Introduction
Chronic pain often lasts months or years, affecting
>20% of adults worldwide and developing into a widespread public
health issue. Chronic pain significantly affects the patients'
ability to sleep, work and study, which lowers their quality of
life and ability to function (1).
Spontaneous pain and evoked pain in reaction to non-noxious or
noxious stimuli are characteristics of chronic pain (2). Preferred pharmacologic treatments
consist of opioids, acetaminophen and nonsteroidal
anti-inflammatory drugs. Due to gastrointestinal discomfort along
with other side effects of drugs, nearly one-third of patients were
unsatisfied with their treatment (3). Thus, developing new analgesic drugs
with fewer side effects is essential.
Neuroinflammation in the spinal cord participates in
and modulates chronic pain, which is marked by the release of
inflammatory mediators and glial cell activation (4,5).
During pain processing, tissue injury generates a number of
nociceptive stimuli that cause primary afferent fibers. After
transmitting to the spinal cord, these nociceptive signals activate
astrocytes and nociceptors (6).
Activated astrocytes undergo hyperplasia and hypertrophy and
release several pro-inflammatory mediators, for instance IL-1β,
which is known to mediate pain and sensitize nociceptors directly
(7). The Nod-like receptor family
pyrin domain-containing 3 (NLRP3) inflammasome is
well-characterized for IL-1β maturation (8). NLRP3 inflammasome assembly allows the
cleavage of caspase-1 and in turn leads IL-1β secretion and NF-κB
activation (9,10). The pathophysiology of chronic pain
is influenced by the dysregulation of NLRP3 inflammasome (11). IL-1β, apoptosis-associated
speck-like protein (ASC) and caspase-1 are among the NLRP3
inflammasome components whose spinal expression is greatly elevated
in the complete Freund's adjuvant (CFA)-induced inflammatory pain
model (12). MCC950 (a specific
inhibitor of NLRP3) or Nlrp3 gene knockout therapy reduces
inflammatory pain in mice (13).
Hence, targeting NLRP3 inflammation activation is a potential
therapy for chronic pain.
Traditional Chinese medicine uses emodin
(1,3,8-trihydroxy-6-methylanthraquinone), which is a natural
anthraquinone derivative and found in Rheum palmatum and a
number of other plants. Emodin possesses a number of benefits,
including those against cancer, inflammation, viruses and bacteria
(14). In several pain models,
such as capsaicin, acetic acid, carrageenan and formalin-induced
pain, emodin decreases nociceptive and inflammatory responses
(15). Emodin treatment alleviates
hyperalgesia by decreasing the P2X (2/3) expression in chronic
constriction injury neuropathic pain model rats (16). Consequently, targeting HDAC6 is
favorable for managing chronic pain as well as
neuroinflammation.
In order to establish a chronic inflammatory pain
model an intraplantar injection of CFA in the left foot of C57BL/6J
mice was performed. For three successive days intraperitoneal
injections of emodin were performed. The related protein expression
levels, changes in behaviors and spinal inflammation were detected.
It was intended to explain how emodin works to reduce chronic pain
and provide theoretical and data support for emodin application in
chronic pain treatment.
Materials and methods
Animal model and drug
administration
Male C57BL/6J mice (6-8 weeks old; 18-20 g; n=30)
were bought from the Hubei Province Experimental Animal Centre.
Animals were kept under 12 h light/dark environment and unlimited
access to food and water. The experiment was approved by the
Laboratory Animal Ethics Committee of Hubei University of Science
and Technology (approval no 2019-03-021).
Prior to the test, mice were randomly divided into
Control, CFA and CFA + emodin groups, 10 mice for each group. There
was a 7-day period of acclimation to the surroundings. Then on days
0 and 7, left hind paws of mice received intraplantar subcutaneous
injections of 10 µl CFA and the equal amount of saline was
administered in mice from control group (17). On days 0, 7 and 14, behavioral
assessments were conducted. On days 15-17 following the CFA
injection, vehicle and emodin (10 mg/kg) were intraperitoneally
injected in CFA and CFA + emodin groups for 3 days. Prior to use,
emodin was dissolved in DMSO which diluted with 0.9% NaCl. After 4
h of emodin treatment, the behavioral tests were conducted.
Antibodies and reagents
Anti-NLRP3 (cat. no. DF7438), anti-HDAC6 (cat. no.
AF6485), anti-glial fibrillary acidic protein (GFAP, cat. no.
BF0345), anti-IL-1β (cat. no. AF5103), anti-β-actin (cat. no.
AF7018) and anti-cleaved caspase-1 (cat. no. AF4022) antibodies
were purchased from Affinity Biosciences. Anti-caspase-1 (cat. no.
A0964), HRP Goat anti-rabbit IgG (H+L; cat. no. AS014) and HRP Goat
anti-mouse IgG (H+L; cat. no. AS003) antibodies were acquired from
ABclonal Biotech Co., Ltd. H&E staining kit (cat. no. BL735B)
was bought from Biosharp Life Sciences. Emodin was purchased from
Shanghai Yuanye Biotechnology Co., Ltd. Goat Anti-Rabbit IgG
H&L (FITC; cat. no. ab6717) and Goat Anti-Mouse IgG H&L
(TRITC; cat. no. ab6786) were from Abcam.
Mechanical threshold test
Mice were housed in a plexiglass container to
acclimatize for almost 30 min. Then, von Frey filaments (Stoelting
Co.) were applied to stimulate the left hind paw. The filaments
were briefly bent by being pushed firmly vertically on the plantar
surfaces for 3-5 sec. Paw flinching and brisk withdrawal were
regarded as positive responses in this circumstance. The patterns
of withdrawal responses were then translated into mechanical
threshold values (18).
Spontaneous flinch test
Mice were housed for nearly 30 mins in a plexiglass
chamber. Within 5 min, numbers of flinches were counted 3 times
individually (19).
Rotarod test
Mice were trained three days at a fixed pace for 10
min before the examinations. In the experiments, the test was
initially set at a constant speed at 10 revolutions per min for 10
sec, then at an increasing speed to 20 revolutions per min for 30
sec. The latency to fall was measured to evaluate the balance and
motor coordination of the animals (20).
H&E staining
Following the behavior test, mice were given a deep
anesthetic dose of 60 mg/kg sodium pentobarbital and transcardially
perfused using 4% PFA. After collection and post-fixation using 4%
PFA for 12 h at 4˚C, the spinal cords were embedded with paraffin
and cutting into 4-µm sections. The sections were treated with
xylene, 100, 90 and 70% ethanol and dyed with H&E staining kit.
Briefly, paraffin sections were treated with xylene (5 min, twice),
100% ethanol (10 min, twice), 90% ethanol (10 min) and 70% ethanol
(10 min) for dewaxing, then stained with hematoxylin solution for 3
min at 25˚C and eosin for 3 min at 25˚C. The slices were treated
with 70% ethanol (10 sec), 80% ethanol (10 sec), 90% ethanol (30
sec), 100% ethanol (1 min, twice) and xylene (1 min, twice) to
dehydrate the sample and render it transparent, sealed with neutral
balsam, images captured under a fluorescence microscope (IX73;
Olympus Corporation) and analyzed using ImageJ 1.48v (National
Institutes of Health). Inflammatory cell infiltration was
categorized as 0 (normal); 1 (meningeal and perivascular
lymphocytic infiltration); 2 (1-10 lymphocytes present); 3 (11-100
lymphocytes); and 4 (>100 lymphocytes).
Immunofluorescence
The sections of spinal cord tissue were hydrated and
subjected to antigen retrieval using antigen retrieval solution
(cat. no. P0083; Beyotime Institute of Biotechnology) for 10 min at
95˚C. The sections were blocked with immunofluorescence blocking
solution (cat. no. P0102; Beyotime Institute of Biotechnology) for
1h before incubating with primary antibody at 4˚C overnight and
fluorescent secondary antibodies for 1 h at room temperature. The
images were captured by a fluorescence microscope and analyzed
using ImageJ 1.48v (National Institutes of Health). The primary
antibodies were anti-HDAC6, anti-IL-1β, anti-caspsae-1, anti-GFAP
and anti-NLRP3, the dilution ratio was 1:100.
Western blotting
Mice were intraperitoneally injected with 150 mg/kg
pentobarbital sodium after the behavioral tests. The lumbar spinal
cords were homogenized in RIPA lysis buffer containing 1% protease
inhibitors (MilliporeSigma) and centrifuged for 20 min (12,000 x g,
4˚C). The supernatant was quantified using a BCA analysis kit (cat.
no. P0012; Beyotime Institute of Biotechnology). Protein mixture
samples were loaded at 20 µg/lane and separated using 10-15%
SDS-PAGE. The blots were transferred to PVDF membranes. Following a
blocking using QuickBlock Blocking Buffer (cat. no. P0220; Beyotime
Institute of Biotechnology) at 4˚C for 15 min, the membranes were
incubated with primary antibodies overnight at 4˚C and secondary
antibodies (1:5,000) for 1 h (room temperature). The bands were
visualized using ECL solution (cat. no. P0018M; Beyotime Institute
of Biotechnology) and analyzed using ImageJ 1.48v (National
Institutes of Health). β-actin was used as a loading control. The
following primary antibodies were used: anti-GFAP (1:1,000),
anti-IL-1β (1:1,000), anti-cleaved-caspsae-1 (1:1,000), anti-HDAC6
(1:1,000), anti-NLRP3 (1:1,000) and anti-β-actin (1:50,000).
Molecular docking
HDAC6 X-ray crystal structure was acquired through
Protein Data Bank (PDB ID, 5B8D; https://www.rcsb.org/structure/5B8D). The structure of
emodin was optimized and retrieved from the PubChem compound
database (PubChem CID, 3220; https://pubchem.ncbi.nlm.nih.gov/compound/3220).
Docking conformation among emodin and HDAC6 was employed by Auto
Dock Vina 1.2.0 software (Center for Computational Structural
Biology, https://vina.scripps.edu/downloads/). The conformation
was seen using PyMOL 2.2.3(21).
Statistical analysis
All statistical analyses were performed using SPSS
26.0 (IBM Corp.). Data of H&E staining, behaviors, western
blotting and immunofluorescence were examined by a one-way ANOVA
followed by Tukey's post-hoc test. Data for paw withdrawal
threshold (PWT), flinches and latency to fall are expressed as mean
± SEM. Data for histology, morphology and western blotting are
expressed as the mean ± SD.
Results
Emodin decreases pain sensitivity in
CFA-induced mice
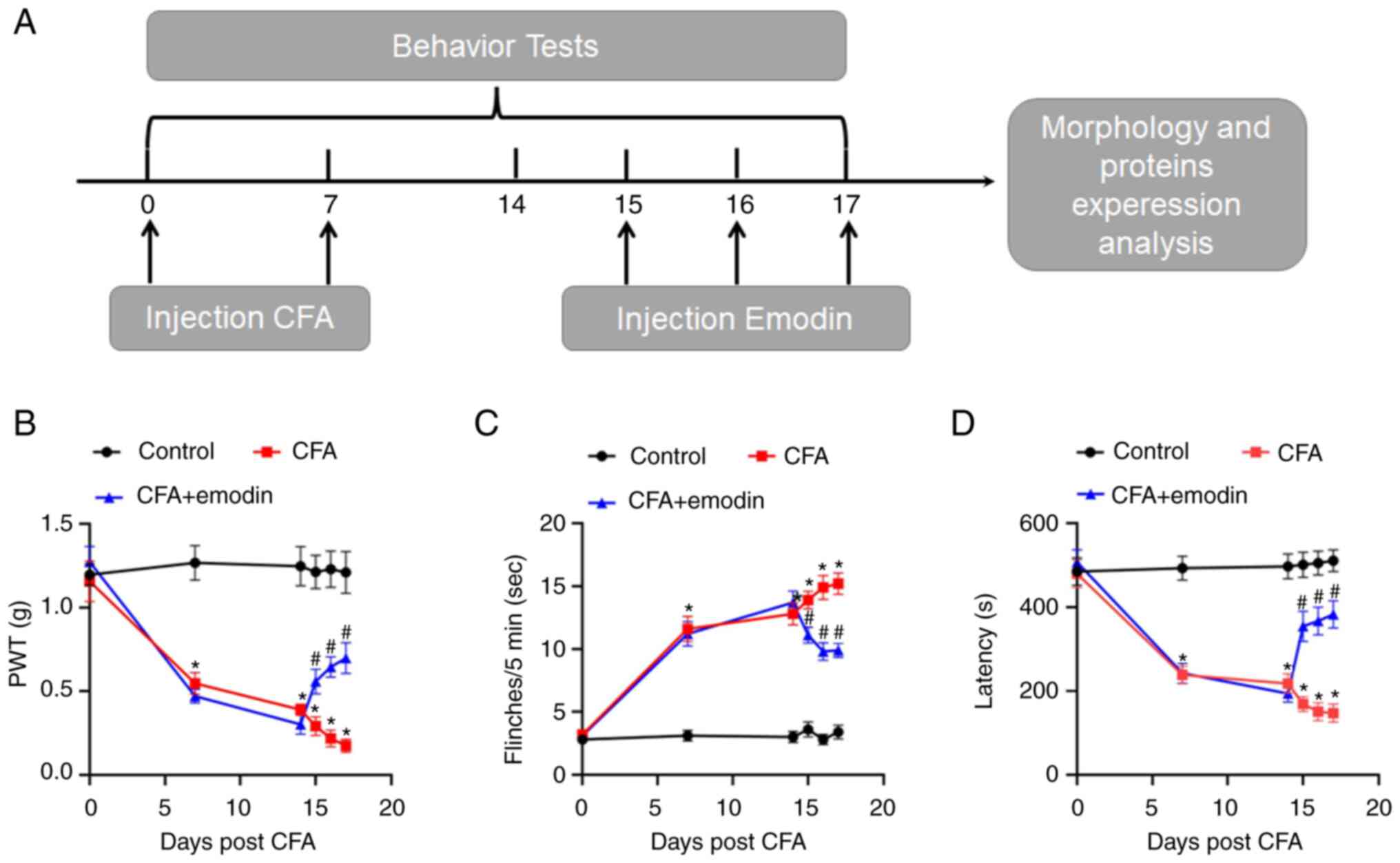
Fig. 1A indicates
the experiment process including the behavioral tests. As shown in
Fig. 1B, mechanical threshold
values in the CFA mice were lowered with the PWT values of day 0, 7
and 14 at 1.15 ± 0.12, 0.55 ± 0.06 and 0.39 ± 0.03, respectively.
The number of flinches (Fig. 1C)
raised in CFA mice, from 3.20 ± 0.36 (day 0) to 11.60 ± 1.01 (day
7), 12.80 ± 0.89 (day 14), compared with control mice. In contrast
to control mice, the latency to fall (Fig. 1D) in CFA mice decreased from 480.03
± 33.27 (day 0) to 238.53 ± 20.75 (day 7) and 217.90 ± 23.67 (day
14). As suggested by the data, CFA results in motor disability as
well as nociceptive hyperalgesia in mice, which indicated that the
mouse model of chronic pain had been constructed effectively. The
impact of emodin on motor function and pain sensitivity was then
evaluated. The values of mechanical threshold in CFA + emodin mice
were higher after emodin treatment on days 15-17, which are 0.56 ±
0.07, 0.64 ± 0.06 and 0.70 ± 0.09, respectively (Fig. 1B). There were significantly fewer
flinches in the CFA + emodin mice, 11.10 ± 0.62 (day 15), 9.80 ±
0.70 (day 16) and 9.90 ± 0.57 (day 17), respectively (Fig. 1C). The emodin treatment also
increased the latency to fall in CFA-induced mice, 354.27 ± 35.95,
367.16 ± 33.36 and 382.72 ± 32.39, on days 15 to 17, respectively
(Fig. 1D). As a result, emodin
alleviated pain behavior and improved motor ability in the CFA
mice.
Emodin reduces spinal cord
inflammatory response by inhibiting spinal cord astrocyte
activation
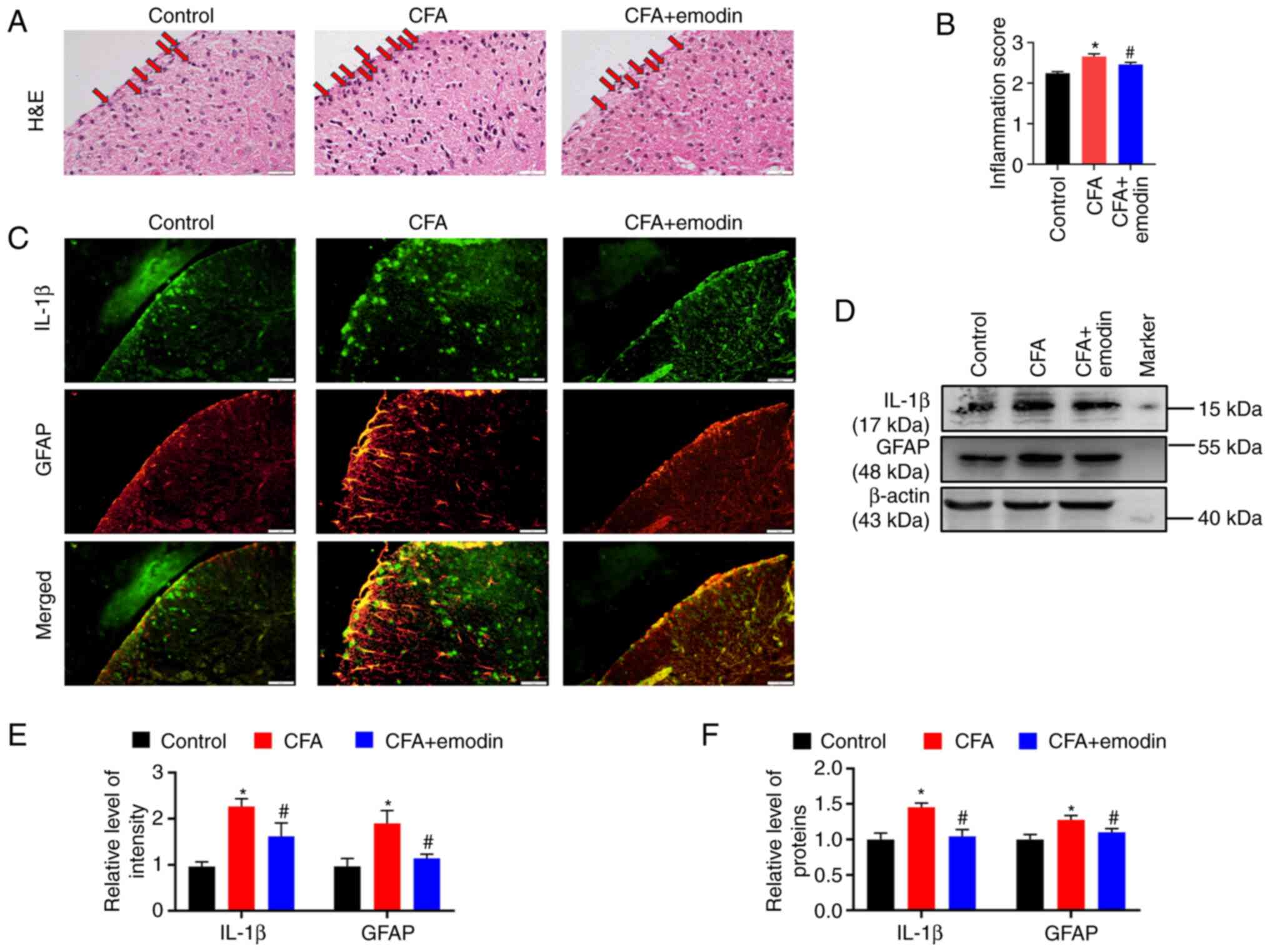
For CFA mice, a significant inflammatory
infiltration was found in the spinal dorsal horn with the
inflammatory scores at 2.65 ± 0.06, whereas emodin treatment
reduced the inflammatory infiltration to 2.45 ± 0.05 (Fig. 2A and B). Activated astrocytes are a major
source of a number of pro-inflammatory cytokines and GFAP is used
as a marker of abnormal proliferation and activation of astrocytes
(22). The fluorescence
intensities of IL-1β and GFAP in spinal dorsal horn of CFA group
were enhanced, with the relative intensity at 2.26 ± 0.17 and 1.90
± 0.28, respectively. The emodin treatment decreased the IL-1β and
GFAP intensity to 1.62 ± 0.29 and 1.14 ± 0.09, respectively
(Fig. 2C and E). As revealed by western blotting, the
CFA group had higher spinal expression levels of IL-1β and GFAP
than the control group, with the relative grey values of 1.45 ±
0.06 and 1.28 ± 0.06, respectively. The levels of IL-1β and GFAP
were reduced to 1.04 ± 0.10 and 1.10 ± 0.05, respectively (Fig. 2D and F) by emodin treatment.
Emodin decreases the activity of
spinal NLPR3 inflammasome
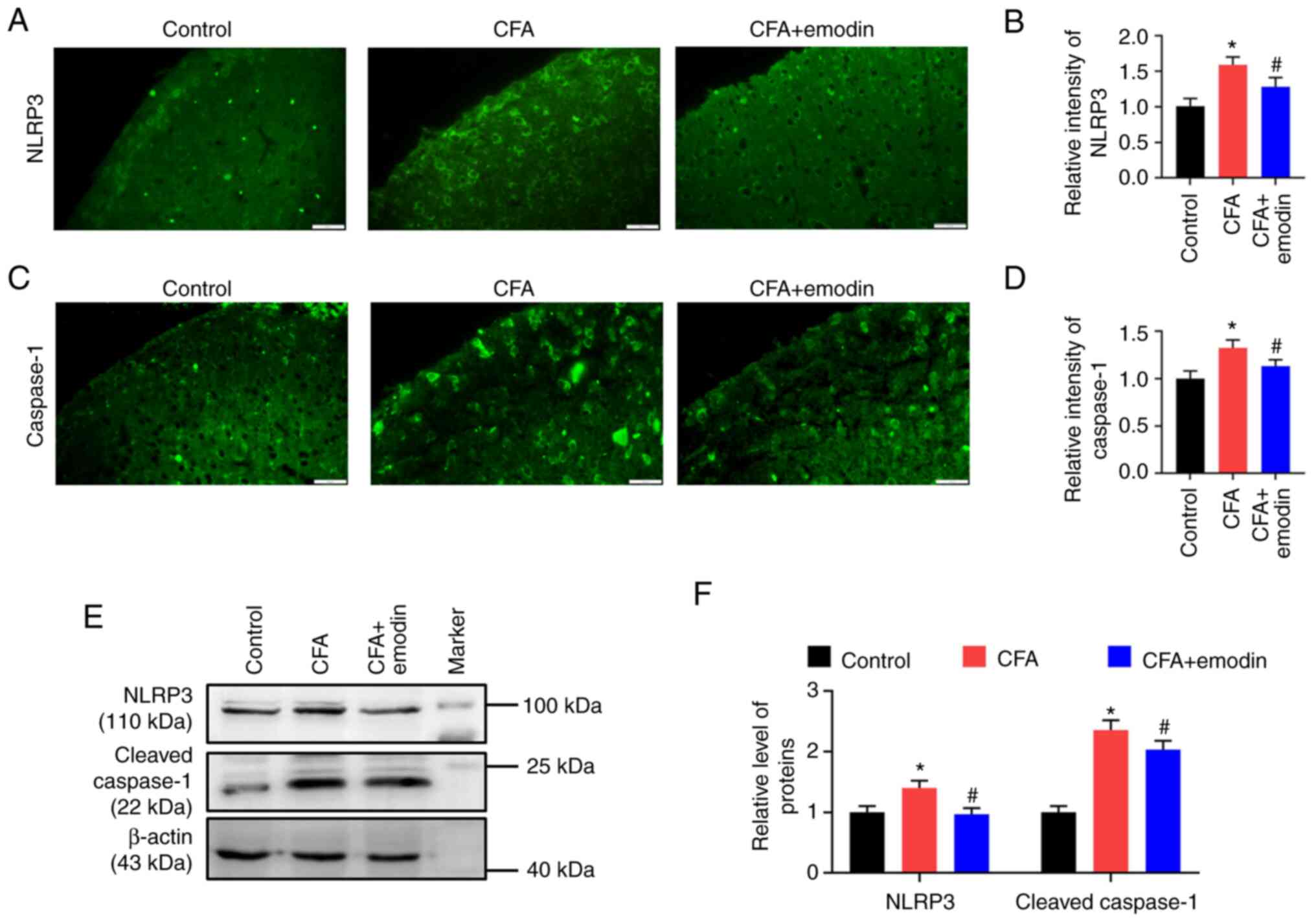
IL-1β maturation is promoted by NLRP3 inflammasome
activation (23). The fluorescence
intensities of NLRP3 and caspase-1 in spinal dorsal horn of CFA
group were enhanced, with the relative intensity at 1.60 ± 0.11 and
1.33 ± 0.08, respectively. The emodin treatment decreased the NLRP3
and caspase-1 intensity to 1.28 ± 0.13 and 1.13 ± 0.07,
respectively (Fig. 3A-D). As
revealed by western blotting, the CFA group had higher spinal
expression levels of NLRP3 and cleaved caspase-1 than control
group, with the relative grey values of 1.40 ± 0.11 and 2.35 ±
0.16, respectively. The levels of NLRP3 and cleaved caspase-1 were
reduced to 0.97 ± 0.10 and 2.03 ± 0.15, respectively (Fig. 3E and F) by emodin administration.
Emodin prevents spinal HDAC6
activity
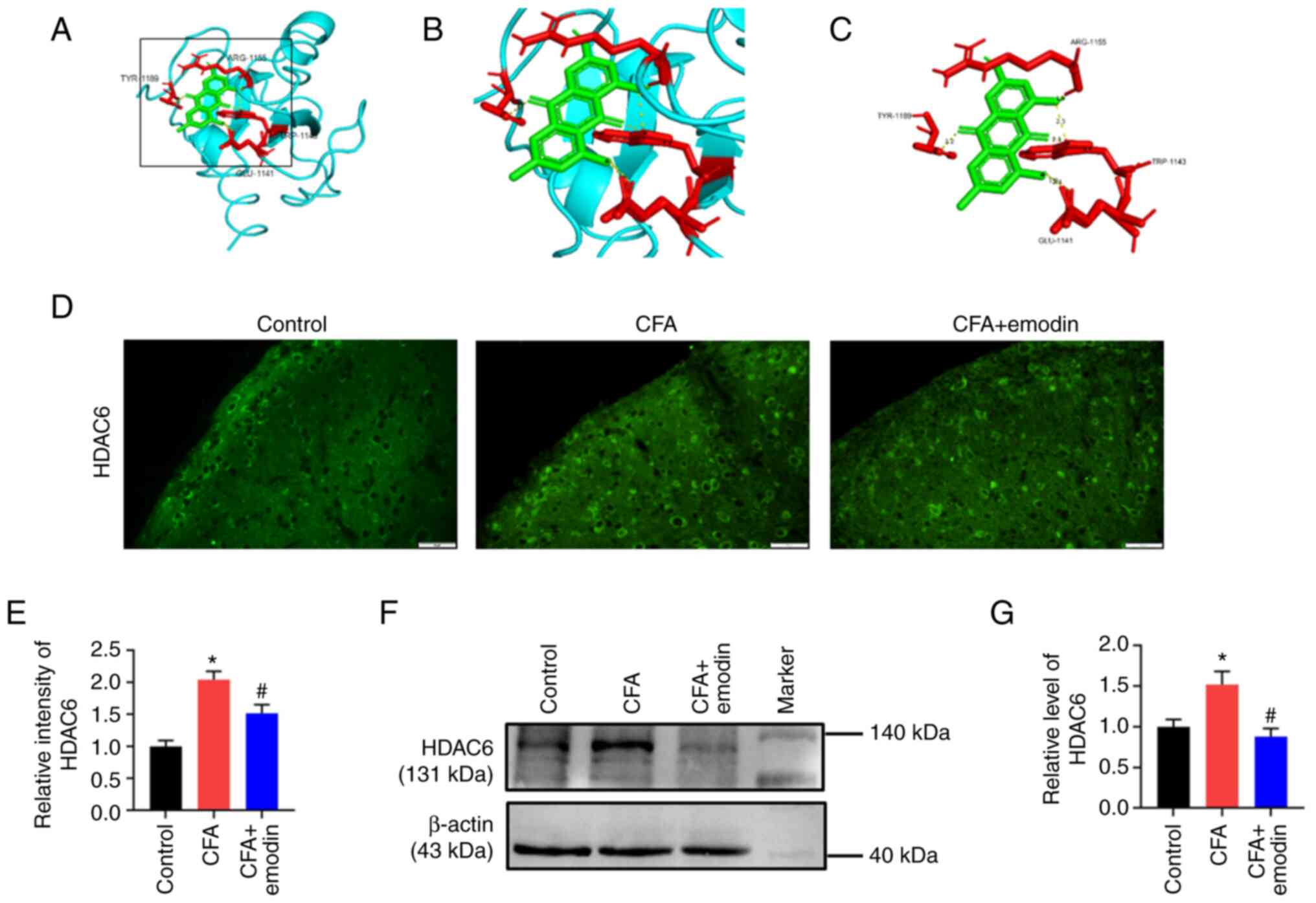
The ligand emodin and the X-ray crystal structures
of HDAC6 were used in a molecular docking test (Fig. 4A-C). According to Auto Dock data,
four electrovalent bonds are produced by emodin and HDAC6 at
residues ARG-1155, TYR-1189, GLU-1141 and TRP-1143. The
electrovalent bond distances were 1.9, 3.2, 2.4 and 2.1 Å between
HDAC6 and emodin, while the binding affinity was -8.7 kcal/mol. The
increased HDAC6 fluorescence intensity in the spinal dorsal horn
was observed in the CFA group, with a relative intensity of 2.04 ±
0.13. HDAC6 intensity was decreased to 1.52 ± 0.13 upon emodin
treatment (Fig. 4D and E). The spinal HDAC6 protein level of CFA
group was higher than the control group; the relative grey value
was 1.52 ± 0.16. HDAC6 protein level was downregulated by emodin
treatment to 0.88 ± 0.10 (Fig. 4F
and G).
Discussion
The present study discovered that emodin reduced
CFA-induced pain by controlling HDAC6. HDAC6 works as a therapeutic
target for chronic pain (24).
HDAC6 inhibitor SW-100 treatment restores deacetylation of
α-tubulin in sciatic nerve and alleviates mechanical allodynia and
hyperalgesia in the peripheral neuropathy mouse model (25). Chemotherapy-induced peripheral
neuropathy model mice treated with HDAC6 inhibitor recover from
mechanical allodynia and spontaneous pain caused by cisplatin
(26). HDAC6 genetic deletion
prevents the mechanical allodynia that cisplatin causes (27). The present study found increased
spinal HDAC6 expression in CFA-induced inflammatory pain model
mice. Emodin binds with HDAC6 at a high binding affinity, producing
a comparatively stable docking outcome (28). In the meantime, in the cardiac
hypertrophy model, emodin inhibits HDAC activity and increases
histone acetylation, while blocking pathological cardiac
hypertrophy (29). Hence, it was
hypothesized that emodin binds with HDAC6 and inhibits its
activity.
Emodin suppresses spinal inflammation by regulating
HDAC6. It is reported that HDAC6 serves as a dynein adaptor for the
purpose of facilitating transport and assembling NLRP3 inflammasome
(30). HDAC6 inhibition decreases
the NLRP3 and IL-1β levels and suppresses nicotine-induced
pyroptosis in atherosclerosis model mice (31). In LPS-induced mice, HDAC6 degrader
application lessened activation of NLRP3 inflammasome (32). In a Parkinson's disease mouse
model, the pharmacological suppression of HDAC6 by tubastatin A
reduces NLRP3 expression and the maturation of IL-1β (33). The present study suggested that
emodin treatment disrupted the binding of HDAC6 and NLRP3 and
suppressed NLRP3 inflammasome activation. Furthermore, HDAC6
participates in pro-inflammatory interleukin expression through
NF-κB signaling, which induces the pro-IL-1β and NLRP3
transcription expression (34). In
arthritis model animals, HDAC6 depletion post-transcriptionally
upregulates NF-κB inhibitor and downregulates NF-κB reporter
activation and prevents the nuclear translocation of NF-κB subunits
(35). HDAC6 mediates
deacetylation of NF-κB and plays an inhibitory effect on invasion
(36). The present study indicated
that emodin administration reduced NF-κB-mediated neuroinflammation
in chronic pain.
During chronic pain processing, NLRP3 inflammasome
is activated and spinal inflammation is triggered. Emodin treatment
binds HDAC6, weakens the HDAC6-NLRP3 interaction, reduces NLRP3
inflammasome reaction and suppresses spinal inflammation while
alleviating chronic inflammatory pain.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Hubei University of
Science and Technology Program (grant nos. BK202213, 2021WG06,
2022YKY02 and 2022YKY09), as well as the Research Project of the
Hubei Provincial Department of Education (grant nos. ZY2023F109,
B2022186 and B2022185).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Each author significantly contributed to the present
study. MX and FZ conceived and designed the study. The experiments
were conducted by DC, YX and TC. SZ, WM and HZ gathered and
analyzed the data. The manuscript was drafted and edited by MX and
FZ. The final manuscript was reviewed and approved by all authors.
DC, YX and TC confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Hubei University of Science and Technology
Ethics Committee approved the present study (approval number
2020-01-900). All experiments were performed in accordance with
international and local guidelines on the ethical use of
animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mills SEE, Nicolson KP and Smith BH:
Chronic pain: A review of its epidemiology and associated factors
in population-based studies. Br J Anaesth. 123:e273–e283.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cohen SP, Vase L and Hooten WM: Chronic
pain: An update on burden, best practices, and new advances.
Lancet. 397:2082–2097. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tobin DG, Lockwood MB, Kimmel PL, Dember
LM, Eneanya ND, Jhamb M, Nolin TD, Becker WC and Fischer MJ: HOPE
Consortium (Corporate Author). Opioids for chronic pain management
in patients with dialysis-dependent kidney failure. Nat Rev
Nephrol. 18:113–128. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Santoni A, Santoni M and Arcuri E: Chronic
cancer pain: Opioids within tumor microenvironment affect
neuroinflammation, tumor and pain evolution. Cancers (Basel).
14(2253)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong ZB, Wang YJ, Wan WJ, Wu J, Wang BJ,
Zhu HL, Xie M and Liu L: Resveratrol ameliorates
oxaliplatin-induced neuropathic pain via anti-inflammatory effects
in rats. Exp Ther Med. 24(586)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ji RR, Nackley A, Huh Y, Terrando N and
Maixner W: Neuroinflammation and central sensitization in chronic
and widespread pain. Anesthesiology. 129:343–366. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Linnerbauer M, Wheeler MA and Quintana FJ:
Astrocyte crosstalk in CNS inflammation. Neuron. 108:608–622.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang YZ, Zhang YL, Huang Q, Huang C,
Jiang ZL, Cai F and Shen JF: AdipoRon alleviates free fatty
acid-induced myocardial cell injury via suppressing Nlrp3
inflammasome activation. Diabetes Metab Syndr Obes. 12:2165–2179.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choudhury SM, Ma X, Zeng Z, Luo Z, Li Y,
Nian X, Ma Y, Shi Z, Song R, Zhu Z, et al: Senecavirus a 3D
interacts with NLRP3 to induce IL-1β production by activating NF-κB
and ion channel signals. Microbiol Spectr.
10(e0209721)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen R, Yin C, Fang J and Liu B: The NLRP3
inflammasome: An emerging therapeutic target for chronic pain. J
Neuroinflammation. 18(84)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu S, Zhao G, Han F, Liang W, Jiao Y, Li Z
and Li L: Muscone relieves inflammatory pain by inhibiting
microglial activation-mediated inflammatory response via abrogation
of the NOX4/JAK2-STAT3 pathway and NLRP3 inflammasome. Int
Immunopharmacol. 82(106355)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khan N, Kuo A, Brockman DA, Cooper MA and
Smith MT: Pharmacological inhibition of the NLRP3 inflammasome as a
potential target for multiple sclerosis induced central neuropathic
pain. Inflammopharmacology. 26:77–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dong X, Fu J, Yin X, Cao S, Li X, Lin L
and Huyiligeqi and Ni J: Emodin: A review of its pharmacology,
toxicity and pharmacokinetics. Phytother Res. 30:1207–1218.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Zhang X, Li J and Guan L: Emodin reduces
inflammatory and nociceptive responses in different pain-and
inflammation-induced mouse models. Comb Chem High Throughput
Screen. 26:989–1000. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao Y, Liu H, Deng L, Zhu G, Xu C, Li G,
Liu S, Xie J, Liu J, Kong F, et al: Effect of emodin on neuropathic
pain transmission mediated by P2X2/3 receptor of primary sensory
neurons. Brain Res Bull. 84:406–413. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun T, Wang J, Li X, Li YJ, Feng D, Shi
WL, Zhao MG, Wang JB and Wu YM: Gastrodin relieved complete
Freund's adjuvant-induced spontaneous pain by inhibiting
inflammatory response. Int Immunopharmacol. 41:66–73.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hao M, Tang Q, Wang B, Li Y, Ding J, Li M,
Xie M and Zhu H: Resveratrol suppresses bone cancer pain in rats by
attenuating inflammatory responses through the AMPK/Drp1 signaling.
Acta Biochim Biophys Sin (Shanghai). 52:231–240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mao Y, Wang C, Tian X, Huang Y, Zhang Y,
Wu H, Yang S, Xu K, Liu Y, Zhang W, et al: Endoplasmic reticulum
stress contributes to nociception via neuroinflammation in a murine
bone cancer pain model. Anesthesiology. 132:357–372.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shi X, Bai H, Wang J, Wang J, Huang L, He
M, Zheng X, Duan Z, Chen D, Zhang J, et al: Behavioral assessment
of sensory, motor, emotion, and cognition in rodent models of
intracerebral hemorrhage. Front Neurol. 12(667511)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seeliger D and de Groot BL: Ligand docking
and binding site analysis with PyMOL and Autodock/Vina. J Comput
Aided Mol Des. 24:417–422. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang D, Hu X, Qian L, O'Callaghan JP and
Hong JS: Astrogliosis in CNS pathologies: Is there a role for
microglia? Mol Neurobiol. 41:232–241. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cao DY, Zhang ZH, Li RZ, Shi XK, Xi RY,
Zhang GL, Li F and Wang F: A small molecule inhibitor of caspase-1
inhibits NLRP3 inflammasome activation and pyroptosis to alleviate
gouty inflammation. Immunol Lett. 244:28–39. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Prior R, Van Helleputte L, Klingl YE and
Van Den Bosch L: HDAC6 as a potential therapeutic target for
peripheral nerve disorders. Expert Opin Ther Targets. 22:993–1007.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Picci C, Wong VSC, Costa CJ, McKinnon MC,
Goldberg DC, Swift M, Alam NM, Prusky GT, Shen S, Kozikowski AP, et
al: HDAC6 inhibition promotes alpha-tubulin acetylation and
ameliorates CMT2A peripheral neuropathy in mice. Exp Neurol.
328(113281)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Krukowski K, Ma J, Golonzhka O, Laumet GO,
Gutti T, van Duzer JH, Mazitschek R, Jarpe MB, Heijnen CJ and
Kavelaars A: HDAC6 inhibition effectively reverses
chemotherapy-induced peripheral neuropathy. Pain. 158:1126–1137.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ma J, Trinh RT, Mahant ID, Peng B,
Matthias P, Heijnen CJ and Kavelaars A: Cell-specific role of
histone deacetylase 6 in chemotherapy-induced mechanical allodynia
and loss of intraepidermal nerve fibers. Pain. 160:2877–2890.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou P, Zhou R, Min Y, An LP, Wang F and
Du QY: Network pharmacology and molecular docking analysis on
pharmacological mechanisms of astragalus membranaceus in the
treatment of gastric ulcer. Evid Based Complement Alternat Med.
2022(9007396)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Evans LW, Bender A, Burnett L, Godoy L,
Shen Y, Staten D, Zhou T, Angermann JE and Ferguson BS: Emodin and
emodin-rich rhubarb inhibits histone deacetylase (HDAC) activity
and cardiac myocyte hypertrophy. J Nutr Biochem.
79(108339)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Magupalli VG, Negro R, Tian Y, Hauenstein
AV, Di Caprio G, Skillern W, Deng Q, Orning P, Alam HB, Maliga Z,
et al: HDAC6 mediates an aggresome-like mechanism for NLRP3 and
pyrin inflammasome activation. Science.
369(eaas8995)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu S, Chen H, Ni H and Dai Q: Targeting
HDAC6 attenuates nicotine-induced macrophage pyroptosis via
NF-κB/NLRP3 pathway. Atherosclerosis. 317:1–9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cao Z, Gu Z, Lin S, Chen D, Wang J, Zhao
Y, Li Y, Liu T, Li Y, Wang Y, et al: attenuation of NLRP3
inflammasome activation by indirubin-derived PROTAC targeting
HDAC6. ACS Chem Biol. 16:2746–2751. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yan S, Wei X, Jian W, Qin Y, Liu J, Zhu S,
Jiang F, Lou H and Zhang B: Pharmacological inhibition of HDAC6
attenuates NLRP3 inflammatory response and protects dopaminergic
neurons in experimental models of parkinson's disease. Front Aging
Neurosci. 12(78)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
He J, Zhou Y, Xing J, Wang Q, Zhu H, Zhu Y
and Zou MH: Liver kinase B1 is required for thromboxane
receptor-dependent nuclear factor-κB activation and inflammatory
responses. Arterioscler Thromb Vasc Biol. 33:1297–1305.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Barter MJ, Butcher A, Wang H, Tsompani D,
Galler M, Rumsby EL, Culley KL, Clark IM and Young DA: HDAC6
regulates NF-κB signalling to control chondrocyte IL-1-induced MMP
and inflammatory gene expression. Sci Rep. 12(6640)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang CJ, Liu YP, Dai HY, Shiue YL, Tsai
CJ, Huang MS and Yeh YT: Nuclear HDAC6 inhibits invasion by
suppressing NF-κB/MMP2 and is inversely correlated with metastasis
of non-small cell lung cancer. Oncotarget. 6:30263–30276.
2015.PubMed/NCBI View Article : Google Scholar
|