Introduction
Alongside global development and population aging,
the incidence of diabetes continues to increase annually (1). Data from the International Diabetes
Federation suggest that ~537 million individuals were affected by
diabetes in 2021, which constituted 10.5% of the global adult
population, and this number is predicted to reach 783 million by
2045, representing 12.2% of the global adult population (2). Pancreatic β-cells play a vital role
in sensing changes in the blood glucose concentration and releasing
insulin in response to regulate it. The mechanism involves
elevation of the ATP/ADP ratio, the closure of ATP-sensitive
K+ channels and activation of voltage-dependent
Ca2+ channels (3,4).
Multiple factors promote pancreatic β-cell dysfunction and
apoptosis, resulting in their inability to produce sufficient
insulin to control blood glucose levels, which is the main feature
of type 2 diabetes mellitus (T2DM) (5). Previous studies have found that the
incidence of T2DM increases linearly with the prevalence of
obesity. This may be due to the greater quantity of free saturated
fatty acids in obese patients compared with patients of normal
weight, which damages β-cell function, increases insulin resistance
and destroys glucose homeostasis (6,7).
Under lipotoxic conditions, the unfolded protein
response (UPR) is activated, which relieves endoplasmic reticulum
(ER) stress by preventing protein translation, increasing the
expression of chaperone proteins that perform refolding, and
accelerating ER-associated degradation processes. However,
sustained lipotoxic stimulation may cause the UPR to be unable to
maintain ER homeostasis, resulting in the disruption of ER
integrity followed by cell apoptosis (8-10).
In addition, lipotoxicity increases the accumulation of
mitochondrial reactive oxygen species (ROS) and induces
mitochondrial DNA mutations, leading to mitochondrial dysfunction
and impairing cellular energy metabolism (11). The impairment of mitochondrial
function may play a key role in the development of insulin
resistance (12). The ER and
mitochondria are two of the most important organelles used by
pancreatic β-cells to carry out their normal functions. The ER is
crucial in insulin synthesis, proper folding of the insulin peptide
and its response to glucose levels, while mitochondria produce ATP
via glucose metabolism, which promotes the secretion of insulin
(13,14).
Research to elucidate the mechanism by which
lipotoxicity damages the function of the ER and mitochondria in
pancreatic β-cells is urgently required. Palmitate (PA) is the most
abundant saturated fatty acid in the body and is often employed to
establish lipotoxicity models. It induces β-cell dysfunction and
apoptosis through molecular mechanisms including oxidative stress,
mitochondrial dysfunction and ER stress, thereby ultimately
impairing glucose-stimulated insulin secretion (GSIS) (15,16).
Protein phosphatase 2A (PP2A) is a serine/threonine
phosphatase consisting of a scaffolding A subunit, regulatory B
subunit and catalytic C subunit. It is an important mediator of key
cellular processes including glycosphingolipid metabolism,
cell-cycle progression, signal transduction, protein translation,
cell proliferation and cell apoptosis (17-19).
The PP2A catalytic subunit (PP2Ac) mainly regulates the degree of
substrate phosphorylation via its catalytic function and alters
PP2A activity. The reduction of PP2A activity via the knockdown of
PP2Ac has been shown to alleviate ER stress in cardiac and hepatic
cells (20,21). In addition, high glucose levels
have been demonstrated to disrupt mitochondrial integrity and
function by modulating the pentose phosphate-PP2A pathway, thus
resulting in mitochondrial disruption and altered mitochondrial
respiration in hepatocytes (22).
Furthermore, PP2Ac regulates insulin resistance in the liver and
insulin secretion by pancreatic β-cells under conditions of glucose
toxicity (23,24). However, the effects of PP2Ac on
β-cell function, proliferation and apoptosis in a lipotoxic
environment are not clear.
Therefore, the present study aimed to elucidate the
role of PP2Ac knockdown in pancreatic β-cells exposed to lipotoxic
conditions. The potential mechanism was evaluated in MIN6 cells and
the effect of PP2Ac knockdown in pancreatic tissue on insulin
resistance in a high-fat diet (HFD)-induced mouse model was
examined.
Materials and methods
Cell culture
The mouse insulinoma 6 (MIN6) cell line was cultured
in modified RPMI (cat. no. SH30809.01; HyClone; Cytiva) containing
10% (v/v) fetal bovine serum (cat. no. 10099-141; Gibco; Thermo
Fisher Scientific, Inc.), 11.1 mM glucose, 2 mM L-glutamine and 1%
penicillin/streptomycin in a humidified atmosphere with 5%
CO2 and 95% air at 37˚C. Cells were treated with PA or
bovine serum albumin (BSA; cat. no. BS114-5g; Biosharp Life
Sciences) at 37˚C in all experiments.
PP2A activity analysis
PP2A activity was measured using a Serine/Threonine
Phosphatase Assay System (cat. no. V2460; Promega Corporation).
Endogenous phosphate was removed by adding cell or tissue lysates
to Sephadex® G-25 in Spin Columns provided with the kit.
The treated samples and PP2A reaction buffer were added to a
96-well plate and incubated for 15 min at room temperature. The
reaction was terminated by adding molybdate dye for 30 min and then
measuring the optical density at 600 nm using a multifunctional
plate reader (VICTOR® Nivo™; PerkinElmer, Inc.). A BCA
Protein Assay Kit (cat. no. P0011; Beyotime Institute of
Biotechnology) was used to calibrate the protein concentrations and
calculate the relative activity of PP2A.
Generation of PP2Ac knockdown
cells
Mouse PP2Ac gene (accession NM_019411) knockdown and
negative control lentiviruses (plasmid backbone GV493) were
designed and synthesized by Shanghai GeneChem Co., Ltd. MIN6 cells
were cultured in a 6-well plate (8x104 cells/well), and
when the degree of cell confluence reached ~30%, the
PP2Acα-interfering or negative control lentiviruses (10
multiplicity of infection) were transfected into the cells,
respectively. Stable PP2Ac knockdown cells and negative control
groups were obtained after 48-72 h by screening with puromycin (13
µg/ml). The sequences of the three PP2Ac small hairpin RNAs
(shRNAs) used for knockdown are listed in Table I. Three PP2Ac knockdown cell groups
(sh1, sh2 and sh3) and a negative short hairpin control group (shc)
were thereby constructed.
 | Table IshRNA sequences. |
Table I
shRNA sequences.
| shRNA | Sequence,
5'-3' |
|---|
| PP2Ac sh1 |
GAGACATTTAATCATGCCAAT |
| PP2Ac sh2 |
TTGACGACACTCTTAAGTATT |
| PP2Ac sh3 |
GACCGGAACGTAGTAACAATT |
| shc |
TTCTCCGAACGTGTCACGT |
Reverse transcription-quantitative PCR
(RT-qPCR)
Transfected MIN6 cells were seeded in 6-well plates
(2x105 cells/well) and cultured until the cell
confluence reached 90%. Total RNA was extracted using an RNA
extraction kit (cat. no. RN07; Aidlab Biotechnologies Co., Ltd.).
RT was then conducted using the ReverTra Ace qPCR RT Kit (cat. no.
FSQ101; Toyobo Life Science). The reaction steps were as follows:
Pre-denaturation at 95˚C for 10 min, followed by 40 cycles of
denaturation at 95˚C at 10 sec, annealing at 60˚C for 30 sec and
extension at 72˚C for 30 sec. Relative expression changes were
calculated using the 2-ΔΔCq method (25). Finally, qPCR was performed using
Magic SYBR Mixture (cat. no. CW3008M; CWBio) in a CFX96 RT-qPCR
Detection System (Bio-Rad Laboratories, Inc.). β-actin mRNA was
used as a control for the experiments. The sequences of the primers
used for PCR were as follows: β-actin forward,
5'-GTGACGTTGACATCCGTAAAGA-3' and reverse,
5'-GTAACAGTCCGCCTAGAAGCAC-3'; and PP2Ac forward,
5'-ATGGACGAGAAGTTGTTCACC-3' and reverse,
5'-CAGTGACTGGACATCGAACCT-3'.
ROS analysis
A master mix was prepared by dissolving
dihydroethidium (cat. no. S0063; Beyotime Institute of
Biotechnology) powder in DMSO to a concentration of 10 mM, and then
diluted by 1:1,000 to reach the working solution concentration upon
usage. MIN6 cells were seeded in 6-well plates (2x105
cells/well), cultured until cell confluence reached 60% and then
treated with 0.5 mM PA or BSA for 24 h. The MIN6 cells were
harvested by trypsinization and resuspended in 1 ml diluted working
solution for 30 min at 37˚C. Next, the cells were washed three
times with PBS and analyzed by the CytoFlex flow cytometry using
the CytExpert software (version 2.4.0.28; Beckman Coulter,
Inc.).
Mitochondrial membrane potential
assay
Mitochondrial membrane potential was measured using
tetramethylrhodamine ethyl ester (TMRE; cat. no. C2001S; Beyotime
Institute of Biotechnology). MIN6 cells were seeded in 6-well
plates (2x105 cells/well), cultured until cell
confluence reached 60% and then treated with 0.5 mM PA or BSA for
24 h. Following treatment, the MIN6 cells were harvested by
trypsinization and then incubated with PBS containing 10 nM TMRE
for 30 min at 37˚C in the dark. The cells were washed with
pre-warmed PBS and analyzed by the CytoFlex flow cytometry using
the CytExpert software (version 2.4.0.28; Beckman Coulter,
Inc.).
ATP measurement
ATP levels were measured using an ATP kit (cat. no.
S0027; Beyotime Institute of Biotechnology). MIN6 cells were seeded
in 6-well plates (2x105 cells/well) and cultured until
cell confluence reached 60%, after which they were treated with 0.5
mM PA or BSA for 24 h. The cells were then lysed, and a supernatant
was obtained via centrifugation at 12,000 x g for 5 min at 4˚C. A
standard curve was established using an ATP standard solution. The
working solution was prepared and the ATP concentration was
measured using a VICTOR Nivo multipurpose microplate reader. To
eliminate errors, the supernatant was collected for BCA protein
quantification analysis to confirm its uniformity.
Determination of apoptosis
An Annexin V-APC/PI Apoptosis Detection Kit (cat.
no. A30813; MultiSciences Biotech Co., Ltd.) was used to detect the
cell apoptosis rate. MIN6 cells were seeded in 6-well plates
(2x105 cells/well) and cultured until cell confluence
reached 60%. Apoptosis was induced using 0.5 mM PA for 24 h at
37˚C, and BSA was used as a control. Annexin V-APC and PI dyes were
added to the cell suspension, which was then incubated for 5 min at
room temperature. Apoptosis was detected by the CytoFlex flow
cytometry using the CytExpert software (version 2.4.0.28; Beckman
Coulter, Inc.). Cells that were double positive for Annexin V and
PI were considered late apoptotic cells, while cells that were
Annexin V positive and PI negative were considered early apoptotic
cells. The cell apoptosis rate was calculated as a percentage as
follows: Sum of the number of cells at early and late
apoptosis/total number of cells per well x100.
RNA sequencing
RNA was extracted and purified from PP2Ac knockdown
and shc MIN6 cells, and a cDNA library was created using the
NEBNext® Ultra™ RNA Library Prep Kit for
Illumina® (cat. no. E7530L; New England Biolabs, Inc.).
The insert size of the library was detected using a bioanalyzer
(Agilent 2100; Agilent Technologies Co. Ltd.) to ensure the quality
of the library. The effective concentration of the final library
was >2 nM. Illumina sequencing (Illumina NovaSeq 6000; Illumina,
Inc.) was performed with paired-end, and the end reading of 150 bp
pairing was generated. The sequenced fragments were converted into
sequencing data using a high-throughput sequencer to obtain raw
reads. The filtered, clean reads were then compared with the
reference genome (Mus Musculus: GRCm38/mm10) using the hisat2
software (version 2.0.5) (26) to
obtain information on the positioning of the reads against the
reference genome. The number of reads in the range from start to
termination was calculated for each gene based on the information
on the position of each gene pair against the reference genome.
Finally, the differentially expressed genes were analyzed and Kyoto
Encyclopedia of Genes and Genomes (KEGG) (27) pathway enrichment analysis was
performed using the clusterProfiler software (version 3.8.1; Padj
<0.05) (28). The RNA
sequencing results are publicly available in the GEO database
(accession number: GSE242538).
Insulin secretion assay
MIN6 cells were seeded in 6-well plates
(2x105 cells/well), cultured until cell confluence
reached 60% and then treated with 0.5 mM PA or BSA for 24 h. The
cells were washed twice with Krebs-Ringer bicarbonate buffer
(KRBB), sugar-free medium (cat. no. PM150122; Procell Life Science
& Technology Co., Ltd.) was added and the cells were incubated
for a further 30 min at 37˚C. KRBB solutions with 2.5 and 20 mM
glucose were used to measure GSIS as previously described (24,29,30).
The cells were incubated with these solutions for 2 h and the
supernatants were obtained via centrifugation at 12,000 x g for 10
min at 4˚C. The insulin concentration was measured using a Mouse
Insulin ELISA Kit (cat. no. E-EL-M2614c; Elabscience Biotechnology,
Inc.). Relative insulin concentrations were calibrated by
measurement of the protein concentration using the BCA method.
Cell viability determination
Cell Counting Kit-8 (CCK-8; cat. no. C0037; Biosharp
Life Sciences) was used to measure cell viability. Cells were
inoculated in 96-well plates at 6,000 cells/well and incubated in a
cell culture incubator for 1 day. Different concentrations of PA
were added, and the cells were further incubated for 24 h. Next, 10
µl CCK-8 working solution and 90 µl culture medium were added to
each well and the plate was incubated for 2 h at 37˚C in the dark.
Finally, the absorbance of each well was measured separately using
a VICTOR Nivo multifunctional microplate reader to calculate cell
viability. When the appropriate concentration of PA was determined,
this concentration was used for the assessment of cell viability
using the aforementioned method at 12, 24, 36 and 48 h of
incubation after the addition of PA, respectively.
Transmission electron microscopy
(TEM)
MIN6 cells were seeded in 10-cm cell culture dishes
(1x106 cells/well) and cultured until cell confluence
reached 60%. They were then treated with 0.5 mM PA or BSA for 24 h.
Pre-chilled electron microscope stationary liquid (Wuhan Servicebio
Technology Co., Ltd.) was added to fix the cells for 1 h at 4˚C,
which were then sequentially dehydrated in a gradient of 50, 70,
80, 90, 95 and 100% ethanol. The cells were then embedded (1%
agarose solution), sectioned (60 nm) and stained with 2% uranium
acetate saturated alcohol solution and 2.6% lead citrate for 8 min
at room temperature, respectively. Finally, images were analyzed by
TEM (Hitachi, Ltd.).
Animal experiments
A total of 40 male 4-week-old C57BL/6J mice weighing
~20 g were purchased from SPF (Beijing) Biotechnology Co., Ltd.
Mice were housed at constant temperature (22-26˚C) and humidity
(50-60%), with a 12-h light/dark cycle during 1 week of adaptive
feeding with free access to food and water. PP2Ac-interfering or
control adeno-associated viruses (AAVs; Shanghai GeneChem Co.,
Ltd.) were respectively injected intraperitoneally into the mice at
a final dose of 2.5x1011 viral genomes (vg) per mouse
and expressed stably in the pancreas after 3 weeks. The AAV
sequences are presented in Table
II. The mice in the interfering and control groups were fed
either a standard diet (SD; 0.2% fat, 71.5% carbohydrate and 18.3%
protein; calorific value, 3.85 kcal/g) or HFD (61.6% fat, 20.3%
carbohydrate and 18.1% protein; calorific value 5.24 kcal/g) for 12
weeks. The mice were finally divided into four groups: SD+AAV-null
group, SD+AAV-PP2Ac group, HFD+AAV-null group and HFD+AAV-PP2Ac
group, with 10 mice in each group. Fasting blood glucose was
measured every 2 weeks. In addition, at the end of the 12-week
feeding period and after a 16-h overnight fast, 2 g/kg glucose (25%
glucose solution) was injected into the mice for the
intraperitoneal glucose tolerance test (IPGTT). Blood glucose
levels in the mice were measured using a glucometer (cat. no.
AB-103G; Glucosure). After a further 3 days, the mice were
anesthetized with 80 mg/kg 1% pentobarbital by intraperitoneal
injection prior to blood collection from the orbital vein for
serological testing. The mice were subsequently euthanized by
CO2 inhalation (flow rate, 40% of the chamber
volume/min). The pancreas was removed by dissection, partially
fixed in 4% paraformaldehyde solution, paraffin-embedded and
histologically analyzed. The remainder of the pancreas was then
frozen in liquid nitrogen and stored in a refrigerator at -80˚C for
subsequent western blotting analysis. The animal experiments were
approved by the Animal Ethics and Experimentation Committee of
Wuhan University (approval no. ZN2022037).
 | Table IIAAV sequences. |
Table II
AAV sequences.
| AAV | Sequence,
5'-3' |
|---|
| AAV-null |
TTCTCCGAACGTGTCACGT |
| AAV-PP2Ac |
TTGACGACACTCTTAAGTATT |
Analysis of glucose tolerance and
serum parameters in mice
Every 2 weeks the mice were fasted overnight for 16
h and their fasting blood glucose was measured the following day.
The IPGTT was performed by injecting glucose into the mice
intraperitoneally at a dose of 2 g/kg and measuring the blood
glucose levels of the mice at 15, 30, 60 and 120 min using a
glucometer. In addition, plasma was isolated from the blood
collected from the orbital vein of the mice at the end of the
experiment. The serum levels of triglycerides and total cholesterol
were analyzed using kits (cat. nos. A110-H and A111-1-1,
respectively; Nanjing Jiancheng Bioengineering Institute) according
to the manufacturer's instructions. Serum insulin levels were
measured using ELISA kits (cat. no. E-EL-M2614c; Elabscience
Biotechnology, Inc.).
Analysis of islet β-cell area and
proliferation rate in mice
After euthanizing each mouse, the pancreas was
removed, washed twice with PBS and fixed in 4% paraformaldehyde
solution at room temperature. After paraffin embedding and
sectioning (4 µm), the pancreas was treated with xylene and
different concentrations of ethanol prior to dewaxing and
dehydration. After blocking with 5% BSA at room temperature for 30
min, primary antibodies against Ki67 (1:200 dilution; cat. no.
GB121141-100; Wuhan Servicebio Technology Co., Ltd.) and insulin
(1:200; cat. no. A19066; ABclonal Biotech Co., Ltd.) were added to
the sections and incubated overnight at 4˚C. The next day,
secondary antibodies conjugated to the fluorescent dyes FITC
(1:100; cat. no. GB22303; Wuhan Servicebio Technology Co., Ltd.)
and Cy3 (1:100; cat. no. GB21301; Wuhan Servicebio Technology Co.,
Ltd.) were incubated with the sections at room temperature for 1 h.
The nuclei were then stained with DAPI reagent at room temperature
for 5 min in the dark before being observed under a fluorescence
microscope and photographed. FITC-labeled insulin appeared green,
while Cy3-labeled Ki67 appeared red and nuclei stained by DAPI
appeared blue. The β-cell proliferation rate was calculated as the
proportion of cells positive for Ki67 labeling among the number of
insulin-positive cells. Cell counting and fluorescence intensity
quantification were performed using ImageJ software (version 1.42;
National Institutes of Health).
Western blotting analysis
Pancreatic tissue or MIN6 cells were homogenized
with RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) containing protease inhibitors. The supernatant was
then obtained via centrifugation at 12,000 x g for 20 min at 4˚C
and the total protein in the supernatant was quantified with a BCA
kit. Equal protein quantities were separated by 12.5% SDS-PAGE and
transferred to PVDF membranes (Millipore; cat. no. IPVH00010;
Sigma-Aldrich (Shanghai) Trading Co., Ltd.). After blocking the
membranes with 5% skimmed milk at room temperature for 2 h, they
were incubated with primary antibodies at 4˚C overnight. The
following day, the membranes were washed and incubated with
secondary antibodies at room temperature for 1 h. The primary
antibodies used were as follows: Anti-β-actin (1:50,000; cat. no.
66009-1-Ig; Proteintech Group, Inc.), anti-PP2Ac (1:2,000; cat. no.
2259s; CST Biological Reagents Co., Ltd.),
anti-microtubule-associated protein 1 light chain 3 b (LC3B;
1:2,000; cat. no. A19665;), anti-glucose-regulated protein 78
(GRP78; 1:2,000; cat. no. A4908), anti-CHOP (1:2,500; cat. no.
A0221), anti-caspase 3 (1:2,000; cat. no. A19654), anti-Bcl-2
(1:2,000; cat. no. A19693), anti-Bax (1:2,000; cat. no. A19684),
anti-pancreatic and duodenal homeobox 1 (Pdx1; 1:2,000; cat. no.
A3070) and anti-insulin (1:2,000; cat. no. A19066), all from
ABclonal Biotech Co., Ltd. β-actin was used as a control for
normalization. Additional primary antibodies were used to detect
cellular signaling pathways, as follows: Anti-ERK1/2 (1:2,000
dilution; cat. no. A16686), anti-phosphorylated (p)-ERK1/2
(1:2,000; cat. no. AP0472), anti-JNK (1:2,000; cat. no. A4867),
anti-p-JNK (1:2,000; cat. no. AP0631), anti-p38 (1:2,000; cat. no.
A0227), anti-p-p38 (1:2,000; cat. no. AP0057) and anti-β-tubulin
(1:1,000; cat. no. A0021), all from ABclonal Biotech Co., Ltd.
β-tubulin was used as a control for normalization. Secondary
antibodies conjugated to horseradish peroxidase were used as
follows: Goat anti-rabbit (1:5,000 dilution; cat. no. AS014;
ABclonal Biotech Co., Ltd.) and goat anti-mouse (1:5,000; cat. no.
AS003; ABclonal Biotech Co., Ltd.). Immunoreactive bands were
visualized with an automatic chemiluminescence image analysis
system (Tanon 5200; Tanon Science and Technology Co., Ltd.), and
the grayscale values of proteins were analyzed with ImageJ software
(version 1.53e).
Statistical analysis
All results are expressed as the mean ± standard
deviation and were analyzed using the SPSS 20.0 software package
(IBM Corp.). Graphs were plotted using GraphPad Prism 8 (GraphPad
Software; Dotmatics). Statistically significant differences between
two experimental conditions were analyzed with the paired Student's
t-test. Comparisons between multiple groups with single independent
variable were analyzed by one-way ANOVA followed by Tukey's post
hoc test, while those with two independent variables were analyzed
by two-way ANOVA with Bonferroni correction. P<0.05 was
considered to indicate a statistically significant difference.
Results
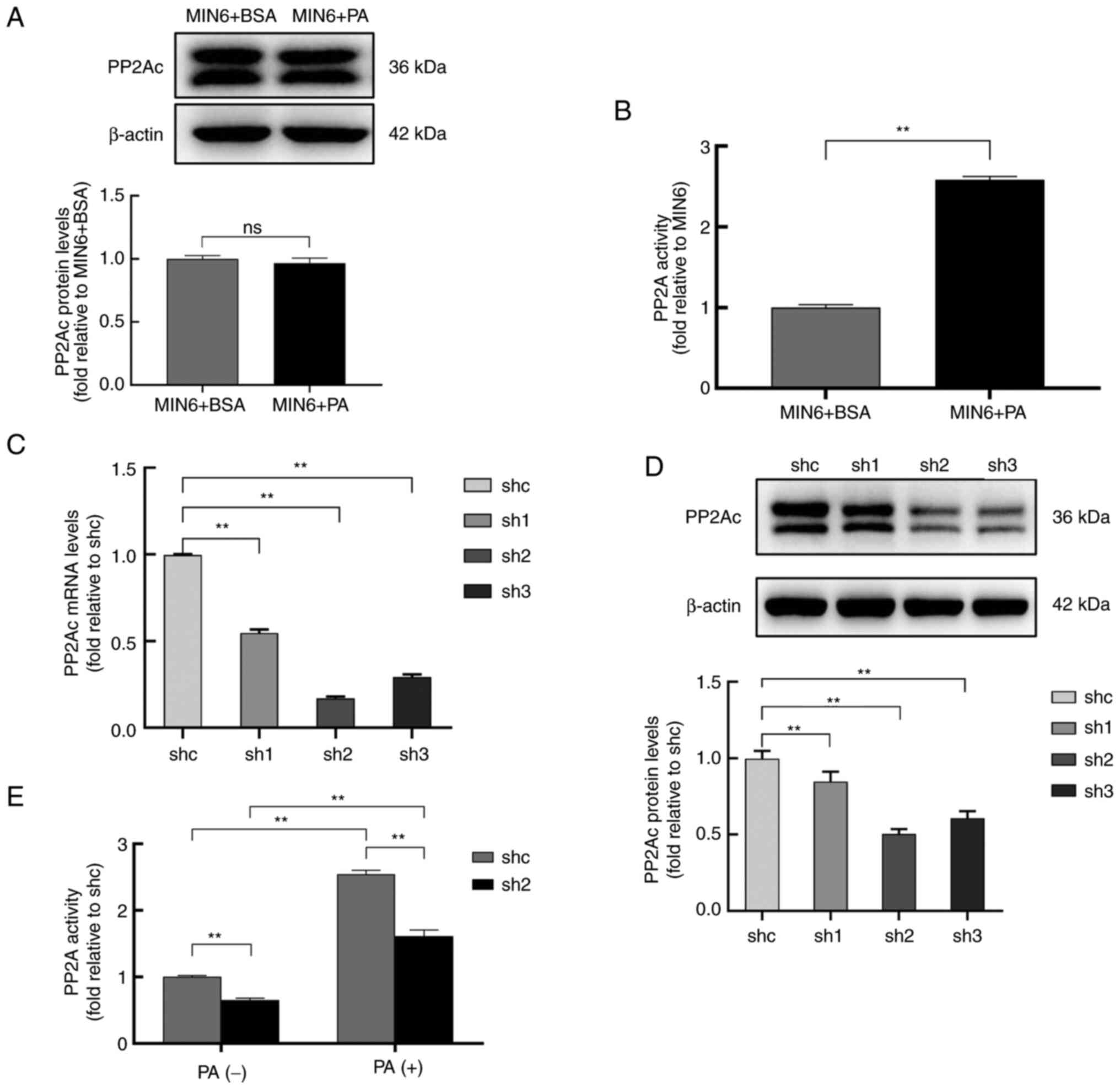
Knockdown of PP2Ac in MIN6 cells
significantly attenuates PA-induced PP2A hyperactivation
To assess the PP2A status in MIN6 cells under
lipotoxic conditions, MIN6 cells were treated with 0.5 mM BSA or PA
for 24 h, and the PP2Ac protein expression level and PP2A activity
of the cells were assayed. The data in Fig. 1A and B indicate that PA exposure significantly
activated PP2A, although it did not change the protein expression
level of PP2Ac in the MIN6 cells. Three PP2Ac knockdown cell groups
and a shc control group were constructed by the transfection of
MIN6 cells with lentiviral vectors. The results showed that,
compared with those in the shc group, the mRNA levels of PP2Ac for
sh1, sh2 and sh3 were reduced to 55, 17 and 19%, respectively
(Fig. 1C), while the PP2Ac protein
expression levels were reduced to 85, 50 and 61%, respectively
(Fig. 1D). As the sh2 cell line
exhibited the greatest knockdown effect, it was selected for the
analysis of PP2A activity under lipotoxic conditions. The results
showed that the knockdown of PP2Ac in MIN6 cells significantly
attenuated PA-induced PP2A hyperactivation (Fig. 1E).
PP2Ac knockdown alleviates PA-induced
mitochondrial dysfunction and ER stress in MIN6 cells
Mitochondria and the ER are crucial organelles for
insulin synthesis and secretion in pancreatic β-cells. Therefore,
the effects of PP2Ac knockdown using shRNAs on the function and
morphology of mitochondria and the ER were studied in MIN6 cells
under lipotoxic conditions. The results revealed that PA
significantly increased endogenous ROS generation and LC3BII
protein expression levels, and decreased the mitochondrial membrane
potential and ATP levels of the cells, whereas PP2Ac knockdown
significantly alleviated these effects (Figs. 2A-D, S1 and S2).
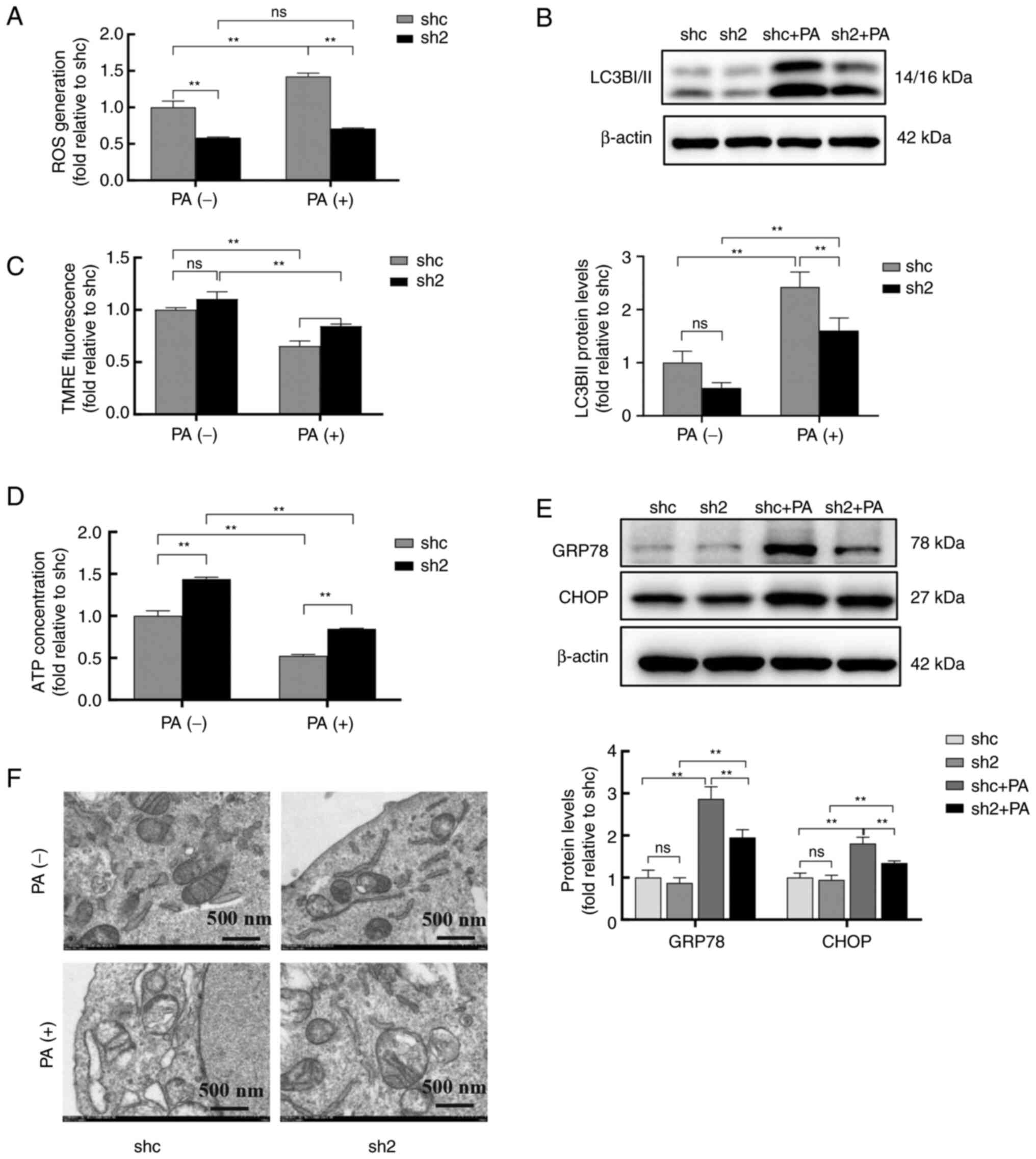 | Figure 2PP2Ac knockdown alleviates PA-induced
mitochondrial dysfunction and endoplasmic reticulum stress in MIN6
cells. MIN6 cells with or without PP2A knockdown were treated with
0.5 mM bovine serum albumin or PA for 24 h. (A) Cellular ROS levels
were detected by labeling with dihydroethidium probe and analysis
by flow cytometry. (B) Protein levels of LC3BI/II were analyzed by
western blotting in MIN6 cells, and β-actin was used as a loading
control. (C) Mitochondrial membrane potential was measured by flow
cytometry using TMRE dye. (D) ATP levels of the transfected cells
were quantified. (E) Protein expression levels of GRP78 and CHOP
were analyzed by western blotting in MIN6 cells, and β-actin was
used as a loading control. (F) Transmission electron microscopy was
used to observe the ultrastructure of the mitochondria and ER in
MIN6 cells. All experiments were performed at least three times,
and values are reported as the mean ± standard deviation.
**P<0.01. PP2Ac, catalytic subunit of protein
phosphatase 2A; PA, palmitate; MIN6, mouse insulinoma 6; ROS,
reactive oxygen species; LC3BI/II, microtubule-associated protein 1
light chain 3 b I/II; TMRE, tetramethylrhodamine ethyl ester;
GRP78, glucose-regulated protein 78; shc, short hairpin RNA
control; sh2, PP2AC short hairpin RNA 2; ns, not significant. |
GRP78 and CHOP are typical markers of ER stress.
After exposure to PA, the protein levels of GRP78 and CHOP were
significantly increased in both the shc and sh2 groups. However, in
the PA-treated cells, the protein levels of GRP78 and CHOP were
lower in the cells transfected with sh2 compared with those
transfected with shc (Fig. 2E).
The ultrastructures of the mitochondria and ER in MIN6 cells were
observed using TEM (Fig. 2F). The
TEM images revealed that exposure to PA promoted mitochondrial
swelling and rupture, and the disappearance of mitochondrial
ridges, while it decreased the quantity of the ER, all of which
were markedly alleviated in the MIN6 cells with PP2Ac knockdown.
These results suggest that PP2Ac knockdown alleviates PA-induced
mitochondrial dysfunction and ER stress in MIN6 cells.
PP2Ac knockdown increases insulin
release in MIN6 cells under PA exposure
Insulin secretion under low glucose (2.5 mM) and
high glucose (20 mM) conditions was examined. PA significantly
reduced insulin secretion when stimulated with both low and high
glucose concentrations, while PP2Ac knockdown effectively increased
insulin secretion (Fig. 3A). After
exposure to 0.5 mM PA, the protein expression levels of insulin and
Pdx1, which are associated with insulin secretion, were reduced in
MIN6 cells, but PP2Ac knockdown effectively alleviated these
reductions (Fig. 3B). These
results suggest that PP2Ac knockdown protected the insulin
secretory function of MIN6 cells against the effects of PA
exposure, which may indicate that the knockdown of PP2Ac is
associated with enhanced resistance to lipotoxicity in MIN6
cells.
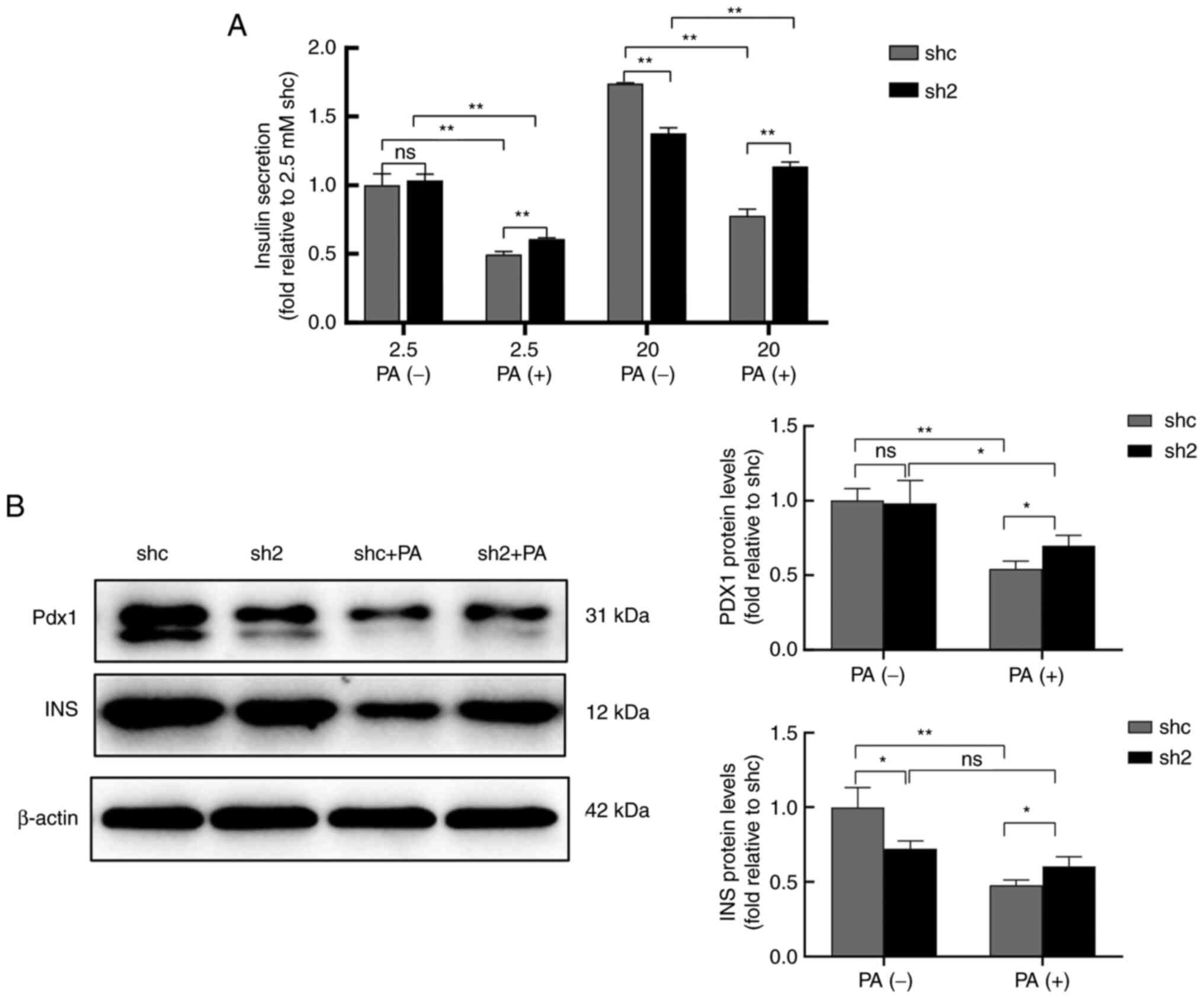 | Figure 3PP2Ac knockdown increases the
secretion of INS by MIN6 cells under PA exposure. (A) INS secretion
of MIN6 cells with or without PP2Ac knockdown stimulated by low
glucose (2.5 mM) and high glucose (20 mM). (B) Expression levels of
the INS secretion-associated proteins Pdx1 and INS were analyzed by
western blotting, and β-actin was used as a loading control. All
experiments were performed at least three times, and values are
reported as the mean ± standard deviation. *P<0.05,
**P<0.01. PP2Ac, catalytic subunit of protein
phosphatase 2A; INS, insulin; MIN6, mouse insulinoma 6; PA,
palmitate; Pdx1, pancreatic and duodenal homeobox 1; shc, short
hairpin RNA control; sh2, PP2AC short hairpin RNA 2; ns, not
significant. |
PP2Ac knockdown attenuates PA-induced
apoptosis in MIN6 cells
CCK-8 analysis revealed that the cell viability of
the sh2 group was markedly lower than that of the shc group at
different concentrations of PA and durations of exposure (Fig. 4A and B). PA increased the apoptosis rate of
MIN6 cells compared with that of the untreated control cells, while
the knockdown of PP2Ac effectively inhibited the PA-induced
increase in the apoptosis rate (Figs.
4C and S3). Previous studies
have shown that caspase 3, Bcl-2 and Bax are important factors in
the endogenous signaling pathway underlying apoptosis (31). Western blotting revealed that
caspase 3 and Bax protein levels were increased, while those of
Bcl-2 were decreased, in MIN6 cells after PA exposure. Compared
with those in the shc cells, the PA-induced changes of protein
expression were alleviated in MIN6 cells with PP2Ac knockdown
(Fig. 4D). These results suggest
that PP2Ac knockdown increased the resistance of MIN6 cells to PA
and reduced apoptosis, which may be associated with inhibition of
the endogenous apoptotic pathway.
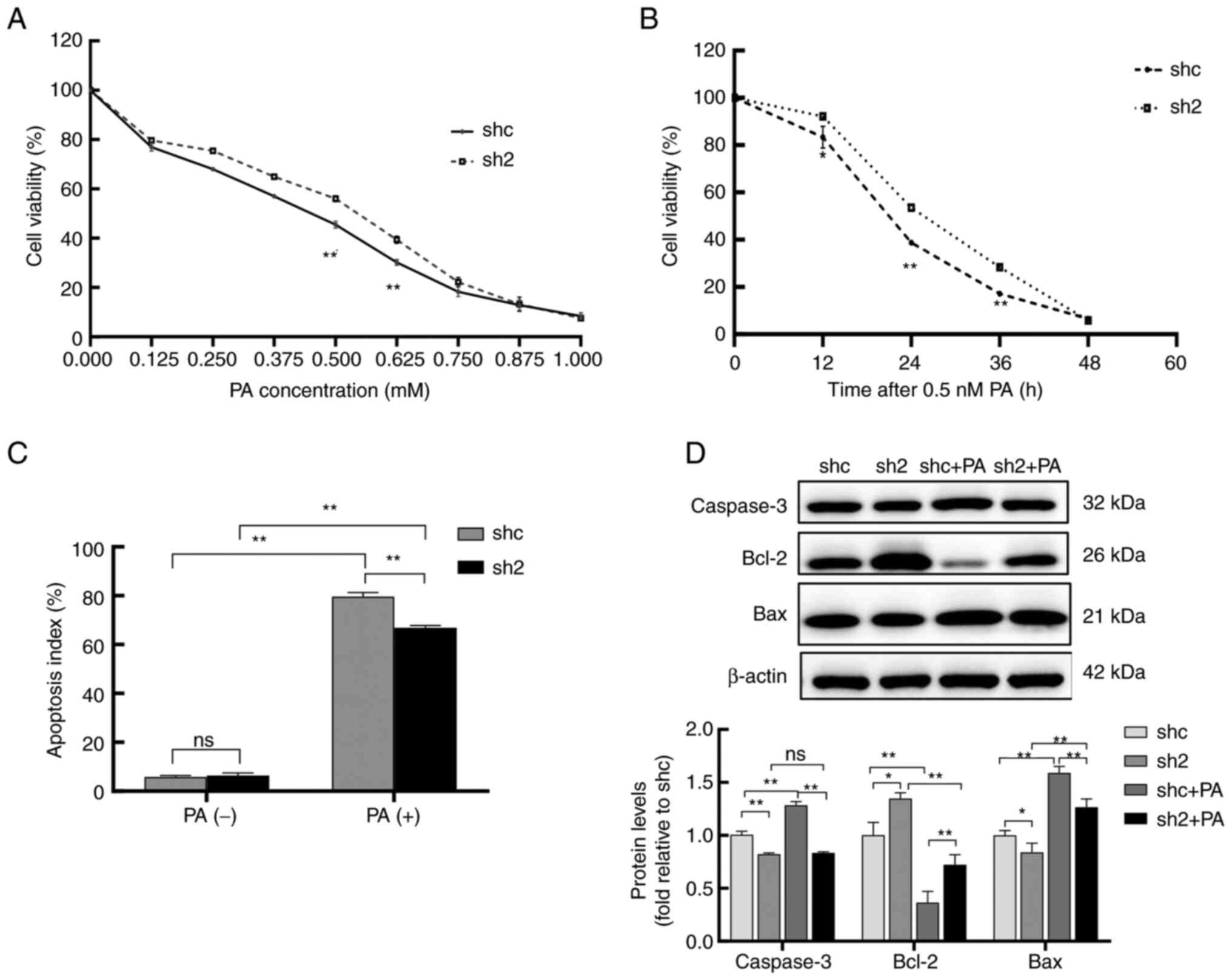 | Figure 4PP2Ac knockdown attenuates PA-induced
apoptosis in MIN6 cells. (A) PP2Ac knockdown increases the
resistance of MIN6 cells to PA; cell viability decreased following
the addition of various concentrations of PA, but the decline in
viability was less in the sh2 group than in the shc group. (B)
Exposure of the cells to 0.5 mM PA for 12, 24, 36 and 48 h, reduced
cell viability, but the cell viability of the sh2 group was always
higher than that of the shc group. (C) Apoptosis of the MIN6 cells
was detected by flow cytometry. (D) Expression levels of the
apoptosis-associated proteins caspase 3, Bcl-2 and Bax in the
transfected MIN6 cells were analyzed by western blotting, using
β-actin as a loading control. All experiments were performed at
least three times, and values are reported as the mean ± standard
deviation. *P<0.05, **P<0.01. PP2Ac,
catalytic subunit of protein phosphatase 2A; PA, palmitate; MIN6,
mouse insulinoma 6; shc, short hairpin RNA control; sh2, PP2AC
short hairpin RNA 2; ns, not significant. |
PP2Ac knockdown is involved in MAPK
pathway changes under lipotoxicity
Sequencing analysis revealed that PP2Ac knockdown
resulted in the upregulation of 1,332 genes and the downregulation
of 1,707 genes compared with those in the shc group. Based on the
results of the KEGG pathway enrichment analysis, the MAPK signaling
pathway was selected for validation (Figs. S4 and S5). Although no significant difference
in the MAPK signaling pathway was detected by mRNA sequencing
analysis, a difference at the protein level was revealed by western
blot analysis; it must be noted that the MIN6 cells were not
treated with PA before sequencing. Western blotting demonstrated
that ERK protein levels were unaffected by PA exposure in both the
shc and sh2 cells, while the PP2Ac knockdown groups exhibited
higher protein levels of ERK than the respective shc groups.
Importantly, PP2Ac knockdown effectively alleviated the reduction
in the phosphorylation levels of ERK caused by PA. Although some
changes in JNK expression were observed, p38 protein levels did not
change significantly in any group following PA treatment or PP2Ac
knockdown, while PP2Ac knockdown attenuated p-JNK levels and the
increase in p-p38 levels induced by PA (Fig. 5). Overall, the results indicate
that PP2Ac knockdown attenuated PA-induced apoptosis, and this may
have occurred through the activation of ERK via the MAPK signaling
pathway, and inhibition of the JNK and p38 pathways.
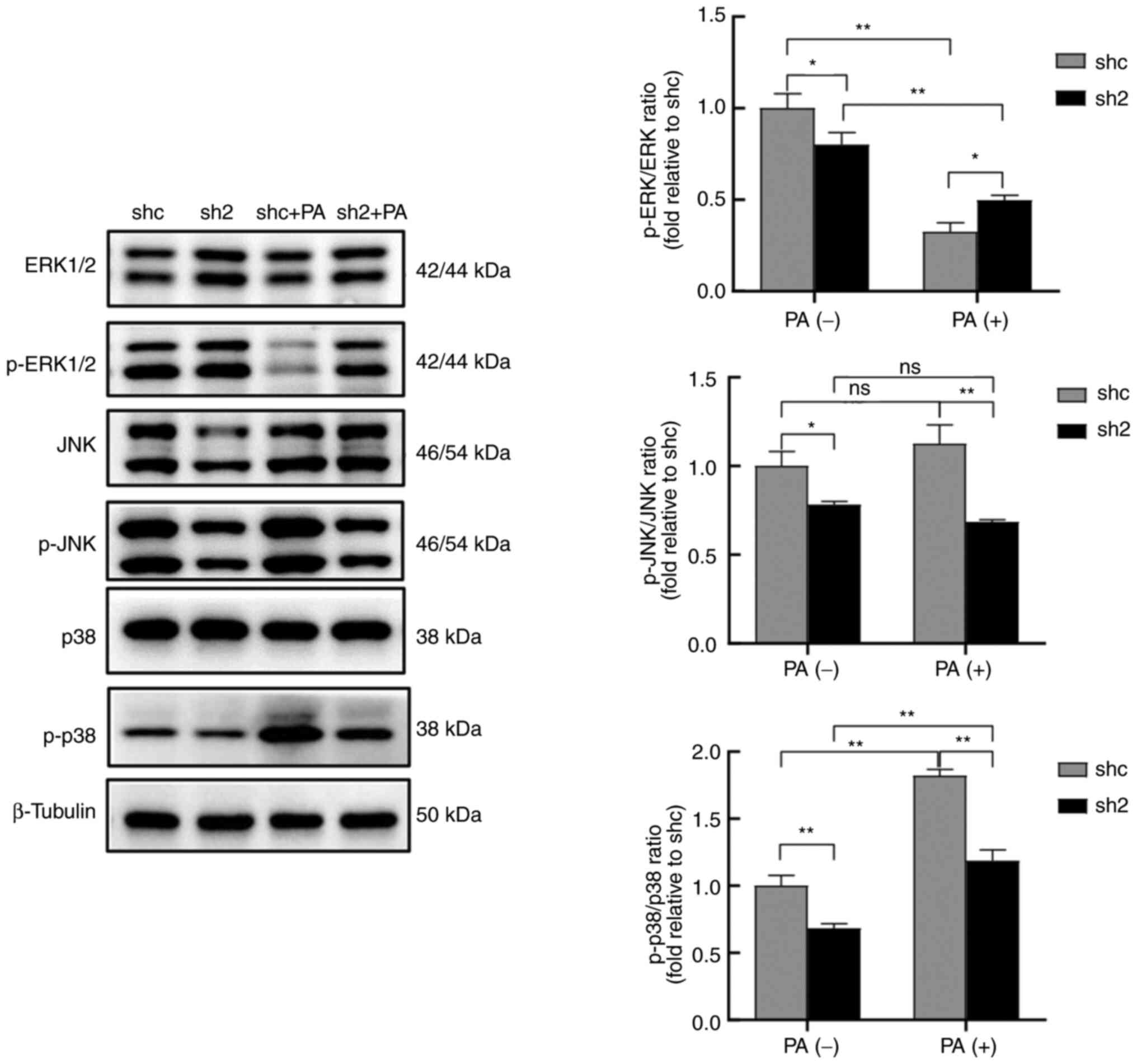 | Figure 5PP2Ac knockdown is associated with
changes to the MAPK pathway in MIN6 cells under lipotoxic
conditions. Protein expression and phosphorylation levels of ERK,
JNK and p38 MAPK pathway proteins in MIN6 cells with or without
PP2Ac knockdown were analyzed by western blotting, using β-tubulin
as a loading control. The ratios of p-ERK/ERK, p-JNK/JNK, p-p38/p38
was analyzed. All experiments were performed at least three times,
and values are reported as the mean ± standard deviation.
*P<0.05, **P<0.01. PP2Ac, catalytic
subunit of protein phosphatase 2A; MIN6, mouse insulinoma 6; PA,
palmitate; shc, short hairpin RNA control; sh2, PP2AC short hairpin
RNA 2; p-, phosphorylated; ns, not significant. |
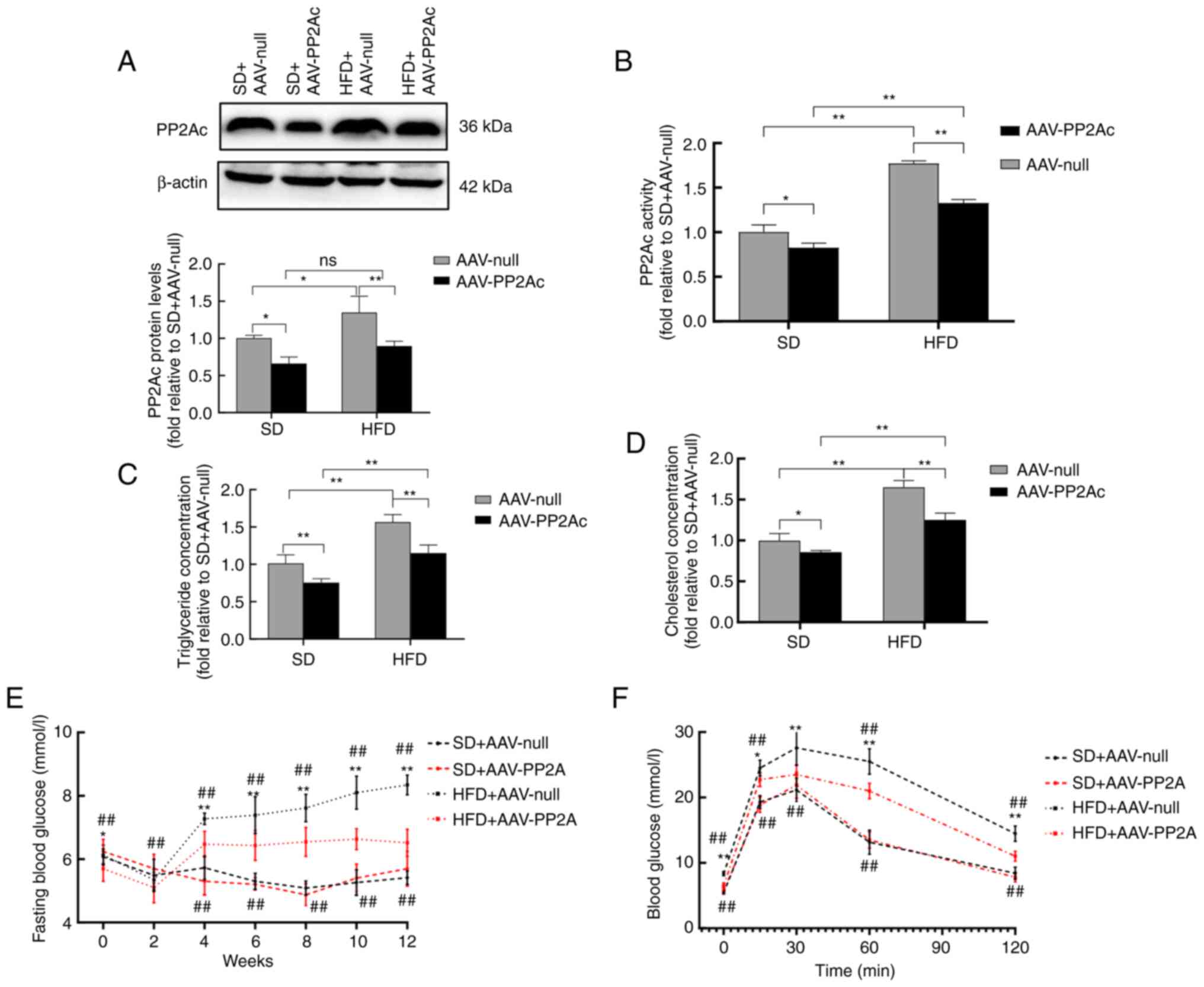
AAVs interfere with PP2Ac gene
expression in mice to improve their metabolic function
To investigate the effect of the PP2Ac gene on
β-cell function in mice, the expression of the PP2Ac gene was
specifically disrupted in pancreatic tissue using an AAV, and mice
were fed with either an SD or a HFD for 12 weeks. Western blotting
results showed that the protein levels of PP2Ac were successfully
reduced in the pancreatic tissues of mice injected with AAV-PP2Ac,
and AAV-PP2Ac significantly inhibited the PP2A activation triggered
by HFD feeding (Fig. 6A and
B). HFD feeding increased the
serum cholesterol and triglyceride levels in mice, while AAV-PP2Ac
treatment reduced the extent of these elevations (Fig. 6C and D). Fasting blood glucose was measured
every 2 weeks in the mice, and the results revealed that
HFD-induced hyperglycemia started in the fourth week, while HFD-fed
mice in the AAV-PP2Ac group maintained lower levels than those in
the AAV-null group (Fig. 6E). In
addition, the IPGTT showed that mice in the AAV-PP2Ac group had
improved glucose tolerance compared with those in the AAV-null
group with HFD feeding (Fig. 6F).
These results indicate that HFD feeding activated PP2A in the
pancreatic tissue, leading to metabolic disorder and impaired
glucose metabolism in mice, while the knockdown of pancreatic PP2Ac
ameliorated these outcomes.
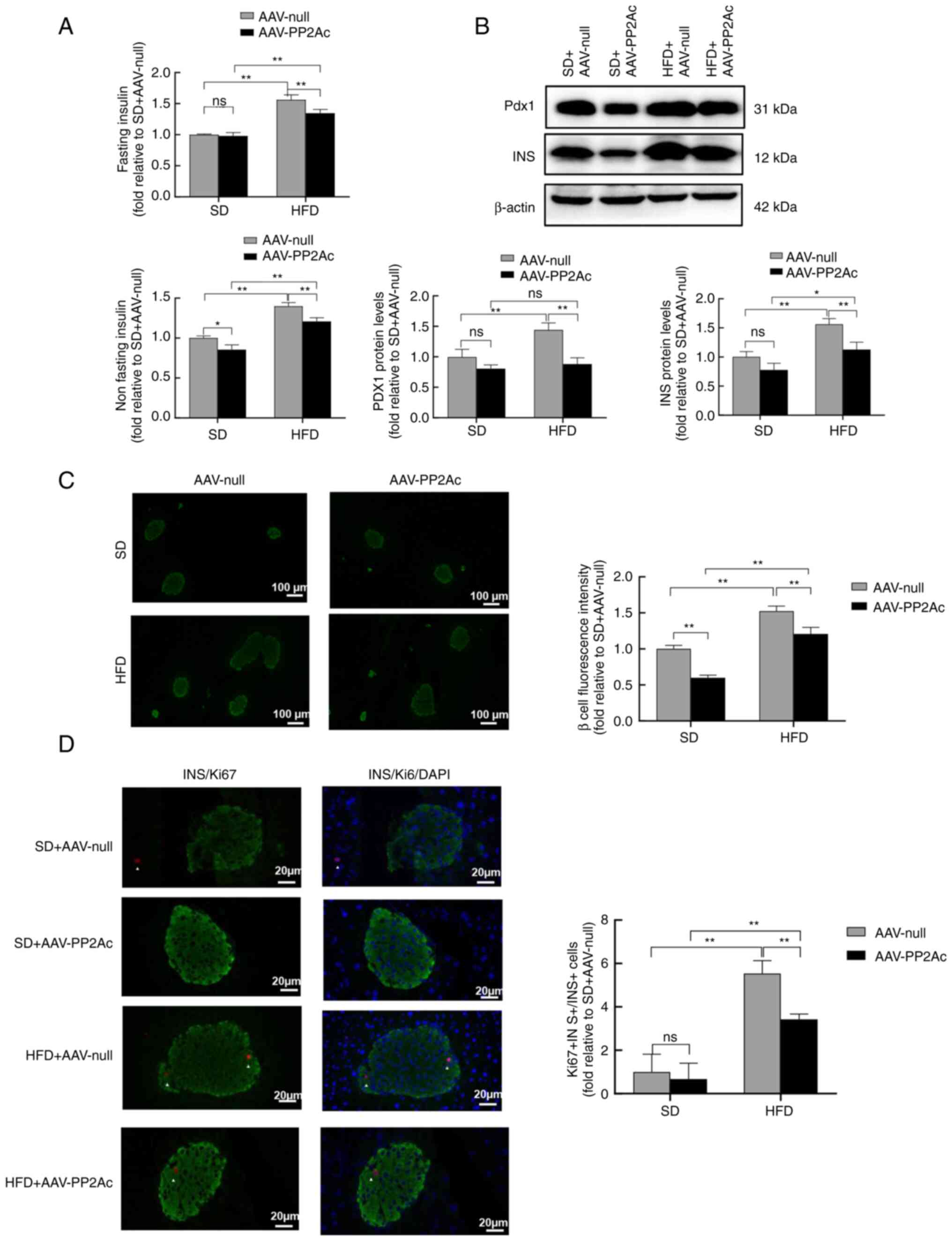
Interference with the PP2Ac gene
attenuates the HFD-induced proliferation of mouse pancreatic
β-cells
To study the effect of HFD on pancreatic β-cell
function in mice, their plasma insulin levels were measured in
fasting and non-fasting states. HFD-fed mice exhibited
hyperinsulinemia and insulin resistance compared with SD-fed mice,
while in the HFD-fed mice the serum insulin levels of the AAV-PP2Ac
mice were significantly reduced compared with those of the AAV-null
mice (Fig. 7A). The western blot
analysis of pancreatic tissue revealed that insulin and Pdx1
protein expression was consistent with this (Fig. 7B). Pancreatic tissues ware
subjected to staining using insulin antibodies for the
identification of β-cells. Evaluation of mouse pancreatic tissue by
immunofluorescence showed an increase in the fluorescence intensity
of pancreatic β-cells in mice fed with a HFD, while the pancreatic
tissue of mice in the AAV-PP2Ac group had a reduced fluorescence
intensity compared with that of the AAV-null group for both SD- and
HFD-fed mice (Fig. 7C). To further
determine the effect of PP2A interference on pancreatic β-cell
proliferation, the mouse pancreatic tissue was subjected to Ki67,
insulin and DAPI costaining, and the percentage increase in
pancreatic β-cell proliferation was calculated. Compared with the
low proliferation rate of pancreatic β-cells in mice fed a SD, the
β-cells exhibited significantly higher proliferation with a HFD in
both AAV-null and AAV-PP2Ac mice, while interfering with PP2Ac gene
expression reduced cell proliferation in the HFD-fed mice (Fig. 7D). These findings suggest that the
HFD induced compensatory pancreatic β-cell proliferation, the
elevation of serum insulin levels and increased insulin resistance
in mice, while the reduction of PP2Ac gene expression effectively
protected pancreatic tissue against the HFD-induced compensatory
proliferation of β-cells.
Discussion
The present study demonstrated that the knockdown of
PP2Ac significantly inhibited PP2A activity, attenuated PA-induced
ER stress, mitochondrial dysfunction and apoptosis, and increased
insulin secretion in MIN6 cells. The specific knockdown of
pancreatic PP2Ac protected against insulin resistance and the
HFD-induced compensatory proliferation of pancreatic β-cells in
mice. Thus, it is hypothesized that PP2Ac may be a novel target for
diabetes treatment.
PP2A is a member of the serine/threonine phosphatase
family, which accounts for ~1% of total cellular proteins and
represents a major class of phosphatases in mammalian cells, the
catalytic action of which is mainly attributable to PP2Ac. Two
common PP2Ac genotypes, PP2Acα and PP2Acβ, have been reported, and
PP2Acα is known to be the major genotype of the two (32). The present study used lentiviral
transfection to interfere with PP2Acα gene expression in MIN6
cells. PP2Ac is closely associated with the development of
diabetes, heart disease, cancer and neurodegenerative diseases
(33). Under glucotoxicity,
lipotoxicity and other physiologically stressful conditions, the
PP2Ac protein is hyperactivated in the pancreas, liver, muscle,
retina and other organs, ultimately leading to the development of
insulin resistance and pancreatic β-cell dysfunction. Notably, PP2A
activity can be restored to near-normal levels either by
interfering with the transcription of the gene encoding PP2Ac or
with the expression of PP2Ac protein (24,34,35).
The present study found that the HFD feeding of mice and the PA
treatment of MIN6 cells induced increases in the PP2A activity of
pancreatic β-cells of ~1.8- and 2.6-fold, respectively, during the
compensatory and decompensated stages of lipotoxicity. Of note,
PP2Ac knockdown reduced PP2A activity and protected pancreatic
β-cells. PA is an abundant saturated fatty acid that is involved in
the lipotoxicity-induced damage of pancreatic β-cells, which leads
to insulin resistance and ultimately, uncompensated T2DM (7,36).
In the present study, exposure to a high glucose concentration (20
mM) stimulated MIN6 cells to produce insulin levels much higher
than those produced under low glucose conditions (2.5 mM),
indicating that the pancreatic β-cells were functioning normally.
Treatment with 0.5 mM PA exacerbated apoptosis and inhibited the
secretion of insulin in pancreatic β-cells, while interference with
PP2Ac expression attenuated the lipotoxicity-induced damage in the
MIN6 cells.
The ER and mitochondria are among most important
organelles required for pancreatic β-cells to carry out their
functions, and they play a crucial role in the synthesis,
processing and secretion of insulin (13,14).
The two organelles closely interact and form the
mitochondria-associated ER membrane, which regulates lipid
synthesis and metabolism, the Ca2+ signaling pathway, ER
stress, mitochondrial function, cell proliferation and apoptosis to
maintain cellular stability (37-40).
A previous study has shown that PA induces apoptosis via the
modulation of multiple pathways, mainly those associated with
oxidative stress, ER stress and mitochondrial dysfunction (41). The inhibition of PP2A activity
achieved through either a reduction in PP2Ac protein levels or
increase in PP2Ac phosphorylation has been shown to attenuate ER
stress in hepatocytes subjected to ischemia-reperfusion injury,
which is suggested to have the potential to protect donor liver
function and improve liver transplant survival (42,43).
In the present study, PP2Ac knockdown was indicated to
significantly reduce the ER stress caused by PA exposure in MIN6
cells, as the protein levels of CHOP and GRP78 were regulated. In
addition, PA exposure has previously been shown to compromise
mitochondrial integrity and function, leading to the significant
production of ROS and a reduction in the mitochondrial membrane
potential (44). PP2Ac knockdown
reduced PA-induced ROS levels and attenuated the PA-induced
reduction in ATP production, indicating that it attenuated the
level of oxidative stress in MIN6 cells. Sustained ER stress and
mitochondrial damage eventually lead to apoptosis (10). This is consistent with the
observation that increasing either the PA concentration or duration
of exposure reduced the viability of MIN6 cells in the present
study, and treatment with PA increased the rate of apoptosis, while
knocking down PP2Ac reduced the rate of cell death. Therefore, it
is speculated that PP2Ac knockdown may reduce the apoptosis of
pancreatic β-cells via the attenuation of ER stress and protection
of the mitochondrial function in MIN6 cells under lipotoxic
conditions.
To investigate the mechanism by which the PP2Ac gene
affects MIN6 cell function and apoptosis, RNA sequencing and KEGG
pathway enrichment analysis were performed, and the MAPK pathway
was selected for immunoblot validation. MAPK family proteins have
been shown to be associated with cell proliferation, apoptosis and
tumor metastasis, and the main MAPK pathways include ERK, JNK and
p38(45). It has been found that
PP2Ac regulates the phosphorylation of proteins in the MAPK
signaling pathway and inhibits the migration of cervical cancer
cells, while the downregulation of PP2Ac expression increases
inflammation in the tumor microenvironment and induces the
infiltration and invasion of pancreatic tumors (46,47).
These findings suggest that PP2A plays an important role in the
inhibition of tumor growth and metastasis and may be a potential
target for cancer therapy, while PP2Ac knockdown can inhibit
apoptosis under specific circumstances. The results of the present
study suggest that knockdown of the PP2Ac gene alleviated the
dysfunction and apoptosis of MIN6 cells under lipotoxic conditions
by activating the ERK signaling pathway and inhibiting the JNK and
p38 pathways.
The current study found that HFD feeding impaired
glucose tolerance and induced insulin resistance in C57BL/6 mice,
leading to the compensatory proliferation of pancreatic β-cells,
but not the decompensated stage. This outcome may be associated
with the use of an insufficient feeding duration or the mouse
strain that was used. In the cell experiments, after exposure to
0.5 mM PA, pancreatic β-cells entered the decompensated stage of
lipotoxicity. Thus, the animal and cellular experiments in the
present effectively demonstrate the role of PP2Ac in pancreatic
β-cells throughout the entire process of lipotoxicity. The current
study suggested that PP2A activity was much higher in the
decompensated stage than in the compensated stage, and that the
knockdown of PP2Ac gene expression protected pancreatic β-cells
from lipotoxicity throughout the process. Notably, PP2Ac knockdown
under normal conditions (without lipotoxicity) has been reported to
inhibit GSIS in pancreatic β-cells (48), which may be due to the low level of
PP2A activity being insufficient to effectively regulate substrate
phosphorylation levels. Therefore, it is hypothesized that the
maintenance of PP2A activity at appropriate levels may sustain
cellular homeostasis, while excessively high or low levels would be
detrimental to cellular function.
In summary, the results of the present study
indicate that PP2Ac knockdown attenuated lipotoxicity-induced ER
stress and mitochondrial dysfunction in pancreatic β-cells, and
protected pancreatic β-cell function, which may have been achieved
via the regulation of PP2A activity. The findings provide a solid
theoretical basis for the clinical treatment of diabetes and offer
new insights for the development of novel drugs targeting PP2Ac in
pancreatic β-cells. However, the study has some limitations, as
follows: Pancreatic-specific gene knockout mice were not used; only
one cell line was used; and the effects of activators and
inhibitors of PP2Ac on MIN6 cell function and proliferation
apoptosis were not investigated. Therefore, future studies should
be designed to overcome these limitations and consolidate the
present findings.
Supplementary Material
Flow cytometric detection of reactive
oxygen species levels in transfected MIN6 cells treated with 0.5 mM
PA or bovine serum albumin for 24 h. The KO525 channel was used to
analyze the MIN6 cells, and representative images are presented.
MIN6, mouse insulinoma 6; PA, palmitate; shc, short hairpin RNA
control; sh2, protein phosphatase 2A short hairpin RNA 2.
Flow cytometric detection of the
mitochondrial membrane potential of transfected MIN6 cells treated
with 0.5 mM PA or bovine serum albumin for 24 h. The PE channel was
used to analyze the MIN6 cells, and representative images are
presented. MIN6, mouse insulinoma 6; PA, palmitate; shc, short
hairpin RNA control; sh2, protein phosphatase 2A short hairpin RNA
2.
Flow cytometric detection of the level
of apoptosis in transfected MIN6 cells treated with 0.5 mM PA or
bovine serum albumin for 24 h. APC and PE dual channels were used
to analyze the MIN6 cells, and representative images are presented.
MIN6, mouse insulinoma 6; PA, palmitate; shc, short hairpin RNA
control; sh2, protein phosphatase 2A short hairpin RNA 2.
Volcano plot showing the up. and
downregulation of genes detected by the mRNA sequencing analysis of
MIN6 cells with PP2A knockdown. MIN6, mouse insulinoma 6; group,
MIN6 cells transfected with PP2A shRNA; control, MIN6 cells
transfected with short hairpin control; padj, adjusted
P-value.
Kyoto Encyclopedia of Genes and
Genomes pathway enrichment analysis of MIN6 cells with protein
phosphatase 2A knockdown. The MAPK pathway was selected for
verification by western blotting of the MIN6 cells. MIN6, mouse
insulinoma 6; padj, adjusted P-value.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81970718).
Availability of data and materials
The RNA sequencing datasets generated and/or
analyzed during the current study are available in the GEO
repository (https://www.ncbi.nlm.nih.gov/gds/?term=GSE242538).
The other datasets used and/or analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ, BT and YX conceived, designed and carried out
the study. JF, LS, HW, JL, CX and MK contributed to performing the
experiments. ZZ, JL and BT conducted data analysis and drafted the
manuscript. ZZ and YX revised the manuscript. ZZ, BT and YX confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by The Ethics and
Scientific Review Board of the Zhongnan Hospital of Wuhan
University (Wuhan, China; approval no. ZN2022037).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Amanat S, Ghahri S, Dianatinasab A,
Fararouei M and Dianatinasab M: Exercise and type 2 diabetes. Adv
Exp Med Biol. 1228:91–105. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sun H, Saeedi P, Karuranga S, Pinkepank M,
Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et
al: IDF Diabetes atlas: Global, regional and country-level diabetes
prevalence estimates for 2021 and projections for 2045. Diabetes
Res Clin Pract. 183(109119)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xiong QY, Yu C, Zhang Y, Ling L, Wang L
and Gao JL: Key proteins involved in insulin vesicle exocytosis and
secretion. Biomed Rep. 6:134–139. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rutter GA, Pullen TJ, Hodson DJ and
Martinez-Sanchez A: Pancreatic β-cell identity, glucose sensing and
the control of insulin secretion. Biochem J. 466:203–218.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Klein S, Gastaldelli A, Yki-Järvinen H and
Scherer PE: Why does obesity cause diabetes? Cell Metab. 34:11–20.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Oh YS, Bae GD, Baek DJ, Park EY and Jun
HS: Fatty acid-induced lipotoxicity in pancreatic beta-cells during
development of type 2 diabetes. Front Endocrinol (Lausanne).
9(384)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ertunc ME and Hotamisligil GS: Lipid
signaling and lipotoxicity in metaflammation: Indications for
metabolic disease pathogenesis and treatment. J Lipid Res.
57:2099–2114. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Lin S, Long H, Hou L, Zhang M, Ting J, Lin
H, Zheng P, Lei W, Yin K and Zhao G: Crosstalk between endoplasmic
reticulum stress and non-coding RNAs in cardiovascular diseases.
Wiley Interdiscip Rev RNA. 14(e1767)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hamacher-Brady A and Brady NR: Mitophagy
programs: Mechanisms and physiological implications of
mitochondrial targeting by autophagy. Cell Mol Life Sci.
73:775–795. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Roszczyc-Owsiejczuk K and Zabielski P:
Sphingolipids as a culprit of mitochondrial dysfunction in insulin
resistance and type 2 diabetes. Front Endocrinol (Lausanne).
12(635175)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thivolet C, Vial G, Cassel R, Rieusset J
and Madec AM: Reduction of endoplasmic reticulum-mitochondria
interactions in beta cells from patients with type 2 diabetes. PLoS
One. 12(e0182027)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ježek P, Holendová B, Jabůrek M, Dlasková
A and Plecitá-Hlavatá L: Contribution of mitochondria to insulin
secretion by various secretagogues. Antioxid Redox Signal.
36:920–952. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Carta G, Murru E, Banni S and Manca C:
Palmitic acid: Physiological role, metabolism and nutritional
implications. Front Physiol. 8(902)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lytrivi M, Castell AL, Poitout V and Cnop
M: Recent insights into mechanisms of β-cell Lipo- and
glucolipotoxicity in type 2 diabetes. J Mol Biol. 432:1514–1534.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Raman D and Pervaiz S: Redox inhibition of
protein phosphatase PP2A: Potential implications in oncogenesis and
its progression. Redox Biol. 27(101105)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Reynhout S and Janssens V: Physiologic
functions of PP2A: Lessons from genetically modified mice. Biochim
Biophys Acta Mol Cell Res. 1866:31–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ronk H, Rosenblum JS, Kung T and Zhuang Z:
Targeting PP2A for cancer therapeutic modulation. Cancer Biol Med.
19:1428–1439. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Binder P, Wang S, Radu M, Zin M, Collins
L, Khan S, Li Y, Sekeres K, Humphreys N, Swanton E, et al: Pak2 as
a novel therapeutic target for cardioprotective endoplasmic
reticulum stress response. Circ Res. 124:696–711. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang L, Jin GH and Zhou JY: The role of
ceramide in the pathogenesis of alcoholic liver disease. Alcohol
Alcohol. 51:251–257. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Theurey P, Tubbs E, Vial G, Jacquemetton
J, Bendridi N, Chauvin MA, Alam MR, Le Romancer M, Vidal H and
Rieusset J: Mitochondria-associated endoplasmic reticulum membranes
allow adaptation of mitochondrial metabolism to glucose
availability in the liver. J Mol Cell Biol. 8:129–143.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen X, Chen S, Shen T, Yang W, Chen Q,
Zhang P, You Y, Sun X, Xu H, Tang Y, et al: Adropin regulates
hepatic glucose production via PP2A/AMPK pathway in
insulin-resistant hepatocytes. FASEB J. 34:10056–10072.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Arora DK, Machhadieh B, Matti A, Wadzinski
BE, Ramanadham S and Kowluru A: High glucose exposure promotes
activation of protein phosphatase 2A in rodent islets and INS-1
832/13 β-cells by increasing the posttranslational
carboxylmethylation of its catalytic subunit. Endocrinology.
155:380–391. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Y, Zhang QY, Sun BF, Ma Y, Zhang Y,
Wang M, Ma C, Shi H, Sun Z, Chen J, et al: Single-cell
transcriptome profiling of the vaginal wall in women with severe
anterior vaginal prolapse. Nat Commun. 12(87)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kanehisa M: Toward understanding the
origin and evolution of cellular organisms. Protein Sci.
28:1947–1951. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dong WW, Feng Z, Zhang YQ, Ruan ZS and
Jiang L: Potential mechanism and key genes involved in mechanical
ventilation and lipopolysaccharide-induced acute lung injury. Mol
Med Rep. 22:4265–4277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Deng S, Yang L, Ma K and Bian W:
Astragalus polysaccharide improve the proliferation and insulin
secretion of mouse pancreatic β-cells induced by high glucose and
palmitic acid partially through promoting miR-136-5p and miR-149-5p
expression. Bioengineered. 12:9872–9884. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wee J, Pak S, Kim T, Hong GS, Lee JS, Nan
J, Kim H, Lee MO, Park KS and Oh U: Tentonin 3/TMEM150C regulates
glucose-stimulated insulin secretion in pancreatic β-cells. Cell
Rep. 37(110067)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Janssens V and Goris J: Protein
phosphatase 2A: A highly regulated family of serine/threonine
phosphatases implicated in cell growth and signalling. Biochem J.
353:417–439. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Baskaran R and Velmurugan BK: Protein
phosphatase 2A as therapeutic targets in various disease models.
Life Sci. 210:40–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kowluru A and Matti A: Hyperactivation of
protein phosphatase 2A in models of glucolipotoxicity and diabetes:
Potential mechanisms and functional consequences. Biochem
Pharmacol. 84:591–597. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kowluru A: Potential roles of PP2A-Rac1
signaling axis in pancreatic β-cell dysfunction under metabolic
stress: Progress and promise. Biochem Pharmacol.
180(114138)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Acosta-Montaño P and García-González V:
Effects of dietary fatty acids in pancreatic beta cell metabolism,
implications in homeostasis. Nutrients. 10(393)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Madec AM, Perrier J, Panthu B and
Dingreville F: Role of mitochondria-associated endoplasmic
reticulum membrane (MAMs) interactions and calcium exchange in the
development of type 2 diabetes. Int Rev Cell Mol Biol. 363:169–202.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee S and Min KT: The interface between ER
and mitochondria: Molecular compositions and functions. Mol Cells.
41:1000–1007. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Szymański J, Janikiewicz J, Michalska B,
Patalas-Krawczyk P, Perrone M, Ziółkowski W, Duszyński J, Pinton P,
Dobrzyń A and Więckowski MR: Interaction of mitochondria with the
endoplasmic reticulum and plasma membrane in calcium homeostasis,
lipid trafficking and mitochondrial structure. Int J Mol Sci.
18(1576)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tubbs E and Rieusset J: Metabolic
signaling functions of ER-mitochondria contact sites: Role in
metabolic diseases. J Mol Endocrinol. 58:R87–R106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu D, Liu L, Zhao Y, Yang L, Cheng J, Hua
R, Zhang Z and Li Q: Melatonin protects mouse testes from palmitic
acid-induced lipotoxicity by attenuating oxidative stress and DNA
damage in a SIRT1-dependent manner. J Pineal Res.
69(e12690)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lan J, Zhong Z, Wang Y, Xiong Y and Ye Q:
Endoplasmic reticulum stress induces liver cells apoptosis after
brain death by suppressing the phosphorylation of protein
phosphatase 2A. Mol Med Rep. 21:567–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xiong Y, Lan J, Huang K, Zhang Y, Zheng L,
Wang Y and Ye Q: PP2Ac upregulates PI3K-Akt signaling and induces
hepatocyte apoptosis in liver donor after brain death. Apoptosis.
24:921–933. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li N, Frigerio F and Maechler P: The
sensitivity of pancreatic beta-cells to mitochondrial injuries
triggered by lipotoxicity and oxidative stress. Biochem Soc Trans.
36:930–934. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zheng HY, Shen FJ, Tong YQ and Li Y: PP2A
Inhibits cervical cancer cell migration by dephosphorylation of
p-JNK, p-p38 and the p-ERK/MAPK signaling pathway. Curr Med Sci.
38:115–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tao M, Liu L, Shen M, Zhi Q, Gong FR, Zhou
BP, Wu Y, Liu H, Chen K, Shen B, et al: Inflammatory stimuli
promote growth and invasion of pancreatic cancer cells through
NF-κB pathway dependent repression of PP2Ac. Cell Cycle.
15:381–393. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jangati GR, Veluthakal R, Susick L, Gruber
SA and Kowluru A: Depletion of the catalytic subunit of protein
phosphatase-2A (PP2Ac) markedly attenuates glucose-stimulated
insulin secretion in pancreatic beta-cells. Endocrine. 31:248–253.
2007.PubMed/NCBI View Article : Google Scholar
|