Introduction
In December 2019, COVID-19, caused by a then-novel
coronavirus, named severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2), caused a global pandemic. According to the World
Health Organization (WHO), COVID-19 cumulatively infected 700
million people and caused 60 million deaths by March 2023
(https://covid19.who.int, accessed on 31 March
2023). SARS-CoV-2 infects host cells by binding to
angiotensin-converting enzyme 2 (ACE2) receptors (1) and stimulating the immune system
resulting in a cytokine storm, reactive oxygen species (ROS)
accumulation, and activation of coagulation components (2). The primary clinical manifestation of
COVID-19 is lung infection, including acute respiratory distress
syndrome and respiratory failure (3). Numerous studies have shown that
COVID-19 affects various systems in the body, including the nervous
system (4). In adult patients, the
severity of COVID-19 is positively correlated with age and
comorbidities (5). The European
Academy of Neurology assessed the predictors of outcomes at the
time of discharge and 6 months post-discharge in 971 patients from
19 countries between July 2020 and March 2021 using the modified
Rankin Scale. They found a predisposition to neurological
complications in patients with COVID-19, and neurological
complications were important predictors of long-term prognosis. In
particular, stroke and ataxia were associated with a poorer 6-month
prognosis (6). Body Mass Index
(BMI) was associated with an increased risk of ARDS and an
increased length of hospital stay in a weight-dependent manner.
ARDS is an independent risk factor for COVID-19. Therefore, obesity
is associated with a poor prognosis for COVID-19 patients (7,8). The
majority of available evidence suggests that patients with
diabetes, especially those with poor glycemic control, experience a
significant 2-4-fold increase in COVID-19 severity,
hospitalization, and mortality compared with non-diabetic patients
(9,10).
Matrix metalloproteinases (MMPs) are zinc-dependent
endopeptidases that are involved in extracellular matrix (ECM)
degradation. MMPs are involved in various physiological processes
such as angiogenesis, apoptosis, and tissue repair, as well as
pathological processes such as hypertension, eclampsia, vascular
inflammation, atherosclerosis, tumor metastasis, cerebral ischemic
stroke, peripheral arterial disease, kidney injury, and lung injury
(11-15).
It has also been reported that MMP-9 is elevated during ascending
aortic aneurysms (16,17) Moreover, it has been shown that
MMP-9 gene expression is upregulated in COVID-19 patients (18).
During the inflammatory process, neutrophils and
macrophages are secreted which activate inflammatory mediators, and
cytokines, such as IL-1 and TNF-α, increase the expression and
activation of MMP-9. MMP-9 degrades the extracellular matrix and
simultaneously results in the release of multiple components, such
as heparin and fibronectin, which can act as chemotactic and
immune-activating proteins (19).
Taken together, MMP-9 contributes to the ‘cytokine storm’ in
patients with COVID-19 by activating cytokines and chemokines
(20).
Higher serum levels of MMP-9 were observed in
patients with severe COVID-19, non-survivors, and ARDS COVID-19
patients (21-31).
Of note, MMP-9 levels were proportional to the risk of respiratory
failure (32). MMP-9 promotes
platelet and neutrophil activation in patients with COVID-19,
resulting in severe thrombotic events (25). Moreover, MMP-9 levels were
increased in COVID-19 patients with comorbidities such as obesity,
diabetes, and neurologic syndrome (22,24,25,30,33)
However, all these studies were conducted with relatively small
sample sizes and/or at single centers. Therefore, the predictive
role of MMP-9 levels in the risk of poor outcomes in COVID-19
patients needs further validation.
MMP-9 may serve a potential role in the diagnosis
and prognostic determination of COVID-19. Here, a comprehensive
meta-analysis of COVID-19 studies was performed to determine the
relationship between MMP-9 and the severity, mortality, and
comorbidities of patients with COVID-19.
Materials and methods
Search strategy, selection criteria,
and quality assessment
This study followed The Preferred Reporting Items
for Systematic Reviews and Meta-Analysis Diagnostic Test Accuracy
(PRISMA-DTA) guidelines (34) and
was registered at the International Prospective Register of
Systematic Reviews (registration no. CRD42022369605). In the
present analysis, two independent authors selected studies by
searching 6 databases including PubMed, EMBASE, Web of Science,
Cochrane, CNKI, and WANFANG. The last retrieval time was March 15,
2023. No restrictions on the language, region of the investigation,
or ethnicity of the study populations were placed. The references
of the retrieved articles were also examined. The search terms
included ‘Matrix Metalloproteinase 9’ and ‘COVID-19’.
The studies that assessed serum MMP-9 levels and had
patients diagnosed with COVID-19 were initially included. The
exclusion criteria were as follows: i) Review articles; ii) case
reports; iii) studies without data on MMP-9; iv) studies with
special subsets of COVID-19 (for example studies with patients all
younger than 18 years old); and v) studies which did not stratify
groups based on COVID-19 severity. The study quality was assessed
using the Newcastle-Ottawa Scale (NOS) (35). Any disagreement between the two
reviewers was resolved by a third investigator (HG).
Data extraction
The first author's name, number of study cases, age,
sex, and region of the study in the different groups (non-ARDS vs.
ARDS; non-survivors vs. survivors; non-severe vs. severe; and with
comorbidities vs. without comorbidities) were extracted from the
eligible studies. The levels and measurement scale (e.g., µg/l,
ng/ml, or pg/ml) of MMP-9 were extracted from the article text,
tables, figures, or the letter from the authors. In a
meta-analysis, categorical variables (such as sex, region of the
study, comorbidities, or outcomes) are treated as dichotomous
variables, while for continuous variables (such as age and MMP-9
levels), median (interquartile range, IQR) or median (range) was
converted to mean ± SD.
Statistical analysis
A random-effects model meta-analysis was performed
using STATA MP version 17 (StataCorp LLC). The effect measure for
comparison between different patient groups used weighted mean
difference (WMD). Heterogeneity among eligible studies was
evaluated using Cochrane's Q-statistic and the
I2-statistic. P>0.10 or I2>50%
indicated significant heterogeneity. Leave-one-out analysis was
used to perform the sensitivity analysis to investigate the
influence of an individual study on the entire risk estimate.
Random effects meta-regression was performed to explore the source
of heterogeneity. The publication bias of the analysis was
performed using an Egger's test.
Results
Literature search and study
characteristics
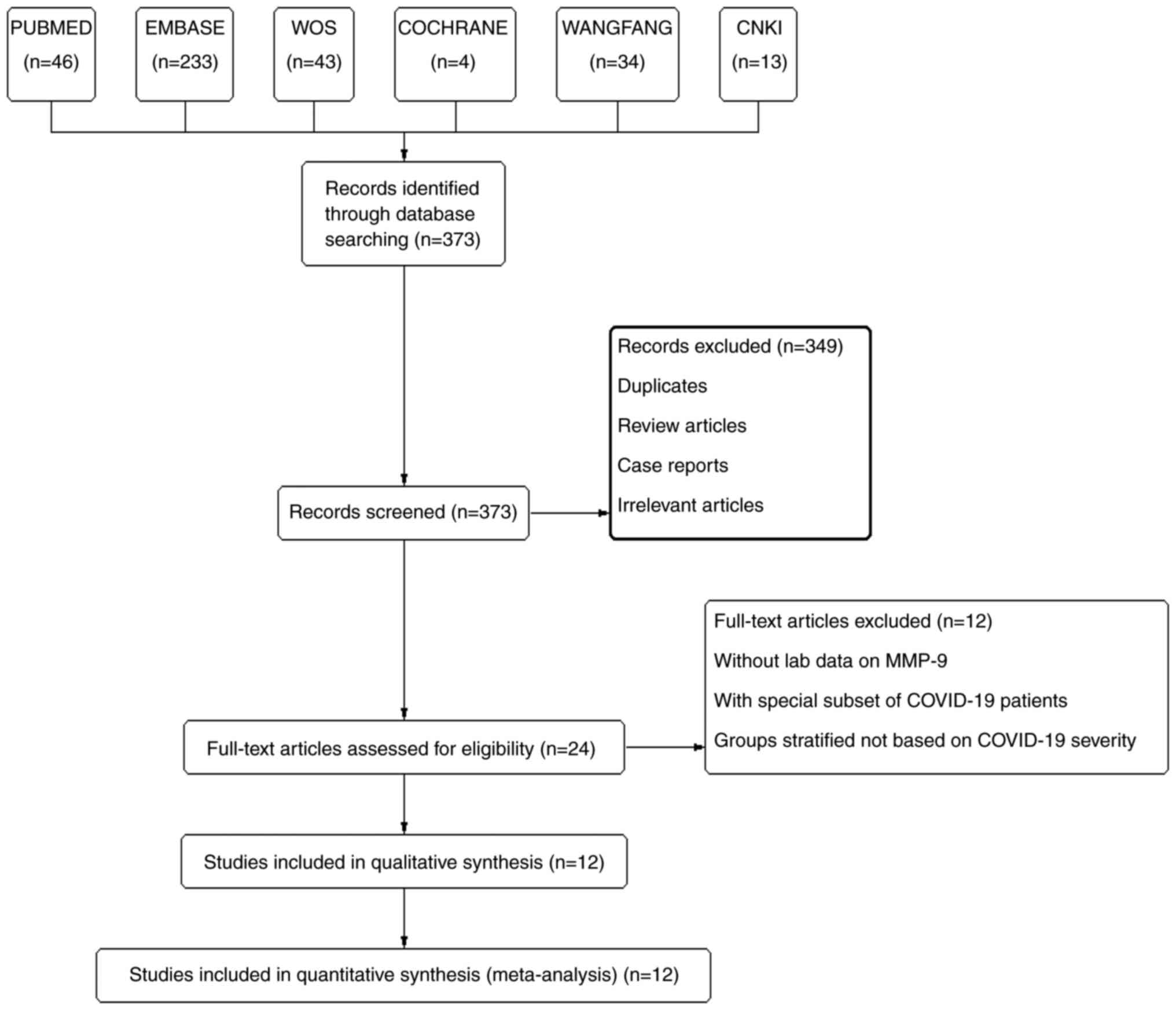
A total of 373 records were retrieved through
searching the database and manual searches. From these, 349 studies
(duplicates, review articles, case reports, and irrelevant
articles) were removed after reading the abstracts and titles; 24
studies remained for a full-text review. After excluding studies
without laboratory data on MMP-9, studies assessing a special
subset of COVID-19 patients, and studies stratifying patients on
criteria other than severity of COVID-19, a total of 12 records
involving 2,062 COVID-19 patients were included in this
meta-analysis (Fig. 1) (21-31,34);
the details of the 12 studies are stated in Table I. Study quality was assessed using
the NOS (Table II). All 12
studies were of a high quality with scores ranging from 7 to 9.
 | Table ICharacteristics of the studies
included in the meta-analysis. |
Table I
Characteristics of the studies
included in the meta-analysis.
| First author,
year | Year | Cases, n | Age, years, mean ±
SD | Male, n (%) | Group
comparison | Country | (Refs.) |
|---|
| Mesa et al,
2021 | 2021 | 60 | 65.45±11.12 | 38 (63.33%) | Non-ARDS vs. ARDS
and Non-survivors vs. survivors | Spain | (21) |
| Ramezani et
al, 2022 | 2023 | 1000 | 55.45±10.15 | 620 (62%) | Non-severe vs.
severe and non-neurologic syndrome vs. neurologic syndrome | Iran | (22) |
| Kassianidis et
al, 2022 | 2022 | 181 | 60.60±13.89 | 128 (70.72%) | Non-severe vs.
severe and non-ARDS vs. ARDS | Greece | (23) |
| Nasr El-Din et
al, 2021 | 2021 | 70 | 58.87±7.05 | 29 (41.4%) | Non-ARDS vs. ARDS
and non-obese diabetic vs. obese diabetic | Egypt | (24) |
| Bonetto et
al, 2022 | 2022 | 228 | 63.73±10.23 | 155 (67.98%) | Non-neurologic
syndrome vs. neurologic syndrome and non-survivors vs.
survivors | Italy | (25) |
| Gelzo et al,
2022 | 2022 | 108 | 43.56±20.82 | 44 (40.74%) | Non-severe vs.
severe | Italy | (26) |
| Mohammadhosayni
et al, 2021 | 2021 | 20 | 60.36±11.13 | 10 (50%) | Non-neurologic
syndrome vs. neurologic syndrome | Iran | (33) |
| Savic et al,
2022 | 2022 | 77 | 58.91±6.74 | 24 (31.17%) | Non-severe vs.
severe | Serbia | (27) |
| Lerum et al,
2021 | 2021 | 108 | 58.0±11.3 | 67 (62%) | Non-severe vs.
severe | Norway | (28) |
| Iwasaki-Hozumi
et al, 2023 | 2023 | 55 | 40.04±47.74 | 40 (73%) | Non-severe vs.
severe | Japan | (29) |
| Moin et al,
2020 | 2020 | 46 | 58.5±9.24 | 23 (50%) | Non-obese diabetic
vs. obese diabetic | Qatar | (30) |
| Springall et
al, 2022 | 2022 | 109 | 54±14.07 | 73 (66%) | Non-severe vs.
severe | Mexico | (31) |
 | Table IIQuality assessment of the included
studies according to the NOS. |
Table II
Quality assessment of the included
studies according to the NOS.
| First author,
year | Is the case
definition adequate? | Representativeness
of the cases | Selection of
controls | Definition of
controls | Comparability of
both groups for age | Comparability of
both groups for sex | Ascertainment of
diagnosis | Same method of
ascertainment for cases and controls | Non-response
rate | Total score | (Refs.) |
|---|
| Martinez Mesa et
al, 2021 | 1 | 1 | 1 | 1 | 1 | | 1 | 1 | 1 | 8 | (21) |
| Ramezani et
al, 2023 | 1 | 1 | 1 | 1 | | | 1 | 1 | 1 | 7 | (22) |
| Kassianidis et
al, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (23) |
| Nasr El-Din et
al, 2021 | 1 | 1 | 1 | 1 | | | 1 | 1 | 1 | 7 | (24) |
| Bonetto et
al, 2022 | 1 | 1 | 1 | 1 | 1 | | 1 | 1 | 1 | 8 | (25) |
| Gelzo et al,
2022 | 1 | 1 | 1 | 1 | 1 | | 1 | 1 | 1 | 8 | (26) |
| Mohammadhosayni
et al, 2021 | 1 | | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (33) |
| Savic et al,
2022 | 1 | 1 | 1 | 1 | 1 | | 1 | 1 | 1 | 8 | (27) |
| Lerum et al,
2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (28) |
| Iwasaki-Hozumi
et al, 2023 | 1 | 1 | 1 | 1 | | 1 | 1 | 1 | 1 | 8 | (29) |
| Moin et al,
2020 | 1 | | 1 | 1 | | 1 | 1 | 1 | 1 | 7 | (30) |
| Springall et
al, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (31) |
MMP-9 is associated with the severity
of COVID-19
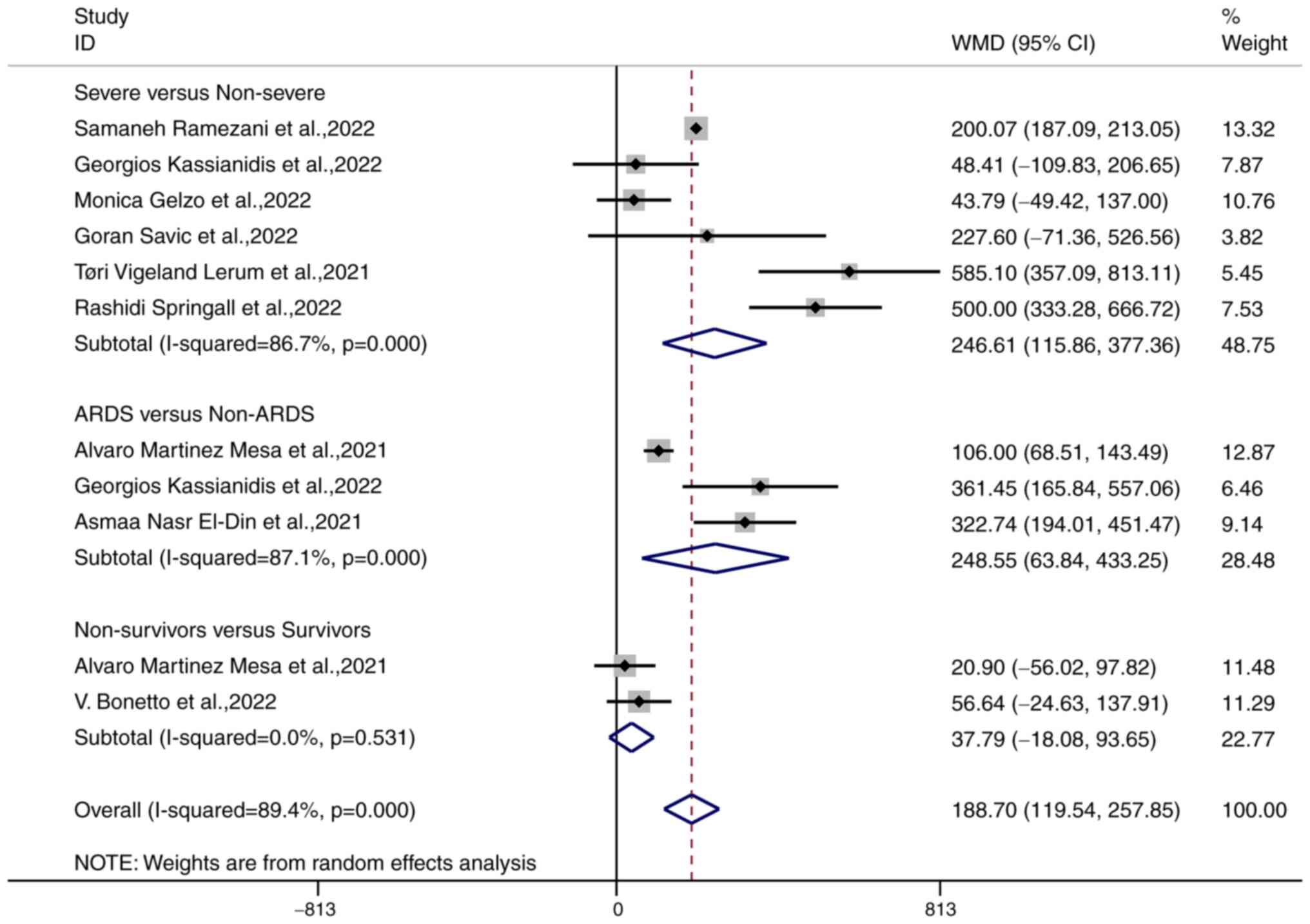
A forest plot of the random effects meta-analysis of
6 studies involving 1,471 patients with COVID-19 showed that the
MMP-9 levels in patients with severe COVID-19 (n=776) were higher
than those in patients with mild to moderate COVID-19 (n=695) [WMD
246.61 (95% CI, 115.86-377.36), P<0.001]; the meta-analysis had
significant heterogeneity (I2=86.7%, P<0.001;
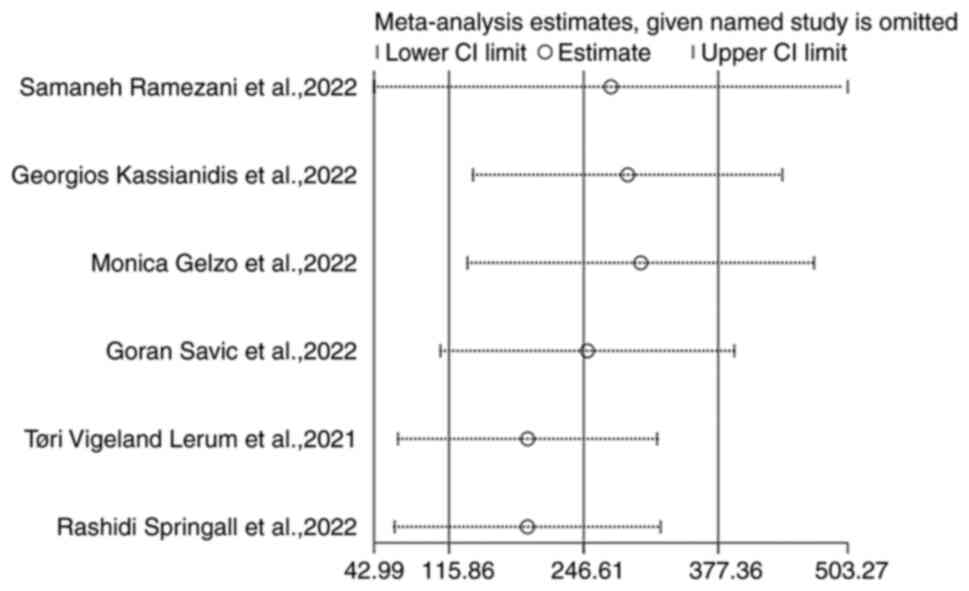
Fig. 2) Sensitivity analysis
showed that the studies had no significant impact on the results
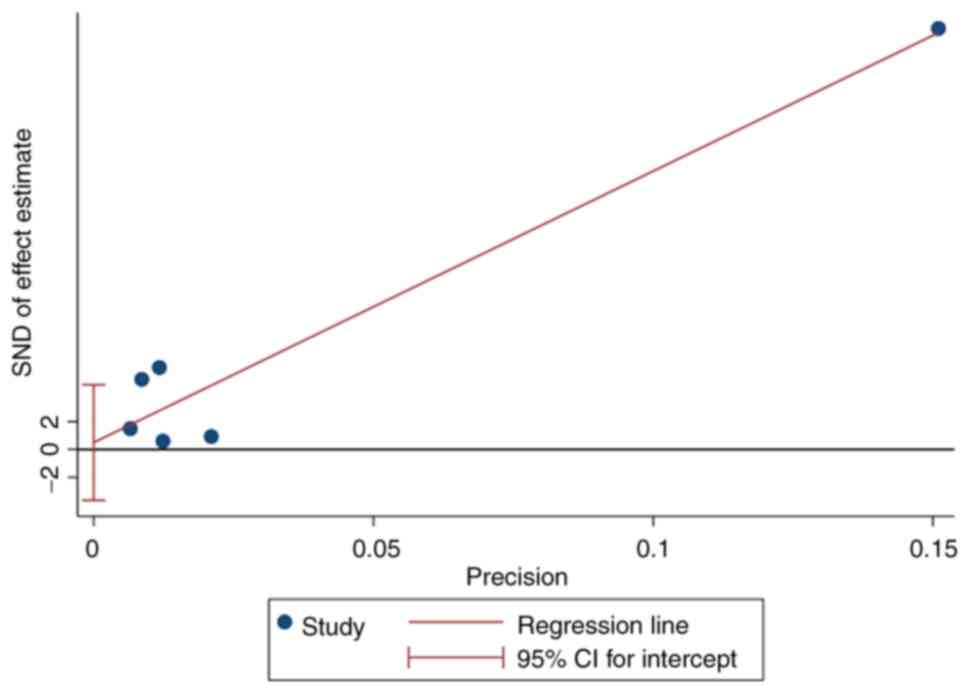
following a leave-one-out analysis (Fig. 3) Egger's test revealed no evidence
of publication bias (P=0.756; Fig.
4) Furthermore, 3 studies involving 263 patients with COVID-19
showed that the MMP-9 levels in patients with ARDS (n=79) were
higher than those in patients without ARDS (n=184) [WMD 248.5 (95%
CI, 63.84-433.25), P=0.008]; the meta-analysis had significant
heterogeneity (I2=87.1%, P<0.001), and 2 studies
involving 87 patients with COVID-19 showed that the MMP-9 levels in
the COVID-19 non-survivors did not significantly differ compared to
those in the COVID-19 survivors [WMD 37.79 (95% CI, -18.08-93.65),
P=0.185], no heterogeneity was observed in this meta-analysis
(I2=0%, P=0.593; Fig.
2). Finally, the MMP-9 levels were positively correlated with
the severity of COVID-19 [WMD 188.70 (95% CI, 119.54-257.85),
P<0.001]; the meta-analysis had significant heterogeneity
(I2=89.7%, P<0.001).
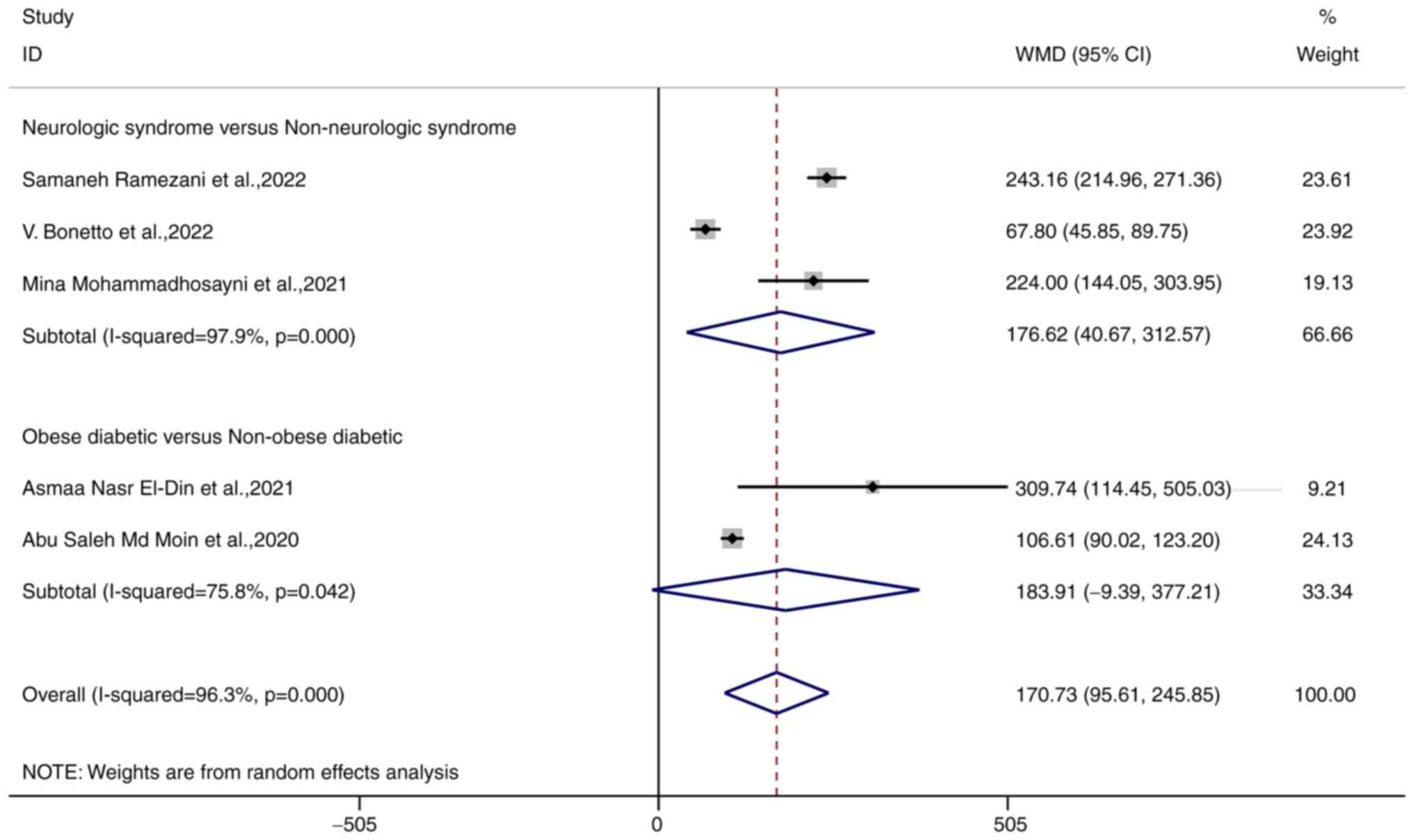
MMP-9 levels are associated with
comorbidities in patients with COVID-19
Patients with comorbidities, including obese
diabetic patients and those with neurological syndromes had
significantly higher MMP-9 levels than those without comorbidities
[WMD 170.73 (95% CI, 95.61-245.85), P<0.001]; the meta-analysis
had significant heterogeneity (I2=96.3%, P<0.001).
Patients with neurological syndromes had higher MMP-9 levels than
those without [WMD 176.62 (95% CI, 40.67-312.57), P=0.011]; the
meta-analysis had significant heterogeneity (I2=98.7%,
P<0.001). Obese diabetic patients did have different levels
compared with non-obese diabetic patients [WMD 183.91 (95% CI
-9.39-377.21), P=0.042]; the results of the meta-analysis had
significant heterogeneity (I2=75.8%, P=0.0042; Fig. 5).
Discussion
The global impact of SARS-CoV-2 was reduced from
2020 to the present due to the emergence of COVID-19 nucleic acid
testing technology and improved epidemic prevention policies
(36). However, the emergence of
novel mutant strains and the daily increase in the number of
confirmed cases and deaths attracted significant attention. It is
important to predict patients who are likely to develop severe
COVID and ARDS through laboratory tests to improve patient
prognosis and reduce mortality. The present meta-analysis included
12 studies that investigated the association between MMP-9 levels
with severe COVID and patients with comorbidities in COVID-19
patients. The results showed that elevated MMP-9 levels were
observed in 1,471 severe COVID-19 patients in 6 studies and 263
patients with ARDS in 3 studies. However, it was not clear whether
elevated MMP-9 levels were significantly associated with patient
mortality in the 87 COVID-19 patients in 2 studies. In addition, 5
studies assessed COVID-19 patients with a poorer prognosis due to
comorbidities, such as diabetic obese patients or neurologic
syndrome, showed significantly higher MMP-9 levels than in patients
without comorbidities.
As one of the most widely studied MMPs, MMP-9 plays
important roles in several biological processes (37). Neutrophils secrete large quantities
of MMP-9 when inappropriately triggered or overactivated, resulting
in cytokine storms and effector cell transfer (38). This results in acute lung injury
and ARDS, exacerbating the condition of COVID-19 patients (39). The present meta-analysis confirmed
that MMP-9 levels were higher in patients with ARDS than in those
without ARDS. Cytokine storms are the primary cause of death in
COVID-19 patients (40): Elevated
MMP-9 levels were correlated (although not significantly) with
mortality in COVID-19 patients in the present meta-analysis, likely
due to the small number of cases and patients included in the
original studies. In addition, MMP-9 was a potential biomarker for
cardiac remodeling in sepsis and hypertension (41,42).
Heart failure due to heart remodeling, a common complication in
elderly patients with COVID-19, poses an additional threat
(43). MMP-9 can bind to
Neutrophil Extracellular Traps (NETs) to induce endothelial cell
dysfunction, leading to a significantly higher risk of thrombosis
in COVID-19 patients with abnormal coagulation (44,45).
MMP-9 is a potential drug target, and effective inhibitors of MMPs
are available for clinical treatment (46). Therefore, detecting the serum MMP-9
levels in COVID-19 patients and providing timely treatment can
effectively improve the prognosis of certain COVID-19 patients.
Desforges et al (47) found that MMP-9 increases the
permeability of the blood-brain barrier, promotes the migration of
monocytes to the central nervous system, and promotes the secretion
of inflammatory mediators, leading to neuronal damage. The
meta-analysis demonstrated a significant increase in the serum
MMP-9 levels of patients with neurologic syndromes. Unal et
al (48) discovered that
elevated MMP-9 levels were associated with obesity and insulin
resistance. The present meta-analysis found that elevated MMP-9
levels were associated with obesity in diabetic patients. The
relationship between MMP-9 levels and COVID-19 patients with type I
diabetes caused by insulin resistance requires further
investigation.
This meta-analysis has several limitations. First,
there was significant heterogeneity in this meta-analysis. A
sensitivity analysis showed that the results of the meta-analysis
were stable and not influenced by factors such as the number of
cases in the combined results. Other factors, including variations
in the species of the virus, changes in the method of measuring
MMP-9, criteria for confirming the diagnosis, and classifying the
severity of the disease; may cause this residual heterogeneity.
Second, most of the included studies were published in high-impact
journals; however, several journals have provided green lanes for
the publication of COVID-19-related articles to combat this
epidemic. Thus, some studies may be at risk of bias. The limited
number of included studies prevented the investigation of study
publication bias in the meta-analysis of comorbidities. Third, the
studies in this meta-analysis were retrospective, and the primary
information was obtained by querying electronic medical records;
therefore, there was a lack of data and information. In summary,
more prospective studies are required to confirm the relationship
between serum MMP-9 levels and severity and mortality in COVID-19
patients. The corresponding therapeutic targets can be identified
by studying the related molecular mechanisms to reduce the severity
of COVID-19 and improve patient prognosis.
In conclusion, the present meta-analysis revealed an
association between serum MMP-9 levels and clinical characteristics
(including severity, mortality, and comorbidities) in patients with
COVID-19. Thus, testing serum MMP-9 levels in COVID-19 patients may
be useful for predicting the deterioration of patients for early
interventions and targeted treatment. Future clinical studies are
required to clarify the mechanism of action of MMP-9 in the
long-term prognosis of patients with COVID-19.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LD made substantial contributions to the conception
and design of the meta-analysis. HJ and XG performed the literature
search and initial screening for the acquisition of data. CZ and TL
performed the full-text reading of the initial screening literature
and decided on the inclusion of articles and analyzed the data. LD
and HG interpreted the data and confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S,
Zhang Q, Shi X, Wang Q, Zhang L and Wang X: Structure of the
SARS-CoV-2 spike receptor-binding domain bound to the ACE2
receptor. Nature. 581:215–220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Catanzaro M, Fagiani F, Racchi M, Corsini
E, Govoni S and Lanni C: Immune response in COVID-19: Addressing a
pharmacological challenge by targeting pathways triggered by
SARS-CoV-2. Signal Transduct Target Ther. 5(84)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Helms J, Kremer S, Merdji H, Clere-Jehl R,
Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana
M, et al: Neurologic Features in Severe SARS-CoV-2 Infection. N
Engl J Med. 382:2268–2270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Richardson S, Hirsch JS, Narasimhan M,
Crawford JM, McGinn T and Davidson KW: The Northwell COVID-19
Research Consortium. Barnaby DP, Becker LB, Chelico JD, et al:
Presenting characteristics, comorbidities, and outcomes among 5700
patients hospitalized with COVID-19 in the New York City Area.
JAMA. 323:2052–2059. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Beghi E, Helbok R, Ozturk S, Karadas O,
Lisnic V, Grosu O, Kovács T, Dobronyi L, Bereczki D, Cotelli MS, et
al: Short- and long-term outcome and predictors in an international
cohort of patients with neuro-COVID-19. Eur J Neurol. 29:1663–1684.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Townsend MJ, Kyle TK and Stanford FC:
Commentary: COVID-19 and obesity: Exploring biologic
vulnerabilities, structural disparities, and weight stigma.
Metabolism. 110(154316)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gong MN, Bajwa EK, Thompson BT and
Christiani DC: Body mass index is associated with the development
of acute respiratory distress syndrome. Thorax. 65:44–50.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gazzaz ZJ: Diabetes and COVID-19. Open
Life Sci. 16:297–302. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Barron E, Bakhai C, Kar P, Weaver A,
Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, et
al: Associations of type 1 and type 2 diabetes with
COVID-19-related mortality in England: A whole-population study.
Lancet Diabetes Endocrinol. 8:813–822. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lind L, Eriksson K and Grahn A: Chemokines
and matrix metalloproteinases in cerebrospinal fluid of patients
with central nervous system complications caused by
varicella-zoster virus. J Neuroinflammation. 16(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Davey A, McAuley DF and O'Kane CM: Matrix
metalloproteinases in acute lung injury: Mediators of injury and
drivers of repair. Eur Respir J. 38:959–970. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X and Khalil RA: Matrix
metalloproteinases, vascular remodeling, and vascular disease. Adv
Pharmacol. 81:241–330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vafadari B, Salamian A and Kaczmarek L:
MMP-9 in translation: From molecule to brain physiology, pathology,
and therapy. J Neurochem. 139 (Suppl 2):S91–S114. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng Z, Limbu MH, Wang Z, Liu J, Liu L,
Zhang X, Chen P and Liu B: MMP-2 and 9 in chronic kidney disease.
Int J Mol Sci. 18(776)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsarouhas K, Tsitsimpikou C, Apostolakis
S, Haliassos A, Tzardi M, Panagiotou M, Tsatsakis A and Spandidos
DA: Homocysteine and metalloprotease-3 and -9 in patients with
ascending aorta aneurysms. Thromb Res. 128:e95–e99. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tsarouhas K, Soufla G, Apostolakis S,
Zaravinos A, Panagiotou M, Khoury M, Hassoulas JA, Tsatsakis AM and
Spandidos DA: Transcriptional regulation of TIMPs in ascending
aorta aneurysms. Thromb Res. 126:399–405. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hazra S, Chaudhuri AG, Tiwary BK and
Chakrabarti N: Matrix metallopeptidase 9 as a host protein target
of chloroquine and melatonin for immunoregulation in COVID-19: A
network-based meta-analysis. Life Sci. 257(118096)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ben Moftah M and Eswayah A: Intricate
relationship between SARS-CoV-2-induced shedding and cytokine storm
generation: A signaling inflammatory pathway augmenting COVID-19.
Health Sci Rev (Oxf). 2(100011)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Charzewski L, Krzysko KA and Lesyng B:
Structural characterisation of inhibitory and non-inhibitory
MMP-9-TIMP-1 complexes and implications for regulatory mechanisms
of MMP-9. Sci Rep. 11(13376)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Martinez Mesa A, Cabrera Cesar E,
Martin-Montanez E, Sanchez Alvarez E, Lopez PM, Romero-Zerbo Y,
Garcia-Fernandez M and Velasco Garrido JL: Acute lung injury
biomarkers in the prediction of COVID-19 severity: Total thiol,
ferritin and lactate dehydrogenase. Antioxidants (Basel).
10(1221)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ramezani S, Ezzatifar F, Hojjatipour T,
Hemmatzadeh M, Shabgah AG, Navashenaq JG, Aslani S, Shomali N,
Arabi M, Babaie F, et al: Association of the matrix
metalloproteinases (MMPs) family gene polymorphisms and the risk of
coronavirus disease 2019 (COVID-19); implications of contribution
for development of neurological symptoms in the COVID-19 patients.
Mol Biol Rep. 50:173–183. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kassianidis G, Siampanos A, Poulakou G,
Adamis G, Rapti A, Milionis H, Dalekos GN, Petrakis V, Sympardi S,
Metallidis S, et al: Calprotectin and Imbalances between
Acute-Phase Mediators Are Associated with Critical Illness in
COVID-19. Int J Mol Sci. 23(4894)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nasr El-Din A, Ata KAE, Abdel-Gawad AR and
Fahmy NF: Impact of high serum levels of MMP-7, MMP-9, TGF-β and
PDGF macrophage activation markers on severity of COVID-19 in
obese-diabetic patients. Infect Drug Resist. 14:4015–4025.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bonetto V, Pasetto L, Lisi I, Carbonara M,
Zangari R, Ferrari E, Punzi V, Luotti S, Bottino N, Biagianti B, et
al: Markers of blood-brain barrier disruption increase early and
persistently in COVID-19 patients with neurological manifestations.
Front Immunol. 13(1070379)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gelzo M, Cacciapuoti S, Pinchera B, De
Rosa A, Cernera G, Scialò F, Comegna M, Mormile M, Fabbrocini G,
Parrella R, et al: Matrix metalloproteinases (MMP) 3 and 9 as
biomarkers of severity in COVID-19 patients. Sci Rep.
12(1212)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Savic G, Stevanovic I, Mihajlovic D,
Jurisevic M, Gajovic N, Jovanovic I and Ninkovic M: MMP-9/BDNF
ratio predicts more severe COVID-19 outcomes. Int J Med Sci.
19:1903–1911. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lerum TV, Maltzahn NN, Aukrust P, Trøseid
M, Henriksen KN, Kåsine T, Dyrhol-Riise AM, Stiksrud B, Haugli M,
Blomberg B, et al: Persistent pulmonary pathology after COVID-19 is
associated with high viral load, weak antibody response, and high
levels of matrix metalloproteinase-9. Sci Rep.
11(23205)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Iwasaki-Hozumi H, Maeda Y, Niki T,
Chagan-Yasutan H, Bai G, Matsuba T, Furushima D, Ashino Y and
Hattori T: Plasma N-Cleaved Galectin-9 is a surrogate marker for
determining the severity of COVID-19 and monitoring the therapeutic
effects of tocilizumab. Int J Mol Sci. 24(3591)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moin ASM, Sathyapalan T, Atkin SL and
Butler AE: Pro-fibrotic M2 macrophage markers may increase the risk
for COVID19 in type 2 diabetes with obesity. Metabolism.
112(154374)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Springall R, Gonzalez-Flores J,
Garcia-Avila C, Juárez-Vicuña Y, Hernández-Diazcouder A,
Márquez-Velasco R, Cásares-Alvarado S, Sánchez-Muñoz F,
Basilio-Gálvez E, Castillo-Salazar M, et al: Elevated levels of
soluble CD147 are associated with hyperinflammation and disease
severity in COVID-19: A proof-of-concept clinical study. Arch
Immunol Ther Exp (Warsz). 70(18)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Petito E, Falcinelli E, Paliani U, Cesari
E, Vaudo G, Sebastiano M, Cerotto V, Guglielmini G, Gori F,
Malvestiti M, et al: Association of neutrophil activation, more
than platelet activation, with thrombotic complications in
coronavirus disease 2019. J Infect Dis. 223:933–944.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mohammadhosayni M, Sadat Mohammadi F,
Ezzatifar F, Mahdavi Gorabi A, Khosrojerdi A, Aslani S, Hemmatzadeh
M, Yazdani S, Arabi M, Marofi F, et al: Matrix metalloproteinases
are involved in the development of neurological complications in
patients with Coronavirus disease 2019. Int Immunopharmacol.
100(108076)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ.
339(b2700)2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wells G, Shea B, O'Connell D, Robertson J,
Peterson J, Welch V, Losos M and Tugwell P: The Newcastle–Ottawa
Scale (NOS) for Assessing the Quality of Non-Randomized Studies in
Meta-Analysis. Enviromental Sci, 2014.
|
|
36
|
Sharma A, Ahmad Farouk I and Lal SK:
COVID-19: A review on the novel coronavirus disease evolution,
transmission, detection, control and prevention. Viruses.
13(202)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 Biosensors: Recent
advances. Sensors (Basel). 18(3249)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sato A, Nishida C, Sato-Kusubata K,
Ishihara M, Tashiro Y, Gritli I, Shimazu H, Munakata S, Yagita H,
Okumura K, et al: Inhibition of plasmin attenuates murine acute
graft-versus-host disease mortality by suppressing the matrix
metalloproteinase-9-dependent inflammatory cytokine storm and
effector cell trafficking. Leukemia. 29:145–156. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hsu AT, Barrett CD, DeBusk GM, Ellson CD,
Gautam S, Talmor DS, Gallagher DC and Yaffe MB: Kinetics and role
of plasma matrix metalloproteinase-9 expression in acute lung
injury and the acute respiratory distress Syndrome. Shock.
44:128–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tang Y, Liu J, Zhang D, Xu Z, Ji J and Wen
C: Cytokine Storm in COVID-19: The current evidence and treatment
strategies. Front Immunol. 11(1708)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Duda I, Krzych L, Jedrzejowska-Szypulka H
and Lewin-Kowalik J: Plasma matrix metalloproteinase-9 and tissue
inhibitor of matrix metalloproteinase-1 as prognostic biomarkers in
critically Ill Patients. Open Med (Wars). 15:50–56. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nemec HM, Ferenczy A, Christie BD III,
Ashley DW and Montgomery A: Correlation of D-dimer and Outcomes in
COVID-19 Patients. Am Surg. 88:2115–2118. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khan E: Heart failure and COVID-19:
Synergism of two inflammatory conditions? Br J Community Nurs.
26:18–25. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Marchesi C, Dentali F, Nicolini E, Maresca
AM, Tayebjee MH, Franz M, Guasti L, Venco A, Schiffrin EL, Lip GY
and Grandi AM: Plasma levels of matrix metalloproteinases and their
inhibitors in hypertension: A systematic review and meta-analysis.
J Hypertens. 30:3–16. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mondal S, Adhikari N, Banerjee S, Amin SA
and Jha T: Matrix metalloproteinase-9 (MMP-9) and its inhibitors in
cancer: A minireview. Eur J Med Chem. 194(112260)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Carmona-Rivera C, Zhao W, Yalavarthi S and
Kaplan MJ: Neutrophil extracellular traps induce endothelial
dysfunction in systemic lupus erythematosus through the activation
of matrix metalloproteinase-2. Ann Rheum Dis. 74:1417–1424.
2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Desforges M, Le Coupanec A, Stodola JK,
Meessen-Pinard M and Talbot PJ: Human coronaviruses: Viral and
cellular factors involved in neuroinvasiveness and
neuropathogenesis. Virus Res. 194:145–158. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Unal R, Yao-Borengasser A, Varma V,
Rasouli N, Labbate C, Kern PA and Ranganathan G: Matrix
metalloproteinase-9 is increased in obese subjects and decreases in
response to pioglitazone. J Clin Endocrinol Metab. 95:2993–3001.
2010.PubMed/NCBI View Article : Google Scholar
|