Introduction
Age-related macular degeneration (AMD) is a prime
cause of visual impairment and severe vision loss and is one of the
top three eye diseases that can result in blindness (1). Subretinal fibrosis (SF) is a pivotal
pathologic trait of neovascular AMD (nAMD) (2). The progression of SF can lead to
vision loss (3). SF is primarily
characterized by an excess of extracellular matrix (ECM) proteins,
including collagen and fibronectin (4). SF and ECM components are mainly
produced by activated myofibroblasts (5). In retinal diseases, oxidative stress
can lead to neurodegeneration and cell loss, which is associated
with early disease progression (6). Thus, it is urgently necessary to
develop novel therapies for the treatment of SF.
Metformin is a biguanide that has been widely used
to treat diabetes since the 1950s (7). In addition to being an antidiabetic
drug with low cost and high safety, metformin has also been shown
to inhibit the proliferation and migration of human retinal
vascular endothelial cells and reduce oxidative stress in cells and
various organs (8,9). Furthermore, metformin has been shown
to reverse existing pulmonary fibrosis in mice (10) and inhibit the migration and
invasion of cancer cells in vitro (11). It has also been shown to have a
protective effect against several age-associated diseases, and may
prevent the development of AMD (12). Currently, metformin is of interest
as a candidate for the treatment of AMD, as it may reduce the
progression of AMD via its antioxidant, anti-inflammatory and
antifibrotic effects (13).
However, the role and mechanism of metformin are intricate, and it
is important to determine the mechanism of metformin in the
treatment of SF.
MicroRNAs (miRNAs) are small noncoding RNAs that
play critical roles in gene regulation and numerous biological
processes (14). The development
of retinal diseases, including AMD, is associated with the
dysregulation of miRNAs and alterations in the mechanisms of miRNA
biogenesis (15). Yi et al
(16) showed that the
overexpression of miR-140-3p significantly alleviated inflammation,
oxidative stress and apoptosis in oxygen-glucose deprivation and
reperfusion models, and exerted a protective effect on cells. In
addition, Al-Modawi et al (17) showed that miR-140-3p prevented
oxidative stress by upregulating the enzyme N(G),
N(G)-dimethylarginine dimethylaminohydrolase 1. Furthermore,
another study showed that the upregulation of miR-140-3p
ameliorated pathological changes and inhibited inflammation,
oxidative stress and fibrosis in rats with rheumatoid arthritis
(18). These findings indicate
that miR-140-3p improves various functions of the body by
alleviating oxidative stress and fibrosis. However, the regulatory
mechanism of miR-140-3p in SF is unknown.
LIN28 is a reprogramming factor and conserved
RNA-binding protein, and the only homolog of LIN28 in humans
(19). LIN28B regulates cell
migration, invasion, apoptosis and fusion (20). In a study by Liang et al
(21), LIN28B was shown to induce
epithelial-mesenchymal transition (EMT) via the inhibition of
let-7d, and the inhibition of LIN28B alleviated TGF-β1-induced
fibrosis. In another study, Zhang and Sui (22) showed that the knockdown of circBPTF
mediated the miR-384/LIN28B axis in human umbilical vein
endothelial cells, thereby preventing the inflammatory injury and
oxidative stress induced by high glucose. Thus, as LIN28B is
indicated to regulate fibrosis, the present study investigated the
effect of LIN28B on SF.
JNK, which is a Ser/Thr kinase, is a member of the
mitogen-activated protein kinase family in mammals. JNK influences
multiple cellular processes, including cell proliferation, survival
and malignant transformation (23). STAT3 is a cytoplasmic transcription
factor that plays a pivotal role in gene expression and is involved
in proliferation and survival (24). Yang et al (25) showed that JNK enhanced the
transcription of TGF-β1 and connective tissue growth factor and
promoted fibrosis in models of acute kidney injury, while the
inhibition of JNK activity protected the kidney from the
development of fibrosis. Du et al (26) demonstrated that the knockdown of
atypical protein kinase C-i inhibited the EMT, migration and
invasion of colorectal cancer cells by inhibiting the Rac1-JNK
pathway. In addition, Zhao et al (27) found that STAT3 was intimately
associated with the occurrence and development of liver fibrosis
induced by multiple factors, and suggested that STAT3 can play an
anti-inflammatory or proinflammatory role in the pathogenesis of
liver fibrosis. Zhao et al (28) also showed that the JNK/STAT3
signaling pathway is involved in the oxidative stress and apoptosis
induced by excessive fluoride in female mice, which reduced the
development of potential oocytes. These studies offer important
information to support exploration of the role of the JNK/STAT3
pathway in fibrotic diseases and serve as a reference for further
study of the mechanism of the JNK/STAT3 pathway in fibrotic
diseases.
Therefore, the present study examined the role and
effect of metformin in SF, and investigated whether its mechanism
involves regulation of the JNK/STAT3 signaling pathway mediated by
LIN28B through miR-140-3p. The study may constitute an academic
reference for the clinical treatment of SF.
Materials and methods
Establishment and grouping of the
animal models
A total of 40 male SPF C57BL/6J mice (18-22 g, 6-8
weeks old) were obtained from the Experimental Animal Center of
Yunnan University. The license number for the use of experimental
animals was SCXK (Dian) K2021-0002. All procedures were approved by
the Experimental Animal Welfare Ethics Committee of the
Experimental Animal Center of Yunnan University (Kunming, China;
ethics no. YNU20220291). Mice were housed individually using a 12-h
light/dark cycle at 22˚C with 50% humidity, with food and water
available at will, and were domesticated and housed for 1 week
prior to the experiment. The mice were randomly divided into 4
groups (n=10/group): Normal control group (mice without laser
irradiation), SF group, SF + metformin (SF + Met) group and SF +
metformin + miR-140-3p inhibitor group. The mice were anesthetized
with pentobarbital sodium (30 mg/kg, intraperitoneal injection),
and the laser-induced SF model was examined for 35 days as
previously described (29).
Briefly, on day 0, the mice were anesthetized with pentobarbital,
and 4-6 laser spots (532 nm, 200 mW, 100 msec, 75 µm;
VISULAS® 532s; Zeiss AG) were created in each fundus
around the optic disc. Immediately after laser irradiation, a
subretinal bubble formed, confirming rupture of the Bruch's
membrane. Mice in the two metformin treatment groups were treated
with 300 mg/kg/day metformin (Beijing Solarbio Science &
Technology Co., Ltd.) by gavage from day 0 to day 35. In addition,
the mice in the SF + metformin + miR-140-3p inhibitor group were
injected with 5 µl miR-140-3p inhibitor lentivirus (Hunan Fenghui
Biotechnology Co., Ltd., China) through the vitreous cavity on day
0 of laser-induced injury. To administer the intravitreal
injection, a 30-gauge needle was first inserted behind the eye
margin to make a cavity. The homologous compounds were injected
into the incipient well cavities with a 34-gauge Hamilton syringe,
and all injections were performed under an operating microscope.
Animal health and behavior were monitored every 2 days.
The mice were euthanized on day 35 by decapitation
using scissors, and were confirmed dead after decapitation by the
loss of heartbeat and breath cessation. Eyeball mouse tissues were
collected and fixed in 5% paraformaldehyde for 1 h. The conditions
for euthanasia were as follows: i) the end of the animal
experiment; ii) the animals were in pain beyond the prespecified
mercy end point (such as marked reduction in pre-test body weight,
markedly coarse fur, accompanied by unresponsive and behavioral
abnormalities, and persistent dyspnea). In addition, meloxicam (1
mg/kg, intragastric administration) was used as an analgesic to
minimize the pain and stress of the animals during the
experiment.
Cell culture and model
construction
The ARPE-19 adult retinal pigment epithelium (RPE)
cell line was purchased from Shenzhen Otwo Biotechnology Co., Ltd.
and cultured in DMEM/F-12 (MilliporeSigma) containing 10% FBS
(MilliporeSigma), 100 U/ml penicillin and 100 µg/ml streptomycin at
37˚C with 5% CO2. The cultured ARPE-19 cells were
treated with 10 ng/ml TGF-β1 for 24 and 48 h to establish the
fibrotic cell model (TGF-β1 group), and the cells were also
cultured with 10 ng/ml TGF-β1 and 2 mM metformin (TGF-β1 + Met
group), or 0 ng/ml TGF-β1, 2 mM metformin and 4 µM anisomycin
(co-treatment) (TGF-β1 + Met + anisomycin group) for 48 h in
subsequent experiments.
Cell transfection
The negative control (NC) mimic, green fluorescent
protein miR-140a-3p mimic, miR-140a-3p inhibitor and NC inhibitor
were obtained from Sangon Biotech Co., Ltd. ARPE-19 cells were
transfected with Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) and the aforementioned nucleic acids (300
ng/µl) for 24 h at 37˚C. The transfection efficiency of the
miR-140a-3p inhibitor was determined by reverse
transcription-quantitative PCR (RT-qPCR) after 36 h. The
transfection efficiency of the miR-140a-3p mimic was determined by
immunofluorescence using a fluorescence microscope (Leica
Microsystems, GmbH) after 36 h.
The lentiviral LIN28B (3rd generation) was obtained
from Shanghai GenePharma Co., Ltd. The 293T cell (The Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences Cell
Bank) used to generate the virions, Packaging plasmids (pMDL, VSVG,
REV) were obtained from Shanghai GenePharma Co., Ltd. A mixture
comprising 1.5 µg LIN28B plasmid and 1.5 µg packaging plasmid
(pMDL:VSVG:REV=5:3:2) was prepared and then added to the culture
dish containing 293T cells for 4-6 h (37˚C, 5% CO2). The
transfection solution was absorbed and discarded, and fresh culture
solution was added for 72 h. Following filtration through a 0.45-µm
filter, the virus was collected by ultracentrifugation (4˚C, 6,000
x g, 2 h), and the titers were determined by a limited dilution
method. One day prior to the experiment, 4x103 ARPE19
cells were inoculated into each well of a 96-well culture plate,
and 10 µl 1x108 TU/ml virus and 5 µg/ml Polybrene were
added to each well (multiplicity of infection, 10) for incubation
at 37˚C for 24 h. Fluorescence was observed after 72 h of
infection. Empty vector was also transfected as the control. The
reagents used for lentiviral transfection were provided by Shanghai
GenePharma Co., Ltd. The sequences of the NC mimic, miR-140-3p
mimic, miR-140-3p inhibitor, NC inhibitor and LIN28B are presented
in Table SI.
Cell Counting Kit-8 (CCK-8) analysis
of cell proliferation
ARPE-19 cells (5x103 cells/well) were
inoculated in a 96-well plate and cultured for 24 h at 37˚C in a 5%
CO2 incubator. Following the relevant treatment, 20 µl
CCK-8 reagent (Beijing Solarbio Science & Technology Co., Ltd.)
was added to the cells in each well. After 2 h of incubation, the
absorbance was measured at 450 nm with a microplate reader.
Reactive oxygen species (ROS)
detection
ARPE-19 cells were seeded in 6-well plates at
2x105 cells/well and incubated with the ROS-specific
fluorescent dye dichloro-dihydro-fluorescein diacetate at 37˚C for
0.5 h in the dark. The cells were washed with PBS to remove the
unbound dye, digested and then resuspended in 0.5 ml PBS. Finally,
ROS levels were determined by FACSCalibur flow cytometry (BD
Biosciences) and analyzed with FlowJ software (v10.8.1; BD
Biosciences).
The retinal and choroidal tissues of C57BL/6J mice
were immediately placed into precooled PBS solution to remove the
blood and other pollutants. The tissue was cut into
1-mm3 pieces with ophthalmic scissors and rinsed in
precooled PBS to remove the cell fragments. Enzyme digestion
solution was added, and digestion was performed in a 37˚C constant
temperature water bath for 30 min with intermittent oscillation.
Digestion was then stopped with precooled PBS, tissue pellets were
removed with a 300-mesh nylon mesh filter, and the filtered cells
were collected to examine the ROS levels using the aforementioned
method.
Western blot analysis
Proteins were isolated from cells or retinochoroidal
tissue with RIPA buffer (Beyotime Institute of Biotechnology)
containing 1% protease inhibitors. Protein concentrations were
determined using a bicinchoninic acid assay (Beijing Solarbio
Science & Technology Co., Ltd.,) according to the
specifications of the kit. A 50-µg quantity of total protein was
loaded per lane, and the proteins were separated by SDS-PAGE (5%
stacking gel and 10% separating gel), transferred to PVDF membranes
(MilliporeSigma) and then blocked with 5% non-fat milk powder for
1.5 h at room temperature. The following diluted primary antibodies
were added and incubated overnight at 4˚C: E-cadherin (1:1,000;
ab231303), vimentin (1:2,000; ab92547), fibronectin (1:3,000;
ab2413), N-cadherin (1:5,000; ab76011), JNK (1:1,000; ab76125),
phosphorylated (p)-JNK (1:1,000; ab124965), STAT3 (1:1,000;
ab68153), p-STAT3 (1:1,000; ab267373), collagen I (1:5,000;
ab138492), collagen III (1:1,000; ab184993), LIN28B (1:2,000;
ab191881) and β-actin (1:1,000; ab8226) from Abcam and α-smooth
muscle actin (SMA; 1:1,000; #192455) Cell Signaling Technology,
Inc.), Next, the membranes were incubated with goat anti-rabbit IgG
H&L HRP-conjugated secondary antibodies (1:4,000; ab97051;
Abcam) for 1 h at room temperature and developed with an ECL kit
(Abcam). Finally, the bands were semiquantitatively analyzed using
ImageJ 1.52a software (National Institutes of Health).
Superoxide dismutase (SOD),
malondialdehyde (MDA), glutathione peroxidase (GSH-PX) and catalase
(CAT) assays
An SOD kit (cat. no. BC0170), GSH-PX kit (cat. no.
BC1195) and CAT kit (cat. no. BC0200) all from Beijing Solarbio
Science & Technology Co., Ltd., and MDA kit (Nanjing Jiancheng
Bioengineering Institute), were used to detect the levels of SOD,
MDA, GSH-PX and CAT in cells and retinal choroid tissue after
processing according to the instructions of the kit
manufacturers.
Detection of apoptosis by flow
cytometry
Cells in each group were digested with trypsin and
rinsed twice with precooled PBS. The apoptosis rate was then
determined using an Annexin-V-FITC/PI apoptosis kit (Absin
Bioscience, Inc.).
Scratch test
ARPE-19 cells in the logarithmic growth phase were
inoculated on 6-well plates after normal digestion and passage. The
cells were scratched with a pipette tip when the cell density
reached 90%. The cells were not serum starved, as 1% FBS medium was
used to culture with the cells. After 0 and 24 h of culture, cell
migration was observed under a light microscope and photographic
images were captured.
Transwell assay of cell invasion
Cell invasion experiments were performed using a
Transwell chamber (Corning, Inc.). The cell concentration was
adjusted to 1x105 cells/ml with serum-free DMEM. Then,
200 µl cell suspension was added to the upper chamber of the
Transwell chamber, which was coated with Matrigel (3 h, 37˚C) and
600 µl DMEM containing 10% FBS was added to the lower chamber of
the 24-well plate. The cells in the lower chamber were immobilized
with 4% paraformaldehyde and stained with crystal violet (Beijing
Solarbio Science & Technology Co., Ltd.) after 24 h (37˚C) of
incubation. The cells were placed under an inverted light
microscope, the number of cells at a fixed position in each well
were observed, and 5 visual fields were selected for photography
and counting.
Coimmunoprecipitation
Cultured cells were homogenized and lysed for 15 min
on ice with a mixture of protease inhibitors (Beyotime Institute of
Biotechnology) in hypotonic lysis buffer (Beyotime Institute of
Biotechnology). The cell lysates were centrifuged at 4,000 x g for
10 min at 4˚C. Following the removal of a small amount of lysate
for input western blot analysis, 1 µg corresponding antibody,
anti-Lin28B (1:100; cat. no. ab191881; Abcam) was added to the
rest, and the sample was incubated overnight at 4˚C with slow
shaking. Cell lysates containing 10 µl protein A agarose beads
(Beyotime Institute of Biotechnology) and antibodies were incubated
overnight at 4˚C. The beads were collected by centrifugation (1,000
x g, 3 min, 4˚C) and washed three times with lysis buffer (Beyotime
Institute of Biotechnology). Protein elution was followed by
immunoblot analysis.
Dual-luciferase assay
The target binding sites of miR-140-3p and LIN28B
were predicted by a bioinformatics database (http://starbase.sysu.edu.cn/). Luciferase reporter
plasmid (pGL3-Basic/LIN28B WT, pGL3-Basic/LIN28B MUT) was purchased
form Shanghai GenePharma Co., Ltd. The LIN28B 3'-untranslated
region containing the miR-140-3p binding site was cloned into a
pGL3 vector (Promega Corporation) to establish the wild-type LIN28B
vector (WT). A site-directed mutagenesis kit was used to generate a
mutant LIN28B vector (MUT). WT or MUT was cotransfected with a
vector overexpressing miR-140-3p or NC mimic into 293T cells using
Lipofectamine™ 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The luciferase activity was determined using a
Promega luciferase reporter system after 48 h, compared with
Renilla luciferase activity. The Dual-Lumi™ dual
luciferase reporter gene detection kit (cat. no. RG088S; Beyotime
Institute of Biotechnology) was used, and the experiment was
conducted following the manufacturer's instructions.
RT-qPCR
Total RNA was isolated from eye tissues and cells
with TRIzol® reagent (cat. no. 15596026; Invitrogen;
Thermo Fisher Scientific, Inc.), and cDNA was synthesized by
reverse transcription using a First-Strand cDNA synthesis kit
(GeneCopoeia, Inc.), with the following temperature protocol: 25˚C
for 5 min, 42˚C for 15 min, 85˚C for 5 min and hold at 4˚C. RT-qPCR
was performed using a SYBR Green real-time fluorescence
quantitative PCR kit (Beijing Solarbio Science & Technology
Co., Ltd.). The conditions of PCR were as follows: 95˚C
predenaturation for 30 sec; 40 cycles of 95˚C for 15 sec and 60˚C
for 30 sec; and 72˚C extension for 30 sec. The internal reference
genes were U6 for miR-140-3p and GAPDH for the other RNAs. Relative
expression of miRNA was analyzed by the 2-ΔΔCq method
(30). The primer sequences are
shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Target | Sequences
(5'-3') |
|---|
| miR-140-3p | F:
GCGCGTACCACAGGGTAGAA |
| | R:
AGTGCAGGGTCCGAGGTATT |
| hsa-LIN28B | F:
TAGGAAGTGAAAGAAGACCCAA |
| | R:
CTGAGGAAACGGTGGTGA |
| mmu-LIN28B | F:
TGTGGACTGTGCGAGAAGAAGA |
| | R:
CCTGTCTGAGTGCTCTGCCATT |
| U6 | F:
GCTTCGGCAGCACATATACTAAAAT |
| | R:
CGCTTCACGAATTTGCGTGTCAT |
| β-actin | F:
CACTGTGCCCATCTACGAGG |
| | R:
TAATGTCACGCACGATTTCC |
RNA pull-down
Using a Magnetic RNA Protein Pull Down Kit (Thermo
Fisher Scientific, Inc.), an RNA pull-down assay was performed in
accordance with the manufacturer's instructions. In brief, RNA
probes and miR-140-3p were incubated with cell lysate at room
temperature. Then magnetic beads were added to form a compound with
the probe, which was detected by immunoprecipitation, washing,
purification and western blotting.
Hematoxylin and eosin (H&E)
staining
After 35 days of treatment, the eyeballs were
isolated, enucleated and fixed in FAS eyeball fixative (Wuhan
Servicebio Technology Co., Ltd.) for >48 h and then dehydrated
and embedded in paraffin. Sections (20-µm thick) were dewaxed,
stained with hematoxylin for 5 min (at room temperature), washed
with tap water, stained with eosin for 1 min (at room temperature)
and then dehydrated before sealing with neutral gum for observation
(light microscopy) and analysis.
Masson staining
Paraffin sections (20-µm thick) were dewaxed,
hydrated with gradient ethanol, stained with hematoxylin for 5 min,
and fully washed with water. The sections were then washed with
Masson ponceau acid fuchsin solution for 5 min, soaked in 2%
glacial acetic acid water, differentiated with 1% phosphomolybdic
acid aqueous solution for 3 min without washing and then directly
dyed with aniline blue or light green solution for 5 min. After
washing with 0.2% glacial acetic acid aqueous solution for 5 sec,
95% alcohol, absolute alcohol, xylene and transparent neutral gum
were applied (at room temperature). A light microscope was used to
observe and images were captured.
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software; Dotmatics)
was used to analyze the experimental data and plot the graphs. The
data are from ≥3 replicates of all experiments. One-way ANOVA was
used for analysis followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant result.
Results
Metformin inhibits laser-induced SF
and oxidative stress in mice
To examine the effect of metformin on laser-induced
SF in mice, the pathology of optic cup tissue was observed using
H&E staining. The results showed that compared with the control
group, the SF group had more subretinal fibers and fibrocytes and a
thickened choroid. However, the subretinal fibers and choroidal
thickness were reduced by metformin (Fig. 1A). Masson staining of the optic cup
tissue showed that the amount of collagen fiber synthesis in the SF
group was markedly higher than that in the control group, and
metformin prominently reduced the synthesis of collagen fibers
(Fig. 1B). Western blot analysis
showed that the levels of α-SMA, collagen I, collagen III,
vimentin, fibronectin and N-cadherin were upregulated in the SF
group in comparison with those in the control group, while
E-cadherin expression was downregulated. Metformin reversed the
effects of SF on these proteins (Fig.
1C and D). Markers of
oxidative stress were examined, and the results showed that, in
comparison with those in the control group, SOD and GSH-PX activity
and CAT content in the SF group were prominently reduced, while the
MDA level was increased. The effects in the SF group were reversed
by metformin (Fig. 1E). These
results indicate that metformin inhibited EMT and
fibrosis-associated protein expression, increased the levels of
SOD, GSH-PX and CAT, inhibited MDA formation, and alleviated the
progression of SF in the mice.
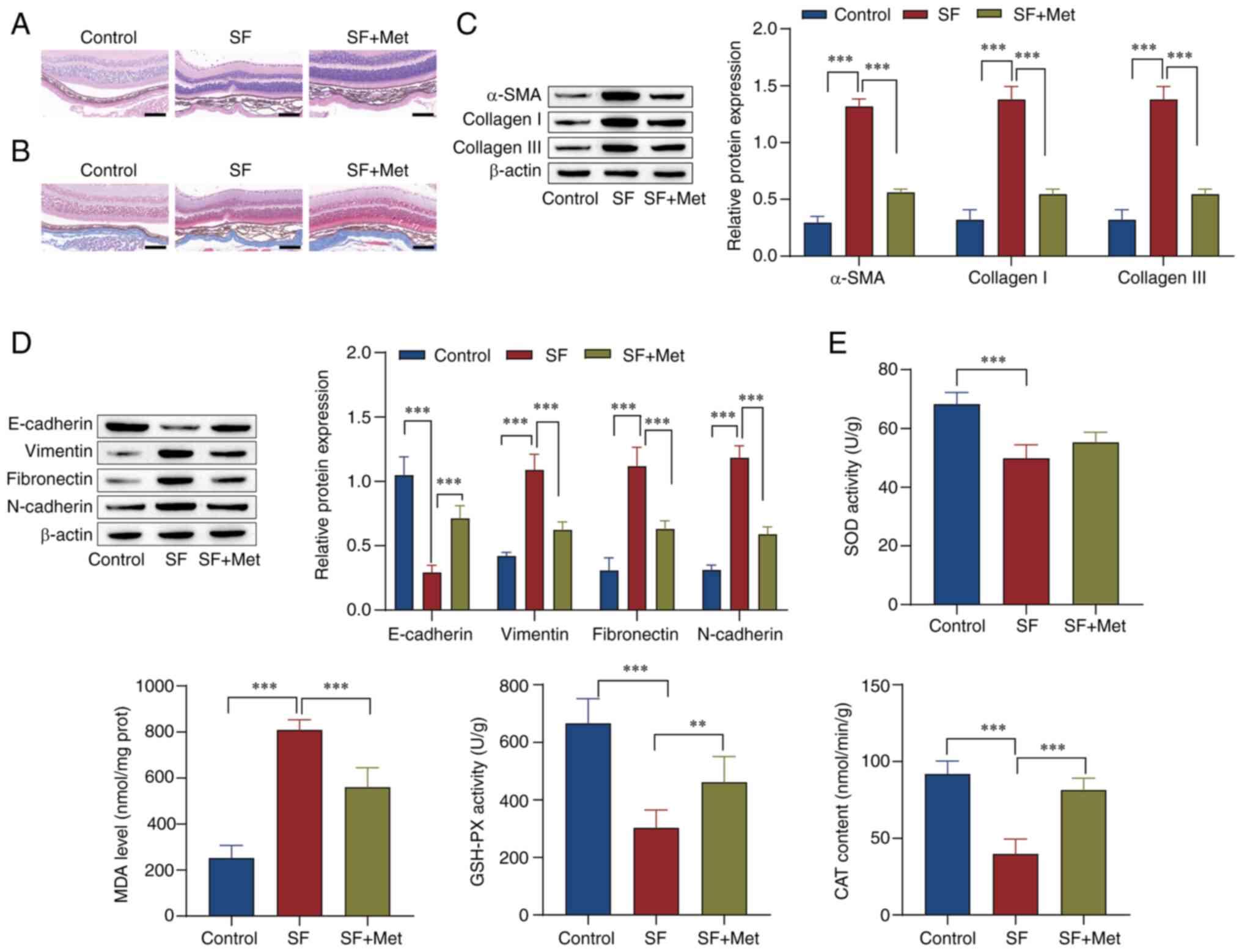 | Figure 1Metformin inhibits the progression of
laser-induced subretinal fibrosis in mice. (A) Hematoxylin and
eosin staining and (B) Masson staining were used to examine optic
cup tissue (scale bar, 100 µm). Western blotting was performed to
examine the levels of (C) fibrosis- and (D) epithelial-mesenchymal
transition-associated proteins. (E) Kits were used to examine the
levels of SOD, MDA, GSH-PX and CAT. **P<0.01,
***P<0.001. Statistical analysis was by one-way ANOVA
followed by Tukey's post hoc tests. SOD, superoxide dismutase; MDA,
malondialdehyde; GSH-PX, glutathione peroxidase; CAT, catalase; SF,
subretinal fibrosis; Met, metformin; SMA, smooth muscle actin;
prot, protein. |
Metformin inhibits EMT in RPE cells
through the JNK/STAT3 signaling pathway
To examine if the effects of metformin are achieved
through the JNK/STAT3 signaling pathway, cell proliferation was
examined by CCK-8 assay and ROS levels were measured using flow
cytometry. The results showed that cell proliferation and ROS
levels were increased in the TGF-β1 group compared with the NC
group. Metformin reversed the effect of TGF-β1, and anisomycin, an
activator of the JNK/STAT3 pathway, attenuated the effect of
metformin in both assays (Fig. 2A
and B). The oxidative stress level
was also examined, and the results showed that in comparison with
those in the untreated control group, the activity of SOD and
GSH-PX and the CAT content were reduced, and the levels of MDA were
increased in the TGF-β1 group. The effect of TGF-β1 was reversed by
metformin, while the effect of metformin was attenuated by
anisomycin (Fig. 2C). In addition,
the results of scratch and Transwell assays showed that the
migration and invasion of cells in the TGF-β1 group were markedly
elevated compared with those in the NC group, the effect of TGF-β1
was reversed by metformin, while the effect of metformin was
attenuated by anisomycin (Fig. 2D
and E). Furthermore, western
blotting showed that TGF-β1 downregulated the level of E-cadherin
and upregulated the levels of vimentin, fibronectin, N-cadherin,
p-STAT3/STAT3, p-JNK/JNK, α-SMA, collagen I and collagen III. The
effect of TGF-β1 was reversed by the addition of metformin, and the
effect of metformin was attenuated (Fig. 2F-H). These results indicate that
the effect of metformin on retinal fibrosis and oxidative stress
may involve the JNK/STAT3 pathway.
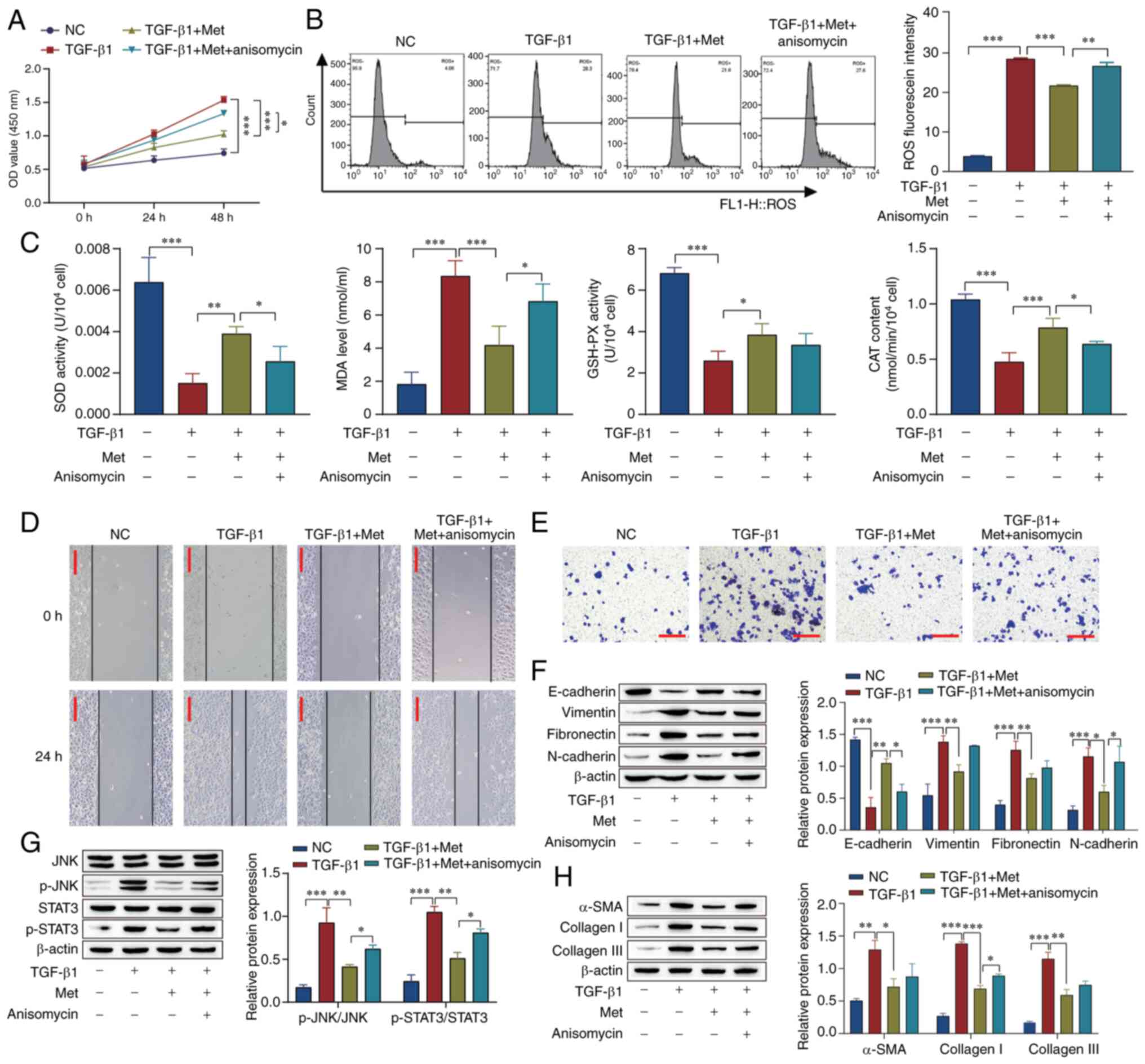 | Figure 2Metformin inhibits fibrosis in
retinal pigment epithelial cells through the JNK/STAT3 signaling
pathway. (A) A Cell Counting Kit-8 assay was used to examine cell
proliferation. (B) ROS levels were detected by flow cytometry. (C)
SOD, MDA, GSH-PX and CAT levels in the cells were detected using
kits. (D) Scratch tests were used to examine the migration of the
cells (scale bar, 100 µm). (E) Cell invasion was measured using
Transwell assays (scale bar, 100 µm). The expression of (F)
epithelial-mesenchymal transition-associated proteins, (G)
JNK/STAT3 pathway-associated proteins and (H) fibrosis-related
proteins. *P<0.05, **P<0.01,
***P<0.001. Statistical analysis was by one-way ANOVA
followed by Tukey's post hoc tests. ROS, reactive oxygen species;
SOD, superoxide dismutase; MDA, malondialdehyde; GSH-PX,
glutathione peroxidase; CAT, catalase; NC, negative control; Met,
metformin; OD, optical density; p-, phosphorylated; SMA, smooth
muscle actin. |
Metformin inhibits JNK/STAT3 signaling
pathway-mediated EMT in RPE cells via miR-140-3p
The levels of miR-140-3p in the RPE cells were
measured by RT-qPCR, and the results showed that metformin promoted
miR-140-3p expression in the presence of TGB-β1 (Fig. 3A). Furthermore, the knockdown of
miR-140-3p was performed to explore the mechanism of miRNA in the
cell model (Fig. 3B). In
comparison with that in the TGF-β1 group, the cell proliferation
activity and ROS levels were decreased in the TGF-β1 + Met group.
The effect of TGF-β1 + Met was attenuated by the miR-140-3p
inhibitor (Fig. 3C and D). Compared with those in the TGF-β1
group, the activity of SOD and GSH-PX and the CAT content were
increased, while the MDA levels were decreased in the TGF-β1 + Met
group. The addition of the miR-140-3p inhibitor attenuated the
effect of metformin (Fig. 3E). The
scratch assay and Transwell results showed that in comparison with
those in the NC group, the migration and invasion of cells in the
TGF-β1 group were markedly increased, and the effect of TGF-β1 was
reversed by metformin. The effect observed in the TGF-β1 + Met
group was attenuated by the miR-140-3p inhibitor (Fig. 3F and G). Western blotting showed that in
comparison with those in the NC group, the levels of E-cadherin
were downregulated and the levels of vimentin, fibronectin,
N-cadherin, p-STAT3/STAT3, p-JNK/JNK, α-SMA, collagen I and
collagen III were upregulated in the TGF-β1 group, and the effect
of TGF-β1 was reversed by metformin. Following transfection of the
miR-140-3p inhibitor, the effect of metformin in the TGF-β1 + Met
group was reversed (Fig. 3H-J). In
summary, these results indicate that metformin inhibits the
JNK/STAT3 signaling pathway by promoting miR-140-3p expression,
thereby inhibiting RPE cell fibrosis and oxidative stress.
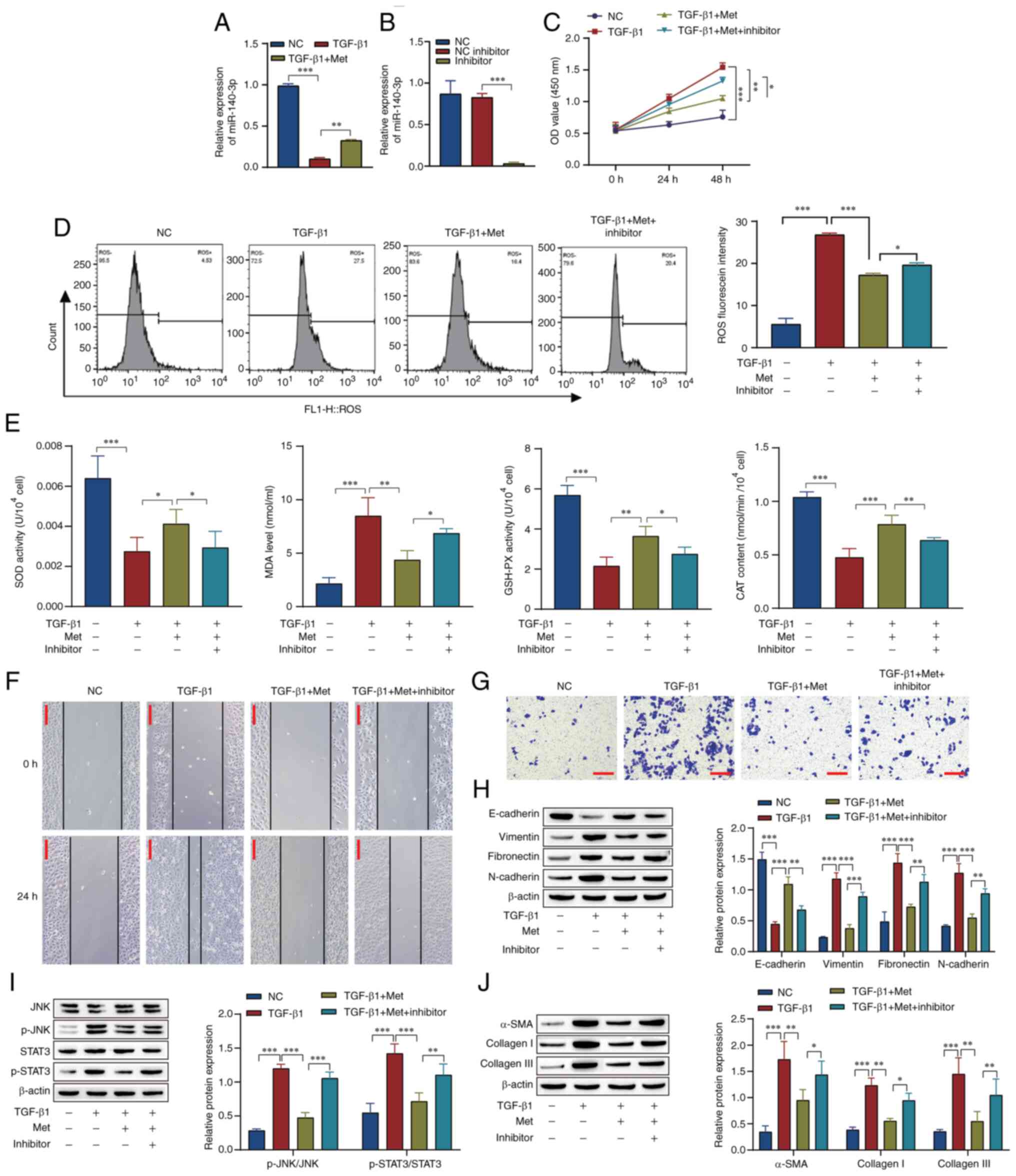 | Figure 3Metformin inhibits JNK/STAT3
signaling pathway-mediated fibrosis in retinal pigment epithelial
cells through miR-140-3p. Reverse transcription-quantitative PCR
was used to examine miR-140-3p expression in (A) the NC, TBF-b1 and
TBF-b1 + Met groups and (B) cells transfected with miR-140-3p
inhibitor or NC inhibitor. (C) A Cell Counting Kit-8 assay was used
to examine cell proliferation. (D) ROS levels were detected by flow
cytometry. (E) SOD, MDA, GSH-PX and CAT levels in the cells were
detected using kits. (F) Scratch tests were used to examine cell
migration (scale bar, 100 µm). (G) Transwell assays were used to
examine cell invasion (scale bar, 100 µm). Western blot analysis of
proteins associated with (H) epithelial-mesenchymal transition, (I)
the JNK/STAT3 pathway and (J) fibrosis. *P<0.05,
**P<0.01, ***P<0.001 Statistical
analysis was performed by one-way ANOVA followed by Tukey's post
hoc tests. NC, negative control; Met, metformin; miR, microRNA;
inhibitor, miR-140-3p inhibitor; ROS, reactive oxygen species; SOD,
superoxide dismutase; MDA, malondialdehyde; GSH-PX, glutathione
peroxidase; CAT, catalase; OD, optical density; p-, phosphorylated;
SMA, smooth muscle actin. |
miR-140-3p affects JNK/STAT3 signaling
through LIN28B
To further investigate the downstream regulatory
mechanism of miR-140-3p, the cells were transfected with miR-140-3p
mimic to explore the mechanism of this miRNA in ARPE19 cells. The
green fluorescence of the miR-140-3p mimic showed that miR-140-3p
was successfully transfected (Fig.
4A). The results of an RNA pull-down assay show that miR-140-3p
bonds with LIN28B (Fig. 4B). The
binding sites of LIN2B targeted by miR-140-3p were predicted using
a bioinformatics website. A dual-luciferase reporter assay
confirmed that miR-140-3p downregulated LIN28B (Fig. 4C). To verify whether there was an
interactive relationship between LIN28B and JNK,
coimmunoprecipitation experiments were performed. The results
showed that LIN28B and JNK proteins were precipitated in cells
incubated with LIN28B antibody compared with those incubated with
IgG antibody (Fig. 4D). RT-qPCR
and western blotting results showed transfection with miR-140-3p
mimic reduced Lin28B expression (Fig.
4E and F).
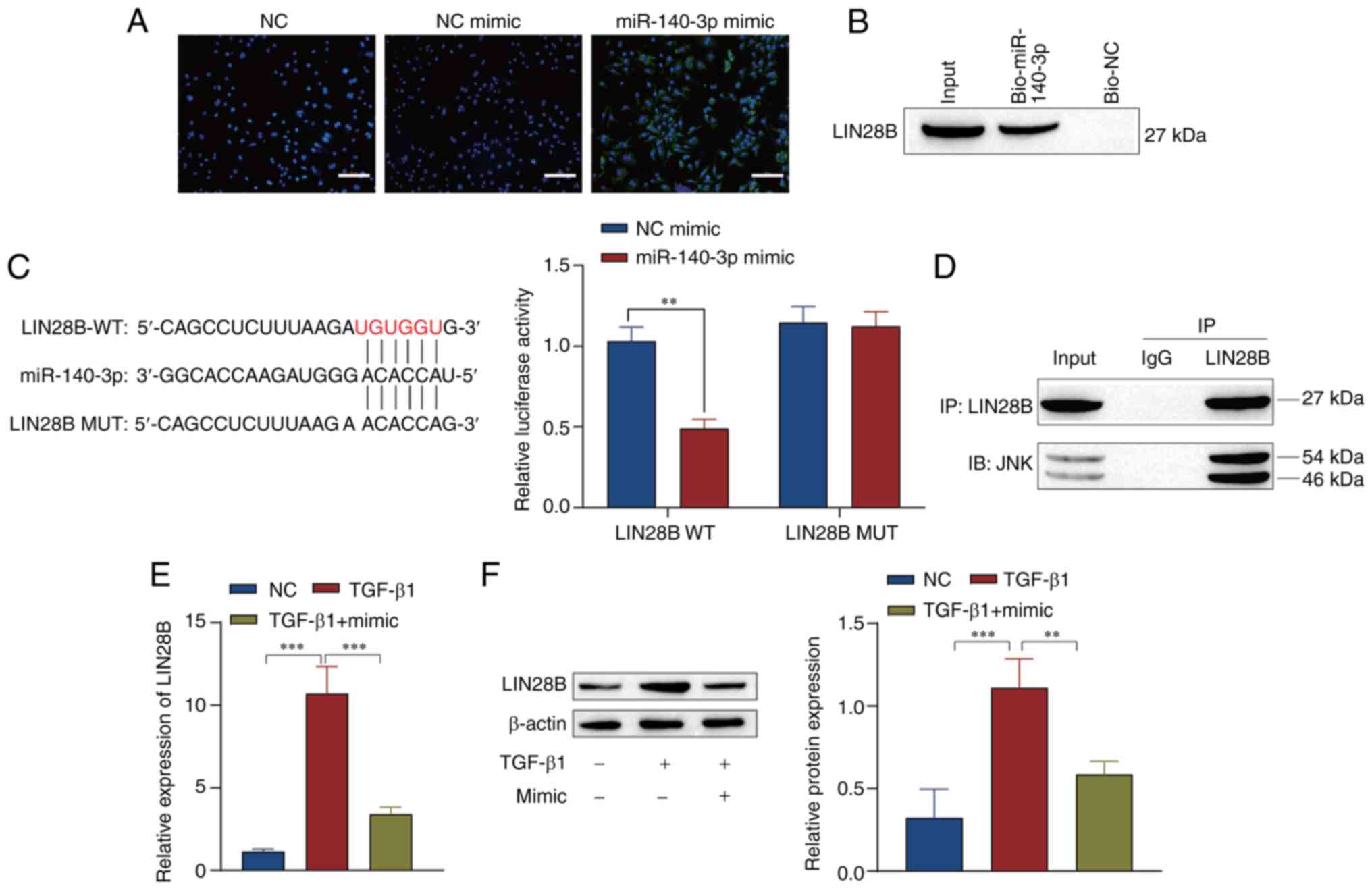 | Figure 4miR-140-3p affects the JNK/STAT3
signaling pathway through LIN28B. (A) Immunofluorescence was used
to confirm transfection with miR-140-3p mimic (scale bar, 100 µm).
(B) RNA pull-down assay confirmed the targeting relationship
between miR-140-3p and LIN28B. (C) StarBase was used to predict the
sequences of the binding sites of miR-140-3p and LIN28B, and a
dual-luciferase assay was performed to confirm the targeting
relationship between miR-140-3p and LIN28B. (D) The interaction
between LIN28B and JNK was verified by coimmunoprecipitation assay.
(E) Reverse transcription-quantitative PCR and (F) western blot
analysis of the expression of LIN28B. **P<0.01,
***P<0.001. Statistical analysis was performed by (C)
two-way ANOVA and (E and F) one-way ANOVA followed by Tukey's post
hoc tests. miR, microRNA; NC, negative control; WT, wild type; MUT,
mutant; IP, immunoprecipitation; IB, immunoblotting; mimic,
miR-140-3p mimic. |
miR-140-3p affects TGF-β1-induced
fibrosis in RPE cells through the JNK/STAT3 signaling pathway via
LIN28B
ARPE19 cells were transfected with LIN28B
overexpression vector, and western blotting results showed that the
overexpression of LIN28B was successful (Fig. 5A). In comparison with those in the
TGF-β1 group, the miR-140-3p mimic inhibited cell viability and
decreased ROS levels, while LIN28B overexpression attenuated the
effect of the mimic in the TGF-β1 + miR-140-3p mimic group
(Fig. 5B and C). In comparison with those in the TGF-β1
group, the activity of SOD and GSH-PX and the content of CAT were
increased, while the levels of MDA were decreased in the cells
treated with TGF-β1 and miR-140-3p mimic. The overexpression of
LIN28B reversed the effect of the mimic in the TGF-β1 + miR-140-3p
mimic group (Fig. 5D). As before,
the scratch assay and Transwell results showed that cell migration
and invasion were increased in the TGF-β1 group compared with the
NC group. Transfection with miR-140-3p mimic reversed the effect of
TGF-β1, while the overexpression of LIN28B attenuated the effect of
the mimic in the TGF-β1 + miR-140-3p mimic group (Fig. 5E and F). Western blotting results indicated
that the miR-140-3p mimic inhibited EMT, activation of the
JNK/STAT3 pathway and the progression of fibrosis, and the
overexpression of LIN28B attenuated these effects (Fig. 5G-I). These results show that
overexpression of miR-140-3p inhibited the JNK/STAT3 pathway,
thereby inhibiting EMT, the JNK/STAT3 pathway, fibrosis-associated
proteins, oxidative stress, cell migration and proliferation, and
these effects were inhibited by the overexpression of LIN28B.
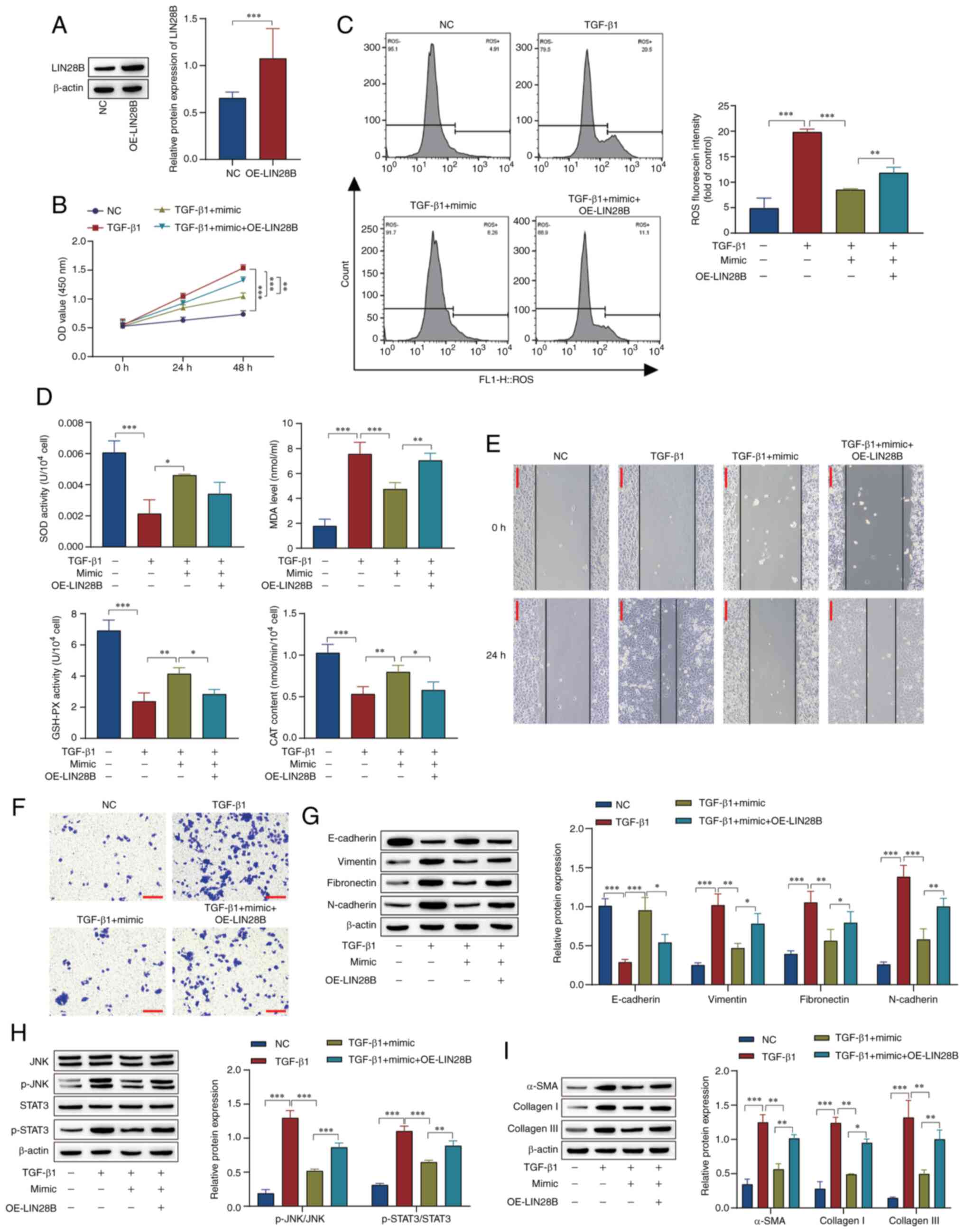 | Figure 5miR-140-3p affects TGF-β1-induced
retinal pigment epithelial cell fibrosis through the JNK/STAT3
signaling pathway via LIN28B. (A) Expression of LIN28B in
transfected cells was measured by western blotting. (B) Cell
Counting Kit-8 analysis of cell proliferation. (C) Flow cytometric
analysis of ROS levels. (D) SOD, MDA, GSH-PX and CAT levels were
detected using kits. (E) Scratch tests were used to examine cell
migration (scale bar, 100 µm). (F) Transwell assays were used to
examine cell invasion (scale bar, 100 µm). Western blot analysis of
proteins associated with (G) epithelial-mesenchymal transition, (H)
the JNK/STAT3 signaling pathway and (I) fibrosis.
*P<0.05, **P<0.01,
***P<0.001. Statistical analysis was performed by
one-way ANOVA followed by Tukey's post hoc tests. miR, microRNA;
ROS, reactive oxygen species; SOD, superoxide dismutase; MDA,
malondialdehyde; GSH-PX, glutathione peroxidase; CAT, catalase; NC,
negative control; OE, overexpression; mimic, miR-140-3p mimic; OD,
optical density; p-, phosphorylated; SMA, smooth muscle actin. |
Metformin affects SF through
miR-140-3p in vivo
Experiments were performed to confirm that the
effect of metformin on SF was mediated via miR-140-3p in mice. The
RT-qPCR results showed that the miR-140-3p inhibitor was
successfully transfected in the mouse eye, and that metformin
promoted miR-140-3p expression in SF model mice. In addition, the
expression of miR-140-3p was markedly decreased by the miR-140-3p
inhibitor (Fig. 6A and B). Western blot analysis showed that the
expression levels of α-SMA, collagen I, collagen III, vimentin,
fibronectin and N-cadherin were upregulated in the SF group, while
E-cadherin expression was downregulated. Metformin reversed the
effect of SF on these proteins, while the knockdown of miR-140-3p
attenuated the effect of metformin in the the SF + Met group
(Fig. 6C and D). H&E staining was performed to
observe the pathology of the optic cup tissue of the mice. The
results showed that compared with the control group, the SF group
had greater number of subretinal fibers and fibrocytes, and a
thickened choroid. The number of subretinal fibers and choroidal
thickness were reduced by metformin. Following the knockdown of
miR-140-3p, the formation of subretinal fibers was increased
compared with that in the SF + Met group (Fig. 6E). Masson staining of the optic cup
tissue showed that the synthesis of collagen fibers in the SF group
was markedly higher than that in the control and SF + Met groups,
and metformin notably reduced the synthesis of collagen fibers.
After miR-140-3p knockdown, collagen fiber synthesis was markedly
elevated compared with that in the SF + Met group (Fig. 6F). These results suggest that
metformin induced miR-140-3p expression, inhibited EMT -and
fibrosis-associated protein expression, thereby alleviating SF in
mice.
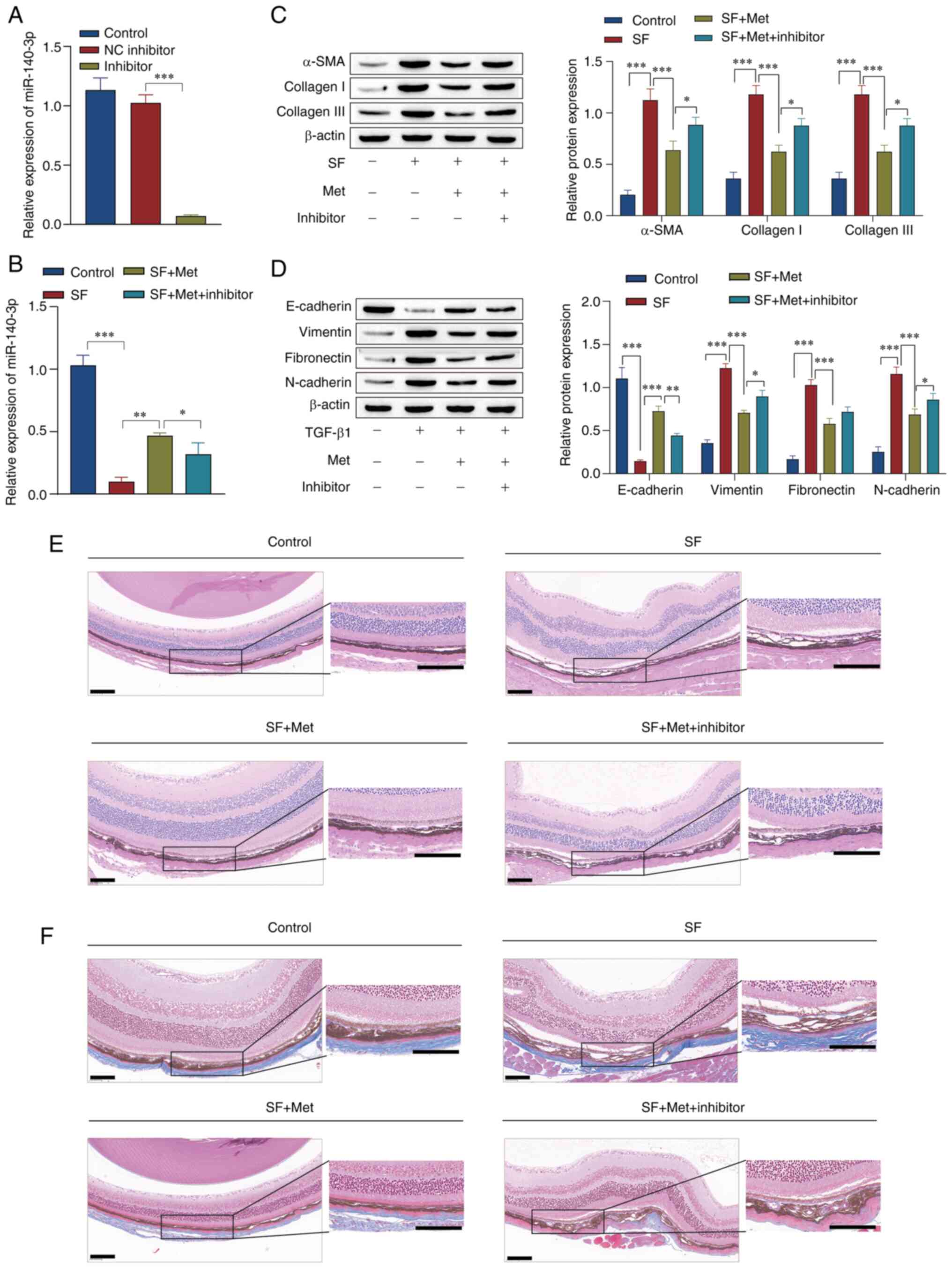 | Figure 6Experiments using mice verified that
metformin affects subretinal fibrosis through miR-140-3p. Reverse
transcription-quantitative PCR was used to measure the level of
miR-140-3p to (A) confirm transfection with miR-140-3p inhibitor
and (B) compare its expression in different groups. Western blot
analysis of the expression levels of proteins associated with (C)
fibrosis and (D) epithelial-mesenchymal transition. (E) Hematoxylin
and eosin staining was used to observe pathological changes in
optic cup tissue (scale bar, 100 µm). (F) Masson staining of optic
cup tissue (scale bar, 100 µm). *P<0.05,
**P<0.01, ***P<0.001. Statistical
analysis was performed by one-way ANOVA followed by Tukey's post
hoc tests. miR, microRNA; NC, negative control; inhibitor,
miR-140-3p inhibitor; SF, subretinal fibrosis; Met, metformin; SMA,
smooth muscle actin. |
Metformin affects the JNK signaling
pathway and oxidative stress in SF through miR-140-3p in vivo
Whether the effect of metformin on the JNK signaling
pathway and oxidative stress in SF is mediated via miR-140-3p was
examined in vivo. The RT-qPCR results showed that metformin
inhibited LIN28B expression, and the expression level of LIN28B was
increased by miR-140-3p inhibitor treatment (Fig. 7A). Western blot analysis showed
that p-STAT3 and p-JNK were upregulated in the SF group compared
with the control group, and metformin attenuated STAT3 and JNK
phosphorylation levels compared with those in the SF group. In
addition, the knockdown of miR-140-3p reversed the effect of
metformin in the SF + Met group (Fig.
7B). Flow cytometry results showed that metformin reversed the
effect of SF modeling on ROS levels, while the knockdown of
miR-140-3p reversed the effect of metformin in the SF + Met group
(Fig. 7C). In addition, the
activity of SOD and GSH-PX and the content of CAT were reduced
while the levels of MDA were increased in the SF group compared
with the control group. The effects observed in the SF group were
reversed by metformin, and those observed the SF +Met group were
attenuated by the knockdown of miR-140-3p (Fig. 7D). These results indicate that
metformin inhibited the JNK signaling pathway and oxidative stress
via the inhibition of LIN28B expression, and the changes induced by
metformin were reversed by knocking down miR-140-3p.
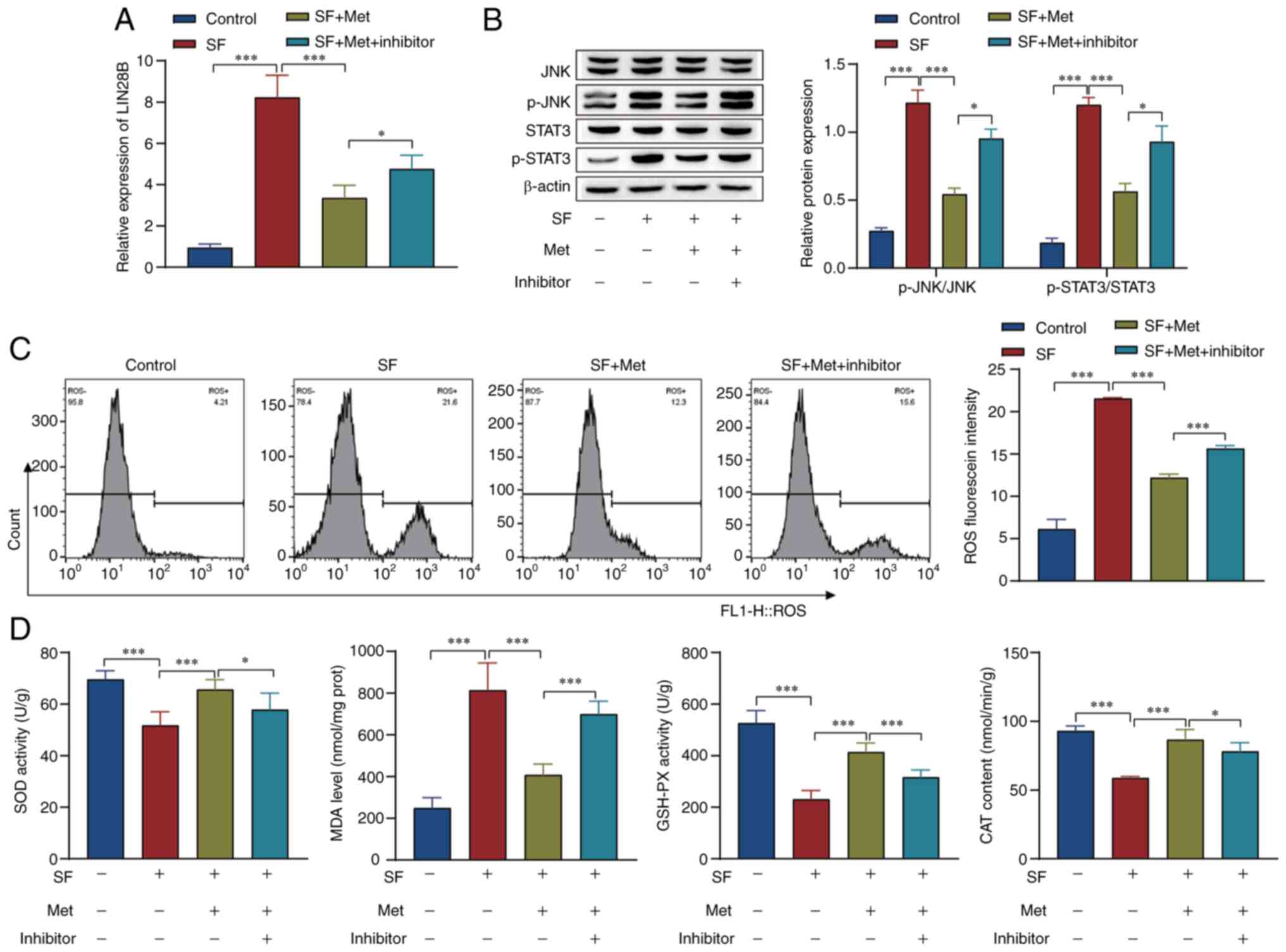 | Figure 7Experiments using mice verified that
metformin affects the JNK signaling pathway and oxidative stress
levels in subretinal fibrosis through miR-140-3p. (A) Reverse
transcription-quantitative PCR was used to examine the level of
LIN28B in the different treatment groups. (B) Western blot analysis
of the levels of JNK/STAT3 pathway-associated proteins. (C) Levels
of ROS were detected by flow cytometry. (D) Kits were used to
examine the levels of SOD, MDA, GSH-PX and CAT.
*P<0.05, ***P<0.001. Statistical
analysis was performed by one-way ANOVA followed by Tukey's post
hoc tests. Inhibitor, miR-140-3p inhibitor; miR, microRNA; OD,
superoxide dismutase; MDA, malondialdehyde; GSH-PX, glutathione
peroxidase; CAT, catalase; SF, subretinal fibrosis; Met, metformin;
p-, phosphorylated; ROS, reactive oxygen species; prot,
protein. |
Discussion
Fibrosis is a pathologic trait of most chronic
inflammatory diseases, which affects almost all tissues and can
eventually lead to organ dysfunction and death (31). SF is a vascularized lesion with
abundant immune cells, myofibroblasts, ECM protein sedimentation
and EMT (6,32,33).
SF damages photoreceptors, the RPE and choroidal capillaries,
leading to irreversible loss of central vision (5). To date, no effective antifibrotic
therapy to reduce SF formation in patients with nAMD has been
discovered (5). Therefore, it is
imperative to further study the pathogenesis of SF and identify
novel and more effective treatments for SF.
Metformin, which is a potent AMP-activated protein
kinase (AMPK) activator, has emerged as a promising agent for the
reduction or reversal of fibrosis (34). This agent also has antioxidant and
anti-inflammatory effects (35).
Kheirollahi et al (36)
showed that metformin accelerated the regression of fibrosis in the
lung by inducing the transdifferentiation of myofibroblasts into
adipofibroblasts. Metformin has also been shown to attenuate renal
fibrosis induced by unilateral ureteral obstruction in
AMPKα2-deficient mice (37). In
the present study, a laser-induced SF model was established in mice
and the optic cup tissue of the mice was examined by H&E and
Masson staining. The results indicated that metformin effectively
alleviated SF in mice.
JNK and STAT3 mediate cell transformation,
proliferation, survival and migration (23,38).
The JNK/STAT3 pathway has been reported to contribute to numerous
diseases; for example, Wang et al (39) showed that the JNK/STAT3 pathway is
involved in the formation of ECM in scar tissue after tendon
injury. In addition, Yan et al (40) and Zhao et al (28) showed that the JNK/STAT3 pathway
plays a role in the regulation of oxidative stress and apoptosis.
Furthermore, Dong et al (41) showed that metformin significantly
attenuated the fibrotic activity of hepatic stellate cells induced
by injury in hepatocellular carcinoma cells by targeting JNK in
vivo. In the present study, the results showed that JNK/STAT3
pathway proteins were highly activated in SF mouse and cell models,
suggesting that the JNK/STAT3 pathway may be involved in the
occurrence and development of SF. Further experiments indicated
that metformin inhibited EMT and fibrosis-associated protein
expression, cell migration, invasion and proliferation, and reduced
ROS and MDA levels by inhibiting the JNK/STAT3 signaling pathway.
The levels of SOD, GSH-PX and CAT were also increased, which may be
associated with the inhibition of fibrosis in RPE cells.
miRNAs have been shown to regulate organ fibrosis by
modulating the activity of relevant signaling pathways (42). For example, it has been shown that
miR-144-3p and miR-328 are involved in the regulation of cardiac
fibrosis, and miR-34a and miR-17-5p contribute to the development
of liver fibrosis under different conditions (42). Wu et al (18) indicated that miR-140-3p is a
mediator of hepatic stellate cell proliferation, and its knockdown
inhibited the TGF-β1-induced proliferation and fibrosis of HSC-T6
cells. In addition, Zhang et al (43) showed that the overexpression of
miR-140-3p inhibited EMT, invasion and metastasis in hepatocellular
carcinoma. In the present study, it was found that miR-140-3p was
expressed at low levels in SF mouse and cell models, and the
expression level of miR-140-3p was markedly elevated after
metformin treatment. The upregulation of miR-140-3p expression by
metformin was associated with inhibition of JNK/STAT3 pathway
activation and EMT- and fibrosis-associated protein expression,
increases in the levels of SOD, GSH-PX and CAT, and inhibition of
MDA production and SF in cells and animals.
LIN28 is a highly conserved RNA-binding protein that
has two homologs: LIN28A and LIN28B. Lin28B deficiency has been
shown to increase let-7a/let-7b expression and decrease hepatic
stellate cell activation and liver fibrosis in mice with alcoholic
liver injury (44). In addition, a
study by Lu et al (45)
showed that the overexpression of LIN28B activated the STAT3
signaling pathway in lung cancer, thus promoting EMT and
accelerating migration and invasion. In the present study, the
relationship between LIN28B and the JNK/STAT3 pathway was examined.
The results demonstrated an interaction between LIN28B and JNK.
Overexpression of LIN28B was also shown to upregulate the
phosphorylation of JNK/STAT3 pathway-related proteins and induce
activation of the JNK/STAT3 pathway. Moreover, the results of the
double luciferase experiment indicated that miR-140-3p targets
LIN28B.
In summary, the present study indicated that
metformin inhibits SF by facilitating miR-140-3p expression and
inhibiting LIN28B and the JNK/STAT3 pathway at the cellular and
organismal levels. The study may be considered as a novel academic
reference for those interested the treatment of SF. However, the
mechanism was only examined in cell and mouse experiments, and it
remains to be further studied whether there are any adverse
reactions or side effects of this treatment in clinical use.
Supplementary Material
Sequences of the nucleic acids used
for transfection.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by The Applied Basic Research
Foundation of the Department of Science, Technology of Yunnan
Province, Yunnan, China (grant no. 202201AY070001-036), the
National Natural Science Foundation Project (grant no. 82260207)
and Scientific Research Fund of Education Department of Yunnan
Province (grant no. 2023J0036).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH, WY, YG and DL were responsible for
conceptualization of the study. ZH, WY and YC were responsible for
methodology. ZH and WY performed validations. ZH, WY, LS and LR
carried out the formal analysis. ZH, WY, YG and LY performed
investigations, wrote the original draft of the manuscript,
reviewed and edited the manuscript and supervised the study. LY
provided resources and acquired funding. YZ, QZ and WZ were
responsible for visualization and data analysis. ZH and LY confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Laboratory Animal Welfare Ethics Committee of Yunnan University
(approval no. YNU20220291), and the animal procedures are reported
according to ARRIVE guidelines 2.0.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mitchell P, Liew G, Gopinath B and Wong
TY: Age-related macular degeneration. Lancet. 392:1147–1159.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ma X, Takahashi Y, Wu W, Chen J, Dehdarani
M, Liang W, Shin YH, Benyajati S and Ma JX: Soluble very
low-density lipoprotein receptor (sVLDLR) inhibits fibrosis in
neovascular age-related macular degeneration. FASEB J.
35(e22058)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou L, Shi DP, Chu WJ, Yang LL and Xu HF:
LRG1 promotes epithelial-mesenchymal transition of retinal pigment
epithelium cells by activating NOX4. Int J Ophthalmol. 14:349–355.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang C, Qin S, Xie H, Qiu Q, Wang H and
Zhang J, Luo D and Zhang J: RO4929097, a selective γ-secretase
inhibitor, inhibits subretinal fibrosis via suppressing notch and
ERK1/2 signaling in laser-induced mouse model. Invest Ophthalmol
Vis Sci. 63(14)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tenbrock L, Wolf J, Boneva S, Schlecht A,
Agostini H, Wieghofer P, Schlunck G and Lange C: Subretinal
fibrosis in neovascular age-related macular degeneration: Current
concepts, therapeutic avenues, and future perspectives. Cell Tissue
Res. 387:361–375. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mueller-Buehl AM, Doepper H, Grauthoff S,
Kiebler T, Peters L, Hurst J, Kuehn S, Bartz-Schmidt KU, Dick HB,
Joachim SC and Schnichels S: Oxidative stress-induced retinal
damage is prevented by mild hypothermia in an ex vivo model of
cultivated porcine retinas. Clin Exp Ophthalmol. 48:666–681.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Flory J and Lipska K: Metformin in 2019.
JAMA. 321:1926–1927. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Han J, Li Y, Liu X, Zhou T, Sun H, Edwards
P, Gao H, Yu FS and Qiao X: Metformin suppresses retinal
angiogenesis and inflammation in vitro and in vivo. PLoS One.
13(e0193031)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang G, Chen S, Shao Z, Li Y, Wang W, Mao
L, Li J and Mei X: Metformin alleviates hydrogen peroxide-induced
inflammation and oxidative stress via inhibiting P2X7R signaling in
spinal cord tissue cells neurons. Can J Physiol Pharmacol.
99:768–774. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rangarajan S, Bone NB, Zmijewska AA, Jiang
S, Park DW, Bernard K, et al: Metformin reverses established lung
fibrosis in a bleomycin model. Nature Medicine. 24:1121–7.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lv Z and Guo Y: Metformin and its benefits
for various diseases. Front Endocrinol (Lausanne).
11(191)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Blitzer AL, Ham SA, Colby KA and Skondra
D: Association of metformin use with age-related macular
degeneration: A case-control study. JAMA Ophthalmol. 139:302–309.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Romdhoniyyah DF, Harding SP, Cheyne CP and
Beare NAV: Metformin, a potential role in age-related macular
degeneration: A systematic review and meta-analysis. Ophthalmol
Ther. 10:245–260. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang L, Chen T, Yin Y, Zhang CY and Zhang
YL: Dietary microRNA-A novel functional component of food. Adv
Nutr. 10:711–721. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Askou AL, Alsing S, Holmgaard A, Bek T and
Corydon TJ: Dissecting microRNA dysregulation in age-related
macular degeneration: New targets for eye gene therapy. Acta
Ophthalmol. 96:9–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yi M, Li Y, Wang D, Zhang Q, Yang L and
Yang C: KCNQ1OT1 exacerbates ischemia-reperfusion injury through
targeted inhibition of miR-140-3P. Inflammation. 43:1832–1845.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Al-Modawi RN, Brinchmann JE and Karlsen
TA: Multi-pathway protective effects of MicroRNAs on human
chondrocytes in an in vitro model of osteoarthritis. Mol Ther
Nucleic Acids. 17:776–790. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu SM, Li TH, Yun H, Ai HW and Zhang KH:
miR-140-3p knockdown suppresses cell proliferation and fibrogenesis
in hepatic stellate cells via PTEN-mediated AKT/mTOR signaling.
Yonsei Med J. 60:561–569. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou J, Ng SB and Chng WJ: LIN28/LIN28B:
An emerging oncogenic driver in cancer stem cells. Int J Biochem
Cell Biol. 45:973–978. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang Q, Niu Y, Song L, Huang J, Wang C
and Ma T: Does LIN28B gene dysregulation make women more likely to
abort? Reprod Fertil. 2:211–220. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liang H, Liu S, Chen Y, Bai X, Liu L, Dong
Y, Hu M, Su X, Chen Y, Huangfu L, et al: miR-26a suppresses EMT by
disrupting the Lin28B/let-7d axis: Potential cross-talks among
miRNAs in IPF. J Mol Med (Berl). 94:655–665. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang W and Sui Y: CircBPTF knockdown
ameliorates high glucose-induced inflammatory injuries and
oxidative stress by targeting the miR-384/LIN28B axis in human
umbilical vein endothelial cells. Mol Cell Biochem. 471:101–111.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Miyazaki T, Bub JD and Iwamoto Y: c-Jun
NH(2)-terminal kinase mediates leptin-stimulated
androgen-independent prostate cancer cell proliferation via signal
transducer and activator of transcription 3 and Akt. Biochim
Biophys Acta. 1782:593–604. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guo ZL, Li JZ, Ma YY, Qian D, Zhong JY,
Jin MM, Huang P, Che LY, Pan B, Wang Y, et al: Shikonin sensitizes
A549 cells to TRAIL-induced apoptosis through the JNK, STAT3 and
AKT pathways. BMC Cell Biol. 19(29)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang L, Besschetnova TY, Brooks CR, Shah
JV and Bonventre JV: Epithelial cell cycle arrest in G2/M mediates
kidney fibrosis after injury. Nat Med. 16:535–543, 1p, 143.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Du GS, Qiu Y, Wang WS, Peng K, Zhang ZC,
Li XS, Xiao WD and Yang H: Knockdown on aPKC-ι inhibits
epithelial-mesenchymal transition, migration and invasion of
colorectal cancer cells through Rac1-JNK pathway. Exp Mol Pathol.
107:57–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao J, Qi YF and Yu YR: STAT3: A key
regulator in liver fibrosis. Ann Hepatol. 21(100224)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao WP, Wang HW, Liu J, Tan PP, Lin L and
Zhou BH: JNK/STAT signalling pathway is involved in
fluoride-induced follicular developmental dysplasia in female mice.
Chemosphere. 209:88–95. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ishikawa K, Kannan R and Hinton DR:
Molecular mechanisms of subretinal fibrosis in age-related macular
degeneration. Exp Eye Res. 142:19–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yi C, Liu J, Deng W, Luo C, Qi J, Chen M
and Xu H: Macrophage elastase (MMP12) critically contributes to the
development of subretinal fibrosis. J Neuroinflammation.
19(78)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li D, Zhang J, Liu Z, Gong Y and Zheng Z:
Human umbilical cord mesenchymal stem cell-derived exosomal miR-27b
attenuates subretinal fibrosis via suppressing
epithelial-mesenchymal transition by targeting HOXC6. Stem Cell Res
Ther. 12(24)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu M, Xu H, Liu J, Tan X, Wan S, Guo M,
Long Y and Xu Y: Metformin and fibrosis: A review of existing
evidence and mechanisms. J Diabetes Res.
2021(6673525)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Khateeb J, Fuchs E and Khamaisi M:
Diabetes and lung disease: A neglected relationship. Rev Diabet
Stud. 15:1–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kheirollahi V, Wasnick RM, Biasin V,
Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J,
Zhang JS, Kwapiszewska G, et al: Metformin induces lipogenic
differentiation in myofibroblasts to reverse lung fibrosis. Nat
Commun. 10(2987)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Feng Y, Wang S, Zhang Y and Xiao H:
Metformin attenuates renal fibrosis in both AMPKα2-dependent and
independent manners. Clin Exp Pharmacol Physiol. 44:648–655.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Y, He G, Tang H, Shi Y, Kang X, Lyu
J, Zhu M, Zhou M, Yang M, Mu M, et al: Aspirin inhibits
inflammation and scar formation in the injury tendon healing
through regulating JNK/STAT-3 signalling pathway. Cell Prolif.
52(e12650)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yan QL, Wang XY, Bai M, Zhang X, Song SJ
and Yao GD: Sesquiterpene lactones from Elephantopus scaber exhibit
cytotoxic effects on glioma cells by targeting GSTP1. Bioorg Chem.
129(106183)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dong G, Ma M, Lin X, Liu H, Gao D, Cui J,
Ren Z and Chen R: Treatment-damaged hepatocellular carcinoma
promotes activities of hepatic stellate cells and fibrosis through
GDF15. Exp Cell Res. 370:468–477. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ghafouri-Fard S, Abak A, Talebi SF,
Shoorei H, Branicki W, Taheri M and Akbari Dilmaghani N: Role of
miRNA and lncRNAs in organ fibrosis and aging. Biomed Pharmacother.
143(112132)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang QY, Men CJ and Ding XW: Upregulation
of microRNA-140-3p inhibits epithelial-mesenchymal transition,
invasion, and metastasis of hepatocellular carcinoma through
inactivation of the MAPK signaling pathway by targeting GRN. J Cell
Biochem. 120:14885–14898. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
McDaniel K, Huang L, Sato K, Wu N, Annable
T, Zhou T, Ramos-Lorenzo S, Wan Y, Huang Q, Francis H, et al: The
let-7/Lin28 axis regulates activation of hepatic stellate cells in
alcoholic liver injury. J Biol Chem. 292:11336–11347.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lu YY, Lin Y, Ding DX, Su S, Chi QQ, Zhang
YC, Sun J, Zhang X, Zhu HM, Huang QS, et al: MiR-26a functions as a
tumor suppressor in ambient particulate matter-bound
metal-triggered lung cancer cell metastasis by targeting
LIN28B-IL6-STAT3 axis. Arch Toxicol. 92:1023–1035. 2018.PubMed/NCBI View Article : Google Scholar
|





















