Introduction
Lead (Pb) is a toxicant prevalent in industrial
settings and a significant contributor to environmental pollution.
Advancements in industrial civilization have resulted in widespread
Pb poisoning, which has emerged as a covert epidemic in the 21st
century (1). In our daily lives,
Pb primarily enters the organism through ingestion or inhalation
from sources such as soil, food, Pb dust and other media (2). Upon entering the human body, Pb can
induce various diseases that affect the neurological, respiratory,
urinary and cardiovascular systems (3). Extensive research has demonstrated
that exposure to Pb disrupts the delicate balance between oxidants
and antioxidants, leading to vascular and cardiac damage (4-6).
Breviscapine (Bre) is a crude extract of several
flavonoids from Erigeron breviscapus (Vant.). It possesses a
range of biological functions, including anti-inflammatory,
antioxidant, anti-apoptotic and myocardial protection (7). Several studies have assessed its
utility in the treatment of cardiovascular diseases, such as
cardiac hypertrophy (8) and
doxorubicin (DOX)-induced cardiotoxicity (9). Additionally, Bre has been shown to
inhibit myocardial inflammation and apoptosis in rats with coronary
embolisms (10) and exhibit
protective effects against myocardial injuries induced by
streptozotocin, thereby enhancing the antioxidant capacity of
myocardial tissues (11). However,
the effect of Bre on Pb-induced myocardial injury remains
unclear.
Nuclear factor erythroid-2 related factor 2 (Nrf2)
is a key transcription factor in oxidative stress regulation that
augments the expression of antioxidant enzymes, thereby protecting
against cell damage caused by reactive oxygen species (ROS)
(12). Nrf2 maintains cellular
homeostasis by regulating the expression of NAD(P)H dehydrogenase
quinone 1 (NQO1) and heme oxygenase-1 (HO-1) (13,14).
Furthermore, a review reported that Nrf2 protects the heart from
oxidative stress damage (15). A
previous study indicated that activation of the Nrf2 pathway can
ameliorate Pb-induced kidney injury (16). Consequently, it was hypothesized
that Bre mitigates Pb-induced myocardial injury by activating the
Nrf2 pathway.
In the present study, both animal and cellular
models of Pb poisoning were established to investigate whether BRE
confers myocardial protection through the Nrf2 pathway. The present
study aimed to identify potential therapeutic agents for treating
patients with Pb poisoning.
Materials and methods
Cell culture and treatment
Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) was
used to culture rat cardiomyocyte-derived H9C2 cells (cat. no.
CRL-1446; American Type Culture Collection). These cells were
maintained at 37˚C under 5% CO2. For modeling Pb
poisoning, H9C2 cells were exposed to 10 µM lead acetate (PbA; cat.
no. 467863; MilliporeSigma) for 24 h. Cells were pre-incubated with
1, 5 and 10 µM of Bre (cat. no. 116122362; Shanghai Yuanye
Bio-Technology Co., Ltd.; high-performance liquid chromatography
≥95%) for 1 h prior to PbA exposure. To evaluate the influence of
Bre on Pb-induced oxidative stress, PbA-treated cells were
pretreated with 10 µM of Bre, either alone or in combination with
10 mM N-acetylcysteine (NAC; cat. no. T5518; TargetMol), a ROS
scavenger, for 1 h. To elucidate the mechanistic action of Bre,
PbA-exposed cells were pre-incubated with 20 µM Nrf2 inhibitor
ML385 (cat. no. T4360; TargetMol) for 2 h prior to a 1 h treatment
with 10 µM of Bre.
Cell viability was assessed using the Cell Counting
Kit-8 (Beyotime Institute of Biotechnology). The assay was
performed with a cell density of 5x103 cells/well, using
10 µl CCK-8 reagent per well. The plates were then incubated at
37˚C for 2 h, and absorbance was detected at a wavelength of 450
nm.
Cell apoptosis
After trypsinization, H9C2 cells (1x105)
were resuspended in binding buffer (195 µl) and incubated with
Annexin V-FITC (5 µl) and propidium iodide (PI, 10 µl) for 20 min
at 25˚C in the dark. Apoptosis was analyzed using a BD LSRFortessa
X-20 flow cytometry (BD Biosciences). Data analysis was conducted
using FlowJo software version 7.6 (FlowJo LLC).
ROS detection
Collected cells (2x105) were incubated
with 2',7'-dichlorfluoresceindiacetate (DCFH-DA) at 37˚C for 20
min. ROS levels were quantitatively measured using flow cytometry
at a wavelength of 488 nm and analyzed using FlowJo software
version 7.6 (FlowJo LLC).
Establishment and processing of animal
models
Six-week-old Sprague Dawley (SD) rats (male; weight,
185±25 g) were purchased from GemPharmatech Co. Ltd. (Nanjing,
China) and housed in a controlled environment at a temperature of
22±2˚C, under a 12/12-h light/dark cycle, 50-60% humidity, and
ad libitum access to food and distilled water. A total of 12
SD rats were randomly assigned to two groups (n=6): control and
model. Subsequently, 24 SD rats were randomly assigned to four
groups (n=6): model, model + 10 mg/kg Bre, model + 20 mg/kg Bre,
and model + 40 mg/kg Bre. The drinking water of the rats in the
model group contained PbA (0.5 g/l). In the subgroup treated with
Bre, PbA was also included in the drinking water in conjunction
with daily administration of Bre through gavage at doses of 10, 20
and 40 mg/kg. After 56 days of continuous consumption, blood
samples were collected from the abdominal arteries under ether
anesthesia. During the administration of ether anesthesia, the
following parameters were rigorously monitored to ensure the
animals were solely anesthetized and not euthanized:
i) Respiratory rate: Maintained within the range of
66-114 breaths per min.
i) Heart rate: Monitored via electrocardiogram or a
dedicated heart rate monitor, and kept within the range of 370-580
beats per min.
iii) Body temperature: Controlled within a range of
37.8-38.7˚C.
iv) Anesthetic dosage: Administered at 30-50 mg/kg
(3-5%), adjusted according to individual animal weight and response
to anesthesia.
Following euthanasia through cervical dislocation,
the heart tissues were collected for follow-up experiments. All
animal procedures were conducted in compliance with the protocols
approved by the Institutional Animal Care and Use Committee of The
Third People's Hospital of Yunnan (approval no. 2020-029; Kunming,
China).
Measurement of blood Pb levels
In accordance with a previous study (17), blood Pb levels were measured using
a graphite furnace atomic absorption spectrophotometer (TAS-990;
Beijing General Analytical Instrument) operating at a wavelength of
283.3 nm.
Histopathology
After 4% formaldehyde fixation for 24 h at 4˚C,
ethanol dehydration, and paraffin embedding, the collected
myocardial tissues were sliced into 5-µm sections. For hematoxylin
and eosin (H&E) staining, the sections were incubated with
hematoxylin for 5 min at room temperature, followed by incubation
with eosin for 2 min at room temperature. For immunohistochemical
staining, the underwent antigen retrieval in a microwave oven and
were soaked in 3% H2O2 for 20 min. Following
incubation with goat serum (Beijing Solarbio Science &
Technology Co., Ltd.) for 10 min, slices were incubated with B-cell
lymphoma 2 (Bcl-2; 1:200; cat. no. ab196495; Abcam),
Bcl-2-associated X protein (Bax; 1:250; cat. no. ab32503; Abcam),
and cleaved caspase-3 (1:400; cat. no. 9661; Cell Signaling
Technology, Inc.) overnight at 4˚C, then incubated with the goat
anti-rabbit IgG HRP-conjugated secondary antibody (1:2,000; cat.
no. ab205718; Abcam) for 15 min at room temperature. After staining
with 3,3-diaminobenzidine (DAB), the tissues were counterstained
with hematoxylin for 3 min, and images were captured using a light
microscope (Olympus Corporation).
Enzyme-linked immunosorbent assay
(ELISA)
Levels of the myocardial injury biomarkers aspartate
aminotransferase (AST; cat. no. Y120639; Shanghai Jining
Industrial), alanine aminotransferase (ALT; cat no. Y122657;
Shanghai Jining Industrial), lactate dehydrogenase (LDH; cat. no.
ml059178; Shanghai Enzyme-linked Biotechnology Co., Ltd.), cardiac
troponin I (cTnI; cat. no. Y120631; Shanghai Jining Industrial),
creatine kinase (CK; cat. no. ml026272; Shanghai Enzyme-linked
Biotechnology Co., Ltd.), and creatine kinase-MB (CK-MB; cat no.
Y118621; Shanghai Jining Industrial) in serum and cells were
measured using the corresponding ELISA kits, in accordance with the
manufacturer's instructions. Levels of the inflammatory cytokines
interleukin (IL)-1β (cat. no. ml037361), IL-6 (cat. no. ml064292),
and tumor necrosis factor-α (TNF-α; cat. no. ml002859) in cells
were determined using ELISA kits (Shanghai Enzyme-linked
Biotechnology Co., Ltd.). Oxidative stress factors, including the
ratio of glutathione (GSH) to oxidized glutathione (GSSG; cat. no.
ab138881; Abcam), as well as the content of malondialdehyde (MDA;
cat. no. E-00579; Shanghai Jining Industrial), superoxide dismutase
(SOD) and catalase (CAT; cat. no. BC0205; Solarbio, Beijing, China)
in the cells and myocardial tissues were detected using commercial
kits.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Myocardial tissue apoptosis was measured using a
TUNEL kit (cat. no. C1086; Beyotime Institute of Biotechnology).
Briefly, myocardial tissue slices were incubated with proteinase K
at 37˚C for 30 min, followed by incubation with TUNEL reaction
solution in the dark at 37˚C for 1 h. Finally, images were captured
using a fluorescence microscope (Olympus Corporation). A total of
five random fields of view per tissue section were examined for
each sample to ensure robust statistical analysis. Fields of view
were selected randomly to minimize bias.
Western blotting
Total protein from myocardial tissue or cells was
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology). The protein sample concentration was determined
using a bicinchoninic acid (BCA) kit (Beyotime Institute of
Biotechnology). After gel electrophoresis on a 10% sodium dodecyl
sulfate-polyacrylamide gel, protein samples (25 µg) were
transferred onto polyvinylidene difluoride membranes and blocked
with fat-free milk for 1 h at 25˚C. Membranes were incubated
overnight with primary antibodies at 4˚C, followed by incubation
with the goat anti-rabbit IgG HRP-conjugated secondary antibody
(1:2,000; cat. no. ab205718; Abcam) for 1 h at 25˚C. Protein bands
were visualized using the ChemiDoc XRS System (Bio-Rad
Laboratories, Inc.). Densitometric analysis was performed using
Image Lab software (Version 6.1; Bio-Rad Laboratories, Inc.). The
primary antibodies used in the present study included Bcl-2
(1:1,000), Bax (1:1,000), cleaved caspase-3 (1:1,000), Nrf2
(1:1,000; cat. no. ab92946; Abcam), heme oxygenase 1 (HO-1;
1:10,000; ab68477; Abcam), NAD(P)H quinone dehydrogenase 1 (NQO1;
1:10,000; cat. no. ab80588; Abcam), glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; 1:10,000; cat. no. ab181602; Abcam), and
lamin B1 (0.1 µg/ml; cat. no. ab16048; Abcam).
Statistical analysis
The SPSS 25.0 software (IBM Corp.) was used for
experimental data analysis, and the experimental data are displayed
as the mean ± standard deviation (SD). Prior to inferential
analyses, a test for normality of data distribution was conducted
using the Shapiro-Wilk test. The unpaired t-test was used for
comparison between groups, whereas one-way analysis of variance
(ANOVA) followed by Tukey's post hoc test was used for the
comparison of multiple groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Bre reduces Pb-induced rat
cardiomyocyte injury
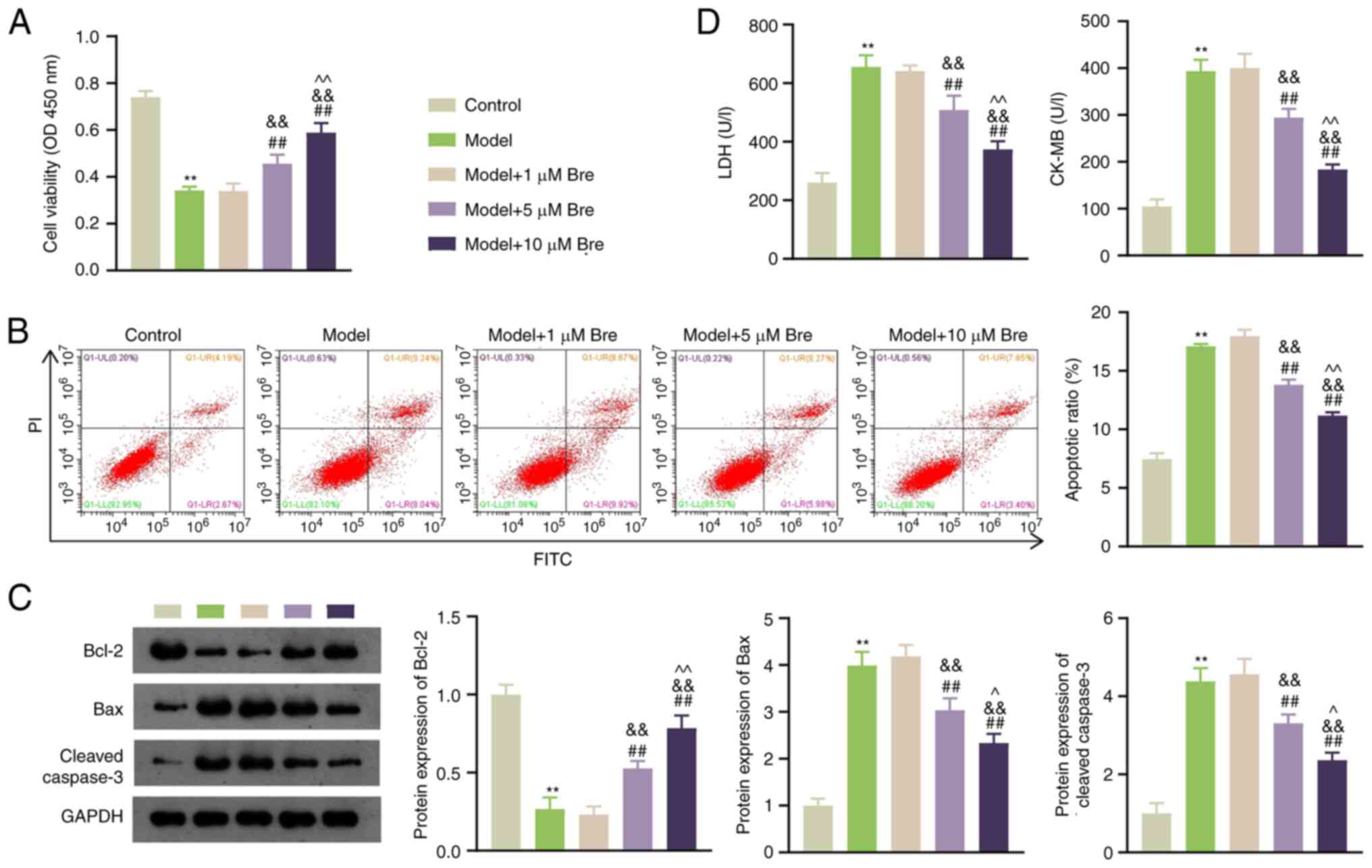
To ascertain the effect of Bre on apoptosis in rat
cardiomyocytes after Pb poisoning, H9C2 cells were pretreated with
1, 5 and 10 µM Bre for 1 h before exposure to PbA. As shown in
Fig. 1A, compared with the control
group, PbA stimulation significantly reduced the viability of H9C2
cells. Bre treatment significantly promoted H9C2 cell viability in
a dose-dependent manner (P<0.01). Moreover, PbA induction
significantly increased apoptosis compared with that of normal
cells, whereas 5 and 10 µM Bre administration decreased apoptosis
(P<0.01; Fig. 1B). The
expression levels of the pro-apoptotic protein Bax and the
downstream protein caspase-3 were higher, and that of the
anti-apoptotic protein Bcl-2 was lower in the model group than in
the control group. These trends were mitigated by Bre
administration (P<0.01; Fig.
1C). LDH and CK-MB levels are indicators of cardiomyocyte
injury (18). Bre dose-dependently
attenuated the PbA-induced upregulation of LDH and CK-MB levels
(P<0.01; Fig. 1D).
Consequently, 10 µM Bre was selected for subsequent
experiments.
Bre alleviates Pb-induced oxidative
stress in rat cardiomyocytes
To elucidate the effect of Bre on oxidative stress
in Pb-induced rat cardiomyocytes, H9C2 cells were pretreated with
NAC (a ROS scavenger) with or without Bre for 1 h before PbA
treatment. ROS production was significantly increased after PbA
exposure in H9C2 cells, and this effect was significantly decreased
by NAC or Bre treatment (P<0.01). The NAC-induced inhibition of
ROS production was further amplified by Bre treatment (P<0.01;
Fig. 2A). Simultaneously, GSH, SOD
and CAT levels, as well as the GSH/GSSG ratio, were lower, and MDA
levels were higher in the model group than in the control group
(P<0.01). Bre administration effectively reversed these changes,
and this effect was further potentiated by NAC (P<0.01; Fig. 2B). Additionally, Bre administration
substantially lowered the levels of inflammatory cytokines IL-1β,
IL-6 and TNF-α induced by PbA (P<0.01; Fig. 2C).
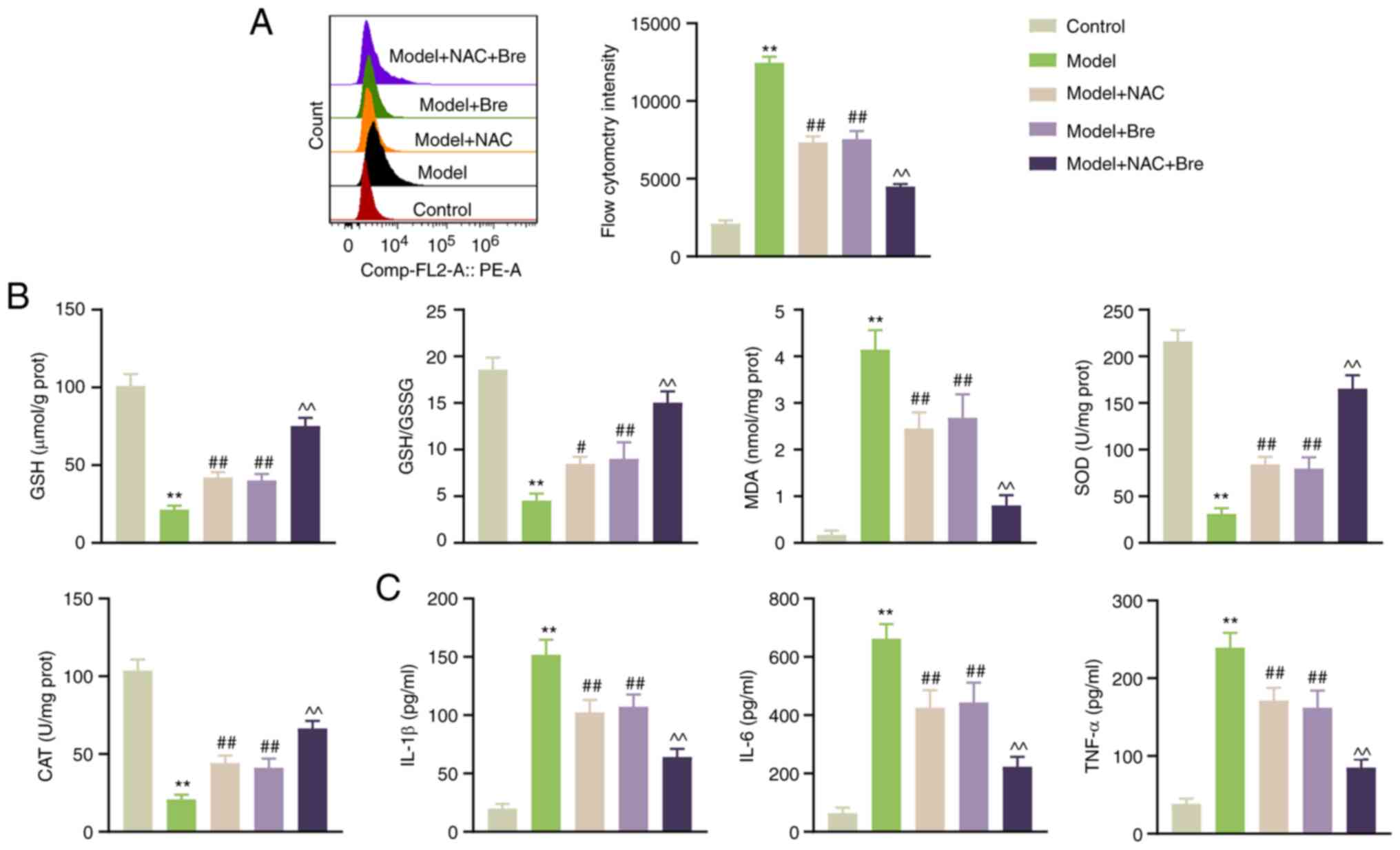 | Figure 2Bre alleviates Pb-induced oxidative
stress in rat cardiomyocytes. (A) Flow cytometry was used for
detection of reactive oxygen species. (B) GSH/GSSG ratio as well as
GSH, MDA, SOD and CAT content in H9C2 cells were measured using
ELISA. (C) IL-1β, IL-6 and TNF-α levels in H9C2 cells were
determined using ELISA. H9C2 cells were pretreated with 10 mM NAC
with or without 10 µM Bre for 1 h before lead acetate treatment.
**P<0.01 vs. control group; #P<0.05 and
##P<0.01 vs. Model; ^^P<0.01 vs. model + Bre
group. Post-hoc analyses were conducted using Tukey's test
following one-way ANOVA. Bre, Breviscapine; GSH, reduced
glutathione; GSSG, oxidized glutathione; MDA, malondialdehyde; SOD,
superoxide dismutase; CAT, catalase; NAC, N-acetylcysteine. |
Bre attenuates Pb-induced myocardial
injury by activating the Nrf2 pathway
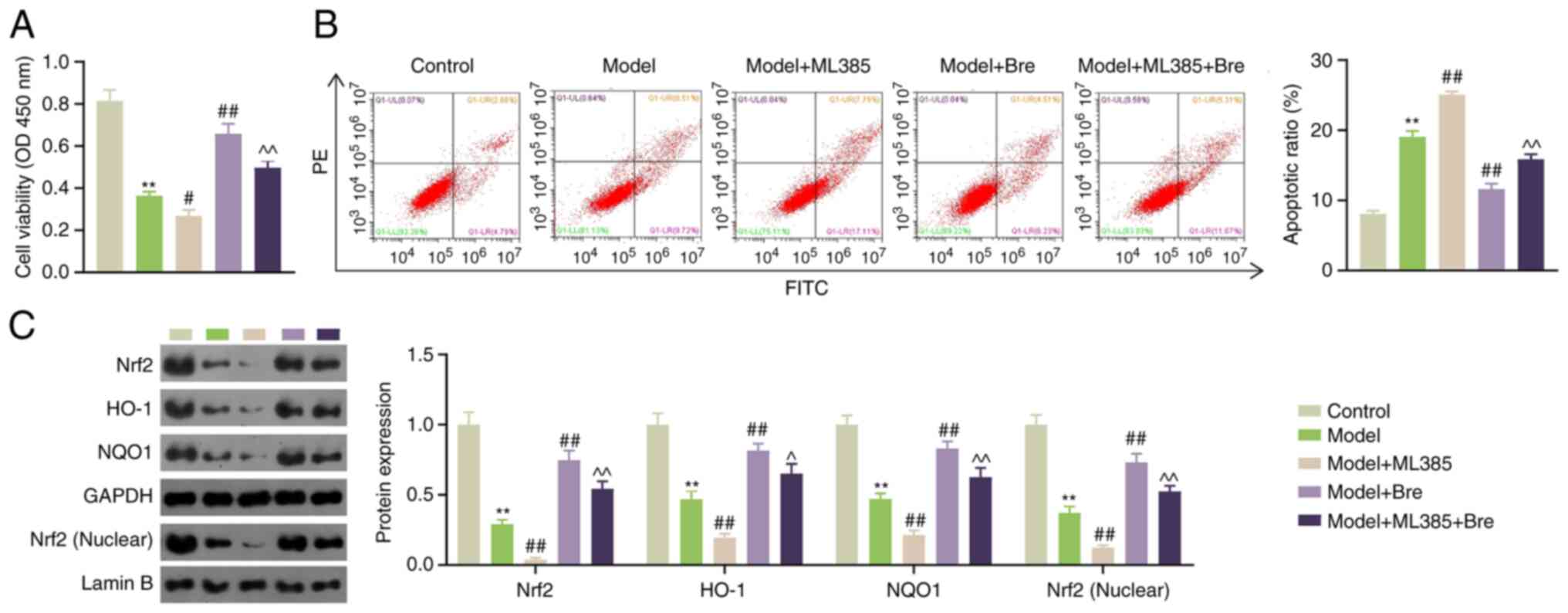
Activation of the Nrf2 pathway attenuates Pb-induced
kidney injury (16). To
investigate whether Bre alleviates Pb-induced myocardial injury by
activating the Nrf2 pathway, H9C2 cells were pretreated with Bre
and ML385 (an Nrf2 inhibitor) prior to Pb exposure. Compared with
the model group, ML385 impaired the viability of PbA-treated H9C2
cells, whereas Bre enhanced cell viability (P<0.05). ML385 also
negated the Bre-induced enhancement of cell viability (P<0.01;
Fig. 3A). Similarly, ML385 and Bre
enhanced and attenuated the apoptotic ratio of rat cardiomyocytes
exposed to Pb, respectively (P<0.01). ML385 treatment resulted
in the Bre-induced inhibition of apoptosis (P<0.01; Fig. 3B). Furthermore, western blotting
demonstrated that the expression of Nrf2, nuclear Nrf2, HO-1 and
NQO1 was downregulated in PbA-induced H9C2 cells (P<0.01). ML385
significantly reduced the expression of Nrf2, HO-1 and NQO1 in
PbA-induced rat cardiomyocytes (P<0.01). Bre administration
significantly increased the expression of Nrf2, nuclear Nrf2, HO-1
and NQO1 in PbA-induced rat cardiomyocytes, which was impaired by
the addition of ML385 (P<0.05; Fig.
3C).
Bre relieves myocardial injury in
Pb-poisoned rats
To further elucidate whether Bre can ameliorate
Pb-induced myocardial damage, a rat model of Pb poisoning was
constructed and the pathological changes and levels of Nrf2
pathway-related proteins in rat myocardial tissues were recorded.
As revealed in Fig. S1A, rats in
the control group survived and showed enhanced grasping power,
whereas rats in the model group exhibited signs of depression,
weaker grasping power, discoloration and hair loss. Importantly,
the administration of 20 and 40 mg/kg Bre significantly attenuated
these adverse effects (Fig. 4A).
Blood Pb levels in the model rats were substantially elevated
compared with those in the control rats, and this elevation was
mitigated by the addition of 20 and 40 mg/kg Bre (P<0.01;
Figs. S1B and 4B). H&E staining showed orderly
arranged myocardial cells with minimal inflammatory infiltration in
the control rats, whereas the model rats displayed partial myogenic
fiber lysis, myocardial fiber breakage and disorganized myocardial
fiber arrangement, in addition to increased inflammatory
infiltration (Fig. S1C). However,
this damage was relieved by Bre administration (Fig. 4C). ELISA results showed that the
serum levels of the myocardial injury biomarkers AST, ALT, LDH,
cTnI, CK and CK-MB increased following PbA exposure compared with
those in the controls, which were weakened by Bre (P<0.01;
Figs. S1D and 4D).
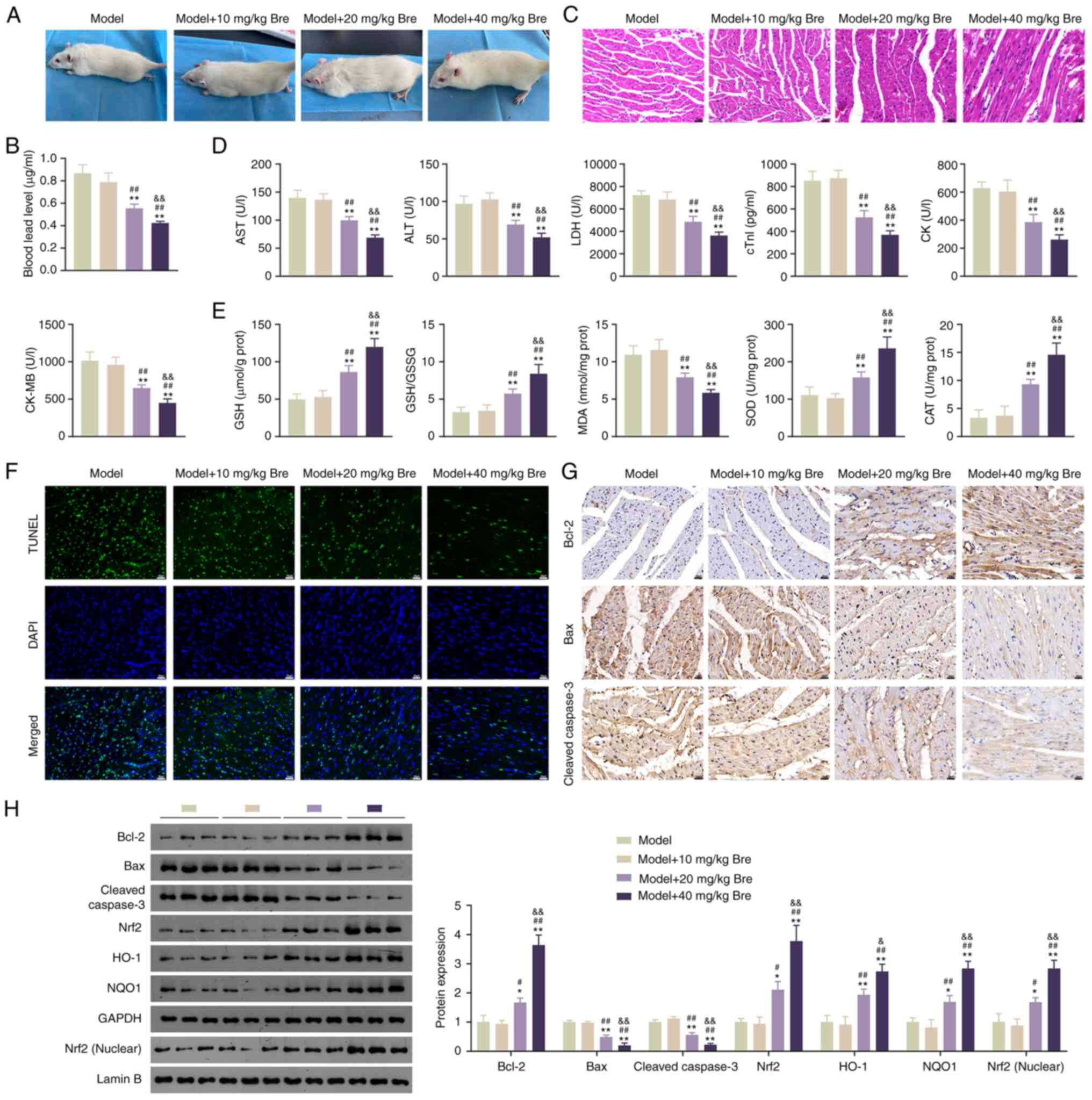 | Figure 4Bre relieves myocardial injury by
activating the Nrf2 pathway in Pb-poisoned rats. (A) The appearance
of Pb-poisoned rats. (B) Determination of Pb content in blood. (C)
H&E staining was used to assess pathological changes in rat
myocardial tissues. Scale bar, 20 µm. (D) ELISA was used to
determine AST, ALT, LDH, cTnI, CK and CK-MB levels in rat
myocardial tissues. (E) ELISA was used to determine GSH/GSSG as
well as MDA, SOD and CAT content in rat myocardial tissues. (F)
TUNEL assay was used to assess apoptosis of rat myocardial tissues.
Scale bar, 20 µm. (G) Immunohistochemistry was used to analyze
Bcl-2, Bax and cleaved caspase-3 expression in rat myocardial
tissues. Scale bar, 20 µm. (H) Western blotting was used to
determine Bcl-2, Bax, cleaved caspase-3, Nrf2, HO-1, NQO1 and
nuclear Nrf2 expression in rat myocardial tissues.
*P<0.05 and **P<0.01 vs. model group;
#P<0.05 and ##P<0.01 vs. model + 10
mg/kg Bre group; &&P<0.01 vs. model + 20
mg/kg Bre group. Post-hoc analyses were conducted using Tukey's
test following one-way ANOVA. Bre, Breviscapine; AST, aspartate
aminotransferase; ALT, alanine aminotransferase; LDH, lactate
dehydrogenase; cTnI, cardiac troponin I; CK, creatine kinase;
CK-MB, creatine kinase-MB; GSH, reduced glutathione; GSSG, oxidized
glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; CAT,
catalase; Nrf2, Nuclear factor erythroid-2 related factor 2; HO-1,
heme oxygenase-1; NQO1, NAD(P)H dehydrogenase quinone 1. |
Further investigations were conducted to assess the
effects of Bre on oxidative stress and apoptosis of myocardial
histiocytes in Pb-exposed rats. The results demonstrated that the
levels of GSH, SOD and CAT, as well as the GSH/GSSSG ratio,
decreased in the model group. By contrast, MDA levels were elevated
compared with those in the controls (P<0.01; Fig. S1E). These phenomena were reversed
by Bre treatment (P<0.01; Fig.
4E). Additionally, the apoptosis-associated green fluorescence
signal increased in the model group compared with the control group
and was attenuated by Bre in a dose-dependent manner (Figs. S1F and 4F). Immunochemical staining revealed that
Bre administration reversed PbA-induced upregulation of Bax and
cleaved caspase-3 and downregulation of Bcl-2 (Figs. S1G and 4G). Western blotting corroborated these
findings (P<0.05; Figs. S1H
and 4H). Furthermore, Bre addition
reversed the PbA-induced downregulation of Nrf2, nuclear Nrf2, HO-1
and NQO1 (P<0.05; Figs. S1H
and 4H).
Discussion
Early studies have delineated the toxic effects of
chronic Pb exposure on the cardiovascular system (19). In the present study, it was
determined that Bre ameliorates Pb-induced myocardial injury. The
underlying mechanism may involve activation of the Nrf2 pathway,
which mitigates Pb-induced oxidative stress.
Apoptosis is the primary cause of tissue damage
(20). The anti-apoptotic protein
Bcl-2, pro-apoptotic protein Bax, and downstream protein caspase-3
play important roles in the apoptotic process (21). Yuan et al (22) reported that Bre promotes
cardiomyocyte proliferation and alleviates apoptosis in hypoxic
environments. In the present study, Pb exposure reduced H9C2 cell
viability, decreased Bcl-2 expression, and increased Bax and
cleaved caspase-3 expression relative to controls. These changes
were reversed by Bre supplementation. The findings of the present
study extend the current understanding of the effects of BRE on
cell viability and proliferation. Moreover, AST, ALT, cTnI, LDH, CK
and CK-MB have been identified as reliable biomarkers of myocardial
injury (23). The present results
corroborated that Bre treatment attenuates Pb-induced upregulation
of AST, ALT, cTnI, LDH, CK and CK-MB in myocardial tissues, which
is consistent with the findings of a previous study (24). The results suggested that Bre
ameliorated Pb-induced myocardial injury.
Toxic metals induce excessive ROS production in the
tissues, thereby triggering oxidative stress (3). This is a hallmark phenomenon in the
development of various diseases, such as cardiovascular diseases
(25). Endogenous antioxidant
enzyme systems act as defense mechanisms against internal oxidative
stress (26). The GSH/GSSG ratio
maintains redox homeostasis in cardiomyocytes by reducing excess
ROS production (27,28). The present study revealed that PbA
exposure in rats significantly decreased antioxidant parameters,
including GSH, CAT and SOD levels, as well as the GSH/GSSG ratio in
myocardial tissues, while concomitantly elevating oxidative
parameters and MDA content. These findings were consistent with a
previous study (29). Excessive
ROS production is responsible for DOX-induced endothelial toxicity
and cardiotoxicity (30,31). Bre treatment increased the contents
of GSH, CAT and SOD, as well as the GSH/GSSG ratio, and decreased
the MDA content. In addition, in vitro experiments showed
that NAC, acting as a ROS scavenger, diminished ROS and MDA levels
while augmenting GSH and SOD levels and the GSH/GSSG ratio. Bre
treatment exhibited an antioxidant effect comparable to that of
NAC, and their combination provided synergistic benefits. These
results indicated that Bre can attenuate oxidative stress in
Pb-exposed cells and rat models.
ROS also facilitates inflammation and cytokine
production. The inflammatory response participates in essential
physiological processes, including the elimination of injured cells
and tissues and the facilitation of cell and tissue repair
(32). The ELISA results of the
present study showed that PbA treatment increased the levels of
inflammatory cytokines in H9C2 cells, which were alleviated by Bre
administration. Bre reduced inflammatory cell infiltration in the
myocardial tissue of Pb-exposed rats. Collectively, these findings
suggested that Bre can inhibit the inflammatory response in the
myocardial tissues, thereby mitigating myocardial damage. This
supports the growing interest in BRE as a promising
anti-inflammatory agent.
Nrf2 is a central regulator of oxidative stress
(33). Numerous studies have
reported that Nrf2 maintains redox homeostasis and prevents tissue
damage caused by oxidative stress following exposure to heavy
metals (34-37).
In the present study, Bre treatment significantly enhanced the
expression of Nrf2 pathway-related proteins (Nrf2, HO-1 and NQO1),
corroborating the results of a previous study (16). Interestingly, the addition of the
Nrf2 inhibitor ML385 increased apoptosis, which was suppressed by
Bre treatment. These findings suggested that Bre attenuated
Pb-induced myocardial injury by activating the Nrf2 pathway.
A notable contribution of the present study is the
examination of the potentially multifaceted pharmacological roles
of Bre. The current results suggested that Bre functions as a
multifunctional agent, implementing the activation of specific
signaling pathways such as the Nrf2 pathway, in addition to its
known anti-inflammatory and antioxidant effects. These interactions
between Bre and these signaling pathways require further
investigation to elucidate novel therapeutic applications.
In conclusion, the present study showed that Bre
alleviated Pb-induced myocardial injury by activating the Nrf2
pathway. This insight enriches the understanding of the mechanisms
by which Bre mitigates oxidative stress and highlights its
potential use in therapeutic interventions for Pb poisoning.
However, certain limitations exist in the present study, such as
that the focus was solely on the Nrf2 pathway as a mechanism for
alleviating Pb-induced myocardial injury, without exploring
alternative pathways. This introduces specific scientific gaps,
which should be addressed in future studies. The present study
serves as a foundational framework for future Pb poisoning
treatment approaches.
Supplementary Material
Pb poisoning induces myocardial injury
in rats. (A) Observation of the appearance of Pb-poisoned rats. (B)
Determination of Pb content in blood. (C) H&E staining was used
to assess pathological changes in rat myocardial tissues. Scale
bar, 20 μm. (D) ELISA was used to measure AST, ALT, LDH,
cTnI, CK and CK-MB levels in rat myocardial tissues. (E) ELISA was
used to measure GSH, GSH/GSSG, MDA, SOD and CAT contents in rat
myocardial tissues. (F) TUNEL assay was used to assess apoptosis of
rat myocardial tissues. Scale bar, 20 μm. (G)
Immunohistochemical staining was used to measure the expression of
Bcl-2, Bax and cleaved caspase-3 in rat myocardial tissues. Scale
bar, 20 μm. (H) Western blotting was used to analyze Bcl-2,
Bax, cleaved caspase-3, Nrf2, HO-1, NQO1 and nuclear Nrf2
expression in rat myocardial tissues. *P<0.05 and
**P<0.01 vs. control group. Post-hoc analyses were
conducted using the unpaired t-test. AST, aspartate
aminotransferase; ALT, alanine aminotransferase; LDH, lactate
dehydrogenase; cTnI, cardiac troponin I; CK, creatine kinase;
CK-MB, creatine kinase-MB; GSH, reduced glutathione; GSSG, oxidized
glutathione; MDA, malondialdehyde; CAT, catalase; Nrf2, Nuclear
factor erythroid-2 related factor 2; HO-1, heme oxygenase-1; NQO1,
NAD(P)H dehydrogenase quinone 1.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Special Basic
Cooperative Research Programs of Yunnan Provincial Undergraduate
Universities' Association (grant no. 2018FH001-089) and the Fund of
Research Institution in Yunnan Provincial Medical and Health Units
(grant no. 2018NS0218).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL, ZX and YL were responsible for study
conceptualization. DL, ZX, YH, JY, HL and BZ contributed to data
curation, formal analysis, investigation and methodology. YL and YH
were responsible for project administration, resources and
supervision. DL analyzed and interpreted the software results. ZX,
YH, JY and BZ repeated the experiments and confirm the authenticity
of all the raw data. DL and ZX contributed to visualization and
writing of the original draft. YL reviewed and edited the
manuscript. DL was responsible for funding acquisition. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Research related to animal use complied with all
relevant national regulations and institutional policies for animal
care and use. The present study was approved (approval no.
2020-029) by the Institutional Animal Care and Use Committee of the
Third People's Hospital of Yunnan (Kunming, China).
Patient consent for publication.
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanna-Attisha M, Lanphear B and Landrigan
P: Lead poisoning in the 21st century: The silent epidemic
continues. Am J Public Health. 108(1430)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Charkiewicz AE and Backstrand JR: Lead
Toxicity and Pollution in Poland. Int J Environ Res Public Health.
17(4385)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Balali-Mood M, Naseri K, Tahergorabi Z,
Khazdair MR and Sadeghi M: Toxic mechanisms of five heavy metals:
Mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol.
12(643972)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Babiker F, Al-Kouh A and Kilarkaje N: Lead
exposure induces oxidative stress, apoptosis, and attenuates
protection of cardiac myocytes against ischemia-reperfusion injury.
Drug Chem Toxicol. 42:147–156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang D, Li S, Gao L, Lv Z, Bing Q, Lv Q,
Zheng X, Li R and Zhang Z: Dietary grape seed procyanidin extract
protects against lead-induced heart injury in rats involving
endoplasmic reticulum stress inhibition and AKT activation. J Nutr
Biochem. 62:43–49. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferreira G, Santander A, Chavarría L,
Cardozo R, Savio F, Sobrevia L and Nicolson GL: Functional
consequences of lead and mercury exposomes in the heart. Mol
Aspects Med. 87(101048)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gao J, Chen G, He H, Liu C, Xiong X, Li J
and Wang J: Therapeutic effects of breviscapine in cardiovascular
diseases: A review. Front Pharmacol. 8(289)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yan L, Huang H, Tang QZ, Zhu LH, Wang L,
Liu C, Bian ZY and Li H: Breviscapine protects against cardiac
hypertrophy through blocking PKC-alpha-dependent signaling. J Cell
Biochem. 109:1158–1171. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li MJ, Sun WS, Yuan Y, Zhang YK, Lu Q, Gao
YZ, Ye T and Xing DM: Breviscapine remodels myocardial glucose and
lipid metabolism by regulating serotonin to alleviate
doxorubicin-induced cardiotoxicity. Front Pharmacol.
13(930835)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen ZQ, Zhou Y, Chen F, Huang JW, Zheng
J, Li HL, Li T and Li L: Breviscapine pretreatment inhibits
myocardial inflammation and apoptosis in rats after coronary
microembolization by activating the PI3K/Akt/GSK-3β Signaling
pathway. Drug Des Devel Ther. 15:843–855. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang M, Zhang WB, Zhu JH, Fu GS and Zhou
BQ: Breviscapine ameliorates cardiac dysfunction and regulates the
myocardial Ca(2+)-cycling proteins in streptozotocin-induced
diabetic rats. Acta Diabetol. 47 (Suppl 1):S209–S218.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang M, Yu X, Li D, Ma N, Wei Z, Ci X and
Zhang S: Nrf2 signaling pathway mediates the protective effects of
daphnetin against D-Galactose induced-premature ovarian failure.
Front Pharmacol. 13(810524)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Latella G: Redox imbalance in intestinal
fibrosis: Beware of the TGFβ-1, ROS, and Nrf2 connection. Dig Dis
Sci. 63:312–320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tonelli C, Chio IIC and Tuveson DA:
Transcriptional regulation by Nrf2. Antioxid Redox Signal.
29:1727–1745. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen QM: Nrf2 for cardiac protection:
Pharmacological options against oxidative stress. Trends Pharmacol
Sci. 42:729–744. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Song Y, Sun H, Gao S, Tang K, Zhao Y, Xie
G and Gao H: Saikosaponin a attenuates lead-induced kidney injury
through activating Nrf2 signaling pathway. Comp Biochem Physiol C
Toxicol Pharmacol. 242(108945)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen F, Zhou CC, Yang Y, Liu JW and Yan
CH: GM1 ameliorates lead-induced cognitive deficits and brain
damage through activating the SIRT1/CREB/BDNF pathway in the
developing male rat hippocampus. Biol Trace Elem Res. 190:425–436.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ding S, Liu D, Wang L, Wang G and Zhu Y:
Inhibiting MicroRNA-29a protects myocardial ischemia-reperfusion
injury by targeting SIRT1 and suppressing oxidative stress and
NLRP3-Mediated pyroptosis pathway. J Pharmacol Exp Ther.
372:128–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zawadzki M, Poreba R and Gać P: Mechanisms
and toxic effects of lead on the cardiovascular system. Med Pr.
57:543–549. 2006.PubMed/NCBI(In Polish).
|
|
20
|
Yao L, Chen H, Wu Q and Xie K:
Hydrogen-rich saline alleviates inflammation and apoptosis in
myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med.
44:1048–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vukojevic K, Carev D, Sapunar D, Petrovic
D and Saraga-Babic M: Developmental patterns of caspase-3, bax and
bcl-2 proteins expression in the human spinal ganglia. J Mol
Histol. 39:339–349. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yuan Y, He P, Tang X, Li J and Mu J:
Effect of breviscapine on proliferation and apoptosis of H9c2
cardiomyocytes and activation of ERK1/2 signaling pathway. J Pract
Med. 36:1611–1615. 2020.(In Chinese).
|
|
23
|
Afsar T, Razak S, Batoo KM and Khan MR:
Acacia hydaspica R: Parker prevents doxorubicin-induced cardiac
injury by attenuation of oxidative stress and structural
Cardiomyocyte alterations in rats. BMC Complement Altern Med.
17(554)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang J, Ji SY, Liu SZ, Jing R and Lou WJ:
Cardioprotective effect of breviscapine: Inhibition of apoptosis in
H9c2 cardiomyocytes via the PI3K/Akt/eNOS pathway following
simulated ischemia/reperfusion injury. Pharmazie. 70:593–597.
2015.PubMed/NCBI
|
|
25
|
Hayden J and Bostick B: Western diet
induced obesity increases oxidative stress in the heart by
impairing the Nrf2 antioxidant response pathway. J Am Coll Cardiol.
73 (9 Suppl)(S896)2019.
|
|
26
|
Prasai PK, Shrestha B, Orr AW and Pattillo
CB: Decreases in GSH:GSSG activate vascular endothelial growth
factor receptor 2 (VEGFR2) in human aortic endothelial cells. Redox
Biol. 19:22–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Quintana-Cabrera R, Fernandez-Fernandez S,
Bobo-Jimenez V, Escobar J, Sastre J, Almeida A and Bolaños JP:
γ-Glutamylcysteine detoxifies reactive oxygen species by acting as
glutathione peroxidase-1 cofactor. Nat Commun.
3(718)2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Giustarini D, Tsikas D, Colombo G, Milzani
A, Dalle-Donne I, Fanti P and Rossi R: Pitfalls in the analysis of
the physiological antioxidant glutathione (GSH) and its disulfide
(GSSG) in biological samples: An elephant in the room. J Chromatogr
B Analyt Technol Biomed Life Sci. 1019:21–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jiang X, Xing X, Zhang Y, Zhang C, Wu Y,
Chen Y, Meng R, Jia H, Cheng Y, Zhang Y and Su J: Lead exposure
activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and
induces reproductive damage in female mice. Ecotoxicol Environ Saf.
207(111231)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He H, Wang L, Qiao Y, Zhou Q, Li H, Chen
S, Yin D, Huang Q and He M: Doxorubicin induces endotheliotoxicity
and mitochondrial dysfunction via ROS/eNOS/NO pathway. Front
Pharmacol. 10(1531)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qiao Y, Hu T, Yang B, Li H, Chen T, Yin D,
He H and He M: Capsaicin alleviates the deteriorative mitochondrial
function by upregulating 14-3-3η in anoxic or anoxic/reoxygenated
cardiomyocytes. Oxid Med Cell Longev. 2020(1750289)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guo J, Pu Y, Zhong L, Wang K, Duan X and
Chen D: Lead impaired immune function and tissue integrity in
yellow catfish (Peltobargus fulvidraco) by mediating oxidative
stress, inflammatory response and apoptosis. Ecotoxicol Environ
Saf. 226(112857)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yu L, Li HX, Guo JY, Huang YQ, Wang H,
Talukder M and Li JL: Di (2-ethyl hexyl) phthalate (DEHP)-induced
spleen toxicity in quail (Coturnix japonica) via disturbing
Nrf2-mediated defense response. Environ Pollut. 251:984–989.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang D, Lv Z, Zhang H, Liu B, Jiang H, Tan
X, Lu J, Baiyun R and Zhang Z: Activation of the Nrf2 Signaling
pathway involving KLF9 plays a critical role in allicin resisting
against arsenic trioxide-induced hepatotoxicity in rats. Biol Trace
Elem Res. 176:192–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ursini F, Maiorino M and Forman HJ: Redox
homeostasis: The Golden Mean of healthy living. Redox Biol.
8:205–215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li J, Zheng X, Ma X, Xu X, Du Y, Lv Q, Li
X, Wu Y, Sun H, Yu L and Zhang Z: Melatonin protects against
chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2
pathway. J Inorg Biochem. 197(110698)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu B, Jiang H, Lu J, Baiyun R, Li S, Lv
Y, Li D, Wu H and Zhang Z: Grape seed procyanidin extract
ameliorates lead-induced liver injury via miRNA153 and
AKT/GSK-3β/Fyn-mediated Nrf2 activation. J Nutr Biochem.
52:115–123. 2018.PubMed/NCBI View Article : Google Scholar
|