Introduction
Elective percutaneous coronary intervention (PCI) is
a widely used revascularization strategy in the treatment of
patients with chronic coronary syndrome (CCS) (1). Evidence from a clinical trial has
demonstrated that elective PCI, compared with medical therapy, can
provide symptom relief and survival benefits in lowering the risk
of adverse events such as cardiac death and myocardial infarction
(MI) (2). In-stent restenosis
(ISR) is a progressive re-narrowing of the coronary lesion after
stent implantation in PCI. Clinically, ISR commonly presents as
unstable angina pectoris and is associated with an increased risk
for acute coronary syndrome (ACS) (3). While the advance of the drug-eluting
stent (DES) has reduced the prevalence of ISR, ISR remains a
significant clinical problem and accounts for ~10% of coronary
revascularization, with associated mortality and morbidity
(4). Considering that
>1,000,000 PCIs are performed in China annually among the CCS
population, identifying prognostic factors for ISR is important in
informing the disease burden and risk stratification (5).
Inflammatory responses to implanted stents are the
driving force and primary pathophysiology mechanism of ISR
(6). Previous studies have
established that systemic inflammation, indicated by the count of
white blood cell (WBC) and its subsets, is closely associated with
the risk of ISR (7,8). In addition, WBC and its subsets are
positively related to adverse clinical endpoints in patients
undergoing elective PCI (9).
Recently, apolipoprotein A-I, a major protein component of
high-density lipoprotein (HDL), has been revealed to improve the
predictive value of WBC for coronary artery disease (CAD) (10). Given that HDL is directly involved
in immunoregulation by altering the membrane lipid contents of
immune cells, the combined effects of HDL with WBC and its subsets
have been explored to assess the inflammatory risk (11). It has been revealed that the
HDL-related inflammatory indices, including monocyte-to-HDL ratio
(MHR), neutrophil-to-HDL ratio (NHR), WBC-to-HDL ratio (WHR) and
C-reactive peptide-to-HDL ratio (CHR), can independently predict
the risk of adverse cardiac events both in the short term and
long-term (12,13). Moreover, the MHR has been
identified to be an independent predictor in the ISR of DES and
bare-mental stents (BMS) among patients with different CAD
manifestations (14). However,
data regarding the association of HDL-related inflammatory indices
with ISR among the CCS population undergoing elective PCI with DES
implantation is currently limited.
To address this, the present study aimed to
investigate the associations between HDL-related inflammatory
indices and ISR after elective PCI in patients with CCS, which
might provide significant prognostic information in this
population.
Materials and methods
Ethics statement
The present retrospective, single-center and
observational study conformed to the Declaration of Helsinki and
was authorized by the Ethics Committee of Fuwai Hospital, Chinese
Academy of Medical Sciences (Beijing, China; approval no.
2016-786). All participants provided written/oral informed
consent.
Study population
The present study followed the methods of Guo et
al (15). A total of 25,776
patients admitted with suspected CAD were retrospectively screened
in Fuwai Hospital, Chinese Academy of Medical Sciences, from
January 2017 to December 2017. The inclusion criteria were: i) Age
>18 years; ii) significant coronary stenosis (≥50%) in baseline
coronary angiography (CAG); iii) successful DES implantation at
baseline; iv) no history of coronary artery bypass grafting (CABG);
v) receiving follow-up coronary evaluation, including CAG and
coronary CT angiography (CCTA). The exclusion criteria were: i)
Patients presenting with ACS; ii) patients with missing
measurements for HDL-related inflammatory indices; iii) considering
the high specificity but relatively lower sensitivity of CCTA,
patients with suspected ISR on CCTA but absence of CAG
confirmation; and iv) patients with concurrent inflammatory
diseases. Finally, 1,471 patients were included in the analysis.
All participants were divided into three groups according to the
tertiles of the HDL-related inflammatory indices (Fig. 1).
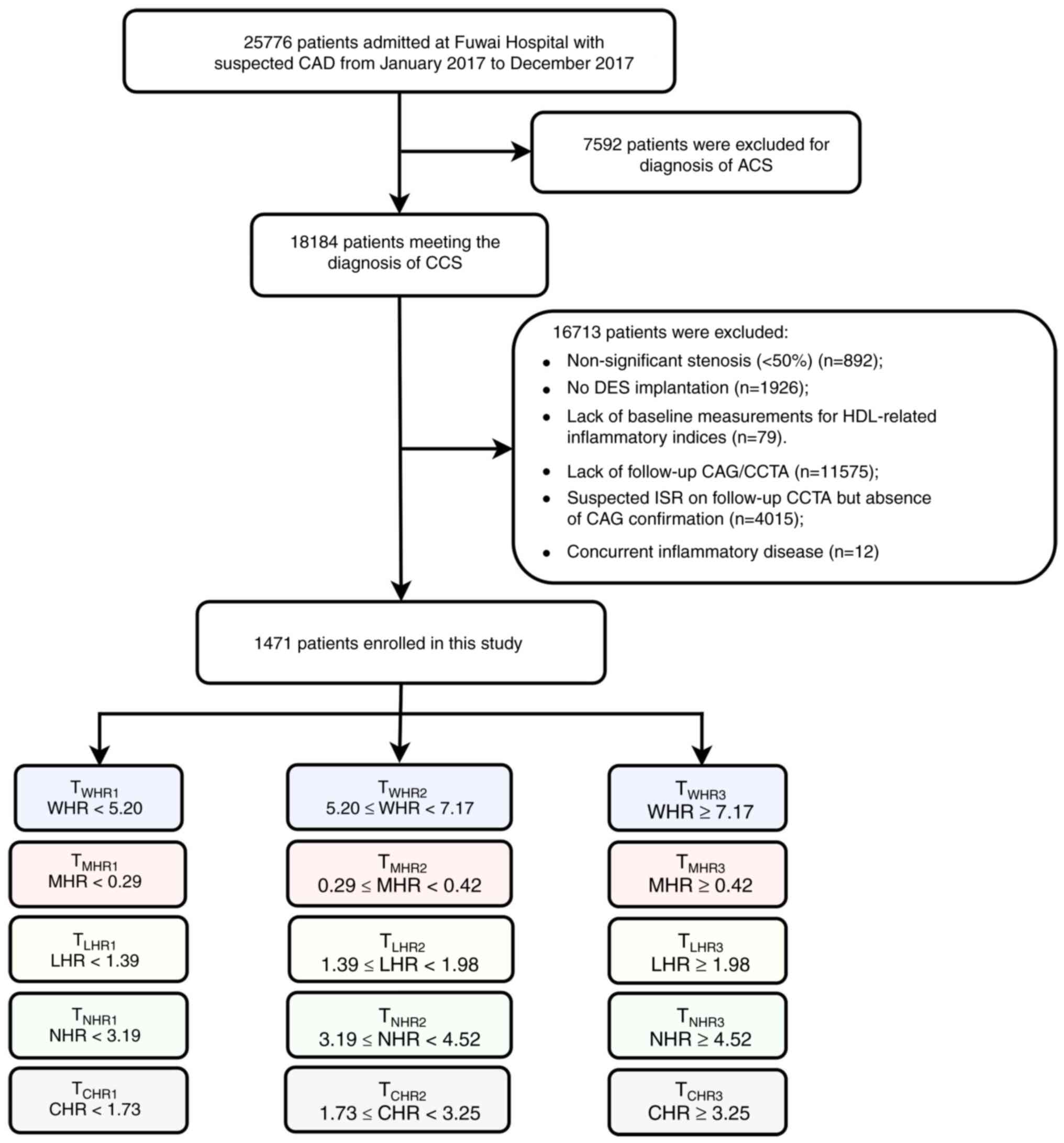 | Figure 1Flowchart of study participants. ACS,
acute coronary syndrome; CAD, coronary artery disease; CCS, chronic
coronary syndrome; CAG, coronary angiography; CCTA, coronary
computed tomography angiography; CHR, C-reactive
protein-to-high-density lipoprotein ratio; DES, drug-eluting stent;
ISR, in-stent restenosis; LHR, lymphocyte-to-high-density
lipoprotein ratio; MHR, monocyte-to-high-density lipoprotein ratio;
NHR, neutrophil-to-high-density lipoprotein ratio; WHR, white blood
cell-to-high-density lipoprotein ratio. |
Endpoints and follow-up
The follow-up period lasted until October 2022. The
primary endpoint was ISR and was assessed as enrolled time to the
first event or until October 2022. ISR was defined as ≥50%
re-narrowing over the entire length of the stent or involving its
5-mm edges. After the baseline successful PCI, all participants
underwent follow-up CAG or CCTA in Fuwai Hospital, Chinese Academy
of Medical Sciences. The outpatient and emergency records were
reviewed during the follow-up to exclude patients with symptoms of
ISR who declined coronary evaluation. To avoid counting endpoints
in patients with early stent thrombosis, ISR within 30 days was
excluded. Of note, CAG and CCTA were interpreted by experienced
radiologists and interventional cardiologists. All participants
received guideline-directed medical therapy.
Measurements and definitions
Data on sociodemographic characteristics, clinical
history and laboratory tests were collected from medical records or
interviews with the participants. The sociodemographic
characteristics included age, sex, height, weight and smoking
status. Clinical history of diabetes, hypertension, dyslipidemia,
leukemia, inflammatory disease, previous PCI and peripheral artery
disease (PAD) were recorded. The laboratory tests consisting of
WBC, neutrophil, lymphocyte, monocyte, total cholesterol,
triglyceride, low-density lipoprotein cholesterol, HDL-cholesterol
(HDL-C), high-sensitive CRP (hs-CRP), fasting blood glucose and
glycated hemoglobin (HbA1c), were performed under standardized
instructions and assaying system in the laboratory of Fuwai
Hospital, Chinese Academy of Medical Sciences. To ensure the
parameters of each participant were at the same temporal window,
all blood samples were obtained after overnight fasting before the
elective PCI.
The diagnosis of CCS was based on the current
guidelines of the European Society of Cardiology (16). Body mass index (BMI) was calculated
as weight/height squared (kg/m2). The estimated
glomerular filtration rate (eGFR) was evaluated according to the
modified Modification of Diet in Renal Disease equation: 186x
Plasma creatine -1.154x age -0.203x0.742 (if female) x1.233 (if
Chinese) (17). WHR was calculated
as WBC/HDL-C. MHR was calculated as monocyte/HDL-C.
Lymphocyte-to-HDL ratio (LHR) was calculated as lymphocyte/HDL-C.
NHR was calculated as neutrophil/HDL-C. CHR was calculated as
hs-CRP/HDL-C.
Statistical analysis
The random forest method was used to impute the
missing data (18). The normality
of the continuous variables was tested by the Kolmogorov-Smirnov
test, in which data with normal distribution were described as mean
± standard deviation, otherwise as median and interquartile range
(IQR). Categorical variables were presented as numbers and
percentages. The one-way analysis of variance (ANOVA) was performed
to assess the data with normal distribution, while the
Kruskal-Wallis ANOVA on ranks was used for categorical variables
and variables following skew distribution. Post-hoc analyses were
implemented when appropriate with the Tukey-Kramer post-hoc test
(homoscedasticity) or Dunnett's T3 post-hoc test
(heteroscedasticity).
The incidence of ISR among the groups was shown by
Kaplan-Meier (KM) method and compared by log-rank tests. To control
the false discovery rate at the level of 5%, the Benjamin-Hochberg
procedure was used to correct the P-values in the pairwise
comparisons. The multivariable Cox proportional hazards regression
analysis was further utilized to estimate the hazard ratio (HR) and
the 95% confidence interval (CI) of the HDL-related inflammatory
indices in developing ISR. According to the clinical significance
and findings from previous studies, the following covariates were
included in the multivariable Cox regression model: Age
(continuous), sex, BMI (continuous), prior PCI, presence of PAD,
presence of multivessel CAD, eGFR (continuous), hs-CRP
(continuous), presence of lesion's length ≥20 mm, stent length
(continuous), presence of restenotic lesions and stent number
(continuous). Proportionality of hazards was assessed for each
variable, and Schoenfeld residuals were visually inspected for
potential time-variant biases. The Schoenfeld residual test showed
that none were significant based on a P-value threshold 0.05.
Moreover, the trend analysis was conducted by entering the tertiles
of the HDL-related inflammatory indices as a continuous variable
and rerunning the corresponding regression models. The potential
non-linear relationship was further explored through the
multivariable Cox proportional hazards model with restricted cubic
splines (RCS) (19). To balance
the effects of best fitting and overfitting in the RCS, the Akaike
information criterion (AIC) was used, and the median of the
HDL-related inflammatory indices was assigned as the reference
value (20).
All statistical analyses were performed using R
software (version 4.2.2) (21).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics
The baseline characteristics of the study population
according to the development of ISR were displayed in Table I. The average age was 58.10±9.31
years (age range, 25-86 years), and 1,151 (78.25%) were men. It was
shown that in comparison with patients without ISR, patients
experiencing ISR were more likely to have a history of prior PCI,
concurrent PAD, multivessel CAD and use of β-blockers (all
P<0.05). For the angiographic details, the presence of
restenotic lesions, lesions ≥20 mm and TIMI grade 0/1 were more
frequent in patients with ISR (all P<0.05). Moreover, patients
with ISR had higher proportions of target vessel territory in the
right coronary artery but lower proportions the in left anterior
descending coronary artery (all P<0.05). Decreased HDL-C and
elevated levels of WBC, neutrophils, lymphocytes, triglycerides and
HbA1c were exhibited in patients who developed ISR (all P<0.05).
Of note, the values of WHR, LHR and NHR were significantly higher
in the ISR group, in which greater proportions of patients were
observed in T2 and T3 tertiles. The characteristics of participants
by the HDL-related inflammatory indices were presented in Table SI, Table SII, Table SIII, Table SIV and Table SV.
 | Table IBaseline characteristics according to
the primary endpoint. |
Table I
Baseline characteristics according to
the primary endpoint.
| Variable | Total (n=1471) | Non-ISR
(n=1220) | ISR (n=251) | P-value |
|---|
| Demographics | | | | |
|
Age, years
(mean ± SD) | 58.10±9.31 | 58.08±9.31 | 58.22±9.34 | 0.836 |
|
Male sex, n
(%) | 1151 (78.25) | 951 (77.95) | 200 (79.68) | 0.602 |
|
Median BMI,
kg/m2 (IQR) | 25.78 (23.89,
27.78) | 25.80 (23.89,
27.78) | 25.69 (23.90,
27.71) | 0.551 |
| Risk factors, n
(%) | | | | |
|
Cigarette
smoking | 898 (61.05) | 740 (60.66) | 158 (62.95) | 0.544 |
|
Diabetes | 613 (41.67) | 506 (41.48) | 107 (42.63) | 0.789 |
|
Hypertension | 955 (64.92) | 782 (64.10) | 173 (68.92) | 0.166 |
|
Dyslipidemia | 1458 (99.12) | 1211 (99.26) | 247 (98.41) | 0.343 |
|
Prior
PCI | 440 (29.91) | 342 (28.03) | 98 (39.04) | <0.001 |
|
PAD | 200 (13.60) | 154 (12.62) | 46 (18.33) | 0.022 |
| Clinical
presentations | | | | |
|
Multi-vessel
CAD, n (%) | 1189 (80.83) | 972 (79.67) | 217 (86.45) | 0.017 |
|
Median LVEF,
% (IQR) | 64 (60, 66) | 64 (60, 66) | 63 (60, 66) | 0.010 |
| Laboratory
measurements | | | | |
|
WBC,
109/l (mean ± SD) | 6.70±1.69 | 6.64±1.67 | 6.98±1.78 | 0.003 |
|
Neutrophil,
109/l (mean ± SD) | 4.28±1.38 | 4.24±1.34 | 4.46±1.51 | 0.025 |
|
Lymphocyte,
109/l (mean ± SD) | 1.86±0.60 | 1.84±0.60 | 1.93±0.64 | 0.033 |
|
Monocyte,
109/l (mean ± SD) | 0.39±0.13 | 0.39±0.13 | 0.40±0.15 | 0.093 |
|
TC, mmol/l
(mean ± SD) | 4.06±1.04 | 4.03±1.04 | 4.17±1.04 | 0.063 |
|
LDL-C,
mmol/l (mean ± SD) | 2.41±0.85 | 2.39±0.84 | 2.50±0.87 | 0.079 |
|
HDL-C,
mmol/l (mean ± SD) | 1.10±0.31 | 1.11±0.32 | 1.06±0.27 | 0.045 |
|
Triglycerides,
mmol/l (mean ± SD) | 1.76±1.22 | 1.73±1.23 | 1.91±1.20 | 0.041 |
|
HbA1c, %
(mean ± SD) | 6.38±1.20 | 6.31±1.14 | 6.70±1.43 | <0.001 |
|
Median eGFR,
ml/min per 1.73 m2 (IQR) | 110.99 (97.27,
125.57) | 110.46 (97.07,
124.54) | 114.18 (98.54,
129.67) | 0.088 |
|
hs-CRP, mg/l
(mean ± SD) | 4.03±5.87 | 4.03±5.81 | 4.03±6.18 | 0.994 |
| Medications at
discharge, n (%) | | | | |
|
DAPT | 1468 (99.80) | 1217 (99.75) | 251 (100.00) | 0.985 |
|
Statins | 1441 (97.96) | 1195 (97.95) | 246 (98.01) | 1.000 |
|
ACEI/ARBs | 736 (50.03) | 598 (49.02) | 138 (54.98) | 0.099 |
|
β-blockers | 1236 (84.02) | 1009 (82.70) | 227 (90.44) | 0.003 |
| Angiographic
findings | | | | |
|
Target
vessel territory | | | | |
|
LM,
n (%) | 44 (2.99) | 38 (3.11) | 6 (2.39) | 0.682 |
|
LAD,
n (%) | 824 (56.02) | 704 (57.70) | 120 (47.81) | 0.005 |
|
LCX,
n (%) | 368 (25.02) | 301 (24.67) | 67 (26.69) | 0.553 |
|
RCA,
n (%) | 564 (38.34) | 452 (37.05) | 112 (44.62) | 0.030 |
|
Restenotic
lesions, n (%) | 80 (5.44) | 38 (3.11) | 42 (16.73) | <0.001 |
|
Trifurcation/bifurcation
lesions, n (%) | 801 (54.45) | 667 (54.67) | 134 (53.39) | 0.762 |
|
Lesions ≥20
mm long, n (%) | 1038 (70.56) | 846 (69.34) | 192 (76.49) | 0.029 |
|
Median
number of stents (IQR) | 2 (1, 2) | 2 (1, 2) | 2 (1, 3) | 0.195 |
|
Median
length of stent, mm (IQR) | 30 (21, 48) | 30 (20, 45) | 33 (23, 54) | 0.014 |
|
TIMI grade
0/1, n (%) | 263 (17.88) | 206 (16.89) | 57 (22.71) | 0.036 |
| Median WHR
(IQR) | 6.16 (4.79,
7.93) | 6.08 (4.75,
7.86) | 6.54 (5.21,
8.25) | 0.003 |
| WHR tertiles, n
(%) | | | | 0.006 |
|
T1 | 484 (32.90) | 423 (34.67) | 61 (24.30) | |
|
T2 | 486 (33.04) | 395 (32.38) | 91 (36.25) | |
|
T3 | 501 (34.06) | 402 (32.95) | 99 (39.44) | |
| Median MHR
(IQR) | 0.35 (0.26,
0.46) | 0.35 (0.26,
0.45) | 0.36 (0.28,
0.51) | 0.035 |
| MHR tertiles, n
(%) | | | | 0.097 |
|
T1 | 458 (31.14) | 389 (31.89) | 69 (27.49) | |
|
T2 | 512 (34.81) | 430 (35.25) | 82 (32.67) | |
|
T3 | 501 (34.06) | 401 (32.87) | 100 (39.84) | |
| Median LHR
(IQR) | 1.67 (1.24,
2.21) | 1.65 (1.22,
2.18) | 1.74 (1.35,
2.31) | 0.008 |
| LHR tertiles, n
(%) | | | | 0.046 |
|
T1 | 485 (32.97) | 417 (34.18) | 68 (27.09) | |
|
T2 | 485 (32.97) | 402 (32.95) | 83 (33.07) | |
|
T3 | 501 (34.06) | 401 (32.87) | 100 (39.84) | |
| Median NHR
(IQR) | 3.87 (2.92,
5.10) | 3.82 (2.88,
5.05) | 4.08 (3.14,
5.32) | 0.014 |
| NHR tertiles, n
(%) | | | | 0.064 |
|
T1 | 482 (32.77) | 414 (33.93) | 68 (27.09) | |
|
T2 | 487 (33.11) | 403 (33.03) | 84 (33.47) | |
|
T3 | 502 (34.13) | 403 (33.03) | 99 (39.44) | |
| Median CHR
(IQR) | 2.37 (1.48,
4.17) | 2.36 (1.48,
4.11) | 2.44 (1.50,
4.34) | 0.742 |
| CHR tertiles, n
(%) | | | | 0.773 |
|
T1 | 481 (32.70) | 400 (32.79) | 81 (32.27) | |
|
T2 | 490 (33.31) | 410 (33.61) | 80 (31.87) | |
|
T3 | 500 (33.99) | 410 (33.61) | 90 (35.86) | |
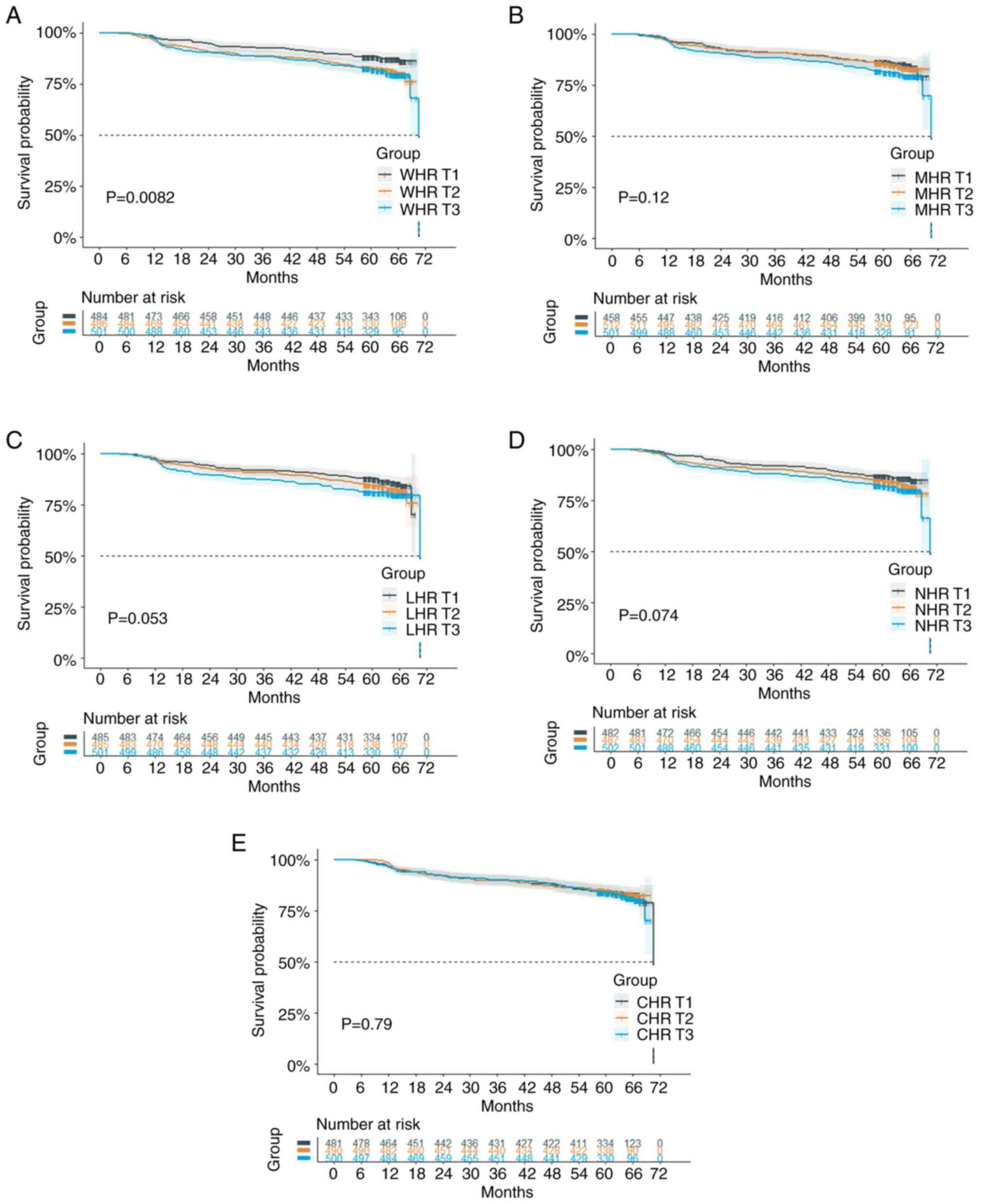
Association between WHR and ISR
A total of 251 (17.06%) patients experienced ISR
during the median follow-up time of 62.27 months (IQR, 58.78-65.67
months). In the KM survival analyses, the incidence of ISR was
significantly higher in the T2 and T3 groups of WHR (overall
P=0.0082, adjusted pairwise P between T1 and T2=0.0150; T1 and
T3=0.0098) (Fig. 2A).
The risk estimates for the associations between
HDL-related inflammatory indices and ISR in the multivariable Cox
regression analyses were presented in Table II. By classifying the patients
into WHR tertiles, the T2 (HR, 1.524; 95% CI 1.102-2.108; P=0.011)
and T3 (HR, 1.608; 95% CI 1.168-2.214; P=0.004) groups of WHR were
found at increased risk of ISR in the unadjusted model 1. After
adjusting for demographic characteristics (age, sex, BMI), clinical
presentations (prior PCI, concurrent PAD, multivessel CAD),
laboratory measures (hs-CRP, eGFR) and angiographic presentations
(lesion's length ≥20 mm, stent length) in model 2, the risk of the
ISR remained increased in T2 (HR, 1.514; 95% CI, 1.090-2.102;
P=0.013) and T3 (HR, 1.567; 95% CI, 1.127-2.179; P=0.008) in
contrast to the T1 group. Specifically, after additionally
adjusting the presence of restenotic lesions and stent number in
model 3, it was found that the risk for ISR increased by 54.7% in
T2 (HR, 1.547; 95% CI 1.114-2.148; P=0.009) and 60.3% in T3 (HR,
1.603; 95% CI 1.152-2.231; P=0.005) compared with T1 of WHR. The
trend analyses for the three models were all statistically
significant (all P for trend <0.05).
 | Table IIAssociations between the HDL-related
inflammatory indices and ISR. |
Table II
Associations between the HDL-related
inflammatory indices and ISR.
| | Model 1 | Model 2 | Model 3 |
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| WHR | 1.03 | 1.004-1.057 | 0.026 | 1.029 | 1.001-1.058 | 0.045 | 1.03 | 1.002-1.060 | 0.037 |
| WHR tertiles | | | | | | | | | |
|
T1 | Reference | | | Reference | | | Reference | | |
|
T2 | 1.524 | 1.102-2.108 | 0.011 | 1.514 | 1.090-2.102 | 0.013 | 1.547 | 1.114-2.148 | 0.009 |
|
T3 | 1.608 | 1.168-2.214 | 0.004 | 1.567 | 1.127-2.179 | 0.008 | 1.603 | 1.152-2.231 | 0.005 |
| P for trend | | | 0.004 | | | 0.01 | | | 0.006 |
| MHR | 1.301 | 0.862-1.964 | 0.21 | 1.248 | 0.796-1.956 | 0.335 | 1.24 | 0.777-1.979 | 0.366 |
| MHR tertiles | | | | | | | | | |
|
T1 | Reference | | | Reference | | | Reference | | |
|
T2 | 1.055 | 0.766-1.454 | 0.742 | 1.051 | 0.755-1.461 | 0.769 | 0.992 | 0.711-1.383 | 0.96 |
|
T3 | 1.341 | 0.986-1.823 | 0.061 | 1.329 | 0.962-1.835 | 0.084 | 1.295 | 0.937-1.791 | 0.118 |
| P for trend | | | 0.054 | | | 0.071 | | | 0.091 |
| LHR | 1.051 | 0.982-1.126 | 0.153 | 1.052 | 0.980-1.129 | 0.164 | 1.051 | 0.977-1.132 | 0.181 |
| LHR tertiles | | | | | | | | | |
|
T1 | Reference | | | Reference | | | Reference | | |
|
T2 | 1.231 | 0.893-1.696 | 0.204 | 1.202 | 0.870-1.661 | 0.265 | 1.188 | 0.861-1.641 | 0.295 |
|
T3 | 1.462 | 1.074-1.991 | 0.016 | 1.455 | 1.062-1.995 | 0.02 | 1.412 | 1.031-1.933 | 0.032 |
| P for trend | | | 0.016 | | | 0.019 | | | 0.031 |
| NHR | 1.061 | 1.009-1.115 | 0.022 | 1.056 | 1.001-1.114 | 0.045 | 1.061 | 1.006-1.120 | 0.029 |
| NHR tertiles | | | | | | | | | |
|
T1 | Reference | | | Reference | | | Reference | | |
|
T2 | 1.241 | 0.902-1.709 | 0.185 | 1.241 | 0.898-1.713 | 0.19 | 1.192 | 0.862-1.649 | 0.288 |
|
T3 | 1.431 | 1.050-1.950 | 0.023 | 1.394 | 1.016-1.914 | 0.04 | 1.447 | 1.053-1.988 | 0.023 |
| P for trend | | | 0.023 | | | 0.041 | | | 0.022 |
| CHR | 1.003 | 0.984-1.022 | 0.753 | 1.018 | 0.953-1.086 | 0.6 | 1.009 | 0.955-1.066 | 0.75 |
| CHR tertiles | | | | | | | | | |
|
T1 | Reference | | | Reference | | | Reference | | |
|
T2 | 0.993 | 0.728-1.354 | 0.965 | 0.969 | 0.710-1.324 | 0.845 | 0.97 | 0.710-1.325 | 0.848 |
|
T3 | 1.09 | 0.805-1.473 | 0.575 | 1.078 | 0.763-1.522 | 0.669 | 1.186 | 0.844-1.666 | 0.325 |
| P for trend | | | 0.57 | | | 0.696 | | | 0.357 |
Similarly, the WHR as a continuous variable was
shown to be significantly associated with the ISR (HR, 1.030; 95%
CI, 1.004-1.057; P=0.026) in the unadjusted model 1 and could serve
as an independent predictor of ISR after adjusting the potential
confounders in model 2 (HR, 1.029; 95% CI, 1.001-1.058; P=0.045)
and model 3 (HR, 1.030; 95% CI, 1.002-1.060; P=0.037).
To further identify the associations between the
HDL-related inflammatory indices with the risk of ISR, the RCS
based on the multivariable-adjusted Cox regression model 3 with
three knots at the 10, 50 and 90th centiles according to AIC was
performed. It was identified that the WHR was associated with the
risk of ISR in a non-linear and dose-dependent manner (non-linear
P=0.034; P overall=0.019). As illustrated in Fig. 3A, the risk of developing ISR
increased rapidly before WHR of 6.16 and turned to a flat trend
afterward. Taking the WHR of 6.16 as the cut-off point, the HR per
unit increase in WHR was 0.857 (95% CI, 0.744-0.988; P=0.034) below
the point and 1.206 (95% CI, 1.045-1.392; P=0.010) above the
point.
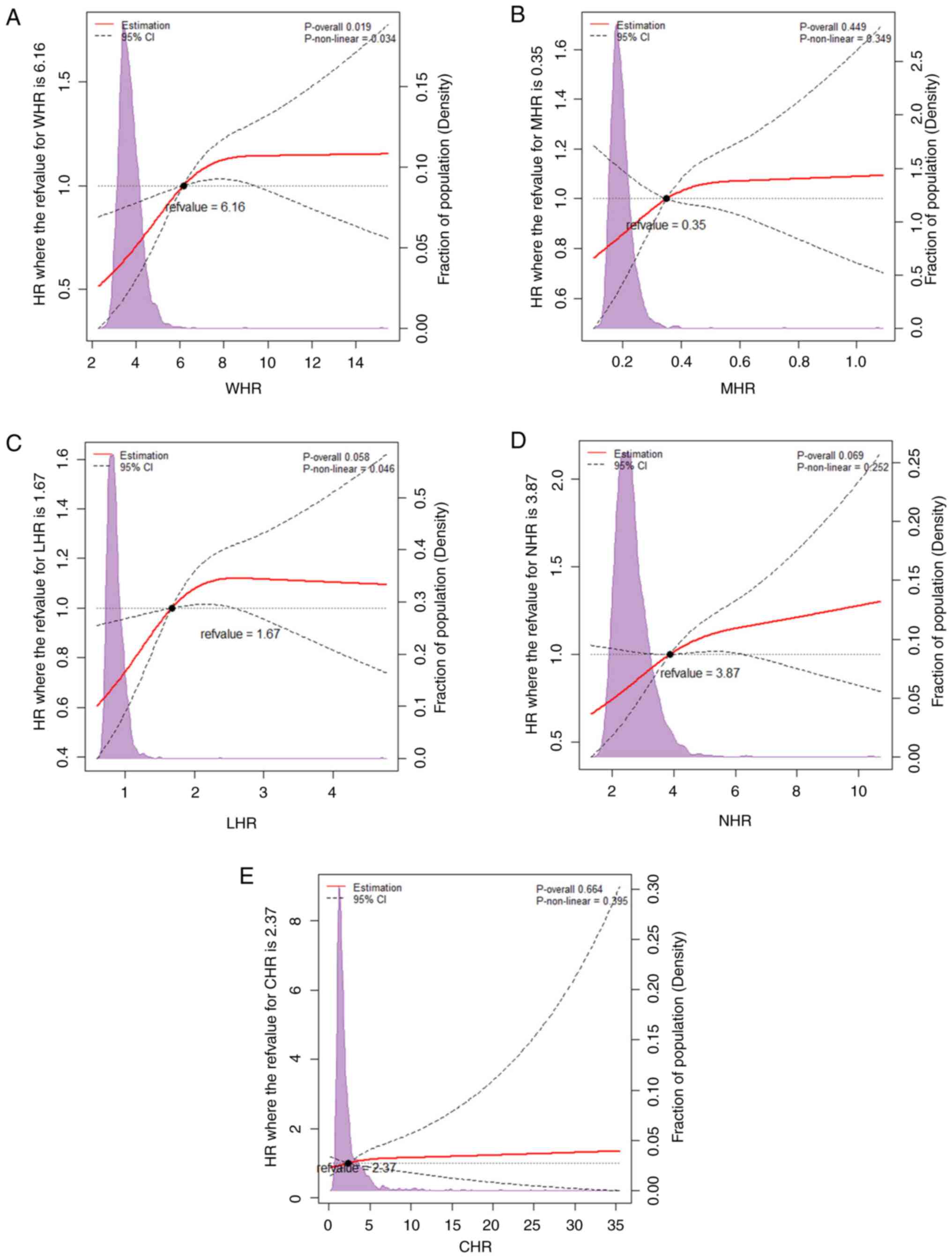 | Figure 3Restricted cubic splines for the
adjusted dose-response associations between the HDL-related
inflammatory indices and ISR. All data were fitted with a linear
regression model using restricted cubic spines with three knots at
the 5, 50 and 95th percentiles. Y-axis represents the odds ratio,
and the dashed lines are 95% confidence intervals. (A) Association
between WHR and ISR; (B) association between MHR and ISR; (C)
association between LHR and ISR; (D) association between NHR and
ISR; (E) association between CHR and ISR. CI, confidence interval;
CHR, C-reactive peptide-to-HDL ratio; HDL, high-density lipoprotein
cholesterol; HR, hazards ratio; LHR, lymphocyte-to-HDL ratio; MHR,
monocyte-to-HDL ratio; NHR, neutrophil-to-HDL ratio; ISR, in-stent
restenosis; WHR, white blood cell-to-HDL ratio. |
Association between LHR and ISR
After classifying the participants according to the
tertiles of LHR, no significant differences were demonstrated
across the tertiles of LHR in the KM analyses (overall P≥0.05)
(Fig. 2C).
Notably, in the multivariable Cox regression
analyses, participants in the T3 group of LHR were identified to
have an increased risk of 46.2% in developing ISR in model 1 (HR,
1.462; 95% CI 1.074-1.991; P=0.016) in contrast to participants of
T1. After adjusting for potential confounders in model 2 and model
3, the risk of ISR remained at 1.455-fold (HR, 1.455; 95% CI
1.062-1.995; P=0.020) and 1.412-fold (HR, 1.412; 95% CI
1.031-1.933; P=0.032) in T3, respectively. The trend analyses were
all statistically significant (all P<0.05).
The potential non-linear relationship between LHR
and ISR was also investigated in the RCS. However, the present
study failed to demonstrate a significant association between them
(P overall ≥0.05) (Fig. 3C).
Association between NHR and ISR
The KM analysis showed non-significant differences
across the tertiles of NHR for the ISR incidence (overall P≥0.05)
(Fig. 2D).
In the multivariable Cox regression analyses, as a
continuous scale, NHR was associated with ISR with an adjusted HR
of 1.056 (HR, 1.056; 95% CI 1.001-1.114; P=0.045) in model 2 and
1.061 (HR, 1.061; 95% CI 1.006-1.120; P=0.029) in model 3. For the
risk of ISR across the NHR tertiles, the T3 was at elevated risk in
all three models (model 1: HR, 1.431; 95% CI 1.050-1.950; P=0.023;
model 2: HR, 1.394; 95% CI 1.016-1.914; P=0.040; model 3: HR,
1.447; 95% CI 1.053-1.988; P=0.023). The trend analyses for the
three models were all statistically significant in NHR (all
P<0.05).
Additionally, the possible non-linear relationships
of NHR with ISR failed to demonstrate significance (P overall
≥0.05) (Fig. 3D).
Discussion
To the best of our knowledge, the present study is
the first to investigate the associations between HDL-related
inflammatory indices and the risk of ISR in patients with elective
PCI. The main findings of this study are as follows: i) The WHR and
NHR, as a continuous or categorical variable, were significantly
associated with ISR; ii) patients with higher values of WHR, NHR
and LHR were more likely to develop ISR after the baseline elective
PCI; and iii) the HDL-related inflammatory indices could act as
independent predictors in the prognosis of elective PCI which might
provide clinical significance in the risk stratification of ISR at
an early stage.
Vascular inflammation of stented lesions is the
primary contributor to ISR (6). In
the early stage following PCI (6-12 months), balloon expansion and
stent implantation cause endothelial injury and activation of
inflammatory cells, resulting in stimulation and proliferation of
vascular smooth muscle cells, eventually neointima hyperplasia
(22). In the late stage (>12
months), chronic inflammation within the neointima could cause
neoatherogenesis and consequent ISR (22). The inflammatory infiltration of ISR
has been confirmed in in vivo coronary imaging and
human-derived restenotic samples (23). By altering the content and
structure of membrane lipids and functional proteins in immune
cells through reverse cholesterol transport (RCT), HDL is recently
recognized to modulate the inflammatory response. Additionally,
components of HDL have been identified to have direct immunological
roles independent of RCT (24).
The anti-inflammatory property of HDL has been verified in clinical
studies in which decreased levels of HDL are inversely correlated
with amplified systemic inflammation and autoimmune disorders
(25,26). Therefore, combining HDL with
inflammatory biomarkers is of great significance in assessing the
residual inflammatory risk under the guideline of PCI management.
To date, the HDL-related inflammatory indices have been found to
contribute to the increased risk of ISR in diverse CAD cohorts.
Specifically, the MHR is positively associated with ISR in patients
with ST-elevation MI undergoing BMS stenting and in patients with
CAD after successful BMS implantation (27,28).
Additionally, the MHR is significantly correlated with the risk of
ISR in non-ST-elevation MI patients with DES (29). Among participants presented with
angina pectoris receiving BMS, the MHR has been found to positively
predict the risk of ISR as well (30).
In line with the previous findings, the present
study further revealed that WHR, LHR and NHR were independently
associated with the risk of ISR among patients receiving elective
PCI. After adjusting for potential confounders involving clinical
presentation, laboratory measures and angiographic manifestation,
WHR values above the second and third tertiles were related to an
increased ISR risk of 60.3 and 54.7%, respectively. Besides,
participants in the third tertiles of LHR and NHR were also at
greater risk of having ISR. Notably, the WHR was associated with
ISR in a non-linear way in which the value of 6.16 might serve as a
cut-off point for the increasing trend in ISR risk. In this
context, the present study extended the association between
HDL-related inflammatory indices and ISR, indicating the potential
for improved risk stratification among CCS patients undergoing
elective PCI. However, different from previous studies, the present
study failed to demonstrate significant associations between MHR
and ISR. Given that the present study focused on patients
clinically presented with CCS and DES implantation, we hypothesized
that the differences in study population, implanted stents and
duration of follow-up might account for the inconsistent findings.
Moreover, experimental data has suggested different modes of
monocyte trafficking between acute and chronic inflammation and the
inflammatory response of monocytes varies in relation to type of
stents (31,32). Therefore, the present study might
provide preliminary evidence for the associations between
HDL-related inflammatory indices and the risk of ISR in patients
with CCS undergoing DES stenting.
Notably, although HDL is generally considered an
atherosclerosis protective factor for its cholesterol efflux
capacity (CEC), results from extensive epidemiological studies do
not support the cardiovascular benefits of HDL in patients with CAD
(33,34). It was found that inflammatory
cytokines in particular circumstances, such as ACS and DM, can
affect the role of RCT in HDL by impairing lipid constituents and
structure (35,36). Furthermore, HDL has been revealed
to promote the inflammatory process in atherosclerosis with a gain
of dysfunction (37). Therefore,
increasing attention has been focused on functional measurements of
HDL instead of concentrations (38). Evaluated by radioisotopic or
fluorimetric bioassays, the CEC is currently used to estimate the
RCT efficiency of HDL (39).
Accumulating data from clinical studies has demonstrated that
higher HDL CEC is inversely associated with the risk of cardiac
outcomes (40,41). Considering this, the prognostic
significance of WHR might be further improved with HDL CEC.
There are several limitations to the present study.
First, this was a retrospective and single-center study which might
affect the generalizability of the findings. Second, there might be
information bias as the patients without follow-up coronary imaging
were excluded. Third, potential confounding factors affecting the
inflammatory condition and activity of HDL, such as food intake and
training habits, were not recorded and included in the analysis.
Lastly, the laboratory parameters were measured only once at the
baseline, leaving a potential bias due to measurement mistakes.
In conclusion, the present study first demonstrated
that higher HDL-related inflammatory indices, including WHR, LHR
and NHR, were significantly and independently associated with an
increased risk of ISR among patients receiving elective PCI. In
addition, a non-linear relationship with a cut-off point being 6.16
was identified between the WHR and the risk of developing ISR. The
current study indicated that the interplay between lipid metabolism
disorder and inflammation contributed to the development of ISR.
Furthermore, the assessment of HDL-related lipoprotein indices
might potentially aid in identifying patients with high risk for
ISR. To validate the present findings, prospective, multi-center
studies are required.
Supplementary Material
Baseline characteristics according to
tertiles of WHR.
Baseline characteristics according to
tertiles of MHR.
Baseline characteristics according to
tertiles of LHR.
Baseline characteristics according to
tertiles of NHR.
Baseline characteristics according to
tertiles of CHR.
Acknowledgements
The authors would like to thank Dr Siyu Yan (Fuwai
Hospital, Chinese Academy of Medical Sciences and Peking Union
Medical College, Beijing, China) for help with collection of data
and revision of the manuscript.
Funding
Funding: This work was supported by the National Key R&D
Program of China (grant no. 2021ZD0111003), Capital's Funds for
Health Improvement and Research from the Beijing Municipal Health
Commission (grant no. SF 2022-2-4035) and the Capital Health
Development Project of China grant (grant no. SHF-2016-2-4032).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM and XG conceptualised the study. XG and PL
designed the methodology. XG and RS performed the statistical
analyses. RS collected the data, interpreted the data and wrote the
original draft. PL reviewed the manuscript. LM acquired funding. XG
and RS confirm the authenticity of all the raw data. All authors
contributed important intellectual content to this study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was performed in line with the
Declaration of Helsinki and was authorized by the Ethics Committee
of Fuwai Hospital, Chinese Academy of Medical Sciences (approval
no. 2016-786). All participants provided written/oral informed
consent for participating.
Patient consent for publication
All participants provided written/oral informed
consent for publication, which the Ethics Committee of Fuwai
Hospital, Chinese Academy of Medical Sciences has approved.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lawton JS, Tamis-Holland JE, Bangalore S,
Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM,
Don CW, et al: 2021 ACC/AHA/SCAI guideline for coronary artery
revascularization: A report of the American college of
cardiology/American heart association joint committee on clinical
practice guidelines. Circulation. 145:e18–e114. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maron DJ, Hochman JS, Reynolds HR,
Bangalore S, O'Brien SM, Boden WE, Chaitman BR, Senior R,
López-Sendón J, Alexander KP, et al: Initial invasive or
conservative strategy for stable coronary disease. N Engl J Med.
382:1395–1407. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alfonso F, Coughlan JJ, Giacoppo D,
Kastrati A and Byrne RA: Management of in-stent restenosis.
EuroIntervention. 18:e103–e123. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shlofmitz E, Iantorno M and Waksman R:
Restenosis of drug-eluting stents: A new classification system
based on disease mechanism to guide treatment and state-of-the-art
review. Circ Cardiovasc Interv. 12(e007023)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
The Writing Committee of the Report on
Cardiovascular Health and Diseases in China. Report on
cardiovascular health and diseases in China 2021: An updated
summary. Chin Circ J. 37:553–578. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pelliccia F, Zimarino M, Niccoli G,
Morrone D, De Luca G, Miraldi F and De Caterina R: In-stent
restenosis after percutaneous coronary intervention: Emerging
knowledge on biological pathways. Eur Heart J Open.
3(oead083)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Clare J, Ganly J, Bursill CA, Sumer H,
Kingshott P and de Haan JB: The mechanisms of restenosis and
relevance to next generation stent design. Biomolecules.
12(430)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Niccoli G, Montone RA, Ferrante G and Crea
F: The evolving role of inflammatory biomarkers in risk assessment
after stent implantation. J Am Coll Cardiol. 56:1783–1793.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gabbasov Z, Kozlov S, Melnikov I, Byazrova
S, Saburova O, Prokofieva L, Caprnda M, Curilla E, Gaspar L,
Rodrigo L, et al: Novel biomarkers for coronary restenosis
occurrence after drug-eluting stent implantation in patients with
diabetes having stable coronary artery disease. Clin Appl Thromb
Hemost. 24:1308–1314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pan Y, Zhang J, Wu TT, Hou XG, Yang Y, Ma
X, Ma YT, Zheng YY and Xie X: Baseline white blood cell
count-to-apolipoprotein A1 ratio as a novel predictor of long-term
adverse outcomes in patients who underwent percutaneous coronary
intervention: A retrospective cohort study. Lipids Health Dis.
19(43)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Catapano AL, Pirillo A, Bonacina F and
Norata GD: HDL in innate and adaptive immunity. Cardiovasc Res.
103:372–383. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Çiçek G, Kundi H, Bozbay M, Yayla C and
Uyarel H: The relationship between admission monocyte HDL-C ratio
with short-term and long-term mortality among STEMI patients
treated with successful primary PCI. Coron Artery Dis. 27:176–184.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang JB, Chen YS, Ji HY, Xie WM, Jiang J,
Ran LS, Zhang CT and Quan XQ: Neutrophil to high-density
lipoprotein ratio has a superior prognostic value in elderly
patients with acute myocardial infarction: A comparison study.
Lipids Health Dis. 19(59)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu TT, Zheng YY, Xiu WJ, Wang WR, Xun YL,
Ma YY, Kadir P, Pan Y, Ma YT and Xie X: White blood cell counts to
high-density lipoprotein cholesterol ratio, as a novel predictor of
long-term adverse outcomes in patients after percutaneous coronary
intervention: A retrospective cohort study. Front Cardiovasc Med.
8(616896)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guo X, Shen R, Yan S, Su Y and Ma L:
Triglyceride-glucose index for predicting repeat revascularization
and in-stent restenosis in patients with chronic coronary syndrome
undergoing percutaneous coronary intervention. Cardiovasc Diabetol.
22(43)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Knuuti J, Wijns W, Saraste A, Capodanno D,
Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C,
Cuisset T, et al: 2019 ESC guidelines for the diagnosis and
management of chronic coronary syndromes. Eur Heart J. 41:407–477.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y,
Xu JS, Huang SM, Wang LN, Huang W, et al: Modified glomerular
filtration rate estimating equation for Chinese patients with
chronic kidney disease. J Am Soc Nephrol. 17:2937–2944.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shah AD, Bartlett JW, Carpenter J,
Nicholas O and Hemingway H: Comparison of random forest and
parametric imputation models for imputing missing data using MICE:
A CALIBER study. Am J Epidemiol. 179:764–774. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Govindarajulu US, Spiegelman D, Thurston
SW, Ganguli B and Eisen EA: Comparing smoothing techniques in Cox
models for exposure-response relationships. Stat Med. 26:3735–3752.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Johannesen CDL, Langsted A, Mortensen MB
and Nordestgaard BG: Association between low density lipoprotein
and all cause and cause specific mortality in Denmark: Prospective
cohort study. BMJ. 371(m4266)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing,
Vienna, Austria, 2022. URL: https://www.R-project.org/.
|
|
22
|
Borovac JA, D'Amario D, Vergallo R, Porto
I, Bisignani A, Galli M, Annibali G, Montone RA, Leone AM, Niccoli
G and Crea F: Neoatherosclerosis after drug-eluting stent
implantation: A novel clinical and therapeutic challenge. Eur Heart
J Cardiovasc Pharmacother. 5:105–116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pinheiro LFM, Garzon S, Mariani J Jr,
Prado GFA, Caixeta AM, Almeida BO and Lemos PA: Inflammatory
phenotype by OCT coronary imaging: Specific features among de novo
lesions, in-stent neointima, and in-stent neo-atherosclerosis. Arq
Bras Cardiol. 119:931–937. 2022.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
24
|
Pérez-Morga D, Vanhollebeke B,
Paturiaux-Hanocq F, Nolan DP, Lins L, Homblé F, Vanhamme L, Tebabi
P, Pays A, Poelvoorde P, et al: Apolipoprotein L-I promotes
trypanosome lysis by forming pores in lysosomal membranes. Science.
309:469–472. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Madsen CM, Varbo A, Tybjærg-Hansen A,
Frikke-Schmidt R and Nordestgaard BG: U-shaped relationship of HDL
and risk of infectious disease: Two prospective population-based
cohort studies. Eur Heart J. 39:1181–1190. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Madsen CM, Varbo A and Nordestgaard BG:
Low HDL cholesterol and high risk of autoimmune disease: Two
population-based cohort studies including 117341 individuals. Clin
Chem. 65:644–652. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Avci II, Sahin I, Gungor B, Karatas MB,
Ozcan KS, Canga Y, Keskin M, Hayiroglu MI, Karadeniz FO and Sungur
A: Association of monocyte to high-density lipoprotein ratio with
bare-metal stent restenosis in STEMI patients treated with primary
PCI. North Clin Istanb. 6:393–400. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yilmaz S, Akboga MK, Sen F, Balcı KG, Aras
D, Temizhan A and Aydogdu S: Usefulness of the
monocyte-to-high-density lipoprotein cholesterol ratio to predict
bare metal stent restenosis. Biomark Med. 10:959–966.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nan J, Meng S, Hu H, Jia R, Chen C, Peng J
and Jin Z: The predictive value of monocyte count to high-density
lipoprotein cholesterol ratio in restenosis after drug-eluting
stent implantation. Int J Gen Med. 13:1255–1263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tok D, Turak O, Yayla Ç, Ozcan F, Tok D
and Çağlı K: Monocyte to HDL ratio in prediction of BMS restenosis
in subjects with stable and unstable angina pectoris. Biomark Med.
10:853–860. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ingersoll MA, Platt AM, Potteaux S and
Randolph GJ: Monocyte trafficking in acute and chronic
inflammation. Trends Immunol. 32:470–477. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kochiadakis GE, Marketou ME, Panutsopulos
D, Arfanakis DA, Skalidis EI, Igoumenidis NE, Hamilos MI, Sourvinos
G, Chlouverakis G, Spandidos D and Vardas PE: Vascular endothelial
growth factor protein levels and gene expression in peripheral
monocytes after stenting: A randomized comparative study of
sirolimus: Eluting and bare metal stents. Eur Heart J. 29:733–740.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ko DT, Alter DA, Guo H, Koh M, Lau G,
Austin PC, Booth GL, Hogg W, Jackevicius CA, Lee DS, et al:
High-density lipoprotein cholesterol and cause-specific mortality
in individuals without previous cardiovascular conditions: The
CANHEART study. J Am Coll Cardiol. 68:2073–2083. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wilkins JT, Ning H, Stone NJ, Criqui MH,
Zhao L, Greenland P and Lloyd-Jones DM: Coronary heart disease
risks associated with high levels of HDL cholesterol. J Am Heart
Assoc. 3(e000519)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Frej C, Mendez AJ, Ruiz M, Castillo M,
Hughes TA, Dahlbäck B and Goldberg RB: A shift in ApoM/S1P between
HDL-particles in women with type 1 diabetes mellitus is associated
with impaired anti-inflammatory effects of the ApoM/S1P complex.
Arterioscler Thromb Vasc Biol. 37:1194–1205. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Djekic S, Vekic J, Zeljkovic A,
Kotur-Stevuljevic J, Kafedzic S, Zdravkovic M, Ilic I, Hinic S,
Cerovic M, Stefanovic M, et al: HDL subclasses and the distribution
of paraoxonase-1 activity in patients with ST-segment elevation
acute myocardial infarction. Int J Mol Sci. 24(9384)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rosenson RS, Brewer HB Jr, Ansell BJ,
Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR and Webb NR:
Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat
Rev Cardiol. 13:48–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Barter PJ and Rye KA: HDL cholesterol
concentration or HDL function: Which matters? Eur Heart J.
38:2487–2489. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ouimet M, Barrett TJ and Fisher EA: HDL
and reverse cholesterol transport. Circ Res. 124:1505–1518.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Adorni MP, Ronda N, Bernini F and Zimetti
F: High density lipoprotein cholesterol efflux capacity and
atherosclerosis in cardiovascular disease: Pathophysiological
aspects and pharmacological perspectives. Cells.
10(574)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tall AR and Rader DJ: Trials and
tribulations of CETP inhibitors. Circ Res. 122:106–112.
2018.PubMed/NCBI View Article : Google Scholar
|