Introduction
Seawater drowning is a main cause of outdoor death
all over the world. Seawater aspiration can lead to acute lung
injury (ALI) (1-3).
Our previous study found that inflammatory cells can infiltrate in
alveolar after the pulmonary microvascular endothelial cells
injured and then the edema fluid forms in alveolar cells (4). In addition, seawater aspiration
induced apoptosis of endothelial cells is a major cause of
endothelial barrier injury (5-7).
In alveolar endothelial cells, studies have
confirmed that there is a local angiotensin (ANG) system existing
(8,9). This system contain ANGII and its
counter regulatory axis angiotensin converting enzyme
(ACE)-2/ANG1-7/Mas (receptor of ANG1-7). The local ANG system
[ACE-2/ANG1-7/Mas axis and ANGII/angiotensin II receptor type 1
(AT1)] play an important role in apoptosis (10-13).
AT1 inhibitor can decrease apoptosis of alveolar epithelial cells
in bleomycin-induced pulmonary fibrosis. However, the
ACE-2/ANG1-7/Mas axis shows significant anti-apoptotic effects in
pulmonary fibrosis (14-17).
MicroRNAs are a class of small noncoding RNAs.
Previous studies have revealed that miR-200c-3p is a biomarker for
predicting the treatment outcome and mortality in sepsis-induced
ALI (18,19). In addition, previous study defined
miR-200c-3p as an endogenous inhibitor of ACE2 (19,20).
However, its role and underlying mechanism in the seawater
aspiration-induced ALI remain to be elucidated. The present study
aimed to clarify the effects of miR-200c-3p/ACE2/ANG1-7 axis on
seawater aspiration induced ALI.
Materials and methods
Animals
Sprague-Dawley (SD) rats (male, 5-7 weeks old;
200±20 g; n=32) were provided by Animal Center of Fourth Military
Medical University. The rats were kept in a temperature-controlled
house (temperature 20-26˚C; humidity 50-60%) with free access to
standard laboratory diet and water ad libitum and a 12-h
light/dark cycle. All the animal experiments were approved by the
Animal Care and Use Committee of the Fourth Military Medical
University (approval no. 2023-66378) and in accordance with the
Declaration of the National Institutes of Health Guide for Care and
Use of Laboratory Animals (Publication No. 85-23, revised 1985)
(21). First, rats were
anesthetized by pentobarbital sodium (50 mg/kg intraperitoneally).
Then a 1 cm syringe was gently inserted into the trachea until 1.5
cm above the carina after exposing the trachea. Next, seawater (4
ml/kg) was instilled into the lung with steady speed in 4 min. Rats
were intravenously treated with miR-200c-3p antagomir (80
mg/kg/day) or the negative controls (antagomir negative control and
antagomir NC for miR-200c-3p antagomir; synthesized by Sangon
Biotech Co., Ltd.) for 3 consecutive days before the seawater
operation. After 4 h of seawater stimulation, the rats were
exsanguinated by aortic transection (4). Then, thorax was rapidly opened and
lungs were processed in the manner described below. Each group
(Normal group; Seawater group; Seawater + miR-200c-3p antagomir
group; and Seawater + antagomir NC group) contained 8 rats.
Drug and reagents
Seawater (osmolality 1300 mmol/l, pH 8.2, SW 1.05,
NaCl 6.518 g/l, MgSO4 3.305 g/l, MgCl2 2.447
g/l, CaCl2 1.141 g/l, KCl 0.725 g/l, NaHCO3
0.202 g/l, NaBr 0.083 g/l) was prepared according to the major
composition of the East China Sea provided by the Chinese Ocean
Bureau.(https://www.nmdis.org.cn/). ELISA kits
for ANGII (cat. no. DANG20) and ANG1-7 (cat. no. 1562/1) were
obtained from R&D Systems. ANG1-7 and A779 (Mas antagonist)
were purchased from GenScript. AT1 antagonist saralasin (SAR) was
purchased from MilliporeSigma. Anti-cleaved-caspase3, anti-Bax,
anti-Bcl-2, anti-ACE-2 and anti-CD31 antibodies were purchased from
Cell Signaling Technology, Inc. Anti-β-actin antibody was purchased
from Santa Cruz Biotechnology, Inc. The binding relationship of
miR-200c-3p and ACE2 was determined from TargetScan (https://www.targetscan.org/vert_80/).
Lung wet/dry (W/D) ratios
Lung tissue of the same part (the whole right lung)
of each rat was taken and the wet weight of the lung tissue was
immediately weighed after wiping off the blood stains. The lung
tissue sample was placed in a 50˚C drying oven for 72 h to constant
weight and the wet-dry ratio calculated.
Evans blue dying
The Evans blue method was used to detect the
permeability of lung tissue. Evans blue solution (20 mg/kg) was
injected into the vein 30 min before anesthesia. After the
experiment, normal saline was injected into the right ventricular
lavage of rats until the left atrial outflow became clear. The
middle lobe of the right lung was removed and dried at 60˚C for 72
h, then soaked in polyformaldehyde at room temperature for 24 h to
extract the Evans blue. The concentration of Evans blue in the
supernatant was measured at 620 nm by spectrophotometer. Evans blue
(µg/g tissue) was calculated against the generated Evans blue
standard absorbance curves.
Primary rat pulmonary micro vascular
cells (RPMVECs) isolation, culture and treatment
First, the outer edges of fresh rat lung lobe were
cut off. Then, 1.5 mm3 specimens of tissue cut from the
lung outer edges were carefully plated into cell culture dishes
(containing DMEM supplemented with 20% FBS, 25 µg/ml of endothelial
cell growth supplement and 100 U/ml of penicillin-streptomycin;
purchased from Sangon Biotech Co., Ltd.) at 37˚C with 5%
CO2 and 95% air. The residue specimens were removed
after 60 h. The cells were passed when a cell monolayer was
achieved (Fig. S1A). In some of
these experiments, RPMVECs were pre-treated with 50 µg/ml SAR,
10-7M ANG1-7 and 10-7M A779 for 2 h before
stimulation. In addition, after incubated (37˚C) in the presence or
absence of miR-200c-3p antagomir (100 nmol/l) for 48 h, seawater
(0.25 ml per 1 ml total volume) were added to cells for 4 h.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from the cells (7,000
cells/cm2) with TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instruction. The purity and concentration of the RNA was analyzed
using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher
Scientific, Inc.) at an optical density of 260/280 nm. Total RNA
was reverse transcribed into cDNA using random primers from the
Transcriptor First Strand cDNA Synthesis kit (Takara Biotechnology
Co., Ltd.). Amplification and detection were carried out by using
Bio-Rad My iQ detection system (Edinburgh Biological Science and
Technology Development co. Berkeley, CA, USA). SYBR GREEN Mastermix
(Takara Bio, Inc.) fluorophore was used for the qPCR. The
thermocycling conditions were: Initial denaturation at 95˚C for 10
min; followed by 40 cycles of 95˚C for 10 sec, 60˚C for 60 sec and
95˚C for 15 sec. The relative expression levels were quantified
using the 2-∆∆Cq method (13), using U6 as the controls to
normalize the expression levels of mRNAs and miRNAs, respectively
(4). The sequences of the rat
miR-200c-3p primers (linear polyA tailed addition method) were
5'-TAATACTGCCGGGTAATGATG-3' (forward);
5'-CAGTGCAGGGTCCGAGGTCAGAGCCACCTGGGCAATTTTTTTTTTTVN-3' (reverse;
universal). The sequences of the U6 primers were
5'-GGAACGATACAGAGAAGATTAGC-3' (forward); 5'-CAGTGCAGGGTCCGA
GGTCAGAGCCACCTGGGCAATTTTTTTTTTTVN-3' (reverse; universal)
ELISA analysis
ANGII and ANG1-7 obtained from lung tissue
supernatant and culture medium were detected with ELISA kits
according to the manufacturer's protocol. The concentration of
ANGII and ANG1-7 was detected in each sample by microplate reader
at 450 nm.
Dual-luciferase reporter assay
RPMVECs were seeded into 24-well plates and after 24
h incubation the confluence reached 60-70%. Wild type (WT) 3'-UTR
of ACE2 and mutant (MT) 3'-UTR of ACE2 reporter plasmids were
constructed in advance. According to the manufacturer's
instruction, cells were transiently co-transfected with miR-200c-3p
mimics or NC mimics together with 0.1 µg reporter plasmids using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After incubation at 37˚C for 48 h,
Dual-luciferase Reporter Assay System (cat. no. E1910; Promega
Corporation) was used to detect firefly and Renilla
luciferase activities and imaged using GloMax 96 Microplate
Luminometer (Promega Corporation).
Western blot analysis
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Proteins were
quantified using the BCA method. The samples were extracted by
centrifugation at 12,000 x g for 20 min at 4˚C. Then 30 µg proteins
were boiled in loading buffer, separated by 10% SDS-polyacrylamide
gels, electrotransferred to nitrocellulose membranes and washed
with 5% non-fat milk in TBST for 1 h at 4˚C (0.1% Tween). The
membrane was incubated overnight at 4˚C with rabbit monoclonal
antibodies for β-actin (1:5,000 dilution; cat. no. sc-47778, Santa
Cruz Biotechnology, Inc.), cleaved-caspase3 (1:1,000 dilution; cat.
no. 9579 Cell Signaling Technology, Inc.), Bax (1:1,000 dilution;
cat. no. 41162 Cell Signaling Technology, Inc.), Bcl-2 (1:1,000
dilution; cat. no. 4223 Cell Signaling Technology, Inc.), ACE-2
(1:1,000 dilution; cat. no. 92485 Cell Signaling Technology, Inc.).
Following the primary antibody incubation, the membranes were
washed with TBST and incubated with HRP conjugated secondary
antibodies goat anti-rabbit (1:1,000; cat. no. GTX213110-01;
GeneTex International Corporation) for 2 h at 4˚C. The membrane was
incubated with the secondary antibody and the relative content of
proteins were tested with chemiluminescent (ECL) detection system
(Beyotime Institute of Biotechnology). The band intensity was
analyzed using ImageJ (1.5.0) software (National Institutes of
Health).
Immunofluorescence (IF) method
IF assays were conducted to determine the cellular
location of CD31 protein expression (Fig. S1C). Cells were seeded on to cover
slips at 5x104/ml density and then fixed in 4%
paraformaldehyde (Beyotime Institute of Biotechnology) for 15 min
at 37˚C, permeabilized using 0.1% Triton X-100 (Beyotime Institute
of Biotechnology) for 20 min and blocked using 5% bovine serum
albumin (MilliporeSigma) for 1 h at 37˚C. Heat-mediated antigen
retrieval was performed with Tris/EDTA buffer (Gibco; Thermo Fisher
Scientific, Inc.). IF was performed with CD31 (5 µg/ml; cat. no.
77699; Cell Signaling Technology, Inc.) antibody for 12 h at 4˚C in
the dark, followed by incubation with Alexa Fluor 488 conjugated,
goat anti-rabbit IgG (1:1,000; cat. no. Ab150077; Abcam.). A total
of five fluorescence images were captured using a fluorescence
microscope (Leica DMi8; Leica Microsystems GmbH) with different
excitation wavelengths for the same field (Alexa Fluor 488 maximum
emission is 518 nm).
Statistical analysis
All data were expressed with mean ± SD. The
statistical significance of the differences between the groups was
determined using GraphPad Pro Prism 6.0 (GraphPad Software;
Dotmatics). Mann-Whitney U-test or one-way ANOVA was used to
compare the differences between groups (Tukey's was used as a post
hoc test). P<0.05 was considered to indicate a statistically
significant difference. The binding relationship of miR-200c-3p and
ACE2 were used Biological website TargetScan (https://www.targetscan.org/vert_80/).
Results
Effects of seawater stimulation on the
expression of miR-200c-3p
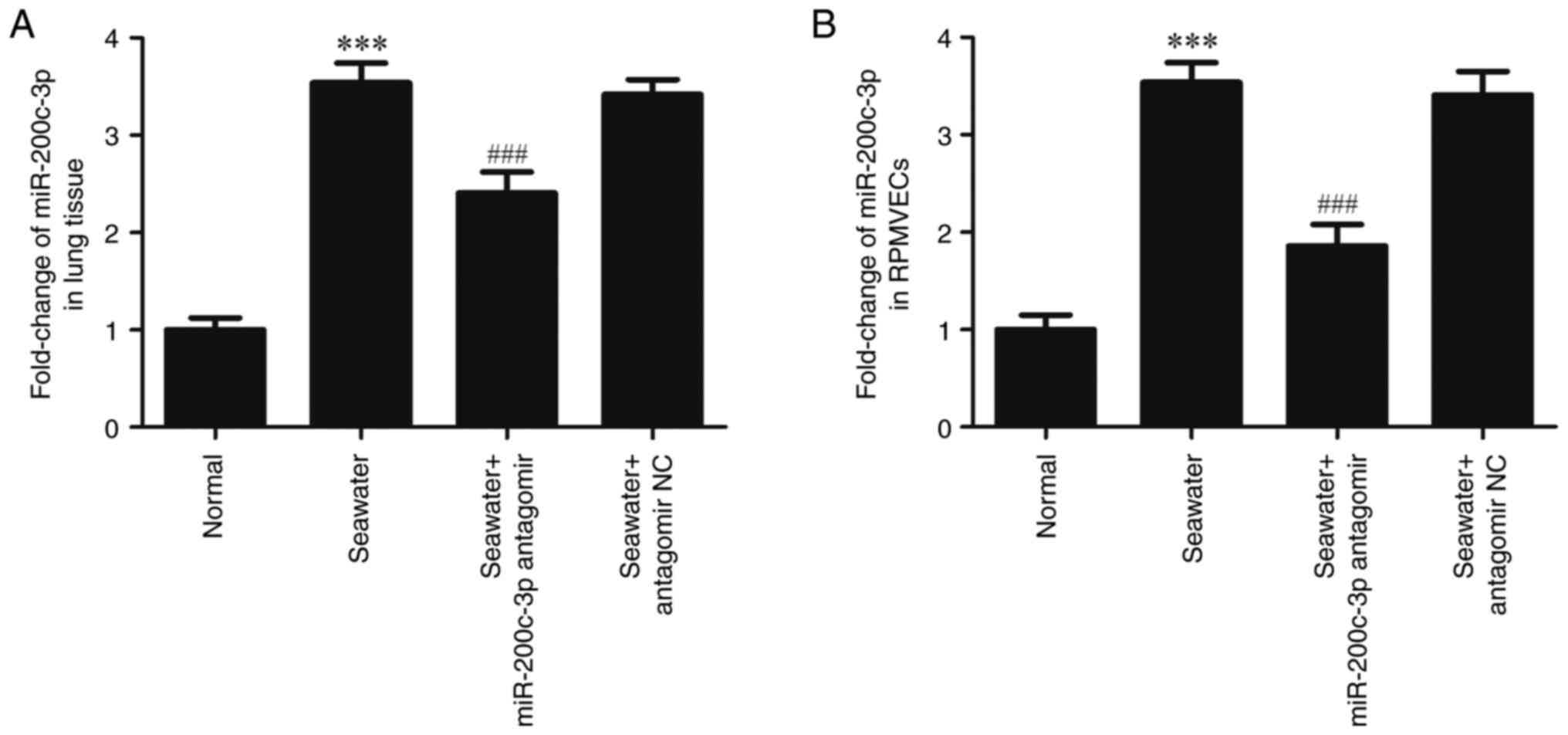
As detected by RT-qPCR, the expression of
miR-200c-3p was significantly upregulated both in lung tissue
(Fig. 1A) and RPMVECs (Fig. 1B) following seawater stimulation.
However, the expression of miR-200c-3p was inhibited following the
miR-200c-3p antagomir (miR-200c-3p inhibitor) administration
(P<0.001).
Effects of miR-200c-3p inhibitor on
the seawater induced lung edema and vascular leakage in lung
tissue
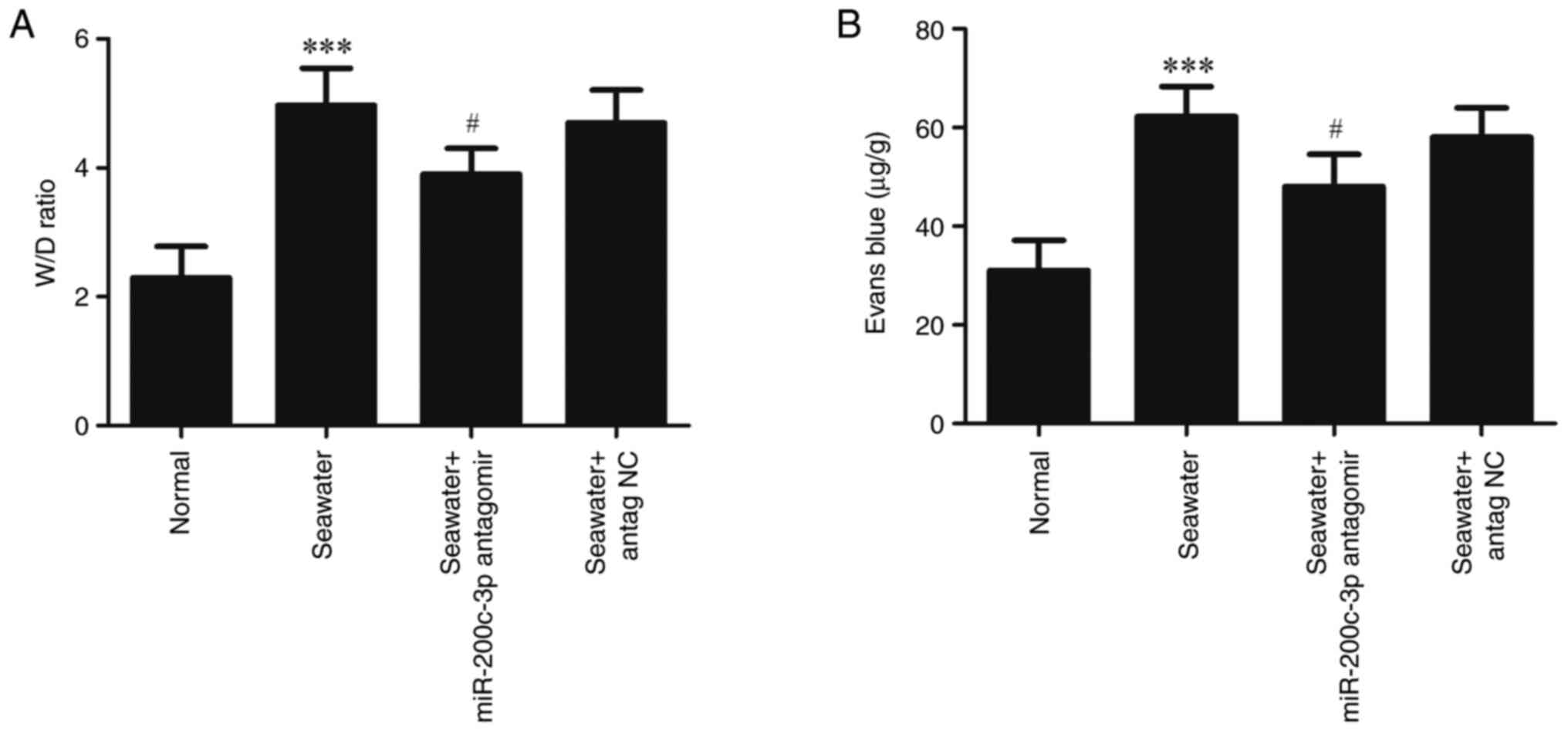
To evaluate lung edema and vascular leakage, the W/D
weight ratios and leak index of Evans blue were measured (Fig. 2). Compared with the Normal group,
seawater administration significantly increased the W/D ratios and
Evans blue leakage (P<0.001). Administration of miR-200c-3p
antagomir significantly suppressed the edema and vascular
leakage.
Effects of miR-200c-3p inhibitor on
apoptosis following seawater stimulation in lung tissue and
RPMVECs
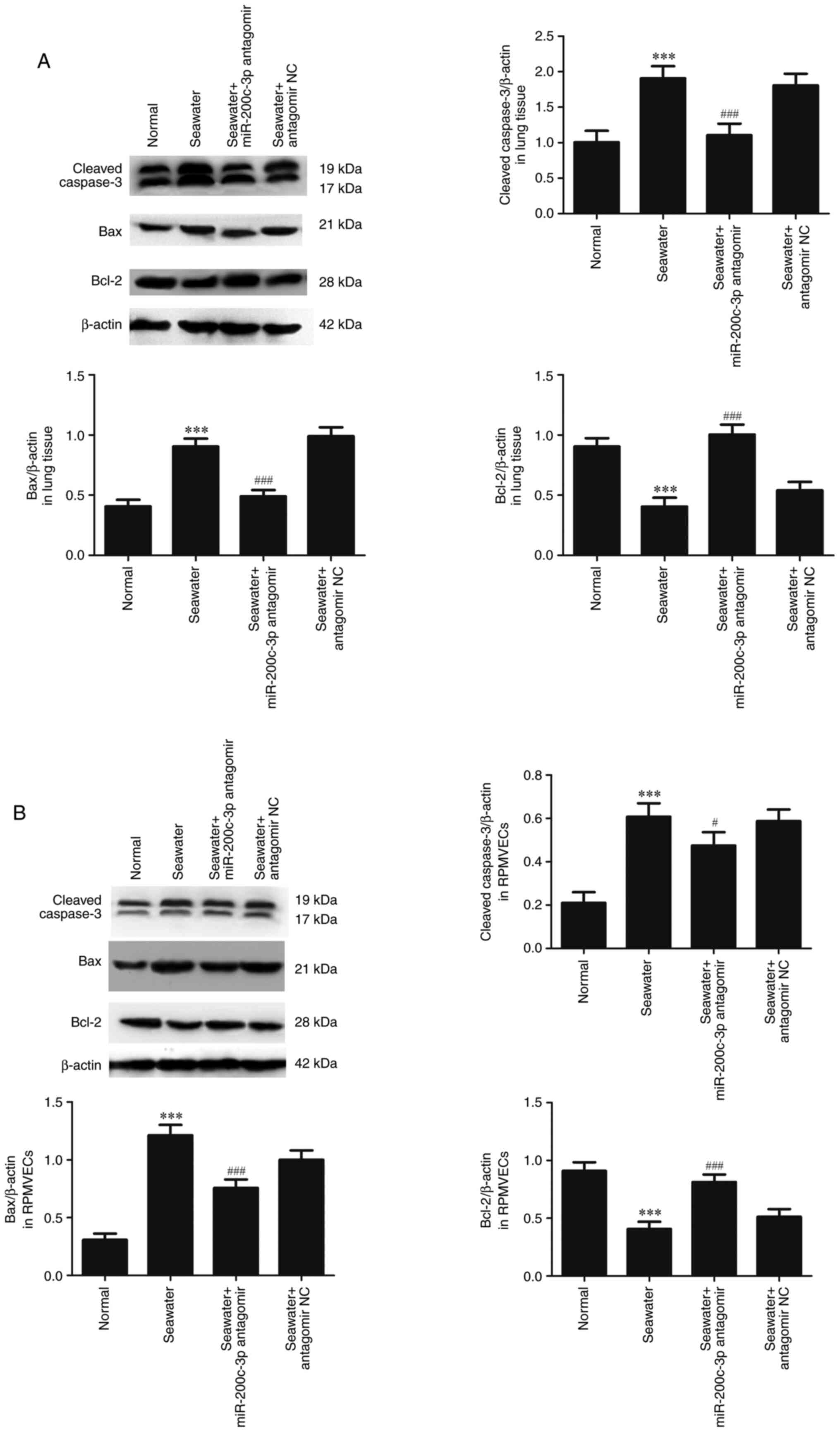
The capacity of miR-200c-3p inhibitor on apoptosis
following seawater stimulation in lung tissue (Fig. 3A) and RPMVECs (Fig. 3B) was further assessed. Seawater
administration promoted the expression of cleaved-caspase3 and Bax
and decreased the expression of anti-apoptosis protein Bcl-2.
However, pretreatment with miR-200c-3p antagomir significantly
reduced the expression of cleaved caspase3 and Bax, and increased
the expression of Bcl-2 both in lung tissue and RPMVECs.
ACE2 is directly targeted by
miR-200c-3p
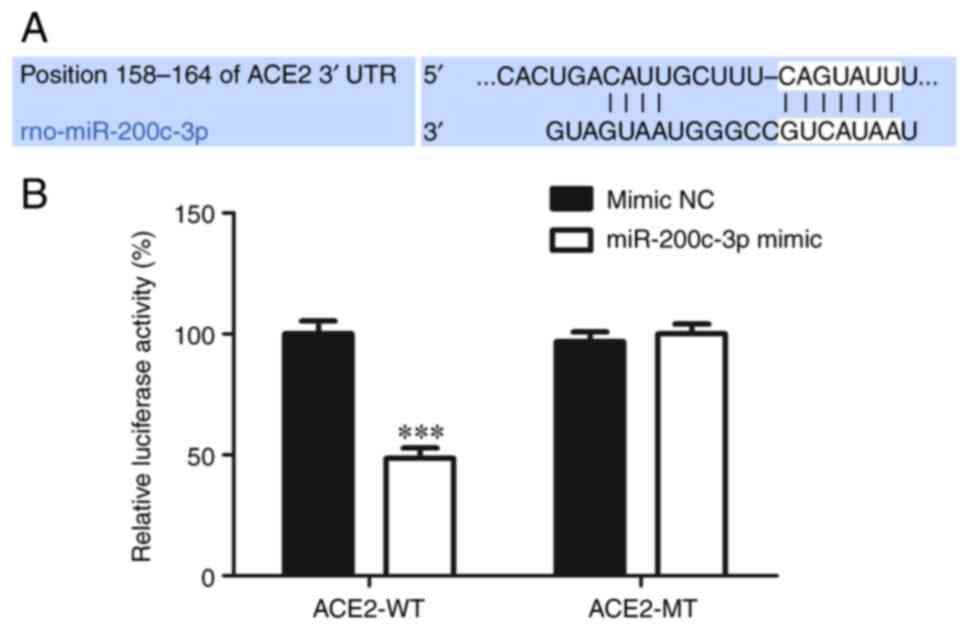
Biological website TargetScan (https://www.targetscan.org/vert_80/) revealed
that miR-200c-3p could bind to ACE2 (Fig. 4A). Dual luciferase reporter gene
assay was used to confirm this and the result showed that
miR-200c-3p mimic clearly inhibited the luciferase activity of the
reporter containing the WT 3'-UTR of ACE2 compared to mimic NC,
However, the luciferase activity change was not detected in the MT
3'-UTR of ACE2 group (Fig. 4B),
indicating that ACE2 was a direct target of miR-200c-3p.
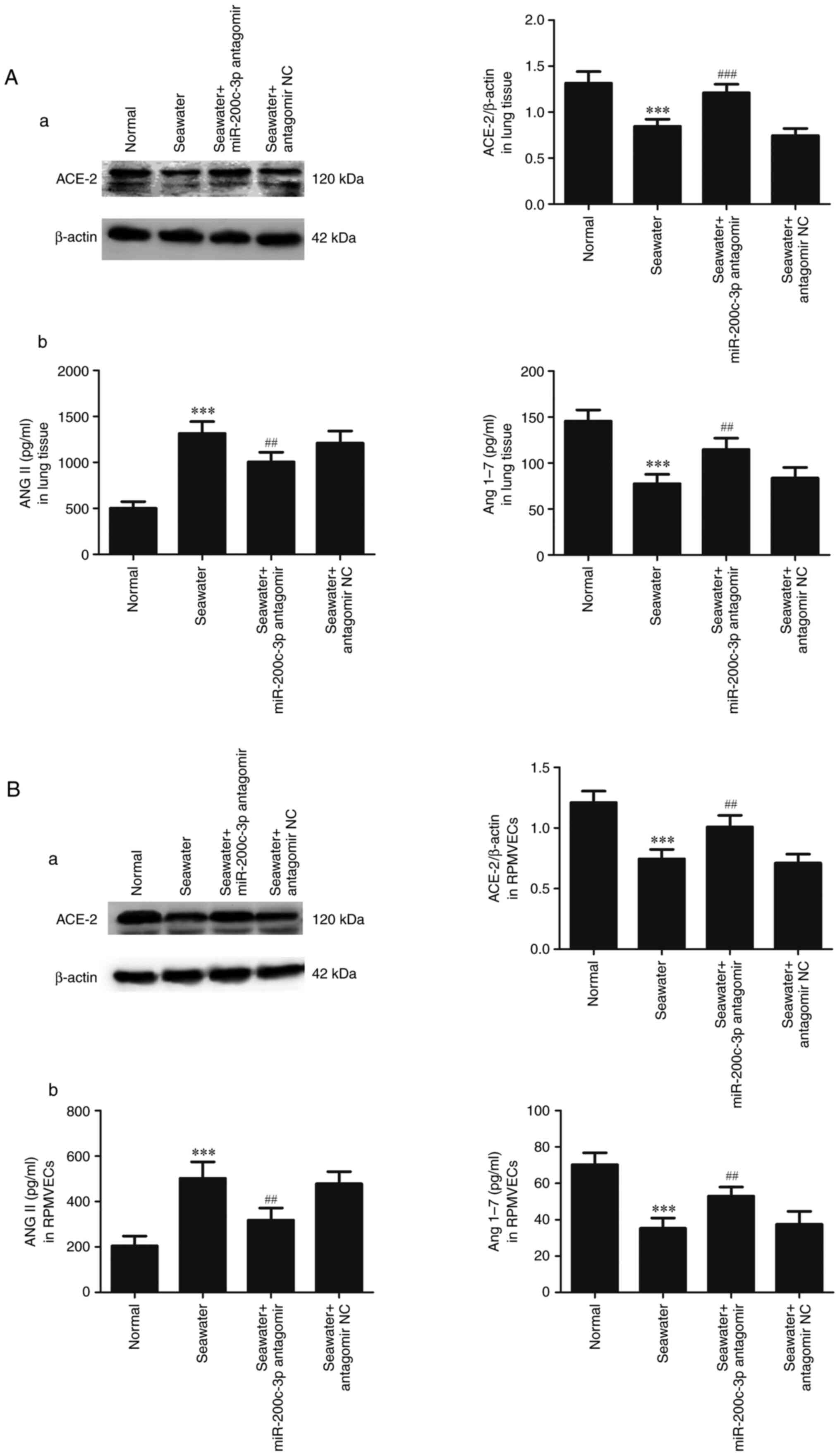
Effects of miR-200c-3p inhibitor on
expression of ACE2, ANGII and ANG1-7 in lung tissue and
RPMVECs
To assess the capacity of miR-200c-3p inhibitor on
the expression of ACE2, ANGII and ANG1-7 in lung tissue (Fig. 5A) and RPMVECs (Fig. 5B), ACE2 was measured by western
blotting and ANGII and ANG1-7 were measured by ELISA kits. Compared
with normal group, expression of ACE2, ANG1-7 were decreased and
ANGII was significantly enhanced in seawater group both in lung
tissue and RPMVECs. In addition, administration of miR-200c-3p
antagomir significantly increased the expression of ACE2, ANG1-7
and inhibited the ANGII expression.
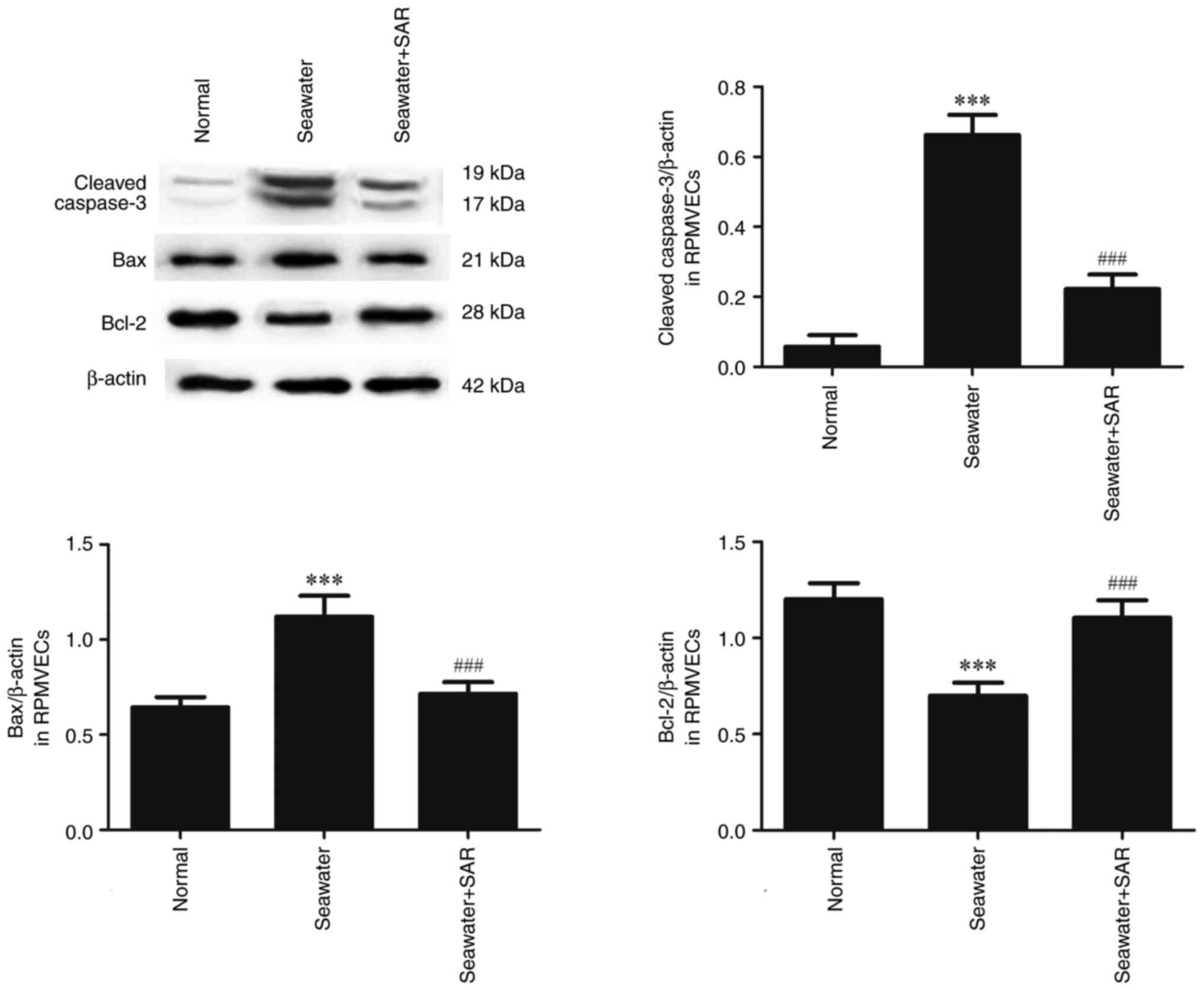
The effect of ANGII on the apoptosis
following seawater stimulation in RPMVECs
AT1 antagonist SAR was used to explore the effect of
ANGII on apoptosis (Fig. 6).
Seawater administration promoted the expression of cleaved-caspase3
and Bax, and decreased the expression of anti-apoptosis protein
Bcl-2. However, pretreatment with SAR significantly reduced the
expression of cleaved caspase3 and Bax, and increased the
expression of Bcl-2.
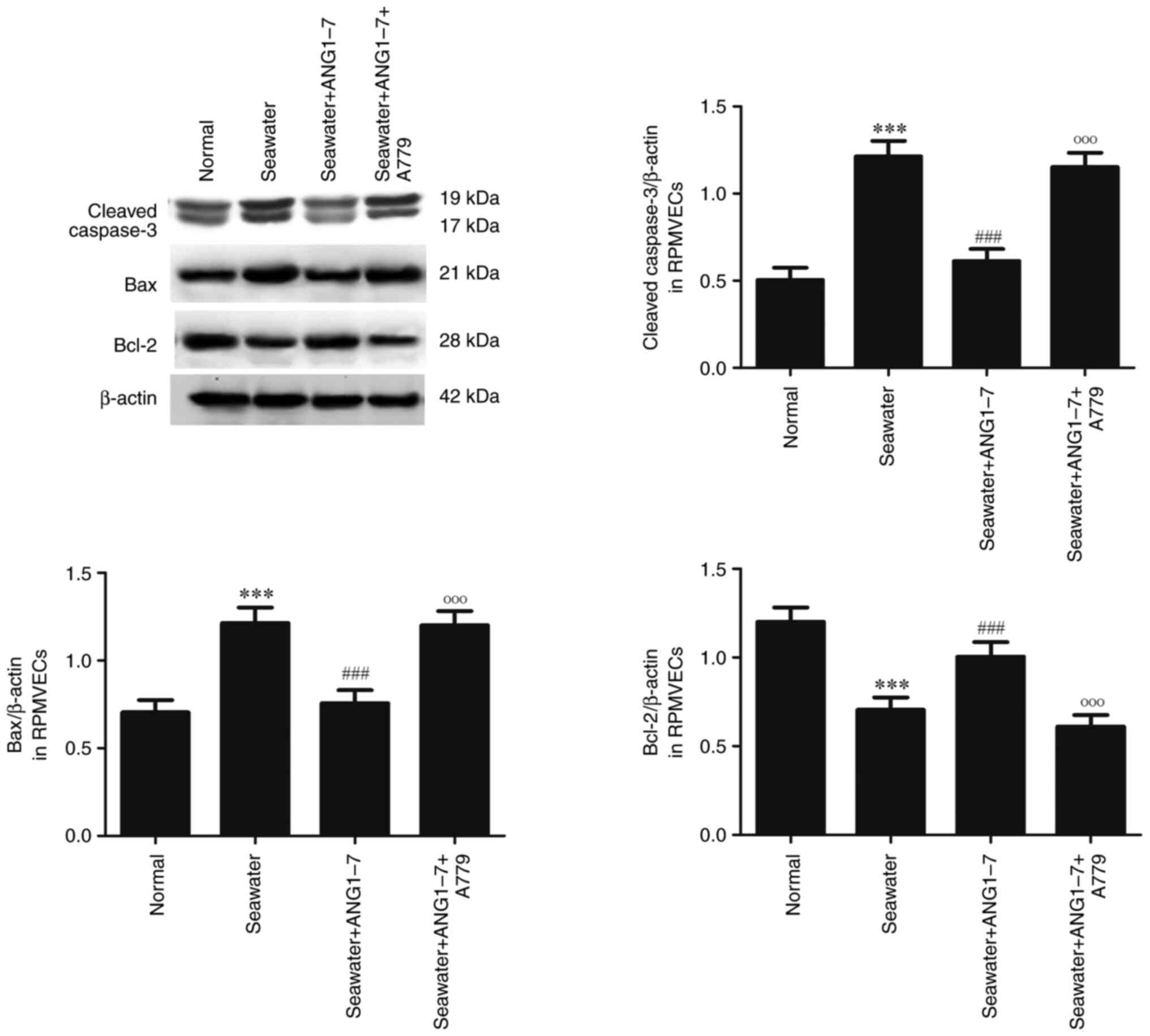
The effect of ANG1-7 on the apoptosis
following seawater stimulation in RPMVECs
ANG1-7 and its receptor Mas antagonist A779 were
used to explore the effect of ANG1-7 on the apoptosis (Fig. 7). Administration of ANG1-7 can
inhibit the expression of cleaved caspase3 and Bax and increase the
expression of Bcl-2 following seawater stimulation. However, adding
A779 significantly promoted the expression of cleaved caspase3, Bax
and inhibited the expression of Bcl-2 following seawater
stimulation.
Discussion
The present study explored the role of miR-200c-3p
in apoptosis of seawater aspiration-induced ALI. The results showed
that expression of miR-200c-3p was significantly upregulated both
in lung tissue and RPMVECs following seawater stimulation. Seawater
stimulation promoted ANGII expression and decreased ACE-2/ANG1-7
expression and induced changes of apoptosis-related protein
expression. Apoptosis can be inhibited by AT1 blocker and abrogated
by adding ANG1-7 following seawater stimulation. Inhibition of
miR-200c-3p suppressed apoptosis and decreased the expression of
ANGII, but increased the ACE-2/ANG1-7 expression.
Apoptosis of endothelial cells following seawater
stimulation is a major cause of endothelial barrier injury.
Seawater aspiration induced apoptosis of endothelial cells is a
major cause of endothelial barrier injury. Inflammatory cells can
infiltrate in alveolar after the pulmonary microvascular
endothelial cells are injured and then the edema fluid forms in
alveolar cells (3-5).
Previous studies suggest that suppression of apoptosis of pulmonary
microvascular endothelial cells can significantly alleviate the
degree of lung injury (4-7).
In alveolar endothelial cells, several studies have confirmed that
there is a local ANG system existing (8,9).
This system includes ANGII and its counter-regulatory axis
ACE-2/ANG1-7/Mas. If the local ANG system in the lung is a response
to injury inducers such as bleomycin, Fas ligand or TNFα,
angiotensinogen (AGT) mRNA and protein are produced. AGT is then
cleaved by proteases to generate the effector peptide ANGII, which
acts by binding to the AT1 receptor. The heptapeptide ANG1-7 is
produced by cleavage of the octapeptide ANGII by ACE-2, which is
also expressed constitutively by alveolar epithelial and
endothelial cells. ANG1-7 acts through its receptor Mas, which
belongs to the G-protein coupled receptor family (22,23).
The local ANG system (ACE-2/ANG1-7/Mas axis and ANGII/AT1) plays an
important role in apoptosis (10-13).
AT1 inhibitor can decrease apoptosis of alveolar epithelial cells
in bleomycin-induced pulmonary fibrosis. In addition, the
ACE-2/ANG1-7/Mas axis shows significant anti-apoptotic effect in
pulmonary fibrosis (14-17).
The present study also found that seawater induced ANGII expression
and decreased ACE-2/ANG1-7 expression. In addition, ANGII receptor
blocker and addition of ANG1-7 can also inhibit seawater induced
apoptosis in RPMVECs.
Previous studies have revealed that miR-200c-3p is a
biomarker for predicting the treatment outcome and mortality of
sepsis-induced ALI. A previous study defined miR-200c-3p as an
endogenous inhibitor of ACE2(20).
The present study also found that ACE2 was a direct target of
miR-200c-3p through dual luciferase reporter gene assay. In
addition, miR-200c-3p inhibitor was used to explore its role in the
seawater aspiration-induced ALI. Inhibition of miR-200c-3p
decreased the apoptosis-related protein expression and decreased
the expression of ANGII, but increased the ACE-2/ANG1-7 expression.
These results showed that miR-200c-3p can regulated seawater
induced apoptosis which may through modulation of the ACE2/ANG1-7
axis.
In conclusion, miR-200c-3p inhibitor pre-treatment
mitigated seawater aspiration-induced lung microvascular
endothelial cell apoptosis, which is probably associated with
induction of ACE2/ANG1-7 signaling. Thus, miR-200c-3p may provide
therapeutic benefits in seawater aspiration-induced ALI
prevention.
Supplementary Material
The identification of the primary
RPMVECs (magnification, x200). (A) passage 1 cells; (B) passage 2
cells; (C) cells stained with anti-CD31 (one of the vascular
endothelial cell markers) antibody. RPMVECs, primary rat pulmonary
micro vascular cells.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by 8th Medical Centre,
Chinese PLA General Hospital Key Research Projects (approval no.
QN202211004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ performed the experiments. MZ and LX analyzed
data, interpreted results of experiments, prepared figures,
drafted, edited and revised the manuscript. MZ and LX confirm the
authenticity of all the raw data. The authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All the animal experiments were approved by the
Animal Care and Use Committee of the Fourth Military Medical
University (approval no. 2023-66378) and in accordance with the
Declaration of the National Institutes of Health Guide for Care and
Use of Laboratory Animals (Publication No. 85-23, revised
1985).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reizine F, Delbove A, Dos Santos A,
Bodenes L, Bouju P, Fillâtre P, Frérou A, Halley G, Lesieur O,
Jonas M, et al: Clinical spectrum and risk factors for mortality
among seawater and freshwater critically ill drowning patients: A
French multicenter study. Crit Care. 25(372)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu W, Pan L, Zhang M, Bo L, Li C, Liu Q,
Wang L and Jin F: Identification of distinct genes associated with
seawater aspiration-induced acute lung injury by gene expression
profile analysis. Mol Med Rep. 14:3168–3178. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jin F and Li C: Seawater-drowning-induced
acute lung injury: From molecular mechanisms to potential
treatments. Exp Ther Med. 13:2591–2598. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang M, Yan X, Liu W, Sun R, Xie Y and
Jin F: Endothelial semaphorin 7A promotes seawater
aspiration-induced acute lung injury through plexin C1 and β1
integrin. Mol Med Rep. 16:4215–4221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang M, Gao Y, Zhao W, Yu G and Jin F:
ACE-2/ANG1-7 ameliorates ER stress-induced apoptosis in seawater
aspiration-induced acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 315:L1015–L1027. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li C, Bo L, Li P, Lu X, Li W, Pan L, Sun
Y, Mu D, Liu W and Jin F: Losartan, a selective antagonist of AT1
receptor, attenuates seawater inhalation induced lung injury via
modulating JAK2/STATs and apoptosis in rat. Pulm Pharmacol Ther.
45:69–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Han F, Luo Y, Li Y, Liu Z, Xu D, Jin F and
Li Z: Seawater induces apoptosis in alveolar epithelial cells via
the Fas/FasL-mediated pathway. Respir Physiol Neurobiol. 182:71–80.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang L, Li Y, Qin H, Xing D, Su J and Hu
Z: Crosstalk between ACE2 and PLGF regulates vascular permeability
during acute lung injury. Am J Transl Res. 8:1246–1252.
2016.PubMed/NCBI
|
|
9
|
Gao YL, Du Y, Zhang C, Cheng C, Yang HY,
Jin YF, Duan GC and Chen SY: Role of renin-angiotensin system in
acute lung injury caused by viral infection. Infect Drug Resist.
13:3715–3725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ye R and Liu Z: ACE2 exhibits protective
effects against LPS-induced acute lung injury in mice by inhibiting
the LPS-TLR4 pathway. Exp Mol Pathol. 113(104350)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu Y, Yang X, Ju Y and Zhao F: Fraxinol
attenuates LPS-induced acute lung injury by equilibrating ACE-Ang
II-AT1R and ACE2-Ang (1-7)-Mas and inhibiting NLRP3. Pharm Bio.
60:979–989. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen Y, Qu L, Li Y, Chen C, He W, Shen L
and Zhang R: Glycyrrhizic acid alleviates lipopolysaccharide
(LPS)-Induced acute lung injury by regulating
angiotensin-converting enzyme-2 (ACE2) and Caveolin-1 signaling
pathway. Inflammation. 45:253–266. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ali RM, Al-Shorbagy MY, Helmy MW and
El-Abhar HS: Role of Wnt4/β-catenin, Ang II/TGFβ, ACE2, NF-κB, and
IL-18 in attenuating renal ischemia/reperfusion-induced injury in
rats treated with Vit D and pioglitazone. Eur J Pharmacol.
831:68–76. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang L, Wang Y, Yang T, Guo Y and Sun T:
Angiotensin-Converting Enzyme 2 attenuates bleomycin-induced lung
fibrosis in mice. Cell Physiol Biochem. 36:697–711. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gupta D, Kumar A, Mandloi A and Shenoy V:
Renin angiotensin aldosterone system in pulmonary fibrosis:
Pathogenesis to therapeutic possibilities. Pharmacol Res.
174(105924)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang
MX, Li W, Li Y, Zhang WY and Li X: The angiotensin-converting
enzyme 2/angiotensin (1-7)/Mas axis protects against lung
fibroblast migration and lung fibrosis by inhibiting the
NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxid Redox
Signal. 22:241–258. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Abdul-Hafez A, Mohamed T, Omar H, Shemis M
and Uhal BD: The renin angiotensin system in liver and lung: Impact
and therapeutic potential in organ fibrosis. J Lung Pulm Respir
Res. 5(00160)2018.PubMed/NCBI
|
|
18
|
He S, Guo Y, Zhao J, Xu X, Wang N and Liu
Q: Ferulic acid ameliorates lipopolysaccharide-induced barrier
dysfunction via MicroRNA-200c-3p-Mediated Activation of PI3K/AKT
Pathway in Caco-2 Cells. Front Pharmacol. 11(376)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shen Y, Zhu Y and Rong F: miR-200c-3p
regulates the proliferation and apoptosis of human trabecular
meshwork cells by targeting PTEN. Mol Med Rep. 22:1605–1612.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Soltani S and Zandi M: miR-200c-3p
upregulation and ACE2 downregulation via bacterial LPS and LTA as
interesting aspects for COVID-19 treatment and immunity. Mol Biol
Rep. 48:5809–5810. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Health N.I.O., Guide for the care and use
of laboratory animals. Publication, 1985.
|
|
22
|
Gopallawa I and Uhal BD: Molecular and
cellular mechanisms of the inhibitory effects of ACE-2/ANG1-7/Mas
axis on lung injury. Curr Top Pharmacol. 18:71–80. 2014.PubMed/NCBI
|
|
23
|
Abuohashish HM, Ahmed MM, Sabry D, Khattab
MM and Al-Rejaie SS: The ACE-2/Ang1-7/Mas cascade enhances bone
structure and metabolism following angiotensin-II type 1 receptor
blockade. Eur J Pharmacol. 807:44–55. 2017.PubMed/NCBI View Article : Google Scholar
|