Introduction
Peucedanum praeruptorum Dunn is a perennial
herb, which is primarily found distributed on hillside forest
edges, the roadside, or semi-negative hillside grass at an altitude
of 250-2,000 meters in China. The dry root donor-part of
Peucedanum praeruptorum Dunn is currently used in
traditional Chinese medicine, exerting an effect on exogenous
wind-heat; lung heat and phlegm depression; cough, asthma, and
phlegm; and tightness in the chest and diaphragm (1). The root of Peucedanum
praeruptorum Dunn contains a variety of coumarin compounds and
these active substances are also independently used in health
products (2-5).
Free radicals and reactive oxygen species, such as
the superoxide anion, peroxy ion, hydroxyl radical, hydrogen
peroxide, and peroxynitrite, are metabolic byproducts produced by
organisms during aerobic respiration. Excessive accumulation of ROS
in the body can cause oxidative stress in cells and tissues,
particularly within renal epithelial cells, and this is considered
to be one of the major pathogenic factors leading to renal disease
(6). It is important to highlight
that among the various ROS, hydrogen peroxide
(H2O2) can easily penetrate the cell membrane
and enter the cytoplasm, leading to lipid peroxidation of cell
membranes. This ultimately results in renal cell injury and
subsequent necrosis of renal tissue (7). Under physiological conditions, the
body possesses endogenous antioxidant systems, such as catalase
(CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px),
and non-enzymatic glutathione (GSH). These endogenous antioxidants
play a crucial role in scavenging free radicals and ROS, thereby
reducing oxidative stress-induced damage. Furthermore, consuming
antioxidant-rich foods or supplements can enhance the body's
intrinsic antioxidant capacity and mitigate the detrimental effects
of oxidative stress on kidney function (8,9).
In the present study, pig renal epithelial LLC-PK1
cells were cultured in vitro to establish an oxidative
damage model of epithelial cells induced by
H2O2. The aim of this study was to
investigate the in vitro free radical scavenging ability of
PPDE and its protective mechanism against oxidative stress-induced
injury in porcine renal epithelial cells stimulated with
H2O2. These findings may serve as a
theoretical foundation for exploring the potential health benefits
and disease prevention properties of PPDE in oxidative
stress-related renal diseases.
Materials and methods
PPDE extraction
Freshly collected Peucedanum praeruptorum
Dunn (Hebei Anguo Ruiqi Traditional Chinese Medicine Co., Ltd,
Anguo, Hebei, China) was freeze-dried and then milled into a fine
powder. PPDE powder and an 80% ethanol solution were mixed in a
ratio of 1:9 before being extracted at 50˚C for 1 h and then
filtered. The filter residue was collected for another subsequent
extraction. The two extracts were combined and evaporated by rotary
evaporation to obtain PPDE.
DPPH radical scavenging
A volume of 0.5 ml PPDE with different
concentrations (0-2.0 mg/ml) was added to 2 ml DPPH ethanol
solution (0.33 mM), mixed, and allowed to stand in the dark at room
temperature for 30 min. The absorbance of the tested solutions was
determined at 517 nm with three in each group in parallel (10). The calculation formula of DPPH
radical scavenging rate (%) was
[1-(Ae-Af)/Ag] x100 where Ae is
the OD value of the DPPH working solution + PPDE solution; Af is
the blank correction, the OD value of PPDE solution + ethanol; and
Ag is the blank control, the OD value of DPPH working solution +
ethanol. Under a concentration range of 0-1.2 mg/ml, the DPPH free
radical scavenging ability of PPDE showed a positive correlation
with the concentration. However, no significant difference in
effect was observed beyond a concentration of 1.2 mg/ml (Fig. S1). Therefore, for the purpose of
conducting the free radical scavenging experiments, experimental
concentrations of 0.2, 0.5, and 1.0 mg/ml were selected.
Hydroxyl radical scavenging
Salicylic acid colorimetry was used (11). First, a 10-ml test tube with a
stopper was used to collect the following experimental solution.
First, 2 ml 0.2, 0.5, or 1.0 mg/ml PPDE solution was added. Next, 2
ml 9 mmol/l ethanol salicylic acid solution and 1 ml 9 mmol/l
ferrous sulfate solution (prepared by ferrous sulfate heptahydrate)
were added, and finally, 2 ml 8.8 mmol/l H2O2
was added to initiate the reaction. Mixtures were incubated in a
37˚C water bath for 30 min, and OD values were measured at 510 nm,
with three in each group in parallel. The calculation formula of
the hydroxyl radical scavenging rate (%) was
[1-(Ab-Ac)/Ad] x100 where Ab is
the OD value of the working solution after the addition of PPDE; Ac
is the double-distilled water instead of H2O2
as the background colorimetric OD value; and Ad is the OD value of
the blank control solution.
ABTS radical scavenging
A volume of 5 ml was taken out from the plugged test
tube, 1 ml ABTS free radical working solution was added, followed
by 0.4 ml 0.2, 0.5, or 1.0 mg/ml PPDE solution. The solvent was
placed in the dark for 30 min and then the OD value was determined
at 734 nm with three in each group in parallel. The ABTS free
radical working solutions were prepared as follows: Solution A, 3
mg ABTS was added to 0.8 ml double distilled water, mixed and
dissolved; and solution B, 1 mg potassium persulfate was added to
1.5 ml double distilled water, mixed and dissolved (12). The calculation formula of ABTS
radical scavenging rate (%) was
[1-(Ai-Aj)/Ao] x100 where Ai is
the OD value of ABTS working fluid + PPDE fluid; Aj is the blank
correction, the OD value of PPDE solution + absolute ethanol; and
Ao is the blank control, ABTS working solution + absolute ethanol
OD value.
Superoxide anion scavenging
For the superoxide anion scavenging experiment, the
pyrogallol autoxidation method was adopted (13). The SOD activity of samples was
detected using a kit (cat. no. A001-3-2; Nanjing Jiancheng
Bioengineering Institute), and the absorbance value was tested at
320 nm with three in each group in parallel. The calculation
formula used for the superoxide anion radical scavenging rate (%)
was [1-(Am-An)/Ap] x100 where Am
is the OD value of the superoxide anion working solution + PPDE
solution; An is the pyrogallol replaced with deionized water as the
background OD value of PPDE; and Ap is the OD value of deionized
water instead of PPDE.
LLC-PK1 cell culture
Pig renal epithelial LLC-PK1 cells were removed from
the cryopreservation tubes from the liquid nitrogen and transferred
to a 37˚C water bath for 15 min to facilitate cell recovery.
Subsequently, the cells were placed in a demethylated medium
(Beijing Solarbio Science & Technology Co., Ltd.), which is a
high-sugar solution supplemented with 10% FBS and 1%
penicillin-streptomycin double antibiotic solution. Cells were
maintained in a humidified incubator at 37˚C supplied with 5%
CO2 air. The medium was changed 2-3 times per week. When
the confluency reached 90%, trypsin (0.25%) was used to digest
cells and subculture. Cells in the logarithmic growth phase cells
were used in all experiments.
MTT assay
The cytotoxicity of the PPDE solution was assessed.
LLC-PK1 cell suspensions (1x104 cells/ml) were plated
into 96 well cell culture plates (60 µl cell + 100 µl medium)
cultured at 37˚C for 24 h before 20 µl of the different
concentrations of PPDE solutions were added (0, 50, 100, or 200
µg/ml) for 24 h. Then, 20 µl MTT (5 mg/ml) was added, mixed, and
cultured for a further 4 h. Subsequently, the media was removed,
and 150 µl DMSO was added and incubated at 37˚C on a shaker for 30
min in the dark. The OD value was measured at 490 nm (14).
The protective effect of PPDE on oxidative damage of
LLC-PK1 cells induced by H2O2 was assessed.
The LLC-PK1 cell suspensions (1x104 cells/ml) were
plated in 96 well cell culture plates (60 µl cell + 100 µl medium),
cultured at 37˚C for 24 h, and then 20 µl
H2O2 (0.3 mmol/l) was added. Cells were
cultured for 4 h to allow for the establishment of the oxidative
damage model. A total of 20 µl of the PPDE solutions was added
(final concentration, 0-300 µg/ml) was added to cells and cultured
for 24 h. Next, 20 µl MTT solution was added, and cells were
incubated for a further 4 h. The media was removed and 150 µl DMSO
was added before incubation at 37˚C in the dark on a shaker for 30
min. The OD value was measured at 490 nm (15). At concentrations ranging from 0-250
µg/ml, the cellular antioxidant capacity of PPDE exhibited a
positive correlation with the concentration used. However, no
significant difference in effect was observed when the
concentration exceeded 250 µg/ml (Fig. S2). Therefore, for subsequent
experiments, the experimental concentrations of PPDE used were 50,
100, and 200 µg/ml.
Determination of intracellular
contents of MDA, SOD, GSH, GSH-Px, and CAT
LC-PK1 cells in the logarithmic growth stage were
digested using 0.25% trypsin and plated into 6-well cell culture
plates (1x105 cells/ml). A total of 2 ml DMEM (Beijing
Solarbio Science & Technology Co., Ltd) was added to cells and
cultured for 24 h. Next, 200 µl H2O2 (0.3
mmol/l) was added. After cells had adhered, cells were treated with
H2O2 and cultured for 4 h to establish the
oxidative damage model. Next, 200 µl of different concentrations of
PPDE solution (0, 50, 100, or 200 µg/ml) per well and PBS buffer
(0.1 M) were added to cells, and incubated for 24 h.
LLC-PK1 cells treated with PPDE were washed with
precooled PBS and had 200 µl trypsin added. Detached cells were
collected, transferred to a 1.5 ml centrifuge tube, and centrifuged
(1,006 x g, 4˚C, 5 min) to remove the supernatant. Precooled PBS
was used to wash the cells again, and centrifuged again at 1,006 x
g for 15 min at 4˚C, and the supernatant was removed. A total of
800 µl normal saline was added and the levels of MDA (cat. no.
A003-1-2), SOD (cat. no. A001-3-2), GSH (cat. no. A006-1-1), GSH-Px
(cat. no. A005-1-2), and CAT (cat. no. A007-1-1) in cell
homogenates were measured using the specific kits (Nanjing
Jiancheng Bioengineering Institute).
Reverse transcription-quantitative
(RT-q)PCR
LC-PK1 cells were plated in a 10 cm cell culture
dish. After treatment as described above, TRIzol®
reagent was used to extract total RNA. After purity detection using
the UV method (measurement of absorbance at 260 and 280 nm, and
calculating the absorbance ratio of
OD260/OD280), the concentration of total RNA
in all sample groups was adjusted to 1 µg/µl. An equal amount of
RNA (2 µg) was added to each group with oligodT18, RNase, dNTP, and
1 µl MLV enzyme, and 10 µl 5x Buffer. The cDNA was synthesized in a
total volume of 20 µl at 37˚C for 120 min, 99˚C for 4 min, and 4˚C
for 3 min. After reverse transcription, qPCR was performed. The
thermocycling conditions were: Pre-denaturation at 95˚C for 5 min;
followed by 35 cycles of annealing at 58˚C for 50 sec and extension
at 72˚C for 90 sec; and a final extension at 72˚C for 10 min. GAPDH
was used as an internal reference, and the expression was
calculated using the 2-∆∆Cq method (16,17).
The sequences of the primers used for qPCR are listed in Table I.
 | Table ISequences of the primers. |
Table I
Sequences of the primers.
| Gene name | Sequence |
|---|
| SOD1 | |
|
Forward |
5'-TATGGGGACAATACACAAGGCT-3' |
|
Reverse |
5'-CGGGCCACCATGTTTCTTAGA-3' |
| CAT | |
|
Forward |
5'-GGAGGCGGGAACCCAATAG-3' |
|
Reverse |
5'-GTGTGCCATCTCGTCAGTGAA-3' |
| GSS | |
|
Forward |
5'-GAATTGCTTGCTACGGCCTG-3' |
|
Reverse |
5'-TCCAGATGGCGTCTTCACAC-3' |
| GSH-Px | |
|
Forward |
5'-CCACCGTGTATGCCTTCTCC-3' |
|
Reverse |
5'-AGAGAGACGCGACATTCTCAAT-3' |
| GAPDH | |
|
Forward |
5'-TGGCCTTCCGTGTTCCTAC-3' |
|
Reverse |
5'-GAGTTGCTGTTGAAGTCGCA-3' |
Western blotting
The cells were washed three times with pre-cooled
PBS before being homogenized with protein lysate and centrifuged at
11,180 x g for 15 min at 4˚C. Protein concentration was determined
using a BCA protein assay. Next, sample buffer was added to the
protein samples and heated at 100˚C for 10 min. On a PAGE Bis-Tris
pre-gel, the pre-mixed samples and protein ladder were loaded (1
µg). Following SDS-PAGE, proteins were transferred to PVDF
membranes, which were blocked for 1 h with 5% skimmed milk in TBST.
The PVDF membranes were then incubated with primary antibodies
against SOD1 (cat. no. MA1-105; monoclonal antibody; 1:100
dilution), CAT (cat. no. MA5-37595; monoclonal antibody; 1:1,600
dilution) or β-actin (cat. no. MA1-140; monoclonal antibody;
1:5,000 dilution) (all Thermo Fisher Scientific, Inc.) at 25˚C for
2 h. The PVDF membranes were then washed five times with TBST for 5
min each, followed by a 1-h incubation with the secondary antibody
(cat. no. A-11001; goat anti-mouse IgG; 1:1,000 dilution; Thermo
Fisher Scientific, Inc.) at 25˚C. Finally, to visualize signals, an
enhanced chemiluminescence analysis kit (GE Healthcare) on a
LAS3000 luminescent image analyzer (Fujifilm Corporation).
HPLC
The test solution was established by dissolving
standards and sample extracts in methanol. The chemical composition
of the PPDE was then determined using the following chromatographic
apparatus: AcclaimTM120 C18 column, 4.6x150 mm, 5 µm in length;
mobile phase, methanol-0.5% glacial acetic acid; detection
wavelength, 359 nm; column temperature, 35˚C; flow rate, 0.6
ml/min; PPDE intake, 20 µl (UltiMate 3000, Thermo Fisher
Scientific, Inc.).
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using SAS 9.2 (SAS Institute, Inc.). Differences were
compared using a one-way ANOVA followed by a Duncan's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Free radical scavenging ability of
PPDE in vitro
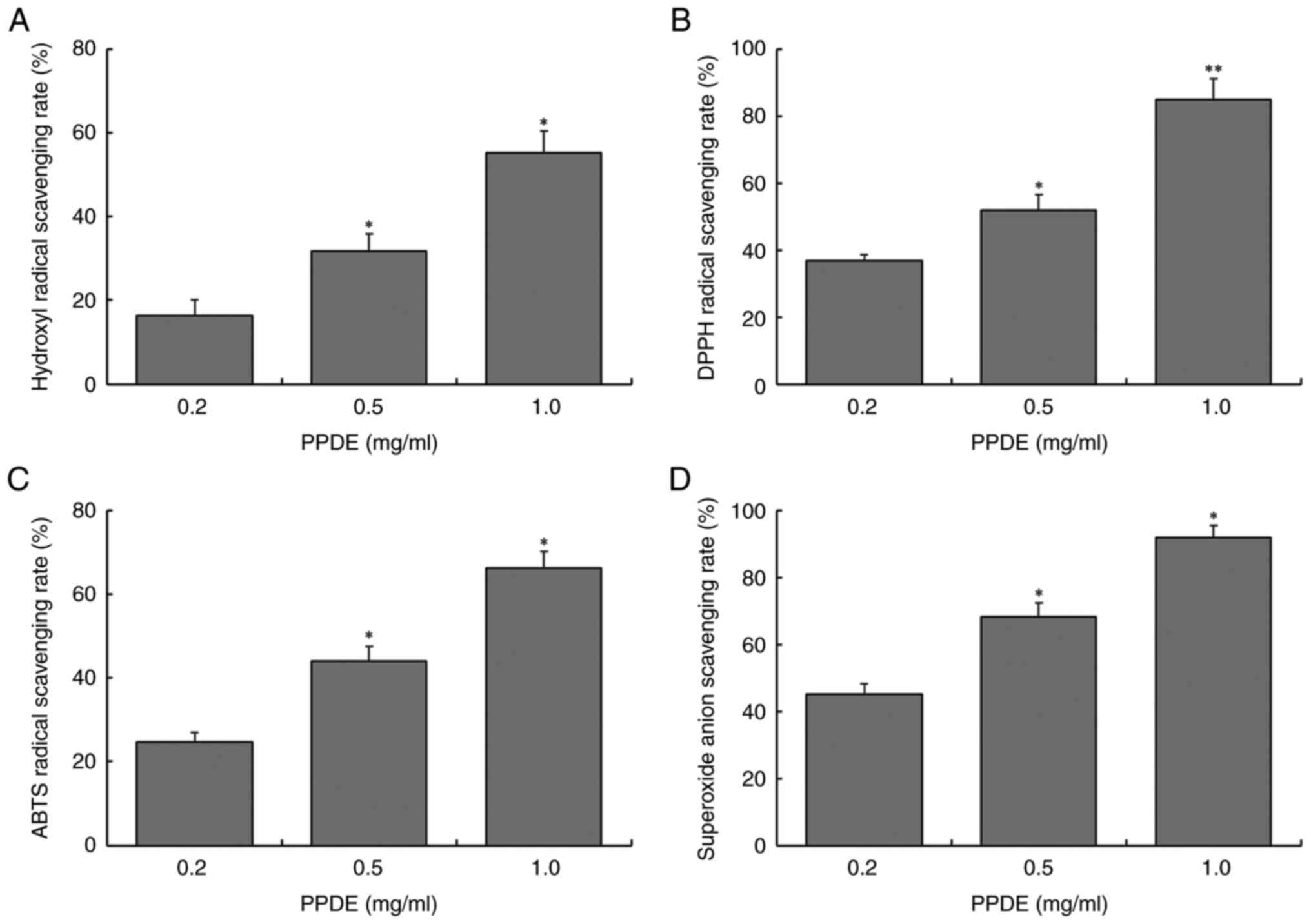
As shown in Fig. 1,
at concentrations of 0.2, 0.5, or 1.0 mg/ml, the scavenging rates
of PPDE on hydroxyl radicals (16.3, 31.8, and 55.2%, respectively),
DPPH radicals (36.8, 51.9, and 84.9%, respectively), ABTS radicals
(24.7, 43.9, and 66.3%, respectively), and superoxide anion
radicals (45.0, 68.3, and 91.8%, respectively) were significantly
enhanced, indicating that the scavenging capacity of PPDE on four
free radicals was dose-dependent and there were significant
differences between different concentrations (P<0.05).
LLC-PK1 cell survival rate
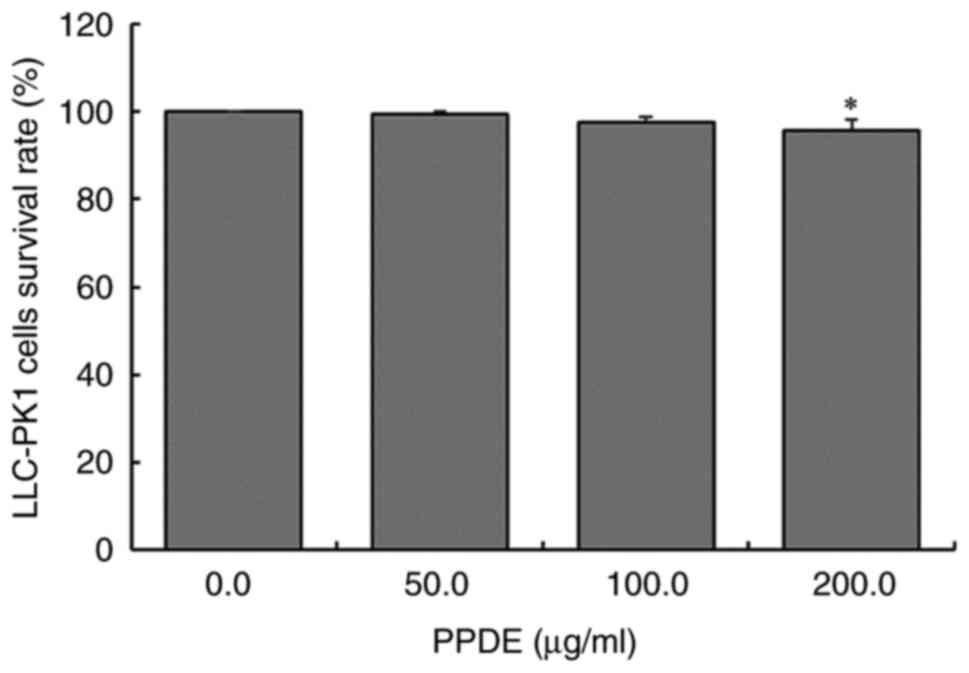
As shown in Fig. 2,
after 0, 50, 100, or 200 µg/ml PPDE treatment, the survival rate of
LLC-PK1 cells was almost 96%. This suggested that PPDE had no
notable toxic effects on normal cells at concentrations at or below
200 µg/ml. Therefore, 50, 100, and 200 µg/ml PPDE were used for all
subsequent experiments.
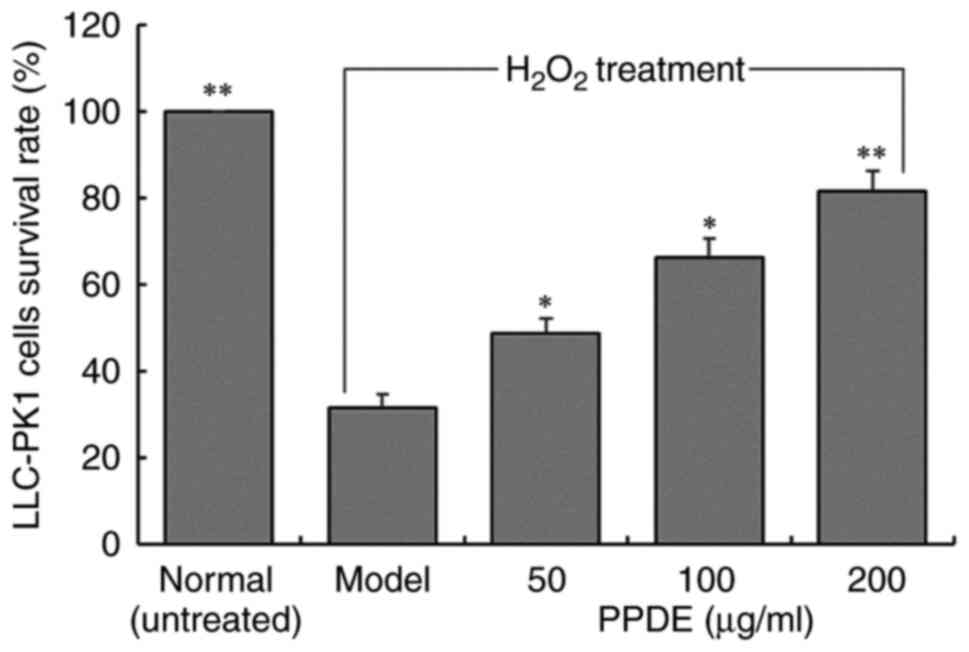
The survival rates of LLC-PK1 cells with
H2O2-induced oxidative damage were decreased
compared with those of untreated normal cells (Fig. 3). Compared with the injured cells,
after 50, 100, or 200 µg/ml PPDE treatments, the cell survival
rates were significantly improved, and a concentration of 200 µg/ml
exhibited a more significant protective effect (P<0.05).
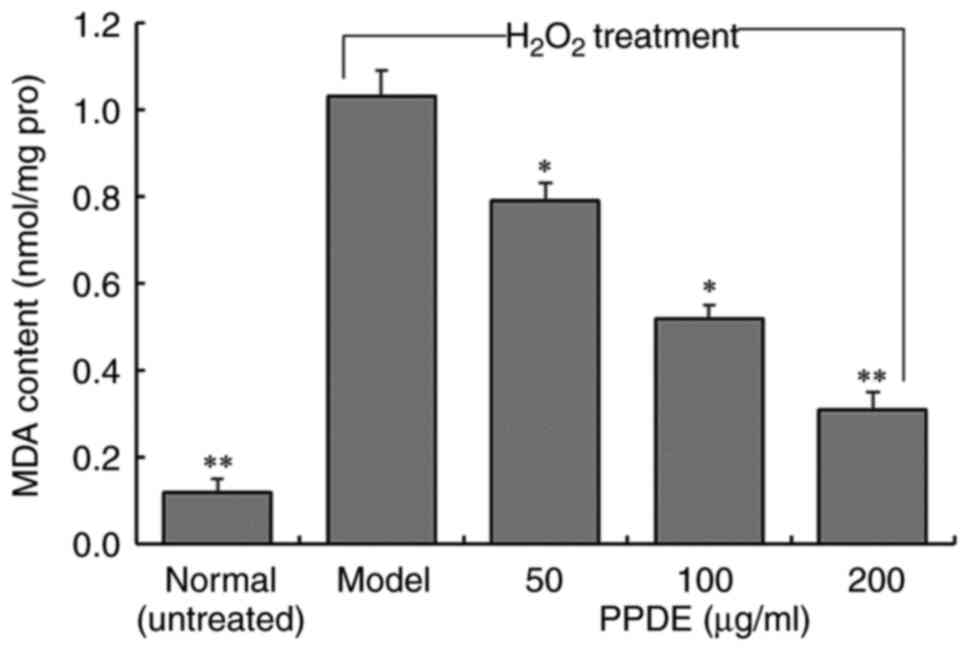
MDA content in oxidation-damaged
LLC-PK1 cells following PPDE treatment
The MDA content of
H2O2-treated LLC-PK1 cells was significantly
higher than untreated normal cells (Fig. 4, P<0.05). At different
concentrations of PPDE, the MDA content of the cells was reduced in
a dose-dependent manner, and the effect of 200 µg/ml PPDE had the
most notable effect on MDA content.
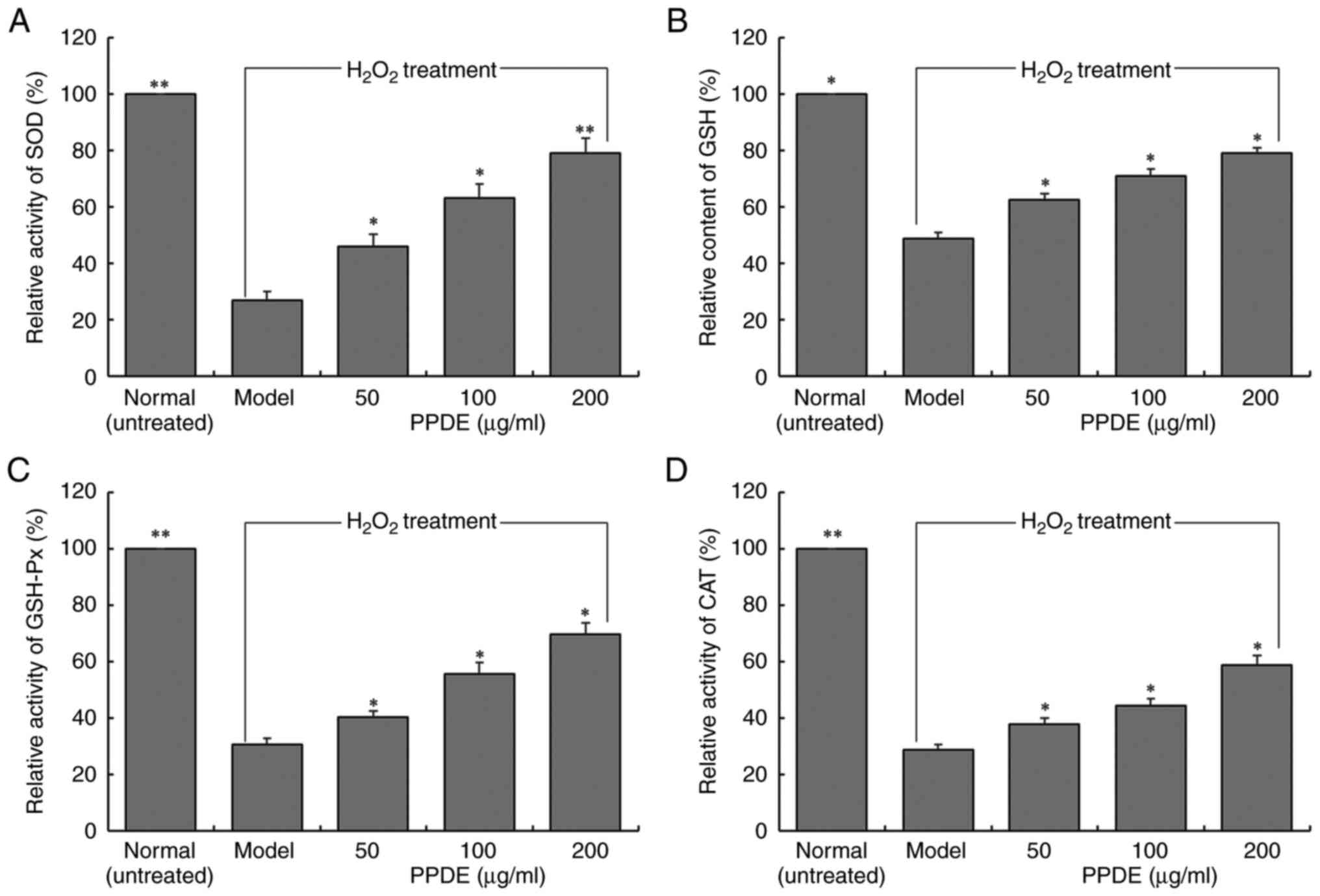
Changes in SOD, GSH, GSH-Px, and CAT
levels in oxidation-damaged LLC-PK1 cells treated with PPDE
The enzymatic activities of SOD, GSH-Px, and CAT and
the content of GSH in LLC-PK1 cells in the normal group were
significantly higher than those in the model group (Fig. 5, P<0.05). Treatment with PPDE
significantly increased the SOD, GSH-Px, and CAT enzyme activities
and the GSH content of cells (P<0.05), and it was positively
correlated with the dose.
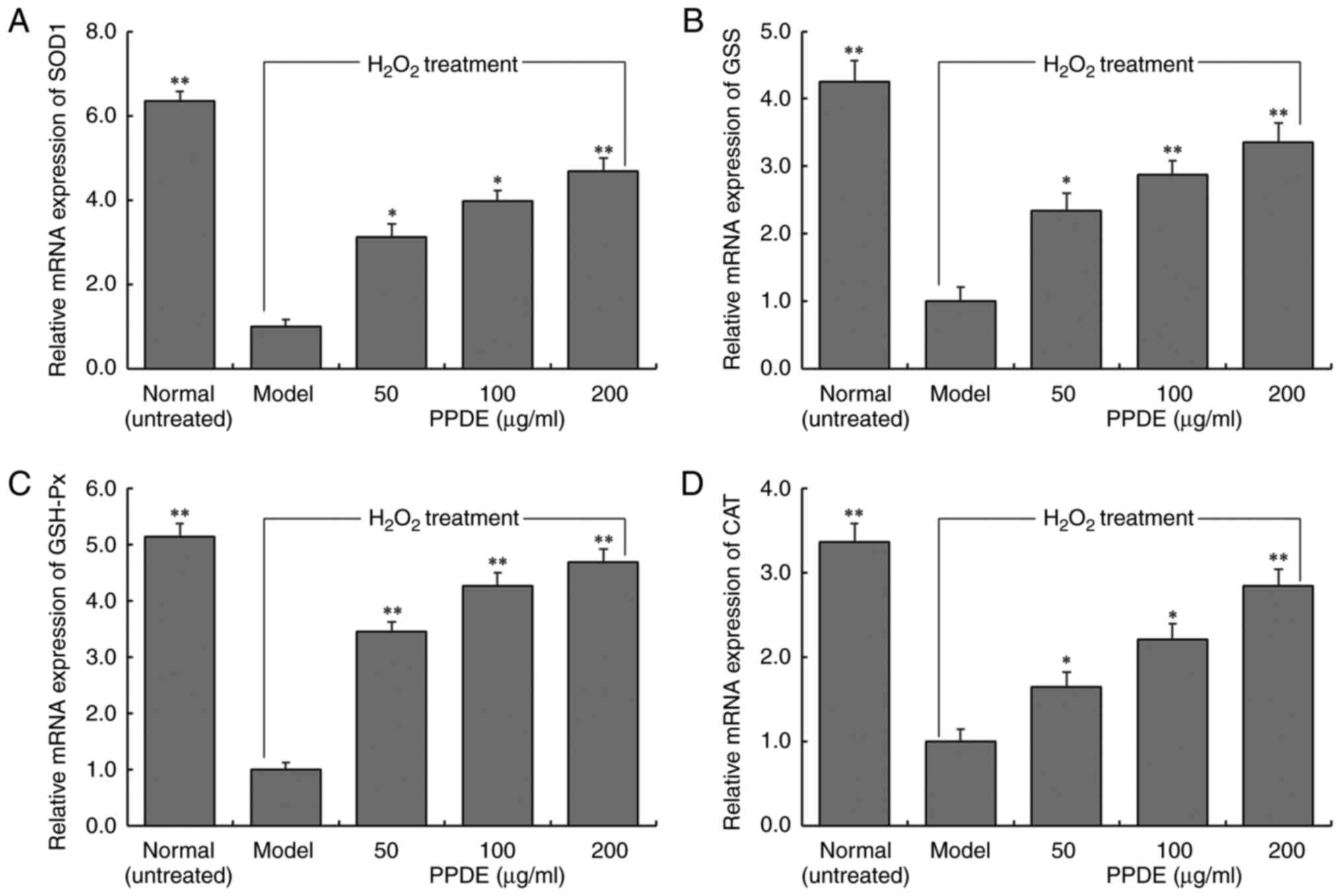
Effect of PPDE on mRNA expression in
damaged LLC-PK1 cells
The mRNA expression levels of SOD1, GSS, GSH-Px, and
CAT in normal cells decreased significantly following induced
oxidation (Fig. 6, P<0.05). The
effect of PPDE was found to significantly upregulate the expression
of SOD1, GSH, GSH-Px, and CAT in oxidation-damaged cells
(P<0.05). These results were consistent with the results of the
changes in the enzymatic activities of these enzymes determined
above.
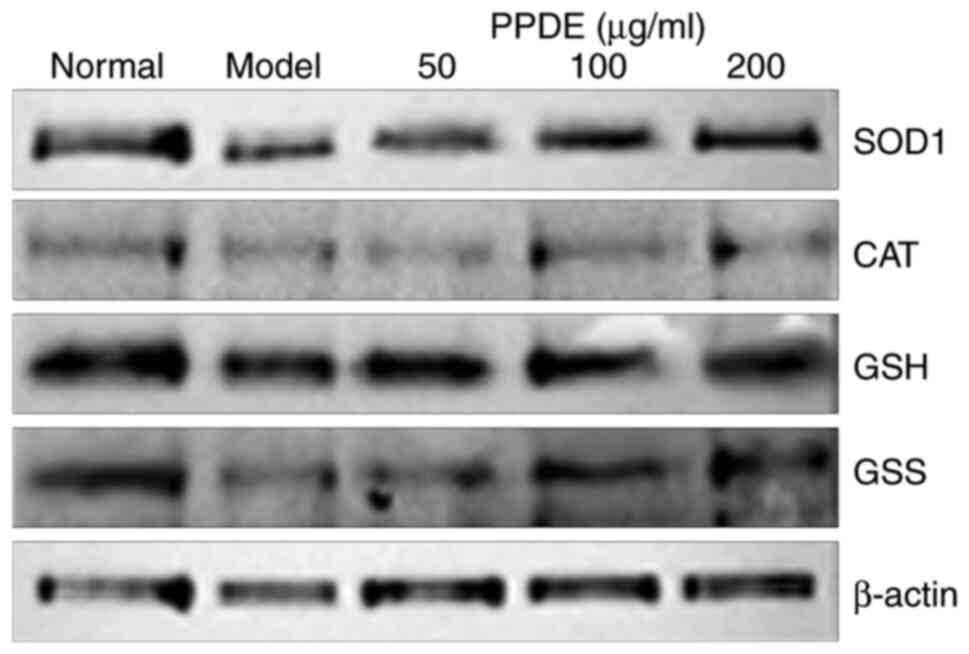
Effect of PPDE on protein expression
in damaged LLC-PK1 cells
Western blotting showed that the normal group had
higher protein expression levels of CAT, SOD1, GSH, and GSS
compared with the model group. Compared to the model group, PPDE
notably upregulated the protein expression levels of CAT, SOD1,
GSH, and GSS, and it was positively correlated with concentration
(Fig. 7).
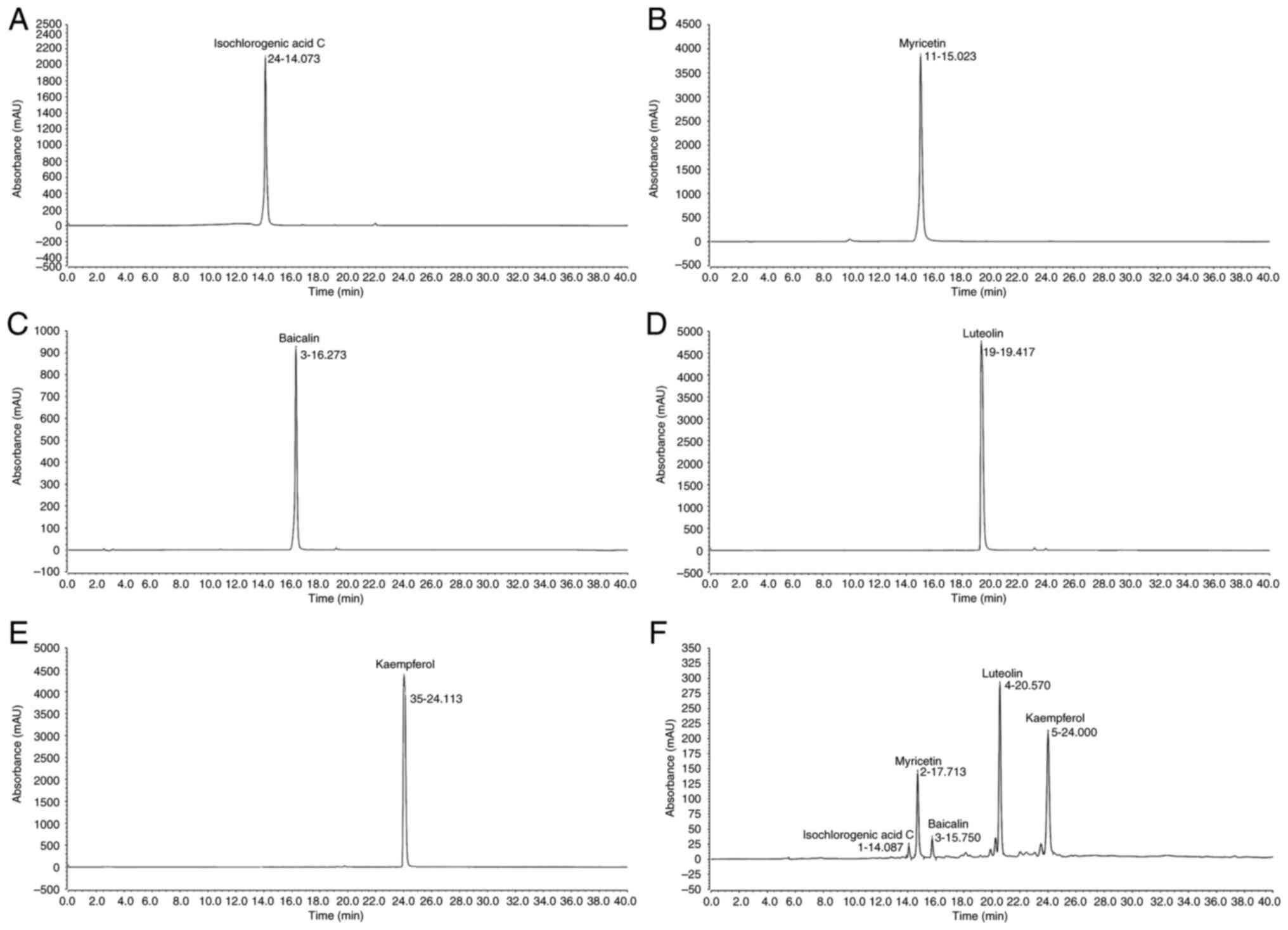
Composition of PPDE
Component analysis revealed that the primary PPDE
constituents were: Isochlorogenic acid, myricetin, baicalin,
luteolin, and kaempferol (Fig.
8).
Discussion
In the preliminary experiments, the toxicity of PPDE
on LLC-PK1 cells, human umbilical vein endothelial cells (HUVECs)
and melan-a mouse melanocytes was examined (data not shown). The
results demonstrated that PPDE did not exhibit any significant
toxic effects on these three types of cells. Although further
investigation is necessary to determine the underlying mechanism.
Similar to a previous study, LLC-PK1 cells have been shown to be
responsive to antioxidant treatments and are considered to be a
well-established cell line for assessing antioxidant effects
(18). In the present study, the
effect of PPDE on LLC-PK1 cells was primarily investigated, and our
future studies will focus on validating these findings and
elucidating the underlying mechanisms in other cell lines.
Oxygen free radicals have the potential to attack
polyunsaturated fatty acids present in biofilms, leading to lipid
peroxidation and subsequent oxidative damage to cells (19). To counteract this oxidative stress,
the body possesses intrinsic antioxidant systems. There are two
primary defense mechanisms against oxidative stress in humans. The
first is the enzymatic antioxidant system, consisting of vital
enzymes such as SOD, GSH-Px, and CAT. The second line of defense is
the non-enzymatic antioxidant system, primarily relying on dietary
antioxidants such as vitamins C and E, glutathione, carotenoids,
copper, selenium, and zinc, amongst others. Antioxidant compounds
in food, such as proanthocyanidins found in blueberries and citrus
fruits, in addition to dietary nutritional supplements, which are
often derived from active components of foods or plants, play a
significant role in enhancing the antioxidant activity and
scavenging of free radicals (20).
The rapid in vitro antioxidant evaluation method used in the
present study can be employed to initially assess the antioxidant
effect of natural products. Exogenous antioxidants found in
foodstuffs are the most reliable method to improve the antioxidant
capacity of cells (21). In
vitro antioxidant activity is measured in a laboratory setting
outside of living organisms, using direct reactions with free
radical reagents. One of the most commonly used in vitro
antioxidant experiments is the DPPH method (10,22,23).
DPPH is a stable free radical compound that is soluble in ethanol
and has a dark purple color. Ultraviolet detection is used to
assess the antioxidant potential of substances added to a reaction;
if the color of the solution becomes lighter, it indicates the
presence of in vitro antioxidant capacity. If in
vitro antioxidant capacity is observed, further in vivo
experiments are warranted (22).
Hydroxyl radicals can cause oxidative damage to DNA, proteins, and
lipids. The effects of antioxidants on oxidative damage can be
detected by capturing ·OH in a Fenton reaction system using
salicylic acid (23). The chemical
ABTS can be oxidized into blue-green ABTS·+ by oxidants,
and its color can become faded under the action of antioxidants.
Under alkaline conditions, pyrogallol can rapidly self-oxidize,
release O2-, and generate colored
intermediates. After the reaction is initiated, the reaction liquid
first turns yellow and brown, then turns green after a few minutes,
and ultimately turning yellow again after a few hours, which is due
to the continuous oxidation of the intermediates generated
(24). Evaluation of the
scavenging ability of a single free radical cannot comprehensively
evaluate the antioxidant activity of any given antioxidant, and
hence the scavenging ability of various free radicals should be
comprehensively evaluated in such studies. In the present study,
PPDE had a scavenging effect on DPPH, ·OH,
O2- and ABTS free radicals, and the effect
was notable.
MDA is one of the final products of membrane lipid
peroxidation. Its concentration can serve as an indicator to assess
the severity of cellular stress. The content of MDA is an important
parameter that reflects the potential antioxidant capacity of the
body, indicating the rate and intensity of lipid peroxidation and
the extent of peroxidation damage (25). Due to its possession of a single
electron, MDA can react with various cellular components, including
normal cells, proteins, and lipids, resulting in changes in their
structure and characteristics. For instance, lipids can be oxidized
by free radicals to produce MDA, which is considered a highly
destructive substance. The presence of MDA can lead to the
polymerization of nucleic acids, proteins, and other polymers,
resulting in the formation of insoluble lipofuscin. This series of
oxidative events accelerates the aging process in the body
(26). In the present study, the
MDA levels in LLC-PK1 cells increased after
H2O2-treated injury and decreased after PPDE
treatment. The antioxidant enzymes or non-enzyme antioxidant
substances CAT, SOD, GSH-Px, and GSH can play either their
respective roles or play a combined role in inhibiting oxidative
damage in the body. As different metal ions bind with the auxiliary
groups of SOD, SOD is present in three forms: Mn-SOD, Cu/Zn-SOD,
and Fe-SOD. Research has shown that SOD can convert excess
O2- into H2O2 and then
be converted into H2O by either CAT or GSH-Px (27). The antioxidant CAT has a specific
affinity for H2O2 and can react with
H2O2 to generate H2O, which is
then transformed into a non-toxic substance (28). GSH-Px is an important peroxidase
decomposition enzyme ubiquitously present throughout the body. The
active center of GSH-Px is selenocysteine, and its activity can
reflect the selenium (Se) levels of the body. Se is a component of
the GSH-Px enzyme system, and it can catalyze GSH into GSSG, reduce
toxic peroxide into non-toxic hydroxyl compounds, and promote the
decomposition of H2O2, ultimately protecting
the structure and function of cell membranes from oxide
interference and damage. Glutathione reductase can catalyze GSSG to
produce GSH using NADPH (29). In
addition, PPDE can also help to enhance its antioxidant activity
and alleviate cell injury by upregulating the transcription of CAT,
SOD, and GSH-Px antioxidant enzymes, as well as the non-enzymatic
antioxidant GSH in mRNA in cells damaged by oxidative stress. The
present study found that after treatment with different
concentrations of PPDE, the contents of the antioxidant enzymes
CAT, SOD, and GSH-Px and the non-enzymatic antioxidant GSH in
damaged cells increased significantly. The increase in the content
of these important antioxidant enzymes contributed to the
inhibition to inhibition of lipid peroxidation and alleviated
further cell damage.
Isochlorogenic acid C, myricetin, baicalin,
luteolin, and kaempferol are all active substances with antioxidant
effects. They also showed bioactive effects on cells (30-34).
In the present study, PPDE also exerted a potent protective effect
on normal cells from oxidative stress damage. However, PPDE is a
complex mixture, and the present study was not able to ascertain
whether its effects were due to a combination of multiple compounds
or a significant impact of a single component. Based on the results
of the present study, it was hypothesized that the substantial
presence of luteolin and kaempferol may be the primary contributing
factors to its effects.
In conclusion, the antioxidant activity of PPDE was
evaluated using in vitro experiments, and its beneficial
effects and potential active components involved in reducing
oxidative stress were preliminarily analyzed. The results showed
that PPDE had significant effects on DPPH, ·OH, and
O2- levels and that ABTS showed these
radicals to have been subjected to significant scavenging
activities by PPDE administration. Additionally, PPDE also
increased the contents of the major antioxidant enzymes CAT, SOD,
and GSH-Px as well as the non-enzymatic antioxidant GSH. qPCR
showed that PPDE could also upregulate the mRNA levels of
antioxidant enzymes, and this may underlie its ability to alleviate
oxidative stress. Peucedanum praeruptorum may protect the
kidney and inhibit nephropathy by reducing the levels of oxidative
stress in renal cells, which is conducive to the development of
novel antioxidant substances and renal-protective functional
foodstuffs. In future research, other detection methods, including
flow cytometry, and more in-depth animal experiments need to be
further used to evaluate the efficacy of PPDE.
Supplementary Material
DPPH scavenging effect of 0-2
μg/ml PPDE. PPDE< Peucedanum praeruptorum Dunn
extract.
Effect of 0-300 μg/ml PPDE
treatment on the survival of oxidatively damaged LLC-PK1 renal
tubular epithelial cells. PPDE, Peucedanum praeruptorum Dunn
extract.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the Science and Technology
Project of Chongqing Education Commission (grant no.
KJZD-M201901601) and Chongqing University Innovation Research Group
Project (grant no. CXQTP20033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH and PW performed the majority of the experiments
and wrote the manuscript. JK, JH, CW and JL contributed to the data
analysis. SC designed the study. All authors have read and approved
the final manuscript. SH and SC confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu PJ, Jin H, Zhang JY, Wang GF, Li JR,
Zhu ZG, Tian YX, Wu SY, Xu W, Zhang JJ and Wu SG: Pyranocoumarins
isolated from Peucedanum praeruptorum Dunn suppress
lipopolysaccharide-induced inflammatory response in murine
macrophages through inhibition of NF-κB and STAT3 activation.
Inflammation. 35:967–977. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gao M, Bian C, Zhou W, Liu L, Li B and
Tang L: Dissipation of tiafenacil in five types of citrus orchard
soils using the HPLC-MS coupled with the quick, easy, cheap,
effective, rugged, and safe method. J Separat Sci. 44:1950–1960.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xiong YY, Wu FH, Wang JS, Li J and Kong
LY: Attenuation of airway hyperreactivity and T helper cell type 2
responses by coumarins from Peucedanum praeruptorum Dunn in
a murine model of allergic airway inflammation. J Ethnopharmacol.
141:314–321. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee J, Lee YJ, Kim JH and Bang OS:
Pyranocoumarins from root extracts of Peucedanum
praeruptorum Dunn with multidrug resistance reversal and
anti-inflammatory activities. Molecules. 20:20967–20978.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bi J, Gao Z, Zhang G and Chen Y: Research
progress on anticancer effects and molecular mechanisms of osthole.
Asian J Tradit Med. 16:53–62. 2021.
|
|
6
|
Liu J, Jia H, Zhu K, Zhao S and Lichtfouse
E: Formation of environmentally persistent free radicals and
reactive oxygen species during the thermal treatment of soils
contaminated by polycyclic aromatic hydrocarbons. Environ Chem
Lett. 18:1329–1336. 2020.
|
|
7
|
Bauer G: Inhibition of membrane-associated
catalase, extracellular ROS/RNS signaling and
aquaporin/H2O2-mediated intracellular
glutathione depletion cooperate during apoptosis induction in the
human gastric carcinoma cell line MKN-45. Antioxidants.
10(1585)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ibrahim M, Ismail A, Al-Sheraji SH, Azlan
A and Hamid AA: Effects of Mangifera pajang Kostermans juice
on plasma antioxidant status and liver and kidney function in
normocholesterolemic subjects. J Funct Foods. 5:1900–1908.
2013.
|
|
9
|
de Haan A, Eijgelsheim M, Vogt L, van der
Zwaag B, van Eerde AM, Knoers NVAM and de Borst MH: Diagnostic
yield of massively parallel sequencing in patients with chronic
kidney disease of unknown etiology: Rationale and design of a
national prospective cohort study. BMJ Open.
12(e057829)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao X, Wang Q, Li G, Chen F, Qian Y and
Wang R: In vitro antioxidant, anti-mutagenic, anti-cancer and
anti-angiogenic effects of Chinese Bowl tea. J Funct Foods.
7:590–598. 2014.
|
|
11
|
Wang LX, Li CY, Hu C, Gong PS and Zhao SH:
Purification and structural characterization of Dendrobium
officinale polysaccharides and its activities. Chem Biodivers.
18(e2001023)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Takatsuka M, Goto S, Kobayashi K, Otsuka Y
and Shimada Y: Evaluation of pure antioxidative capacity of
antioxidants: ESR spectroscopy of stable radicals by DPPH and ABTS
assays with singular value decomposition. Food Biosci.
48(101714)2022.
|
|
13
|
Xu SJ, Zhu Q, Wang ZH, Xu Y and Zou SJ:
Scavenging activity of superoxide free radical of hesperidin and
hesperetin. Acta Chinese Med Pharmacol. 2015:56–58. 2015.
|
|
14
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao X, Yi RK, Feng X and Song JL:
Protective effect of Korean sesame sauce on AAPH-induced oxidative
stress in LLC-PK1 cells. Food Sci. 38:213–218. 2017.
|
|
16
|
Long X, Wu H, Zhou Y, Wan Y, Kan X, Gong J
and Zhao X: Preventive effect of Limosilactobacillus
fermentum SCHY34 on lead acetate-induced neurological damage in
SD rats. Front Nutr. 9(852012)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu TT, Chen R, Qian Y, Ye K, Long XY, Park
KY and Zhao X: Antioxidant effect of Lactobacillus fermentum
HFY02-fermented soy milk on D-galactose-induced aging mouse model.
Food Sci Human Wellness. 11:1362–1372. 2022.
|
|
18
|
Pine MD, Greer K and Busbee D: Comparison
of reactive oxygen scavenging systems between a cetacean (DKN1) and
a porcine renal epithelial cell line (LLC-PK1). Comp Biochem
Physiol A Mol Integr Physiol. 147:550–555. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu Y, Liu K, Jin R, Jiang D and Fang D:
Dynamic visualization of free radicals at single oxygen bubbles
using chemiluminescence. Chem Asian J. 16:4049–4052.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang F, Yang Q, Zhao Q and Li X: Cold
shock treatment with oxalic acid could alleviate chilling injury in
green bell pepper by enhancing antioxidant enzyme activity and
regulating proline metabolism. Sci Horticult. 295(110783)2022.
|
|
21
|
Zhang H, Zong R, He H and Huang T: Effects
of hydrogen peroxide on Scenedesmus obliquus: Cell growth,
antioxidant enzyme activity and intracellular protein
fingerprinting. Chemosphere. 287(132185)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huong DQ, Bay MV and Nam PC: Antioxidant
activity of thiourea derivatives: An experimental and theoretical
study. J Mol Liquids. 340(117149)2021.
|
|
23
|
Liao CY, Yang SF, Wu TJ, Chang H, Huang
CYF, Liu YF, Wang CH, Liou JC, Hsu SL, Lee H, et al: Novel function
of PERP-428 variants impacts lung cancer risk through the
differential regulation of PTEN/MDM2/p53-mediated antioxidant
activity. Free Radic Biol Med. 167:307–320. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li C, Chen S, Sha J, Cui J, He J, Fu J and
Shen Y: Extraction and purification of total flavonoids from
Eupatorium lindleyanum DC and evaluation of their
antioxidant and enzyme inhibitory activities. Food Sci Nutr.
9:2349–2363. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Le G, Yang L, Du H, Hou L, Ge L, Sylia A,
Muhmood A, Chen X, Han B and Huang K: Combination of zinc and
selenium alleviates ochratoxin A-induced fibrosis via blocking
ROS-dependent autophagy in HK-2 cells. J Trace Elem Med Biol.
69(126881)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ni CX, Gong H, Liu Y, Qi Y, Jiang CL and
Zhang JP: Green tea consumption and the risk of liver cancer: A
meta-analysis. Nutr Cancer. 69:211–220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Du W, Zhai P, Liu S, Zhang Y and Lu L: The
copper chaperone CcsA, coupled with superoxide dismutase SodA,
mediates the oxidative stress response in Aspergillus fumigatus.
Appl Environ Microbiol. 87(e0101321)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ren H, Li J, Liu P, Ren X, Song T, Gao G,
Li D and Liu S: Cloning of catalase gene and antioxidant genes in
Scophthalmus maximus response to metalloprotease of
Vibrio anguillarum stress. J Oceanol Limnol. 40:322–335.
2022.
|
|
29
|
Gao Z, Gao X, Fan W, Liu S, Li M, Miao Y,
Ding C, Tang Z, Yan L, Liu G, et al: Bisphenol A and genistein have
opposite effects on adult chicken ovary by acting on
ERα/Nrf2-Keap1-signaling pathway. Chem-Biol Interact.
347(109616)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang HN, Shen Z, Liu Q, Hou XY, Cao Y, Liu
DH, Jiang HJ and Du HZ: Isochlorogenic acid (ICGA): Natural
medicine with potentials in pharmaceutical developments. Chinese J
Nat Med. 18:860–871. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gao C, Zhou Y, Li H, Cong X, Jiang Z, Wang
X, Cao R and Tian W: Antitumor effects of baicalin on ovarian
cancer cells through induction of cell apoptosis and inhibition of
cell migration in vitro. Mol Med Rep. 16:8729–8734.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu B, Zhang Q, Shen W and Zhu J:
Anti-proliferative and chemosensitizing effects of luteolin on
human gastric cancer AGS cell line. Mol Cell Biochem. 313:125–132.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2012.PubMed/NCBI View Article : Google Scholar
|