Introduction
GLOBOCAN 2020 showed lung cancer is one of leading
causes of cancer-associated mortalities (1.8 million mortalities
worldwide and 710 thousand mortalities in China) (1). The majority of lung cancer is
non-small cell lung cancer (NSCLC), accounting for 80-85% of cases
(2). Anaplastic lymphoma kinase
(ALK) fusion genes, which occur in 3-7% of patients with
NSCLC, are important oncogenic drivers (3-5).
Echinoderm microtubule associated protein-like 4-ALK was the
first reported fusion gene in NSCLC (5) and TRK-fused gene-ALK and
kinesin family member 5B-ALK have subsequently been found
(6,7).
The first-generation ALK tyrosine kinase inhibitor
(TKI) crizotinib is a potent targeted drug for patients with
ALK-positive NSCLC (8).
Nevertheless, most patients eventually develop progressive disease
(PD). The main reason for crizotinib resistance is poor penetration
into the central nervous system (9). Second-generation ALK TKIs, such as
iruplinalkib (WX-0593), alectinib and brigatinib, can overcome
crizotinib resistance (10-14).
In 2018, Dickson et al (15) first reported a rare alteration,
sperm antigen with calponin homology and coiled-coil domains 1 like
(SPECC1L)-ALK fusion, in epithelioid fibrous
histiocytoma. Crizotinib can inhibit the proliferation and survival
of SPECC1L-ALK transfected HEK293 cells in vitro
(16), indicating ALK TKI may
serve as a treatment option for NSCLC with SPECC1L-ALK
fusion. However, there is a lack of clinical evidence. A NSCLC case
with SPECC1L-ALK fusion, treated with crizotinib ±
bevacizumab for 23 months and iruplinalkib for 2.5 months, has been
previously reported (17). The
patient maintained partial response (PR) as of the reporting date
(January 2020). The present study reports subsequent follow-up of
this case.
Case report
Demographic and tumour characteristics and the
course of treatment with crizotinib have been reported previously
(17). In brief, the patient was a
female non-smoker aged 44 years. The patient attended Beijing Chest
Hospital (Beijing, China) for worsened cough and expectoration in
August 2017. Stage IV lung adenocarcinoma with lymph node
metastases and pleural effusion but no brain metastasis (cT4N3M1c)
was diagnosed according to computed tomography (CT) and lung tissue
and retroperitoneal lymph node puncture biopsy. Lung tissue was
tested using immunohistochemistry (IHC; Ventana ALK D5F3 CDx Assay,
Roche), showing positive ALK expression (17). Further, next-generation sequencing
was performed, and SPECC1L-ALK fusion was identified with a
mutant allele frequency (MAF) of 28.66% (17). In October 2017, crizotinib
treatment (250 mg, orally, twice daily) was initiated. In December
2017, PR was achieved according to Response Evaluation Criteria in
Solid Tumors version 1.1 (RECIST v1.1) (18). In March 2018, bevacizumab was added
to the regimen because tumour size increased and stable disease was
reached. In September 2018, the patient developed PD, with
progression-free survival (PFS) of nearly 23 months on crizotinib ±
bevacizumab. A second biopsy indicated increased ALK expression in
IHC and the same SPECC1L-ALK fusion with a lower MAF (1.5%)
(17). In October 2019,
combination of crizotinib and bevacizumab was discontinued.
The patient participated in the single-arm phase II
INTELLECT study of iruplinalkib (10) at Beijing Chest Hospital in October
2019. In the INTELLECT study, patients with ALK-positive
crizotinib-resistant advanced NSCLC aged ≥18 years with Eastern
Cooperative Oncology Group performance status (19) of 0-2 were included. The primary
endpoint was the independent review committee-assessed objective
response rate. At baseline, the patient had lymph node and brain
metastases and pleural effusion. The brain metastasis was first
found at screening of the INTELLECT study. According to the
protocol of the INTELLECT study, oral iruplinalkib 180 mg once
daily with a 7-day lead-in phase at 60 mg once daily was given.
Tumour was assessed every 6 weeks in the first 24 weeks after
iruplinalkib treatment initiation, every 9 weeks after 24 weeks and
every 12 weeks after 1 year until disease progression, withdrawal
of consent, loss of follow-up, start of other antitumor treatment
or death. For this patient, chest and upper abdomen CT and brain
magnetic resonance imaging were used. Systemic and intracranial
response was evaluated according to RECIST v1.1 and Response
Assessment in Neuro-Oncology (RANO) brain metastases criteria
(20), respectively. Adverse
events were classified and graded according to National Cancer
Institute Common Terminology Criteria for Adverse Events, version
4.03(21).
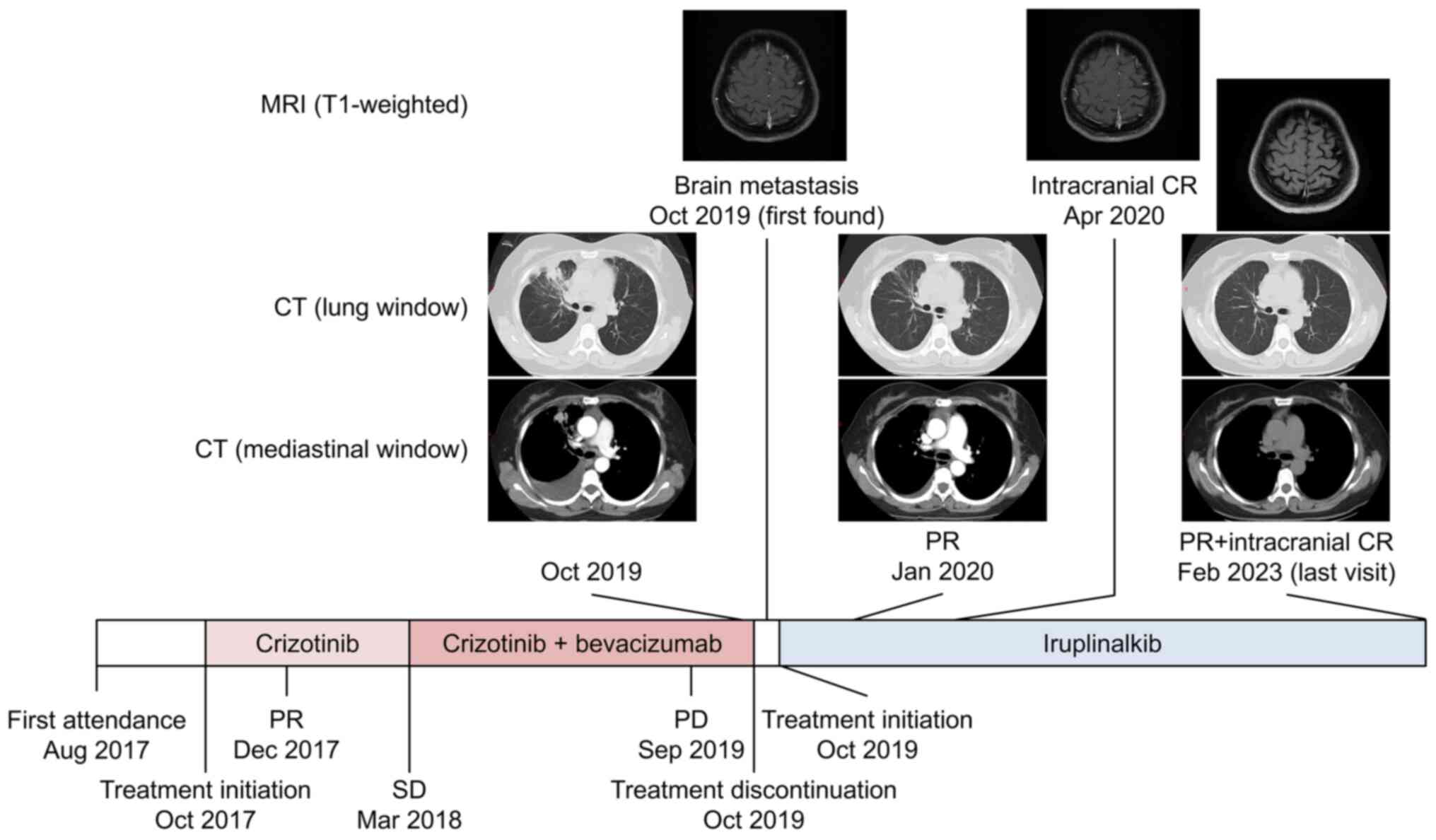
PR was achieved one month later (November 2019).
This patient was followed-up at 4th week but not 6th week.
Additionally, the intracranial lesion disappeared and intracranial
complete response was achieved in April 2020. As of February 2023,
systemic and intracranial exhibited PR and complete response,
respectively (Fig. 1). The PFS on
iruplinalkib lasted for 39.3 months, with right censoring.
No notable treatment-related adverse event (TRAE)
was observed except grade 3 hypertriglyceridaemia complicated with
grade 2 hypercholesterolaemia. These TRAEs were first reported in
November 2019. Oral fenofibrate 200 mg twice daily was given and
triglyceride and cholesterol levels reverted to baseline values
(Table I). There was no dose
interruption, reduction or discontinuation due to TRAE.
 | Table ITRAEs. |
Table I
TRAEs.
| TRAE | Time of occurrence
after treatment initiation, days | Grade | Management | Duration, days | Outcome |
|---|
| Nausea | 8 | 1 | Observation | 3 | Recovered without
sequelae |
| Vomiting | 8 | 1 | Observation | 3 | Recovered without
sequelae |
| Diarrhoea | 10 | 1 | Observation | 3 | Recovered without
sequelae |
|
Hypercholesterolaemia | 10 | 2 | Fenofibrate (200 mg,
orally, twice daily) | 156 | Recovered without
sequelae |
|
Hypertriglyceridaemia | 10 | 3 | Fenofibrate (200 mg,
orally, twice daily) | 444 | Recovered without
sequelae |
| Hypertension | 13 | 2 | Fosinopril (10 mg,
orally, once daily) Then switched to nifedipine (30 mg, orally,
once daily) and irbesartan and hydrochlorothiazide (150 mg/12.5 mg,
orally, once daily) | Up to cut-off date
(February-2023) | Not recovered |
| Hepatic function
abnormal | 20 | 1 | Bicyclol (25 mg,
orally, thrice daily) | 42 | Recovered without
sequelae |
| Anaemia | 116 | 1 | Observation | 50 | Recovered without
sequelae |
| Hyperuricaemia | 1,028 | 1 | Observation | Up to cut-off date
(February-2023) | Not recovered |
Discussion
SPECC1L-ALK fusion is a rare alteration first
reported in epithelioid fibrous histiocytoma in 2018(15). In 2020 and 2021, this fusion was
identified in NSCLC and glioma, respectively (17,22).
The present study reported subsequent follow-up of a patient with
NSCLC harbouring SPECC1L-ALK.
Generally, as oncogenic drivers, ALK fusion proteins
can result in continuous ALK and downstream signalling pathway
activation, inducing tumour proliferation and survival (23). However, the mechanism of
SPECC1L-ALK fusion proteins in ALK and downstream signalling
pathway activation is unclear. ALK TKIs are recommended targeted
drugs for ALK-positive disease (8). In vitro, crizotinib, a
first-generation ALK TKI, inhibits growth and survival of
SPECC1L-ALK transfected HEK293 cells in a dose-dependent
manner (16). Nevertheless, to the
best of our knowledge, there is a lack of studies of novel ALK TKIs
for patients with NSCLC and SPECC1L-ALK.
The present case received crizotinib as the
first-line treatment and achieved PR. Then, bevacizumab was added
due to increased tumour size. First-line PFS reached ~23 months.
After PD on crizotinib + bevacizumab, second-generation ALK TKIs
may be ideal treatment options (10-14,24-26).
Iruplinalkib is a second-generation ALK TKI
developed by Qilu Pharmaceutical Co., Ltd. (Jinan, China) with
potent preclinical and clinical efficacy and acceptable safety
profiles in ALK-positive NSCLC (10,27-29).
The phase II INTELLECT study (10)
of iruplinalkib showed an objective response rate and a PFS
superior or similar to other second-generation ALK TKIs (for
example, ceritinib and brigatinib) (11-14,24-26).
The present patient had systemic PR and intracranial CR, which
continued as of cut-off date (February 2023). The PFS was 39.3
months with right censoring, notably longer than the
investigator-assessed median PFS of 14.5 months in the INTELLECT
study. In terms of TRAE, most were grade 1 to 2 and not notable in
the present patient. Grade 3 hypertriglyceridaemia and grade 2
hypercholesterolaemia occurred during iruplinalkib treatment.
Triglyceride and cholesterol levels reverted to baseline after oral
fenofibrate 200 mg twice daily was administered. No
treatment-related dose interruption, reduction or discontinuation
occurred.
In summary, iruplinalkib had promising systemic and
intracranial efficacy for NSCLC harbouring SPECC1L-ALK gene,
with acceptable and manageable safety profiles. Iruplinalkib may be
used for patients with rare ALK fusions. The oncogenic mechanisms
of SPECC1L-ALK fusion protein and anti-tumour activity of ALK TKI
on tumour with SPECC1L-ALK need further investigation.
Acknowledgements
The authors would like to thank Mr. Yunjie Yu for
medical writing support and Ms. Fengcai Sun and Ms. Jing Wang for
assisting with data collection (all Qilu Pharmaceutical Co., Ltd.,
Jinan, China).
Funding
Funding: The present study was supported by Qilu Pharmaceutical
Co., Ltd.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW, YH and SZ were responsible for conception and
design of the study. QZ, JL, XL, HZ, MS, YH and SZ acquired and
interpreted medical images and advised on patient treatment. QZ and
CZ analysed and interpreted the data. QZ and JL drafted the
manuscript. XL, HZ, CZ, MW, MS, YH and SZ reviewed and revised the
manuscript. All authors confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent to
participate in the INTELLECT study, which was approved by the
ethics committee of Beijing Chest Hospital (Beijing, P.R. China;
Approval no. 2019-40-01).
Patient consent for publication
The patient provided written informed consent for
the publication of any potentially identifiable images or data.
Competing interests
CZ, MW and MS are employees of Qilu Pharmaceutical
Co., Ltd. (Jinan, China). All other authors declare that they have
no competing interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Planchard D, Popat S, Kerr K, Novello S,
Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD,
et al: Metastatic non-small cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 29 (Suppl 4):iv192–iv237. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dearden S, Stevens J, Wu YL and Blowers D:
Mutation incidence and coincidence in non small-cell lung cancer:
Meta-analyses by ethnicity and histology (mutMap). Ann Oncol.
24:2371–2376. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Koivunen JP, Mermel C, Zejnullahu K,
Murphy C, Lifshits E, Holmes AJ, Choi HG, Kim J, Chiang D, Thomas
R, et al: EML4-ALK fusion gene and efficacy of an ALK kinase
inhibitor in lung cancer. Clin Cancer Res. 14:4275–4283.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rikova K, Guo A, Zeng Q, Possemato A, Yu
J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al: Global survey
of phosphotyrosine signaling identifies oncogenic kinases in lung
cancer. Cell. 131:1190–1203. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Takeuchi K, Choi YL, Togashi Y, Soda M,
Hatano S, Inamura K, Takada S, Ueno T, Yamashita Y, Satoh Y, et al:
KIF5B-ALK, a novel fusion oncokinase identified by an
immunohistochemistry-based diagnostic system for ALK-positive lung
cancer. Clin Cancer Res. 15:3143–3149. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang S, Anjum R, Squillace R, Nadworny S,
Zhou T, Keats J, Ning Y, Wardwell SD, Miller D, Song Y, et al: The
potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of
resistance to first- and second-generation ALK inhibitors in
preclinical models. Clin Cancer Res. 22:5527–5538. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi Y, Chen J, Zhang H, Zhang Z, Zhang Y,
Wang Z, Zhang S, Zhao J, Liu C, Wang X, et al: Efficacy and safety
of iruplinalkib (WX-0593) in ALK-positive crizotinib-resistant
advanced non-small cell lung cancer patients: A single-arm,
multicenter phase II study (INTELLECT). BMC Med.
21(72)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang JCH, Ou SHI, De Petris L, Gadgeel S,
Gandhi L, Kim DW, Barlesi F, Govindan R, Dingemans AC, Crino L, et
al: Pooled systemic efficacy and safety data from the pivotal phase
II studies (NP28673 and NP28761) of alectinib in ALK-positive
non-small cell lung cancer. J Thorac Oncol. 12:1552–1560.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wolf J, Helland Å, Oh IJ, Migliorino MR,
Dziadziuszko R, Wrona A, de Castro J, Mazieres J, Griesinger F,
Chlistalla M, et al: Final efficacy and safety data, and
exploratory molecular profiling from the phase III ALUR study of
alectinib versus chemotherapy in crizotinib-pretreated ALK-positive
non-small-cell lung cancer. ESMO Open. 7(100333)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang JCH, Liu G, Lu S, He J, Burotto M,
Vincent S, Yin J, Ma X and Popat S: 319O ALTA-3: A randomized trial
of brigatinib (BRG) vs alectinib (ALC) in crizotinib
(CRZ)-refractory advanced ALK+ NSCLC. Ann Oncol. 33(S1564)2022.
|
|
14
|
Gettinger SN, Huber RM, Kim DW, Bazhenova
L, Hansen KH, Tiseo M, Langer CJ, Paz-Ares Rodríguez LG, West HL,
Reckamp KL, et al: Long-term efficacy and safety of brigatinib in
crizotinib-refractory ALK+ NSCLC: Final results of the phase 1/2
and randomized phase 2 (ALTA) trials. JTO Clin Res Rep.
3(100385)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dickson BC, Swanson D, Charames GS,
Fletcher CD and Hornick JL: Epithelioid fibrous histiocytoma:
Molecular characterization of ALK fusion partners in 23 cases. Mod
Pathol. 31:753–762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen YL, Chen WL, Cheng YC, Lin MC, Yang
SC, Tsai HW, Lin CC, Su WC, Chow NH and Ho CL: Development of a
novel ALK rearrangement screening test for non-small cell lung
cancers. PLoS One. 16(e0257152)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma L, Zhang Q, Dong Y, Li H and Wang J:
SPECC1L-ALK: A novel gene fusion response to ALK inhibitors in
non-small cell lung cancer. Lung Cancer. 143:97–100.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
20
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
National Cancer Institute. Common
terminology criteria for adverse events (CTCAE). 2010. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Accessed October 31, 2023.
|
|
22
|
Bagchi A, Orr BA, Campagne O, Dhanda S,
Nair S, Tran Q, Christensen AM, Gajjar A, Furtado LV, Vasilyeva A,
et al: Lorlatinib in a child with ALK-fusion-positive high-grade
glioma. N Engl J Med. 385:761–763. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chiarle R, Voena C, Ambrogio C, Piva R and
Inghirami G: The anaplastic lymphoma kinase in the pathogenesis of
cancer. Nat Rev Cancer. 8:11–23. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Shaw AT, Kim TM, Crinò L, Gridelli C,
Kiura K, Liu G, Novello S, Bearz A, Gautschi O, Mok T, et al:
Ceritinib versus chemotherapy in patients with ALK-rearranged
non-small-cell lung cancer previously given chemotherapy and
crizotinib (ASCEND-5): A randomised, controlled, open-label, phase
3 trial. Lancet Oncol. 18:874–886. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu YL, Shi Y, Tan DSW, Xiaoqing L, Cheng
Y, Zhou J, An TT, Lu Y, Zhu B, Bai C, et al: Phase 1/2 study of
ceritinib in Chinese patients with advanced anaplastic lymphoma
kinase-rearranged non-small cell lung cancer previously treated
with crizotinib: Results from ASCEND-6. Lung Cancer. 150:240–266.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang Y, Zhou J, Zhou J, Feng J, Zhuang W,
Chen J, Zhao J, Zhong W, Zhao Y, Zhang Y, et al: Efficacy, safety,
and biomarker analysis of ensartinib in crizotinib-resistant,
ALK-positive non-small-cell lung cancer: A multicentre, phase 2
trial. Lancet Respir Med. 8:45–53. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu X, Zhang L, Wan H, Zhu Z, Jin J, Qin
Y, Mao W, Yan K, Fang D, Jiang W, et al: Discovery and preclinical
evaluations of WX-0593, a novel ALK inhibitor targeting
crizotinib-resistant mutations. Bioorg Med Chem Lett.
66(128730)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shi Y, Fang J, Hao X, Zhang S, Liu Y, Wang
L, Chen J, Hu Y, Hang X, Li J, et al: Safety and activity of
WX-0593 (Iruplinalkib) in patients with ALK- or ROS1-rearranged
advanced non-small cell lung cancer: A phase 1 dose-escalation and
dose-expansion trial. Signal Transduct Target Ther.
7(25)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang Y, Zheng Q, Wang X, Zhao S, Huang W,
Jia L, Ma C, Liu S, Zhang Y, Xin Q, et al: Iruplinalkib (WX-0593),
a novel ALK/ROS1 inhibitor, overcomes crizotinib resistance in
preclinical models for non-small cell lung cancer. Invest New
Drugs. 41:254–266. 2023.PubMed/NCBI View Article : Google Scholar
|