Introduction
Musculoskeletal disorders are on the rise worldwide due to the aging and growing population (1). In particular, osteoarthritis (OA) is a major cause of health problems globally, and worldwide estimates suggest that 250 million individuals are affected, and is the main reason for functional decline and pain of the knee (2). It is particularly prevalent in developed countries where a greater number of individuals are reaching an advanced age, and it was estimated that the number of patients with OA in the US would exceed 60 million in 2020(3). OA is caused by the gradual wear and tear of cartilage over numerous decades, and the accompanying pain reduces quality of life (4). Severe cases of OA can be treated with knee osteotomy or knee replacement, and sodium hyaluronate (SH), paracetamol, nonsteroidal anti-inflammatory drugs or corticosteroids can be administered by intraarticular injection as a conservative treatment. However, surgery or regular hospital visits can be a burden on patients (5,6). Control of body weight with appropriate exercise, a balanced diet and care for the knees using dietary supplementation (such as glucosamine or chondroitin) are recommended to prevent severe knee discomfort (7-9).
In Japan, ~10 million individuals perform annual runs and ~3 million run at least twice a week. Furthermore, 250,000 have been reported to run full-length marathons at least once a year (10). However, numerous runners who are middle-aged and elderly experience issues centered around knee joints, such as gonarthrosis, due to physical decline from aging and overwork (11). To prevent the progression of running disorders, such as gonarthrosis, it is desirable to treat them as easily as possible at a relatively mild stage, such as when the affected individuals feel discomfort in the knees, before they become serious due to excessive training (12). Therefore, in the present study, the functionality of oral dietary supplements to alleviate knee pain and discomfort in healthy recreational runners who have yet to experience any running disorders was assessed.
Sodium hyaluronate (SH) is a high molecular-weight polysaccharide (~100-10,000 kDa) composed of repeating polymeric disaccharides of D-glucuronic acid and N-acetyl-D-glucosamine (13). SH is present in every connective tissue and organ with synovial fluid having the highest concentration of SH in the body at 3-4 mg/ml (14). There have been multiple studies on the effect of oral SH on gonarthrosis; however, most of them include severe cases such as patients with osteoarthritis of the knee, rated as grade ≥2 based on the Kellgren-Lawrence (KL) classification, and there are no reports of studies conducted only on healthy individuals (15-21). Therefore, a 12-week, randomized, double-blinded, placebo-controlled parallel study was performed, which recruited healthy adult men and women, rated as grade ≤1 based on the KL classification, meaning the individuals knees were healthy, with primary endpoints scored using visual analog scales (VAS) to evaluate the influence of oral SH on discomfort and pain in the knees (22).
Materials and methods
Study samples
In the present study, SH [Hyabest® (S) LF-P; Kewpie Corp.] was used with dextrin as a placebo (Matsutani Chemical Industry Co., Ltd.), as dextrin is a safe food ingredient that has no effect on the knee (15,23). Both SH and the placebo (dextrin) were developed into capsule supplements by Aliment Industry Co., Ltd. and had the same taste and appearance. SH capsules had an SH content of 111 mg/capsule based on a qualitative analysis using high-performance liquid chromatography performed by the Japan Food Research Laboratories. The daily intake was one capsule.
Study design and ethics
The present study was performed at Hokkaido University of Education (Hokkaido, Japan) between December 2021 and March 2022 under the administration of an internal medicine specialist. A total of 56 subjects, recruited specifically for the present study after obtaining written informed consent, were divided into two groups using block randomization to ensure there was no bias in sex or age (stratified randomization by sex and age). Grouping information was kept sealed and confidential from the time-point of grouping until the study was completed (24). The subjects, investigators, supplements provider and the outcome assessors were all blinded. The placebo group and the SH group both took one capsule per day over a period of 12 weeks. A VAS questionnaire regarding their knees was administered before the start of the study, at 6 weeks and at 12 weeks after starting treatment with the capsules. The duration of the study was chosen according to previous studies (25). A locomotive syndrome risk test (26-29), one-leg standing time with eyes open test (30), a blood test and physician interview were performed before the start of the study and at 12 weeks after starting treatment with the capsules. X-ray examination of the subjects' knees was performed during the study period in small groups and in no particular order at Hokkaido University Hospital (Hokkaido, Japan) under the administration of an orthopedic specialist. The present study complies with the Helsinki Declaration and the Ethical guidelines for Medical and Health Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labor and Welfare; and Ministry of Economy, Trade and Industry, Japan) (31) and was approved by Hokkaido University of Education Ethics Review Committee (approval no. 2021035001; March 26, 2021). The study protocol was registered with the University Hospital Medical Information Network Clinical Trial Registry in advance (registration no. UMIN000045980, November 4, 2021).
Subjects
The subjects were 56 healthy adults (39 men and 17 women; mean age, 60.1±1.2 years). The inclusion criteria were as follows: i) Adult men and women aged 20-65 years; ii) determined to be healthy by the principal investigator of the present study, an internal medicine specialist, through blood tests assessing the levels of interleukin-10 (IL-10), aspartate aminotransferase (AST), alanine transaminase (ALT), γ-glutamyl transferase (γGTP), lactate dehydrogenase (LDH), creatine kinase (CK) and C-reactive protein (CRP), and medical interviews regarding the medical history of the individual; iii) experienced knee discomfort in the past year. The exclusion criteria were as follows: i) Taking medication or regularly going to the hospital; ii) a history of serious illness; iii) participation in another clinical trial within the past month; iv) habitual excessive eating or drinking, or irregular diet using a self-reporting method; v) deemed unsuitable by the screening physician (such as due to knee osteoarthritis or knee injury); vi) drug and food allergies; and vii) assessed as grade ≥2 based on KL classification, which indicates the individual has knee osteoarthritis. Participants were required to record their daily routines before (for 1 week within 1 month prior to intake) and during the study period; these were verified using diaries, which were used to confirm that there were no changes in lifestyle (such as diet and daily routines) before and during the study. The trial was explained in detail to the subjects and those who gave their written informed consent were enrolled in the study. The sample size was determined based on a similar previous study (32). As α=0.05 and power (1-β)=80%, the required sample size was ≥30. The Cancer Research and Biostatistics Statistical Tools (https://stattools.crab.org/) was used to calculate the sample size.
VAS
A total of 21 items were selected from the Japanese Knee OA Measure (JKOM) and the Western Ontario and McMaster Universities OA Index that were suitable for the evaluation of healthy subjects (33,34). Knee pain, stiffness and discomfort were evaluated using VAS in the following situations: Upon awaking; climbing stairs; descending stairs; going to bed; bending and stretching the knee joint; walking for a longer distance or duration than normal; jogging for a longer distance or duration than normal. For VAS, 100-mm lines were used, with the left end of each line indicating the best condition without any symptoms and the right end indicating the worst condition ever experienced by the patient. Subjects evaluated their condition before they started taking the capsules, and at 6 and 12 weeks after they started taking them.
Locomotive syndrome risk test
The locomotive syndrome risk test consisted of two physical examinations, the two-step test and the stand-up test, and the 25-question geriatric locomotive function scale (GLFS-25) questionnaire (26-29). The stand-up test evaluated whether the subjects could stand up from a sitting position in a chair at four different heights (40, 30, 20 and 10 cm) on one or both legs. The results of the stand-up test were scored as follows: 1, 10 cm on one leg; 2, 20 cm on one leg; 3, 30 cm on one leg; 4, 40 cm on one leg; 5, 10 cm on both legs; 6, 20 cm on both legs; 7, 30 cm on both legs; and 8, 40 cm on both legs. The scores were converted and used for analysis. A lower score on the stand-up test indicated a better result. The GLFS-25 were 25 questions that were answered on a 5-point Likert-type scale to assess the degree of locomotion. These tests were performed before the start of the supplement regimen and at 12 weeks after the start of the supplement regimen.
One-leg standing time with eyes open test
The time a subject could stand on one leg with their eyes open (30) was measured using a digital stopwatch with a maximum length of 120 sec prior to the start of the supplement regimen and 12 weeks after the start of the regimen.
Blood test
Blood samples from subjects were tested for levels of IL-10, AST, ALT, γGTP, LDH, CK and CRP prior to the start of the supplement regimen and at 12 weeks after the start of the regimen. The physician collected the blood samples, and the blood analysis was performed by Hoken Kagaku, Inc.
Statistical analysis
Values are presented as the mean ± standard error and P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS Statistics 28.0 (IBM Corp.). Intergroup comparisons of changes in VAS were analyzed using two-way mixed analysis of covariance (ANCOVA) with initial values as covariates followed by a Sidak post-hoc test, and difference in the interaction effects, if significant. The intergroup comparisons applied ANCOVA with initial values as covariates, and comparisons before and after 6 and 12 weeks of the start of the supplement regimen were performed using a Sidak post-hoc test. Intergroup comparisons of the two-step test [two-step value=two-step length (cm)/height (cm)], one-leg standing time with eyes open test [duration of standing on one leg (sec)] and blood test were analyzed using two-way mixed ANOVA followed by a Sidak post-hoc test. Differences in the results of the stand-up test and GLFS-25 between the groups were assessed using the Mann-Whitney U-test with Bonferroni correction applied. In addition, before the start of the supplement regimen and at 12 weeks after the start of the regimen, the results were compared using the Wilcoxon signed-rank test with Bonferroni correction applied. Differences between groups for background characteristics such as age, height, body weight and body mass index (BMI) were assessed using the unpaired t-test and differences in the number of participants (men and women) between the groups were assessed using the Chi-squared test.
Results
Characteristics of subjects
Background characteristics of the study participants are presented in Table I. No significant differences in sex (placebo group, male:female=23:6; SH group, male:female ratio=16:11; P=0.103), age (placebo group, 61.50±1.59 years; SH group, 58.50±1.81 years), body height, weight and BMI were determined between the placebo and SH groups. None of the participants dropped out during the study period. Among the 56 study participants in the full analysis set, 25 were excluded who were found to have gonarthrosis of KL grade ≥2 based on knee X-rays or who had made errors when filling in the VAS. This resulted in a per protocol set consisting of 31 subjects for analysis (Figs. 1 and 2). The ingestion rate of the trial supplements was 98%. The diary records completed by the participants confirmed that the subjects included in the analysis did not change their diet or daily routine to a large extent during the trial period from the time before the trial, and they continued their training within the scope of daily life.
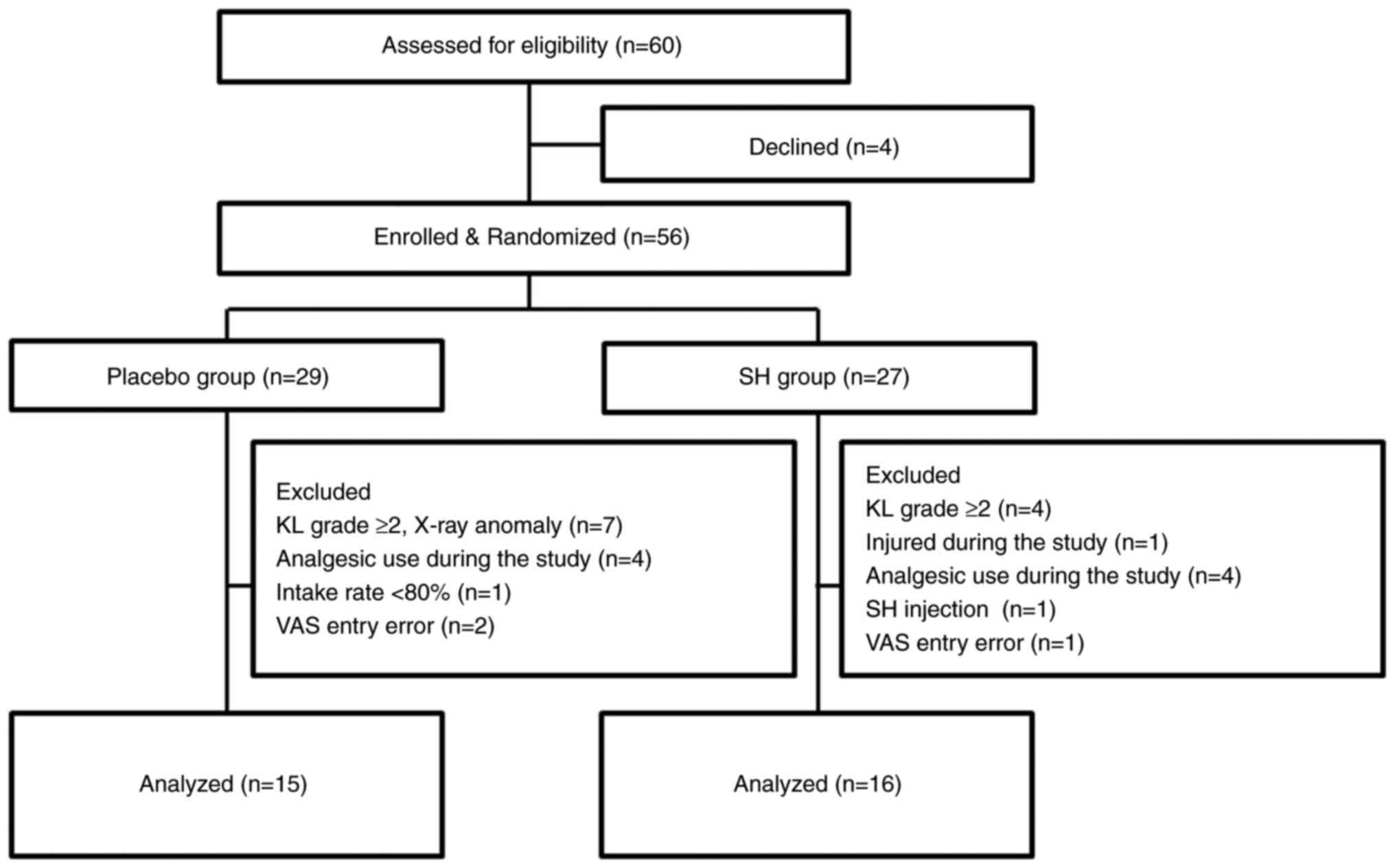 |
Figure 1
Flowchart of subject recruitment and assignment to the study groups. SH, sodium hyaluronate; KL, Kellgren-Lawrence classification; VAS, visual analog scale.
|
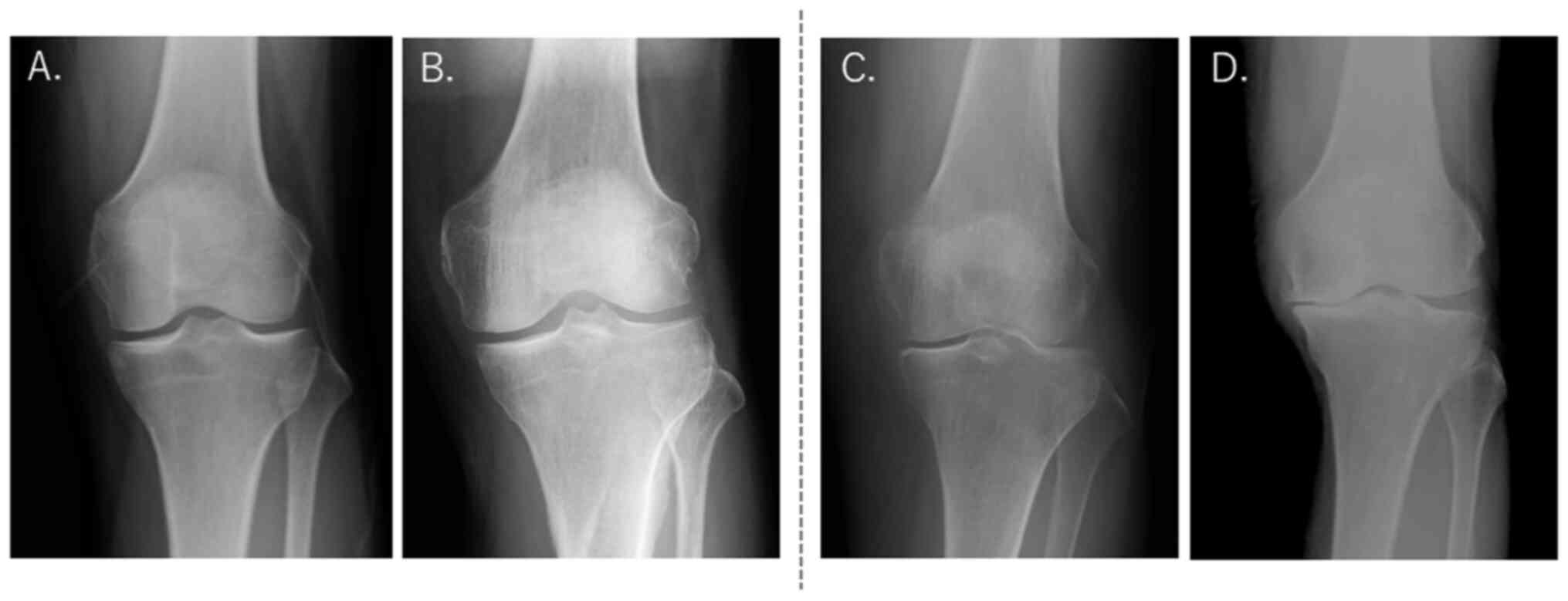 |
Figure 2
Representative X-ray images of knees from subjects who were (A) KL grade 0, (B) KL grade 1, (C) KL grade 2 and (D) KL grade 4. Selected for analysis were KL grades 0-1 and excluded from analysis were KL grades ≥2. KL, Kellgren-Lawrence classification.
|
 |
Table I
Background characteristics of the subjects in the placebo and sodium hyaluronate groups.
|
Table I
Background characteristics of the subjects in the placebo and sodium hyaluronate groups.
| Characteristic |
Placebo (n=29) |
SH (n=27) |
P-value |
| Sex |
|
|
|
| Male |
23 |
16 |
0.103 |
| Female |
6 |
11 |
|
| Age, years |
61.50±1.59 |
58.50±1.81 |
0.219 |
| Height, cm |
167.00±1.17 |
166.00±1.46 |
0.509 |
| Body weight, kg |
60.80±1.71 |
61.00±2.28 |
0.966 |
| BMI, kg/m2 |
21.70±0.44 |
21.90±0.59 |
0.742 |
VAS
Changes in VAS compared with the baseline are presented in Table II. Total scores for pain in the knees were significantly lower in the SH group than those in the placebo group at six weeks after the start of the supplement regimen and at 12 weeks after the start of the regimen (P<0.001). Total scores for knee pain significantly increased in the placebo group (P<0.001) when comparing the time before the start of the study and 12 weeks later; however, there was no significant difference in the SH group, which maintained a healthy condition. Total scores for knee stiffness in the SH group were significantly lower (P=0.017) at 12 weeks after the start of the regimen in comparison with the placebo group. The total score for knee stiffness significantly increased in the placebo group (P=0.030) before the regimen compared with after 12 weeks; however, there was no significant difference in the SH group, which maintained a healthy condition. The total score for knee discomfort in the SH group was significantly lower (P<0.001) at 12 weeks after the start of the regimen than that in the placebo group. Total scores for knee discomfort significantly increased (P=0.015) in the placebo group in a comparison of scores before the start of the regimen and 12 weeks after the start of the regimen, but no significant difference was observed in the SH group, which maintained a healthy condition. Discomfort in the knee when descending stairs at 12 weeks after the start of the regimen was significantly lower in the SH group (P=0.045) than in the placebo group. The score for pain after a longer walk at 12 weeks after the start of the regimen was significantly lower in the SH group (P=0.023) than that in the placebo group. There were no significant differences between the groups with regard to the condition of the knees when bent and extended; however, scores significantly increased in the placebo group (P=0.038) at 12 weeks after the start of the regimen in comparison to the time-point before the start of the regimen. The SH group maintained a healthy condition and exhibited no significant differences. There were no significant differences between the groups with regard to the condition of the knees upon waking, climbing stairs, going to bed and jogging an increased distance or duration compared with the average of the individual.
 |
Table II
Changes from baseline on visual analog scales for knee pain, stiffness and discomfort during certain activities after 6 and 12 weeks of ingestion of placebo or SH.
|
Table II
Changes from baseline on visual analog scales for knee pain, stiffness and discomfort during certain activities after 6 and 12 weeks of ingestion of placebo or SH.
| |
6 weeks |
12 weeks |
| Item |
Placebo (n=15) |
SH (n=16) |
Placebo (n=15) |
SH (n=16) |
| Total |
|
|
|
|
| Pain |
-0.11±0.06 |
0.09±0.08a |
0.44±0.18b |
-0.18±0.10a |
| Stiffness |
0.18±0.08 |
0.21±0.10 |
0.21±0.08c |
-0.08±0.09d |
| Discomfort |
0.13±0.10 |
0.09±0.08 |
0.33±0.13c |
-0.17±0.09a |
| Upon waking |
|
|
|
|
| Pain |
-0.27±0.25 |
0.19±0.14 |
0.18±0.42 |
0.07±0.26 |
| Stiffness |
0.38±0.16 |
0.31±0.15 |
0.19±0.35 |
0.16±0.25 |
| Discomfort |
0.29±0.25 |
0.16±0.20 |
0.83±0.63 |
0.04±0.25 |
| Climbing stairs |
|
|
|
|
| Pain |
-0.19±0.15 |
0.22±0.22 |
0.30±0.24 |
0.03±0.25 |
| Stiffness |
0.44±1.30 |
0.17±0.18 |
0.37±0.18 |
-0.08±0.31 |
| Discomfort |
0.34±1.35 |
0.04±0.20 |
0.38±0.35 |
-0.25±0.30 |
| Descending stairs |
|
|
|
|
| Pain |
-0.10±0.10 |
0.10±1.25 |
0.71±0.51 |
-0.29±0.23 |
| Stiffness |
0.02±0.09 |
0.17±0.16 |
0.29±0.16 |
-0.22±0.23 |
| Discomfort |
-0.16±0.17 |
0.02±0.17 |
0.34±0.27 |
-0.44±0.25d |
| Going to bed |
|
|
|
|
| Pain |
0.01±0.06 |
0.11±0.11 |
0.25±0.16 |
0.06±0.12 |
| Stiffness |
0.05±0.12 |
0.15±0.13 |
0.15±0.11 |
0.03±0.09 |
| Discomfort |
-0.06±0.07 |
0.11±0.16 |
0.09±0.12 |
-0.03±0.08 |
| Bending and stretching knees |
|
|
|
|
| Pain |
-0.05±0.22 |
0.30±0.47 |
0.62±0.29c |
0.03±0.17 |
| Stiffness |
0.45±0.27 |
0.64±1.24 |
0.35±0.14 |
0.03±0.18 |
| Discomfort |
0.26±0.19 |
0.25±0.29 |
0.37±0.31 |
-0.19±0.24 |
| Walking for a longer distance/time compared with usual |
|
|
|
|
| Pain |
-0.13±0.30 |
-0.03±0.23 |
0.53±0.33 |
-0.38±0.20d |
| Stiffness |
0.13±0.31 |
0.07±0.25 |
0.04±0.21 |
-0.21±0.17 |
| Discomfort |
0.23±0.31 |
0.06±0.25 |
0.27±0.35 |
-0.21±0.19 |
| Jogging for a longer distance/time compared with usual |
|
|
|
|
| Pain |
-0.07±0.24 |
-0.26±0.33 |
0.44±0.42 |
-0.85±0.45 |
| Stiffness |
-0.31±0.22 |
-0.03±0.35 |
0.12±0.33 |
-0.25±0.31 |
| Discomfort |
-0.01±0.22 |
0.02±0.35 |
0.01±0.32 |
-0.11±0.32 |
Locomotive syndrome risk test
There were no significant differences between the placebo and SH groups, and no significant differences between before and after 12 weeks of the start of the supplement regimen (Table III).
 |
Table III
Results of the locomotive syndrome risk test and one-leg standing time with eyes open test at baseline and at 12 weeks after starting treatment.
|
Table III
Results of the locomotive syndrome risk test and one-leg standing time with eyes open test at baseline and at 12 weeks after starting treatment.
| |
0 weeks |
12 weeks |
| Test |
Placebo (n=15) |
SH (n=16) |
Placebo (n=15) |
SH (n=16) |
| Stand-up test score |
3.67±0.45 |
3.38±0.27 |
3.20±0.39 |
2.63±0.29 |
| Two-step test, 2 step valuea |
1.56±0.03 |
1.58±0.04 |
1.63±0.03 |
1.63±0.05 |
| GLFS-25 score |
7.47±1.90 |
6.25±1.27 |
5.21±1.09 |
5.50±1.08 |
| One-leg standing time with eyes open test, sec |
|
|
|
|
| Right leg |
111±4.48 |
101±10.0 |
116±4.00 |
98.6±9.83 |
| Left leg |
100±8.76 |
96.3±9.37 |
108±6.72 |
107±8.31 |
One-leg standing time with eyes open
There were no significant differences between the placebo and SH groups, and no significant differences between before and after 12 weeks of the start of the supplement regimen (Table III).
Blood test
There were no significant differences between the placebo and SH groups, and no significant differences between before and after 12 weeks of the start of the supplement regimen (Table IV). No adverse events were caused by the intake of SH during the trial.
 |
Table IV
Results of blood tests at baseline and at 12 weeks after the start of the supplement regimen.
|
Table IV
Results of blood tests at baseline and at 12 weeks after the start of the supplement regimen.
| |
0 weeks |
12 weeks |
| Parameter |
Normal reference values |
Placebo (n=15) |
SH (n=16) |
Placebo (n=15) |
SH (n=16) |
| AST, U/l |
10-40 |
32.5±3.37 |
28.3±1.58 |
31.2±3.14 |
26.3±1.66 |
| ALT, U/l |
5-45 |
26.9±3.33 |
22.7±2.15 |
26.7±2.93 |
23.0±2.59 |
| LDH, U/l |
120-220 |
202±8.36 |
216±8.05 |
214±9.33 |
213±9.81 |
| γGT, U/l |
Male: 0-80 |
47.5±10.1 |
43.3±9.17 |
51.6±15.8 |
49.7±16.8 |
| |
Female: 0-30 |
|
|
|
|
| CK, U/l |
Male: 60-250 |
213±32.4 |
283±66.9 |
381±163.8 |
164±18.3 |
| |
Female: 50-170 |
|
|
|
|
| CRP, mg/dl |
≤0.35 |
0.06±0.01 |
0.06±0.02 |
0.12±0.03 |
0.06±0.01 |
| IL-10, pg/ml |
<8 |
5.67±1.24 |
5.19±0.88 |
5.76±0.99 |
5.68±0.79 |
Discussion
The present study was a randomized, double-blinded, placebo-controlled parallel-group study that assessed the effectiveness and safety of the test supplement (SH) on knee discomfort when taken for a period of 12 weeks. Subjects were middle- and old-aged healthy individuals, who had an exercise routine that mostly included jogging and were able to continue with their normal training whilst feeling discomfort in their knees. Subjects with severe gonarthrosis, namely those who scored grade ≥2 based on the KL classification, an indicator of the severity of knee OA, were excluded.
A study evaluating the use of oral SH for individuals with knee OA have reported using paracetamol (an analgesic) as a positive control (17). In addition, crystalline cellulose, dextrin, fatty acid sugar esters and cornstarch have been used as placebo controls (16,23,35). These placebo controls have been reported to have no influence on the knee and no safety concerns. The present study adopted dextrin as a common placebo control in a food supplement study. As it is not a positive control, it could not be compared with medicinal products; however, it was considered to be an appropriate control, as the participants of the present study were considered to be healthy and did not require the administration of medicinal products.
Under the KL classification, a significant association is usually recognized between the severity of gonarthrosis and the JKOM scores, but the degree of such association declines when the severity decreases in relatively mild cases, such as those of grade 1(36). Therefore, as the participants of the present study were graded as ≤1, the association with JKOM scores was reduced and the effect of oral SH was hypothesized to be lower. However, the total points for each VAS used in the present study (pain, stiffness and discomfort) demonstrated that the knee discomfort of the subjects were significantly relieved by oral intake of SH, and this highlights the potential improvement function of SH.
Furthermore, oral intake of SH also alleviated discomfort in the knees when descending stairs and pain in the knees when walking for a longer distance or duration than normal. It is hypothesized that discomfort in the knees when descending stairs was easy to recognize as a subjective symptom, as the body's center of gravity is accelerated downward with the downward motion of the body, which puts a particularly heavy burden on the knee joints (37).
A grade of ≥2 in the KL classification usually increases the rate of knee deformity, articular crepitus and decreased range of motion of the knee (38). Previous SH intake studies on OA have had an 8-week to 12-month duration (23,25,32). The effectiveness of oral SH has been reported to take 12 months in serious cases of grades 2 and 3, demonstrated through an improvement in JKOM scores for elderly subjects aged ≤70 years (23). Conversely, similar previous studies in individuals with mild knee pain or discomfort had a 12-week study period (25,32). In a study of participants with knee OA with an 8-week duration of SH supplementation, a stratified analysis of pain scores of ≥10 determined significant relief of knee pain and discomfort in the SH group compared with the placebo-treated group, but the overall analysis failed to confirm a clear efficacy (16). It was hypothesized that long-term intake is required in KL grade ≥2 with knee OA and 12 weeks of intake is effective in relatively healthy study participants. In the present trial, if individuals with a KL classification of grade ≥2 had been examined, the functionality of SH may have been demonstrated more clearly, even in the locomotive syndrome risk test. However, it is worth noting that a subjective assessment, such as VAS, demonstrated that oral SH alleviates pain and discomfort for healthy individuals who are classified as mild cases.
SH binds to toll-like receptor-4 (TLR-4), present on the mucosal epithelium of the intestine, and suppresses inflammation by promoting the expression of SOCS3 and the production of the regulatory cytokine IL-10 (25,39). The results of the present trial suggested that SH increased the levels of IL-10 (although not statistically significant) as well as an ameliorative effect on knee joint pain, stiffness and discomfort; therefore, this could have possibly involved an anti-inflammatory effect mediated by TLR-4(40). As there is no genetic variation in TLR4 due to ethnicity, the potential function of SH intake on the knee via TLR-4-mediated signal transmission may be extrapolated to other ethnicities, which should be investigated further in the future (41).
Dietary supplements that have been reported to be useful for the improvement of knee discomfort include muco-polysaccharides such as chondroitin, glucosamine and SH. Studies have reported the improvement of knee discomfort using 350 mg/day chondroitin sulfate and 1,500 mg/day glucosamine with healthy adults (42,43). In contrast, reports on the effectiveness of different SH doses range from 48-240 mg/day, which are relatively easy doses to maintain (17,25,44). The present study demonstrated the effect of SH on the knees at a dose of 111 mg/day, which is within the effective range of SH reported in the aforementioned studies, thereby supporting the results of previous research.
An increase in the incidence of OA and a decrease in the amount of endogenous SH are expected in the future due to aging, even for healthy runners such as the subjects of the present study (45,46). Knee OA in particular is the most frequent cause of knee pain in the elderly in Japan (47). This is due to the structural characteristics of the knee joint, which make it inherently unstable, and the biomechanical environment being prone to large loads, making it one of the joints most susceptible to sports injuries and knee OA (47). For middle-aged and elderly individuals who have adopted physical activities centered around running as part of their daily routine, such as the subjects in the present study, training whilst experiencing knee pain or discomfort can further increase the risk of worsening knee pain or discomfort. Therefore, considering the beneficial effect on knee joint pain from the oral intake of SH reported (35,48), effectively utilizing SH as a supplement aimed at alleviating discomfort and suppressing inflammation may contribute to enhanced quality of life for middle-aged and elderly runners who are at risk of such issues.
A limitation of the present study is the lack of objective indicators for objective comparisons, such as before-and-after comparisons of X-ray images, were not performed. To minimize the burden on the study participants, images were taken only once during the study period and were used to select subjects for analysis. Future studies that include objective indices such as imaging are needed.
To conclude, the present study demonstrated that continuous oral intake of SH (111 mg/day) for 12 weeks reduced total VAS scores for pain, stiffness and discomfort in the knees of healthy adults with a KL grade of ≤1, particularly with regard to alleviating discomfort when descending stairs and pain in the knees after walking longer than normal. Furthermore, SH was demonstrated to be safe for use as a functional food with no adverse effects based on blood tests measuring levels of IL-10, AST, ALT, γGTP, LD, CK and CRP. Therefore, the continued intake of SH could potentially contribute to knee health and function. Future studies should investigate whether a synergistic effect can be obtained by combining SH with glucosamine and chondroitin.
Acknowledgements
Not applicable.
Funding
Funding: The present research was funded by Kewpie Corporation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KS and RM conceptualized the study. KS, RM, MO, TT and KO contributed to the methodology, software, validation, formal analysis and investigation. MR and YT arranged the resources. KS, MO and TT performed data curation. KS and MO contributed to the preparation of writing the original draft. RM, YT and MK interpretated the data for the manuscript. KS and MO contributed to visualization. KS and KO contributed to supervision. RM contributed to the project administration and funding acquisition. All authors have read and approved the final manuscript. MO and TT confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was performed according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Hokkaido University of Education Ethics Review Committee (approval no. 2021035001). The subjects included in the study provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
MO, TT, RM, YT and MK are employees of Kewpie Corporation. Kewpie Corporation provided the study capsules used in the present study. The remaining authors have no other competing interests to report regarding the present work.
References
|
1
|
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet. 396:1204–1222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hunter DJ and Bierma-Zeinstra S: Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Centers for Disease Control and Prevention (CDC). Prevalence of arthritis-United States, 1997. MMWR Morb Mortal Wkly Rep. 50:334–336. 2001.PubMed/NCBI
|
|
4
|
Loeser RF Jr: Aging and the etiopathogenesis and treatment of osteoarthritis. Rheum Dis Clin North Am. 26:547–567. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Peyron JG and Balazs EA: Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. Pathol Biol (Paris). 22:731–736. 1974.PubMed/NCBI
|
|
6
|
Kilincoglu V, Yeter A, Servet E, Kangal M and Yildirim M: Short term results comparison of intraarticular platelet-rich plasma (prp) and hyaluronic acid (ha) applications in early stage of knee osteoarthritis. Int J Clin Exp Med. 8:18807–18812. 2015.PubMed/NCBI
|
|
7
|
Øiestad BE, Juhl CB, Culvenor AG, Berg B and Thorlund JB: Knee extensor muscle weakness is a risk factor for the development of knee osteoarthritis: An updated systematic review and meta-analysis including 46 819 men and women. Br J Sports Med. 56:349–355. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wei N and Dai Z: The role of nutrition in osteoarthritis: A literature review. Clin Geriatr Med. 38:303–322. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brophy RH and Fillingham YA: aaos clinical practice guideline summary: Management of osteoarthritis of the knee (Nonarthroplasty), third edition. J Am Acad Orthop Surg. 30:e721–e729. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tanaka T: Questionnaire survey on marathon life of civil runner. Tokushima University Bulletin. 22:19–34. 2013.(In Japanese).
|
|
11
|
Castillo B, Sepúlveda F and Micheo W: Conservative management and rehabilitation in the older runner with knee osteoarthritis: An evidence-based review. Am J Phys Med Rehabil. 98:416–421. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shipway R and Holloway I: Health and the running body: Notes from an ethnography. Int Rev Sociol Sport. 51:78–96. 2016.
|
|
13
|
Fraser JR, Laurent TC and Laurent UB: Hyaluronan: Its nature, distribution, functions and turnover. J Intern Med. 242:27–33. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Balazs EA, Watson D, Duff IF and Roseman S: Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 10:357–376. 1967.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ogura M, Takabe W, Yagi M, Wakayama S and Yonei Y: Study for investigation of symptomatic improvement and safety of the ingestion of rooster comb degradation product containing low-molecular hyaluronic acid (INJUV) in individuals with knee and lower back pain; open label trial with no control group. Glycative Stress Res. 5:55–67. 2018.
|
|
16
|
Sato T and Iwaso H: An effectiveness study of hyaluronic acid Hyabest® (J) in the treatment of osteoarthritis of the knee on the patients in the United State. J New Rem Clin. 58:551–558. 2009.
|
|
17
|
Möller I, Martinez-Puig D and Chetrit C: LB012 oral administration of a natural extract rich in hyaluronic acid for the treatment of knee OA with synovitis: A retrospective cohort study. Clin Nutr Suppl. 4:171–172. 2009.
|
|
18
|
Yoshimura M, Aoba Y, Watari T, Momomura R, Watanabe K, Tomonaga A, Matsunaga M, Suda Y, Lee WY, Asai K, et al: Evaluation of the effect of a chicken comb extract-containing supplement on cartilage and bone metabolism in athletes. Exp Ther Med. 4:577–580. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Martinez-Puig D, Möller I, Fernández C and Chetrit C: Efficacy of oral administration of yoghurt supplemented with a preparation containing hyaluronic acid (Mobilee™) in adults with mild joint discomfort: A randomized, double-blind, placebo-controlled intervention study. Mediterr J Nutr Metab. 6:63–68. 2013.
|
|
20
|
Nelson FR, Zvirbulis RA, Zonca B, Li KW, Turner SM, Pasierb M, Wilton P, Martinez-Puig D and Wu W: The effects of an oral preparation containing hyaluronic acid (Oralvisc®) on obese knee osteoarthritis patients determined by pain, function, bradykinin, leptin, inflammatory cytokines, and heavy water analyses. Rheumatol Int. 35:43–52. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jensen GS, Attridge VL, Lenninger MR and Benson KF: Oral intake of a liquid high-molecular-weight hyaluronan associated with relief of chronic pain and reduced use of pain medication: Results of a randomized, placebo-controlled double-blind pilot study. J Med Food. 18:95–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kohn MD, Sassoon AA and Fernando ND: Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 474:1886–1893. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tashiro T, Seino S, Sato T, Matsuoka R, Masuda Y and Fukui N: Oral administration of polymer hyaluronic acid alleviates symptoms of knee osteoarthritis: A double-blind, placebo-controlled study over a 12-month period. ScientificWorldJournal. 2012(167928)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sato T: Human nutrition lecture series: Introduction to randomized clinical trials part 4: Methods of randomization. Jpn J Nutr Diet. 65:255–260. 2007.(In Japanese).
|
|
25
|
Oe M, Tashiro T, Yoshida H, Nishiyama H, Masuda Y, Maruyama K, Koikeda T, Maruya R and Fukui N: Oral hyaluronan relieves knee pain: A review. Nutr J. 15(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Muranaga S and Hirano K: Development of a convenient way to predict ability to walk, using a two-step test. J Showa Med Assoc. 63:301–308. 2003.(In Japanese).
|
|
27
|
Muranaga S: Evaluation of the muscular strength of the lower extremities using the standing movement and clinical application. J Showa Med Assoc. 61:362–367. 2001.(In Japanese).
|
|
28
|
Seichi A, Hoshino Y, Doi T, Akai M, Tobimatsu Y and Iwaya T: Development of a screening tool for risk of locomotive syndrome in the elderly: The 25-question geriatric locomotive function scale. J Orthop Sci. 17:163–172. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yamada K, Ito YM, Akagi M, Chosa E, Fuji T, Hirano K, Ikeda S, Ishibashi H, Ishibashi Y, Ishijima M, et al: Reference values for the locomotive syndrome risk test quantifying mobility of 8681 adults aged 20-89 years: A cross-sectional nationwide study in Japan. J Orthop Sci. 25:1084–1092. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Murata S, Oyama M, Otao H, Murata J, Toyota K, Fujino H, Yumioka M and Takeda I: Relationship between one-leg standing time with eyes open and physical function among community-dwelling elderly females. Rigakuryoho Kagaku. 23:79–83. 2008.(In Japanese).
|
|
31
|
Ethical guidelines for Medical and Health Research Involving Human Subjects: Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labor and Welfare; and Ministry of Economy, Trade and Industry, Japan, 2015.
|
|
32
|
Takamizawa N, Shioya N, Nagaoka H and Uchino T: Effects and safety of a dietary supplement containing hyaluronic acid derived from chicken combs on knee pain, stiffness and discomfort-A randomized, double-blind, placebo-controlled, parallel-group comparison study. Jpn Pharmacol Ther. 44:207–217. 2016.(In Japanese).
|
|
33
|
Akai M, Doi T, Fujino K, Iwaya T, Kurosawa H and Nasu T: An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol. 32:1524–1532. 2005.PubMed/NCBI
|
|
34
|
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J and Stitt LW: Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 15:1833–1840. 1988.PubMed/NCBI
|
|
35
|
Hatayama T, Nagano M, Yamaguchi N, Kumagai S and Oonuki K: The effect of a supplement on knee pain and discomfort evaluated by visual analogue scale (VAS): A randomized, double-blind, placebo-controlled study. Jpn J Health Promot. 10:13–17. 2008.(In Japanese).
|
|
36
|
Watanabe H, Urabe K, Kamiya K, Hamazaki N, Miida K, Suda K, Hendona T, Fujita M, Aikawa J, Itoman M, et al: Relationship between quality of life using the Japan knee osteoarthritis measure (JKOM) and physical function in patients with osteoarthritis of the knee. Jpn Phys Ther Assoc. 34:67–73. 2007.(In Japanese).
|
|
37
|
Tanabe Y and Mizukami M: Lower limb biomechanics during stair descent are related to the descent velocity of healthy young individuals. J Phys Ther Sci. 30:207–212. 2015.
|
|
38
|
Bagge E, Bjelle A, Eden S and Svanborg A: Osteroarthritis in elderly: Clinical and radio-logical findings in 79 and 85 years olds. Ann Rheum Dis. 50:535–539. 1991.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Asari A, Kanemitsu T and Kurihara H: Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via toll-like receptor 4 in the intestinal epithelium. J Biol Chem. 285:24751–24758. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kimura M: Absorption of orally administered hyaluronan. J Funct Foods. 14:30–35. 2018.(In Japanese).
|
|
41
|
Taylor BD, Darville T, Ferrell RE, Ness RB and Haggerty CL: Racial variation in toll-like receptor variants among women with pelvic inflammatory disease. J Infect Dis. 207:940–946. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ostojic SM, Arsic M, Prodanovic S, Vukovic J and Zlatanovic M: Glucosamine administration in athletes: Effects on recovery of acute knee injury. Res Sports Med. 15:113–24. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sugino T, Fujita M, Sugawara Y and Kajimoto O: Pig-derived chondroitin sulfate attenuates mild knee joint pain in healthy middle and old aged people-a randomized, double-blind, placebo-controlled, parallel-group comparison trial-. JPT. 47:2045–2058. 2019.
|
|
44
|
Iwaso H and Sato T: Examination of the efficacy and safety of oraladministration of Hyabest® (J)-highly-pure hyaluronic acid for knee joint pain. J Jpn Soc Clin Sports Med. 58:566–572. 2009.(In Japanese).
|
|
45
|
Martin JA and Buckwalter JA: Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J. 21:1–7. 2001.PubMed/NCBI
|
|
46
|
Okamoto A: Hyaluronic acid and joint fluid. B&R. 13:14–23. 1999.(In Japanese).
|
|
47
|
Miura H: The merits and demerits of sports in osteoarthritis of the knee. Jpn J Phys Fit Sports Med. 67:168–174. 2018.(In Japanese).
|
|
48
|
Goto M, Hosako Y, Katayama M and Yamada T: Biochemical analysis of rheumatoid synovial fluid after serial intra-articular injection of high molecular weight sodium hyaluronate. Int J Clin Pharmacol Res. 13:161–166. 1993.PubMed/NCBI
|
















