Nasopharyngeal carcinoma (NPC) is a malignancy that
is commonly observed in Southern China, South-East Asia and North
Africa. NPC is closely associated with genetic factors (such as HLA
genes residing at the major histocompatibility complex region on
chromosome 6p21), infection with the Epstein-Barr Virus (EBV) and
environmental factors (1).
Radiotherapy and chemotherapy are the main therapeutic options
applied for NPC treatment (1).
With the advances in radiotherapy technologies and chemotherapy
treatments over the past decade, the 5-year overall survival rate
of patients with NPC has improved to >80% for early-stage and
50-60% for locally-advanced disease patients (2). However, relapse and metastasis remain
an issue in ~30% patients after standard care
(radiotherapy/chemotherapy) (1,3).
Cisplatin-based chemotherapy is the standard first-line treatment
method for inoperable, recurrent and metastatic NPC (4). However, responses to such regimen do
not endure and tend to reach a plateau, particularly in heavily
pre-treated (radiotherapy/chemotherapy) disease. Targeted therapies
and immunotherapy demonstrate efficacy for recurrent and/or
metastatic NPC (RM-NPC) (1).
In the present review, the evidence and potential
value of targeted therapies and immunotherapy for the clinical
management of RM-NPC were comprehensively summarized. The current
review aimed to provide suggestions to facilitate the optimal
tailoring of treatment modalities, in addition to highlighting
important future research directions and gaps in the knowledge in
the field.
Over the past decade, gene sequencing technologies
have been evolving, which advanced the understanding into the
molecular signaling pathways involved in tumors (5,6).
This has stimulated an interest in molecular-targeted therapies. To
date, a number of clinical trials have evaluated the feasibility of
targeted therapies for the treatment of RM-NPC, including
anti-epidermal growth factor receptor (EGFR) and anti-vascular
endothelial growth factor (VEGF) (7,8).
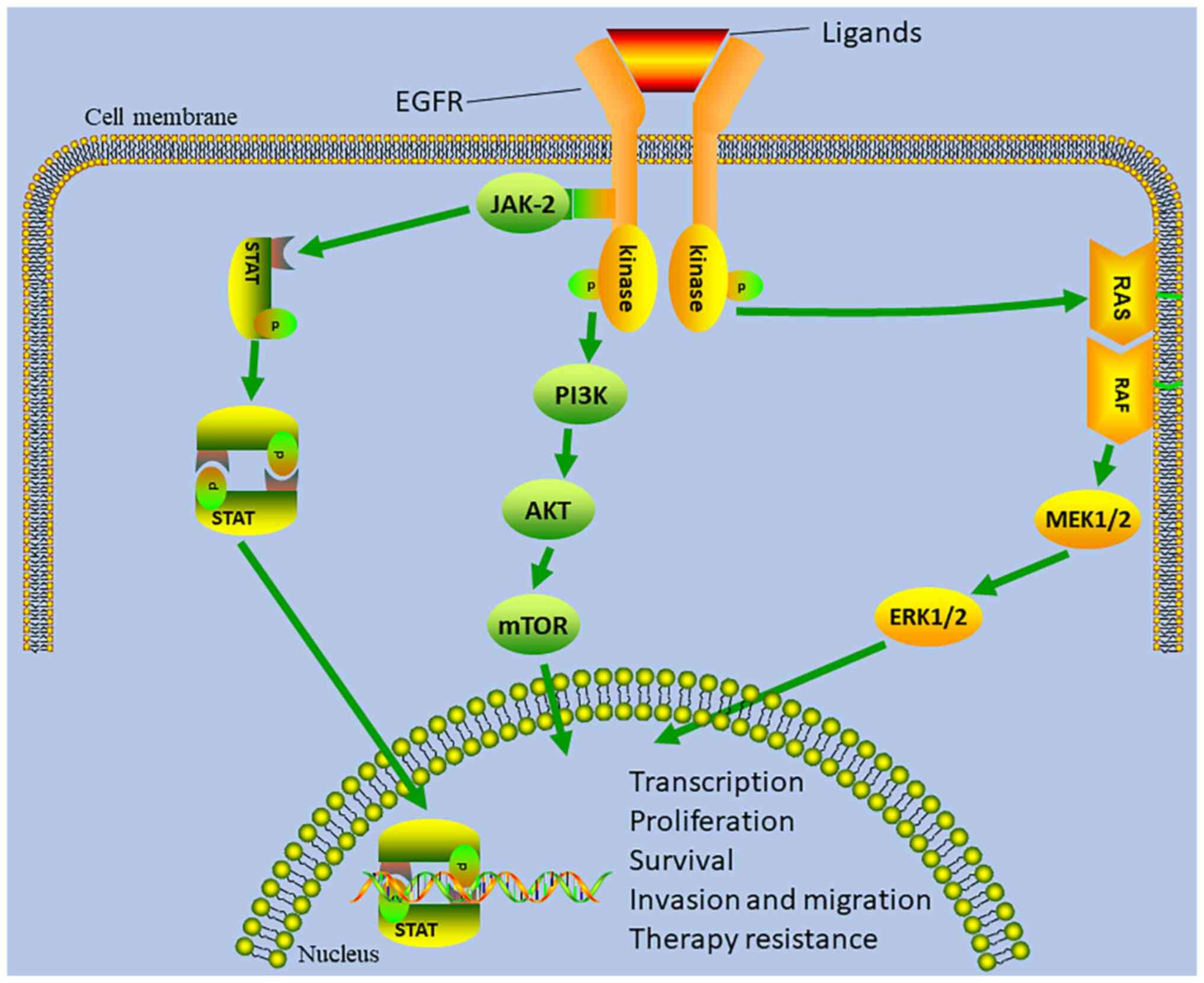
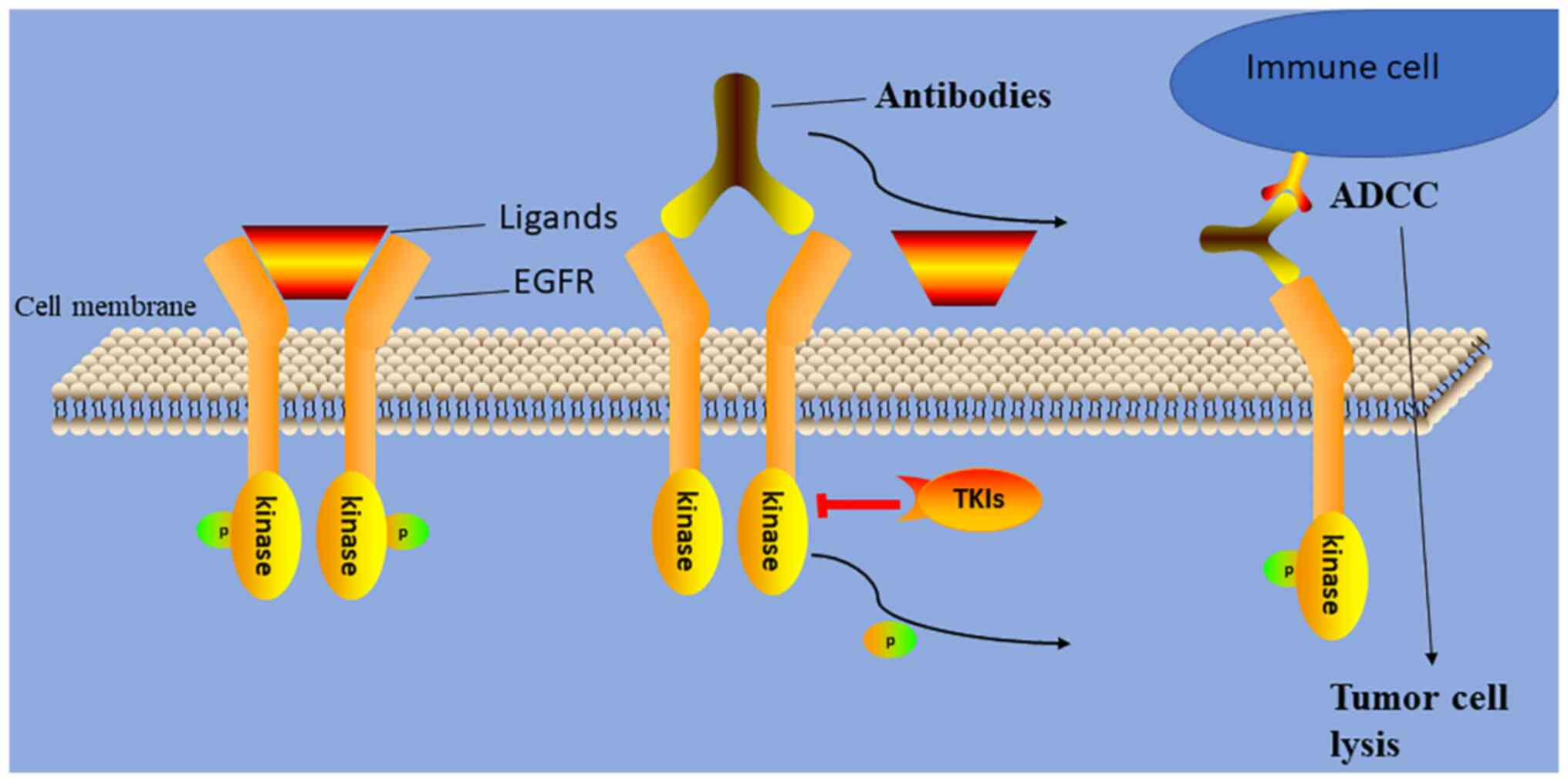
EGFR is highly expressed in most (>82%) NPCs and
contributes to tumor development (Fig.
1) (5,6). As a result, a number of
investigations have assessed the efficacy and toxicity of anti-EGFR
monoclonal antibodies (Table I)
and EGFR tyrosine kinase inhibitors (TKIs) for patients with RM-NPC
(Fig. 2). Chan et al
(7) reported that the combination
of cetuximab (a monoclonal antibody targeting EGFR) and carboplatin
displayed clinical efficacy in patients with RM-NPC who have
already been treated with platinum-based chemotherapy. The overall
response rate (ORR) was 11.7% (7/60 patients), the median
progression-free survival (PFS) was 81 days, and the median overall
survival (OS) was 233 days. For adverse events, 51.7% (31/60) of
the cases had grade 3/4 toxicities (7). Another retrospective study evaluating
the toxicity and efficacy of the combination of paclitaxel,
carboplatin and cetuximab for the first-line treatment for patients
with RM-NPC revealed that this regimen was feasible and potentially
effective, with a 58.3% (7/12 patients) ORR and a 4.1-month PFS
(8). Furthermore, the
aforementioned data were confirmed by another previous
retrospective study, which revealed that an anti-EGFR monoclonal
antibody (nimotuzumab or cetuximab) combined with gemcitabine and
platinum achieved a 10.3-month median PFS, a 42.8-month median OS
and a 67.9% (57/84 patients) ORR. The most common grade 3/4 adverse
events were leukopenia (35.7%; 30/84 patients) and thrombocytopenia
(26.2%; 22/84 patients) (9).
Furthermore, using cetuximab alongside chemoradiotherapy for the
treatment of patients with RM-NPC was found to improve the survival
of patients, with an ORR of 70.0% (21/30), median PFS of 12.2
months and median OS of 23.6 months (10). However, the aforementioned studies
included small sample sizes, resulting in limited reproducibility.
In addition, these studies did not evaluate the difference in the
efficacy and safety profile between standard platinum-based
chemotherapy plus monoclonal antibody and chemotherapy alone. A
retrospective study revealed that there was no difference between
anti-EGFR therapy plus chemotherapy (62 cases) and chemotherapy
alone (248 cases) in the outcomes of patients with de novo
metastatic NPC (11). Therefore,
the use of anti-EGFR monoclonal antibodies warrants consideration
for the treatment of early-stage metastatic NPCs.
Nimotuzumab is an IgG1 humanized anti-EGFR
monoclonal antibody. A multicenter, phase II study (12) explored the effects of nimotuzumab
combined with cisplatin and 5-fluorouracil (PF) on patients with
RM-NPC after standard chemoradiotherapy. The results indicated that
ORR was 71.4% (25/35 patients), the median PFS was 7.0 months and
OS was 16.3 months. However, leukopenia was also observed, which is
a severe side effect (62.9% of patients had grade 3/4) (12). In another clinical trial, compared
with chemotherapy alone, nimotuzumab plus chemotherapy prolonged
the survival and did not exacerbate the toxicity of RM-NPC, with
7.5 vs. 8.5 months in median PFS and 25.6 vs. 48.6 months in median
OS, respectively (13).
Chemoradiotherapy is a treatment option for locally recurrent NPC.
However, the combination of radiation and chemotherapy may increase
the toxicity. Additionally, when combined with chemotherapy,
patients become less tolerant to repeated irradiation (1). A previous study suggested that
compared with chemoradiotherapy, radiotherapy combined with
nimotuzumab achieved similar local control rates and OS for
patients with RM-NPC (14).
Furthermore, the nimotuzumab treatment group had a lower incidence
of acute and late toxicities (14). The addition of nimotuzumab to
radiotherapy may be a promising strategy for patients who cannot
tolerate chemoradiotherapy. Given that both cetuximab and
nimotuzumab demonstrated clinical efficacy for RM-NPC to an extent,
a retrospective study by Chen et al (15) aimed to determine which drug would
be more effective. It was revealed that cetuximab plus palliative
chemotherapy had a longer PFS time compared with nimotuzumab plus
palliative chemotherapy (9.7 vs. 7.9 months), but there was no
difference in the OS time (15).
However, these findings need to be verified by future head-to-head
randomized trials.
VEGF and its receptor VEGFR serve an important role
in NPC, being associated with angiogenesis and metastasis (22,23).
Therefore, targeting VEGF signaling has been considered potentially
beneficial for patient outcome. Sorafenib, an oral multi-kinase
inhibitor, offered only modest efficacy (ORR of 3.8%; 1/26
patients) for recurrent or metastatic squamous cell carcinoma of
the head and neck and NPC (24).
However, only a small percentage of patients (26.9%; 7/26 patients)
were diagnosed with NPC in this aforementioned study (24). In addition, Xue et al
(25) previously revealed a high
ORR of 77.8% (42/54 patients), a median PFS of 7.2 months and an OS
of 11.8 months after treatment with sorafenib plus PF. Compared
with the OS of patients treated with PF (19.5 months) demonstrated
in another previous study (26),
this OS was shorter despite the higher ORR (77.8 vs. 60.2%)
(25). Furthermore, 83.3% (45/54)
of patients exhibited hand-foot skin reactions [18.5% (10/54) of
grade 3/4] (25). Consequently,
whether sorafenib can provide additional benefits for patients with
RM-NPC requires further exploration, as does the difference between
sorafenib plus PF and the standard dose of PF alone.
Sunitinib is another multi-kinase inhibitor of
VEGFR1-3, platelet-derived growth factor receptor (PDGFR), stem
cell factor receptor and fms-like tyrosine kinase
receptor-3(27). Although
sunitinib demonstrated modest anticancer activity (an ORR of 10%)
in patients with RM-NPC who had been previously treated with
high-dose (curative) radiation, 64.3% (9/14) patients hemorrhaged
(epistaxis, hemoptyses and hematemesis) [29% (4/14) in grade 3/4
and 14.3% (2/14) in grade 5] (27). Pazopanib is also a multi-kinase
inhibitor of VEGFR-1, -2, and -3, platelet-derived growth factor
(PDGF)-a, PDGF-b and c-kit tyrosine kinases. Pazopanib displayed
promising efficacy and acceptable side effects in patients with
RM-NPC who had already been heavily pre-treated (after ≥2 lines of
therapy), as 6.1% (2/33) cases achieved partial responses (PRs) and
48.5% (16/33 patients) achieved stable disease. However, 15.2%
(5/33) patients had grade 3/4 hand-foot syndrome and 1 patient
succumbed to epistaxis and myocardial infarction (28).
Lucitanib is a novel multi-target inhibitor of
fibroblast growth factor receptors 1-3, VEGFRs 1-3 and PDGFRα/β
(31). A previous Phase Ib study
found that lucitanib has promising anticancer activity and
tolerable toxicity in patients with RM-NPC who had already been
heavily pretreated. However, the tolerability and efficacy of
lucitanib in patients with RM-NPC should be evaluated in further
phase II/III studies (31). Given
the modest efficacy in patients with RM-NPC, a further study of
angiogenesis inhibitors (sorafenib and sunitinib) as a single
treatment for this disease is not likely to yield beneficial
results. However, these inhibitors are generally well-tolerated and
easy to deliver (oral administration). Therefore, the combination
of these inhibitors with other agents or radiation may yet prove be
a viable option for patients with RM-NPC.
Apart from the EGFR and VEGF pathways, the PI3K/AKT
signaling pathway has also been found to be activated in >40% of
cases with NPC (32,33). However, MK-2206, an oral AKT
inhibitor, demonstrated a limited effect on patients with RM-NPC
who had already been heavily pretreated. Only 4.8% (1/21) of
patients had PR, whereas the median PFS of all patients was 3.5
months (34). The reason for this
may be the activation of compensatory pathways, such as the MAPK
signaling pathway (35).
Immunotherapy, especially immune checkpoint
inhibitors, has become an intensively researched topic in the field
of tumor therapy. It has been previously reported that there are
various types of immune cells in NPC tissues, such as natural
killer cells and T lymphocytes (36,37).
However, the immunogenic effects of these cells are typically
suppressed, such that the tumor cells can evade immunosurveillance
(37). Therefore, the mechanisms
by which tumor cells can evade this surveillance and how immune
cells can be activated to destroy cancer cells have garnered the
interest of researchers. Based on the findings of previous studies
(38-41),
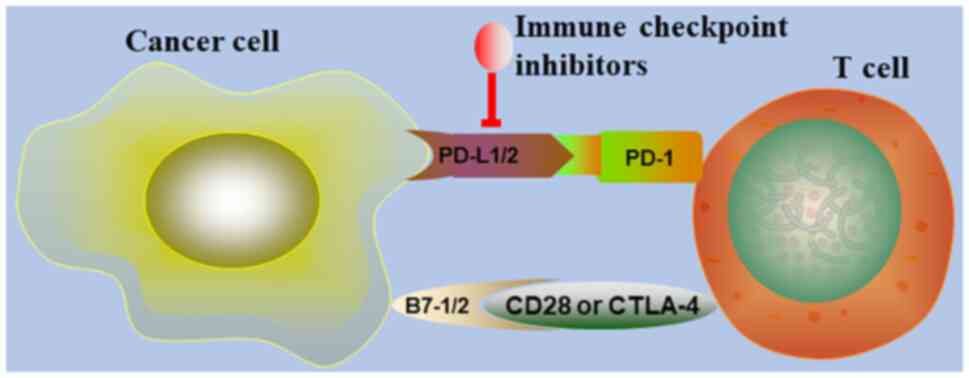
cytotoxic T-lymphocyte protein 4, programmed death-1 (PD-1)
(Fig. 3) and EBV are potential
targets for circumventing the evasion of the immune system by tumor
cells. In the present review, the prospect of targeting the
aforementioned components and using EBV-related vaccines or
cytotoxic T-lymphocytes (CTLs) was evaluated in the context of
RM-NPC. It appears to be a valid option for patients because of the
promising effectiveness and safety profile reported.
To date, immune checkpoint inhibitors that have been
assessed for NPC are pembrolizumab, nivolumab, camrelizumab and
toripalimab (Table II). In 2017,
a phase Ib trial (KEYNOTE-028) preliminarily reported that
pembrolizumab possessed antitumor activity in programmed
death-ligand 1 (PD-L1)-positive patients with RM-NPC (40). It was then revealed that 25.9%
(7/27) patients obtained a PR over a median follow-up time of 20
months whereas 51.9% (14/27) patients had stable disease. However,
29.6% (8/27) cases suffered from grade 3-5 toxicities, including
hepatitis (7.4%; 2/27 patients) and pneumonitis (7.4%; 2/27
patients), whilst 1 patient succumbed to sepsis (40).
Given that EBV serves a crucial role in NPC
progression, vaccines encoding part of an EBV component or
EBV-related adoptive and active T lymphocytes were proposed
treatment options before the emergence of immune checkpoint
inhibitors (1). Chia et al
(50) explored the ability of a
dendritic cell vaccine against the EBV antigens, namely latent
membrane proteins 1 and 2, which are expressed in NPC cells.
Although no adverse events were observed, there was limited
efficacy for patients with metastatic NPC, as the ORR was found to
be 6.3% (1/16 patients) for 7.5 months, the median PFS was 1.92
months and the 1-year OS was 18.8% (3/16 patients) (50). AdE1-LMPpoly, an adenoviral
vector-based vaccine encoding EBV nuclear antigen-1, possessed the
property of stimulating a T lymphocyte response in the majority of
RM-NPC cases (51). After the
adoptive transfer of responsive T lymphocytes to patients, the
median time to progression was 136 days and 71.4% (10/14) patients
obtained stable disease from 38 to 420 days (51). Therefore, AdE1-LMPpoly may provide
benefit for patients with NPC.
EBV-specific CTL (EBV-CTL) therapy was also
evaluated in NPC. In 2010, of the 15 recurrent/refractory
EBV-positive NPC cases, 5 patients achieved CRs and 2 patients had
PRs, but no patients suffered from severe toxicities after
treatment with EBV-CTLs. In addition, of the 8 recurrent patients
who were in remission at the time of EBV-CTL application, 5 cases
were disease-free from 17 to 75 months after treatment (52). In another study, despite an ORR of
4.8% (1/21 patients with CR), the patient was kept in remission for
>8 years after EBV-CTL infusion. The median PFS and OS were 2.2
and 16.7 months, respectively. However, 2/21 cases that previously
failed chemotherapy became sensitized to chemotherapy drugs again
(53). As a consequence,
investigating how to increase the efficacy and predict patient
response to EBV-CTL treatment may form another direction for future
studies. Furthermore, a combination of EBV-CTLs and chemotherapy as
a first-line therapy could benefit patients. A phase II trial
(54) found the ORR to be 71.4%
(25/35) (8.6% of CR, 62.8% of PR) in a total of 35 patients, and
the 2-year OS rate was 62.9%, which was higher compared with that
following chemotherapy monotherapy (29.5%) in a previously
published study (1).
Platinum-based chemotherapy has been the standard
treatment of RM-NPC for over a decade. Although chemotherapy agents
and treatment modalities have advanced during this time, the
efficacy has reached a plateau. Furthermore, the strategy of how to
select a second- or third-line treatment after the failure of
first-line treatment remains unclear. Therefore, the survival of
patients with RM-NPC, especially in heavily pretreated (after ≥2
lines of therapy) patients with NPC, remains poor. As understanding
into the molecular mechanisms underlying tumor progression deepens,
precision therapies (including targeted therapy and immunotherapy)
have emerged over the past years. For targeted therapy, anti-EGFR
monoclonal antibodies and inhibitors against VEGF/VEGFR have
demonstrated benefits for patients with RM-NPC, where the
associated adverse events are also reversible and manageable.
However, future large randomized trials are required before the
wider clinical application of such targeted therapy. At present,
EGFR-TKIs are not recommended for further large-scale studies in
patients with RM-NPC due to the limited efficacy in previous
investigations. In addition, immunotherapy is emerging as an option
for RM-NPC in tumor therapy. Immune checkpoint inhibitors and
adoptive EBV-CTL monotherapy or in combination with chemotherapy
have demonstrated promising outcomes in patients with RM-NPC.
However, additional studies are warranted to consolidate these
findings in the future. In addition, searching for biomarkers that
can accurately predict the response to adoptive EBV-CTL therapy may
be a next research step.
Not applicable.
Funding: This study was supported by the Sanming Project of
Medicine in Shenzhen (grant nos. SZXK013 and SZSM201612063),
Shenzhen High-level Hospital Construction Fund and the National
Cancer Center, Chinese Academy of Medical Sciences and Peking Union
Medical College (grant no. E010322023).
Not applicable.
RL contributed to the study design and prepared the
manuscript. The author has read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Carioli G, Negri E, Kawakita D, Garavello
W, La Vecchia C and Malvezzi M: Global trends in nasopharyngeal
cancer mortality since 1970 and predictions for 2020: Focus on
low-risk areas. Int J Cancer. 140:2256–2264. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xiao WW, Huang SM, Han F, Wu SX, Lu LX,
Lin CG, Deng XW, Lu TX, Cui NJ and Zhao C: Local control, survival,
and late toxicities of locally advanced nasopharyngeal carcinoma
treated by simultaneous modulated accelerated radiotherapy combined
with cisplatin concurrent chemotherapy: Long-term results of a
phase 2 study. Cancer. 117:1874–1883. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang L, Huang Y, Hong S, Yang Y, Yu G,
Jia J, Peng P, Wu X, Lin Q, Xi X, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin in recurrent or metastatic
nasopharyngeal carcinoma: A multicentre, randomised, open-label,
phase 3 trial. Lancet. 388:1883–1892. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ciardiello F and Tortora G: A novel
approach in the treatment of cancer: Targeting the epidermal growth
factor receptor. Clin Cancer Res. 7:2958–2970. 2001.PubMed/NCBI
|
|
6
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW,
Millward MJ, Hong RL, Whang-Peng J, Ma BB, To KF, et al:
Multicenter, phase II study of cetuximab in combination with
carboplatin in patients with recurrent or metastatic nasopharyngeal
carcinoma. J Clin Oncol. 23:3568–3576. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ueda Y, Enokida T, Okano S, Fujisawa T,
Ito K and Tahara M: Combination treatment with paclitaxel,
carboplatin, and cetuximab (PCE) as first-line treatment in
patients with recurrent and/or metastatic nasopharyngeal carcinoma.
Front Oncol. 10(571304)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen C, Zhang X, Zhou Y, Fu S, Lin Z, Hong
S and Zhang L: Treatment of recurrent or metastatic nasopharyngeal
carcinoma by targeting the epidermal growth factor receptor
combined with gemcitabine plus platinum. Cancer Manag Res.
12:10353–10360. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu T, Ou X, Shen C and Hu C: Cetuximab in
combination with chemoradiotherapy in the treatment of recurrent
and/or metastatic nasopharyngeal carcinoma. Anticancer Drugs.
27:66–70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun XS, Liang YJ, Li XY, Liu SL, Chen QY,
Tang LQ and Mai HQ: Palliative chemotherapy with or without
anti-EGFR therapy for de novo metastatic nasopharyngeal carcinoma:
A propensity score-matching study. Drug Des Devel Ther.
13:3207–3216. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao C, Miao J, Shen G, Li J, Shi M, Zhang
N, Hu G, Chen X, Hu X, Wu S, et al: Anti-epidermal growth factor
receptor (EGFR) monoclonal antibody combined with cisplatin and
5-fluorouracil in patients with metastatic nasopharyngeal carcinoma
after radical radiotherapy: A multicentre, open-label, phase II
clinical trial. Ann Oncol. 30:637–643. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu Y, Yang S, Zhou S, Yang J, Qin Y, Gui
L, Shi Y and He X: Nimotuzumab plus platinum-based chemotherapy
versus platinum-based chemotherapy alone in patients with recurrent
or metastatic nasopharyngeal carcinoma. Ther Adv Med Oncol.
12(1758835920953738)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zong JF, Liang QD, Lu QJ, Liu YH, Xu HC,
Chen BJ, Guo QJ, Xu Y, Hu CR, Pan JJ and Lin SJ: Comparison of
radiotherapy combined with nimotuzumab vs. chemoradiotherapy for
locally recurrent nasopharyngeal carcinoma. BMC Cancer.
21(1274)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen C, Zhou Y, Zhang X, Fu S, Lin Z, Fang
W, Yang Y, Huang Y, Zhao H, Hong S and Zhang L: Anti-epidermal
growth factor receptor monoclonal antibody plus palliative
chemotherapy as a first-line treatment for recurrent or metastatic
nasopharyngeal carcinoma. Cancer Med. 9:1721–1732. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chua DT, Wei WI, Wong MP, Sham JS,
Nicholls J and Au GK: Phase II study of gefitinib for the treatment
of recurrent and metastatic nasopharyngeal carcinoma. Head Neck.
30:863–867. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma B, Hui EP, King A, To KF, Mo F, Leung
SF, Kam M, Lo YM, Zee B, Mok T, et al: A phase II study of patients
with metastatic or locoregionally recurrent nasopharyngeal
carcinoma and evaluation of plasma Epstein-Barr virus DNA as a
biomarker of efficacy. Cancer Chemother Pharmacol. 62:59–64.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
You B, Le Tourneau C, Chen EX, Wang L,
Jarvi A, Bharadwaj RR, Kamel-Reid S, Perez-Ordonez B, Mann V and
Siu LL: A Phase II trial of erlotinib as maintenance treatment
after gemcitabine plus platinum-based chemotherapy in patients with
recurrent and/or metastatic nasopharyngeal carcinoma. Am J Clin
Oncol. 35:255–260. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee SC, Lim SG, Soo R, Hsieh WS, Guo JY,
Putti T, Tao Q, Soong R and Goh BC: Lack of somatic mutations in
EGFR tyrosine kinase domain in hepatocellular and nasopharyngeal
carcinoma. Pharmacogenet Genomics. 16:73–74. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wakisaka N, Wen QH, Yoshizaki T, Nishimura
T, Furukawa M, Kawahara E and Nakanishi I: Association of vascular
endothelial growth factor expression with angiogenesis and lymph
node metastasis in nasopharyngeal carcinoma. Laryngoscope.
109:810–814. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Murono S, Inoue H, Tanabe T, Joab I,
Yoshizaki T, Furukawa M and Pagano JS: Induction of
cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is
involved in vascular endothelial growth factor production in
nasopharyngeal carcinoma cells. Proc Natl Acad Sci USA.
98:6905–6910. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Elser C, Siu LL, Winquist E, Agulnik M,
Pond GR, Chin SF, Francis P, Cheiken R, Elting J, McNabola A, et
al: Phase II trial of sorafenib in patients with recurrent or
metastatic squamous cell carcinoma of the head and neck or
nasopharyngeal carcinoma. J Clin Oncol. 25:3766–3773.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xue C, Huang Y, Huang PY, Yu QT, Pan JJ,
Liu LZ, Song XQ, Lin SJ, Wu JX, Zhang JW, et al: Phase II study of
sorafenib in combination with cisplatin and 5-fluorouracil to treat
recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol.
24:1055–1061. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jin Y, Shi YX, Cai XY, Xia XY, Cai YC, Cao
Y, Zhang WD, Hu WH and Jiang WQ: Comparison of five cisplatin-based
regimens frequently used as the first-line protocols in metastatic
nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 138:1717–1725.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hui EP, Ma BBY, King AD, Mo F, Chan SL,
Kam MKM, Loong HH, Ahuja AT, Zee BCY and Chan ATC: Hemorrhagic
complications in a phase II study of sunitinib in patients of
nasopharyngeal carcinoma who has previously received high-dose
radiation. Ann Oncol. 22:1280–1287. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lim WT, Ng QS, Ivy P, Leong SS, Singh O,
Chowbay B, Gao F, Thng CH, Goh BC, Tan DS, et al: A Phase II study
of pazopanib in Asian patients with recurrent/metastatic
nasopharyngeal carcinoma. Clin Cancer Res. 17:5481–5489.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hu-Lowe DD, Zou HY, Grazzini ML, Hallin
ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu
EY, et al: Nonclinical antiangiogenesis and antitumor activities of
axitinib (AG-013736), an oral, potent, and selective inhibitor of
vascular endothelial growth factor receptor tyrosine kinases 1, 2,
3. Clin Cancer Res. 14:7272–7283. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hui EP, Ma BBY, Loong HHF, Mo F, Li L,
King AD, Wang K, Ahuja AT, Chan CML, Hui CWC, et al: Efficacy,
safety, and pharmacokinetics of axitinib in nasopharyngeal
carcinoma: A preclinical and phase II correlative study. Clin
Cancer Res. 24:1030–1037. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Luo F, Ma YX, Liu QW, Yang YP,
Fang WF, Huang Y, Zhou T, Li J, Pan HM, et al: A Phase Ib study of
lucitanib (AL3810) in a cohort of patients with recurrent and
metastatic nasopharyngeal carcinoma. Oncologist. 27:e453–e462.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Or YY, Hui AB, To KF, Lam CN and Lo KW:
PIK3CA mutations in nasopharyngeal carcinoma. Int J Cancer.
118:1065–1067. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yip WK, Leong VC, Abdullah MA, Yusoff S
and Seow HF: Overexpression of phospho-Akt correlates with
phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal
carcinoma. Oncol Rep. 19:319–328. 2008.PubMed/NCBI
|
|
34
|
Ma BB, Goh BC, Lim WT, Hui EP, Tan EH,
Lopes Gde L, Lo KW, Li L, Loong H, Foster NR, et al: Multicenter
phase II study of the AKT inhibitor MK-2206 in recurrent or
metastatic nasopharyngeal carcinoma from patients in the mayo phase
II consortium and the cancer therapeutics research group (MC1079).
Invest New Drugs. 33:985–991. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma BB, Lui VW, Hui CW, Lau CP, Wong CH,
Hui EP, Ng MH, Cheng SH, Tsao SW, Tsang CM, et al: Preclinical
evaluation of the mTOR-PI3K inhibitor BEZ235 in nasopharyngeal
cancer models. Cancer Lett. 343:24–32. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lu J, Chen XM, Huang HR, Zhao FP, Wang F,
Liu X and Li XP: Detailed analysis of inflammatory cell
infiltration and the prognostic impact on nasopharyngeal carcinoma.
Head Neck. 40:1245–1253. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee AZE, Tan LSY and Lim CM:
Cellular-based immunotherapy in Epstein-Barr virus induced
nasopharyngeal cancer. Oral Oncol. 84:61–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Brunner MC, Chambers CA, Chan FK, Hanke J,
Winoto A and Allison JP: CTLA-4-Mediated inhibition of early events
of T cell proliferation. J Immunol. 162:5813–5820. 1999.PubMed/NCBI
|
|
39
|
Wherry EJ: T cell exhaustion. Nat Immunol.
12:492–499. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Hsu C, Lee SH, Ejadi S, Even C, Cohen RB,
Le Tourneau C, Mehnert JM, Algazi A, van Brummelen EMJ, Saraf S, et
al: Safety and antitumor activity of pembrolizumab in patients with
programmed death-ligand 1-Positive nasopharyngeal carcinoma:
Results of the KEYNOTE-028 study. J Clin Oncol. 35:4050–4056.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stoker SD, Novalić Z, Wildeman MA, Huitema
AD, Verkuijlen SA, Juwana H, Greijer AE, Tan IB, Middeldorp JM and
de Boer JP: Epstein-Barr virus-targeted therapy in nasopharyngeal
carcinoma. J Cancer Res Clin Oncol. 141:1845–1857. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fang W, Yang Y, Ma Y, Hong S, Lin L, He X,
Xiong J, Li P, Zhao H, Huang Y, et al: Camrelizumab (SHR-1210)
alone or in combination with gemcitabine plus cisplatin for
nasopharyngeal carcinoma: Results from two single-arm, phase 1
trials. Lancet Oncol. 19:1338–1350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou
T, Shen L, Wu H, Lang J, et al: Camrelizumab versus placebo in
combination with gemcitabine and cisplatin as first-line treatment
for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st):
A multicentre, randomised, double-blind, phase 3 trial. Lancet
Oncol. 22:1162–1174. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW,
Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AYC, et al:
Antitumor activity of nivolumab in recurrent and metastatic
nasopharyngeal carcinoma: An International, multicenter study of
the mayo clinic phase 2 consortium (NCI-9742). J Clin Oncol.
36:1412–1418. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ma Y, Fang W, Zhang Y, Yang Y, Hong S,
Zhao Y, Tendolkar A, Chen L, Xu D, Sheng J, et al: A Phase I/II
open-label study of nivolumab in previously treated advanced or
recurrent nasopharyngeal carcinoma and other solid tumors.
Oncologist. 24:891–e431. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wei XL, Ren C, Wang FH, Zhang Y, Zhao HY,
Zou BY, Wang ZQ, Qiu MZ, Zhang DS, Luo HY, et al: A phase I study
of toripalimab, an anti-PD-1 antibody, in patients with refractory
malignant solid tumors. Cancer Commun (Lond). 40:345–354.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu
XC, Liao W, Jiang Y, Lin XY, Zhang QY, et al: Efficacy, safety, and
correlative biomarkers of toripalimab in previously treated
recurrent or metastatic nasopharyngeal carcinoma: A phase II
clinical trial (POLARIS-02). J Clin Oncol. 39:704–712.
2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen
J, Li J, Shi YR, Jin F, Xu R, et al: Toripalimab or placebo plus
chemotherapy as first-line treatment in advanced nasopharyngeal
carcinoma: A multicenter randomized phase 3 trial. Nat Med.
27:1536–1543. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hua Y, You R, Wang Z, Huang P, Lin M,
Ouyang Y, Xie Y, Zou X, Liu Y, Duan C, et al: Toripalimab plus
intensity-modulated radiotherapy for recurrent nasopharyngeal
carcinoma: An open-label single-arm, phase II trial. J Immunother
Cancer. 9(e003290)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chia WK, Wang WW, Teo M, Tai WM, Lim WT,
Tan EH, Leong SS, Sun L, Chen JJ, Gottschalk S and Toh HC: A phase
II study evaluating the safety and efficacy of an
adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients
with advanced metastatic nasopharyngeal carcinoma. Ann Oncol.
23:997–1005. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Smith C, Tsang J, Beagley L, Chua D, Lee
V, Li V, Moss DJ, Coman W, Chan KH, Nicholls J, et al: Effective
treatment of metastatic forms of Epstein-Barr virus-associated
nasopharyngeal carcinoma with a novel adenovirus-based adoptive
immunotherapy. Cancer Res. 72:1116–1125. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Louis CU, Straathof K, Bollard CM,
Ennamuri S, Gerken C, Lopez TT, Huls MH, Sheehan A, Wu MF, Liu H,
et al: Adoptive transfer of EBV-specific T cells results in
sustained clinical responses in patients with locoregional
nasopharyngeal carcinoma. J Immunother. 33:983–990. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Huang J, Fogg M, Wirth LJ, Daley H, Ritz
J, Posner MR, Wang FC and Lorch JH: Epstein-Barr virus-specific
adoptive immunotherapy for recurrent, metastatic nasopharyngeal
carcinoma. Cancer. 123:2642–2650. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chia WK, Teo M, Wang WW, Lee B, Ang SF,
Tai WM, Chee CL, Ng J, Kan R, Lim WT, et al: Adoptive T-cell
transfer and chemotherapy in the first-line treatment of metastatic
and/or locally recurrent nasopharyngeal carcinoma. Mol Ther.
22:132–139. 2014.PubMed/NCBI View Article : Google Scholar
|