Introduction
Oral squamous cell carcinoma (OSCC) represents the
most frequent form of head and neck squamous cell carcinoma,
accounting for 90% of all oral cancer cases worldwide. In addition,
OSCC is associated with a poor prognosis with a 5-year overall
survival rate of only ~50% globally (1,2). A
previous study reported that >30% of patients with OSCC may
develop multiple tumors within 5-10 years, which further aggravates
this prognosis (3). Although great
progress has been made in surgical techniques, radiotherapy and
chemotherapy, the survival rate remains relatively low, due to the
local recurrence and metastasis of the disease (4,5).
Therefore, an in-depth understanding of the molecular mechanism
underlying the occurrence and development of OSCC is of crucial
importance for the development of new therapeutic strategies.
Cancerous inhibitor of protein phosphatase 2A
(CIP2A), originally named KIAA1524 or p90, has been reported to act
as an oncogene by promoting tumor growth in several types of
cancer, including bladder cancer, non-small cell lung cancer and
colorectal cancer (6-8).
Notably, CIP2A is also highly expressed in OSCC tissues, and high
CIP2A expression is significantly related to poor prognosis and
short survival time (9,10). A previous study indicated that
CIP2A participates in the regulation of tumor angiogenesis
(11), and downregulation of CIP2A
has been shown to suppress the proliferation and vascularization of
renal clear cell carcinoma cells (12). In addition, the glycogen synthase
kinase (GSK)-3β/β-catenin pathway serves a crucial role in
regulating the proliferation, migration and angiogenesis of cancer
cells (13,14). Inhibiting GSK-3β/β-catenin
signaling has been demonstrated to suppress the progression of OSCC
(15). Whether CIP2A can affect
the malignant phenotypes and angiogenesis of OSCC by regulating the
GSK-3β/β-catenin pathway remains to be investigated. The STRING
database predicted that there may be an interaction between CIP2A
and AKT1. It has also been reported that CIP2A regulates cell
proliferation via the AKT signaling pathway in human lung cancer
(16), and that CIP2A
downregulation may induce apoptosis via inhibition of the AKT
signaling pathway in breast cancer (17). AKT1 has been reported to be
involved in accelerating the malignant progression of various types
of cancer, including pancreatic cancer, epithelial ovarian cancer
and colorectal cancer (18-20).
In particular, AKT1 expression has been shown to be significantly
elevated in OSCC tissues (21).
Furthermore, AKT1 has been demonstrated to promote tumor
angiogenesis (22) and to regulate
GSK-3β/β-catenin signaling to participate in the progression of
various types of cancer, including prostate cancer, glioma and
breast cancer (23-25).
Therefore, the present study aimed to assess the relationship
between CIP2A and AKT1, and to determine their roles in the
malignant progression and angiogenesis of OSCC.
In the present study, the expression of CIP2A and
AKT1 in OSCC cell lines was detected. Subsequently, further
experiments analyzed the effects of CIP2A silencing on the
malignant progression and angiogenesis of OSCC cells, and explored
the relationship between CIP2A and AKT1 to reveal the mechanisms
underlying OSCC.
Materials and methods
Cell culture
Three human OSCC cell lines, HN-4, SCC-9 and CAL-27,
provided by the American Type Culture Collection were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.). The human normal oral
keratinocytes (HOKs; cat. no. 2610) and human umbilical vein
endothelial cells (HUVECs; cat. no. 8000) were obtained from
ScienCell Research Laboratories, Inc. The HOK cells were grown in
oral keratinocyte growth medium (ScienCell Research Laboratories,
Inc.) and the HUVECs were cultured in endothelial cell medium
(Gibco; Thermo Fisher Scientific, Inc.). All media were
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and the cells were cultured in a humidified
atmosphere containing 5% CO2 at 37˚C.
Transfection
For transfection, the short hairpin RNAs (shRNAs)
targeting CIP2A (shRNA-CIP2A-1, sense, 5'-GCAGTGCATTCAACTTCTACA-3',
antisense, 5'-TGTAGAAGTTGAATGCACTGC-3'; shRNA-CIP2A-2, sense,
5'-CGCTGGTTAAGCCAACCTTTG-3', antisense,
5'-CAAAGGTTGGCTTAACCAGCG-3'), the empty shRNA plasmid (shRNA-NC,
sense, 5'-TTCTCCGAACGTGTCACGT-3', antisense,
5'-ACGTGACACGTTCGGAGAA-3'), pc-DNA3.1 vectors containing the
complete sequence of AKT1 (Ov-AKT1) and the empty vector plasmid
(Ov-NC) were supplied by Shanghai GenePharma Co., Ltd. CAL-27 cells
at the logarithmic phase were seeded in a 6-well plate
(1x105 cells/well) and were incubated at 37˚C for 24 h.
A total of 100 nM recombinant was transfected into cells at 70%
confluence using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h at 37˚C. The transfected cells
were collected 48 h post-transfection for subsequent
experiments.
Cell viability assay
The transfected CAL-27 cells were inoculated in
96-well plates at a density of 2,000 cells/well. After incubation
for 24, 48 and 72 h, 10 µl Cell Counting Kit-8 (CCK-8) solution
(Beijing Solarbio Science & Technology Co., Ltd.) was added to
each well. The 96-well plate was cultured in the incubator for 1 h
and the optical density at 450 nm was detected using an
enzyme-labeled instrument.
5-ethynyl-2'-deoxyuridine (EdU)
staining
An EdU kit (BeyoClick™ EdU Cell Proliferation Kit
with Alexa Fluor 488; Beyotime Institute of Biotechnology) was used
to detect cell proliferation. Following incubation for 3 days in
24-well plates, CAL-27 cells were incubated with 50 µM EdU, fixed
with 4% paraformaldehyde for 30 min at 37˚C and stained with DAPI
in the dark at room temperature for 30 min. Finally, the
proliferation of cells was observed and images were captured using
an inverted fluorescence microscope (Nikon Corporation).
TUNEL staining
The apoptosis of transfected cells was assessed
using the TUNEL assay (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. CAL-27 cells
(2x104 cells/well) were cultured on glass coverslips
overnight. After being fixed with 4% paraformaldehyde at 37˚C for 1
h, the cells were blocked with 3% H2O2 for 20
min at room temperature. After permeabilization with 0.1% Triton
X-100 at 4˚C for 2 min, the slides were incubated with 50 µl TUNEL
reaction mixture for 1 h at 37˚C, mounted with
Vectashield® mounting medium containing 1 mg/ml DAPI
solution for 5 min at 37˚C in the dark, and cell samples in five
randomly selected fields were analyzed using an inverted
fluorescence microscope (Nikon Corporation).
Wound healing assay
The CAL-27 cells were seeded into six-well plates at
a density of 2x105/well and cultured until the cells
reached ~100% confluence. The confluent cells were scratched using
a sterilized 10-µl pipette tip. The detached cells were removed by
washing with PBS. Subsequently, the medium was replaced with
serum-free DMEM and the cells were incubated at 37˚C. After 24 h,
images of cell migration were captured using an inverted light
microscope (Nikon Corporation) and the wound area was measured
using ImageJ software version 1.7.0 (National Institutes of
Health).
Transwell invasion assay
After transfection, CAL-27 cells were collected and
5x104 cells were added to 200 µl serum-free medium in
the upper chambers precoated with Matrigel (BD Biosciences) at 37˚C
for 30 min. Normal medium containing 10% FBS was added to the lower
chamber. After culturing for 24 h, the invasive cells were fixed
with 4% paraformaldehyde at room temperature for 30 min and stained
with 0.1% crystal violet at room temperature for 10 min.
Observation of invasive cells was performed using an inverted light
microscope (Nikon Corporation).
HUVECs tube formation assay
Matrigel (200 µl) was pipetted into each well of
24-well plates and melted overnight at 4˚C. HUVECs
(5x104) were serum-starved in medium overnight at 37˚C
and then seeded into plates precoated with Matrigel. After
incubation at 37˚C for 30 min, the starved HUVECs were cultured
with 200 µl conditioned medium for 8 h at 37˚C with 5%
CO2. The conditioned medium was collected from the
supernatants of transfected CAL-27 cells through centrifugation at
500 x g at room temperature for 10 min. Tube formation was viewed
and images were captured under an inverted light microscope (Nikon
Corporation).
Co-immunoprecipitation (Co-IP)
assay
The transfected cells were lysed on ice for 30 min
in RIPA lysis buffer (Beyotime Institute of Biotechnology)
containing protease inhibitors. The supernatant was collected after
centrifugation at 13,000 x g for 10 min at 4˚C. Then, 0.2 mg
protein A agarose beads (Thermo Fisher Scientific, Inc.) were
exposed to 500 µg lysis buffer and incubated with 2 µg IgG antibody
(cat. no. ab313801; 1:30; Abcam) or CIP2A antibody (cat. no.
#14805; 1:100; Cell Signaling Technology) or AKT1 antibody (cat.
no. ab182729; 1:40; Abcam) overnight at 4˚C. Following the IP
reaction and centrifugation at 1,000 x g at 4˚C for 2 min, the
agarose beads were rinsed utilizing lysis buffer and boiled for 5
min at 100˚C. Western blot analysis was performed to analyze the
expression of target proteins.
Western blot analysis
The cells were lysed in RIPA lysis buffer (Beyotime
Institute of Biotechnology) and the lysates were centrifuged at
12,000 x g for 10 min at 4˚C to obtain the proteins, the
concentration of which was determined using the bicinchoninic acid
method. Protein samples (40 µg/per lane) were separated by SDS-PAGE
on 10% gels and then transferred to PVDF membranes. After blocking
in 5% non-fat milk for 1 h at room temperature, these membranes
were probed with primary antibodies overnight at 4˚C, followed by
incubation with a HRP-conjugated secondary antibody (cat. no.
7074P2; 1:5,000; Cell Signaling Technology, Inc.) at room
temperature for 1 h. Chemiluminescence was used to expose the
immunoreactive protein bands on the membrane using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc.). The band
intensities were semi-quantified relative to GAPDH and gray
intensity analysis was performed using ImageJ software version
1.8.0 (National Institutes of Health). Anti-CIP2A (cat. no. 14805S;
1:1,000), anti-Bcl2 (cat. no. 4223T; 1:1,000), anti-Bax (cat. no.
41162S; 1:1,000), anti-AKT1 (cat. no. 75692S; 1:1,000),
anti-phosphorylated (p)-GSK-3β (cat. no. 5558T; 1:1,000),
anti-GSK-3β (cat. no. 12456T; 1:1,000), anti-β-catenin (cat. no.
8480T; 1:1,000) and anti-GAPDH (cat. no. 5174T; 1:1,000) antibodies
were obtained from Cell Signaling Technology, Inc.
Bioinformatics tools
STRING database (https://string-db.org/) was used to predict the
proteins that can interact with CIP2A.
Statistical analysis
GraphPad Prism 8.0 (Dotmatics) was used for the
statistical analysis. All experimental data are presented as the
mean ± standard deviation of three experiments. One-way analysis of
variance followed by Tukey's post hoc test was performed to compare
the data from multiple groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
CIP2A is highly expressed in OSCC
cells and interference with CIP2A inhibits the proliferation of
OSCC cells
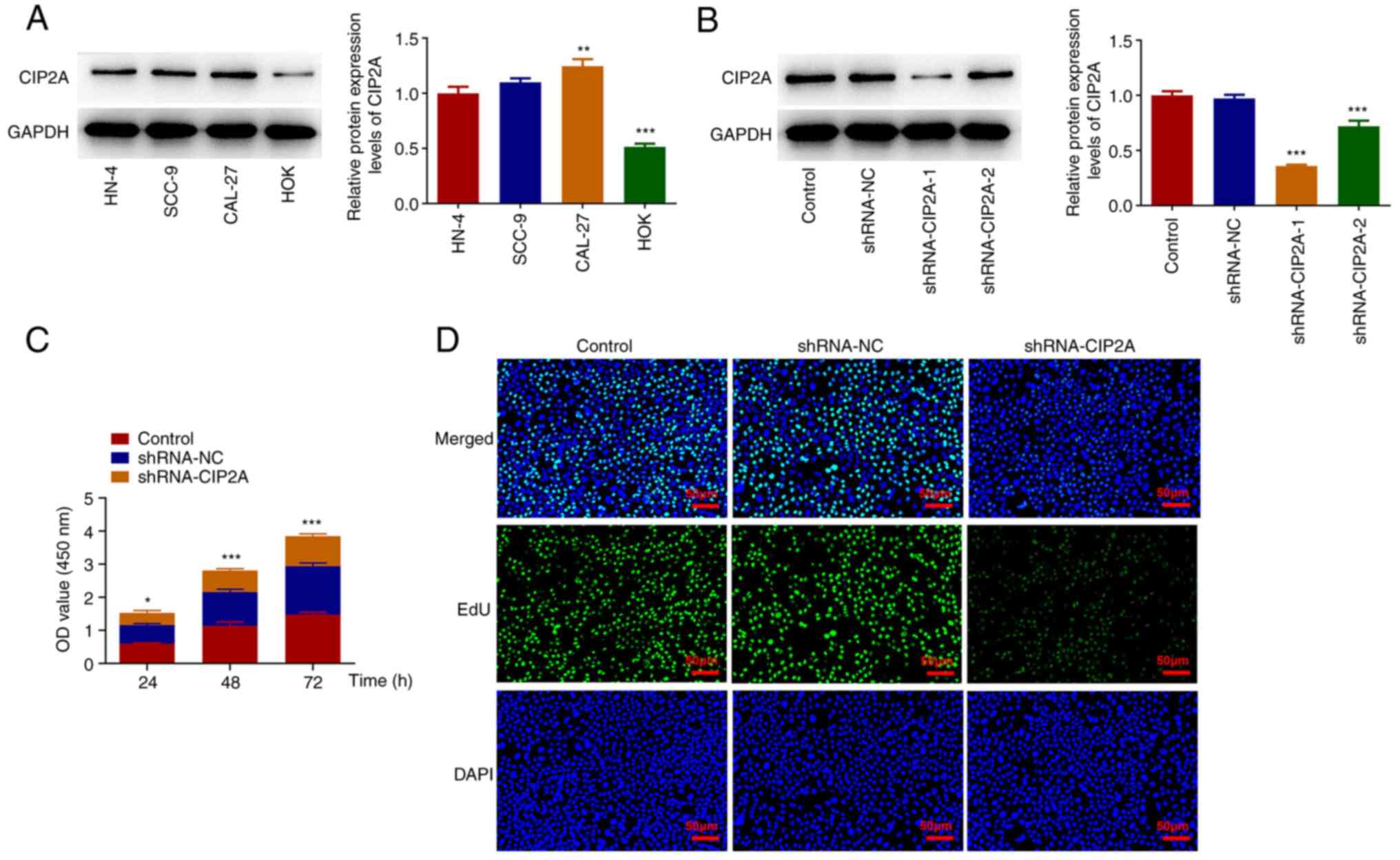
Firstly, CIP2A expression in three human OSCC cell
lines and HOKs was assessed by western blotting. As shown in
Fig. 1A, CIP2A protein expression
levels were significantly elevated in HN-4, SCC-9 and CAL-27 cells
compared with those in HOKs. CAL-27 cells were chosen to perform
the subsequent experiments, as they had the highest expression of
CIP2A in all of the aforementioned OSCC cell lines. CIP2A was
silenced in CAL-27 cells to observe its effect on cell viability
and cell proliferation. CIP2A expression was significantly
downregulated post-transfection with shRNA-CIP2A-1/2 compared with
that in the shRNA-NC group. The lowest CIP2A expression was
observed in the shRNA-CIP2A-1 group; therefore, shRNA-CIP2A-1 was
selected for the subsequent experiments. As shown in Fig. 1C, knockdown of CIP2A reduced the
viability of CAL-27 cells relative to the shRNA-NC group.
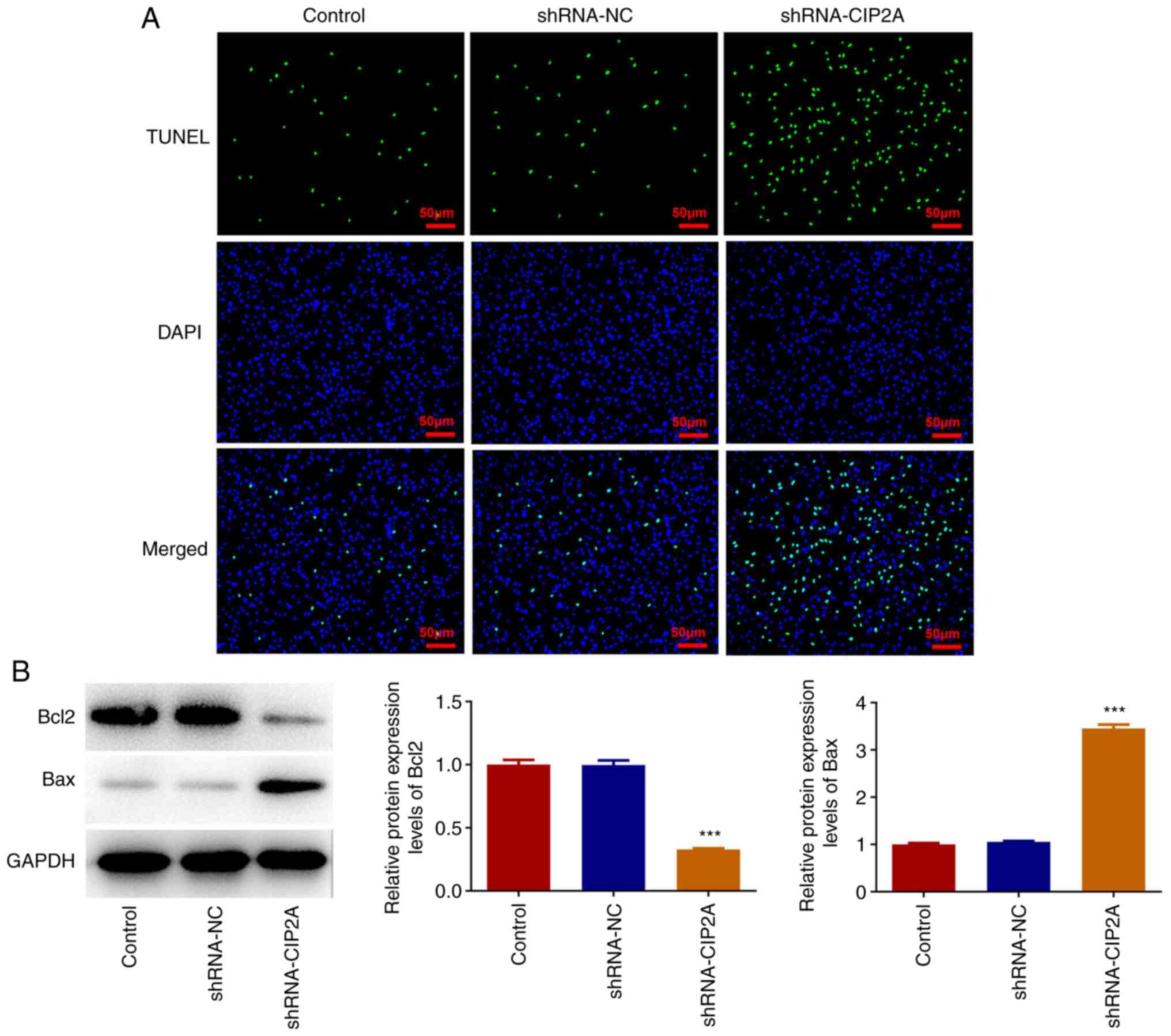
Consistently, the marked decrease in EdU fluorescence intensity and
increase in TUNEL fluorescence intensity of the shRNA-CIP2A group
suggested the inhibitory effect of CIP2A silencing on proliferation
and the promoting effect of CIP2A silencing on the apoptosis of
CAL-27 cells (Figs. 1D and
2A). In addition, CIP2A knockdown
significantly downregulated Bcl2 expression and upregulated Bax
expression when compared with the shRNA-NC group (Fig. 2B). These results indicated that
knockdown of CIP2A may inhibit the proliferation of OSCC cells.
Knockdown of CIP2A alleviates the
migration and invasion of OSCC cells, and restricts angiogenesis of
HUVECs
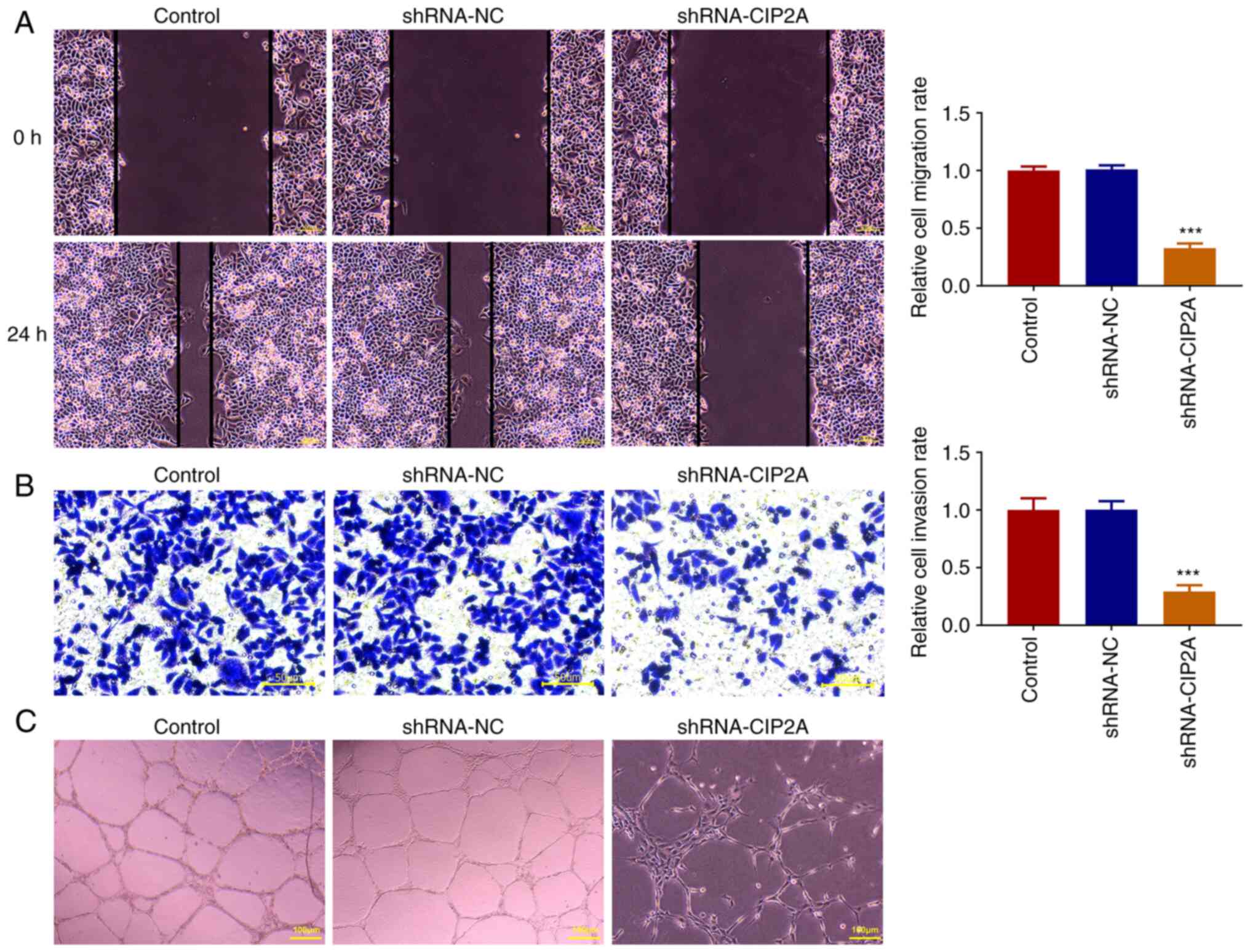
The wound healing assay was used to evaluate the
migration of CAL-27 cells after transfection with shRNA-CIP2A.
CIP2A silencing significantly reduced the relative cell migration
rate compared with the shRNA-NC group, with an apparent decrease in
wound closure (Fig. 3A). The
Transwell assay also revealed the same trend in cell invasion as
that of cell migration (Fig. 3B).
Additionally, tube formation was significantly restricted, with a
decreased number of nodes in the CIP2A-silenced group compared with
that in the shRNA-NC group (Fig.
3C). Together, these data indicated that knockdown of CIP2A may
attenuate the migration and invasion of OSCC cells, and restrict
the angiogenesis of HUVECs.
AKT1 can interact with CIP2A in OSCC
cells
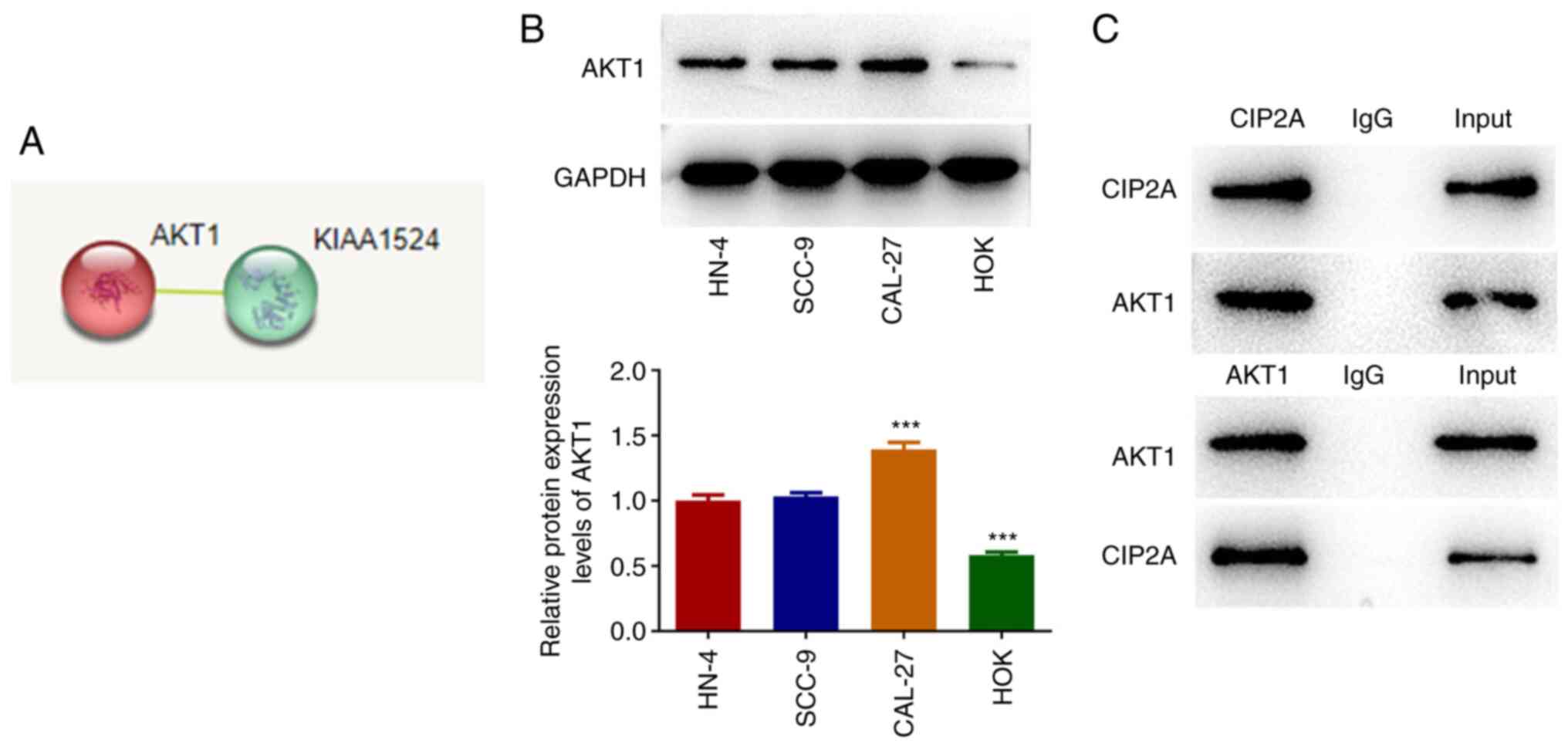
To explore the potential mechanism of CIP2A in the
regulation of CAL-27 cells, the STRING database was used to predict
the proteins that could interact with CIP2A, and AKT1 was found to
interact with CIP2A (Fig. 4A).
Compared with in HOK cells, AKT1 expression was significantly
elevated in the HN-4, SCC-9 and CAL-27 cell lines (Fig. 4B). Co-IP assay confirmed the
interaction between AKT1 and CIP2A in CAL-27 cells (Fig. 4C).
AKT1 overexpression reverses the
inhibitory effects of CIP2A silencing on the proliferation,
migration and invasion of OSCC cells, and angiogenesis of
HUVECs
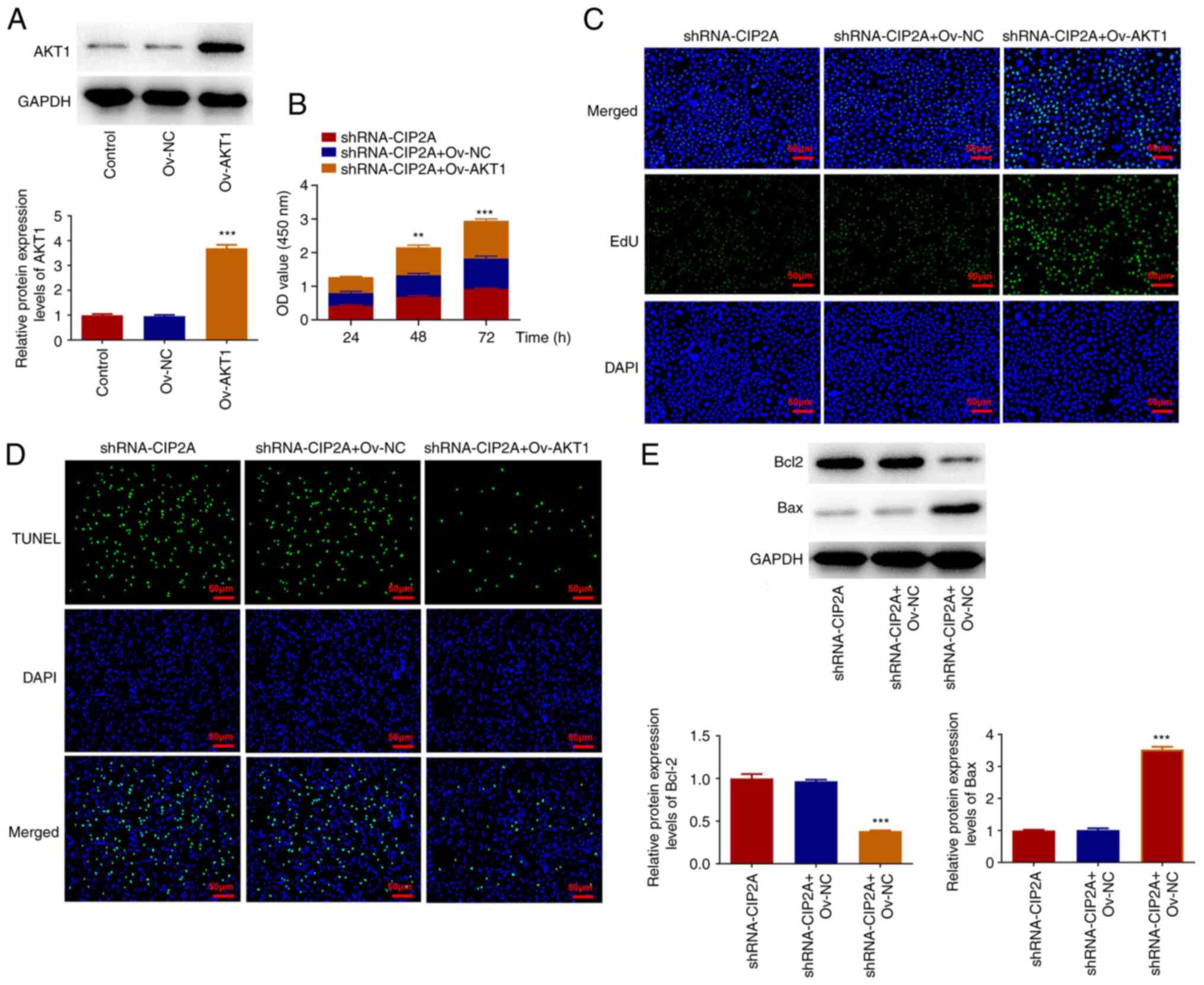
Subsequently, AKT1 was overexpressed to analyze its
effects on malignant phenotypes of CAL-27 cells transfected with
shRNA-CIP2A. As shown in Fig. 5A,
AKT1 expression was significantly increased in the Ov-AKT1 group
compared with that in the Ov-NC group. The results of the CCK-8
assay suggested that AKT1 overexpression enhanced the viability of
CAL-27 cells compared with that in the shRNA-CIP2A + Ov-NC group
(Fig. 5B). Furthermore, the EdU
fluorescence intensity was strengthened and TUNEL fluorescence
intensity was reduced after AKT1 overexpression in CIP2A-silenced
CAL-27 cells (Fig. 5C and D). Bcl2 expression was downregulated,
whereas Bax expression was upregulated in the shRNA-CIP2A + Ov-AKT1
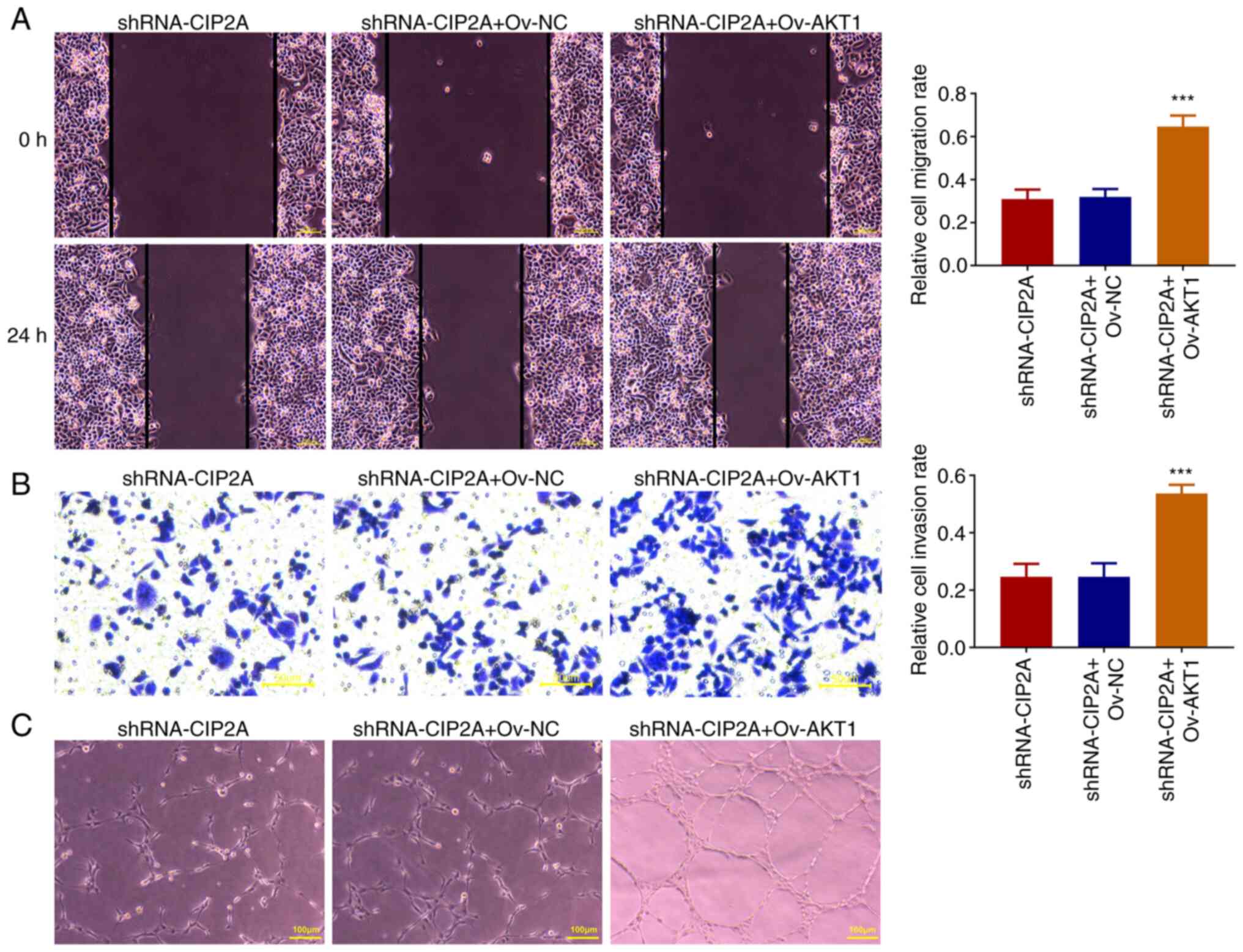
group compared with the shRNA-CIP2A + Ov-NC group (Fig. 5E). The results of wound healing and
Transwell invasion assays suggested that AKT1 overexpression
elevated the migration and invasion of CAL-27 cells when compared
with the shRNA-CIP2A + Ov-NC group (Fig. 6A and B). Furthermore, tube formation was
markedly increased with an elevated number of nodes in the Ov-AKT1
group as compared with that in the shRNA-CIP2A + Ov-NC group
(Fig. 6C). These data indicated
that AKT1 overexpression may relieve the effects of CIP2A silencing
on the viability, proliferation, migration and invasion of OSCC
cells, and angiogenesis of HUVECs.
AKT1 overexpression alleviates
inactivation of the GSK-3β/β-catenin signaling induced by CIP2A
knockdown in OSCC cells
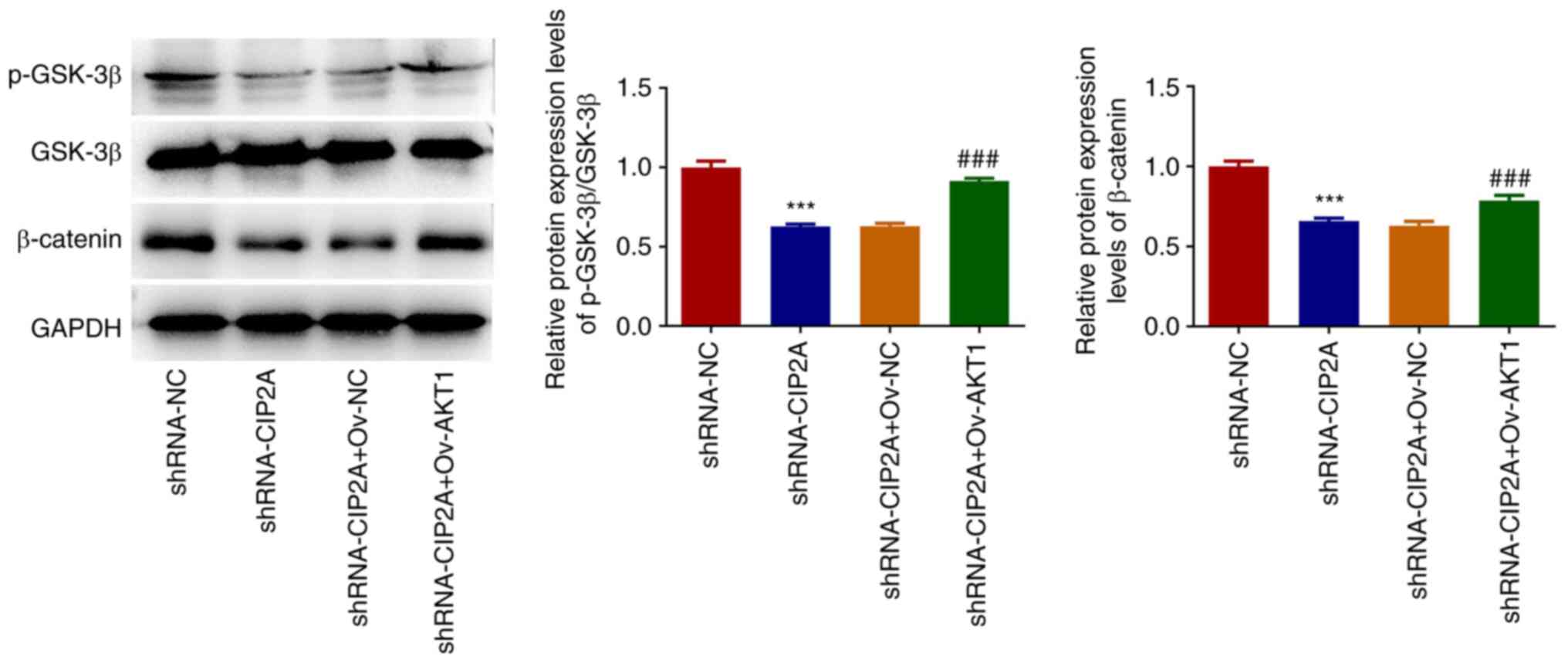
To further clarify the downstream mechanism of CIP2A
and AKT1 in the regulation of OSCC malignant biological behaviors,
the expression levels of proteins in the GSK-3β/β-catenin signaling
pathway were detected by western blotting. As shown in Fig. 7, CIP2A knockdown significantly
reduced the expression levels of p-GSK-3β and β-catenin compared
with that in the shRNA-NC group. However, AKT1 overexpression
alleviated inactivation of the GSK-3β/β-catenin signaling pathway
induced by CIP2A knockdown in CAL-27 cells.
Discussion
OSCC is the most common malignant tumor of the oral
cavity. Due to the high invasiveness of OSCC, a large number of
patients often present with the advanced stages of the disease at
the time of diagnosis, and therefore face complex surgical
procedures and poor prognosis (26). Research aimed at understanding the
molecular mechanism underlying distant metastasis of OSCC has
garnered attention, with the aim of identifying potential target
molecules and achieving targeted therapy. The present study
detected upregulation of CIP2A in OSCC cell lines. Furthermore,
knockdown of CIP2A markedly attenuated the proliferation, migration
and invasion of OSCC cells, and the angiogenesis of HUVECs.
Notably, it was revealed that AKT1 could interact with CIP2A to
reverse the effects of CIP2A knockdown on the malignant biological
behaviors of OSCC cells. Considering these results, CIP2A/AKT1
could be developed as potent antitumor agents targeting OSCC.
Abnormal and accelerated proliferation of cells is a
characteristic of tumors. Malignant tumors require nutrients and
oxygen to survive and proliferate; therefore, they need to grow
near blood vessels to enter the bloodstream. The more
neovascularization in the tumor, the higher the malignant degree
(27). A critical event
contributing to this metastasis is tumor-related angiogenesis
(28). Tumor cell proliferation,
invasion and metastasis are achieved through angiogenesis (29). Continuous angiogenesis is
increasingly being recognized as having a crucial role in the
growth and metastasis of solid tumors, including OSCC, as it can
support tumor growth as well as the metastasis of malignant cells
from the primary tumor site to distant organs (30,31).
As a well-known oncoprotein, the expression of which is elevated in
multiple human solid tumor types, abnormal CIP2A expression in
cancer has been demonstrated in previous studies (32). For example, CIP2A expression has
been reported to be higher in renal cell carcinoma (RCC) cells
compared with that in renal tubular epithelial cells (HK-2 cells),
and CIP2A gain-of-function can strengthen the proliferation and
invasion of RCC cells (33). By
enhancing the proliferative capacity and restraining the apoptotic
ability, CIP2A has also been considered a promising therapeutic
strategy for patients with multiple myeloma (34). Significantly elevated CIP2A
expression has also been found in osteosarcoma, endometrioid
adenocarcinoma and laryngeal carcinoma, in which CIP2A can promote
the malignant biological behaviors of these tumor cells (35-37).
Notably, CIP2A is highly expressed in OSCC tissues, and high CIP2A
expression has been reported to be related to poor prognosis and
short survival time (9,10). Our previous study indicated that
CIP2A is implicated in the regulation of tumor angiogenesis
(11). Similarly, the present
study revealed that the expression of CIP2A was significantly
elevated in OSCC cell lines, and silencing of CIP2A exerted
inhibitory effects on the proliferation, migration and invasion of
OSCC cells, and the angiogenesis of HUVECs.
To assess the mechanisms underlying the regulatory
effects of CIP2A on OSCC progression, the STRING database was used
to predict the proteins that could interact with CIP2A. AKT1 was
revealed to interact with CIP2A in the present study.
CIP2A-mediated AKT activation serves a role in bortezomib-induced
apoptosis in head and neck squamous cell carcinoma cells (38). Furthermore, inhibition of CIP2A and
its downstream AKT/mTOR signaling cascade can potentiate the
chemosensitivity of A549/cisplatin cells to cisplatin by enhancing
apoptosis (39). A number of
studies have suggested that AKT1, which is the predominantly
expressed and best-characterized isoform of AKT in numerous types
of cancer, serves as the target protein of a number of genes to
exert carcinogenic effects in various types of cancer by
accelerating malignant progression, including breast cancer,
gastric cancer and papillary thyroid cancer (40-42).
A previous study demonstrated that AKT1 is responsible for the
growth and survival of endothelial cells, which serve a crucial
role in the angiogenesis (43).
Notably, AKT1 expression has been shown to be significantly
elevated in OSCC tissues (21).
Similar to the previous results reported in the aforementioned
literature, the present study also revealed that AKT1 expression
was markedly upregulated in OSCC cells. Notably, it was
demonstrated that AKT1 overexpression reversed the inhibitory
effects of CIP2A knockdown on the proliferation, migration and
invasion of OSCC cells, and angiogenesis of HUVECs. These findings
suggested the importance of the CIP2A/AKT1 axis in regulation of
the malignant biological behaviors of OSCC.
To further investigate the downstream signaling
pathway that could be regulated by CIP2A/AKT1, the expression
levels of proteins in the GSK-3β/β-catenin signaling pathway were
evaluated by western blotting in CAL-27 cells transfected with
shRNA-CIP2A and Ov-AKT1. β-catenin acts as a central regulator in
multiple physiological processes, and overexpression of β-catenin
has been reported to be associated with various types of cancer,
and to have an important role in cancer metastasis and angiogenesis
(44-46).
Degradation of β-catenin is mediated by the phosphokinase activity
of GSK-3β, a serine/threonine kinase (47-49).
Evidence has documented that the GSK-3β/β-catenin pathway is
implicated in various types of cancer, such as hepatocellular
carcinoma, breast cancer and colorectal cancer (50-52).
Notably, inhibiting the GSK-3β/β-catenin signaling has been
demonstrated to suppress the proliferation and promote the
apoptosis of OSCC cells (15). By
modulating the GSK-3β/β-catenin signaling pathway, the
microRNA-203/SNAI2 axis can regulate prostate tumor growth,
migration and angiogenesis (13).
Galectin-3 favors tumor angiogenesis in hepatocellular carcinoma
via activation of the AKT/GSK-3β/β-catenin signaling cascade
(46). Moreover, SAMD9 promotes
tumor stemness and angiogenesis of esophageal squamous cell
carcinoma by stimulating MYH9-mediated GSK3β/β-catenin signaling
(53). Notably, AKT1 has been
reported to regulate GSK-3β/β-catenin signaling to accelerate the
progression of gastric cancer, colorectal cancer and pancreatic
carcinoma (49,54,55).
In the present study, CIP2A knockdown markedly downregulated
p-GSK-3β and β-catenin expression, which was reversed by the
overexpression of AKT1 in CAL-27 cells. These results demonstrated
that CIP2A/AKT1 could promote the malignant behaviors of OSCC by
activating the GSK-3β/β-catenin pathway.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that CIP2A knockdown
suppresses the proliferation, migration and invasion of OSCC cells,
and the angiogenesis of HUVECs. Mechanistically, CIP2A can interact
with AKT1 to promote the malignant biological behaviors of OSCC by
upregulating the GSK-3β/β-catenin pathway. These findings may
provide a new insight into the mechanism underlying OSCC and
provide potential therapeutic targets for OSCC. Nevertheless, the
present study has a limitation; the regulatory effects of CIP2A and
AKT1 were only discussed on the progression of OSCC cells. Further
in vivo experiments involving transgenic animals will be
further explored in future investigations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and XW designed the study, and drafted and
revised the manuscript. HZ and HL analyzed the data and searched
the literature. YC and XW confirm the authenticity of all the raw
data. All authors performed the experiments. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Aerospace Center Hospital
(Beijing, China) waived the requirement for ethics approval for
using the purchased human normal oral keratinocytes and human
umbilical vein endothelial cells.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Montero PH and Patel SG: Cancer of the
oral cavity. Surg Oncol Clin N Am. 24:491–508. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ren ZH, Hu CY, He HR, Li YJ and Lyu J:
Global and regional burdens of oral cancer from 1990 to 2017:
Results from the global burden of disease study. Cancer Commun
(Lond). 40:81–92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gonzalez-Moles MA, Warnakulasuriya S,
Gonzalez-Ruiz I, González-Ruiz L, Ayén Á, Lenouvel D, Ruiz-Ávila I
and Ramos-García P: Clinicopathological and prognostic
characteristics of oral squamous cell carcinomas arising in
patients with oral lichen planus: A systematic review and a
comprehensive meta-analysis. Oral Oncol. 106(104688)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Colevas AD, Yom SS, Pfister DG, Spencer S,
Adelstein D, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ,
et al: NCCN Guidelines Insights: Head and Neck Cancers, Version
1.2018. J Natl Compr Canc Netw. 16:479–490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Panzarella V, Pizzo G, Calvino F,
Compilato D, Colella G and Campisi G: Diagnostic delay in oral
squamous cell carcinoma: The role of cognitive and psychological
variables. Int J Oral Sci. 6:39–45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao F, Wang X, Chen S, Xu T, Wang X, Shen
Y, Dong F, Zhong S and Shen Z: CIP2A depletion potentiates the
chemosensitivity of cisplatin by inducing increased apoptosis in
bladder cancer cells. Oncol Rep. 40:2445–2454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen D, Fan S, Wang J, Liang Y, Li P, Lv
X, Sun Y, Wang Q, Liu H, Zhang C and Yi Y: Cip2a induces arginine
biosynthesis and promotes tumor progression in non-small cell lung
cancer. Mol Carcinog. 62:561–572. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Jin L, Si Y, Hong X, Liu P, Zhu B, Yu H,
Zhao X, Qin S, Xiong M, Liu Y, et al: Ethoxysanguinarine inhibits
viability and induces apoptosis of colorectal cancer cells by
inhibiting CIP2A. Int J Oncol. 52:1569–1578. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Velmurugan BK, Wang HK, Chung CM, Lee CH,
Huang LR, Yeh KT and Lin SH: CIP2A overexpression in Taiwanese oral
cancer patients. Cancer Manag Res. 11:2589–2594. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alzahrani R, Alrehaili AA, Gharib AF,
Anjum F, Ismail KA and Elsawy WH: Cancerous inhibitor of protein
phosphatase 2A as a molecular marker for aggressiveness and
survival in oral squamous cell carcinoma. J Cancer Prev. 25:21–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guenebeaud C, Goldschneider D, Castets M,
Guix C, Chazot G, Delloye-Bourgeois C, Eisenberg-Lerner A, Shohat
G, Zhang M, Laudet V, et al: The dependence receptor UNC5H2/B
triggers apoptosis via PP2A-mediated dephosphorylation of DAP
kinase. Mol Cell. 40:863–876. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao H, Li Y, Lin T, Cheng Y and Ma Y:
Downregulation of CIP2A inhibits cancer cell proliferation and
vascularization in renal clear cell carcinoma. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 164:196–202. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tian X, Tao F, Zhang B, Dong JT and Zhang
Z: The miR-203/SNAI2 axis regulates prostate tumor growth,
migration, angiogenesis and stemness potentially by modulating
GSK-3β/β-CATENIN signal pathway. IUBMB Life. 70:224–236.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Fei HR, Cui LY, Zhang ZR, Zhao Y and Wang
FZ: Caudatin inhibits carcinomic human alveolar basal epithelial
cell growth and angiogenesis through modulating
GSK3beta/beta-catenin pathway. J Cell Biochem. 113:3403–3410.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li J, Sun S, Li J, Zhao X, Li Z, Sha T and
Cui Z: NCAPG, mediated by miR-378a-3p, regulates cell
proliferation, cell cycle progression, and apoptosis of oral
squamous cell carcinoma through the GSK-3β/β-catenin signaling.
Neoplasma. 68:1201–1211. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lei N, Peng B and Zhang JY: CIP2A
regulates cell proliferation via the AKT signaling pathway in human
lung cancer. Oncol Rep. 32:1689–1694. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Laine A, Sihto H, Come C, Rosenfeldt MT,
Zwolinska A, Niemelä M, Khanna A, Chan EK, Kähäri VM,
Kellokumpu-Lehtinen PL, et al: Senescence sensitivity of breast
cancer cells is defined by positive feedback loop between CIP2A and
E2F1. Cancer Discov. 3:182–197. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu L, Liang D, Zheng Q, Zhao M, Lv R,
Tang J and Chen N: Berbamine dihydrochloride suppresses the
progression of colorectal cancer via RTKs/Akt axis. J
Ethnopharmacol. 303(116025)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang W, Yu S, Li W, Hu H and Zou G:
Silencing of lncRNA SNHG17 inhibits the tumorigenesis of epithelial
ovarian cancer through regulation of miR-485-5p/AKT1 axis. Biochem
Biophys Res Commun. 637:117–126. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li N, Yang F, Liu DY, Guo JT, Ge N and Sun
SY: Scoparone inhibits pancreatic cancer through PI3K/Akt signaling
pathway. World J Gastrointest Oncol. 13:1164–1183. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun EC, Dong SS, Li ZJ and Li CX:
Clinicopathological significance of AKT1 and PLK1 expression in
oral squamous cell carcinoma. Dis Markers.
2022(7300593)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun X, Hu F, Hou Z, Chen Q, Lan J, Luo X,
Wang G, Hu J and Cao Z: SIX4 activates Akt and promotes tumor
angiogenesis. Exp Cell Res. 383(111495)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sagredo AI, Sagredo EA, Cappelli C, Báez
P, Andaur RE, Blanco C, Tapia JC, Echeverría C, Cerda O, Stutzin A,
et al: TRPM4 regulates Akt/GSK3-β activity and enhances β-catenin
signaling and cell proliferation in prostate cancer cells. Mol
Oncol. 12:151–165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Deng YQ, Kong GY, Li S, Li F and Wen SL:
Upregulation of lnc-ZNF281 Inhibits the Progression of Glioma via
the AKT/GSK-3β/ β-Catenin Signaling Pathway. J Immunol Res.
2021(5573071)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Roy A, Ansari SA, Das K, Prasad R,
Bhattacharya A, Mallik S, Mukherjee A and Sen P: Coagulation factor
VIIa-mediated protease-activated receptor 2 activation leads to
β-catenin accumulation via the AKT/GSK3β pathway and contributes to
breast cancer progression. J Biol Chem. 292:13688–13701.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dave K, Ali A and Magalhaes M: Increased
expression of PD-1 and PD-L1 in oral lesions progressing to oral
squamous cell carcinoma: A pilot study. Sci Rep.
10(9705)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fanelli M, Locopo N, Gattuso D and
Gasparini G: Assessment of tumor vascularization:
Immunohistochemical and non-invasive methods. Int J Biol Markers.
14:218–231. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Marla V, Hegde V and Shrestha A:
Relationship of Angiogenesis and Oral Squamous Cell Carcinoma.
Kathmandu Univ Med J (KUMJ). 13:178–185. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Soofiyani SR, Hejazi MS and Baradaran B:
The role of CIP2A in cancer: A review and update. Biomed
Pharmacother. 96:626–633. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Y, Fang L, Zang Y, Ren J and Xu Z:
CIP2A promotes proliferation, invasion and chemoresistance to
cisplatin in renal cell carcinoma. J Cancer. 9:4029–4038.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zheng Z, Qiao Z, Chen W, Gong R, Wang Y,
Xu L, Ma Y, Zhang L, Lu Y, Jiang B, et al: CIP2A regulates
proliferation and apoptosis of multiple myeloma cells. Mol Med Rep.
14:2705–2709. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhai M, Cong L, Han Y and Tu G: CIP2A is
overexpressed in osteosarcoma and regulates cell proliferation and
invasion. Tumour Biol. 35:1123–1128. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yu N, Zhang T, Zhao D, Cao Z, Du J and
Zhang Q: CIP2A is overexpressed in human endometrioid
adenocarcinoma and regulates cell proliferation, invasion and
apoptosis. Pathol Res Pract. 214:233–239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen XD, Tang SX, Zhang JH, Zhang LT and
Wang YW: CIP2A, an oncoprotein, is associated with cell
proliferation, invasion and migration in laryngeal carcinoma cells.
Oncol Rep. 38:1005–1012. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lin YC, Chen KC, Chen CC, Cheng AL and
Chen KF: CIP2A-mediated Akt activation plays a role in
bortezomib-induced apoptosis in head and neck squamous cell
carcinoma cells. Oral Oncol. 48:585–593. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Feng F, Cheng P, Wang C, Wang Y and Wang
W: Polyphyllin I and VII potentiate the chemosensitivity of
A549/DDP cells to cisplatin by enhancing apoptosis, reversing EMT
and suppressing the CIP2A/AKT/mTOR signaling axis. Oncol Lett.
18:5428–5436. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang G, Liu Z, Xu H and Yang Q:
miR-409-3p suppresses breast cancer cell growth and invasion by
targeting Akt1. Biochem Biophys Res Commun. 469:189–195.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Deng T, Shen P, Li A, Zhang Z, Yang H,
Deng X, Peng X, Hu Z, Tang Z, Liu J, et al: CCDC65 as a new
potential tumor suppressor induced by metformin inhibits activation
of AKT1 via ubiquitination of ENO1 in gastric cancer. Theranostics.
11:8112–8128. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tan J, Li C, Ren L, Zhu X, Hua F and Fu Y:
miR-451a suppresses papillary thyroid cancer cell proliferation and
invasion and facilitates apoptosis through targeting DCBLD2 and
AKT1. Mol Cell Probes. 66(101863)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Alwhaibi A, Verma A, Adil MS and Somanath
PR: The unconventional role of Akt1 in the advanced cancers and in
diabetes-promoted carcinogenesis. Pharmacol Res.
145(104270)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang F, Fang E, Mei H, Chen Y, Li H, Li D,
Song H, Wang J, Hong M, Xiao W, et al: Cis-Acting circ-CTNNB1
Promotes β-Catenin Signaling and Cancer Progression via
DDX3-Mediated Transactivation of YY1. Cancer Res. 79:557–571.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang X, Wang L and Qu Y: Targeting the
β-catenin signaling for cancer therapy. Pharmacol Res.
160(104794)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Song M, Pan Q, Yang J, He J, Zeng J, Cheng
S, Huang Y, Zhou ZQ, Zhu Q, Yang C, et al: Galectin-3 favours
tumour metastasis via the activation of beta-catenin signalling in
hepatocellular carcinoma. Br J Cancer. 123:1521–1534.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3beta to the
APC-beta-catenin complex and regulation of complex assembly.
Science. 272:1023–1026. 1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chua HH, Tsuei DJ, Lee PH, Jeng YM, Lu J,
Wu JF, Su DS, Chen YH, Chien CS, Kao PC, et al: RBMY, a novel
inhibitor of glycogen synthase kinase 3β, increases tumor stemness
and predicts poor prognosis of hepatocellular carcinoma.
Hepatology. 62:1480–1496. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pan J, Fan Z, Wang Z, Dai Q, Xiang Z, Yuan
F, Yan M, Zhu Z, Liu B and Li C: CD36 mediates palmitate
acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin
pathway. J Exp Clin Cancer Res. 38(52)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang L, Tan W, Wei Y, Xie Z, Li W, Ma X,
Wang Q, Li H, Zhang Z, Shang C and Chen Y: CircLIFR suppresses
hepatocellular carcinoma progression by sponging miR-624-5p and
inactivating the GSK-3β/β-catenin signaling pathway. Cell Death
Dis. 13(464)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang CH, Liu H, Zhao WL, Zhao WX, Zhou HM
and Shao RG: G3BP1 promotes human breast cancer cell proliferation
through coordinating with GSK-3β and stabilizing β-catenin. Acta
Pharmacol Sin. 42:1900–1912. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mao D, Zhang X, Wang Z, Xu G and Zhang Y:
TMEM97 is transcriptionally activated by YY1 and promotes
colorectal cancer progression via the GSK-3β/β-catenin signaling
pathway. Hum Cell. 35:1535–1546. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li Q, Luo H, Dai FQ, Wang RT, Fan XQ, Luo
YY, Deng MS, Wang Y, Long T, Guo W, et al: SAMD9 promotes
postoperative recurrence of esophageal squamous cell carcinoma by
stimulating MYH9-Mediated GSK3β/β-Catenin signaling. Adv Sci
(Weinh). 10(e2203573)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu
C, Liu D, Zheng M, Sun J, Feng H and Lu A: Tumor-derived CXCL5
promotes human colorectal cancer metastasis through activation of
the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer.
16(70)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhang Y, Li JH, Yuan QG and Yang WB:
Restraint of FAM60A has a cancer-inhibiting role in pancreatic
carcinoma via the effects on the Akt/GSK-3β/β-catenin signaling
pathway. Environ Toxicol. 37:1432–1444. 2022.PubMed/NCBI View Article : Google Scholar
|