Introduction
Postoperative radiotherapy (RT) forms a significant
component in the standard treatment regimen of breast cancer (BC).
Postoperative RT for early-stage BC can not only reduce
postoperative recurrence, but it can also improve the survival rate
(1,2). However, increased cardiac irradiation
dose during postoperative RT in patients with left-sided BC has
been reported to cause cardiac injury, which is classified as a
late adverse event and can lead to a decreased survival rate
(3). Therefore, the deep
inspiration breath-hold technique (DIBH) is becoming an
increasingly preferred method for reducing the mean heart dose
(MHD) in patients with left-sided BC (4-6).
In DIBH, the patients are requested to hold their breath with deep
inspiration during irradiation, so that the lungs between the
anterior chest wall and heart are filled with air (7). This causes the heart to move away
from the anterior chest wall, which then reduces the irradiation
dose to the heart (4-6).
However, treatment planning for DIBH is time-consuming, laborious
and costly for both the patient and the radiation therapist in
terms of treatment planning and implementation (8). In particular, Asian countries tend to
have fewer personnel and facilities for RT compared with the United
States (9,10), limiting the application of DIBH.
Furthermore, although MHD is greatly influenced by breast volume
(11,12), patients of Asian ethnicities such
as Japanese (12), Korean
(13) and Chinese (14) tend to have less breast volume
(12-14)
compared with patients in the United States and Europe (11,15,16).
Therefore, the MHD also tends to be lower in patients of Asian
ethnicities (12) compared with
that in the United States and Europe, limiting the number of
patients with left BC with high MHD who require DIBH. Only ~26% of
Asian patients with left-sided BC receive high MHD (>300 cGy)
after left-sided BC RT, with an average MHD of 304 cGy following
calculation by the wedge method (W) and 251 cGy by the
field-in-field method (FIF) (12).
Therefore, it is desirable to select Asian patients who may not
require DIBH prior to RT planning for left-sided BC. DIBH can be
used more efficiently if MHD can be predicted in advance, where
patients with MHD who do not require DIBH can be accurately
selected.
Over the past decade, artificial intelligence (AI)
and machine learning (ML) techniques are increasing being applied
in the field of RT (17-20).
However, few studies have applied ML for predicting the MHD during
RT using patient information (21-23).
Therefore, the purpose of the present study is to compare the
various ML models for predicting MHD to select patients who may not
require DIBH and to present the optimal model for predicting
MHD.
Materials and methods
Patients and RT
The present study included 577 female (a mean age of
55 years and standard deviation of 11 years) patients with BC who
received RT at Okayama University Hospital (Okayama, Japan) between
April 2009 and March 2016. Fifteen patients were excluded based on
the exclusion criteria of some missing data. All patients underwent
whole-breast irradiation after partial breast resection, where 167
patients underwent the wedge method and 395 patients underwent the
FIF method. In FIF, two types of methods were used. The
one-reference point FIF method (FIF-1RP) was used for 142 patients,
where one reference point (RP) was set at the mid-level between the
upper and lower edges of the irradiation field or 2 cm apart from
the deepest point and upper edge of the irradiation field. The
other method was the FIF with two RP (FIF-2RP) method (24), which was applied on 253 patients.
The FIF-2RP method involves 2RPs set for each patient, specifically
one RP for the main beam at a point 2 cm apart from the deepest
point and upper edge of the irradiation field, the other RP for the
FIF at the mid-level between the upper and lower edges of the
irradiation field (24). All
patients were irradiated with 2 Gy per fraction, 25 fractions, for
a total of 50 Gy. Some patients were irradiated with an additional
10 Gy boost on the tumor bed. The heart dose during the 50-Gy
whole-breast irradiation (12) was
the subject of the present study.
Data collection
As explanatory variables, data including right-left,
tumor site (upper-inner quadrant, lower-inner quadrant, upper-outer
quadrant, lower-outer quadrant and central portions) (25), chest wall thickness (CWT),
irradiation method (W, FIF-1RP and FIF-2RP), body mass index (BMI),
separation (SEP), age, height and weight were collected
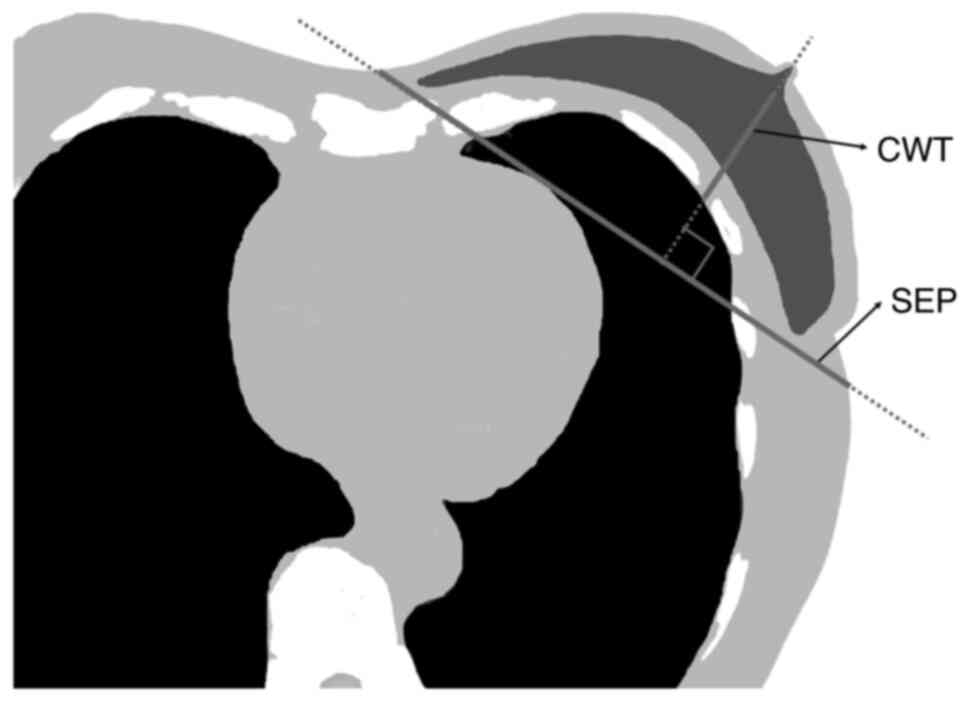
retrospectively, whilst as an objective variable, MHD (12) was collected retrospectively. CWT
and SEP were measured using a nipple-level one-slice simulated CT
image for treatment planning (Fig.
1). SEP was defined as the distance along the posterior edge of
the tangent fields at the nipple level. CWT was defined as the
distance from the nipple surface to the lung, on a perpendicular
line of breast separation. Data on the right and left sides, tumor
site, irradiation method and BMI were collected from clinical
records, whilst MHD was collected from the RT planning system. In
the present study, MHD ≥300 cGy was defined as high MHD, whereas
MHD <300 cGy would be defined as low MHD, following the QUANTEC
cardiac guidelines (3). There were
76 patients (14%) of high MHD and 486 patients (86%) of low
MHD.
Instruments used for ML
Python (version 3.8, Python Software Foundation) and
the Python open source ML library; scikit-learn (version 0.24.1,
https://scikit-learn.org/stable/index.html),
TensorFlow (version 1.15.3, https://www.tensorflow.org/install?hl=ja) and extreme
gradient boosting (XGB, version 1.4.2) (26), were used.
Data partitioning and model
building
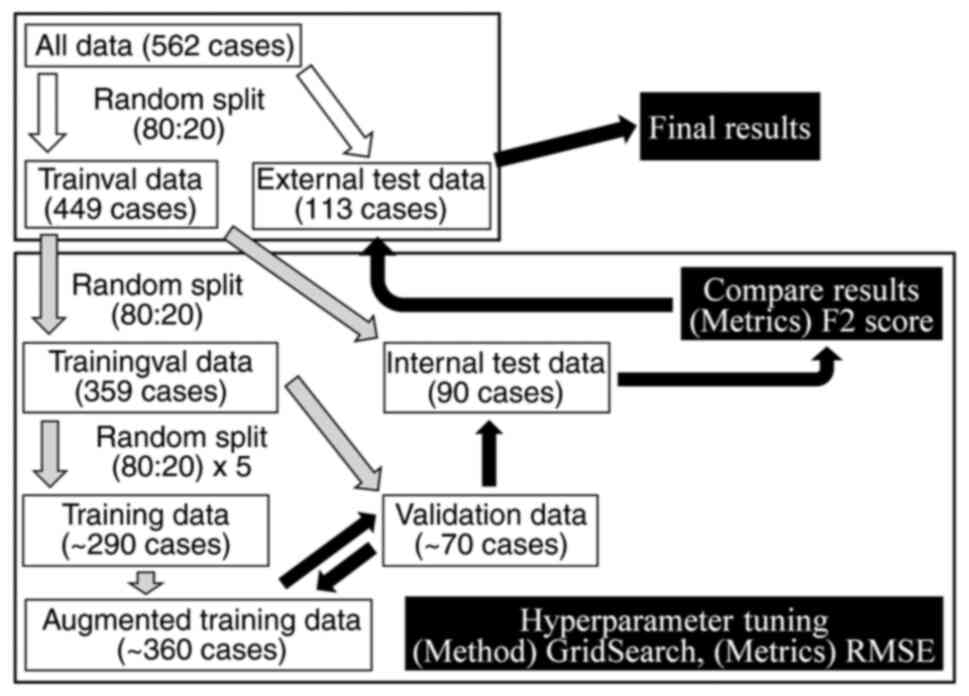
Fig. 2 shows the
data preparation process. All data were split into datasets used
for model building using Python by ML, hereafter defined as the
‘trainval’ dataset and data used for evaluating the prediction
model of final MHD, hereafter defined as the ‘external test’
dataset. The trainval dataset and the external test dataset were
achieved by random splitting, to a ratio of 80:20(27). A ratio of 80:20 is generally
recommended in machine learning. In the pre-study, when the
trainval dataset was reduced to other ratios such as 70:30 and the
external test dataset was increased, the prediction performance
deteriorated (data not shown) due to the occurrence of learning
loss, so a ratio of 80:20 was selected.
Correlation analysis of the
explanatory and objective variables
For the analysis of correlation between the
explanatory and objective variables, the statistical software SPSS
(v27.0, IBM Corp.) was used to calculate the Spearman's correlation
coefficient (rs) for interval scaled variables and the
Eta analysis correlation coefficient (η) for nominal scaled
variables. Correlation coefficients of 1.0-0.8, 0.8-0.6, 0.6-0.4,
0.4-0.2 and 0.2-0.0 would be adjudged to be ‘very strong’,
‘strong’, ‘normal’, ‘weak’ and ‘no’, respectively, for the
correlation strength. Since right-left, tumor site and irradiation
method are categorical variables, they were converted to 0 or 1 and
used as dummy variables. The six explanatory variables used for ML
were right-left, tumor site, CWT, irradiation method, BMI and
SEP.
Process of building models in ML. ML
algorithms
In total, four supervised ML algorithms were used:
i) decision tree; ii) random forest (RF); iii) XGB; and iv) deep
neural network (DNN).
Dealing with unbalanced data sets
Since the MHD data, the collected objective
variable, are unbalanced data, the Python library synthetic
minority oversampling with Gaussian noise (SMOGN) (28) was used to augment the number of
patients of high MHD in the training dataset (Fig. 2). SMOGN is a common machine
learning method for increasing the number of small number of high
MHD cases (28).
Hyper-parameter tuning
The trainval dataset was randomly divided into the
‘trainingval’ dataset and ‘internal test’ dataset at a ratio of
80:20. The training dataset was randomly divided into the training
dataset and validation dataset using 5-fold cross validation
(5-fold CV) at a ratio of 80:20 to avoid overfitting. The training
dataset was used to increase the number of patients of high MHD
using SMOGN, hereafter defined as the ‘augmented’ training dataset.
Using the augmented training dataset and validation dataset,
hyperparameter tuning, which is a process of selecting the optimal
parameters for each algorithm, was performed. GridSearchCV in
scikit-learn (version 0.24.1, https://scikit-learn.org/stable/index.html) was used
for all algorithms except for DNN. In DNN, hyperparameter tuning
was performed manually. The root mean squared error (RMSE) was used
as the evaluation metric for prediction. Hyperparameters with the
best RMSE were determined for each algorithm.
Creating a model using the F2 score as
the evaluation index
In the present study, a predictive model and RMSE as
the evaluation metric were used. Since the aim of the present study
was to select patients with low MHD to whom DIBH are not
applicable, emphasis was placed on learning to minimize false
negatives (FN), preventing the false reporting of high MHD as low
MHD. Therefore, the F2 score obtained using the confusion matrix
was used in conjunction with RMSE as the evaluation metric. The
model was created to have the best F2 score using the optimal value
of hyperparameter tuning with RMSE and the internal test data.
Final model validation using the
external test data
The final model was validated using external test
data from 113 patients who were not used to train and build the
model. In the final model validation, RMSE, MSE, MAE,
rs, accuracy, precision, recall, specificity, AUC-ROC,
F1 score, and F2 score were evaluated for each algorithm using the
confusion matrix.
Results
Correlations between explanatory and
objective variables
Table I shows the
rs- and η-values between the explanatory variables and
MHD as the objective variable. Among the explanatory variables, a
strong correlation was found between right-left and MHD
(P<0.001). The correlation coefficients for the other variables
were low, but a significant correlation was found between CWT and
MHD (P=0.005).
 | Table ISpearman's correlation coefficient
(rs) and Eta correlation ratio (η) between explanatory
variables and MHD. |
Table I
Spearman's correlation coefficient
(rs) and Eta correlation ratio (η) between explanatory
variables and MHD.
| Variables | rs or
η | P-value |
|---|
| Right and left | 0.780a | <0.001 |
| Tumor site | 0.126a | 0.063 |
| Chest wall
thickness | 0.118b | 0.005 |
| Irradiation
method | 0.103a | 0.051 |
| Age | -0.039b | 0.357 |
| Body mass
index | 0.038b | 0.370 |
| Separation | -0.027b | 0.522 |
| Weight | 0.025b | 0.560 |
| Height | -0.024b | 0.572 |
Parameter optimization through
hyperparameter tuning
Table II shows the
optimal values of the hyperparameters for each algorithm. Each
model with these optimal hyperparameters was then evaluated with an
internal test dataset.
 | Table IIHyperparameters-tuning results. |
Table II
Hyperparameters-tuning results.
| Machine learning
algorithm | Hyperparameter
name | Best value |
|---|
| Decision tree | max_depth | 15 |
| |
min_samples_leaf | 3 |
| | max_leaf_nodes | 15 |
| Random forest | max_depth | 5 |
| | max_features | auto |
| |
min_samples_split | 2 |
| | n_estimators | 100 |
| Extreme gradient
boosting |
colsample_bytree | 0.700 |
| | eta | 0.05 |
| | eval_metric | RMSE |
| | max_depth | 11 |
| |
min_child_weight | 6 |
| | subsample | 0.500 |
| Deep neural
network | Number of hidden
layers | 4 |
| | Number of neurons
in each hidden layer | 256 |
| | Activation function
in the hidden layers | ReLU |
| | Activation function
in the output layer | linear |
| | Loss function | MSE |
| | Optimizer | Adam |
Evaluation of models in each algorithm
using internal test data
Table III
summarizes the optimal results out of 5-fold CV for each algorithm
using the internal test dataset, with F2 score as the evaluation
metric. In addition to the F2 score, the results of other
evaluation metrics, such as RMSE, were also indicated. The results
of RMSE were the lowest for XGB at 67.4, followed by DNN at 69.5
and RF at 81.2. For the results of F2 scoring, DNN was the highest
at 0.64, followed by RF at 0.60 and decision tree at 0.48.
 | Table IIIBest evaluation results of each
algorithm in 5 fold-cross validation using internal test data. |
Table III
Best evaluation results of each
algorithm in 5 fold-cross validation using internal test data.
| Metrics | Decision tree | Random forest | Extreme gradient
boosting | Deep neural
network |
|---|
| RMSE | 85.5 | 81.2 | 67.4 | 69.5 |
| MSE | 7311 | 6600 | 4545 | 4834 |
| MAE | 56.2 | 57.1 | 47.2 | 49.9 |
| rs | 0.722 | 0.770 | 0.749 | 0.804 |
| Accuracy | 0.75 | 0.72 | 0.79 | 0.76 |
| Precision | 0.35 | 0.34 | 0.30 | 0.40 |
| Recall | 0.53 | 0.73 | 0.20 | 0.75 |
| Specificity | 0.80 | 0.72 | 0.91 | 0.76 |
| AUC-ROC | 0.67 | 0.73 | 0.55 | 0.75 |
| F1 score | 0.42 | 0.47 | 0.24 | 0.52 |
| F2 score | 0.48 | 0.60 | 0.21 | 0.64 |
Final evaluation of the model of each
algorithm using external test data
Table IV shows the
results of the final evaluation of the model for each algorithm
using the external test dataset. For RMSE, DNN had the lowest score
of 77.5, followed by XGB with 85.6. For the F2 score, DNN had the
highest score of 0.80, whilst RF had 0.64.
 | Table IVThe final evaluation results of each
algorithm using external test data. |
Table IV
The final evaluation results of each
algorithm using external test data.
| Metrics | Decision tree | Random forest | Extreme gradient
boosting | Deep neural
network |
|---|
| RMSE | 97.6 | 88.8 | 85.6 | 77.5 |
| MSE | 9527 | 7878 | 7325 | 6000 |
| MAE | 57.9 | 58.6 | 50.8 | 48.7 |
| rs | 0.645 | 0.724 | 0.701 | 0.770 |
| Accuracy | 0.77 | 0.75 | 0.81 | 0.88 |
| Precision | 0.38 | 0.37 | 0.36 | 0.57 |
| Recall | 0.67 | 0.78 | 0.22 | 0.89 |
| Specificity | 0.79 | 0.75 | 0.93 | 0.87 |
| AUC-ROC | 0.73 | 0.76 | 0.57 | 0.88 |
| F1 score | 0.48 | 0.50 | 0.28 | 0.70 |
| F2 score | 0.58 | 0.64 | 0.24 | 0.80 |
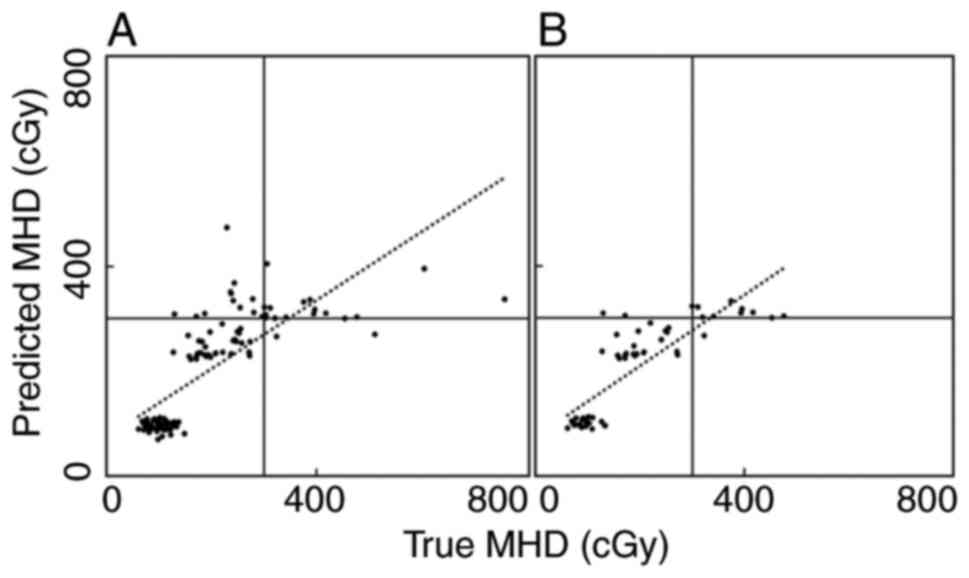
Fig. 3A shows the
correlation between the true and predicted MHDs in the external
test dataset using the DNN, where a strong correlation was observed
with a rs of 0.77. Fig.
3B shows the correlation between the true and predicted MHDs
for the FIF-2RP patients among the external test data using DNN,
where a potent correlation was observed, with a rs of
0.83.
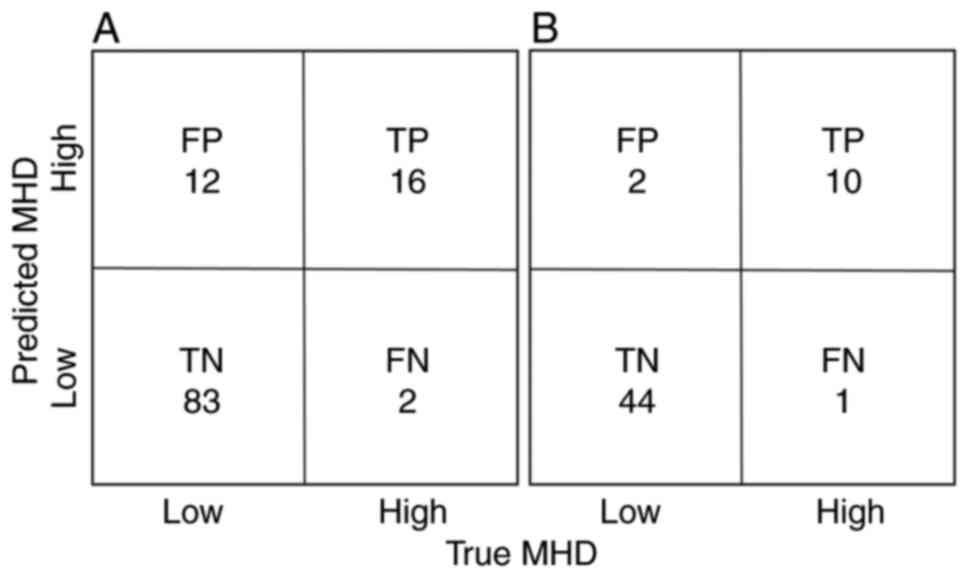
Fig. 4A shows the
confusion matrix for the predicted MHD in the external test dataset
using DNN, with 16 true positives and only 2 FN in the DNN, with a
F2 score of 0.80. Fig. 4B shows
the confusion matrix for predicted MHD in the FIF-2RP patients of
the external test dataset using the DNN. There were 10 true
positives and only one FN, with a F2 score of 0.89.
Discussion
In the present study, four different ML algorithms
were used based on the factors obtained from a single CT image
slice and clinical factors to create models for predicting the MHD.
Specific focus was placed on low MHD, which is not an indication
for DIBH. The prediction performance of each model was then
evaluated and compared. Among the algorithms tested, DNN was found
to show the highest performance, with an F2 score of 0.80 and an
area under the curve-receiver operating characteristic score of
0.88. The present study revealed that DNN is the optimal model for
predicting MHD to select patients who are less likely to require
DIBH. Previously, FIF-2RP was reported as a novel method of FIF
that can significantly reduce the incidence of adverse skin events
whilst slightly reducing MHD, compared with conventional FIF-1RP
(24). In DNN, which was the
optimal predictor of MHD, the prediction accuracy for FIF-2RP was
higher compared with the analysis accuracy for all irradiation
methods, where DNN appeared to be useful for selecting patients for
whom DIBH was not applicable even for FIF-2RP.
In the postoperative treatment of BC, RT contributes
to the reduction of postoperative local recurrence and improves
survival (1,2). However, RT for BC can also reduce the
survival rate due to late cardiac adverse events in some patients
with left BC (3). DIBH, which
reduces MHD, is becoming used more frequently in clinical practice
for reducing cardiac adverse events in left BC treatment (4-6).
DIBH involves asking the patient to hold their breath with deep
inspiration during irradiation, causing the lung to expand with air
and to enter between the heart and the chest wall (7). This dislodges the heart from the
irradiation field, with the resultant reduction of heart dose
(4-6).
However, DIBH imposes several burdens on both patients and the RT
staff, such as the additional breath-held CT imaging with deep
inspiration and irradiation with respiratory synchronization,
complex RT planning, extension treatment time and increasing costs
(8). Furthermore, in Asian women,
for FIF, the MHD of left BC is 257±90 cGy for FIF-1RP and 248±76
cGy for FIF-2RP (24), such that
only ~14% of the patients have high MHD requiring DIBH (24). Therefore, a simple and accurate MHD
prediction method prior to RT planning is needed for selecting
patients for DIBH in Asian women. The present study revealed the
highly effective utility of DNN among the ML models tested.
In recent years, AI use is becoming increasingly
common in radiological practice (17,18),
including AI-assisted imaging, RT planning and contouring,
radiation exposure reduction and quality assurance. A number of
studies have attempted the prediction of MHD in RT for BC (21-23).
Koide et al (21) used a
convolutional neural network to predict the difference between MHD
with and without DIBH and MHD without DIBH, using preoperative
frontal and lateral chest radiographs of 103 patients with BC. The
advantage of using chest radiographs is that they are simpler
compared with CT. However, in this report, the correlation
coefficient between true and predicted MHD without DIBH was 0.46
and the specificity was 0.77(21),
suggesting that results from the present study using the DNN-based
method were superior. Another report (22) of the use of ML for the RT of BC
showed the dose distribution of volumetric modulated arc therapy
was well predicted by deep learning, with resultant improvement of
the radiation treatment process by reducing the time required for
planning, while maintaining plan quality. For using ML, CT at DIBH
was synthesized without imaging, where the effect of MHD reduction
by DIBH was examined using MHD at DIBH calculated based on the
synthesized CT (23).
A unique feature of the present study was the use of
different ML models for prediction, which was able to predict the
absolute value of MHD for each individual patient. In addition, the
evaluation of the confusion matrix used in the classification model
was incorporated into the model creation process during learning.
To reduce the number of FNs that incorrectly predicted patients
with high MHD to be low MHD and to minimize the number of missed
patients with high MHD for the selection of patients with low MHD
who are not candidates for DIBH, the F2 score was used as the
metric of the confusion matrix in the learning process.
Using the model created in the present study, the
proposed RT flow for Asian women with left BC is shown in Fig. 5, where only a portion of patients
have high MHD requiring DIBH. First, a simulated CT is taken during
free breathing, before the MHD is predicted using the explanatory
variables from a nipple-level, one-slice simulated CT image and the
present model. If the patient is predicted to have low MHD, which
is expected to be the case in >50% of all patients, then
treatment planning should be done by free-breathing CT. If the
treatment planning result is low MHD, RT without DIBH would be
administered according to the plan. If the treatment plan results
in a rare FN with high MHD, then a simulated CT imaging for DIBH
would be added and DIBH would be performed according to the DIBH
treatment planning. For patients with high MHD, who are predicted
to represent <50% of all patients, a simulated CT for DIBH would
be taken and DIBH would be performed according to the DIBH
treatment planning. This RT flow should assist in reducing the
number of patients who will undergo both DIBH and free-breathing
treatment planning, increasing the cost and time effectiveness.
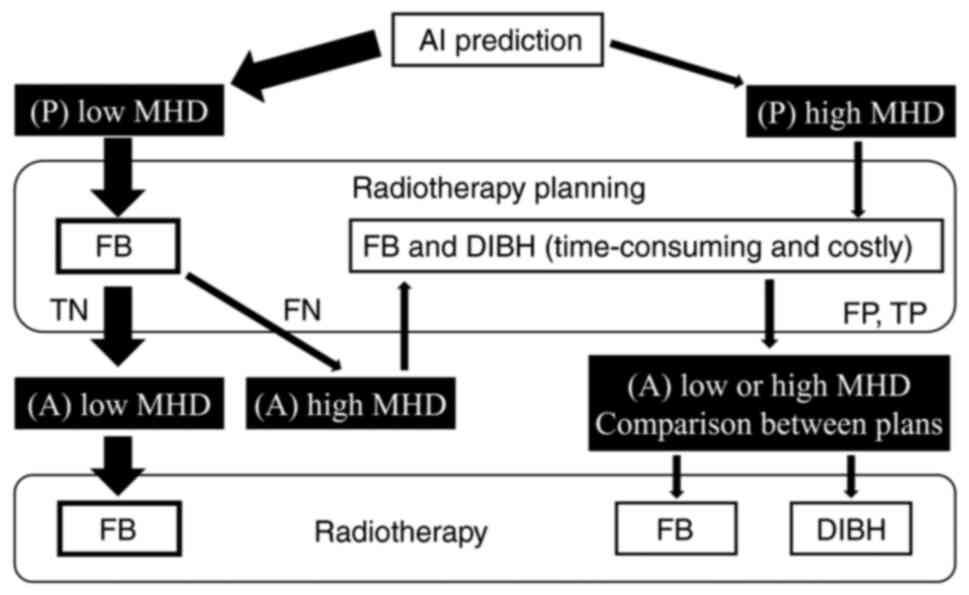 | Figure 5Radiotherapy flow using AI models in
clinical practice. MHD ≥300 cGy was defined as high MHD, whereas
MHD <300 cGy would be defined as low MHD. AI, artificial
intelligence; P, predictive; MHD, mean heart dose; FB,
free-breathing; DIBH, deep inspiration breath-hold; TN, true
negative; FN, false negative; FP, false positive; TP, true
positive; A, actual. |
The first limitation of the present study is the
relatively small number of patients (562 patients). In existing
reports on MHD prediction, even fewer patients were included
compared with the present study, such as 103(21) and 94(23). In addition, the data of the present
study were unbalanced, with a high number of patients with low MHD.
In response to this, data augmentation using SMOGN was performed,
but it may be necessary to study with additional patients with high
MHD to restore the balance.
To conclude, the present study enables the accurate
prediction of MHD prior to RT planning by DNN using factors
obtained from a single CT image slice and factors based on patient
information. The present method is expected to be beneficial for
selecting Asian patients with low MHD who do not require DIBH.
Acknowledgements
The authors would like to thank Professor Ken'ichi
Morooka (Faculty of Environmental, Life, Natural Science and
Technology, Okayama University, Okayama, Japan) and assistant
Professor Ryohei Fukui (Faculty of Health Sciences, Okayama
University, Okayama, Japan) for their useful advice.
Funding
Funding: The present study was partially supported by the
Grant-in-Aid for Scientific Research (grant no. 23K07063) from the
Ministry of Health, Labour and Welfare of Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RK, MK and WEHA participated in research design. RK,
MK, WEA, NT, HinI, KK, KS, MO, YT, YN, MH, YM, HirI and SS
performed the experiments and collected data. RK, MK and WEA
analyzed the data and were major contributors in writing the
manuscript. RK, MK, WEA, MB and IS analyzed data and confirm the
authenticity of all the raw data. All the authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Okayama University Graduate School of Medicine,
Dentistry and Pharmaceutical Sciences, and Okayama University
Hospital (approval no. 2103-024). Patients provided written
informed consent for undergoing RT and for the anonymous use of
their data for scientific studies. The institutional informed
consent forms for treatment included consent for the use of patient
data and materials for research purposes. The present study was
conducted in accordance with The Declaration of Helsinki, as
revised in 2013.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Darby S, McGale P, Correa C, Taylor C,
Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et
al: Effect of radiotherapy after breast-conserving surgery on
10-year recurrence and 15-year breast cancer death: Meta-analysis
of individual patient data for 10,801 women in 17 randomised
trials. Lancet. 378:1707–1716. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Beaton L, Bergman A, Nichol A, Aparicio M,
Wong G, Gondara L, Speers C, Weir L, Davis M and Tyldesley S:
Cardiac death after breast radiotherapy and the QUANTEC cardiac
guidelines. Clin Transl Radiat Oncol. 19:39–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu Y, Yang D, Zhang X, Teng Y, Yuan W,
Zhang Y, He R, Tang F, Pang J, Han B, et al: Comparison of deep
inspiration breath hold versus free breathing in radiotherapy for
left sided breast cancer. Front Oncol. 12(845037)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Falco M, Masojć B, Macała A, Łukowiak M,
Woźniak P and Malicki J: Deep inspiration breath hold reduces the
mean heart dose in left breast cancer radiotherapy. Radiol Oncol.
55:212–220. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yamauchi R, Mizuno N, Itazawa T, Saitoh H
and Kawamori J: Dosimetric evaluation of deep inspiration breath
hold for left-sided breast cancer: Analysis of patient-specific
parameters related to heart dose reduction. J Radiat Res.
61:447–456. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stowe HB, Andruska ND, Reynoso F, Thomas M
and Bergom C: Heart sparing radiotherapy techniques in breast
cancer: A focus on deep inspiration breath hold. Breast
Cancer-Targets Ther. 14:175–186. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Darapu A, Balakrishnan R, Sebastian P,
Hussain MR, Ravindran P and John S: Is the deep inspiration
breath-hold technique superior to the free breathing technique in
cardiac and lung sparing while treating both left-sided
post-mastectomy chest wall and supraclavicular regions? Case Rep
Oncol. 10:37–51. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Teshima T, Owen JB, Hanks GE, Sato S,
Tsunemoto H and Inoue T: A comparison of the structure of radiation
oncology in the United States and Japan. Int J Radiat Oncol Biol
Phys. 34:235–242. 1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nakamura K, Konishi K, Komatsu T, Sasaki T
and Shikama N: Patterns of radiotherapy infrastructure in Japan and
in other countries with well-developed radiotherapy
infrastructures, Jpn J Clin. Oncol. 48:476–479. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Morganti AG, Cilla S, de Gaetano A,
Panunzi S, Digesù C, Macchia G, Massaccesi M, Deodato F, Ferrandina
G, Cellini N, et al: Forward planned intensity modulated
radiotherapy (IMRT) for whole breast postoperative radiotherapy. Is
it useful? When? J Appl Clin Med Phys. 12(3451)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ishizaka H, Kuroda M, Tekiki N, Khasawneh
A, Barham M, Hamada K, Konishi K, Sugimoto K, Katsui K, Sugiyama S,
et al: Investigation into the effect of breast volume on
irradiation dose distribution in Asian women with breast cancer.
Acta Med Okayama. 75:307–314. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim S and Kim M and Kim M: The affecting
factors of breast anthropometry in Korean women. Breastfeed Med.
9:73–78. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li X, Zhou C, Wu Y and Chen X: .
Relationship between formulaic breast volume and risk of breast
cancer based on linear measurements. BMC Cancer.
20(989)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tortorelli G, Di Murro L, Barbarino R,
Cicchetti S, di Cristino D, Falco MD, Fedele D, Ingrosso G,
Janniello D, Morelli P, et al: Standard or hypofractionated
radiotherapy in the postoperative treatment of breast cancer: A
retrospective analysis of acute skin toxicity and dose
inhomogeneities. BMC Cancer. 13(230)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim T, Reardon K, Trifiletti DM, Geesey C,
Sukovich K, Crandley E, Read PW and Wijesooriya K: How dose sparing
of cardiac structures correlates with in-field heart volume and
sternal displacement. J Appl Clin Med Phys. 17:60–68.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Siddique S and Chow JCL: Artificial
intelligence in radiotherapy. Rep Pract Oncol Radiother.
25:656–666. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kang J, Schwartz R, Flickinger J and
Beriwal S: Machine learning approaches for predicting radiotherapy
outcomes: A clinician's perspective. Int J Radiat Oncol Biol Phys.
93:1127–1135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Luo Y, Chen S and Valdes G: . Machine
learning for radiation outcome modeling and prediction. Med Phys.
47:e178–e184. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Brodin NP, Schulte L, Velten C, Martin W,
Shen S, Shen J, Basavatia A, Ohri N, Garg MK, Carpenter C and Tomé
WA: Organ-at-risk dose prediction using a machine learning
algorithm: Clinical validation and treatment planning benefit for
lung SBRT. J Appl Clin Med Phys. 23(e13609)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Koide Y, Aoyama T, Shimizu H, Kitagawa T,
Miyauchi R, Tachibana H and Kodaira T: . Development of deep
learning chest X-ray model for cardiac dose prediction in
left-sided breast cancer radiotherapy. Sci Rep.
12(13706)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahn SH, Kim E, Kim C, Cheon W, Kim M, Lee
SB, Lim YK, Kim H, Shin D, Kim DY, et al: Deep learning method for
prediction of patient-specific dose distribution in breast cancer.
Radiat Oncol. 16(154)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koide Y, Shimizu H, Wakabayashi K,
Kitagawa T, Aoyama T, Miyauchi R, Tachibana H and Kodaira T:
Synthetic breath-hold CT generation from free-breathing CT: A novel
deep learning approach to predict cardiac dose reduction in
deep-inspiration breath-hold radiotherapy. J Radiat Res.
62:1065–1075. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tekiki N, Kuroda M, Ishizaka H, Khasawneh
A, Barham M, Hamada K, Konishi K, Sugimoto K, Katsui K, Sugiyama S,
et al: New field-in-field with two reference points method for
whole breast radiotherapy: Dosimetric analysis and
radiation-induced skin toxicities assessment. Mol Clin Oncol.
15(193)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
International Classification of Diseases
for Oncology. Available from: https://apps.who.int/iris/bitstream/handle/10665/96612/9789241548496_eng.pdf.
|
|
26
|
Chen T and Guestrin C: XGBoost: A Scalable
Tree Boosting System. In proceedings of the 22nd ACM SIGKDD
international conference on knowledge discovery and data mining.
pp785-794, 2016.
|
|
27
|
Joseph VR: Optimal ratio for data
splitting. Stat Anal Data Min. 15:531–538. 2022.
|
|
28
|
Branco P, Torgo L and Ribeiro RP: SMOGN: A
pre-processing approach for imbalanced regression. Proc Mach Learn
Res. 74:36–50. 2017.
|