Introduction
The thin structure of the layers of the head often
results in the loss of multiple layers of tissue and skull after
trauma, and even the exposure of important structures, such as
brain tissue (1). However, the
limited amount of tissue in the head itself makes it difficult to
provide an adequate donor site in the face of large defects. This
situation is even more prominent in young children, thus forcing
the consideration of free flaps. A free flap refers to a flap that
is completely removed from the donor site and incorporates segments
of vascularized mucosa, bone or nerve, as well as skin, which
allows reconstruction of complex composite defects ‘with like
tissue’ (2). Nutrition is supplied
to the flap through microvascular anastomosis surgery and vascular
anastomosis in the recipient area. The advantages of free flap
reconstruction include selection of well-matched tissue, shape
plasticity and reliable vascularity (3). Although free flap surgery in children
is challenging, several studies have shown the same or even higher
success rates as in adults (4,5). The
present report describes the case of a 2-year-old child weighing 9
kg who experienced a skull fracture with encephalocele and
life-threatening infection after a car accident. An artificial dura
mater combined with bone cement was used to repair the skull, and
the wound was then covered with a free latissimus dorsi muscle flap
(LDMF) combined with a split-thickness skin graft (STSG), thus
achieving a satisfactory result.
Case report
In July 2020, a 2-year-old boy who was hit by a car
and dragged for >100 m was taken to a local hospital for
treatment in Tengzhou, China. Imaging revealed multiple fractures
to the skull and body. Contusions were noted in the right frontal
and temporal lobes of the skull, with a subdural hematoma, but the
rest of the brain tissue was normal. The red blood cell count
(3.03x1012/l; reference range,
4.3-5.8x1012/l) and hemoglobin levels (88 g/l; reference
range, 130-175 g/l) were decreased, and various inflammatory
indicators [white blood cells, 9.88x109/l (reference
range, 3.5-9.5x109/l); D-dimer, 22.27 mg/l (reference
range, 0-0.5 mg/l); C-reactive protein, 98.3 mg/l (reference range,
0-10 mg/l); interleukin-6, 287.5 pg/ml (reference range, 0-7
pg/ml)] were markedly increased. The child weighed only 9 kg, which
was below the normal range for their age (reference range,
12.54-14.15 kg). The local hospital managed the wound debridement
and provided allograft skin coverage of the wound. Tracheal
intubation was preserved after surgery, and the child was
transferred to the pediatric intensive care unit (PICU) of Shandong
Provincial Hospital (Jinan, China). After admission, the parents of
the child were given a critically ill notification. The patient was
treated with linazolamide (10 mg/kg, three times a day, intravenous
injection for 14 days), a blood transfusion (300 ml plasma, 2U red
blood cells suspension leukocyte reduced and 4U cryoprecipitate)
and mechanical ventilation. First, the fractures and trauma of the
extremities were treated cooperatively using a multidisciplinary
team (MDT) approach. The left upper limb underwent debridement and
skin grafting, and the left femur fracture underwent open reduction
and internal fixation. The head, although the most damaged, having
been debrided and covered, was temporarily more stable and did not
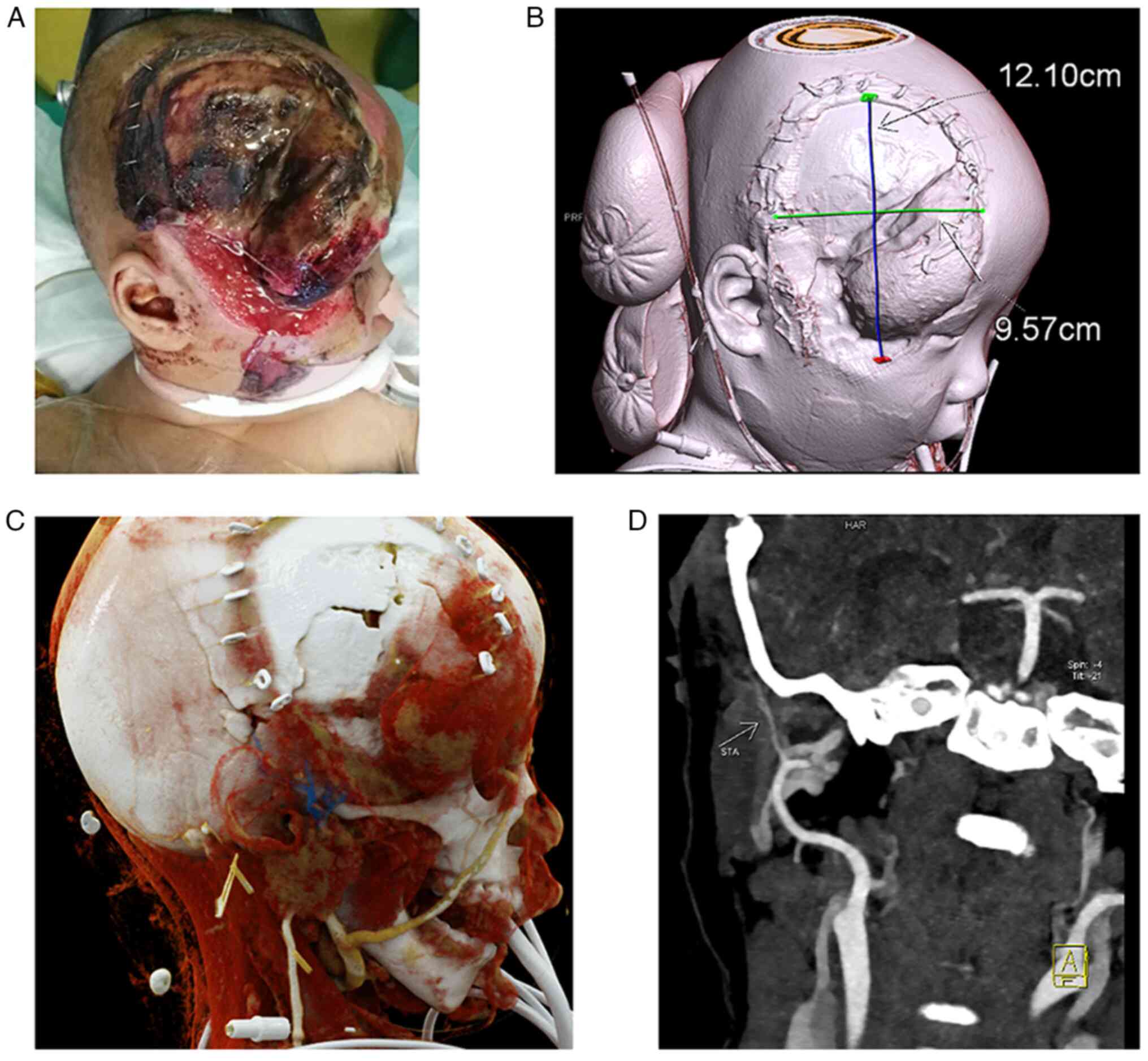
require MDT treatment. However, after ~1 week, the allograft showed
signs of infection and decay (Fig.
1A). The neurosurgeon chose to repair the skull using bone
cement and requested that the plastic and reconstruction team cover
the wound. Three-dimensional computed tomography angiography (CTA)
showed partial leakage of brain tissue within a defect area of
12.10x9.57 cm2 (Fig.
1B). The child was also in a poor physical condition and would
most likely not be able to tolerate the cranium restoration
surgery; therefore, LDMF plus STSG was considered to yield better
results. The superficial temporal artery was selected as the
recipient vessel in the head. The superficial temporal vein is
shown in blue in Fig. 1C, whereas
the stump of the superficial temporal artery is visible in Fig. 1D.
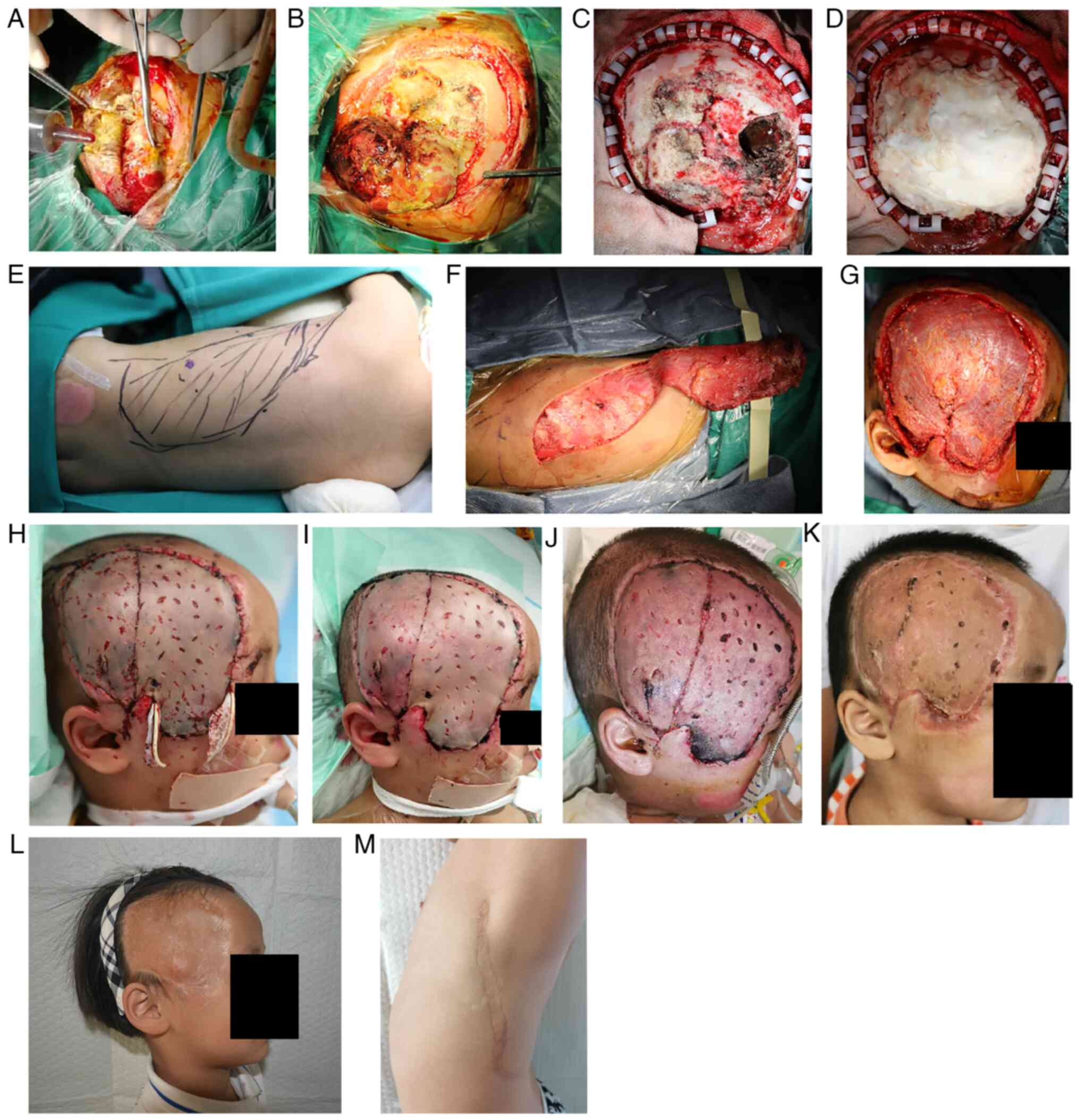
Surgery was performed on day 9 post-trauma,
beginning with removal of the infected leaking brain tissue by the
neurosurgeon. The allograft skin was opened and the brain tissue
was observed to be expanding along the defect (Fig. 2A). The size of the bulge was ~4x5
cm2 (Fig. 2B). After an
incision was made into the dura mater of the brain tissue at the
bulge, the necrotic brain tissue and the subdural hematoma were
removed (Fig. 2C). The dural
defect was repaired with an artificial dura and bone cement shaping
(Fig. 2D). The LDM contours were
marked preoperatively (Fig. 2E). A
left LDMF was created during surgery; it was separated from the
deep fascia layer and deep muscular layer, and was detached keeping
the latissimus dorsi muscle intact. The thoracodorsal artery was
preserved and the anterior serratus branch of the thoracodorsal
artery was dissected (Fig. 2F).
Next, split-thickness skin measuring ~15x10 cm2 was
harvested from the right dorsal side. The dorsal thoracic and
superficial temporal arteries were anastomosed (Fig. 2G). The muscle flap was matched to
the shape of the wound. After the blood supply and venous return
were clear, the flap was sutured and covered with an STSG.
The flap was in good condition on the first day
after a dressing change (Fig. 2H).
Some necrosis was noted at the distal end of the flap on
postoperative day 3, but the overall flap tissue survival rate was
excellent (Fig. 2I). A total of 1
week post-surgery, it was recommended that the PICU withdraw the
ventilator. The child then remained stable, and their physical
condition gradually recovered until they were considered out of
critical condition. The red blood cell count
(3.96x1012/l) and hemoglobin levels (123 g/l) increased,
whereas their inflammatory marker levels (white blood cells,
13x109/l; D-dimer, 1.22 mg/l; C-reactive protein,
<4.00 mg/l) decreased. The sutures were removed at 9 days
post-surgery (Fig. 2J). The child
was discharged from the hospital 19 days after surgery. A total of
1 month after surgery, the patient returned for follow-up, and the
overall recovery was excellent except for partial necrosis of the
distal portion of the flap (Fig.
2K). This part also healed after dressing change treatment. At
1 year later, the internal fixation of the left femur was
removed.
After a 3-year follow-up period, both the recipient
(Fig. 2L) and donor (Fig. 2M) sites of the patient had
recovered well. The surgery did not affect limb development,
including differences in upper limb strength compared with children
of the same age. In October 2023, the patient had mild scoliosis
caused by the missing latissimus dorsi muscle, and the child's
parents have been informed to pay attention to daily exercise and
fixation of orthosis, such as Boston orthosis or Crass Cheneau
orthosis. An intelligence test, the Denver Development Screening
Test (6), was performed and the
result was normal. The patient underwent cranial CT 1 year after
surgery and no abnormalities were found. Considering the economic
situation of the parents, it was suggested that if they detected no
abnormalities, the patient could wait and undergo a cranial CT scan
once the body rapidly grows during adolescence. The child has
already attended kindergarten and the parents have not identified
any other abnormal intellectual or physical activity in their daily
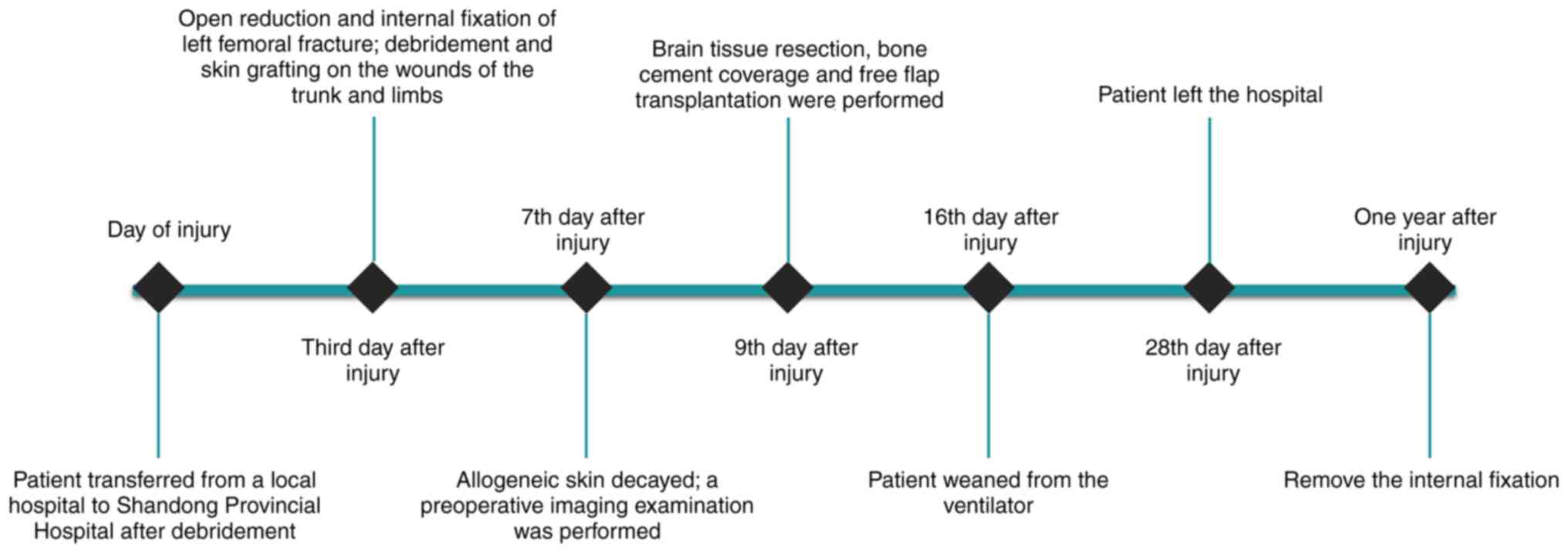
lives. The chronological order of the treatments administered to
the patient is shown in Fig.
3.
Discussion
Free flap surgery in children is usually considered
to be more challenging compared with adults due to the smaller
vessels that may be more prone to vasospasm and difficulties in
postoperative care. Recovery of the donor area and the impact on
patient growth must also be considered (7). However, a number of studies have
shown a high success rate of free flap surgery in children. Five
articles, including 646 children with a total of 694 free flaps,
were reviewed in a previous study, with an overall survival rate of
96.4% of the free flaps (8).
Reasons for the high success rate may include the absence of a
history of smoking and of underlying diseases affecting the
vasculature, and the fact that the vessels are not small relative
to the flap volume (9). There may
also be a relatively subjective reason, as surgeons who perform
free flap surgery in children usually have abundant microsurgical
experience (5), which may ensure a
certain degree of success. However, pediatric free flap surgery is
usually a selective operation, and the child is in good underlying
condition to tolerate the surgery and recover quickly. By contrast,
in the present case, the child had a life-threatening condition,
which posed a significant risk to the surgery.
The success of the procedure also requires adequate
preoperative preparation and careful postoperative care. Imaging is
important before surgery, including ultrasound, CTA, magnetic
resonance angiography, infrared imaging and indocyanine green
fluorescein angiography. CTA has excellent specificity and
sensitivity in microsurgery, and also has the advantages of being
frequently available and cost-effective (10). Notably, the three-dimensional
reconstruction of CTA images displays the recipient and donor sites
well. In addition, for selective operations, it is possible to
screen and detect obesity, hereditary diseases or syndromes, atopic
diseases, psychiatric disorders and the presence of a history of
radiotherapy (11). As well as
preoperative preparation, postoperative care and monitoring are
crucial; Li et al (4)
reported that sedation or general anesthesia could be withheld if
the child could cooperate to keep quiet with the parents. However,
other studies have suggested that pain medication and sedation can
be used appropriately depending on the mental status and tolerance
of the child (5,12). Some studies have also suggested
that children should be placed in the ICU within 24 h of surgery to
ensure regular monitoring of the flap (13). The child in the present case was
admitted to the PICU due to a poor underlying condition, thus they
were maintained in the PICU post-surgery. Another potential issue
is whether the blood vessels of children are more prone to
vasospasm (13,14). Although there is no uniform answer,
there is a general agreement that monitoring is critical for 3 days
post-surgery, a period when vasospasm or embolism are more likely
to occur (5). Although necrosis
occurred distal to the flap in the present case, no postoperative
vascular problems were identified. It may be hypothesized that the
necrosis is more likely related to the weak physical condition of
the patient and the presence of curvature in the flap.
Donor site selection is also an important issue for
free flap surgery in children. As the child grows, the donor site
may have growth disturbances or developmental asymmetry, especially
in areas where muscle has been excised (9). Although prospective studies are
lacking, numerous retrospective studies have shown a low incidence
of growth disturbances at the donor site (9,15).
In the present case, the wound area had reached 3-4% of the total
body surface area. For a child with such a low body weight, the
anterolateral femoral flap and the deep inferior epigastric
perforator flap do not have as much tissue volume as in adults, and
the latissimus dorsi flap was thus considered the most suitable.
Moreover, from the infection point of view, this muscle has a
stronger resistance to infection. From the perspective of damage to
the child, the child had multiple fractures and trauma throughout
the body, and their underlying condition was poor. Therefore,
taking a muscle flap combined with an STSG could reduce trauma and
may be easier to plasticize. Upton and Guo (14) used muscle combined with an STSG
when covering injuries in children with trauma and infected wounds.
Due to its extensive coverage, thin thickness, flexibility and
minimal donor area injury, the LDMF combined with skin graft is
considered the best choice for subtotal or total scalp
reconstruction (16,17).
In the treatment of the child reported in the
present case, cranial trauma coverage was only part of the process.
The whole body treatment also relied on the combined efforts of
several departments, including PICU, neurosurgery, pediatric
orthopedics and imaging. Thus, a MDT treatment approach is very
important in the treatment of large complex injuries in children.
Currently, in addition to cancer, more pediatric diseases require
MDT intervention for safe and considerate treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Clinical Medical
Science Innovation Program of Jinan (grant no. 202019076), Taishan
Scholars (grant no. ts201511100) and the National Natural Science
Foundation of China (grant no. 82172227).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
ZL and GX designed the study, searched the
literature and wrote the first draft of the manuscript. PZ obtained
and processed patient's and CTA images. XY contributed to
acquisition of data and interpreted the relevant information. GX
and ZZ were responsible for formulating the patient's treatment
plan. CF and RH contributed to analysis and interpretation of data,
critical revisions of the intellectual content and confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
guardians of the patient for the publication of any accompanying
images or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xi C, Yao H, Xu Y, Liu Y, Tian H and Hu J:
The emergency epidemiologic characteristics of casualties cases
with head injury in Shanghai. Chin J Emerg Med. 17:1131–1134.
2008.
|
|
2
|
Rassekh CH: Free flap options for common
head and neck defects. Facial Plast Surg. 12:97–101.
1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Archibald H, Stanek J and Hamlar D: Free
Flap donor-site complications and management. Semin Plast Surg.
37:26–30. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li J, Xiong H, Li G, Zhou P, Ai F, Wang K
and Chen J: Free flap reconstruction of extremity defects in
pediatric patients. Handchir Mikrochir Plast Chir. 53:349–355.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu S, Zhang WB, Yu Y, Wang Y, Mao C, Guo
CB, Yu GY and Peng X: Free flap transfer for pediatric head and
neck reconstruction: What factors influence flap survival?
Laryngoscope. 129:1915–1921. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barnes KE and Stark A: The denver
development screening test. A normative study. Am J Public Health.
65:363–369. 1975.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Roasa FV, Castañeda SS and Mendoza DJC:
Pediatric free flap reconstruction for head and neck defects. Curr
Opin Otolaryngol Head Neck Surg. 26:334–339. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Markiewicz MR, Ruiz RL, Pirgousis P, Bryan
Bell R, Dierks EJ, Edwards SP and Fernandes R: Microvascular free
tissue transfer for head and neck reconstruction in children: Part
I. J Craniofac Surg. 27:846–856. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alkureishi LWT, Purnell CA, Park P, Bauer
BS, Fine NA and Sisco M: Long-term outcomes after pediatric free
flap reconstruction. Ann Plast Surg. 81:449–455. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Knitschke M, Baumgart AK, Bäcker C,
Adelung C, Roller F, Schmermund D, Böttger S, Howaldt HP and Attia
S: Computed tomography angiography (CTA) before reconstructive jaw
surgery using fibula free flap: Retrospective analysis of vascular
architecture. Diagnostics (Basel). 11(1865)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Starnes-Roubaud MJ, Hanasono MM, Kupferman
ME, Liu J and Chang EI: Microsurgical reconstruction following
oncologic resection in pediatric patients: A 15-year experience.
Ann Surg Oncol. 24:4009–4016. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Gijn DR, D'Souza J, King W and Bater
M: Free flap head and neck reconstruction with an emphasis on
postoperative care. Facial Plast Surg. 34:597–604. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Duteille F, Lim A and Dautel G: Free flap
coverage of upper and lower limb tissue defects in children: A
series of 22 patients. Ann Plast Surg. 50:344–349. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Upton J and Guo L: Pediatric free tissue
transfer: A 29-year experience with 433 transfers. Plast Reconstr
Surg. 121:1725–1737. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Serletti JM: Current trends in pediatric
microsurgery. Clin Plast Surg. 32:45–52, viii. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Deng K, Xiao H, Wang H and Xu X:
Latissimus dorsi muscle flap for scalp reconstruction and
postoperative ulceration management. J Craniofac Surg.
33:e233–e236. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Desai SC, Sand JP, Sharon JD, Branham G
and Nussenbaum B: Scalp reconstruction: An algorithmic approach and
systematic review. JAMA Facial Plast Surg. 17:56–66.
2015.PubMed/NCBI View Article : Google Scholar
|