Introduction
Epstein-Barr virus (EBV) is a member of the
g-herpesvirus family and is classified within the lymphocryptovirus
genus (1). The EBV genome contains
linear double-stranded DNA of 172k base pairs (2). Primary EBV infection usually takes
place during childhood and the virus subsequently undergoes an
asymptomatic latency phase (2).
The occurrence of certain human malignant tumors has
been closely related to EBV infection, including nasopharyngeal
carcinoma and lymphoid malignancies. EBV-associated
lymphoproliferative disorders have been widely clarified and are
divided into B-cell and T/natural killer (NK) cell disorders
(3). EBV-associated B-cell
lymphoproliferative disorders include the following: i) Burkitt's
lymphoma; ii) a proportion of Hodgkin lymphomas; iii)
post-transplant lymphoproliferative disorders (PTLDs); iv)
HIV-associated lymphoproliferative disorders; and v) other rare
histotypes (3). T/NK-cell
lymphoproliferative disorders that have been reported to be
EBV-associated include: i) A proportion of peripheral T-cell
lymphomas; ii) angioimmunoblastic T-cell lymphoma; iii) extranodal
nasal type NK/T-cell lymphoma; and iv) other rare histotypes,
including lymphomatoid granulomatosis, pyothorax-associated
lymphoma and senile EBV-associated B-cell lymphoproliferative
disorders (3).
EBV targets lymphocytes and achieves latent
infection in a circular episomal form (4). Different latency patterns are
recognized based on latent gene expression patterns. There are
three types of latent gene expression, which have been described as
latency I, II and III encoding genes: i) EBV nuclear antigen
(EBNA)-1, EBV encoded RNA (EBER)-1 and
EBER-2 (latency I, II and III); ii) EBNA-2 and
EBNA-3 (latency III); and iii) latent membrane protein
(LMP)-1 and LMP-2 (latency II and III)
(4). Latency I is generally
associated with EBV-related Burkitt's lymphoma (5,6),
latency II has been associated with classical Hodgkin lymphoma
(cHL) and T-cell non-Hodgkin lymphoma and latency III occurs mainly
in immune-compromised individuals suffering from PTLDs and
HIV-associated lymphoproliferative disorders and in lymphoblastoid
cell lines (5,6).
The programmed cell death (PD)-1/PD-1 ligand 1
(PD-L1) pathway was first reported by Dong et al (7) in 1999. It was indicated that the
PD-1/PD-L1 pathway regulates effector T-cell responses, which are
considered to be involved in the negative regulation of immune
responses, thus protecting tissues from immune-mediated damage
(8,9). However, activation of the PD-1/PD-L1
pathway in tumor cells inhibits effector T-cell function and
activates immunosuppressive regulatory T-cell function, resulting
in tumor evasion of host immune surveillance (10,11).
PD-L1 is expressed in tumor cells and tumor-infiltrating
nonmalignant cells, primarily macrophages, while PD-1 is expressed
by tumor-infiltrating lymphocytes (TILs) (8).
Evidence has suggested that aberrant PD-L1
expression is associated with poor prognosis in certain types of
solid cancer, such as non-small cell lung cancer, advanced melanoma
and renal cell carcinoma (12-14),
and cHL (15). The presence of
large numbers of PD-1 expressing TILs is associated with favorable
overall survival in patients with diffuse large B cell lymphoma
(DLBCL) (16,17).
EBV-infected cells that acquire alterations
involving PD-1/PD-L1 are thought to effectively evade anti-EBV
immune surveillance, which has been associated with
immunotolerance. PD-1/PD-L1 axis checkpoint blockade may provide
effective therapeutics against EBV-related lymphomas compared with
conventional chemotherapy (18,19).
It was also shown that PD-L1 expression was induced by LMP1
promoter activity in EBV-transformed B cells. In addition, >70%
of EBV+ cases of PTLDs express detectable PD-L1(20).
In DLBCL, Kwon et al (21) observed that EBV infection may
contribute to PD-L1 expression in activated B-cell type DLBCL.
However, no consensus has been reached on whether EBV positivity
has a definite impact on PD1/PD-L1 expression in EBV-related
lymphomas and lymphoproliferative disorders. Accordingly, the
present meta-analysis was carried out to elucidate the association
between the PD1/PD-L1 axis and EBV infection.
Materials and methods
Search strategy and selection
criteria
The present meta-analysis was performed according to
the PRISMA guidelines (http://www.prisma-statement.org). Studies were
identified by searching the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) for articles
published up to 30th June, 2022. The following keywords were
searched: ‘lymphoma’ AND ‘lymphoproliferative disorders’ OR ‘LPDs’
AND ‘Epstein-Barr virus’ OR ‘EBV’ AND ‘EBV-encoded RNA’ OR ‘EBER’.
A reference search was also performed and article searches were
restricted to literature written in English.
The inclusion criteria were as follows: i)
Histopathological diagnosis of lymphomas and LPDs according to the
World Health Organization classification (22), including post-transplant or
immunocompromised patients; ii) detailed data sufficient to
evaluate EBV status identification. Tumor cells expressing EBER,
EBNA or LMP-1 confirmed by in situ hybridization and/or
genetic identification should be considered sufficient to confirm a
positive case; iii) an analysis relevant to PD-L1/PD-1 expression
in tumor cells and in the lymphocytes or macrophagocytes of the
tumor microenvironment, PD-L1/PD-1 identification explicitly stated
and justification for positive status provided; iv) studies
including a minimum of 10 participants, 5 of which were in the
EBV+ DLBCL subgroup and 5 in the control group; and v)
inclusion of a control group of patients with EBV-
DLBCL, offering a comparison between EBV+ and
EBV- subgroups.
The exclusion criteria were as follows: i)
Insufficient raw data for estimating EBV and PD-L1/PD-1
identification; ii) review articles, opinion reports, conference
abstracts without original data and case reports; and iii) studies
not written in English. Any disagreements were resolved by
consensus.
Data pooling
Data associated with clinicopathological
characteristics were extracted from each of the eligible studies.
The data extracted were the first author's surname and the
publication year, the pathological diagnosis and the number of
EBV-positive and EBV-negative cases (Table I). The expression of PD-L1 in
neoplastic cells (nPD-L1) and microenvironmental PD-L1 (miPD-L1)
were used for analysis. The positive expression of nPD-L1 and
miPD-L1 had been detected by immunohistochemistry (IHC) using
immunofluorescence staining to calculate the proportion of positive
cells. nPD-L1 positivity (nPD-L1+) was defined as the
presence of PD-L1-positive neoplastic cells among the total tissue
cellularity. miPD-L1 positivity (miPD-L1+) was defined
as PD-L1-positive nonmalignant cells among the total tissue
cellularity. PD-1 is more commonly expressed in TILs (8) and PD-1 TIL positivity
(PD-1+ TILs) was defined as PD-1-positive TILs among the
total tissue cellularity. However, the cut-off values to classify
PD-L1 or PD-1 to be positive have no general agreement, which is
one cause of heterogeneity in the present meta-analysis. The
positive cases of nPD-L1, miPD-L1 and PD-1 TILs and cut-off values
for IHC staining results are presented in Table I.
 | Table IMain characteristics of the eligible
studies. |
Table I
Main characteristics of the eligible
studies.
| | Tumor cells | Immune cells | Cut off | |
|---|
| First author,
year | Pathology | Features | EBV status | Cases | nPD-L1+
cases, n (%) | miPD-L1+
cases, n (%) |
PD-1+TILs cases, n (%) | nPD-L1 positive
cells, % | miPD-L1 positive
cells, % | (Refs.) |
|---|
| Kataoka, 2019 | DLBCL | / | i) +; ii) - | i) 27; ii) 48 | i) 5(19); ii)
1(2) | / | / | 5 | / | (39) |
| Kiyasu, 2015 | DLBCL | / | +; - | i) 114; ii)
1139 | i)
22(19)a; ii) 110
(9.6)a | i) 37(32); ii)
135(12) | i) 58
(0-802)a; ii) 19
(0-802)a | 30 | 20 | (24) |
| Chen, 2013 | DLBCL | Elderly,
HIV-associated | +; - | i) 16; ii) 66 | i) 16(100); ii)
7(11) | i) 16(100); ii)
9(14) | / | 5 | 20 | (25) |
| Anastasiadou,
2019 | DLBCL | / | +; - | / | i) (84); ii)
(28) | / | / | NA | / | (26) |
| Kinch, 2019 | DLBCL | PTLD | +; - | i) 27; ii) 20 | i) 18(67); ii)
8(40) | / | / | 5 | / | (27) |
| Veloza, 2019 | DLBCL | PTLD | +; - | i) 21; ii) 16 | i) 18(86); ii)
6(38) | i) 20(95); ii)
8(50) | i) 3/13(23); ii)
5/12(42) | 5 | 20 | (28) |
| Ishikawa, 2018 | DLBCL | Primary gastric
DLBCL | +; - | i) 25; ii) 215 | i) 0/14 (0); ii)
0/40 (0) | i) 12/14(86); ii)
17/40(43) | / | 5 | 20 | (29) |
| Ishikawa, 2018 | DLBCL | Primary intestinal
DLBCL | +; - | i) 10; ii) 52 | i) 2(20); ii)
1(2) | i) 8/8(100); ii)
31/48(65) | / | 5 | 20 | (30) |
| Quan, 2015 | DLBCL | / | +; - | i) 7; ii) 20 | i) 5(71); ii)
8(40) | / | / | using
FCMa | / | (31) |
| Cohen, 2017 | DLBCL | / | +; - | / | / | / | i) (15.70); ii)
(14.90) | / | / | (32) |
| Kwon, 2016 | DLBCL | / | +; - | i) 6; ii) 107 | i) 4 (66.7); ii) 9
(8.4) | i) 4 (66.7); ii) 10
(9.3) | i) 1 (16.7) ii)
27/103; (26.2) | NA | NA | (21) |
| Sakakibara,
2018 | cHL | / | +; - | i) 11; ii) 16 | i) 11(100); ii)
8(50) | i) 11(100); ii)
11(69) | / | 5 | 20 | (33) |
| Paydas, 2015 | cHL | / | +; - | i) 40; ii) 47 | i) 8(20); ii)
10(21) | / | i) 10(25); ii)
8(17) | 5 | 20 | (34) |
| Ozturk, 2020 | cHL | / | +; - | i) 15; ii) 21 | i) 11(73); ii)
4(19) | / | i) 8(53); ii)
12(57) | 80 | 5 | (35) |
| Antel, 2021 | cHL | 44% HIV
positive | +; - | i) 39; ii) 38 | i) 23(59); ii)
17(45) | / | / | 50 | / | (36) |
| Kohno, 2020 | cHL | MTX-LPD | +; - | i) 8; ii) 1 | i) 7(87); ii)
1(100) | / | / | NA | / | (37) |
| Chen, 2013 | PTLD | / | +; - | i) 10; ii) 7 | i) 6(60); ii)
4(57) | i) 7(70); ii)
4(57) | / | 5 | 20 | (25) |
| Kinch, 2019 | PTLD | / | +; - | i) 43; ii) 37 | i) 24(56); ii)
16(43) | / | / | 5 | / | (27) |
| Veloza, 2019 | PTLD | / | +; - | i) 34; ii) 16 | i) 23(68); ii)
7(44) | i) 29(85); ii)
8(50) | i) 8/25(32); ii)
5/12(42) | 5 | 20 | (28) |
| Laurent, 2016 | PBL | / | +; - | i) 39; ii) 38 | i) 7/9(78); ii)
1/2(50) | i) 16/23(70); ii)
8/18(44) | i) 14/18(78); ii)
6/14(43) | NA | NA | (38) |
Statistical analysis
The meta-analysis was performed using R Studio 4.1.0
(RStudio, Inc.). In brief, effect sizes for each study were
determined by calculating risk ratios (RR) and the corresponding
95% confidence interval (CI). The pooled proportions were
calculated using the Mantel-Haenszel method (23). According to the recommendations
provided by the Cochrane Handbook for Systematic Reviews of
Interventions (https://training.cochrane.org/handbook/current/chapter-10#section-10-10-4-1),
a choice of whether a common-effects or random-effects model
applied should not be made through a statistical test for
heterogeneity and considering that heterogeneity is always expected
for the intervention effects among multiple studies, a
random-effects model was employed. P<0.05 was considered to
indicate a statistically significant difference. Publication bias
was examined by funnel plots and Egger's tests.
Results
Selection and characteristics of the
studies
A literature search in PubMed identified 806
relevant records for screening. Following title and abstract
screening, most records were excluded for one of the following
reasons: Studies not containing any human subjects, insufficient
data, published in a language other than English, review articles
and editorials. A total of 165 studies underwent full text
screening and 17 studies met the inclusion criteria with a further
three articles included through a reference search. A total of 16
studies (21,24-39)
with a total of 2,396 patients were finally included in the present
meta-analysis.
Patients in the studies had a histologically
confirmed diagnosis of lymphoma subtypes, with 11 articles on DLBCL
comprising 1,936 patients (21,24-32,39),
5 articles on cHL including 236 patients (33-37),
3 articles on PTLDs comprising 147 patients (25,27,28)
and 1 article on plasmablastic lymphoma (PBL) including 77 patients
(38). According to the cut-off
values, the included articles described the IHC results of the
immune checkpoint molecules PD-L1 and PD-1. In addition, all
studies were retrospective and reported positive and negative cases
of EBV infection and immune checkpoint molecules. The main
characteristics of the eligible studies are summarized in Table I.
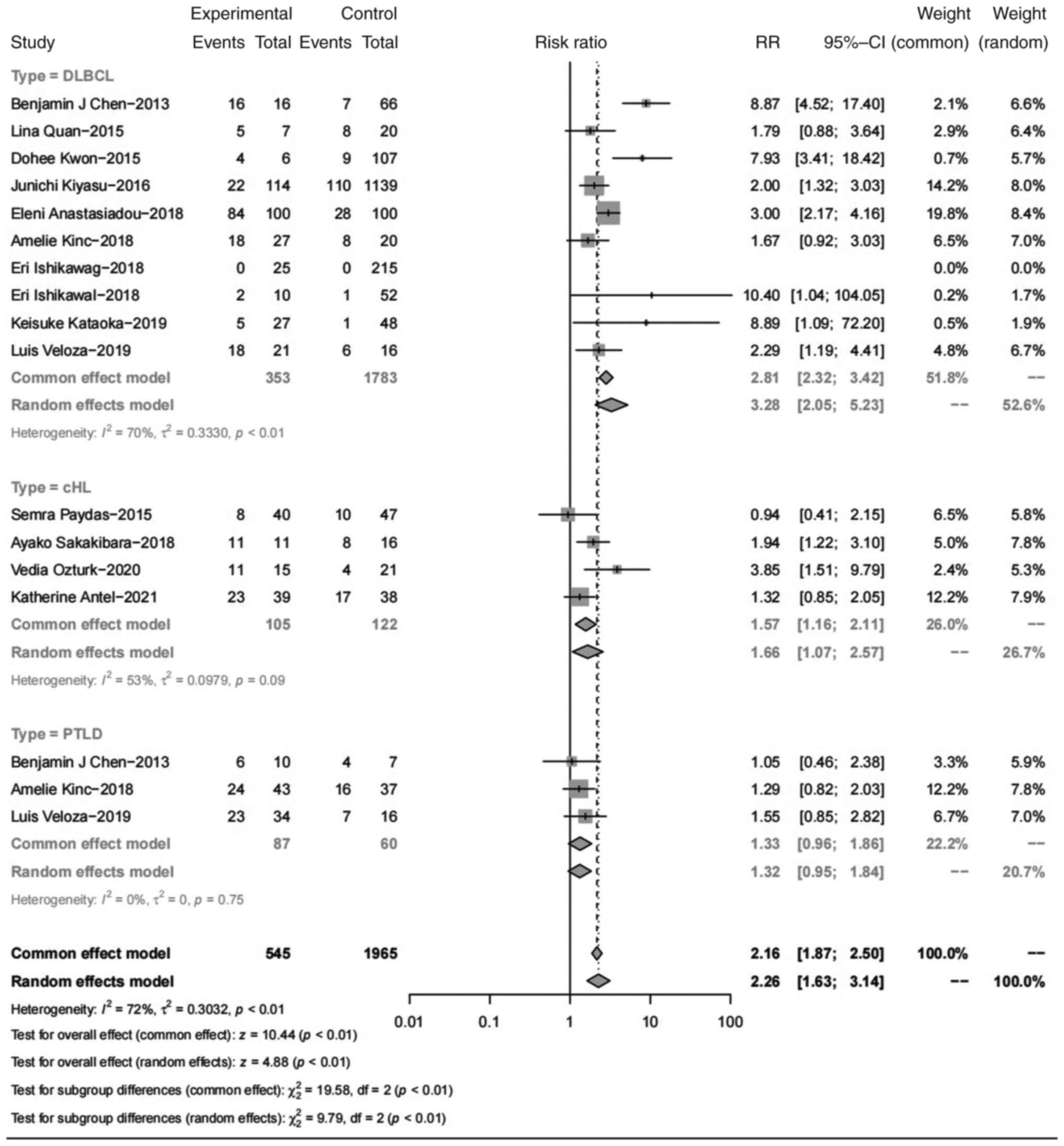
Association between EBV infection and
PD-L1 expression in tumor cells
PD-L1 expression in tumor cells was significantly
higher in EBV+ lymphomas than in EBV-
lymphomas, with a pooled RR of 2.26 (95% CI, 1.63-3.14; P<0.01;
Fig. 1). Specifically, nPD-L1
expression was higher in patients with EBV+ DLBCLs than
in those with EBV- DLBCLs (RR=3.28; 95% CI, 2.05-5.23).
This result was similar in cHLs, as nPD-L1 was higher in
EBV+ cases than in EBV- cases (RR=1.66; 95%
CI, 1.07-2.57). In PTLDs, nPD-L1 expression showed no significant
increase in EBV+ cases, with an RR of 1.32 (95% CI,
0.95-1.84).
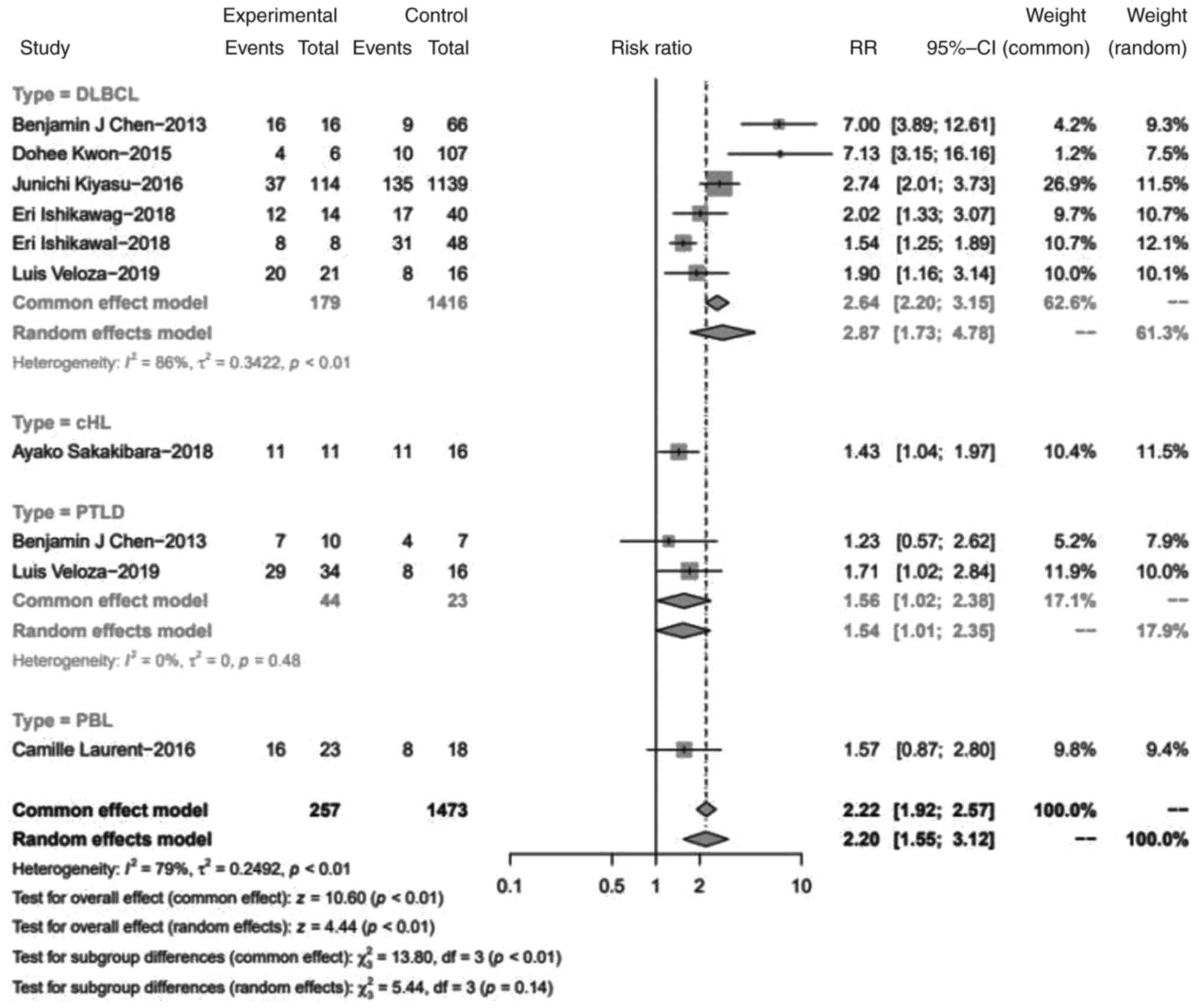
Association between EBV infection and
PD-L1 expression in immune cells
The PD-L1 expression of immune cells in the tumor
microenvironment was compared between EBV+ and
EBV- lymphomas (Fig.
2). Statistically, EBV infection increased the expression of
PD-L1 in immune cells with a pooled RR of 2.20 (95% CI, 1.55-3.12;
P<0.01). Specifically, miPD-L1 expression was higher in
EBV+ DLBCLs than in EBV- DLBCLs (RR=2.87; 95%
CI, 1.73-4.78). In PTLD, a similar result of PD-L1 expression
increasing in immune cells of EBV+ cases was observed,
with an RR of 1.54 (95% CI, 1.01-2.35).
Association between EBV infection and
PD-1 expression in TILs
It was found that PD-1 expression in TILs was not
associated with EBV infection, with a pooled RR of 1.10 (95% CI,
0.81-1.48; P>0.05). Specifically, in the DLBCL and cHL subtypes,
the expression of PD-1 TILs showed no discrimination between
EBV+ and EBV- cases, with RRs of 0.89 (95%
CI, 0.52-1.53) and 1.09 (95% CI, 0.67-1.78) (Fig. 3).
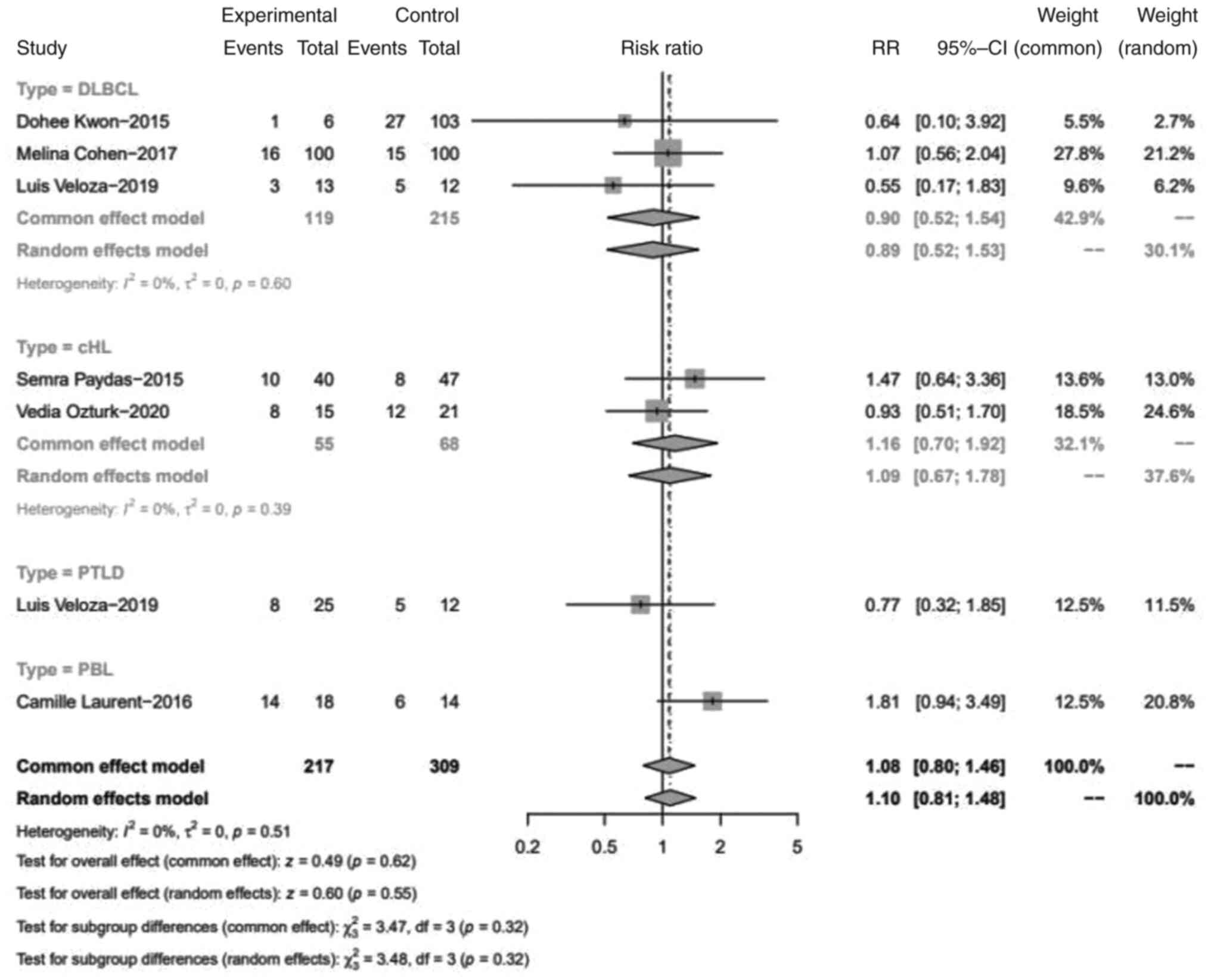 | Figure 3Forest plot of the RR for PD-L1 TILs
positive proportion between EBV+ and EBV-
lymphomas. PD-L1, programmed cell death 1; TILs, tumor-infiltrating
lymphocytes; EBV, Epstein-Barr virus; DLBCL, diffuse large B-cell
lymphoma; df, degrees of freedom; cHL, classical Hodgkin lymphoma;
PTLD, post-transplant lymphoproliferative disorders; RR, relative
risk; CI, confidence interval; PBL, plasmablastic lymphoma. |
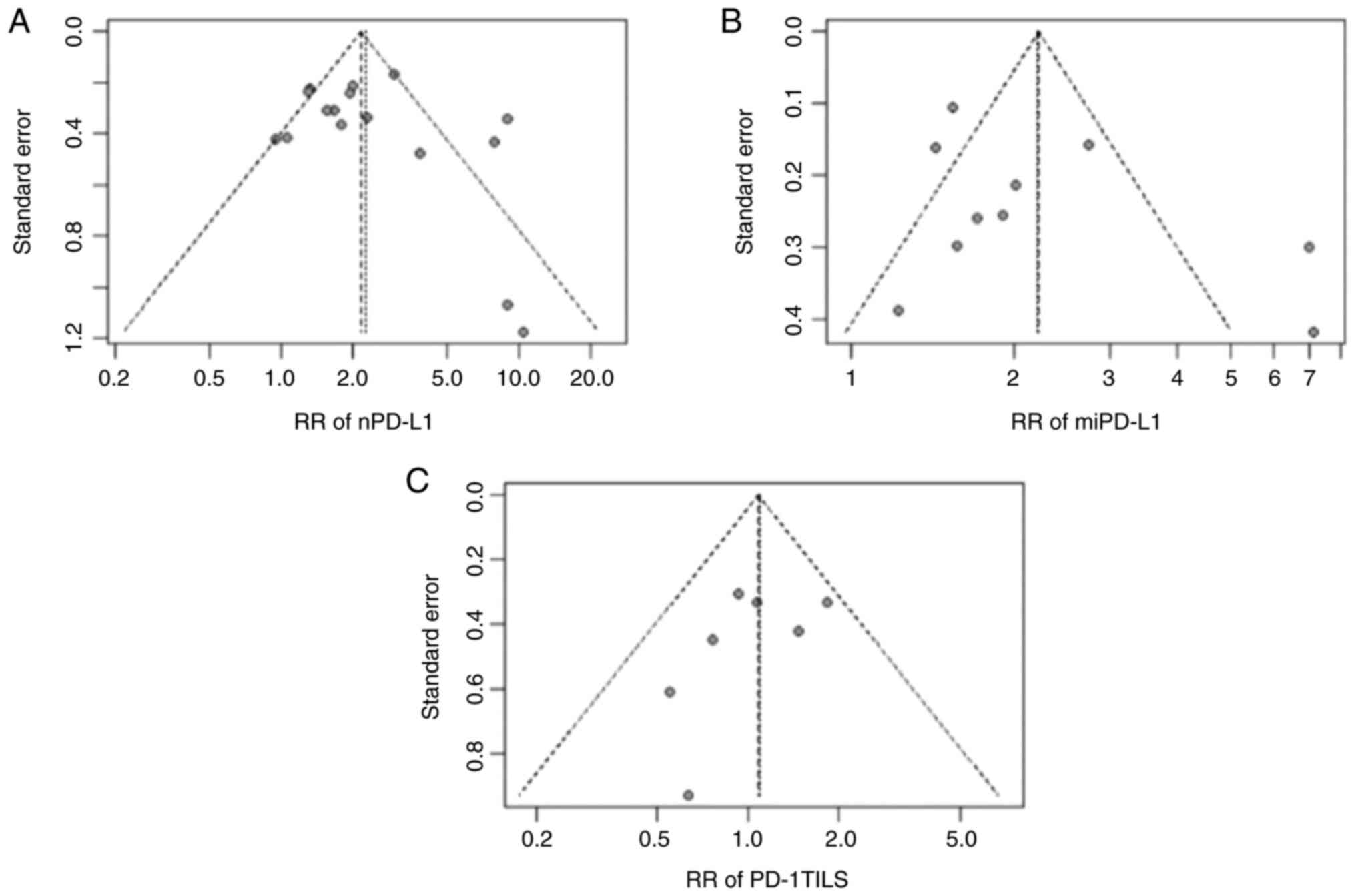
Publication bias
The funnel plots revealed that the RR analyses of
nPD-L1, miPD-L1 and PD-1 TILs may have publication bias and
heterogeneity (Fig. 4).
Discussion
EBV-associated lymphomas and lymphoproliferative
diseases are rare but are often malignant and largely resistant to
current chemotherapeutic regimens. Given their association with the
oncogenic virus and an ‘immune privileged’ milieu, they are
attractive targets for immune-based therapies (40). Certain virus-associated solid
cancers were reported to induce PD-L1 expression (41-43)
and anti-PD-1 and anti-PD-L1 blockades have resulted in durable
clinical responses in various types of cancer (44,45).
However, the efficacy of such immune-targeted therapies in
EBV-associated lymphomas and LPDs has not been fully elucidated. In
the present study, it was demonstrated that EBV infection may have
certain effects on the antitumor immune response in EBV-associated
lymphomas through a mechanism of increasing PD-L1 expression in
tumor cells and TILs.
Several studies have uncovered the functional
mechanism of PD-L1 in EBV+ lymphomas. Green et al
(20) identified an activating
protein-1 (AP-1)-responsive enhancer in the PD-L1 gene. Using
EBV-transformed B cells, it was further demonstrated that the
expression of EBV-encoded LMP-1 promotes PD-L1 expression through
both AP-1 signaling and JAK-STAT signaling activity. Quan et
al (31) also found that the
antitumor immune effects of PD-1 blockade are more effective in
EBV+ DLBCL than in EBV- DLBCL. The results of
the aforementioned studies suggest that PD-1 blockade may restore
T-cell exhaustion and immune escape, resulting in more efficacious
immunotherapy treatment for EBV+ DLBCL.
Barzyk and Sheriff (46) performed a systematic review, which
included 11 studies, to evaluate the association of EBV with PD-L1
expression in DLBCL. A narrative synthesis was conducted using
table summarization and concluded that a non-EBV related mechanism
is likely related to increased PD-L1 expression, with relevance to
the cell of origin. In the present study, statistical methods were
used to analyze the effect of EBV infection on the expression of
immunomodulatory molecules in EBV-associated lymphomas.
Statistically significant results based on abstracted data from 20
studies suggested that antitumor immunity appears to have an
important role in these virus-associated lymphomas. The increased
expression of PD-L1 in tumor cells and the tumor microenvironment
may be a mechanism contributing to the pathogenesis of
EBV+ lymphomas. Further research is needed to elucidate
the intrinsic molecular mechanism of antitumor immunity in EBV
infection.
In the process of collating data for the present
study, it was noticed that the expression of CD30 probably has
relevance to EBV virus infection. Therefore, a meta-analysis was
performed in the present study to determine whether such an
association existed. The results indicated increased CD30
expression in EBV+ DLBCL cases compared to
EBV- cases, with statistical significance (RR=2.36; 95%
CI, 1.60-3.47; P<0.01; Fig.
S1). In a review of the molecular biology of Hodgkin's
lymphoma, the author proposed that the occurrence of Hodgkin's
lymphoma is responsible for constitutive NF-κB activation, which is
induced by CD30 overexpression, EBV LMP-1, and factors of immune
evasion (47). The findings of the
present study also showed the probable relevance of the increased
expression of CD30 and EBV infection in the development of
EBV+ DLBCL, but this still requires further
exploration.
The present study had certain limitations. The
funnel plot estimates suggested substantial statistical
heterogeneity. No sensitivity analysis or meta-regression analysis
was performed to determine which factors affected the results.
Potentially, the following aspects have been present. First, the
detection and determination of PD-L1+ and
PD-1+ expression require standardization. In general,
the threshold for nPD-L1 positivity is ≥5% of the tumor cell
population showing 2+ or 3+ membrane staining for IHC, while
miPD-L1 is considered positive when ≥20% of tumor-infiltrating
immune cells show 2+ or 3+ membrane or cytoplasmic staining. The
positive thresholds of nPD-L1 and miPD-L1 are different in two
articles (23,34), as shown in Table I. While most studies used IHC
methods to detect PD-L1 expression, one article adopted the flow
cytometry method (30). As another
limitation, the small size of included articles may have limited
the strength of the evidence in the present study. The insufficient
number of cases of PBL and PTLD subtypes made it impracticable to
conduct a meta-analysis.
In conclusion, EBV involvement is a distinctive
subtype of lymphoma and the present systematic review indicates
that enhancement of the PD-1/PD-L1 pathway in tumor cells and the
tumor microenvironment may be a potential mechanism in the
development of EBV-associated lymphatic diseases. The impact of EBV
infection on immune-mediated damage and the efficacy of
immune-targeted therapies in these EBV-positive diseases need to be
further explored.
Supplementary Material
Forest plot of the RR for the
CD30-positive proportion between EBV+ and
EBV- DLBCL. EBV, Epstein-Barr virus; DLBCL, diffuse
large B-cell lymphoma; RR, relative risk; CI, confidence
interval.
Acknowledgements
The authors would like to thank Professor Wangjian
Zhang (Department of Medical Statistics, School of Public Health,
Sun Yat-sen University, Guangzhou, China) for his methodological
guidance.
Funding
Funding: The study was supported by The National Natural Science
Foundation of China (grant no. 82000146).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY performed data analysis and wrote the manuscript.
SJ was involved in the acquisition of data and analysis. XY
performed analyses and obtained the funding. HD was involved in the
conception and design of the study. JY and HD confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roschewski M and Wilson WH: EBV-associated
lymphomas in adults. Best Pract Res Clin Haematol. 25:75–89.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Young LS and Murray PG: Epstein-Barr virus
and oncogenesis: From latent genes to tumours. Oncogene.
22:5108–5121. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carbone A, Gloghini A and Dotti G:
EBV-associated lymphoproliferative disorders: Classification and
treatment. Oncologist. 13:577–585. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sbih-Lammali F, Djennaoui D, Belaoui H,
Bouguermouh A, Decaussin G and Ooka T: Transcriptional expression
of Epstein-Barr virus genes and proto-oncogenes in north African
nasopharyngeal carcinoma. J Med Virol. 49:7–14. 1996.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shannon-Lowe C and Rickinson AB: Bell AIL
Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B
Biol Sci. 372(20160271)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vockerodt M, Yap LF, Shannon-Lowe C,
Curley H, Wei W, Vrzalikova K and Murray PG: The Epstein-Barr virus
and the pathogenesis of lymphoma. J Pathol. 235:312–322.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Francisco LM, Salinas VH, Brown KE,
Vanguri VK, Freeman GJ, Kuchroo VK and Sharpe AH: PD-L1 regulates
the development, maintenance, and function of induced regulatory T
cells. J Exp Med. 206:3015–3029. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Ohaegbulam KC, Assal A, Lazar-Molnar E,
Yao Y and Zang X: Human cancer immunotherapy with antibodies to the
PD-1 and PD-L1 pathway. Trends Mol Med. 21:24–33. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma G, Deng Y, Jiang H, Li W, Wu Q and Zhou
Q: The prognostic role of programmed cell death-ligand 1 expression
in non-small cell lung cancer patients: An updated meta-analysis.
Clin Chim Acta. 482:101–107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abdel-Rahman O: PD-L1 expression and
outcome of advanced melanoma patients treated with anti-PD-1/PD-L1
agents: A meta-analysis. Immunotherapy. 8:1081–1089.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Z, Peng S, Xie H, Guo L, Cai Q, Shang
Z, Jiang N and Niu Y: Prognostic and clinicopathological
significance of PD-L1 in patients with renal cell carcinoma: A
meta-analysis based on 1863 individuals. Clin Exp Med. 18:165–175.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Younes A, Santoro A, Shipp M, Zinzani PL,
Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V,
Kuruvilla J, et al: Nivolumab for classical Hodgkin's lymphoma
after failure of both autologous stem-cell transplantation and
brentuximab vedotin: A multicentre, multicohort, single-arm phase 2
trial. Lancet Oncol. 17:1283–1294. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Muenst S, Hoeller S, Willi N, Dirnhofera S
and Tzankov A: Diagnostic and prognostic utility of PD-1 in B cell
lymphomas. Dis Markers. 29:47–53. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ahearne MJ, Bhuller K, Hew R, Ibrahim H,
Naresh K and Wagner SD: Expression of PD-1 (CD279) and FoxP3 in
diffuse large B-cell lymphoma. Virchows Arch. 465:351–358.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tse E and Kwong YL: How I treat NK/T-cell
lymphomas. Blood. 121:4997–5005. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ok CY, Papathomas TG, Medeiros LJ and
Young KH: EBV-positive diffuse large B-cell lymphoma of the
elderly. Blood. 122:328–340. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Green MR, Rodig S, Juszczynski P, Ouyang
J, Sinha P, O'Donnell E, Neuberg D and Shipp MA: Constitutive AP-1
activity and EBV infection induce PD-L1 in Hodgkin lymphomas and
posttransplant lymphoproliferative disorders: Implications for
targeted therapy. Clin Cancer Res. 18:1611–1618. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik
JH, Kim YA, Kim TM, Heo DS, Kim CW and Jeon YK: Clinicopathological
analysis of programmed cell death 1 and programmed cell death
ligand 1 expression in the tumour microenvironments of diffuse
large B cell lymphomas. Histopathology. 68:1079–1089.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the world health
organization classification of haematolymphoid tumours: Lymphoid
neoplasms. Leukemia. 36:1720–1748. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
24
|
Kiyasu J, Miyoshi H, Hirata A, Arakawa F,
Ichikawa A, Niino D, Sugita Y, Yufu Y, Choi I, Abe Y, et al:
Expression of programmed cell death ligand 1 is associated with
poor overall survival in patients with diffuse large B-cell
lymphoma. Blood. 126:2193–2201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen BJ, Chapuy B, Ouyang J, Sun HH,
Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA and Rodig
SJ: PD-L1 expression is characteristic of a subset of aggressive
B-cell lymphomas and virus-associated malignancies. Clin Cancer
Res. 19:3462–3473. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Anastasiadou E, Stroopinsky D, Alimperti
S, Jiao AL, Pyzer AR, Cippitelli C, Pepe G, Severa M, Rosenblatt J,
Etna MP, et al: Epstein-Barr virus-encoded EBNA2 alters immune
checkpoint PD-L1 expression by downregulating miR-34a in B-cell
lymphomas. Leukemia. 33:132–147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kinch A, Sundstrom C, Baecklund E, Backlin
C, Molin D and Enblad G: Expression of PD-1, PD-L1, and PD-L2 in
posttransplant lymphoproliferative disorder after solid organ
transplantation. Leuk Lymphoma. 60:376–384. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Veloza L, Teixido C, Castrejon N, Climent
F, Carrio A, Marginet M, Soldini D, González-Farré B,
Ribera-Cortada I, Lopez-Guillermo A, et al: Clinicopathological
evaluation of the programmed cell death 1 (PD1)/programmed cell
death-ligand 1 (PD-L1) axis in post-transplant lymphoproliferative
disorders: Association with Epstein-Barr virus, PD-L1 copy number
alterations, and outcome. Histopathology. 75:799–812.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ishikawa E, Tanaka T, Shimada K, Kohno K,
Satou A, Eladl AE, Sakakibara A, Furukawa K, Funasaka K, Miyahara
R, et al: A prognostic model, including the EBV status of tumor
cells, for primary gastric diffuse large B-cell lymphoma in the
rituximab era. Cancer Med. 7:3510–3520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ishikawa E, Kato S, Shimada K, Tanaka T,
Suzuki Y, Satou A, Kohno K, Sakakibara A, Yamamura T, Nakamura M,
et al: Clinicopathological analysis of primary intestinal diffuse
large B-cell lymphoma: Prognostic evaluation of CD5, PD-L1, and
Epstein-Barr virus on tumor cells. Cancer Med. 7:6051–6063.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Quan L, Chen X, Liu A, Zhang Y, Guo X, Yan
S and Liu Y: PD-1 blockade can restore functions of T-cells in
epstein-barr virus-positive diffuse large B-cell lymphoma in vitro.
PLoS One. 10(e0136476)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cohen M, Vistarop AG, Huaman F, Narbaitz
M, Metrebian F, De Matteo E, Preciado MV and Chabay PA: Cytotoxic
response against Epstein Barr virus coexists with diffuse large
B-cell lymphoma tolerogenic microenvironment: Clinical features and
survival impact. Sci Rep. 7(10813)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sakakibara A, Kohno K, Eladl AE, Klaisuwan
T, Ishikawa E, Suzuki Y, Shimada S, Nakaguro M, Shimoyama Y,
Takahara T, et al: Immunohistochemical assessment of the diagnostic
utility of PD-L1: A preliminary analysis of anti-PD-L1 antibody
(SP142) for lymphoproliferative diseases with tumour and
non-malignant Hodgkin-Reed-Sternberg (HRS)-like cells.
Histopathology. 72:1156–1163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Paydas S, Bagir E, Seydaoglu G, Ercolak V
and Ergin M: Programmed death-1 (PD-1), programmed death-ligand 1
(PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma.
Ann Hematol. 94:1545–1552. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ozturk V, Yikilmaz AS, Kilicarslan A,
Bakanay SM, Akinci S and Dilek I: The triple positivity for EBV,
PD-1, and PD-L1 identifies a very high risk classical hodgkin
lymphoma. Clin Lymphoma Myeloma Leuk. 20:e375–e381. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Antel K, Chetty D, Oosthuizen J, Mohamed
Z, Van der Vyver L and Verburgh E: CD68-positive tumour associated
macrophages, PD-L1 expression, and EBV latent infection in a high
HIV-prevalent South African cohort of Hodgkin lymphoma patients.
Pathology. 53:628–634. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kohno K, Suzuki Y, Elsayed AA, Sakakibara
A, Takahara T, Satou A, Kato S, Nakamura S and Asano N:
Immunohistochemical assessment of the diagnostic utility of PD-L1
(Clone SP142) for methotrexate-associated lymphoproliferative
disorders with an emphasis of neoplastic PD-L1 (Clone
SP142)-positive classic hodgkin lymphoma type. Am J Clin Pathol.
153:571–582. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Laurent C, Fabiani B, Do C, Tchernonog E,
Cartron G, Gravelle P, Amara N, Malot S, Palisoc MM, Copie-Bergman
C, et al: Immune-checkpoint expression in Epstein-Barr virus
positive and negative plasmablastic lymphoma: A clinical and
pathological study in 82 patients. Haematologica. 101:976–984.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kataoka K, Miyoshi H, Sakata S, Dobashi A,
Couronne L, Kogure Y, Sato Y, Nishida K, Gion Y, Shiraishi Y, et
al: Frequent structural variations involving programmed death
ligands in Epstein-Barr virus-associated lymphomas. Leukemia.
33:1687–1699. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Marques-Piubelli ML, Salas YI, Pachas C,
Becker-Hecker R, Vega F and Miranda RN: Epstein-Barr
virus-associated B-cell lymphoproliferative disorders and
lymphomas: A review. Pathology. 52:40–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M,
Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ and Yang DL:
Immunostaining of PD-1/PD-Ls in liver tissues of patients with
hepatitis and hepatocellular carcinoma. World J Gastroenterol.
17:3322–3329. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Badoual C, Hans S, Merillon N, Van Ryswick
C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A,
Besnier N, et al: PD-1-expressing tumor-infiltrating T cells are a
favorable prognostic biomarker in HPV-associated head and neck
cancer. Cancer Res. 73:128–138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fang W, Zhang J, Hong S, Zhan J, Chen N,
Qin T, Tang Y, Zhang Y, Kang S, Zhou T, et al: EBV-driven LMP1 and
IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma:
Implications for oncotargeted therapy. Oncotarget. 5:12189–12202.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Barzyk GA and Sheriff V: EBV positivity
and programmed death-ligand 1 expression in diffuse large B-cell
lymphoma: A systematic review. Anticancer Res. 40:5951–5968.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Weniger MA and Küppers R: Molecular
biology of Hodgkin lymphoma. Leukemia. 35:968–981. 2021.PubMed/NCBI View Article : Google Scholar
|