and 2.1% at 1, 3 and 12 months, respectively.
Furthermore, 249 out of 437 patients received NSBB after the first
endoscopic treatment. For patients with and without NSBB, the 12
months rebleeding rates were 24.9% (62/249) and 48.4% (91/188),
respectively. A total of 156 of 437 patients only received
endoscopic treatment once during follow-up. For patients who
underwent a single endoscopic treatment and those who received
multiple treatments, the 12 months rebleeding rates were 35.3%
(55/156) and 34.9% (98/281), respectively. PT and INR were higher
in the rebleeding group than the non-rebleeding group and ALB was
lower in the rebleeding group than the non-rebleeding group
(Table I). MELD, ALBI, mALBI and
PALBI scores were higher in the rebleeding than the non-rebleeding
group (Table I).
 | Table IBaseline characteristics of
patients. |
Table I
Baseline characteristics of
patients.
| Variable | All patients
(n=437) | Non-rebleeding
group (n=284) | Rebleeding group
(n=153) |
t/Z/χ2-score | P-value |
|---|
| Sex | | | |
χ2=1.701 | 0.192 |
|
Female | 184 (42.11) | 126 (44.37) | 58 (37.91) | | |
|
Male | 253 (57.89) | 158 (55.63) | 95 (62.09) | | |
| Age, years | 57.21±10.79 | 57.38±10.35 | 56.89±11.58 | t=0.439 | 0.661 |
| Etiology | | | |
χ2=7.553 | 0.056 |
|
HBV | 169 (38.67) | 123 (43.31) | 46 (30.06) | | |
|
HCV | 30 (6.87) | 17 (5.99) | 13 (8.50) | | |
|
Alcohol | 85 (19.45) | 52 (18.31) | 33 (21.57) | | |
|
Other | 153 (35.01) | 92 (32.39) | 61 (39.87) | | |
| Number of
endoscopic treatments | 2 (1,4) | 2 (1,4) | 2 (1,4) | Z=-0.397 | 0.691 |
| Use of NSBB
drugs | 249 (56.98) | 187 (65.85) | 62 (40.52) |
χ2=26.010 | <0.001 |
| PT, sec | 16.21±2.44 | 15.76±2.03 | 17.04±2.90 | t=4.828 | <0.001 |
| INR | 1.32±0.26 | 1.28±0.22 | 1.40±0.30 | t=4.441 | <0.001 |
| Fibrinogen,
g/l | 2.10
(1.64,2.56) | 2.09
(1.63,2.56) | 2.10
(1.64,2.57) | Z=-0.033 | 0.973 |
| WBC count,
x109/l | 3.88
(2.56,5.53) | 3.87
(2.56,5.54) | 3.89
(2.59,5.52) | Z=-0.056 | 0.956 |
| NEUT count, % | 69.80±10.67 | 69.27±10.78 | 70.79±10.41 | t=1.418 | 0.157 |
| HGB, g/l | 93.99±25.90 | 94.33±26.34 | 93.36±25.13 | t=0.375 | 0.708 |
| PLT,
x109/l | 72.00
(51.00,96.50) | 71.00
(51.00,94.00) | 75.00
(54.00,100.50) | Z=-1.089 | 0.276 |
| ALB, g/l | 34.35±5.72 | 34.96±5.74 | 33.22±5.51 | t=3.077 | 0.002 |
| ALT, U/l | 25.00
(16.00,34.00) | 25.00
(16.00,33.00) | 24.00
(16.00,35.00) | Z=-0.583 | 0.560 |
| AST, U/l | 30.00
(21.00,44.00) | 29.00
(20.25,40.75) | 32.00
(21.00,45.00) | Z=-1.471 | 0.141 |
| TBIL, µmol/l | 20.40
(14.45,31.00) | 20.10
(14.3,29.98) | 21.00
(14.75,33.90) | Z=-0.807 | 0.420 |
| BUN, mmol/l | 5.69
(4.24,8.08) | 5.62
(4.14,8.02) | 5.88
(4.51,8.11) | Z=-0.929 | 0.353 |
| Cr, µmol/l | 64.00
(54.00,76.00) | 64.00
(54.00,75.00) | 65.00
(55.00,77.50) | Z=-0.944 | 0.345 |
| Na, mmol/l | 139.20±3.78 | 139.43±3.89 | 138.77±3.53 | t=1.741 | 0.082 |
| GLU, mmol/l | 7.39±3.67 | 7.39±3.57 | 7.40±3.86 | t=0.033 | 0.974 |
|
HBV-DNA/HCV-RNA | | | |
χ2=3.233 | 0.072 |
|
Negative | 349 (79.86) | 234 (82.39) | 115 (75.16) | | |
|
Positive | 88 (20.14) | 50 (17.61) | 38 (24.84) | | |
| Spleen diameter,
cm | 15.27±2.44 | 15.35±2.49 | 15.12±2.35 | t=0.967 | 0.334 |
| Width of portal
vein, mm | 14.03±1.73 | 14.01±1.71 | 14.05±1.79 | t=0.201 | 0.435 |
| PVT | | | |
χ2=3.000 | 0.392 |
|
None | 335 (76.66) | 212 (74.65) | 123 (80.39) | | |
|
Only
trunk | 57 (13.04) | 41 (14.44) | 16 (10.46) | | |
|
Only
branch | 16 (3.66) | 11 (3.87) | 5 (3.27) | | |
|
Trunk and
branches | 29 (6.64) | 20 (7.04) | 9 (5.88) | | |
| CT portosystemic
shunt | | | |
χ2=0.250 | 0.617 |
|
No | 390 (89.24) | 255 (89.79) | 135 (88.24) | | |
|
Yes | 47 (10.76) | 29 (10.21) | 18 (11.76) | | |
| Form of esophageal
varices | | | |
χ2=4.000 | 0.261 |
|
F0 | 25 | 17 | 8 | | |
|
F1 | 24 | 17 | 7 | | |
|
F2 | 97 | 71 | 26 | | |
|
F3 | 291 | 179 | 112 | | |
| Gastric
varices | | | |
χ2=3.000 | 0.392 |
|
GOV-1 | 182 | 110 | 72 | | |
|
GOV-2 | 124 | 85 | 39 | | |
|
GOV-1 and
GOV-2 | 31 | 22 | 9 | | |
|
IGV1 | 25 | 17 | 8 | | |
| CTP grade | | | |
χ2=-1.841 | 0.066 |
|
A | 232 (53.09) | 158 (55.63) | 74 (48.37) | | |
|
B | 173 (39.59) | 111 (39.09) | 62 (40.52) | | |
|
C | 32 (7.32) | 15 (5.28) | 17 (11.11) | | |
| MELD score | 7.11
(4.31,10.36) | 6.52
(4.14,9.70) | 8.09
(4.76,10.98) | Z=-3.198 | 0.001 |
| ALBI grade | | | |
χ2=-3.170 | 0.002 |
|
1 | 66 (15.10) | 54 (19.01) | 12 (7.84) | | |
|
2 | 313 (71.63) | 198 (69.72) | 115 (75.17) | | |
|
3 | 58 (13.27) | 32 (11.27) | 26 (16.99) | | |
| mALBI grade | | | |
χ2=-3.411 | 0.001 |
|
1 | 66 (15.10) | 54 (19.01) | 12 (7.84) | | |
|
2a | 96 (21.97) | 66 (23.24) | 30 (19.61) | | |
|
2b | 217 (49.66) | 132 (46.48) | 85 (55.56) | | |
|
3 | 58 (13.27) | 32 (11.27) | 26 (16.99) | | |
| PALBI grade | | | |
χ2=-2.062 | 0.039 |
|
1 | 129 (29.52) | 96 (33.80) | 33 (21.57) | | |
|
2 | 176 (40.27) | 106 (37.33) | 70 (45.75) | | |
|
3 | 132 (30.21) | 82 (28.87) | 50 (32.68) | | |
| ALBI-FIB-4 | | | |
χ2=1.162 | 0.281 |
|
Low-risk | 221 (50.57) | 149 (52.46) | 72 (47.06) | | |
|
High-risk | 216 (49.43) | 135 (47.54) | 81 (52.94) | | |
| FIB-4 index | 4.97
(3.07,7.78) | 4.95
(3.16,7.69) | 5.15
(2.92,7.86) | Z=-0.019 | 0.984 |
Combined ALBI score and INR in the
training cohort
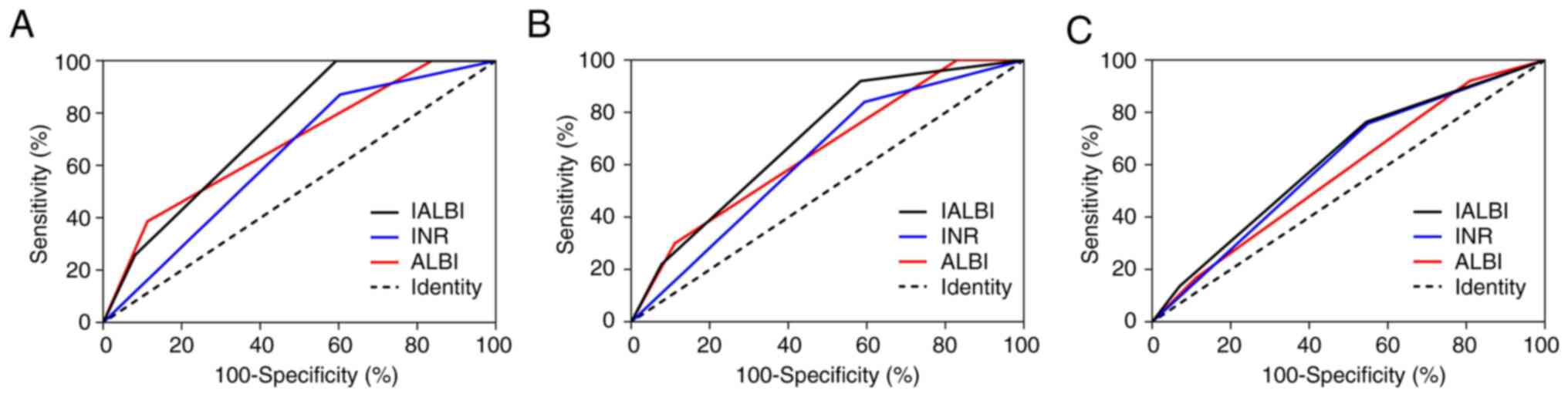
AUCs for IALBI at 1, 3 and 12 months were 0.739,
0.697 and 0.620, respectively; those for INR were 0.634, 0.623 and
0.604, respectively, and those for ALBI were 0.687, 0.654 and
0.573, respectively. The AUC of IALBI predicting 1-month rebleeding
was higher than that of INR and ALBI, AUC for prediction of 3-month
rebleeding was higher than that of INR and the AUC for prediction
of rebleeding at 12 months was higher than that of ALBI (Fig. 2; Table SII).
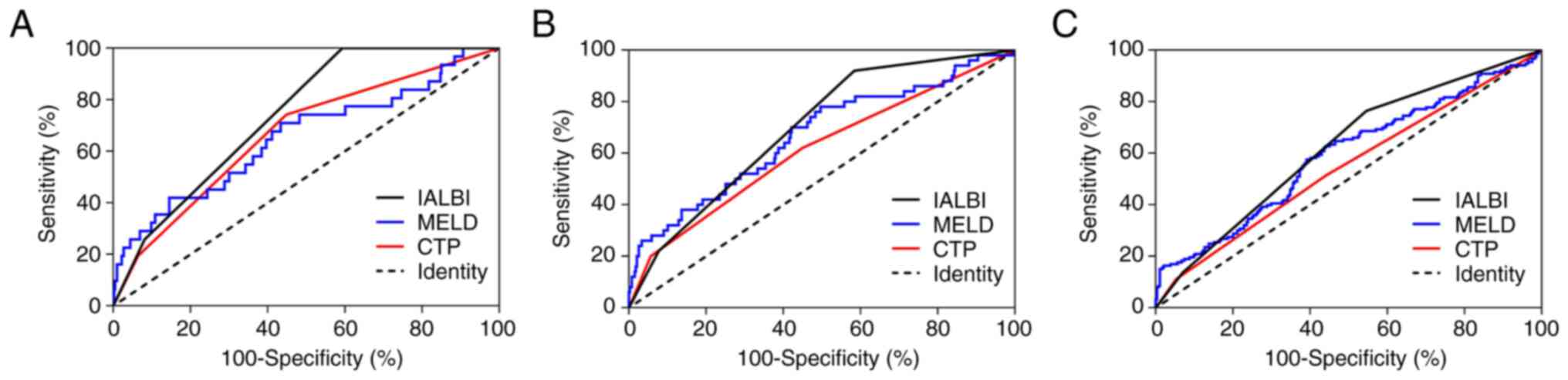
IALBI was compared with other liver function scores
(CTP and MELD). AUCs for CTP at 1, 3 and 12 months were 0.666,
0.613 and 0.547, respectively, and those for MELD were 0.655, 0.668
and 0.593, respectively. AUCs of IALBI to predict rebleeding at 3
and 12 months were higher than those of CTP. At each time-point,
AUCs of IALBI were higher than those of MELD; however, the
differences were not statistically significant. Over time, the
liver function scores showed progressively lower predictive power
for rebleeding and only IALBI had predictive power at 12 months
(Fig. 3; Table SIII).
Risk factors for rebleeding at 12
months in the training cohort
Sex, age, etiology (viral and non-viral), PT, INR,
fibrinogen, WBC, NEUT, HGB, PLT, ALB, ALT, AST, TBIL, BUN, Cr, Na,
CLU, spleen and portal vein diameter, PVT, CT portosystemic shunt,
CTP, MELD, IALBI and FIB-4 were used as independent variables to
establish the Cox proportional risk model. The results indicated a
significant association between rebleeding and PT, INR, ALB, TBIL,
CTP, MELD and IALBI. Significant variables in the univariate
analysis were subjected to multivariate analysis using the Cox
proportional hazards model (excluding variables with VIF >9 to
avoid collinearity). IALBI was the significant variable in the
model and could be used to predict rebleeding (Table II).
 | Table IICompeting risk factors of 12 months
rebleeding in all patients. |
Table II
Competing risk factors of 12 months
rebleeding in all patients.
| | Univariate | Multivariate |
|---|
| Variable | HR (95% CI) | Wald
χ2 | P-value | VIF | HR (95% CI) | Wald
χ2 | P-value |
|---|
| Sex | 1.191
(0.859-1.651) | 1.103 | 0.294 | | - | - | - |
| Age | 0.997
(0.983-1.012) | 0.129 | 0.720 | | - | - | - |
| Etiology | | | | | | | |
|
Viral | 1.000 | - | - | | - | - | - |
|
Non-viral | 1.317
(0.913-1.901) | 2.171 | 0.141 | | - | - | - |
| PT | 1.219
(1.151-1.292) | 45.097 | <0.001 | 9.182 | - | - | - |
| INR | 5.243
(3.094-8.886) | 37.903 | <0.001 | 10.150 | - | - | - |
| Fibrinogen | 1.065
(0.87-1.303) | 0.369 | 0.543 | | - | - | - |
| WBC | 1.005
(0.96-1.053) | 0.054 | 0.817 | | - | - | - |
| NEUT | 1.012
(0.997-1.028) | 2.410 | 0.121 | | - | - | - |
| HGB | 1.000
(0.994-1.006) | 0.002 | 0.964 | | - | - | - |
| PLT | 1.002
(0.998-1.006) | 1.243 | 0.265 | | - | - | - |
| ALB | 0.949
(0.922-0.976) | 12.875 | <0.001 | 2.111 | - | - | - |
| ALT | 1.001
(0.998-1.003) | 0.160 | 0.689 | | - | - | - |
| AST | 1.002
(0.999-1.005) | 2.971 | 0.085 | | - | - | - |
| TBIL | 1.010
(1.005-1.015) | 16.016 | <0.001 | 1.630 | - | - | - |
| BUN | 1.018
(0.976-1.061) | 0.672 | 0.412 | | - | - | - |
| Cr | 1.005
(0.998-1.012) | 2.162 | 0.141 | | - | - | - |
| Na | 0.967
(0.93-1.005) | 2.924 | 0.087 | | - | - | - |
| CLU | 1.001
(0.958-1.045) | 0.001 | 0.975 | | - | - | - |
| Spleen
diameter | 0.969
(0.908-1.034) | 0.887 | 0.346 | | - | - | - |
| Portal vein
diameter | 1.008
(0.921-1.103) | 0.028 | 0.866 | | - | - | - |
| PVT | 0.766
(0.514-1.142) | 1.706 | 0.192 | | - | - | - |
| CT portosystemic
shunt | 1.17
(0.715-1.913) | 0.391 | 0.532 | | - | - | - |
| CTP grade | - | - | - | 1.829 | - | 1.125 | 0.570 |
|
A | 1.000 | - | - | | 1.000 | - | - |
|
B vs. A | 1.193
(0.851-1.672) | 1.049 | 0.306 | | 0.951
(0.669-1.351) | 0.08 | 0.778 |
|
C vs. A | 2.244
(1.324-3.804) | 9.009 | 0.003 | | 1.300
(0.712-2.371) | 0.729 | 0.393 |
| MELD | 1.088
(1.048-1.13) | 19.103 | <0.001 | 2.009 | 1.045
(1.001-1.091) | 4.101 | 0.060 |
| IALBI grade | - | - | - | 2.697 | - | 10.215 | 0.043 |
|
1 | 1.000 | - | - | | 1.000 | - | - |
|
2 vs. 1 | 2.314
(1.577-3.395) | 18.396 | <0.001 | | 1.929
(1.257-2.960) | 9.054 | 0.003 |
|
3 vs. 1 | 3.377
(1.97-5.788) | 19.585 | <0.001 | | 2.416
(1.262-4.626) | 7.092 | 0.008 |
| FIB-4 | 1.019
(0.987-1.051) | 1.362 | 0.243 | | - | - | - |
Predictive value of IALBI based on
etiologies and CTP grades in the training cohort
The patients in the training cohort were divided
into viral and non-viral groups based on etiology. Of 199 patients
in the viral group, 59 patients (29.6%) experienced rebleeding
within 12 months; of 238 patients in the non-viral group, 94
(39.5%) experienced rebleeding within 12 months. In patients with
viral cirrhosis, PT and INR were higher in the rebleeding than the
non-rebleeding group and Na was lower in the rebleeding group than
the non-rebleeding group. A total of 38 patients (64.4%) were
positive for hepatitis B virus DNA or hepatitis C virus RNA in the
rebleeding group, which was higher than that in the non-rebleeding
group [35.7% (50/140)]. CTP, ALBI, mALBI, PALBI and IALBI were
higher in the rebleeding than the non-rebleeding group (Table SIV). Among patients with non-viral
cirrhosis, PT, INR and FIB were higher in the rebleeding than the
non-rebleeding group and IALBI was higher in the rebleeding than
the non-rebleeding group (Table
SIV).
In all of the patients in the training cohort and
viral group, the incidence of rebleeding increased with increasing
IALBI grade at all time-points. At 1 month, no patient experienced
rebleeding in the IALBI grade 1 group. In the non-viral group, the
incidence of rebleeding increased with the increasing IALBI grade
at 1 and 3 months. At 12 months, the incidence of rebleeding
increased with increasing IALBI grade; however, the difference was
not statistically significant. No patient experienced rebleeding in
the IALBI grade 1 group at 1 and 3 months (Fig. 4). The predictive value of IALBI for
rebleeding of patients with different etiology is presented in
Table III.
 | Table IIIPredictive value of IALBI for
rebleeding by CTP grade and etiology. |
Table III
Predictive value of IALBI for
rebleeding by CTP grade and etiology.
| A, 1 month |
|---|
| Group | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|
| All patients | 100.0 | 40.6 | 11.4 | 100.0 |
| CTP A | 100.0 | 54.5 | 14.4 | 100.0 |
| CTP B | 100.0 | 26.9 | 9.5 | 100.0 |
| CTP C | 100.0 | 53.8 | 14.2 | 100.0 |
| Viral | 100.0 | 65.3 | 18.0 | 100.0 |
| Non-Viral | 40.0 | 84.5 | 16.5 | 94.9 |
| B, 3 months |
| Group | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| All patients | 100.0 | 27.5 | 15.1 | 100.0 |
| CTP A | 72.7 | 54.1 | 17.0 | 93.9 |
| CTP B | 100.0 | 27.5 | 15.1 | 100.0 |
| CTP C | 70.0 | 54.5 | 16.6 | 93.4 |
| Viral | 70.0 | 65.4 | 20.7 | 94.4 |
| Non-Viral | 100.0 | 18.8 | 13.7 | 100.0 |
| C, 12 months |
| Group | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| All patients | 91.5 | 32.7 | 42.3 | 87.7 |
| CTP A | 66.7 | 60.7 | 47.8 | 77.2 |
| CTP B | 91.5 | 32.7 | 42.3 | 87.7 |
| CTP C | 64.7 | 60.0 | 46.6 | 75.9 |
| Viral | 63.5 | 72.8 | 55.7 | 78.7 |
| Non-Viral | 90.3 | 20.8 | 38.1 | 79.9 |
The sensitivity and NPV of IALBI for rebleeding
decreased with time in the different CTP stages. For CTP A, B and
C, the sensitivity and NPV of the IALBI score to predict 1-month
rebleeding were all 100% (Table
III).
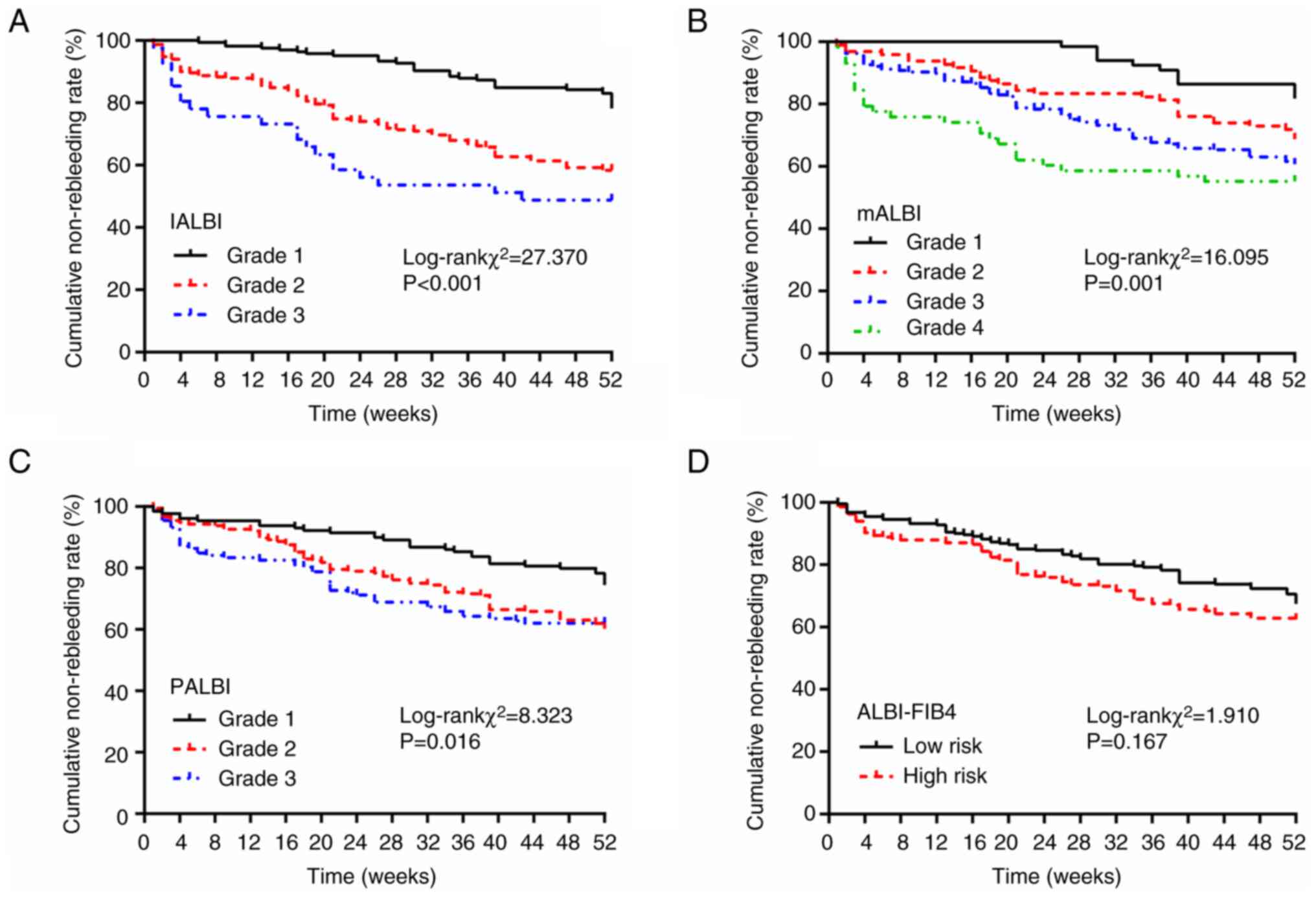
Cumulative rebleeding rate of training
cohort classified by ALBI-associated scoring systems
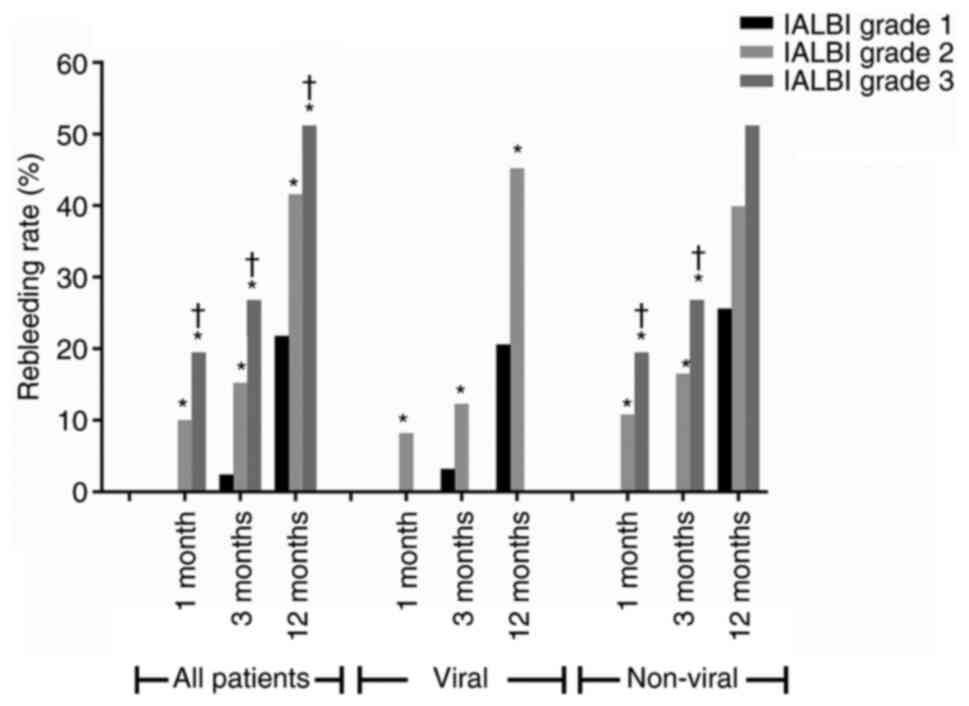
In the training cohort, 165 patients (37.8%) with
IALBI grade 1 had cumulative rebleeding rates of 0.0, 2.4 and 21.8%
at 1, 3 and 12 months, respectively. Overall, 231 (52.9%) patients
with IALBI grade 2 had cumulative rebleeding rates of 10.0, 15.2
and 41.7% at 1, 3 and 12 months, respectively. The cumulative
rebleeding rates at 1, 3 and 12 months were 19.5, 26.8 and 51.2%,
respectively, in patients with IALBI grade 3. The differences in
cumulative rebleeding rates between the three IALBI grades were
statistically significant, and the risk of rebleeding was
significantly lower in patients with IALBI grade 1 than in those
with grade 2 or 3 (Fig. 5;
Table SV).
At 1 month, rebleeding rate of the IALBI grade 2
group was ~10-times higher than that of the grade 1 group and that
of the grade 3 group was ~19.5-times higher than that of the grade
1 group. At 3 months, rebleeding rates of the IALBI grade 2 and 3
groups were ~6.3- and 11.2-times higher than the grade 1 group,
respectively. At 12 months, the rebleeding rates of the IALBI grade
2 and 3 groups were ~1.9- and 2.3-times higher than those of the
grade 1 group, respectively (Table
SV).
The differences in cumulative rebleeding rates for
different grades of mALBI and PALBI scores were significant. For
the ALBI-FIB4 score, the difference in cumulative rebleeding rates
between the low- and high-risk groups was not significant (Fig. 5; Table SV).
Predictive ability of ALBI-associated
scoring systems for rebleeding based on ROC curves in the training
cohort
In the training cohort, IALBI grade showed good
discrimination in predicting rebleeding at all time points (AUC,
0.739, 0.697 and 0.620 at 1, 3 and 12 months, respectively). The
sensitivity, specificity and negative predictive value (NPV) of
IALBI for rebleeding decreased with time (Table III). AUCs of IALBI for predicting
rebleeding at all time points were higher than those of PALBI and
ALBI-FIB4. AUCs of IALBI grade were consistently higher than those
of mALBI grade at all time points; however, the difference was not
significant. Specifically, mALBI grade did not predict 12 months
rebleeding (Fig. 6A-C; Table IV).
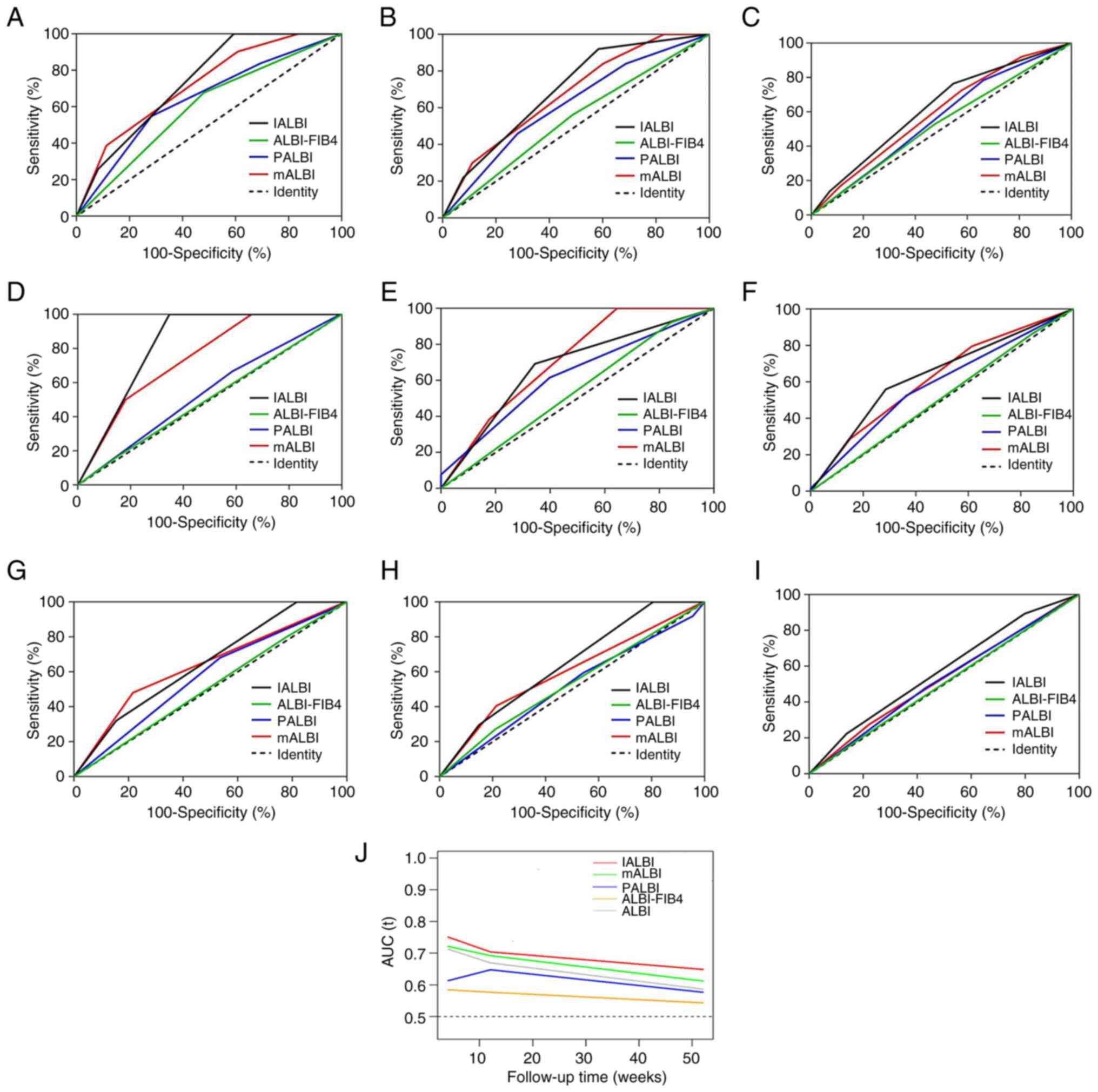 | Figure 6ROC curves for discriminative ability
of mALBI, PALBI, ALBI-FIB4 and IALBI to detect rebleeding. All
patients at (A) 1, (B) 3 and (C) 12 months. Patients with viral
cirrhosis at (D) 1, (E) 3 and (F) 12 months. Patients with
non-viral cirrhosis at (G) 1, (H) 3 and (I) 12 months. (J)
Time-dependent ROC curves for ALBI-related scores. ROC, receiver
operating characteristic; mALBI, modified albumin-bilirubin; PALBI,
platelet-ALBI; FIB-4, fibrosis-4; IALBI, international normalized
ratio-ALBI. |
 | Table IVPredictive value of scoring systems
at different time points. |
Table IV
Predictive value of scoring systems
at different time points.
| A, All
patients |
|---|
| Time, months | Variable | AUC (95% CI) | P1 | P2 | P3 | P4 |
|---|
| 1 | IALBI | 0.739
(0.687-0.792) | Ref. | 0.566 | 0.029 | 0.001 |
| | mALBI | 0.722
(0.645-0.799) | 0.566 | Ref. | 0.057 | 0.002 |
| | PALBI | 0.644
(0.546-0.741) | 0.029 | 0.057 | Ref. | 0.318 |
| | ALBI-FIB4 | 0.599
(0.511-0.686) | 0.001 | 0.002 | 0.318 | Ref. |
| 3 | IALBI | 0.697
(0.638-0.755) | Ref. | 0.355 | 0.024 | <0.001 |
| | mALBI | 0.676
(0.610-0.742) | 0.355 | Ref. | 0.07 | <0.001 |
| | PALBI | 0.616
(0.540-0.692) | 0.024 | 0.07 | Ref. | 0.032 |
| | ALBI-FIB4 | 0.537
(0.463-0.611) | <0.001 | <0.001 | 0.032 | Ref. |
| 12 | IALBI | 0.620
(0.572-0.668) | Ref. | 0.167 | 0.009 | <0.001 |
| | mALBI | 0.592
(0.541-0.642) | 0.167 | Ref. | 0.083 | 0.004 |
| | PALBI | 0.556
(0.504-0.608) | 0.009 | 0.083 | Ref. | 0.226 |
| | ALBI-FIB4 | 0.527
(0.478-0.576) | <0.001 | 0.004 | 0.226 | Ref. |
| B, Viral
cirrhosis |
| Time, months | Variable | AUC (95% CI) | P1 | P2 | P3 | P4 |
| 1 | IALBI | 0.826
(0.793-0.86) | Ref. | 0.283 | 0.001 | <0.001 |
| | mALBI | 0.745
(0.596-0.894) | 0.283 | Ref. | 0.027 | 0.08 |
| | PALBI | 0.459
(0.250-0.667) | 0.001 | 0.027 | Ref. | 0.748 |
| | ALBI-FIB4 | 0.508
(0.343-0.673) | <0.001 | 0.08 | 0.748 | Ref. |
| 3 | IALBI | 0.674
(0.539-0.809) | Ref. | 0.526 | 0.645 | 0.003 |
| | mALBI | 0.713
(0.615-0.810) | 0.526 | Ref. | 0.314 | <0.001 |
| | PALBI | 0.624
(0.473-0.775) | 0.645 | 0.314 | Ref. | 0.095 |
| | ALBI-FIB4 | 0.461
(0.381-0.540) | 0.003 | <0.001 | 0.095 | Ref. |
| 12 | IALBI | 0.637
(0.563-0.711) | Ref. | 0.653 | 0.251 | <0.001 |
| | mALBI | 0.620
(0.542-0.698) | 0.653 | Ref. | 0.398 | 0.002 |
| | PALBI | 0.584
(0.507-0.660) | 0.251 | 0.398 | Ref. | 0.03 |
| | ALBI-FIB4 | 0.489
(0.436-0.543) | <0.001 | 0.002 | 0.03 | Ref. |
| C, Non-viral
cirrhosis |
| Time, months | Variable | AUC (95% CI) | P1 | P2 | P3 | P4 |
| 1 | IALBI | 0.645
(0.562-0.728) | Ref. | 0.758 | 0.142 | 0.006 |
| | mALBI | 0.633
(0.530-0.737) | 0.758 | Ref. | 0.304 | 0.015 |
| | PALBI | 0.571
(0.470-0.673) | 0.142 | 0.304 | Ref. | 0.265 |
| | ALBI-FIB4 | 0.510
(0.426-0.595) | 0.006 | 0.015 | 0.265 | Ref. |
| 3 | IALBI | 0.642
(0.573-0.711) | Ref. | 0.108 | 0.003 | <0.001 |
| | mALBI | 0.597
(0.512-0.682) | 0.108 | Ref. | 0.089 | 0.003 |
| | PALBI | 0.517
(0.424-0.609) | 0.003 | 0.089 | Ref. | 0.319 |
| | ALBI-FIB4 | 0.469
(0.392-0.547) | <0.001 | 0.003 | 0.319 | Ref. |
| 12 | IALBI | 0.575
(0.513-0.636) | Ref. | 0.053 | 0.006 | 0.027 |
| | mALBI | 0.530
(0.473-0.587) | 0.053 | Ref. | 0.121 | 0.307 |
| | PALBI | 0.476
(0.41-0.542) | 0.006 | 0.121 | Ref. | 0.591 |
| | ALBI-FIB4 | 0.496
(0.442-0.55) | 0.027 | 0.307 | 0.591 | Ref. |
In the viral group, IALBI grade showed better
discrimination in predicting rebleeding at all time points (AUC,
0.826, 0.674 and 0.620 at 1, 3 and 12 months, respectively). AUCs
of IALBI grade for predicting rebleeding at all time points were
higher than those of ALBI-FIB4 and AUC of IALBI grade for the
prediction of 1-month rebleeding was higher than that of PALBI.
AUCs of IALBI grade were higher than those of mALBI grade except at
3 months; however, the difference was not statistically significant
(Fig. 6D-F; Table IV).
In the non-viral group, IALBI grade predicted
rebleeding at 1 and 3, but not at 12 months (AUC, 0.645, 0.642 and
0.575 at 1, 3 and 12 months, respectively). AUCs for IALBI to
predict rebleeding at 1 and 3 months were higher than those of
ALBI-FIB4 and PALBI. mALBI grade did not predict rebleeding at 3 or
12 months (Fig. 6G-I; Table IV).
To illustrate the variation of AUCs over time for
ALBI-associated scores, time-dependent ROC curves were plotted.
During follow-up, IALBI performed best compared with other
ALBI-associated scores, while AUCs to predict rebleeding decreased
over time (Fig. 6J).
Validation cohort
Characteristics of the validation cohort (n=159) are
shown in Table SVI. In the
validation cohort, IALBI grade showed good discrimination in
predicting rebleeding (AUC, 0.742, 0.728 and 0.592 at 1, 3 and 12
months, respectively). The sensitivity, specificity and NPV of the
IALBI score to predict 1-month rebleeding were 94.1, 31.0 and
97.8%, respectively (Fig.
S1).
Discussion
Previous studies have revealed that the mortality
rates of acute variceal bleeding in patients with liver cirrhosis
are 10-20% within 6 weeks and ~40% within 1 year (28,29).
Each year, ~12% of cirrhotic patients experience a first variceal
bleeding event and in the absence of secondary prophylaxis, the
risk of rebleeding within 1 year is up to 60% (28,29).
Endoscopic treatment is an effective method for variceal bleeding,
and rebleeding after endoscopic treatment seriously affects
prognosis of patients with liver cirrhosis. In the present training
cohort, the rebleeding rates at 1, 3 and 12 months were 7.1, 11.4
and 35.0%, respectively, and the mortality rate was 2.1% at 12
months. If patients with EGVB and a high risk of rebleeding
following endoscopic therapy are identified early, the mortality
can be reduced by improving monitoring and providing early clinical
intervention. The gold standard tests used to assess PH and
gastroesophageal varices are measuring hepatic venous pressure
gradient (HVPG) via hepatic vein catheterization and EGD,
respectively. However, measurement of HVPG is invasive and not
routinely performed in all centers. Endoscopy carries certain
risks, such as bleeding and perforation, and it may be
uncomfortable (30). Thus, certain
patients refuse regular EGD as recommended by the Baveno VI
consensus (6). Therefore,
non-invasive models have been used to predict esophagogastric
variceal bleeding as an alternative to EGD (7-9).
The ALBI score is a prognostic tool proposed in
2015, involving two parameters, ALB and TBIL, originally applied
for patients with HCC to assess the severity of liver dysfunction
(12). ALBI is associated with
HVPG and can be used to assess in-hospital mortality in patients
with acute upper gastrointestinal bleeding with liver cirrhosis
(31). The ALBI score has
limitations, as a large number of patients are categorized as ALBI
grade 2(13). Thus, mALBI, in
which grade 2 is divided into grade 2a and 2b, has been proposed
(13). mALBI classification is
associated with the severity of esophagogastric varices in patients
with cirrhosis (32).
In 2015, Roayaie et al (14) developed the PALBI score by adding
platelet counts to the ALBI score and adequately stratified the
survival of patients with HCC. Recent studies have suggested that
the PALBI score can predict rebleeding in patients with acute
variceal bleeding in cirrhosis (33,34).
In 2019, Guha et al (15)
combined ALBI and FIB4 scores to assess risk of decompensation,
including gastrointestinal bleeding, ascites and hepatic
encephalopathy, in patients with compensated cirrhosis. Hsu et
al (35) concluded that
ALBI-FIB4 score shows better predictive ability than the ALBI score
for the risk of decompensatory events.
The liver is the site where coagulation factors,
such as I, II, V, VII and X, are synthesized. When severe liver
disease occurs, the ability of the liver to synthesize coagulation
factors is decreased, resulting in elevated PT and INR (36). Elevated INR is associated with poor
prognosis in patients with liver failure. INR is an independent
predictor of liver fibrosis in chronic hepatitis C and predicts
severe esophageal varices in hepatitis C-induced cirrhosis
(37). In the present training
cohort, INR was higher in the rebleeding group than the
non-rebleeding group and INR could predict rebleeding in patients
with EGVB after endoscopic treatment. This was similar to the
findings of Zhang et al (38), who found that INR ≥1.2 is an
independent predictor of first variceal bleeding in cirrhosis.
Recently, Ding et al (39)
reported that the INR-to-platelet ratio could be used to predict
the extent of liver fibrosis in chronic hepatitis B. Farid et
al (37) used α-fetoprotein,
INR and platelets to develop a model that predicted development of
large esophageal varices in cirrhosis of hepatitis C. To the best
of our knowledge, the present study is the first to combine ALBI
and INR to establish the IALBI model to predict rebleeding in
patients with EGVB following endoscopic therapy. By contrast, Majid
et al (40) demonstrated
that neither ALBI nor MELD scores were correlated with esophageal
varices. In the present training cohort, the AUCs of IALBI for the
prediction of rebleeding were 0.739, 0.697 and 0.620 at 1, 3 and 12
months, respectively. In multivariate analysis, IALBI was an
independent risk factor associated with rebleeding in patients with
EGVB following endoscopic therapy. Therefore, IALBI score could be
used to predict rebleeding following endoscopic treatment in
patients with EGVB. For rebleeding prediction, IALBI score was
better than either the INR or ALBI scores alone. Compared with
other liver function scores (CTP and MELD), IALBI showed better
predictive power and the AUCs of IALBI were higher than those of
MELD at all time points; however, the difference was not
statistically significant.
To the best of our knowledge, the present study is
the first to compare all ALBI-related scores (including mALBI,
PALBI and ALBI-FIB4) with a novel scoring system (IALBI) to analyze
predictive ability for early-, intermediate- and long-term
rebleeding in cirrhotic patients with EGVB following endoscopic
therapy. Time-dependent ROC curves showed that, among all
ALBI-associated scores, AUC of the IALBI grade was consistently
higher than that of ALBI, mALBI, PALBI and ALBI-FIB4 grades at all
time points. This indicated that the proposed classification system
had high predictive power.
The present study validated the predictive power of
the IALBI grading system in different populations in the training
cohort. In the viral group, IALBI score exhibited good predictive
power for rebleeding at all time points. IALBI score had the best
predictive power for early rebleeding. In the non-viral group,
IALBI score was able to predict 1- and 3-month rebleeding and was
superior to ALBI-FIB4 and PALBI scores, while mALBI score only
showed predictive power for 1-month rebleeding. Similarly, in the
validation cohort, IALBI predicted rebleeding, particularly early
rebleeding.
IALBI may have the best predictive ability for
rebleeding, especially early rebleeding, while the predictive
efficacy for rebleeding after 3 months was decreased. This was
similar to the findings of Xavier et al (41), who concluded that ALBI is more
appropriate to evaluate the short-term prognosis of patients with
acute upper gastrointestinal bleeding. Chen et al (42) found that ALBI grade is a useful
score to predict not only the development of post-banding ulcer
bleeding (PBUB) but also 6-week mortality after PBUB. The
predictive power of IALBI for rebleeding decreased with time. This
may be due to external factors, such as NSBB drugs and prophylactic
endoscopic treatment. Liver stiffness measurement (LSM) predicts
rebleeding events of hepatitis B-induced liver cirrhosis (43). Adding other factors (such as LSM)
to IALBI may improve the long-term predictive power of the model.
Future studies will further explore how to improve the stability of
IALBI.
To investigate the predictive power of IALBI score,
the IALBI score was divided into grades 1-3 and Kaplan-Meier curves
were plotted in the training cohort. The present study revealed
marked differences of cumulative rebleeding rates between patients
with grade 1 and 3. The incidence of rebleeding increased with
increasing IALBI grade at all time-points. At each time point,
grade 1 patients had lower risk of rebleeding than grade 2 and 3
patients. For example, at 1 month, rebleeding rate of patients with
IALBI grade 2 was ~10-times higher than that of patients with grade
1, and that of patients with grade 3 was ~19.5 times higher than
that of patients with grade 1. Similarly, cumulative rebleeding
rates of mALBI and PALBI scores differed by grade, while those of
the ALBI-FIB4 score did not. Therefore, patients with IALBI grade 1
had lower risk of rebleeding than those with IALBI grade 2 and
3.
In the training cohort, the predictive value of the
IALBI score was evaluated for different CTP classifications and
etiologies and it was revealed that IALBI had excellent NPVs,
especially for 1-month rebleeding (100% for CTP A, B and C and the
viral group, and 94.9% for the non-viral group) and the NPVs
gradually decreased over time. Therefore, the graded treatment of
patients according to IALBI may avoid unnecessary endoscopic
screening.
In the present study, ALBI-associated scores were
compared with the proposed scoring system. IALBI score was found to
be the best predictor of rebleeding in patients with EGVB following
endoscopic treatment, particularly for prediction of early
rebleeding. Additionally, by grading IALBI score, it was revealed
that risk of rebleeding increased with the increasing IALBI grade
and rate of rebleeding was markedly lower in patients with IALBI
grade 1 than in those with grade 2 and 3. No patients with IALBI
grade 1 experienced rebleeding within 1 month in both the viral and
non-viral groups. IALBI showed excellent NPV.
In conclusion, IALBI score may be a simple,
objective and clinically applicable non-invasive tool for
identification of patients at low risk of rebleeding and who may
not require endoscopic secondary prophylaxis and TIPS in the
short-term. This conclusion should be validated in further research
since this was a single-center study.
Supplementary Material
ROC curves of IALBI in the validation
cohort. ROC curve at (A) 1, (B) 3 and (C) 12 months. ROC, receiver
operating characteristic; IALBI, INR.albumin.bilirubin; AUC, area
under ROC curve; NPV, negative predictive value.
Combination of ALBI and INR as
prognostic indices.
INR, ALBI and IALBI for predicting
rebleeding.
IALBI, CTP and MELD for predicting
rebleeding.
Characteristics of patients with viral
and non-viral cirrhosis.
Summary of cumulative rebleeding rates
across different ALBI related scoring systems.
Baseline characteristics of the
training and the validation cohort.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Tianjin Key Medical
Discipline (Specialty) Construction Project (grant no.
TJYXZDXK-034A).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLi, FL and TW designed the study. FL, TW, BQ, FT
and YG performed data extraction and the data were analyzed by FL,
TW, BQ, FT and JLv. FL and TW confirm the authenticity of all the
raw data. The manuscript draft was prepared by JLv and revised by
JLi, FL and TW. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Third Central Hospital (approval no.
IRB2021-028-01) and performed in accordance with the Helsinki
Declaration of 1975. Written informed consent was obtained from all
study participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alqahtani SA and Jang S: Pathophysiology
and management of variceal bleeding. Drugs. 81:647–667.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Drolz A, Ferlitsch A and Fuhrmann V:
Management of coagulopathy during bleeding and invasive procedures
in patients with liver failure. Visc Med. 34:254–258.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Conn HO, Grace ND, Bosch J, Groszmann RJ,
Rodés J, Wright SC, Matloff DS, Garcia-Tsao G, Fisher RL and Navasa
M: Propranolol in the prevention of the first hemorrhage from
esophagogastric varices: A multicenter, randomized clinical trial.
The Boston-New Haven-Barcelona portal hypertension study group.
Hepatology. 13:902–912. 1991.PubMed/NCBI
|
|
4
|
Kezer CA and Gupta N: The role of
therapeutic endoscopy in patients with cirrhosis-related causes of
gastrointestinal bleeding. Curr Gastroenterol Rep.
20(31)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cheung J, Zeman M, van Zanten SV and
Tandon P: Systematic review: Secondary prevention with band
ligation, pharmacotherapy or combination therapy after bleeding
from oesophageal varices. Aliment Pharmacol Ther. 30:577–588.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de*Franchis R and Faculty B: Expanding
consensus in portal hypertension: Report of the Baveno VI consensus
workshop: Stratifying risk and individualizing care for portal
hypertension. J Hepatol. 63:743–752. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kothari HG, Gupta SJ, Gaikwad NR,
Sankalecha TH and Samarth AR: Role of non-invasive markers in
prediction of esophageal varices and variceal bleeding in patients
of alcoholic liver cirrhosis from Central India. Turk J
Gastroenterol. 30:1036–1043. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang SF, Huang YT, Huang CH, Chang SH and
Lin CY: Fibrosis index predicts variceal bleeding and reduces need
for nonselective beta-blocker in compensated cirrhosis with initial
small esophageal varices without red-color sign. Ann Transl Med.
8(1223)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cifci S and Ekmen N: Evaluation of
non-invasive fibrosis markers in predicting esophageal variceal
bleeding. Clin Endosc. 54:857–863. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang J, Zhang Z, Yan X, Li M, Xia J, Liu
Y, Chen Y, Jia B, Zhu L, Zhu C, et al: Albumin-Bilirubin (ALBI) as
an accurate and simple prognostic score for chronic hepatitis
B-related liver cirrhosis. Dig Liver Dis. 51:1172–1178.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oikonomou T, Goulis L, Doumtsis P,
Tzoumari T, Akriviadis E and Cholongitas E: ALBI and PALBI grades
are associated with the outcome of patients with stable
decompensated cirrhosis. Ann Hepatol. 18:126–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hiraoka A, Kumada T, Tsuji K, Takaguchi K,
Itobayashi E, Kariyama K, Ochi H, Tajiri K, Hirooka M, Shimada N,
et al: Validation of modified ALBI grade for more detailed
assessment of hepatic function in hepatocellular carcinoma
patients: A multicenter analysis. Liver Cancer. 8:121–129.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Roayaie S, Jibara G, Berhane S, Tabriz-ian
P, Park JW, Yang J, Yan L, Han G, Izzo F, Chen M, et al: PALBI-an
objective score based on platelets, Albumin & Bilirubin
stratifies HCC patients undergoing resection & ablation better
than child's classification. Hepatology. 62:631A–632A. 2015.
|
|
15
|
Guha IN, Harris R, Berhane S, Dillon A,
Coffey L, James MW, Cucchetti A, Harman DJ, Aithal GP, Elshaarawy
O, et al: Validation of a model for identification of patients with
compensated cirrhosis at high risk of decompensation. Clin
Gastroenterol Hepatol. 17:2330–2338.e2331. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ho SY, Liu PH, Hsu CY, Chiou YY, Su CW,
Lee YH, Huang YH, Lee FY, Hou MC and Huo T: Prognostic performance
of ten liver function models in patients with hepatocellular
carcinoma undergoing radiofrequency ablation. Sci Rep.
8(843)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim HJ, Lee HK and Cho JH: Factor analysis
of the biochemical markers related to liver cirrhosis. Pak J Med
Sci. 31:1043–1046. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tait IS, Krige JE and Terblanche J:
Endoscopic band ligation of oesophageal varices. Br J Surg.
86:437–446. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mishra SR, Sharma BC, Kumar A and Sarin
SK: Endoscopic cyanoacrylate injection versus beta-blocker for
secondary prophylaxis of gastric variceal bleed: A randomised
controlled trial. Gut. 59:729–735. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tajiri T, Yoshida H, Obara K, Onji M, Kage
M, Kitano S, Kokudo N, Kokubu S, Sakaida I, Sata M, et al: General
rules for recording endoscopic findings of esophagogastric varices
(2nd edition). Dig Endosc. 22:1–9. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seo YS: Prevention and management of
gastroesophageal varices. Clin Mol Hepatol. 24:20–42.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Malinchoc M, Kamath PS, Gordon FD, Peine
CJ, Rank J and ter*Borg PC: A model to predict poor survival in
patients undergoing transjugular intrahepatic portosystemic shunts.
Hepatology. 31:864–871. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, Sulkowski MS, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nahm FS: Receiver operating characteristic
curve: Overview and practical use for clinicians. Korean J
Anesthesiol. 75:25–36. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim JH: Multicollinearity and misleading
statistical results. Korean J Anesthesiol. 72:558–569.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Robin X, Turck N, Hainard A, Tiberti N,
Lisacek F, Sanchez JC and Müller M: pROC: An open-source package
for R and S+ to analyze and compare ROC curves. BMC Bioinformatics.
12(77)2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lv Y, Qi X, He C, Wang Z, Yin Z, Niu J,
Guo W, Bai W, Zhang H, Xie H, et al: Covered TIPS versus endoscopic
band ligation plus propranolol for the prevention of variceal
rebleeding in cirrhotic patients with portal vein thrombosis: A
randomised controlled trial. Gut. 67:2156–2168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bari K and Garcia-Tsao G: Treatment of
portal hypertension. World J Gastroenterol. 18:1166–1175.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Levy I and Gralnek IM: Complications of
diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best
Pract Res Clin Gastroenterol. 30:705–718. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hsieh YC, Lee KC, Wang YW, Yang YY, Hou
MC, Huo TI and Lin HC: Correlation and prognostic accuracy between
noninvasive liver fibrosis markers and portal pressure in
cirrhosis: Role of ALBI score. PLoS One.
13(e0208903)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Miyamoto Y, Enomoto H, Nishikawa H,
Nishimura T, Iwata Y, Nishiguchi S and Iijima H: Association of the
modified ALBI grade with endoscopic findings of gastroesophageal
varices. In Vivo. 35:1163–1168. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Faisal MS, Singh T, Amin H and Esfeh JM:
Role of platelet-albumin-bilirubin score in predicting re-bleeding
after band ligation for acute variceal hemorrhage. World J Hepatol.
12:880–882. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Elshaarawy O, Allam N, Abdelsameea E,
Gomaa A and Waked I: Platelet-albumin-bilirubin score-a predictor
of outcome of acute variceal bleeding in patients with cirrhosis.
World J Hepatol. 12:99–107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hsu CY, Parikh ND, Huo TI and Tapper EB:
Comparison of seven noninvasive models for predicting
decompensation and hospitalization in patients with cirrhosis. Dig
Dis Sci. 66:4508–4517. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hollestelle MJ, Geertzen HG, Straatsburg
IH, van Gulik TM and van*Mourik JA: Factor VIII expression in liver
disease. Thromb Haemost. 91:267–275. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Farid K, Omran MM, Farag RE, Arafa MM and
Emran TM: Development and evaluation of a novel score for
prediction of large oesophageal varices in patients with hepatitis
C virus-induced liver cirrhosis. Br J Biomed Sci. 74:138–143.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang S, Song W, Yang B, Jia H, Chen S, Li
J and Yang C: A non-invasive model based on the virtual portal
pressure gradient to predict the first variceal hemorrhage in
cirrhotic patients. Hepatol Int. 16:926–935. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ding R, Zheng J, Huang D, Wang Y, Li X,
Zhou X, Yan L, Lu W, Yang Z and Zhang Z: INR-to-platelet ratio
(INPR) as a novel noninvasive index for predicting liver fibrosis
in chronic hepatitis B. Int J Med Sci. 18:1159–1166.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Majid Z, Khan SA, Akbar N, Khalid MA,
Hanif FM, Laeeq SM and Luck NH: The use of albumin-to-bilirubin
score in predicting variceal bleed: A pilot study from Pakistan.
Euroasian J Hepatogastroenterol. 12:77–80. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xavier SA, Vilas-Boas R, Carvalho PB,
Magalhães JT, Marinho CM and Cotter JB: Assessment of prognostic
performance of albumin-bilirubin, child-pugh, and model for
end-stage liver disease scores in patients with liver cirrhosis
complicated with acute upper gastrointestinal bleeding. Eur J
Gastroenterol Hepatol. 30:652–658. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen CW, Kuo CJ, Lee CW, Kuo T, Chiu CT,
Lin CJ, Lim SN, Yeh CT and Lin WR: Albumin-bilirubin grade as a
novel predictor of the development and short-term survival of
post-banding ulcer bleeding following Endoscopic Variceal Ligation
in Cirrhotic patients. Medicina (Kaunas). 58(1836)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu L, Nie Y, Zhang Y, Liu Q and Zhu X:
Liver stiffness is a predictor of rebleeding in patients with
hepatitis b-related cirrhosis: A real-world cohort study. Front Med
(Lausanne). 8(690825)2021.PubMed/NCBI View Article : Google Scholar
|