Introduction
Parkinson's disease (PD) is a degenerative
neurological disorder that is prevalent worldwide, affecting 3.7%
individuals aged >65 years (1).
At present, the prevalence rate of PD is high and 7-10 million
individuals worldwide are reported to suffer from PD (2). PD has a chronic course, involving
main pathological features including dopamine deficiency and
degeneration of substantia nigra dopamine neurons (3). In addition, PD is characterized by a
high disability rate, impairing the performance of daily
activities, which are mediated by dopamine. Therefore,
understanding the pathogenesis of PD and the development of
effective drug treatments are important for improving the quality
of life of patients with PD whilst reducing the disease burden on
society.
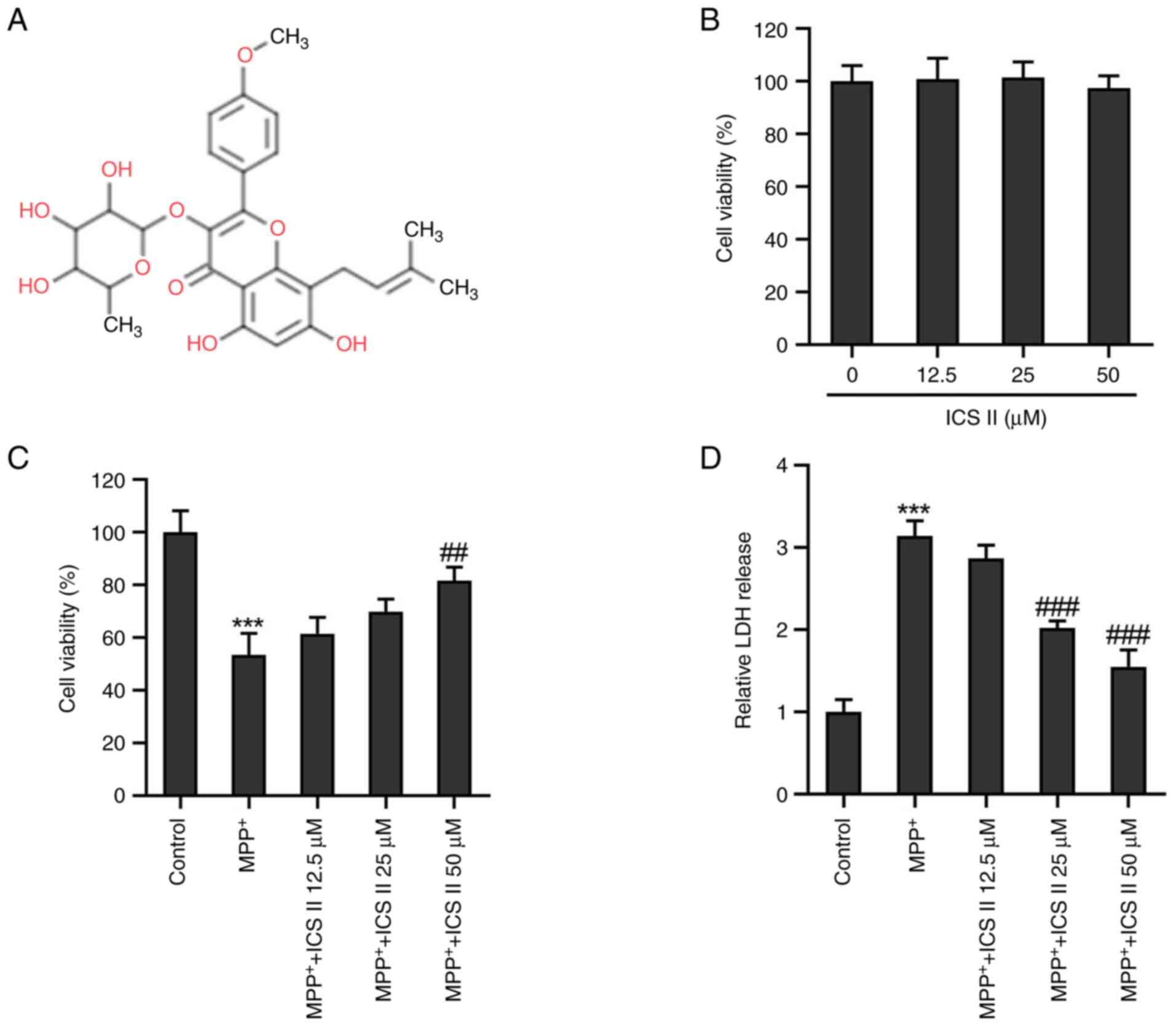
Icariside II (ICS II; Fig. 1A) is an active flavonoid that can
be extracted from the Chinese herb Epimedium, which has been
shown to inhibit the inflammatory response and microcirculation
disturbance, and reduce the damage caused by vascular dementia
(4). In addition, ICS II has been
reported to show protective activity in the central nervous system
(5,6). ICS II can confer therapeutic effects
against certain neurodegenerative diseases, such as Alzheimer's
disease (AD) (7-10).
In a model of streptozotocin-induced rats with AD, ICS II treatment
was found to increase the survival of hippocampal neurons and
inhibit neuroinflammation (7).
Furthermore, ICS II has been demonstrated to inhibit neuronal
apoptosis and neuroinflammation in amyloid β-peptide 25-35-induced
AD rats (8). ICS II has also been
observed to improve neurogenesis and inhibit mitochondrial
division, contributing to cognitive recovery in AD mice (9). In another previous study, ICS II was
shown to improve spatial learning and memory impairment in AD mice
(10). However, to the best of our
knowledge, the potential effects of ICS II on PD remain
unclear.
Histone deacetylases (HDACs) have previously been
reported to be epigenetic targets for treating PD (11). HDAC2 is a class I HDAC that has
been reported to serve an important role in chromosome structure
modification and gene expression regulation (12). Histone deacetylation facilitates
the binding of DNA to the histone octamer, which stabilizes the
nucleosome structure and prevents the specific binding of certain
transcription factors to DNA binding sites, in turn inhibiting gene
expression (13,14). Previous studies have reported that
HDAC2 inhibition can prevent the loss of microglial cells and
dopaminergic neurons in the substantia nigra in PD (15,16).
1-methyl-4-phenylpyridinium (MPP+) is the
neurotoxic form of methyl-4-phenyl-1,2,3,6-tetrahydropyridine
(mPTP). MPP+ can been taken up by dopaminergic neurons,
leading to mitochondrial dysfunction, oxidative stress and
programmed cell death, which simulates the parkinsonian syndrome in
cell and animal models (17). In
the present study, the 1-methyl-4-phenylpyridinium
(MPP+)-induced SK-N-SH cell model was used to simulate
PD in vitro. The present study aimed to evaluate the
potential effects of ICS II on MPP+-induced SK-N-SH cell
injury, in addition to understanding the underlying mechanism of
action.
Materials and methods
Bioinformatics tools
A search of the SuperPreD database (https://prediction.charite.de/subpages/target_prediction.php)
demonstrated that both HDAC2 and HDAC8 were potential targets for
ICS II. Based on a relatively high value of ‘Model accuracy’ (93.99
and 94.75% for HDAC8 and HDAC2, respectively), HDAC2 was selected
for further investigation in the present study.
Cell culture and treatment
The human neuroblastoma cell line SK-N-SH (cat. no.
CL-0214) was purchased from Procell Life Science & Technology
Co., Ltd. SK-N-SH cells were cultured in DMEM (HyClone; Cytiva)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin mixture (Thermo Fisher Scientific, Inc.) at
37˚C with 5% CO2.
SK-N-SH cells were pre-treated with 2 mM
MPP+ (MilliporeSigma) for 24 h at 37˚C to establish the
in vitro PD cell model (18). MPP+-induced and
untreated control SK-N-SH cells were treated with different
concentrations of ICS II (0, 12.5, 25 and 50 µM; MilliporeSigma)
for 24 h at 37˚C (19).
MTT assay
After the aforementioned cell treatments, SK-N-SH
cells were seeded into a 96-well plate at a density of
1x103 cells/well and cultured for 24 h at 37˚C. SK-N-SH
cells were then incubated with 10 µl 5 mg/ml MTT reagent
(MilliporeSigma) for 4 h at 37˚C. After removing the culture
supernatant, SK-N-SH cells were incubated with 250 µl DMSO for 20
min at room temperature to dissolve the formazan crystals. The
absorbance at a 570 nm wavelength was detected using a microplate
reader.
Cell transfection
HDAC2 (NC_000006.12) was cloned into the pcDNA3.1
vector to obtain HDAC2-overexpression (Oe-HDAC2) vector (Guangzhou
Ruibio Co., Ltd.) and the empty pcDNA3.1 vector was used as a
negative control (Oe-NC). SK-N-SH cells were transiently
transfected with 1 µg Oe-NC or 1 µg Oe-HDAC2 for 48 h at 37˚C using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's instructions. At 48 h
post-transfection, subsequent experiments were conducted.
Immunofluorescence
After the aforementioned treatments, SK-N-SH cells
cultured in 24-well plates at a density of 5x104
cells/well were fixed with 4% paraformaldehyde at 4˚C for 15 min
and incubated in 0.1% Triton X-100 in PBS for 15 min at 37˚C. After
blocking with 10% BSA (Gold Biotechnology) for 1 h at room
temperature, SK-N-SH cells were incubated with a primary antibody
against 8-hydroxydesoxyguanosin (8-OHdG; cat. no. sc-393871; 1:100
dilution; Santa Cruz Biotechnology, Inc.) overnight at 4˚C. The
cells were then incubated with FITC-conjugated goat anti-mouse
secondary antibodies (cat. no. ab6785; 1:1,000 dilution; Abcam) for
1 h at room temperature. SK-N-SH cell nuclei were stained with 1
µg/ml DAPI (MilliporeSigma) for 30 min at room temperature, before
being imaged using a fluorescence microscope (Olympus Corp.).
Western blot analysis
After the aforementioned treatments, SK-N-SH cells
were lysed using RIPA lysis buffer (Hunan Auragene Biotech Co.,
Ltd.), before being centrifuged for 10 min at 10,000 x g at 4˚C to
obtain total proteins. The concentration of total proteins was
measured using a BCA assay kit (Beyotime Institute of
Biotechnology). The protein samples (30 µg per lane) were subjected
to 10% SDS-PAGE before being transferred onto PVDF membranes
(MilliporeSigma). After blocking with 5% skimmed milk for 1 h at
room temperature, membranes were incubated with primary antibodies
against γ-H2A histone family member X (γ-H2AX; 1:5,000 dilution;
cat. no. ab81299; Abcam), HDAC2 (1:2,000 dilution; cat. no.
ab32117; Abcam) and GAPDH (1:2,500 dilution; cat. no. ab9485;
Abcam) overnight at 4˚C. The next day, after washing using TBS-0.1%
Tween-20, membranes were incubated with goat anti-rabbit
HRP-conjugated secondary antibodies (1:3,000 dilution; cat. no.
ab6721; Abcam) for 1 h at room temperature. Protein bands were
visualized using an Immobilon Western HRP Substrate
(MilliporeSigma). The band intensity of proteins was
semi-quantified using the ImageJ software (version 1.49; National
Institutes of Health).
JC-1 staining
After the aforementioned cell treatments, the
mitochondrial membrane potential of SK-N-SH cells was detected
using a JC-1 assay kit (cat. no. C2006; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Briefly, SK-N-SH cells were cultured in six-well plates at
3x105/well for 24 h at 37˚C, followed by staining with
JC-1 solution for 20 min at 37˚C in the dark. Finally, SK-N-SH
cells were washed using PBS, before being imaged using a
fluorescence microscope (Olympus Corp.) at an excitation wavelength
of 488 nm and an emission wavelength of 525 nm to determine the
fluorescence intensity.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from SK-N-SH cells using the
TRIzol® reagent (Thermo Fisher Scientific, Inc.), which
was then converted to cDNA using PrimeScript™ RT Master
Mix (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. cDNA was then amplified by RT-qPCR
using SYBR® Premix Ex Taq™ (Takara
Biotechnology Co., Ltd.) in a 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used: Initial denaturation at 94˚C
for 10 min, followed by 40 cycles at 94˚C for 10 sec, 60˚C for 20
sec and 72˚C for 1 min. The primer sequences used in the present
study were as follows: HDAC2 forward (F),
5'-GCTATTCCAGAAGATGCTGTTC-3' and reverse (R),
5'-GTTGCTGAGCTGTTCTGATTTG-3'; and GAPDH F,
5'-CAGGAGGCATTGCTGATGAT-3' and R, 5'-GAAGGCTGGGGCTCATTT-3'. mRNA
expression was quantified using the 2-∆∆Cq method and
normalized to GAPDH (20).
For the measurement of the mitochondrial DNA (mtDNA)
content, total DNA extracted from SK-N-SH cells was purified using
TIANamp Genomic DNA Kit (cat. no. DP304; Tiangen Biotech Co., Ltd.)
according to the manufacturer's instructions. The relative mtDNA
copy number was evaluated via qPCR amplification of the
mitochondrial D-loop using SYBR® Premix Ex
Taq™ (Takara Biotechnology Co., Ltd.) as mentioned above
using the following primers: F, 5'-ATGGCCAACCTCCTACTCCT-3' and R,
5'-GCGGTGATGTAGAGGGTGAT-3', with GAPDH as a normalization
control.
Detection of lactate dehydrogenase
(LDH) release, ATP level and complex I activity
LDH release, ATP levels and Complex I activity of
treated SK-N-SH cells seeded into 96-well plates at a density of
5x104 cells/well were determined using the LDH
Cytotoxicity Assay Kit (cat. no. C0016; Beyotime Institute of
Biotechnology), ATP Assay Kit (cat. no. S0026; Beyotime Institute
of Biotechnology) and Complex I Enzyme Activity Microplate Assay
Kit (colorimetric; cat. no. ab109721; Abcam) according to the
manufacturer's instructions.
Mitochondrial permeability transition
pore (mPTP) opening evaluation
Cellular mPTP opening was measured using an mPTP
Assay Kit (cat. no. C2009S; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. SK-N-SH cells seeded
in 24-well plates (5x105 cells/well) were stained using
5 µM Calcein AM reaction mixture (Santa Cruz Biotechnology, Inc.)
for 30 min at 37˚C in the dark. After washing with PBS, the
fluorescence intensity was observed using a fluorescence microscope
(Olympus Corp.) at 490 nm for excitation and 515 nm for emission.
The loss of calcein fluorescence in SK-N-SH cells indicated the
opening of the mPTP.
Molecular docking
The crystal structure of HDAC2 [protein data bank
(PDB) ID: 4LY1] was downloaded from the PDB website (http://www.rcsb.org/) and saved in PDB format. The 3D
structure of ICS II was obtained from the PubChem database
(https://pubchem.ncbi.nlm.nih.gov/compound/13964067#section=3D-Conformer).
Molecular docking was used to predict the optimal binding site of
ICS II to HDAC2 using AutoDock (version 4.2; Scripps Institute).
The optimal binding mode between ICS II and HDAC2 was acquired
under the minimum binding free energy conformation, before the
output results were visualized in PyMOL (version 2.2.0) software
(Schrödinger, LLC).
Statistical analysis
GraphPad Prism 8 (GraphPad Software; Dotmatics) was
used to perform statistical analysis. The results are expressed as
the mean ± standard deviation. One-way analysis of variance and
Tukey's post-hoc test were used to compare differences among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ICS II mitigates SK-N-SH cytotoxicity
induced by MPP+
To explore the cytotoxicity of ICS II, SK-N-SH cells
were treated with different concentrations (0, 12.5, 25 and 50 µM)
of ICS II. The MTT assay demonstrated that the different
concentrations of ICS II tested did not influence SK-N-SH cell
viability (Fig. 1B), suggesting
that ICS II was not harmful to SK-N-SH cells at the concentrations
tested in the present study. By contrast, treatment with 2 mM
MPP+ significantly decreased the viability of SK-N-SH
cells compared with that in the control group, which was in turn
significantly reversed by treatment with 50 µM ICS II (Fig. 1C). LDH release by
MPP+-induced SK-N-SH cells was found to be significantly
increased compared with that in the control group, but ICS II
treatment (25 and 50 µM) significantly decreased the LDH release
induced by MPP+ in SK-N-SH cells (Fig. 1D). These results suggest that ICS
II restored the viability of SK-N-SH cells treated with
MPP+.
ICS II alleviates DNA damage in
SK-N-SH cells induced by MPP+
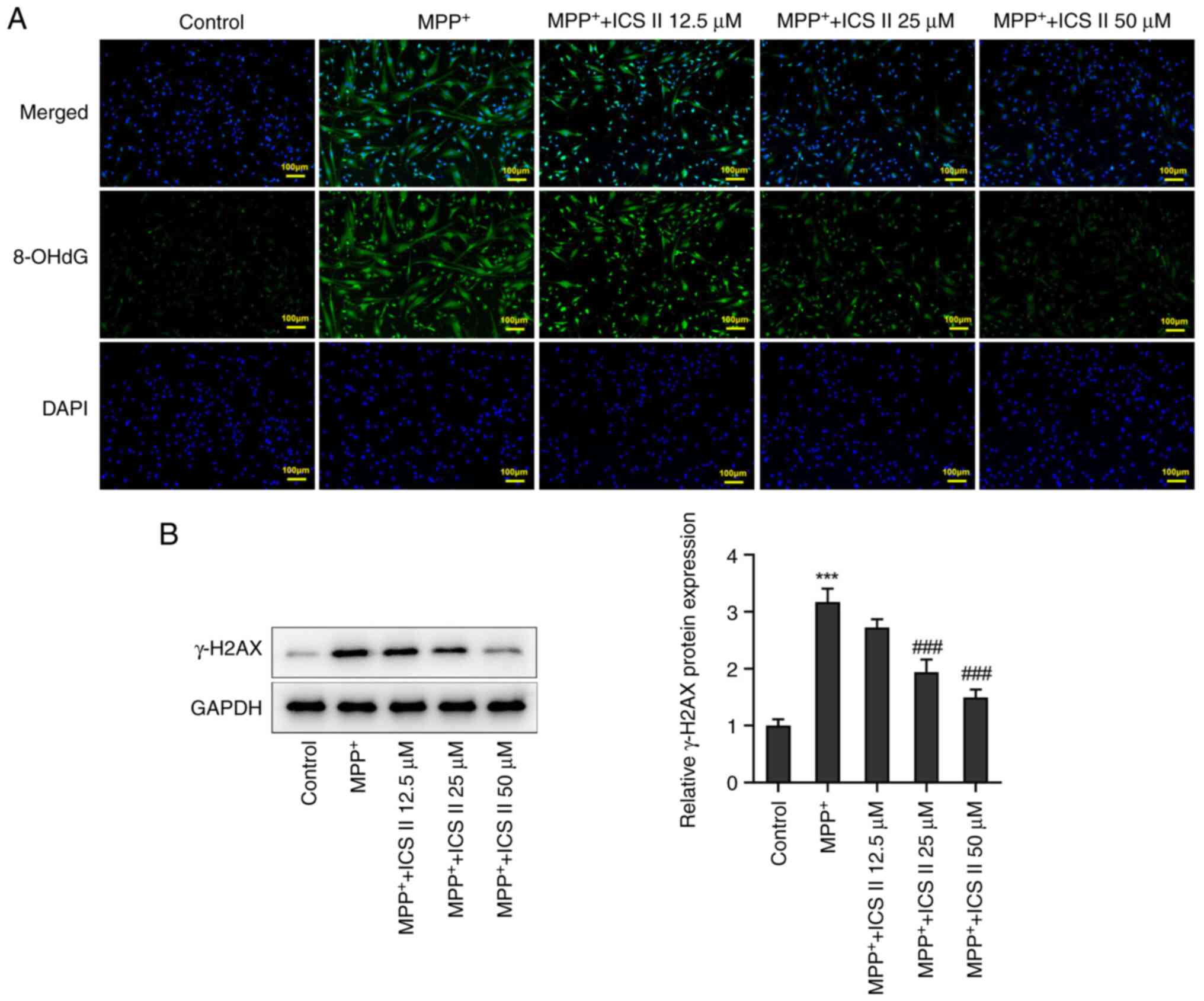
8-OHdG is a marker of free radical-induced oxidative
DNA lesions (21). MPP+
treatment caused a marked increase in the production of 8-OHdG in
SK-N-SH cells compared with that in the control group, suggesting
that MPP+ caused DNA damage in SK-N-SH cells. By
contrast, ICS II reversed the production of 8-OHdG in
MPP+-treated SK-N-SH cells in a dose-dependent manner
(Fig. 2A). The protein expression
level of γ-H2AX was also found to be significantly increased in
MPP+-induced SK-N-SH cells compared with that in the
control group, but this was in turn significantly reversed by 25
and 50 µM ICS II treatment (Fig.
2B). These results suggest that ICS II can protect against DNA
damage in MPP+-treated SK-N-SH cells.
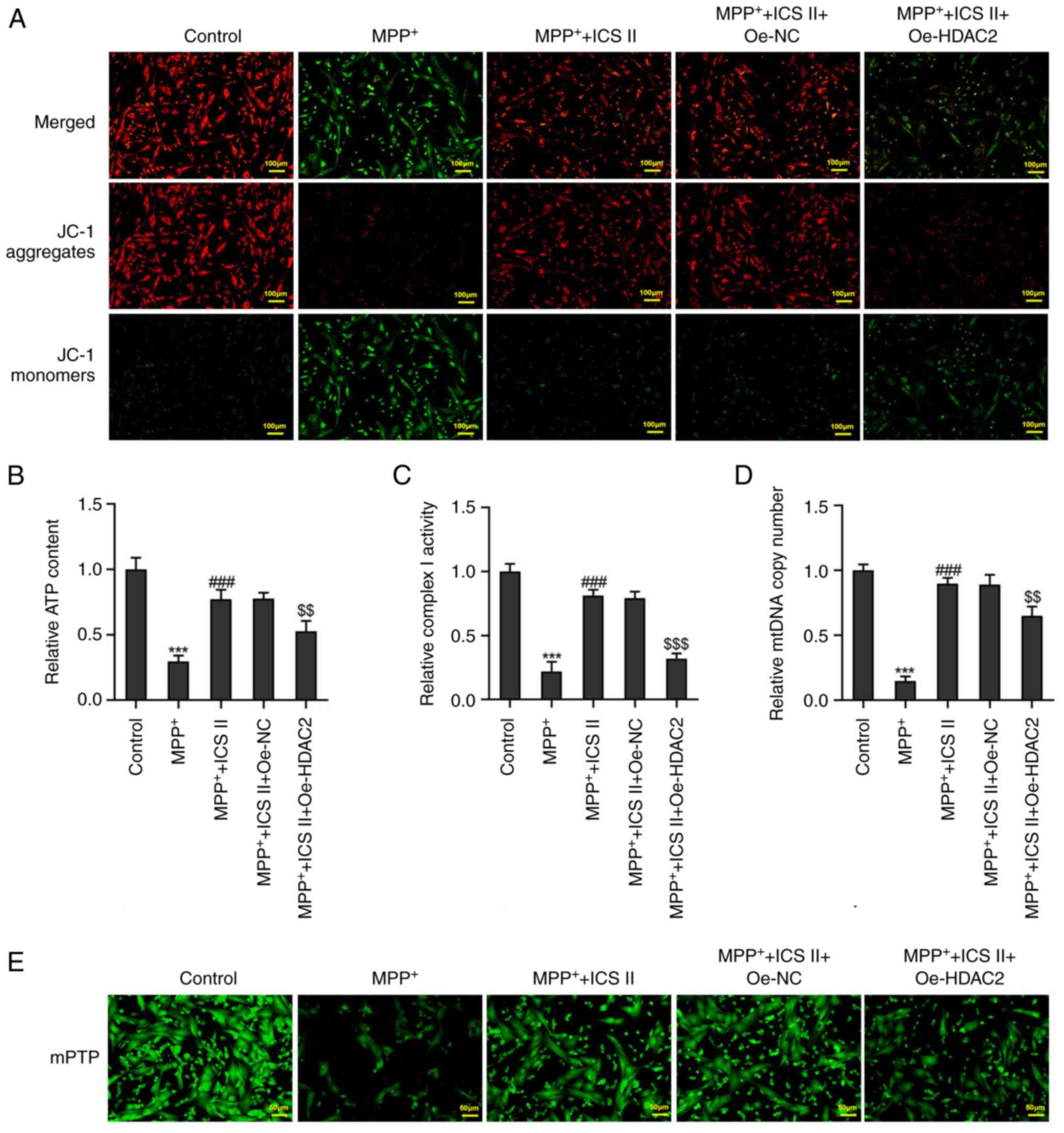
ICS II reverses
MPP+-induced mitochondrial dysfunction in SK-N-SH
cells
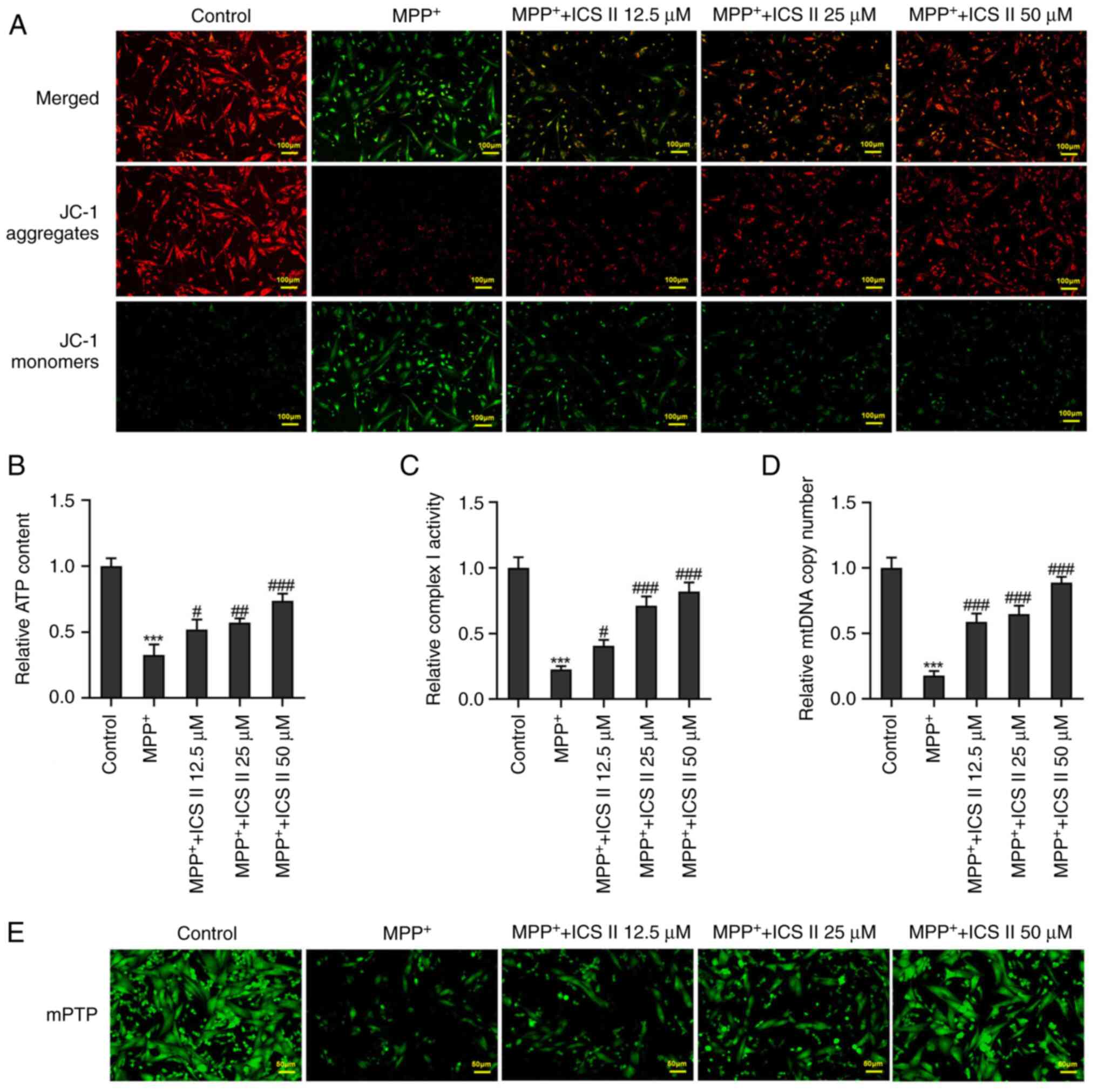
The JC-1 fluorescent probe, which has an excitation
wavelength of 488 nm and a monomer emission wavelength of 525 nm,
is able to enter cells and localize to the mitochondrial membrane
(22). MPP+ was
observed to markedly decrease the mitochondrial membrane potential
in SK-N-SH cells, which was reversed by ICS II treatment (Fig. 3A). The ATP content, complex I
activity and mtDNA copy number were all significantly reduced by
MPP+ treatment in SK-N-SH cells compared with those in
the control group (Fig. 3B-D). By
contrast, ICS II treatment significantly increased the ATP content,
Complex I activity and mtDNA copy number in MPP+-treated
SK-N-SH cells at all concentrations tested (Fig. 3B-D). There is an inverse
correlation between the number of mPTP opening and the calcein-AM
fluorescence intensity (23). ICS
II treatment also markedly decreased mPTP opening in
MPP+-induced SK-N-SH cells in a dose-dependent manner
(Fig. 3E). These results suggest
that ICS II treatment can improve mitochondrial function in
MPP+-treated SK-N-SH cells.
ICS II downregulates HDAC2 expression
in MPP+-induced SK-N-SH cells
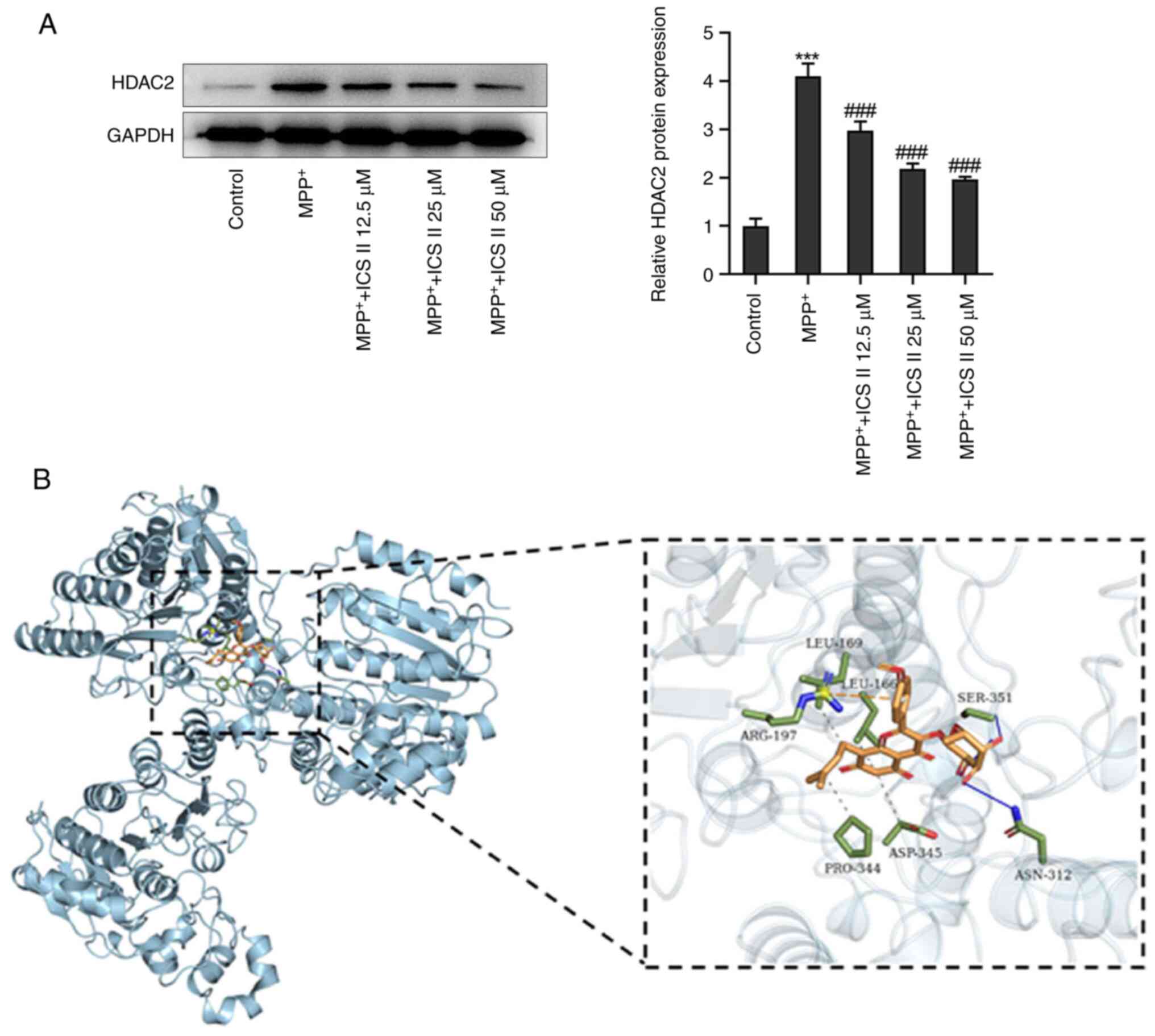
MPP+ was found to significantly increase
the protein expression levels of HDAC2 in SK-N-SH cells, which was
significantly reversed by ICS II at all concentrations tested
(Fig. 4A). Molecular docking was
then performed between HDAC2 and ICS II, where the position with
the lowest free energy (-7.7 kcal/mol) between HDAC2 and ICS II was
selected for visualization. The HDAC2/ICS II complex was found in
the residues LEU-169, LEU-166, SER-351, ARG-197, PRO-344, ASP-345
and ASN-312. Of note, ICS II formed two hydrogen bonds, primarily
with residues (SER-351 and ASN-312) on HDAC2 protein (Fig. 4B). Moreover, ICS II at the
concentration of 50 µM exhibited the most prominent effect, thence
being chosen for the subsequent assays. These results suggest that
ICS II could bind to HDAC2 whilst also decreasing its protein
expression levels.
Overexpression of HDAC2 reverses the
protective effects of ICS II on MPP+-induced SK-N-SH
cells
Transfection of SK-N-SH cells with Oe-HDAC2 was
found to significantly increase the mRNA and protein expression
levels of HDAC2 compared with those in the control group (Fig. 5A and B). In addition, HDAC2 overexpression
significantly increased LDH release in MPP+-induced
SK-N-SH cells treated with 50 µM ICS II compared with that in the
cells transfected with Oe-NC (Fig.
5C). 8-OHdG production was also markedly increased upon the
overexpression of HDAC2, compared with that in
MPP+-induced and ICS II-treated SK-N-SH cells
transfected with Oe-NC (Fig. 5D).
Furthermore, protein expression levels of γ-H2AX in
MPP+-induced SK-N-SH cells treated with ICS II were
significantly increased by the overexpression of HDAC2 compared
with cells transfected with Oe-NC (Fig. 5E).
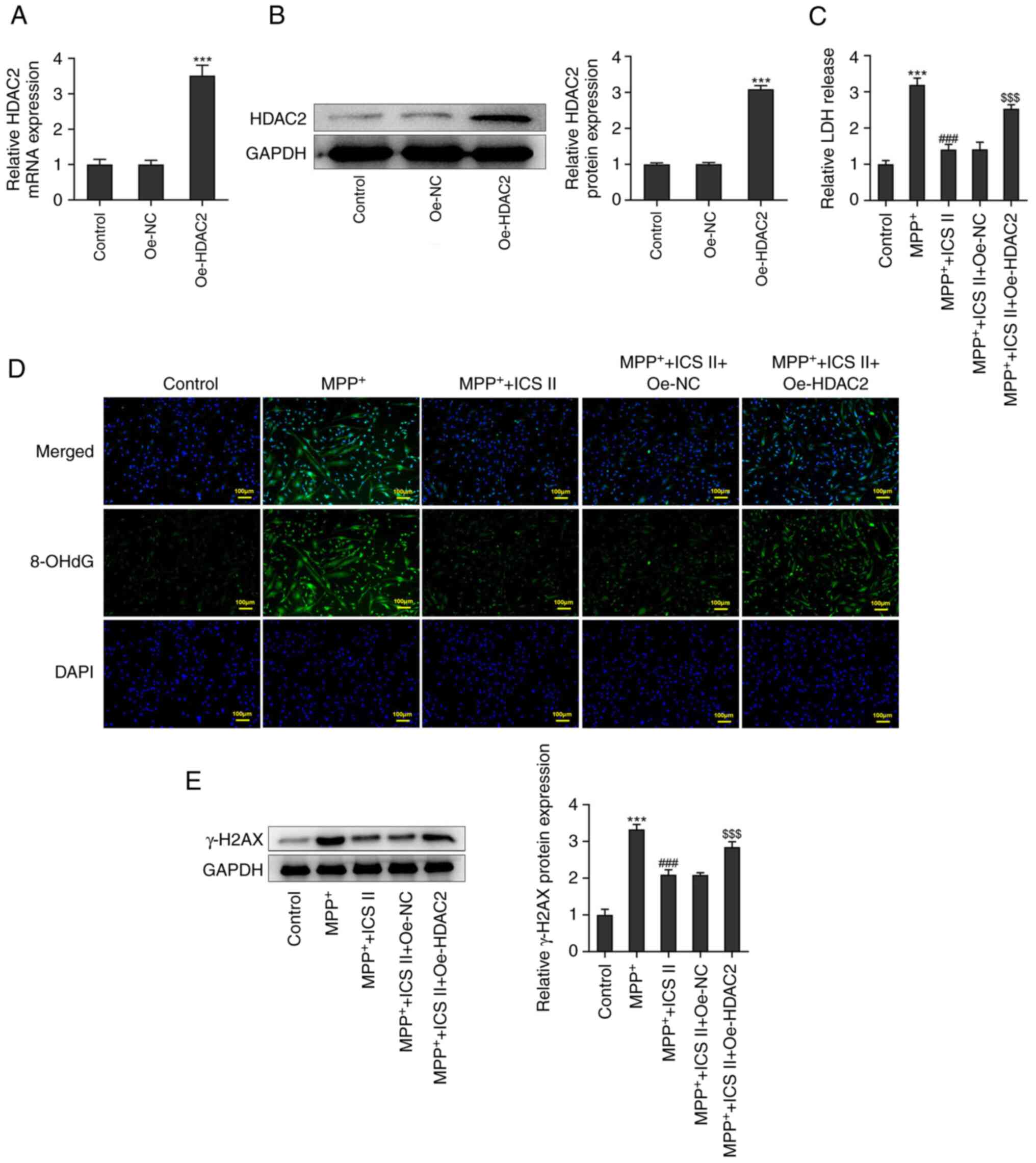 | Figure 5Overexpression of HDAC2 reverses the
protective effects of ICS II on DNA damage in
MPP+-induced SK-N-SH cells. (A) mRNA and (B) protein
expression levels of HDAC2 in SK-N-SH cells transfected with
Oe-HDAC2 were detected by reverse transcription-quantitative PCR
and western blotting, respectively. (C) LDH release of
Oe-HDAC2-transfected SK-N-SH cells treated with MPP+ and ICS II was
detected with an LDH activity assay kit. (D) Production of 8-OHdG
by Oe-HDAC2-transfected SK-N-SH cells treated with MPP+ and ICS II
was detected by immunofluorescence (scale bars, 100 µm). (E)
Protein expression levels of γ-H2AX in Oe-HDAC2-transfected SK-N-SH
cells treated with MPP+ and ICS II were detected by western
blotting. ***P<0.001 vs. Control;
###P<0.001 vs. MPP+;
$$$P<0.001 vs. MPP+ + ICS II + Oe-NC. ICS
II, icariside II; MPP+, 1-methyl-4-phenylpyridinium; 8-OHdG,
8-hydroxydesoxyguanosin; γ-H2AX, γ-H2A histone family member X;
HDAC2, histone deacetylase 2; Oe, overexpression; NC, negative
control; LDH, lactate dehydrogenase. |
HDAC2 overexpression also markedly decreased the
mitochondrial membrane potential in MPP+-induced SK-N-SH
cells treated with ICS II compared with that in cells transfected
with Oe-NC (Fig. 6A).
Additionally, HDAC2 overexpression significantly decreased the ATP
content, complex I activity and the mtDNA copy number in
MPP+-induced SK-N-SH cells treated with ICS II compared
with those in cells transfected with Oe-NC (Fig. 6B-D). HDAC2 overexpression also
markedly increased mPTP opening in MPP+-induced SK-N-SH
cells treated with ICS II compared with that in cells transfected
with Oe-NC (Fig. 6E). These
results suggest that HDAC2 overexpression promoted DNA damage and
mitochondrial dysfunction in MPP+-induced SK-N-SH cells
treated with ICS II.
Discussion
In the present study, the effect of ICS II on DNA
damage and mitochondrial function in MPP+-induced
SK-N-SH cells was investigated. It was demonstrated that ICS II can
increase cell viability whilst alleviating DNA damage and
mitochondrial dysfunction in MPP+-induced SK-N-SH cells.
ICS II treatment was found to inhibit the expression of HDAC2,
whilst HDAC2 overexpression could reverse the effects of ICS II
treatment on SK-N-SH cells. These findings suggest that ICS II can
be used as a potentially promising future treatment method for
PD.
Mitochondrial homeostasis is necessary for
generating energy in the form of ATP, regulating calcium
homeostasis and controlling programmed cell death (24). Imbalance in mitochondrial
homeostasis can lead to the development of progressive pathological
conditions, including Alzheimer's disease, Parkinson's disease
(PD), Huntington's disease and amyotrophic lateral sclerosis,
associated with aging and neurodegeneration (25,26).
In particular, mitochondrial dysfunction serves a key role in the
development of PD. In patients with PD, mitochondrial respiratory
chain complex I activity is decreased and reactive oxygen species
(ROS) production is increased, which leads to the depolarization of
the mitochondrial membrane potential and the increase in membrane
permeability, ultimately causing membrane damage (27). The present study demonstrated that
MPP+ induction was able to decrease the mitochondrial
membrane potential, reduce the intracellular ATP content and
complex I activity whilst increasing mPTP opening in SK-N-SH
cells.
mPTP-induced inflammation and dopaminergic neuronal
death can be alleviated by improving mitochondrial function
(28). A previous study involving
an in vivo PD model of MPP+/mPTP-induced SH-SY5Y
cells reported that MPP+-induced mitochondrial damage
can be reversed by promoting mitophagy and suppressing
mitochondrial fission (29). HDAC2
inhibition may suppress the mitochondrial apoptosis pathway to
protect against acute liver failure (16,30).
In another study, blocking HDAC2 was found to improve neuronal
mitochondrial dynamics to protect neurons against oxidative injury
and apoptosis (31). ICS II has
previously been reported to inhibit mitochondrial division in the
hippocampus of Aβ25-35-induced rats (9). ICS II has also been shown to prevent
myocardial infarction-induced mitochondrial oxidative stress
(32). In PC12 cells that had
underwent oxygen-glucose deprivation and reoxygenation, ICS II was
found to restore the mitochondrial membrane potential by
suppressing the excessive production of mitochondrial ROS (33). The present study demonstrated that
HDAC2 was a potential target of ICS II using SuperPreD database and
provided evidence that ICS II treatment downregulated HDAC2
expression in MPP+-induced SK-N-SH cells. In addition,
ICS II was observed to alleviate DNA damage and restored
mitochondrial function in MPP+-induced SK-N-SH cells by
decreasing HDAC2 expression. Furthermore, rescue experiments were
performed to confirm whether ICS II exerted its protective effects
on MPP+-induced SK-N-SH cells through HDAC2. HDAC2
overexpression was found to negate the protective effect of ICS II
on MPP+-induced SK-N-SH cells, suggesting that ICS II
can protect SK-N-SH cells from MPP+-induced DNA damage
and mitochondrial dysfunction by downregulating HDAC2
expression.
In conclusion, the present study demonstrated that
ICS II exerts a protective role against MPP+-induced
neurotoxicity, where HDAC2 is a potential target of ICS II.
Therefore, ICS II or alternative treatment strategies to reduce the
expression of HDAC2 may provide novel therapeutic options for
restoring mitochondrial function in dopaminergic neurons for the
treatment of PD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WF and JZ designed the study, performed the
experiments and drafted and revised the manuscript. WF analyzed the
data and searched the literature. Both authors read and approved
the final version of the manuscript. WF and JZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma S, Awasthi A and Singh S: Altered
gut microbiota and intestinal permeability in Parkinson's disease:
Pathological highlight to management. Neurosci Lett.
712(134516)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nair AT, Ramachandran V, Joghee NM, Antony
S and Ramalingam G: Gut microbiota dysfunction as reliable
non-invasive early diagnostic biomarkers in the pathophysiology of
Parkinson's disease: A critical review. J Neurogastroenterol Motil.
24:30–42. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Xu X, Wang R, Hao Z, Wang G, Mu C, Ding J,
Sun W and Ren H: DJ-1 regulates tyrosine hydroxylase expression
through CaMKKβ/CaMKIV/CREB1 pathway in vitro and in vivo. J Cell
Physiol. 235:869–879. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yin C, Deng Y, Liu Y, Gao J, Yan L and
Gong Q: Icariside II ameliorates cognitive impairments induced by
chronic cerebral hypoperfusion by inhibiting the amyloidogenic
pathway: Involvement of BDNF/TrkB/CREB signaling and up-regulation
of PPARα and PPARγ in rats. Front Pharmacol. 9(1211)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng Y, Xiong D, Yin C, Liu B, Shi J and
Gong Q: Icariside II protects against cerebral ischemia-reperfusion
injury in rats via nuclear factor-κB inhibition and peroxisome
proliferator-activated receptor up-regulation. Neurochem Int.
96:56–61. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yan BY, Pan CS, Mao XW, Yang L, Liu YY,
Yan L, Mu HN, Wang CS, Sun K, Liao FL, et al: Icariside II improves
cerebral microcirculatory disturbance and alleviates hippocampal
injury in gerbils after ischemia-reperfusion. Brain Res.
1573:63–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yin C, Deng Y, Gao J, Li X, Liu Y and Gong
Q: Icariside II, a novel phosphodiesterase-5 inhibitor, attenuates
streptozotocin-induced cognitive deficits in rats. Neuroscience.
328:69–79. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Deng Y, Long L, Wang K, Zhou J, Zeng L, He
L and Gong Q: Icariside II, a broad-spectrum anti-cancer agent,
reverses beta-amyloid-induced cognitive impairment through reducing
inflammation and apoptosis in rats. Front Pharmacol.
8(39)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xiao HH, Chen JC, Li H, Li RH, Wang HB,
Song HP, Li HY, Shan GS, Tian Y, Zhao YM, et al: Icarisid II
rescues cognitive dysfunction via activation of Wnt/β-catenin
signaling pathway promoting hippocampal neurogenesis in APP/PS1
transgenic mice. Phytother Res. 36:2095–2108. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Yan L, Deng Y, Gao J, Liu Y, Li F, Shi J
and Gong Q: Icariside II effectively reduces spatial learning and
memory impairments in Alzheimer's disease model mice targeting
beta-amyloid production. Front Pharmacol. 8(106)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Gu Z, Lin S, Chen L, Dzreyan V, Eid
M, Demyanenko S and He B: Histone deacetylases as epigenetic
targets for treating Parkinson's disease. Brain Sci.
12(672)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma P and Schultz RM: HDAC1 and HDAC2 in
mouse oocytes and preimplantation embryos: Specificity versus
compensation. Cell Death Differ. 23:1119–1127. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stoddard SV, May XA, Rivas F, Dodson K,
Vijayan S, Adhika S, Parker K and Watkins DL: Design of potent
panobinostat histone deacetylase inhibitor derivatives: Molecular
considerations for enhanced isozyme selectivity between HDAC2 and
HDAC8. Mol Inform. 38(e1800080)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen J, Li N, Liu B, Ling J, Yang W, Pang
X and Li T: Pracinostat (SB939), a histone deacetylase inhibitor,
suppresses breast cancer metastasis and growth by inactivating the
IL-6/STAT3 signalling pathways. Life Sci.
248(117469)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tan Y, Delvaux E, Nolz J, Coleman PD, Chen
S and Mastroeni D: Upregulation of histone deacetylase 2 in laser
capture nigral microglia in Parkinson's disease. Neurobiol Aging.
68:134–141. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Choong CJ, Sasaki T, Hayakawa H, Yasuda T,
Baba K, Hirata Y, Uesato S and Mochizuki H: A novel histone
deacetylase 1 and 2 isoform-specific inhibitor alleviates
experimental Parkinson's disease. Neurobiol Aging. 37:103–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Singer TP and Ramsay RR: Mechanism of the
neurotoxicity of MPTP. An update. FEBS Lett. 274:1–8.
1990.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan X, Wu Y, Lu L and Feng J: Long
noncoding RNA SNHG14 knockdown exerts a neuroprotective role in
MPP+-induced Parkinson's disease cell model through
mediating miR-135b-5p/KPNA4 axis. Metab Brain Dis. 37:2363–2373.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu F, Lv C, Deng Y, Liu Y, Gong Q, Shi J
and Gao J: Icariside II, a PDE5 inhibitor, suppresses
oxygen-glucose deprivation/reperfusion-induced primary hippocampal
neuronal death through activating the PKG/CREB/BDNF/TrkB signaling
pathway. Front Pharmacol. 11(523)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Halczuk KM, Boguszewska K, Urbaniak SK,
Szewczuk M and Karwowski BT: 8-oxo-7,8-dihydro-2'-deoxyguanosine
(8-oxodG) and 8-hydroxy-2'-deoxyguanosine (8-OHdG) as a cause of
autoimmune thyroid diseases (AITD) during pregnancy? Yale J Biol
Med. 93:501–515. 2020.PubMed/NCBI
|
|
22
|
Perelman A, Wachtel C, Cohen M, Haupt S,
Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths
facilitate mitochondrial membrane potential cytometry. Cell Death
Dis. 3(e430)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang DQ, Ma YJ, Wang Y, Lu HX, Mao SH and
Zhao SH: Microglia activation induces oxidative injury and
decreases SIRT3 expression in dopaminergic neuronal cells. J Neural
Transm (Vienna). 126:559–568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Harrington JS, Ryter SW, Plataki M, Price
DR and Choi AMK: Mitochondria in health, disease, and aging.
Physiol Rev. 103:2349–2422. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Golpich M, Amini E, Mohamed Z, Azman Ali
R, Mohamed Ibrahim N and Ahmadiani A: Mitochondrial dysfunction and
biogenesis in neurodegenerative diseases: Pathogenesis and
treatment. CNS Neurosci Ther. 23:5–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Grimm A and Eckert A: Brain aging and
neurodegeneration: From a mitochondrial point of view. J Neurochem.
143:418–431. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park JS, Davis RL and Sue CM:
Mitochondrial dysfunction in Parkinson's disease: New mechanistic
insights and therapeutic perspectives. Curr Neurol Neurosci Rep.
18(21)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim HY, Bae CH, Kim J, Lee Y, Jeon H, Kim
H and Kim S: Rumex japonicus houtt. protects dopaminergic neurons
by regulating mitochondrial function and gut-brain axis in in vitro
and in vivo models of Parkinson's disease. Antioxidants (Basel).
11(141)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu LK, Agarwal S, Kuo CH, Kung YL, Day CH,
Lin PY, Lin SZ, Hsieh DJ, Huang CY and Chiang CY: Artemisia leaf
extract protects against neuron toxicity by TRPML1 activation and
promoting autophagy/mitophagy clearance in both in vitro and in
vivo models of MPP+/MPTP-induced Parkinson's disease.
Phytomedicine. 104(154250)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Y, Wang Y, Chen Q, Jiao F, Wang L and
Gong Z: HDAC2 inhibitor CAY10683 reduces intestinal epithelial cell
apoptosis by inhibiting mitochondrial apoptosis pathway in acute
liver failure. Histol Histopathol. 34:1173–1184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Frankowski H, Yeboah F, Berry BJ,
Kinoshita C, Lee M, Evitts K, Davis J, Kinoshita Y, Morrison RS and
Young JE: Knock-down of HDAC2 in human induced pluripotent stem
cell derived neurons improves neuronal mitochondrial dynamics,
neuronal maturation and reduces amyloid beta peptides. Int J Mol
Sci. 22(2526)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Y, Feng L, Xie D, Lin M, Li Y, Chen N,
Yang D, Gao J, Zhu Y and Gong Q: Icariside II, a naturally
occurring SIRT3 agonist, protects against myocardial infarction
through the AMPK/PGC-1α/apoptosis signaling pathway. Antioxidants
(Basel). 11(1465)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Feng L, Gao J, Liu Y, Shi J and Gong Q:
Icariside II alleviates oxygen-glucose deprivation and
reoxygenation-induced PC12 cell oxidative injury by activating
Nrf2/SIRT3 signaling pathway. Biomed Pharmacother. 103:9–17.
2018.PubMed/NCBI View Article : Google Scholar
|