Introduction
Cluster of differentiation (CD)44 is a cell-surface
glycoprotein in cell-cell interactions. Its expression has been
confirmed in a number of cells including lymphocytes, cancer cells
and mesenchymal stem cells (1-3).
The authors previously revealed that CD44 is involved in
odontoblast differentiation (4,5).
Chemokine-like receptor 1 (CMKLR1), also known as chemerin receptor
23 (ChemR23), has been implicated in tooth development and in the
inhibition of inflammation (6).
Therefore, the present study aimed to determine the detailed
localization of CD44 and CMKLR1 in teeth and to clarify changes in
CD44 and CMKLR1 expression in pulpitis.
A number of studies have investigated inflammation
of the dental pulp (7-10);
however, further clarification of the mechanism of pulp
inflammation is expected to lead to more reliable treatment of
pulpitis. Suppression of pulpal inflammation and induction of
remineralization have also been investigated (7,11-13)
and are considered to be important in dental treatment to preserve
the dental pulp or to regenerate the tooth structure (14-16).
When dental pulp is infected with bacteria as a result of dental
caries or periodontitis and irreversible pulpitis develops, removal
of dental pulp is often performed (17). To remove pulp and perform root
canal treatment, the tooth structure must be opened; however, the
loss of tooth material leads to a higher risk of fracture or tooth
extraction (18). By preserving
uninfected pulp as much as possible, it is possible to prevent
invasion of bacteria into the tooth interior, which is one of the
functions of the pulp. Preserving or regenerating dental pulp is
therefore thought to extend the life of a tooth. The present study
focused on CMKLR1 because it is involved in tooth development and
inflammation. A deeper understanding of CMKLR1 may be critical to
suppressing inflammation in dental pulp.
CMKLR1 is present in ameloblasts and odontoblasts
during tooth development and chemerin and CMKLR1 is expressed in
differentiating tooth epithelial and mesenchymal cells and has an
important role in tooth development (19). Furthermore, chemerin/CMKLR1
interactions suppress excessive inflammation and promote tissue
regeneration in non-dental tissues (20,21).
CD44 is a transmembrane glycoprotein with various biological
functions and is a marker for mesenchymal stem cells (22,23).
It is also strongly expressed in odontoblasts during tooth
development (24,25). CD44-positive cells in teeth are
localized at the tip of the immature root and at the coronal pulpal
corner. CD44 is also expressed in some odontoblasts and is involved
in calcification (26).
These separate studies of CD44 and CMKLR1 indicate
that CD44 is important for odontoblast differentiation and tooth
mineralization and that CMKLR1 acts in the regulation of pulpal
inflammation. Moreover, CD44 and CMKLR1 are localized in
odontoblasts and undifferentiated mesenchymal cells of teeth.
However, it remains unclear whether the two receptors, CD44 and
CMKLR1, coexist in odontoblasts or undifferentiated mesenchymal
cells. Furthermore, it is unclear how CD44 and CMKLR1 interact and
participate in the induction of odontoblast differentiation, tooth
calcification and inhibition of pulpitis. The present study
investigated the localization of CD44- and CMKLR1-expressing cells
in teeth and also observed changes in their expression during
inflammation in pulpitis.
Materials and methods
Tissue samples from animals
Previous studies have attempted animal models of
pulpitis in mice and rats (27,28).
The present study generated a rat pulpitis model based on these
studies. A total of five 12-week-old male Wistar rats (250-300 g)
were purchased from Japan SLC, Inc., and maintained at 23±2˚C and a
60±5% humidity with 12 h light/dark cycles and free access to
sterilized food and water. Animal experiments were approved by the
ethics committee of Asahi University (approval number: 21-009 and
22-045). General anesthesia was induced with 6% isoflurane (MSD
Animal Health) and maintained at 2%. Limbs and tails of rats under
anesthesia were taped. The occlusal surfaces of the bilateral
maxillary first molars were observed under a dissecting microscope
and opened with a 1:5 speed-up contra angle TorqTech attachment
with a round burr (Morita Corp.) to create cavities and expose the
pulp (Fig. S1A). After the
treatment, the animals were monitored and, if there were signs of
pain, the endpoint of comfort treatment was considered. However,
such signs were not observed in this experiment. At 24 h after pulp
exposure, rats were deeply anaesthetized by intraperitoneal
injection of 8% chloral hydrate (400 mg/kg) without signs of
peritonitis. After confirming loss of consciousness, perfusion
fixation was performed with cold 4% paraformaldehyde in 0.1 M
phosphate buffer (pH 7.4) while bleeding (29-31).
Cardiac arrest was confirmed in rats after perfusion fixation.
Micro computed tomography confirmed that the hole in the treated
tooth reached the pulp. (Fig.
S1B). Excised maxillae were immersed in 10% EDTA-2Na (Dojindo
Laboratories, Inc.) solution and decalcified at 4˚C for 4 weeks.
Paraffin embedding was performed by immersing the specimen in
absolute ethanol and xylene and then adding dissolved paraffin
according to the general method. Horizontal 5-µm sections were
prepared using a microtome (REM-700; Yamato Kohki Industrial Co.,
Ltd.). Some sections were stained with 0.03% toluidine blue to
confirm histological morphology.
Tooth samples
Human teeth were extracted from patients for
orthodontic treatment after obtaining their permission. Teeth were
placed in 10% formalin neutral buffer solution (FUJIFILM Wako Pure
Chemical Corporation) and stored at 4˚C. Three normal upper wisdom
teeth and two wisdom teeth with irreversible pulpitis were used.
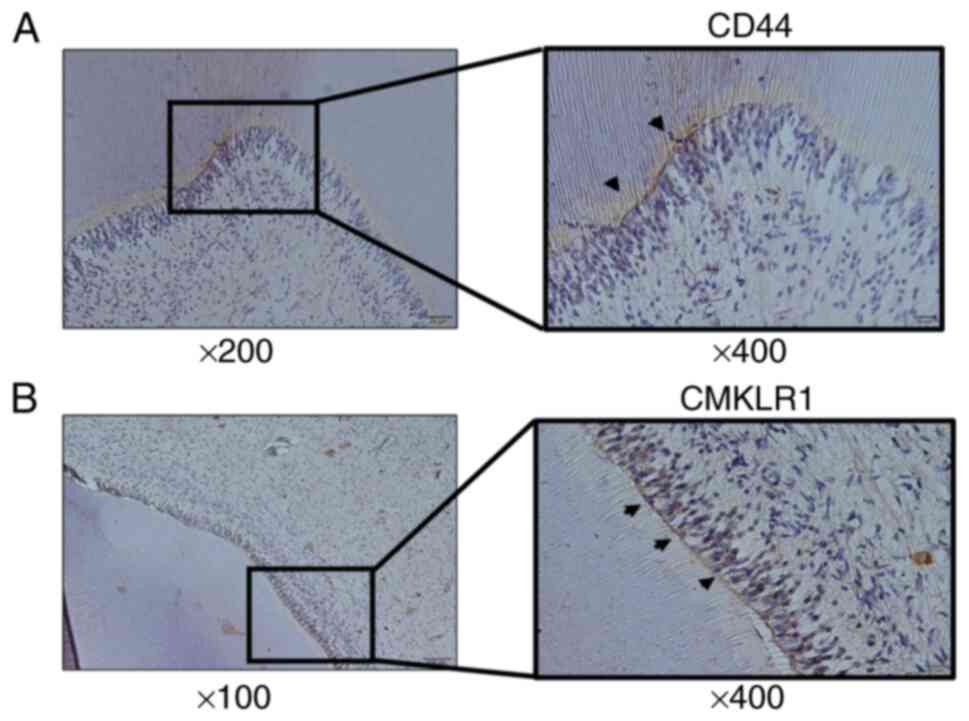
One of the affected teeth was the lower right wisdom tooth of a
55-year-old male (Fig. 4A) and the
other was the upper right wisdom tooth of a 36-year-old male
(Fig. 4B), which had to be
extracted due to irreversible pulpitis associated with dental
caries. The study was approved by the Asahi University Research
Ethics Committee (approval no. 31020) and was conducted according
to the Declaration of Helsinki. Teeth were immersed in 10% EDTA-2Na
(Dojindo Laboratories, Inc.) solution and decalcified at 4˚C for 4
weeks. Thereafter, teeth were immersed in EDT-X (neutral
decalcifying solution, FALMA, Inc.) at room temperature for an
additional 4 weeks. Tissues were then embedded in paraffin and
horizontal 5-µm sections were prepared using a sliding microtome
(REM-700; Yamato Kohki Industrial Co., Ltd.).
Immunohistochemistry
Serial 5-µm paraffin sections were deparaffinized
using xylene and serial ethanol dilutions and then stained with
hematoxylin for 20 min and then eosin for 20 min at room
temperature. Endogenous peroxidase was inactivated by treatment
with 3% hydrogen peroxide solution for 10 min at room temperature.
Sections were then washed with PBS, treated with 1% BSA for 30 min
at room temperature and incubated with anti-CD44 (anti-homing
receptor, cloneA020: MilliporeSigma) antibody at 1:200 dilution for
24 h at room temperature. Antigen retrieval for CMKLR1 antigen was
performed using a microwave rapid sample processor MI-77 (Azumaya)
with settings of 80˚C, 20 min and an output of 6. Sections were
then incubated with anti-chemokine-like receptor 1 polyclonal
antibody (anti-CMKLR1; Cayman Chemical Company) diluted at 1:100,
followed by incubation with peroxidase-labelled goat anti-rat IgG
(Bethyl Laboratories, Inc.) or Nichirei Histofine R (Nichirei
Biosciences Inc.) diluted at 1:500 for 30 min. DAB staining was
then performed and the nuclei were counterstained with hematoxylin
for 1 min at room temperature.
Cells
Human dental pulp stem cells were obtained from
Lonza Group Ltd. Stem cells were cultured at 37˚C in Dental Pulp
Stem Cell Growth Medium (Lonza Group Ltd.) in humidified air
containing 5% CO2.
Western blotting
Whole-cell extracts were obtained using lysis buffer
(10X RIPA buffer; Cell Signaling Technology, Inc. supplemented with
1 mM PMSF and 1X protease inhibitors. Total protein concentration
in the lysates was assayed using PierceTM 660 nm Protein
Assay Reagent (Thermo Fisher Scientific Inc.). A 10 µg protein
sample was separated by 8% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and blotted onto polyvinylidene fluoride
membranes. After blocking with 5% non-fat milk for 1 h at room
temperature, membranes were then incubated with anti-DSPP mouse
monoclonal (cat. no. sc-73632; 1:200; Santa Cruz Biotechnology
Inc.), anti-CD44 mouse monoclonal (cat. no. 5640; 1:1,000; Cell
Signaling Technology, Inc.), anti-COX-2 rabbit polyclonal (cat. no.
SAB4200576; 1:500; MilliporeSigma), or β-actin (cat. no. 5441;
1:10,000; MilliporeSigma) antibodies for 1 h at room temperature. A
peroxidase-conjugated secondary antibody (anti-rabbit IgG; cat. no.
7074; 1:1,000; or anti-mouse IgG; cat. no. 7076; 1:1,000; Cell
Signaling Technology, Inc.) for 1 h at room temperature, and
chemiluminescence (ClarityTM Western ECL substrate;
Bio-Rad Laboratories, Inc.) were then used to visualize
immunoreactive proteins. Images were acquired using a Light-Capture
II instrument (Atto Co., Ltd.). Band intensity was semi-quantified
by densitometry using a CS Analyzer 3.0 (Atto Co., Ltd.). The
abundance of the target protein relative to the abundance of
β-actin was calculated.
Immunofluorescence staining
Samples were blocked with ready-to-use
Immunofluorescence Blocking Buffer (Cell Signaling Technology,
Inc.) for 1 h. The blocking solution was aspirated and anti-mouse
CD44 monoclonal (cat. no. 5640, 1:400; Cell Signaling Technology,
Inc.) and anti-rabbit CMKLR1 polyclonal (1:200; Cayman Chemical)
primary antibodies were then added at the same time and incubated
overnight at 4˚C. Fluorescence-labelled secondary anti-mouse
IgG(H+L), F(ab')2 Fragment (Alexa Fluor R555 conjugate; cat. no.
4409, 1:500; Cell Signaling Technology, Inc.) and anti-rabbit
IgG(H+L), F(ab')2 Fragment (Alexa Fluor R488 conjugate; cat. no.
4412, 1:500; Cell Signaling Technology, Inc.) were added, incubated
in the dark for 1 h and counterstained with DAPI (cat. no. 4083,
1:2,000; Cell Signaling Technology, Inc.) for 5 min in the dark at
room temperature. After dehydration and encapsulation, observation
was performed using an LSM710 confocal laser microscope (Zeiss
GmbH) and images were acquired with LSM780 software ZEN (version
2012 SP1; Zeiss GmbH).
Statistical analysis
Semi-quantitative western blotting data are
presented as the mean ± standard deviation from three independent
experiments and were evaluated using one-way analysis of variance
followed by Dunnett's multiple comparison. P<0.05 was considered
to indicate a statistically significant difference.
Results
Localization of CD44 and CMKLR1 in rat
teeth
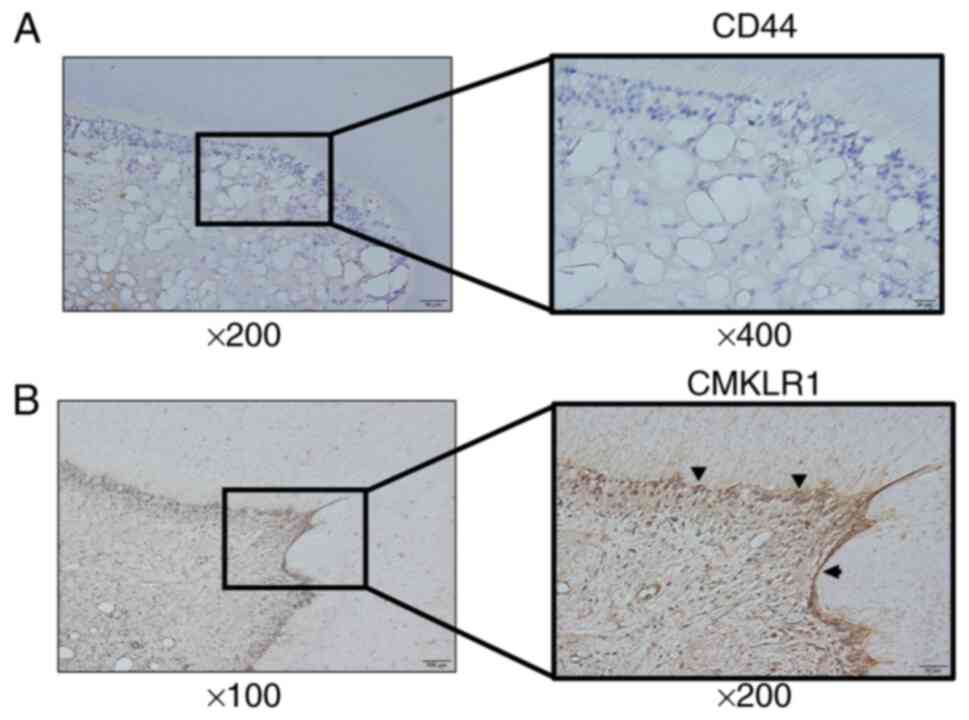
CD44 and CMKLR1 localization in rat teeth was
assessed by immunohistochemical staining. CD44 and CMKLR1 were both
detected in odontoblasts, which connect to dentinal tubules in the
enamel dentin junction (Fig. 1A
and B). CD44- and CMKLR1-positive
cells were confirmed in the odontoblast layer at sites such as the
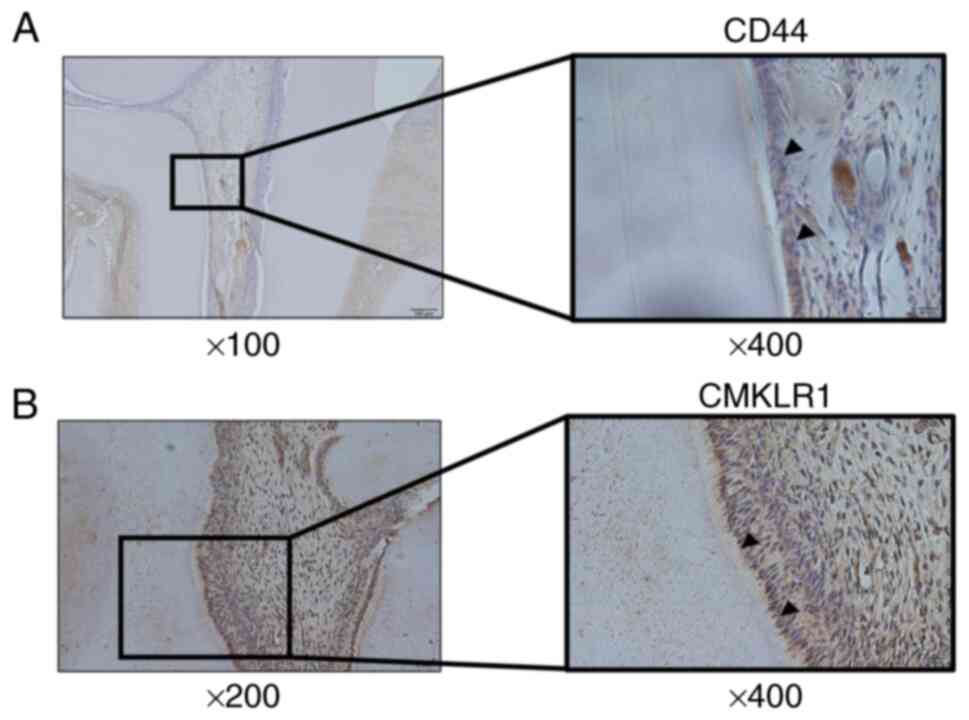
medullary horn and the base of the medulla. In pulp tissue that was
inflamed by drilling a cavity in a tooth, the CD44-positive cells
observed in normal tissue disappeared but CMKLR1-positive cells
remained in the odontoblastic layer (Fig. 2A and B).
Localization of CD44 and CMKLR1 in
human teeth
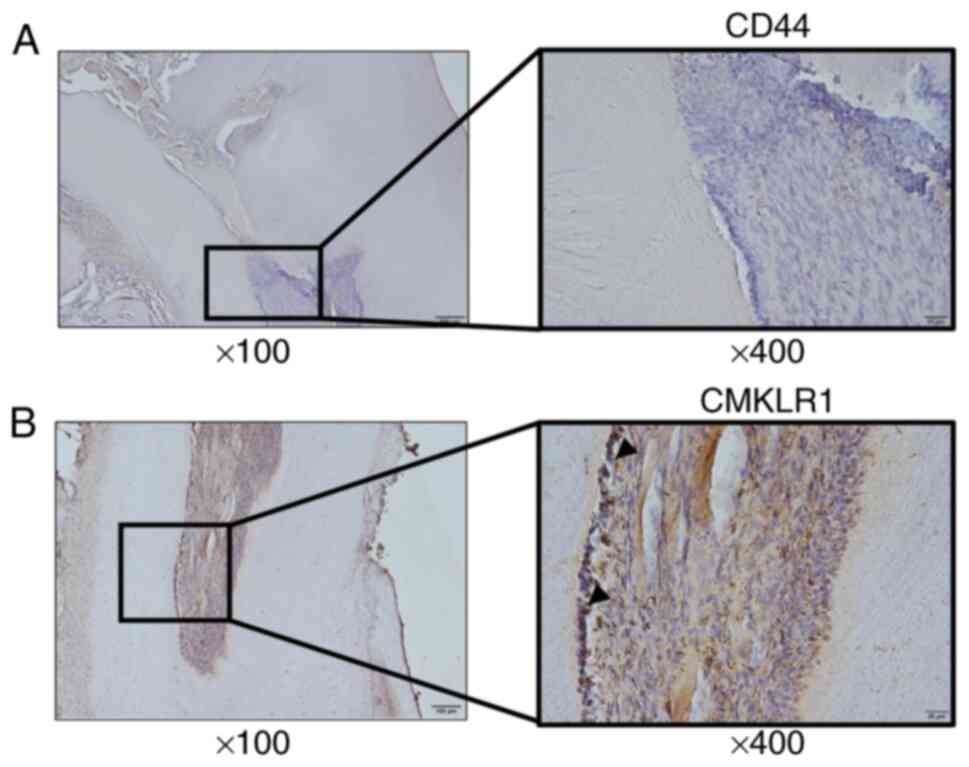
CD44 and CMKLR1 expression in human teeth were next
verified. Immunohistochemical staining confirmed CD44- and
CMKLR1-positive cells in the odontoblast layer around the pulp horn
and at the base of the pulp bed, as in rat teeth (Fig. 3A and B). In odontoblasts from human pulpitis
tissue, CD44-positive cells disappeared but CMKLR1-positive cells
remained, as in rat inflamed pulp tissue (Fig. 4A and B).
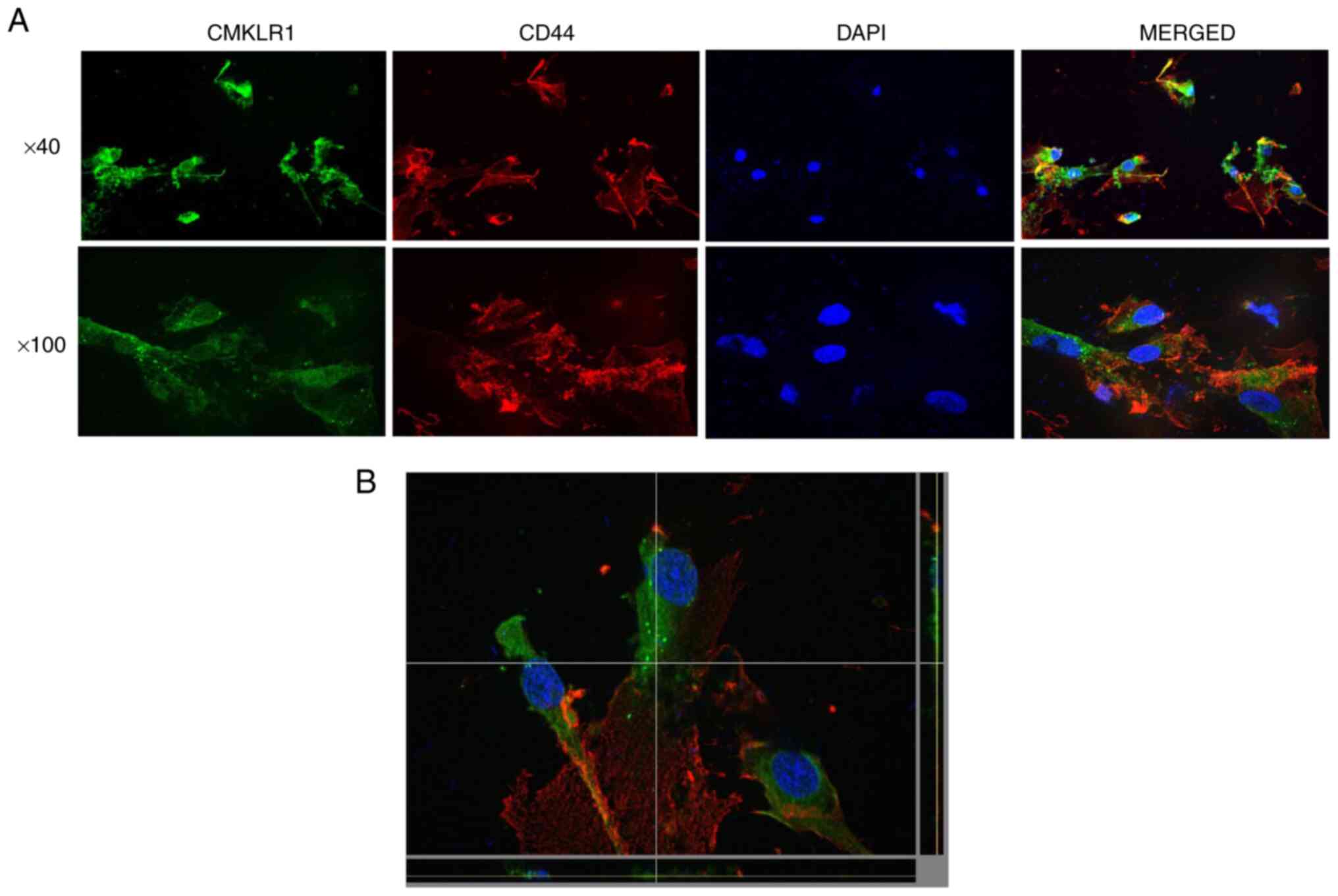
Localization of CD44 and CMKLR1 in
human dental pulp stem cells
The localization of CD44 and CMKLR1 in human dental
pulp stem cells was confirmed by immunostaining. CD44 and CMKLR1
were both detected in different cytoplasmic sites in dental pulp
stem cells but co-localization was not observed. CD44 was mainly
localized in the cell membrane, while CMKLR1 was mainly detected in
the cytoplasm (Fig. 5A and
B).
Effect of resolvinE1 on human dental
pulp stem cells
CMKLR1 regulates pulpal inflammation; therefore, the
expression of cyclooxygenase-2 (COX-2) was examined to confirm
whether stimulation with lipoteichoic acid (LTA), a major component
of the Gram-positive bacterial cell wall (32,33),
causes an inflammatory reaction in dental pulp stem cells. COX-2
expression was confirmed after stimulation with 10 to 50 µg/ml LTA
(Fig. 6A and B). Resolvin E1, a bioactive lipid that
interacts with chemerin/CMKLR1, is produced from ω-3 fatty acids
and has an inflammatory convergence effect; it suppresses the
increase in neutrophil infiltration in inflammatory exudate
(34). Notably, treatment of
dental pulp stem cells with resolvin E1 at 1 to 5 µM resulted in
the expression of dentin sialophosphoprotein (DSPP), a
differentiation marker for odontoblasts, but at 10 µM resolvin E1,
DSPP expression ceased (Fig. 6C
and D). Therefore, an optimum
concentration of resolvin E1 is required to induce the
differentiation of dental pulp stem cells into odontoblasts. Next,
it was examined how the induction of DSPP expression is affected by
the inflammatory response using low concentrations of resolvin E1
at optimum concentration. When the effect of LTA and resolvin E1 on
inflammatory stimulation was examined, COX-2 expression was induced
by LTA plus resolvin E1 but DSPP expression was not suppressed,
while CD44 expression remained unchanged (Fig. 6E and F). This indicated that resolvin E1 may be
able to induce the differentiation of dental pulp stem cells into
odontoblasts even when infected with Gram-positive bacteria.
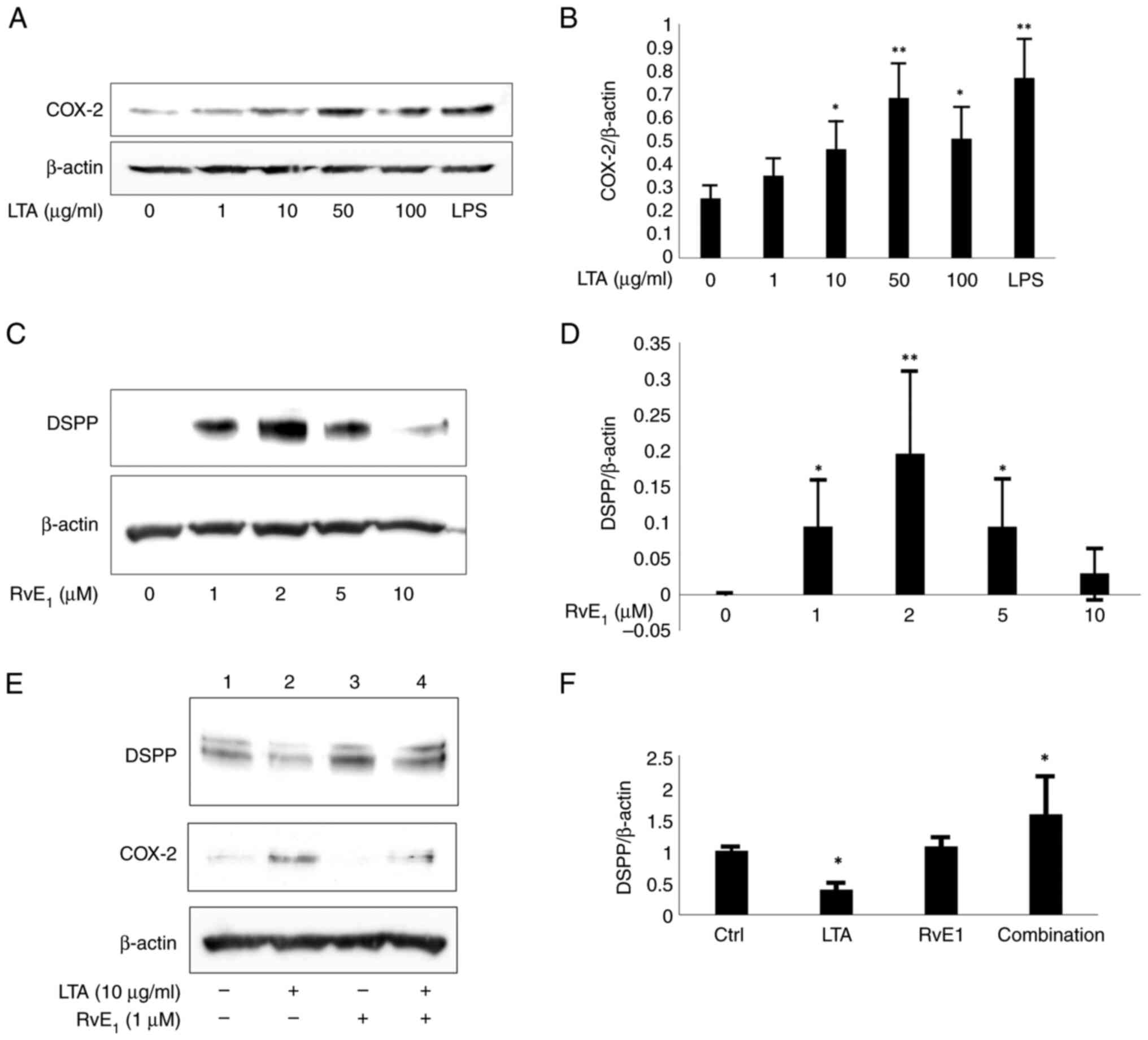 | Figure 6Ability of resolvin E1 to induce
differentiation of dental pulp stem cells into odontoblasts. COX-2
was expressed after stimulation with 10 to 50 µg/ml LTA (A) The
protein level of COX-2 in response to a negative control (lane 1)
was semi-quantified relative to the level of β-actin. (B) Values
were obtained from three independent experiments, with the COX-2
level in the negative control lane (lane 1) set to 1.
*P<0.05, **P<0.01 (lane 1 vs. lane 2,
3, 4, 5 or 6). (C) When dental pulp stem cells were treated with
1-5 µM resolvin E1, DSPP was expressed, but when treated with 10 µM
resolvin E1, DSPP expression ceased. (D) The protein level of DSPP
in response to a negative control (lane 1) was semi-quantified
relative to the level of β-actin. The negative control lane (lane
1) was set to 1. *P<0.05, **P<0.01
(lane 1 vs. lane 2, 3, 4 or 5). (E) COX-2 was expressed and DSPP
expression was not suppressed after LTA and resolvin E1 (1 µM)
treatment, while CD44 expression remained. (F) The protein level of
DSPP in response to a negative control (lane 1) was semi-quantified
relative to the level of β-actin. Values were obtained from three
independent experiments, with the DSPP level in the negative
control lane (lane 1) set to 1. *P<0.05 (lane 1 vs.
lane 2, 3 or 4). COX-2, cyclooxygenase-2; LTA, lipoteichoic acid;
DSPP, dentin sialophosphoprotein; CD44, cluster of differentiation
44. |
Discussion
Endodontic therapy in clinical dentistry is a
treatment that attempts to preserve teeth by removing infected
pulp, protecting the remaining pulp and maintaining an aseptic
environment as far as possible. Therefore, preservation and
regeneration of dental pulp are important areas of research in the
field of endodontics. The control of pulpitis is essential for
preserving pulp. The suppression of pulp inflammation and the
induction of remineralization promote the preservation and
regeneration of dental pulp. In previous research, we focused on
CD44 and found that it is an important molecule for inducing the
differentiation of dental pulp stem cells into odontoblasts. Other
papers have shown that in tooth tissue, CD44 is expressed in the
dental pulp tissue and odontoblast layer, which are in the process
of differentiation (24-26).
When considering the clinical application of research on the
induction of differentiation of dental pulp stem cells into
odontoblasts, it is possible to regenerate dentin by applying
dental pulp capping to tissues that have been clinically inflamed.
Considering this, several points are not yet clear: i) What is the
localization of CD44 in tooth tissue when there is inflammation?
ii) Are there molecules in tooth tissue that control inflammation
and are involved in inducing differentiation of dental pulp stem
cells into odontoblasts? If so, where are they located? Therefore,
the present study focused on CD44, which is thought to be involved
in inducing the differentiation of dental pulp stem cells into
odontoblasts and CMKLR1, which is involved in the regulation of
inflammation. The present study aimed to clarify their localization
in dental tissues.
The present study confirmed that CD44 and CMKLR1
were localized in odontoblasts in the pulp horn or pulp floor. CD44
and CMKLR1 were also expressed in dental pulp stem cells. CD44 was
localized in the cell membrane and CMKLR1 was detected in the
cytoplasm of dental pulp stem cells. Although it is unclear from
immunohistochemical staining alone whether CD44 and CMLR1 are
present in the cytoplasm or cell membrane of the odontoblast layer,
it was evident that CD44 and CMKLR1 were expressed in the
odontoblast layer in the present study. In pulpitis-affected teeth,
odontoblast structures were disrupted and CD44 expression was not
observed but CMKLR1 immunoreactivity remained in the odontoblastic
layer. Furthermore, the expression of COX-2 was not suppressed by
resolving E1, a bioactive lipid that acts on CMKLR1; however, in
the inflammation model of dental pulp stem cells, the
differentiation of dental pulp stem cells into odontoblasts was
still induced. This indicated that targeting CMKLR1 may be useful
for inducing the differentiation of dental pulp stem cells into
odontoblasts in pulpitis.
CD44 is a surface marker of dental pulp stem cells
and is involved in the induction of their differentiation into
odontoblasts. Our previous study revealed the following points: i)
Even when dental pulp stem cells were induced to differentiate into
odontoblasts by hyaluronic acid, a ligand for CD44, there was no
change in the expression of CD44(5); ii) shikonin (a naphthoquinone
compound) induces differentiation of dental pulp stem cells into
odontoblasts. However, knockdown of CD44 in dental pulp stem
cells does not induce their differentiation into odontoblasts
(4); and iii) in the experiment
using dental pulp stem cells, even when inflammatory stimulation
was applied with LTA, there was no significant change in the
expression of CD44 as detected by western blotting analysis.
Considering these three points, even if the expression of CD44 in
the odontoblastic layer disappears because of inflammation, it
cannot be denied that CD44 is important for odontoblastic
differentiation. In dental tissue, CD44-expressing cells are found
in the apical portion of immature roots or in the odontoblast layer
(26). CMKLR1 is involved in
suppressing inflammation (6,21)
and the action of chemerin-CMKLR1 is important for tooth
development (19,35). However, the localization of CMKLR1
in teeth has not been clarified. The present study determined the
detailed localization of CD44 and CMKLR1 in tooth tissue. CD44
expression was not observed in pulpitis, in which the pulp tissue
was degenerated and the arrangement of the odontoblast layer was
disturbed, but CMKLR1 expression was still confirmed in the
degenerated odontoblast layer. It was also observed that CMKLR1 was
expressed in dental pulp stem cells and was mainly localized in the
cytoplasm. Furthermore, it was determined that resolvin E1, which
acts on CMKLR1, did not suppress the induction of differentiation
of dental pulp stem cells into odontoblasts even in the presence of
an inflammatory stimulus.
The present study showed that resolvin E1 induced
the differentiation of dental pulp stem cells into odontoblasts.
This induction was also observed in the presence of an inflammatory
stimulus. This indicated that resolvin E1 may induce odontoblast
differentiation even in the presence of pulpitis. However, these
findings were made in cell-based experiments and it is necessary to
verify the inhibition of pulpitis and the regeneration of dentin by
resolvin E1 treatment in an animal model of pulpitis.
The present study confirmed that CD44 and CMKLR1 are
both present in odontoblasts of dental tissue. CD44 and CMKLR1 were
both observed in the plasma membrane and CMKLR1 was also detected
in the cytoplasm of dental pulp stem cells. In addition, although
resolvin E1, which acts on CMKLR1, did not suppress the expression
of COX-2, it induced the differentiation of dental pulp stem cells
into odontoblasts. It is therefore hypothesized that resolvin E1
may be useful in inducing odontoblast differentiation in inflamed
dental pulp. Although a direct association between CD44 and CMKLR1
was not determined in the present study and exploration of the
interaction between CD44 and CMKLR1 is a future issue, CD44 and
CMKLR1 may play an important role in the preservation and
regeneration of dental pulp.
Supplementary Material
Generation of a rat pulpitis model.
(A) A model of pulpitis was constructed by making holes in the
upper molars of rats under inhalation anesthesia while viewing
under a microscope. (B) After 24 h, the sample was collected and
micro-CT was used to confirm whether the cut hole had reached the
pulp. Arrowheads indicate treated teeth in the micro-CT image.
micro-CT, micro computed tomography.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by JSPS KAKENHI
(grant number: 20K09947).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DY performed the majority of the experiments and
drafted the manuscript. NU contributed to the experimental design,
performed some experiments and drafted the manuscript. YM performed
some experiments and data analyses. NK participated in the study
design and manuscript preparation and critically revised the
manuscript. SK contributed to the experimental conceptualization
and data interpretation and critically revised the manuscript. DY
and NU confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Asahi
University Research Ethics Committee (approval no. 31020) and
written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
NU ORCID ID http://orcid.org/0000-0002-3249-782X/.
References
|
1
|
Orme IM and Henao-Tamayo MI: Trying to see
the forest through the Trees: Deciphering the nature of memory
immunity to mycobacterium tuberculosis. Front Immunol.
9(461)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Murakami K, Umemura N, Adachi M, Motoki M
and Ohkoshi E: ABCG2, CD44 and SOX9 are increased with the
acquisition of drug resistance and involved in cancer stem cell
activities in head and neck squamous cell carcinoma cells. Exp Ther
Med. 24(722)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Modder UI, Roforth MM, Nicks KM, Peterson
JM, McCready LK, Monroe DG and Khosla S: Characterization of
mesenchymal progenitor cells isolated from human bone marrow by
negative selection. Bone. 50:804–810. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kajiura K, Umemura N, Ohkoshi E, Ohta T,
Kondoh N and Kawano S: Shikonin induces odontoblastic
differentiation of dental pulp stem cells via AKT-mTOR signaling in
the presence of CD44. Connect Tissue Res. 62:689–697.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Umemura N, Ohkoshi E, Tajima M, Kikuchi H,
Katayama T and Sakagami H: Hyaluronan induces odontoblastic
differentiation of dental pulp stem cells via CD44. Stem Cell Res
Ther. 7(135)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cash JL, Hart R, Russ A, Dixon JP,
Colledge WH, Doran J, Hendrick AG, Carlton MB and Greaves DR:
Synthetic chemerin-derived peptides suppress inflammation through
ChemR23. J Exp Med. 205:767–775. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Le Fournis C, Jeanneau C, Giraud T, El
Karim I, Lundy FT and About I: Fibroblasts control macrophage
differentiation during pulp inflammation. J Endod. 47:1427–1434.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alvarez-Vasquez JL and Castaneda-Alvarado
CP: Dental pulp fibroblast: A star cell. J Endod. 48:1005–1019.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang J, Qiao J, Ma L, Li X, Wei C, Tian X
and Liu K: Identification of the characteristics of infiltrating
immune cells in pulpitis and its potential molecular regulation
mechanism by bioinformatics method. BMC Oral Health.
23(287)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jungbluth H, Kaiser MLB, Lalaouni D,
Winter J and Jepsen S: Immunohistochemical analysis of s100
proteins in normal and irreversibly inflamed human dental pulps. J
Endod. 49:504–513. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Prati C and Gandolfi MG: Calcium silicate
bioactive cements: Biological perspectives and clinical
applications. Dent Mater. 31:351–370. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bucchi C, Bucchi A and Martinez-Rodriguez
P: Biological properties of dental pulp stem cells isolated from
inflamed and healthy pulp and cultured in an inflammatory
microenvironment. J Endod. 49:395–401 e6. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vaseenon S, Srisuwan T, Chattipakorn N and
Chattipakorn SC: Lipopolysaccharides and hydrogen peroxide induce
contrasting pathological conditions in dental pulpal cells. Int
Endod J. 56:179–192. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xie Z, Shen Z, Zhan P, Yang J, Huang Q,
Huang S, Chen L and Lin Z: Functional dental pulp regeneration:
Basic research and clinical translation. Int J Mol Sci.
22(8991)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Le Fournis C, Jeanneau C, Roumani S,
Giraud T and About I: Pulp fibroblast contribution to the local
control of pulp inflammation via complement activation. J Endod. 46
(9S):S26–S32. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Giraud T, Jeanneau C, Rombouts C, Bakhtiar
H, Laurent P and About I: Pulp capping materials modulate the
balance between inflammation and regeneration. Dent Mater.
35:24–35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Esposito P, Varvara G, Caputi S and
Perinetti G: Catalase activity in human healthy and inflamed dental
pulps. Int Endod J. 36:599–603. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Caplan DJ, Cai J, Yin G and White BA: Root
canal filled versus non-root canal filled teeth: A retrospective
comparison of survival times. J Public Health Dent. 65:90–96.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ohira T, Spear D, Azimi N, Andreeva V and
Yelick PC: Chemerin-ChemR23 signaling in tooth development. J Dent
Res. 91:1147–1153. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hasturk H, Kantarci A, Ohira T, Arita M,
Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN and Van Dyke
TE: RvE1 protects from local inflammation and osteoclast-mediated
bone destruction in periodontitis. FASEB J. 20:401–403.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cash JL, Christian AR and Greaves DR:
Chemerin peptides promote phagocytosis in a ChemR23- and
Syk-dependent manner. J Immunol. 184:5315–5324. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park BW, Kang EJ, Byun JH, Son MG, Kim HJ,
Hah YS, Kim TH, Mohana Kumar B, Ock SA and Rho GJ: In vitro and in
vivo osteogenesis of human mesenchymal stem cells derived from
skin, bone marrow and dental follicle tissues. Differentiation.
83:249–259. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Felszeghy S, Módis L, Tammi M and Tammi R:
The distribution pattern of the hyaluronan receptor CD44 during
human tooth development. Arch Oral Biol. 46:939–945.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Leonardi R, Loreto C, Caltabiano R and
Caltabiano C: Immunolocalization of CD44s in human teeth. Acta
Histochem. 108:425–429. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen KL, Huang YY, Lung J, Yeh YY and Yuan
K: CD44 is involved in mineralization of dental pulp cells. J
Endod. 39:351–356. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li M, Tian J, Xu Z, Zeng Q, Chen W, Lei S
and Wei X: Histology-based profile of inflammatory mediators in
experimentally induced pulpitis in a rat model: Screening for
possible biomarkers. Int Endod J. 54:1328–1341. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Erdogan O, Xia J, Chiu IM and Gibbs JL:
Dynamics of innate immune response in bacteria-induced mouse model
of pulpitis. J Endod: Sep 9, 2023 (Epub ahead of print).
|
|
29
|
Sato F, Wajima D, Takeshima Y, Nakagawa I,
Kim T, Motoyama Y, Park YS and Nakase H: Neuroprotective effects of
pravastatin in cerebral venous infarction in a rat model. IBRO
Neurosci Rep. 14:202–209. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bay V, Iversen NK, Shiadeh SMJ, Tasker RA,
Wegener G and Ardalan M: Tissue processing and optimal
visualization of cerebral infarcts following sub-acute focal
ischemia in rats. J Chem Neuroanat. 118(102034)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tsai TH, Lin SH, Wu CH, Tsai YC, Yang SF
and Lin CL: Mechanisms and therapeutic implications of RTA 408, an
activator of Nrf2, in subarachnoid hemorrhage-induced delayed
cerebral vasospasm and secondary brain injury. PLoS One.
15(e0240122)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ahn KB, Jeon JH, Baik JE, Park OJ, Kang
SS, Yun CH, Park JH and Han SH: Muramyl dipeptide potentiates
staphylococcal lipoteichoic acid induction of cyclooxygenase-2
expression in macrophages. Microbes Infect. 16:153–160.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tominari T, Sanada A, Ichimaru R,
Matsumoto C, Hirata M, Itoh Y, Numabe Y, Miyaura C and Inada M:
Gram-positive bacteria cell wall-derived lipoteichoic acid induces
inflammatory alveolar bone loss through prostaglandin E production
in osteoblasts. Sci Rep. 11(13353)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chiang N and Serhan CN: Specialized
pro-resolving mediator network: An update on production and
actions. Essays Biochem. 64:443–462. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu H, Chen J, Ge J, Xia K, Tao S, Su Y and
Zhang Q: Resolvin E1 ameliorates pulpitis by suppressing dental
pulp fibroblast activation in a chemerin receptor 23-dependent
manner. J Endod. 45:1126–1134 e1. 2019.PubMed/NCBI View Article : Google Scholar
|