Introduction
Over 700 million individuals have been affected by
the coronavirus disease 2019 (COVID-19), which has already resulted
in over 6.5 million deaths (1).
The majority of COVID-19 patients have no or minor symptoms, while
~20% of them experience significant symptoms that necessitate
hospitalization (2). The most
frequent effects of COVID-19 include abnormalities of the
respiratory system, although other organs may also be affected
(3-7).
The host's features, viral dynamics during acute infection and the
host immune response have all been reported to correlate with
disease severity (8,9). A severe COVID-19 course and greater
mortality have been linked to older age, a high body mass index and
a variety of comorbidities, including cardiovascular illnesses,
diabetes, or types of cancer (10-13).
‘Long-COVID’ refers to some of the recovered
patients who have experienced persistent impairments (14-16).
In a retrospective analysis involving 193,113 participants, it was
discovered that there is an elevated risk for several clinical
outcomes, such as respiratory impairment, after COVID-19 disease
(17). Another study found that
>20% of COVID-19 survivors had pulmonary function impairment
after 6 months. In addition, it has been reported that there had
been no significant change in lung function impairment after 2
years in one of the largest research projects, with 349 and 230
subjects who had pulmonary function testing, respectively, 6 months
and 2 years after discharge (18,19).
In the present study, compared with their counterparts who were
matched for age, sex and chronic pulmonary disease after two years,
survivors with a critical illness had a greater risk of diffusion
capacity for carbon monoxide (DLCO) impairment, lower residual
volume (RV) and lower total lung capacity (TLC) (18).
The most prevalent manifestation, DLCO, has been
related to critical courses during acute illness in a number of
studies (20-22).
The variability of study designs and settings across studies,
however, makes it challenging to reach definitive conclusions.
Moreover, most of the studies refer to the first waves of the
pandemic (23,24).
Several studies have reported a significantly
reduced risk of hospitalization, admission to the intensive care
unit, requirement for oxygenation and mortality rate in
omicron-infected patients compared with those infected with other
variants (25-27).
In addition, it has been reported that there is a
reduction in the risk of developing the long COVID-19 syndrome with
the omicron variant compared with the delta variant (28). However, knowledge about lung
function after hospitalization during the omicron variant
predominance period is still scarce. The purpose of the present
study was to describe the pulmonary function at three months after
hospitalization due to COVID-19 pneumonia and to make a comparison
of alpha, delta and omicron variant predominance periods.
Materials and methods
Study population and data
collection
This retrospective study included consecutive,
ambulatory patients who visited the post-COVID-19 Outpatient Clinic
of Laiko General Hospital (Athens, Greece) for respiratory function
evaluation 3 months after their hospitalization for COVID-19
pneumonia. The period of their hospitalization was between February
15, 2021 and May 15, 2022, covering all the periods of predominance
of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Inclusion criteria were: i) The ability to perform pulmonary
function tests (PFTs) satisfactorily; and ii) a stable clinical and
physical condition (vital signs, such as their heart rate, blood
pressure and body temperature, were steady and within normal
limits. Also, the patients were conscious (aware) and comfortable
for at least 4 weeks before evaluation. Exclusion criteria were: i)
History of congestive heart failure; ii) primary lung disease such
as asthma, chronic obstructive pulmonary disease (COPD), or lung
fibrosis; iii) neuromuscular diseases; iv) collagen vascular
diseases; and v) occupational exposure that could probably affect
lung function. In addition, none of the patients had reported a
lung infection 2 weeks before the evaluation.
The patients were subjected to the recording of
their age, sex, medical history, smoking history, height, weight
and body mass index (BMI). The patients were classified into four
groups according to the levels of oxygen required during
hospitalization: Group A, no oxygen requirement; group B, delivery
of oxygen ≤60%; group C, need for delivery of oxygen 60-100% or
high flow nasal cannula or non-invasive mechanical ventilation;
group D, need for mechanical ventilation.
The retrospective design of the present study could
lead to bias from missing data and this was addressed by obtaining
only complete data.
The Institutional Review Board of Laiko General
Hospital in Athens, Greece, approved the present study (protocol
number 765/12-2021). The present study was in accordance with the
Declaration of Helsinki of 1995 (as revised in Edinburgh, 2000).
All subjects gave written informed consent for enrollment in the
present study.
Assessment of lung function
PFTs included spirometry, body plethysmography and
the measurement of DLCO. The Powercube Body+, a new generation body
box (GANSHORN Medizin Electronic GmbH), was used to perform PFTs.
For spirometry, maximal expiratory flow volume estimation was
conducted while participants were seated and wearing nose clips. An
automated spirometer connected to the body box was used for the
measurement of forced expiratory volume in 1 sec (FEV1) and the
measurement of forced vital capacity (FVC). Up to three trials were
performed and the average of two technically acceptable tests was
recorded. The predicted values were those of the European
Respiratory Society (29).
DLCO is the volume of CO that diffuses across the
alveolo-capillary membrane in one unit of time (1 min) with a
certain pressure gradient (1 mmHg) (30). The DLCO was measured with the
single breath holding technique using CH4 and CO as
tracer gases. Corrections were made for the arterial hemoglobin
concentration. Up to four trials were performed and the average of
two technically acceptable trials was recorded. The predicted
values were those of the European Respiratory Society (31).
TLC is the volume of air in the lungs upon the
maximum effort of inspiration (30). The measurement of TLC was performed
using the body plethysmography technique. Breathing at rest and the
shutter maneuver, which uses transient occlusion to purposefully
block the airflow, come first in the estimation of lung function by
body plethysmography. After the opening of the shutter, an
expiratory reserve volume (ERV) effort and an inspiratory vital
capacity (IVC) effort were performed, allowing the computation of
TLC. During measurement, the box is closed with an airtight seal,
with the exception of a small, controlled used for the
stabilization of the internal pressure. One pressure transducer
measures the pressure inside the body box relative to ambient
pressure, while another is placed close to the mouth for monitoring
mouth pressure during the shutter maneuver. Up to three trials were
performed and the average (mean) of two technically acceptable
measurements was recorded. The predicted values were those of the
European Respiratory Society (29). Abnormal values of FEV1, FVC, DLCO
and TLC were considered values <80% of the predicted (29). A summary of the present study
scheme is illustrated in Fig.
1.
Statistical analysis
The assessment of the normal distribution of
variables was performed with the Kolmogorov-Smirnov test. The mean
± standard deviation (normally distributed) is used to present
continuous variables. Categorical variables are displayed as
frequencies or percentages. Comparisons of variables between groups
were performed using the unpaired t-test or one-way ANOVA for
continuous variables and the Chi-squared test or Fischer's exact
test for categorical variables. The Bonferroni test for multiple
comparisons was also used after one-way ANOVA for continuous
variables. Statistical analysis was conducted using IBM
SPSS-Statistics version 29.0 (IBM Corp.). P<0.05 was considered
to indicate a statistically significant difference.
Results
Baseline characteristics
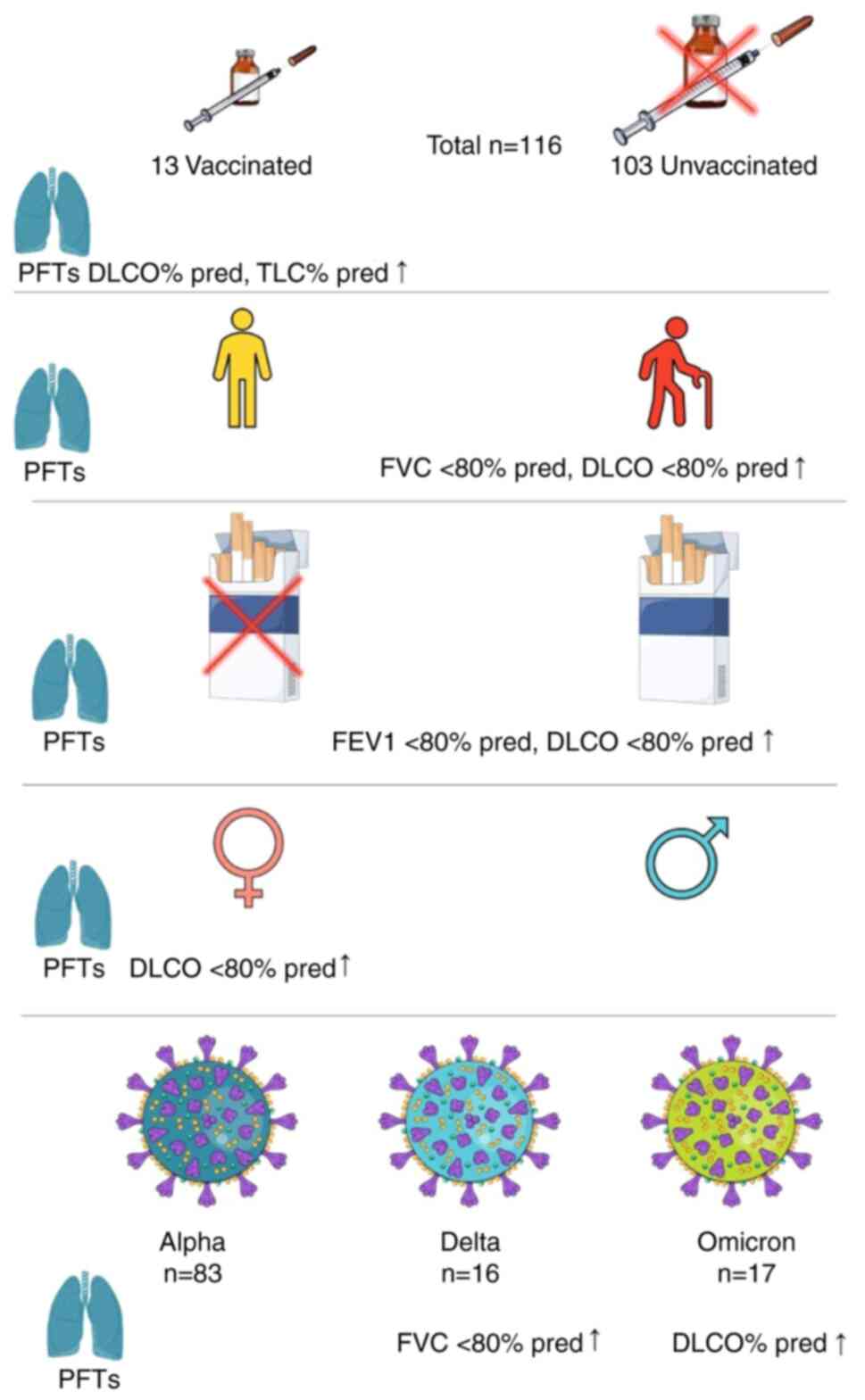
A total of 116 (82 male and 34 female) patients with
a mean age of 57.77±11.45 years (range 19-84) were finally included
in the present study. A total of 83 (71.6%) patients were
hospitalized in the period of alpha variant predominance, 16
(13.8%) in the period of delta variant predominance and 17 (14.6%)
in the omicron variant predominance period. Of note, the majority
of the patients were unvaccinated (103 patients, 88.8%). Abnormal
values of FEV1% predicted (pred) were observed in 9 (7.8%)
patients, abnormal values of FVC% pred were observed in 8 (6.9%)
patients, abnormal values of DLCO% pred were observed in 33
patients (28.4%) and abnormal values of TLC% pred were observed in
24 (20.7%) patients. Baseline characteristics of the present study
populations are displayed in Table
I.
 | Table ICharacteristics of the study
population. |
Table I
Characteristics of the study
population.
| Variable | Frequency | % |
|---|
| Variant | | |
|
Omicron | 17 | 14.6 |
|
Alpha | 83 | 71.6 |
|
Delta | 16 | 13.8 |
| Sex | | |
|
Male | 82 | 70.7 |
|
Female | 34 | 29.3 |
| Vaccination with at
least two doses | | |
|
No | 103 | 88.8 |
|
Yes | 13 | 11.2 |
| FEV1 <80%
pred | | |
|
No | 107 | 92.2 |
|
Yes | 9 | 7.8 |
| FVC <80%
pred | | |
|
No | 108 | 93.1 |
|
Yes | 8 | 6.9 |
| DLCO <80%
pred | | |
|
No | 83 | 71.6 |
|
Yes | 33 | 28.4 |
| TLC <80%
pred | | |
|
No | 92 | 79.3 |
|
Yes | 24 | 20.7 |
| Need for oxygen
during hospitalization | | |
|
Group A | 8 | 6.9 |
|
Group B | 70 | 60.4 |
|
Group C | 33 | 28.4 |
|
Group D | 5 | 4.3 |
| Smoking status | | |
|
No | 59 | 50.9 |
|
Yes | 9 | 7.8 |
|
Ex | 48 | 41.3 |
| | Mean | Standard
deviation |
| Age (years) | 57.77 | 11.45 |
| BMI
(k/m2) | 29.01 | 5.76 |
Characteristics of the study
population in relation to the variant
In the cohort of the present study, there was no
statistically significant difference in age, BMI, smoking status,
sex, or need for oxygen during hospitalization among the patients
infected with SARS-CoV-2 in different variant periods. However,
there was a statistically significant difference in vaccination
status, with the largest percentage of unvaccinated patients
observed during the period of alpha variant predominance (P=0.001)
(Table II).
 | Table IICharacteristics of the study
population in relation to the variant. |
Table II
Characteristics of the study
population in relation to the variant.
| | | Variable | | |
|---|
| | | Age (years) | | P-value |
|---|
| Variant | | Mean | Standard
deviation | | 0.421 |
|---|
|
Omicron | | 60.94 | 10.44 | | |
|
Alpha | | 56.99 | 11.11 | | |
|
Delta | | 58.44 | 14.09 | | |
| | | BMI
(k/m2) | | |
| Variant | | Mean | Standard
deviation | | 0.123 |
|
Omicron | | 26.97 | 4.61 | | |
|
Alpha | | 29.69 | 6.06 | | |
|
Delta | | 27.64 | 4.65 | | |
| | | Sex (no. of
patients) | | |
| Variant | | Males | Females | | 0.267 |
|
Omicron | | 11 | 6 | | |
|
Alpha | | 57 | 26 | | |
|
Delta | | 14 | 2 | | |
| | Smoking status (no.
of patients) | | |
| Variant | No | Yes | Ex | | 0.221 |
|
Omicron | 8 | 1 | 8 | | |
|
Alpha | 41 | 5 | 37 | | |
|
Delta | 10 | 3 | 3 | | |
| | | Vaccination status
(no. of patients) | | |
| Variant | | No | Yes | | 0.001 |
|
Omicron | | 7 | 10 | | |
|
Alpha | | 82 | 1 | | |
|
Delta | | 14 | 2 | | |
| | Need for oxygen
during hospitalization (no. of patients) | |
| Variant | Group A | Group B | Group C | Group D | 0.543 |
|
Omicron | 2 | 11 | 4 | 0 | |
|
Alpha | 5 | 49 | 26 | 3 | |
|
Delta | 1 | 10 | 3 | 2 | |
PFTs results in the different
categories of variants, smoking status, vaccination status, sex and
need for oxygen during hospitalization
In addition, the mean values of FEV1% pred, FVC%
pred, DLCO% pred and TLC% pred were compared in the different
categories of variants, smoking status, vaccination status, sex and
need for oxygen during hospitalization. A statistically significant
difference was observed in the mean value of DLCO% pred among the
patients infected with different variants, with higher values of
DLCO% pred observed in patients infected during the omicron variant
predominance period (P=0.028). In addition, there was a
statistically significant difference in the mean values of FVC%
pred, DLCO% pred and TLC% pred among the patients with different
needs for oxygen during their hospitalization, with higher values
observed in patients of groups A and B (P=0.001, P=0.007 and
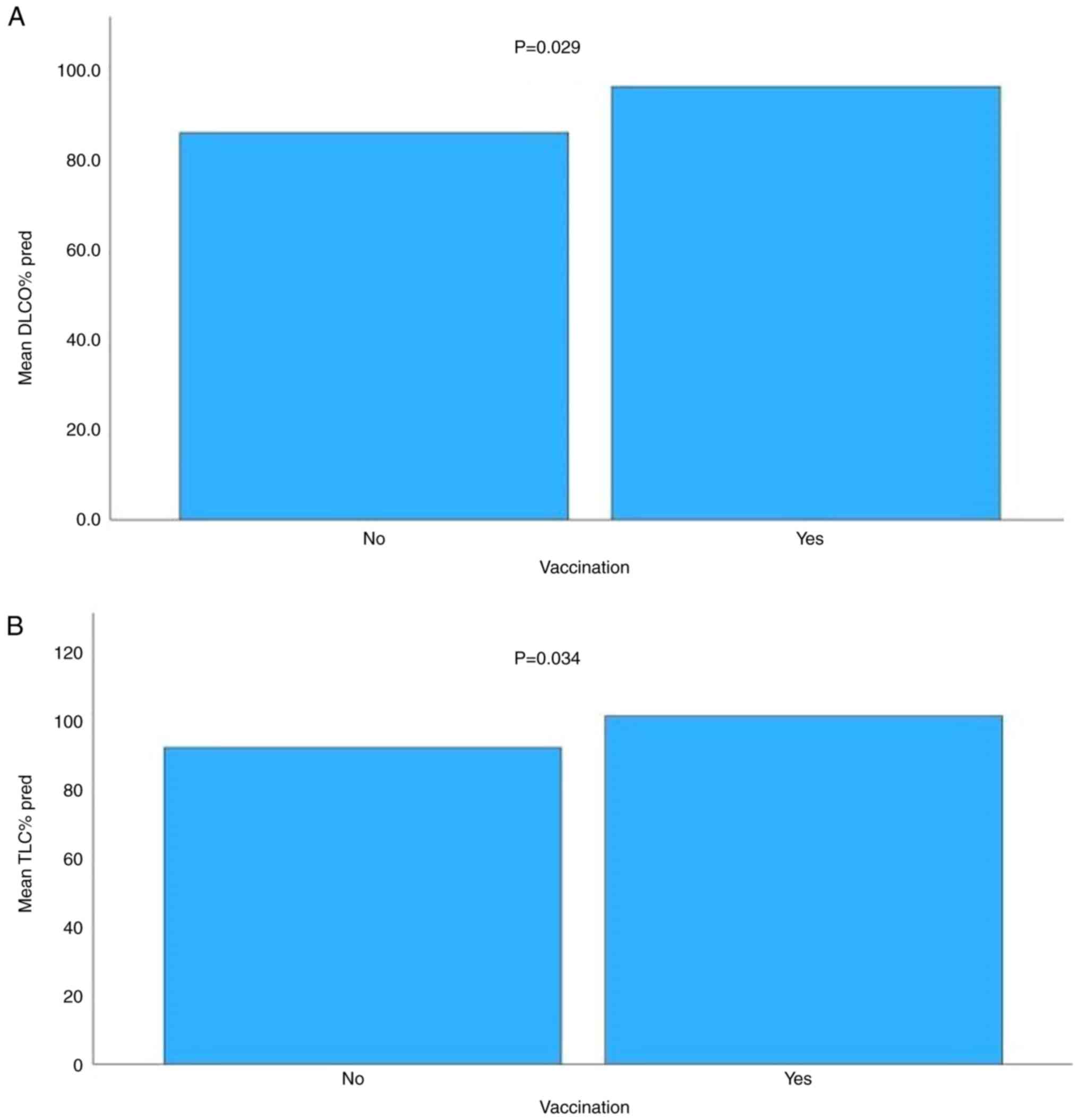
P=0.001, respectively). Moreover, there was a statistically
significant difference in the mean values of DLCO% pred and TLC%
pred between vaccinated and unvaccinated patients, with greater
values observed among vaccinated patients (P=0.029 and P=0.034,
respectively; Table III;
Fig. 2).
 | Table IIIMean values of FEV1% pred, FVC% pred,
DLCO% pred and TLC% pred in the different categories of variants,
smoking status, vaccination status, sex and need for oxygen during
hospitalization. |
Table III
Mean values of FEV1% pred, FVC% pred,
DLCO% pred and TLC% pred in the different categories of variants,
smoking status, vaccination status, sex and need for oxygen during
hospitalization.
| | Variable | |
|---|
| | FEV1% pred | P-value |
|---|
| Variant | Mean | SD | 0.354 |
|---|
|
Omicron | 103.06 | 20.52 | |
|
Alpha | 105.84 | 15.51 | |
|
Delta | 99.44 | 19.26 | |
| | FVC% pred | |
| Variant | Mean | SD | 0.202 |
|
Omicron | 104.12 | 20.19 | |
|
Alpha | 105.63 | 16.16 | |
|
Delta | 97.06 | 20.50 | |
| | DLCO% pred | |
| Variant | Mean | SD | 0.028 |
|
Omicron | 97.11 | 21.08 | |
|
Alpha | 85.31 | 15.35 | |
|
Delta | 82.02 | 25.08 | |
| | TLC% pred | |
| Variant | Mean | SD | 0.226 |
|
Omicron | 98.87 | 15.44 | |
|
Alpha | 92.38 | 14.94 | |
|
Delta | 89.33 | 19.67 | |
| | FEV1% pred | |
| Smoking status | Mean | SD | 0.125 |
|
No | 106.64 | 17.75 | |
|
Yes | 94.44 | 18.09 | |
|
Ex | 103.96 | 15.20 | |
| | FVC% pred | |
| Smoking status | Mean | SD | 0.234 |
|
No | 105.40 | 18.89 | |
|
Yes | 94.67 | 18.40 | |
|
Ex | 104.40 | 15.42 | |
| | DLCO% pred | |
| Smoking status | Mean | SD | 0.314 |
|
No | 87.25 | 19.91 | |
|
Yes | 77.55 | 14.67 | |
|
Ex | 87.23 | 16.63 | |
| | TLC% pred | |
| Smoking status | Mean | SD | 0.158 |
|
No | 94.45 | 17.92 | |
|
Yes | 82.29 | 16.20 | |
|
Ex | 92.49 | 12.38 | |
| | FEV1% pred | |
| Need for oxygen
during hospitalization | Mean | SD | 0.116 |
|
Group A | 109.00 | 19.50 | |
|
Group B | 106.67 | 16.00 | |
|
Group C | 100.82 | 17.29 | |
|
Group D | 92.40 | 16.24 | |
| | FVC% pred | |
| Need for oxygen
during hospitalization | Mean | SD | 0.001 |
|
Group A | 108.50 | 20.20 | |
|
Group B | 108.80 | 15.52 | |
|
Group C | 96.21 | 17.10 | |
|
Group D | 87.00 | 17.21 | |
| | DLCO% pred | |
| Need for oxygen
during hospitalization | Mean | SD | 0.007 |
|
Group A | 86.87 | 18.37 | |
|
Group B | 90.49 | 14.91 | |
|
Group C | 81.18 | 19.05 | |
|
Group D | 67.20 | 35.35 | |
| | TLC% pred | |
| Need for oxygen
during hospitalization | Mean | SD | 0.001 |
|
Group A | 95.50 | 17.13 | |
|
Group B | 98.32 | 12.80 | |
|
Group C | 83.94 | 14.86 | |
|
Group D | 70.00 | 17.26 | |
| | FEV1% pred | |
| Vaccination
status | Mean | SD | 0.947 |
|
No | 104.51 | 15.94 | |
|
Yes | 104.85 | 23.66 | |
| | FVC% pred | |
| Vaccination
status | Mean | SD | 0.638 |
|
No | 103.94 | 16.66 | |
|
Yes | 106.38 | 23.92 | |
| | DLCO% pred | |
| Vaccination
status | Mean | SD | 0.029 |
|
No | 85.45 | 16.99 | |
|
Yes | 95.61 | 25.13 | |
| | TLC% pred | |
| Vaccination
status | Mean | SD | 0.034 |
|
No | 91.93 | 15.24 | |
|
Yes | 101.09 | 18.84 | |
| | FEV1% pred | |
| Sex | Mean | SD | 0.340 |
|
Male | 103.59 | 17.35 | |
|
Female | 106.88 | 15.56 | |
| | FVC% pred | |
| Sex | Mean | SD | 0.057 |
|
Male | 102.12 | 17.70 | |
|
Female | 109.21 | 16.25 | |
| | DLCO% pred | |
| Sex | Mean | SD | 0.052 |
|
Male | 89.06 | 18.22 | |
|
Female | 80.61 | 17.08 | |
| | TLC% pred | |
| Sex | Mean | SD | 0.418 |
|
Male | 92.08 | 16.28 | |
|
Female | 94.81 | 14.55 | |
Regarding the abnormal values of FEV1% pred, FVC%
pred, DLCO% pred and TLC% pred, higher rates of impaired values of
FEV1% pred were observed among the active smokers compared with
ex-smokers and to non-smokers (P=0.011), among older patients
compared with younger ones (P=0.032) and among patients with higher
needs for oxygen during hospitalization compared with those with
lower needs (P=0.038). Higher rates of impaired values of FVC% pred
were also observed among the patients infected during the delta
variant predominance period compared with patients infected during
the alpha and delta variant predominance periods (P=0.008), among
older patients compared with younger ones (P=0.019) and among
patients with higher needs for oxygen during hospitalization
compared with those with lower needs (P=0.012). There were also
higher rates of abnormal values of DLCO% pred among females
compared with males (P=0.004) and among older patients compared
with younger ones (P=0.006). Finally, there were higher rates of
impaired values of TLC% pred among the active smokers compared with
ex-smokers and to non-smokers (P=0.029) and among patients with
higher needs for oxygen during hospitalization compared with those
with lower needs (P=0.001; Table
IV).
 | Table IVAbnormal values of FEV1% pred, FVC%
pred, DLCO% pred and TLC% pred in the different categories of
variants, smoking status, sex, vaccination status and need for
oxygen during hospitalization. |
Table IV
Abnormal values of FEV1% pred, FVC%
pred, DLCO% pred and TLC% pred in the different categories of
variants, smoking status, sex, vaccination status and need for
oxygen during hospitalization.
| | Parameter | |
|---|
| Variable | Alpha | Delta | Omicron | P-value |
|---|
| FEV1 <80%
pred | | | | |
|
No | 79 | 13 | 15 | 0.130 |
|
Yes | 4 | 3 | 2 | |
| FVC <80%
pred | | | | |
|
No | 80 | 12 | 16 | 0.008 |
|
Yes | 3 | 4 | 1 | |
| DLCO <80%
pred | | | | |
|
No | 61 | 9 | 13 | 0.333 |
|
Yes | 22 | 7 | 4 | |
| TLC <80%
pred | | | | |
|
No | 66 | 10 | 15 | 0.163 |
|
Yes | 16 | 6 | 2 | |
| | Smoking status | |
| | No | Yes | Ex | |
| FEV1 <80%
pred | | | | |
|
No | 54 | 6 | 46 | 0.011 |
|
Yes | 4 | 3 | 2 | |
| FVC <80%
pred | | | | |
|
No | 54 | 7 | 46 | 0.148 |
|
Yes | 4 | 2 | 2 | |
| DLCO <80%
pred | | | | |
|
No | 44 | 4 | 34 | 0.152 |
|
Yes | 14 | 5 | 14 | |
| TLC <80%
pred | | | | |
|
No | 48 | 4 | 38 | 0.029 |
|
Yes | 10 | 5 | 9 | |
| | Sex | |
| | Male | Female | |
| FEV1 <80%
pred | | | |
|
No | 74 | 33 | 0.212 |
|
Yes | 8 | 1 | |
| FVC <80%
pred | | | |
|
No | 75 | 33 | 0.279 |
|
Yes | 7 | 1 | |
| DLCO <80%
pred | | | |
|
No | 65 | 18 | 0.004 |
|
Yes | 17 | 16 | |
| TLC <80%
pred | | | |
|
No | 63 | 28 | 0.390 |
|
Yes | 18 | 6 | |
| | Vaccination
status | |
| | No | Yes | |
| FEV1 <80%
pred | | | |
|
No | 96 | 11 | 0.275 |
|
Yes | 7 | 2 | |
| FVC <80%
pred | | | |
|
No | 97 | 11 | 0.200 |
|
Yes | 6 | 2 | |
| DLCO <80%
pred | | | |
|
No | 74 | 9 | 0.844 |
|
Yes | 29 | 4 | |
| TLC <80%
pred | | | |
|
No | 81 | 10 | 0.835 |
|
Yes | 21 | 3 | |
| | Age (years) | |
| | Mean | SD | |
| FEV1 <80%
pred | | | |
|
No | 57.20 | 11.53 | 0.032 |
|
Yes | 64.56 | 8.11 | |
| FVC <80%
pred | | | |
|
No | 57.09 | 11.49 | 0.019 |
|
Yes | 66.88 | 5.81 | |
| DLCO <80%
pred | | | |
|
No | 55.94 | 11.18 | 0.006 |
|
Yes | 62.36 | 10.95 | |
| TLC <80%
pred | | | |
|
No | 57.19 | 11.44 | 0.534 |
|
Yes | 58.79 | 10.29 | |
| | Need for oxygen
during hospitalization | |
| | Group A | Group B | Group C | Group D | |
| FEV1 <80%
pred | | | | | |
|
No | 8 | 66 | 30 | 3 | 0.038 |
|
Yes | 0 | 4 | 3 | 2 | |
| FVC <80%
pred | | | | | |
|
No | 7 | 68 | 30 | 3 | 0.012 |
|
Yes | 1 | 2 | 3 | 2 | |
| DLCO <80%
pred | | | | | |
|
No | 6 | 55 | 20 | 2 | 0.106 |
|
Yes | 2 | 15 | 13 | 3 | |
| TLC <80%
pred | | | | | |
|
No | 6 | 65 | 18 | 2 | 0.001 |
|
Yes | 2 | 5 | 14 | 3 | |
Multivariate logistic regression
analysis for abnormal values of FEV1% pred, FVC% pred, DLCO% pred
and TLC% pred
According to binary logistic regression analysis,
active smoking is an independent predictor of abnormal values of
FEV1% pred and TLC% pred (P=0.038, OR: 8.574, CI 1.124-65.424 and
P=0.004, OR: 14.733, CI 2.323-93.429, respectively), age is an
independent predictor of impaired values of FVC% pred and DLCO%
pred (P=0.027, OR: 1.124, CI 1.014-1.246 and P=0.011, OR: 1.054, CI
1.012-1.098, respectively) and female sex is an independent
predictor of impaired values of DLCO% pred (P=0.009, OR: 1.124, CI
1.014-1.246; Table V). Fig. 3 summarizes the main findings of the
present study.
 | Table VMultivariate logistic regression
analysis for abnormal values of FEV1% pred, FVC% pred, DLCO% pred
and TLC% pred. |
Table V
Multivariate logistic regression
analysis for abnormal values of FEV1% pred, FVC% pred, DLCO% pred
and TLC% pred.
| Logistic regression
analysis for FEV1 <80% pred |
|---|
| | 95% CI for OR |
|---|
| Variable | P-value | OR | Lower | Upper |
|---|
| Group A | Ref | Ref | Ref | Ref |
| Group B | 0.999 | 42.479 | 0.000 | NC |
| Group C | 0.999 | 77.603 | 0.000 | NC |
| Group D | 0.999 | 45.972 | 0.000 | NC |
| Non-smoker | Ref | Ref | Ref | Ref |
| Active smoker | 0.038 | 8.574 | 1.124 | 65.424 |
| Ex-smoker | 0.259 | 0.339 | 0.052 | 2.221 |
| Age per year | 0.060 | 1.086 | 0.997 | 1.182 |
| Logistic regression
analysis for FVC <80% pred |
| | 95% CI for OR |
| Variable | P-value | OR | Lower | Upper |
| Group A | Ref | Ref | Ref | Ref |
| Group B | 0.077 | 0.050 | 0.002 | 1.386 |
| Group C | 0.360 | 0.230 | 0.010 | 5.341 |
| Group D | 0.849 | 1.394 | 0.046 | 42.213 |
| Age per year | 0.027 | 1.124 | 1.014 | 1.246 |
| Omicron
variant | Ref | Ref | Ref | Ref |
| Alpha variant | 0.573 | 0.478 | 0.037 | 6.237 |
| Delta variant | 0.247 | 4.574 | 0.350 | 59.849 |
| Logistic regression
analysis for DLCO <80% pred |
| | 95% CI for OR |
| Variable | P-value | OR | Lower | Upper |
| Age per year | 0.011 | 1.054 | 1.012 | 1.098 |
| Female sex | 0.009 | 3.291 | 1.352 | 8.009 |
| Logistic regression
analysis for TLC <80% pred |
| | 95% CI for OR |
| Variable | P-value | OR | Lower | Upper |
| Group A | Ref | Ref | Ref | Ref |
| Group B | 0.138 | 0.205 | 0.025 | 1.665 |
| Group C | 0.231 | 3.355 | 0.463 | 24.302 |
| Group D | 0.227 | 5.121 | 0.362 | 72.442 |
| Non-smoker | Ref | Ref | Ref | Ref |
| Active smoker | 0.004 | 14.733 | 2.323 | 93.429 |
| Ex-smoker | 0.859 | 0.901 | 0.286 | 2.839 |
Discussion
In the cohort of the present study, abnormal values
of FVC% pred was observed in 8 (6.9%) patients of DLCO% pred in 33
patients (28.4%) and of TLC% pred in 24 (20.7%) patients. These
findings agree with reports from the existing literature (23,32,33).
The role of age in the development of long COVID-19
syndrome has been contentious, with some researchers finding age to
be a remarkable predictor of the syndrome and other studies finding
decreased risk with age or no relation (34). Age has been described as an
independent factor associated with impaired lung function after
hospitalization due to COVID-19 pneumonia in other cohorts from the
first pandemic waves (35). Age
was an independent factor predicting abnormal lung function in the
cohort of the present study, which consisted of patients from
different periods of the pandemic.
The intricate process of lung aging arises from the
cumulative alteration of lung cellular systems caused by damage and
restoration. The incapacity of lung cells to maintain baseline
homeostasis is a result of age-related alterations in intrinsic
processes that support cell regeneration and repair, such as
telomere shortening, increased oxidative stress, mitochondrial
dysfunction and depletion of adult stem cell reserves. A number of
anatomical and functional changes in the respiratory tract are
linked to normal lung aging. Several of these changes worsen lung
function, modify pulmonary remodeling, reduce regeneration and
increase vulnerability to pulmonary illness. The lung has developed
a variety of innate and adaptive defense mechanisms to maintain
homeostasis and react to external stimuli. Oxygen free radicals and
proinflammatory cytokines are produced in greater amounts during
immunosenescence (36,37). Innate and adaptive immune responses
in the lung have been linked to aging-related alterations in
prognosis and recovery in pulmonary inflammatory disorders
(38).
According to several studies, females face a greater
risk of suffering from long term symptoms following SARS-CoV-2
infection (39-41).
Autoimmune-related illnesses have been discovered to affect female
patients much more frequently than males and autoimmunity has been
proposed as a possible mechanism of long COVID-19 syndrome
(42). According to some
scientists, higher Toll-like receptor 7 (TLR7) expression results
in higher IFN signaling in acute COVID-19 and improved viral
clearance in females, but continued IFN signaling may result in
excessive immune activation and persistent inflammation,
predisposing females to a higher risk of autoimmunity and long-term
consequences (43,44). The present study also found that
female sex was an independent factor associated with impaired
values of DLCO% pred, which indicate lung epithelial damage, or
interstitial or pulmonary vascular abnormalities (45,46).
Overall, research indicates that females are more
likely than males to have lung disorders and that their degrees of
severity, rate of exacerbations, hospitalizations and death are all
higher in females than in males. These include pulmonary
hypertension, asthma, COPD and certain forms of lung cancer,
including adenocarcinoma. Moreover, women almost always have
certain uncommon and poorly known lung diseases, such as
lymphangioleiomyomatosis (47).
Smoking is an established risk factor for lung
function decline (48). It has
also been linked to long term consequences after SARS-CoV-2
infection (38,49). In the cohort of the present study,
smoking, except for the abnormal values of FEV1% pred, was
associated with abnormal values of TLC which indicate a restrictive
pattern of pulmonary dysfunction (50). These data suggested that expanding
the promotion of tobacco cessation strategies are new priorities in
this pandemic era.
The present study demonstrated some important
attributes that should be presented. It is one of a handful of
studies that include patients from a long period of the pandemic.
Although the mean values of DLCO% pred were higher in patients
infected during the period of omicron variant predominance, the
percentage of patients with abnormal values of FEV1% pred, FVC%
pred, DLCO% pred and TLC% pred did not differ among patients
infected with the three different variants of SARS-CoV-2,
suggesting that a significant proportion of patients who are
hospitalized due to COVID-19 pneumonia will develop abnormal lung
function regardless of the SARS-CoV-2 variant.
In addition, considering that the majority of the
cohort of the present study was unvaccinated against SARS-CoV-2,
the present study provided indirect evidence that vaccination
against SARS-CoV-2 is not only crucial for preventing severe acute
illness but also to limit post-acute sequelae of this infection.
The findings of the present study indicated that it is important to
build vaccine confidence in the general population and this can
potentially reduce lung impairment rates after hospitalization,
visits to outpatient clinics for abnormal lung function and overall
health costs.
There are several limitations in the present study.
First, the lack of pre-SARS-CoV-2 infection PFTs makes it difficult
to tell to which extent the infection affected the lung function.
Second, this is a single center study on hospitalized COVID-19
patients. Moreover, a low representation of patients with ICU
admission in the cohort of the present study does not permit the
generalizability of the present study findings to this specific
population. In addition, the specific viral variations of patients
were not identified. The prevalent variant at the time the patient
was identified as having SARS-CoV2 infection served as the basis
for the variant assignment.
In conclusion, the present study in a predominantly
unvaccinated cohort of patients hospitalized for COVID-19 pneumonia
revealed independent associations between age, female sex, smoking
and abnormal lung function three months post-acute infection. These
findings underscore the importance of considering these factors in
the post-acute care of COVID-19 survivors. While the percentage of
patients developing abnormal lung function did not vary
significantly across different variant predominance periods, future
research should explore these associations in more diverse cohorts.
These insights contribute to our evolving understanding of the
long-term consequences of COVID-19 and offer valuable
considerations for clinical practice and future investigations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article. The raw data is
available from the corresponding author upon reasonable
request.
Authors' contributions
NVS and VEG conceptualized the present study. VEG,
AG, SM, DAS, MNG, EA, PP, IGL, SP and NVS made a substantial
contribution to data interpretation and analysis and wrote and
prepared the draft of the manuscript. VEG and NVS analyzed the data
and provided critical revisions. VEG and NVS confirm the
authenticity of all the data. All authors contributed to manuscript
revision and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in line with the
Declaration of Helsinki and gained approval by the Institutional
Review Board of Laiko General Hospital (approval no. 765/12-2021).
Written informed consent was obtained from the patients for
participation and publication of the data.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Cascella M, Rajnik M, Aleem A, Dulebohn SC
and Di Napoli R: Features, evaluation, and treatment of coronavirus
(COVID-19) [Updated 2023 Aug 18]. In: StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available
from: https://www.ncbi.nlm.nih.gov/books/NBK554776/.
|
|
2
|
Sheleme T, Bekele F and Ayela T: Clinical
presentation of patients infected with coronavirus disease 19: A
systematic review. Infect Dis (Auckl).
13(1178633720952076)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siafarikas C, Stafylidis C, Tentolouris A,
Samara S, Eliadi I, Makrodimitri S, Spandidos DA, Mathioudakis N,
Karamichalos P, Papalexis P, et al: Radiologically suspected
COVID-19-associated organizing pneumonia responding well to
corticosteroids: A report of two cases and a review of the
literature. Exp Ther Med. 24(453)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Georgakopoulou VE, Gkoufa A, Damaskos C,
Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A,
Asimakopoulou S, Chlapoutakis S, et al: COVID-19-associated acute
appendicitis in adults. A report of five cases and a review of the
literature. Exp Ther Med. 24(482)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cholongitas E, Bali T, Georgakopoulou VE,
Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D,
Basoulis D, Eliadi I, Karamanakos G, et al: Prevalence of abnormal
liver biochemistry and its impact on COVID-19 patients' outcomes: A
single-center Greek study. Ann Gastroenterol. 35:290–296.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Georgakopoulou VE, Avramopoulos P,
Papalexis P, Bitsani A, Damaskos C, Garmpi A, Venetikou MS,
Paramythiotis D, Karlafti E, Sklapani P, et al: COVID-19 induced
hypoparathyroidism: A case report. Exp Ther Med.
23(346)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Georgakopoulou VE, Gkoufa A, Garmpis N,
Makrodimitri S, Papageorgiou CV, Barlampa D, Garmpi A, Chiapoutakis
S, Sklapani P, Trakas N and Damaskos C: COVID-19 and acute
pancreatitis: A systematic review of case reports and case series.
Ann Saudi Med. 42:276–287. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S,
Huang H, Zhang L, Zhou X, Du C, et al: Risk factors associated with
Acute respiratory distress syndrome and death in patients with
coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern
Med. 180:934–943. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cevik M, Tate M, Lloyd O, Maraolo AE,
Schafers J and Ho A: SARS-CoV-2, SARS-CoV, and MERS-CoV viral load
dynamics, duration of viral shedding, and infectiousness: A
systematic review and meta-analysis. Lancet Microbe. 2:e13–e22.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Georgakopoulou VE, Gkoufa A, Bougea A,
Basoulis D, Tsakanikas A, Makrodimitri S, Karamanakos G, Spandidos
DA, Angelopoulou E and Sipsas NV: Characteristics and outcomes of
elderly patients with Parkinson's disease hospitalized due to
COVID-19-associated pneumonia. Med Int (Lond). 3(34)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gkoufa A, Maneta E, Ntoumas GN,
Georgakopoulou VE, Mantelou A, Kokkoris S and Routsi C: Elderly
adults with COVID-19 admitted to intensive care unit: A narrative
review. World J Crit Care Med. 10:278–289. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lempesis IG and Georgakopoulou VE:
Implications of obesity and adiposopathy on respiratory infections;
focus on emerging challenges. World J Clin Cases. 11:2925–2933.
2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thaweethai T, Jolley SE, Karlson EW,
Levitan EB, Levy B, McComsey GA, McCorkell L, Nadkarni GN,
Parthasarathy S, Singh U, et al: Development of a definition of
postacute sequelae of SARS-CoV-2 infection. JAMA. 329:1934–1946.
2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Efstathiou V, Stefanou MI, Demetriou M,
Siafakas N, Makris M, Tsivgoulis G, Zoumpourlis V, Kympouropoulos
SP, Tsoporis JN, Spandidos DA, et al: Long COVID and
neuropsychiatric manifestations (review). Exp Ther Med.
23(363)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Efstathiou V, Stefanou MI, Demetriou M,
Siafakas N, Katsantoni E, Makris M, Tsivgoulis G, Zoumpourlis V,
Kympouropoulos SP, Tsoporis JN, et al: New-onset neuropsychiatric
sequelae and ‘long-COVID’ syndrome (Review). Exp Ther Med.
24(705)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Daugherty SE, Guo Y, Heath K, Dasmariñas
MC, Jubilo KG, Samranvedhya J, Lipsitch M and Cohen K: Risk of
clinical sequelae after the acute phase of SARS-CoV-2 infection:
Retrospective cohort study. BMJ. 373(n1098)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang C, Huang L, Wang Y, Li X, Ren L, Gu
X, Kang L, Guo L, Liu M, Zhou X, et al: 6-Month consequences of
COVID-19 in patients discharged from hospital: a cohort study.
Lancet. 397:220–232. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang L, Li X, Gu X, Zhang H, Ren L, Guo
L, Liu M, Wang Y, Cui D, Wang Y, et al: Health outcomes in people 2
years after surviving hospitalisation with COVID-19: A longitudinal
cohort study. Lancet Respir Med. 10:863–876. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
E E, R F, Öi E, Im L, M L, S R, E W, C J,
M H and A M: Impaired diffusing capacity for carbon monoxide is
common in critically ill Covid-19 patients at four months
post-discharge. Respir Med. 182(106394)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Riou M, MarcoT C, Oulehri W, Enache I,
Pistea C, Chatron E, Labani A, Geny B, Ohana M, De Blay F, et al:
Respiratory follow-up after hospitalization for COVID-19: Who and
when? Eur J Clin Invest. 51(e13603)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shah AS, Wong AW, Hague CJ, Murphy DT,
Johnston JC, Ryerson CJ and Carlsten C: A prospective study of
12-week respiratory outcomes in COVID-19-related hospitalisations.
Thorax. 76:402–404. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee JH, Yim JJ and Park J: Pulmonary
function and chest computed tomography abnormalities 6-12 months
after recovery from COVID-19: A systematic review and
meta-analysis. Respir Res. 23(233)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu T, Wu D, Yan W, Wang X, Zhang X, Ma K,
Chen H, Zeng Z, Qin Y, Wang H, et al: Twelve-month systemic
consequences of coronavirus disease 2019 (COVID-19) in patients
discharged from hospital: A prospective cohort study in Wuhan,
China. Clin Infect Dis. 74:1953–1965. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang L, Berger NA, Kaelber DC, Davis PB,
Volkow ND and Xu R: COVID infection rates, clinical outcomes, and
racial/ethnic and gender disparities before and after omicron
emerged in the US medRxiv: Feb 22, 2022 (Epub ahead of print).
|
|
26
|
Abdullah F, Myers J, Basu D, Tintinger G,
Ueckermann V, Mathebula M, Ramlall R, Spoor S, de Villiers T, Van
der Walt Z, et al: Decreased severity of disease during the first
global omicron variant covid-19 outbreak in a large hospital in
Tshwane, South Africa. Int J Infect Dis. 116:38–42. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arabi M, Al-Najjar Y, Mhaimeed N, Salameh
MA, Paul P, AlAnni J, Abdelati AA, Laswi I, Khanjar B, Al-Ali D, et
al: Severity of the omicron SARS-CoV-2 variant compared with the
previous lineages: A systematic review. J Cell Mol Med.
27:1443–1464. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Antonelli M, Pujol JC, Spector TD,
Ourselin S and Steves CJ: Risk of long COVID associated with delta
versus omicron variants of SARS-CoV-2. Lancet. 399:2263–2264.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Quanjer PH, Tammeling GJ, Cotes JE,
Pedersen OF, Peslin R and Yernault JC: Lung volumes and forced
ventilatory flows. Report working party standardization of lung
function tests, European community for steel and coal. Official
statement of the European respiratory society. Eur Respir J Suppl.
16:5–40. 1993.PubMed/NCBI
|
|
30
|
Georgakopoulou VE, Asimakopoulou S and
Cholongitas E: Pulmonary function testing in patients with liver
cirrhosis (review). Med Int (Lond). 3(36)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cotes JE, Chinn DJ, Quanjer PH, Roca J and
Yernault JC: Standardization of the measurement of transfer factor
(diffusing capacity). Eur Respir J. 6 (Suppl 16):S41–S52.
1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Karampitsakos T, Sotiropoulou V, Katsaras
M, Tsiri P, Georgakopoulou VE, Papanikolaou IC, Bibaki E, Tomos I,
Lambiri I, Papaioannou O, et al: Post-COVID-19 interstitial lung
disease: Insights from a machine learning radiographic model. Front
Med (Lausanne). 9(1083264)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang
Y, Hu P, Guo L, Liu M, Xu J, et al: 1-Year outcomes in hospital
survivors with COVID-19: A longitudinal cohort study. Lancet.
398:747–758. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang C, Ramasamy A, Verduzco-Gutierrez M,
Brode WM and Melamed E: Acute and post-acute sequelae of SARS-CoV-2
infection: A review of risk factors and social determinants. Virol
J. 20(124)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tarraso J, Safont B, Carbonell-Asins JA,
Fernandez-Fabrellas E, Sancho-Chust JN, Naval E, Amat B, Herrera S,
Ros JA, Soler-Cataluña JJ, et al: Lung function and radiological
findings 1 year after COVID-19: A prospective follow-up. Respir
Res. 23(242)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schneider JL, Rowe JH, Garcia-de-Alba C,
Kim CF, Sharpe AH and Haigis MC: The aging lung: Physiology,
disease, and immunity. Cell. 184:1990–2019. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aghali A, Koloko Ngassie ML, Pabelick CM
and Prakash YS: Cellular senescence in aging lungs and diseases.
Cells. 11(1781)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cho SJ and Stout-Delgado HW: Aging and
lung disease. Annu Rev Physiol. 82:433–459. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tsampasian V, Elghazaly H, Chattopadhyay
R, Debski M, Naing TKP, Garg P, Clark A, Ntatsaki E and Vassiliou
VS: Risk factors associated with post-COVID-19 condition: A
systematic review and meta-analysis. JAMA Intern Med. 183:566–580.
2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bai F, Tomasoni D, Falcinella C,
Barbanotti D, Castoldi R, Mulè G, Augello M, Mondatore D, Allegrini
M, Cona A, et al: Female gender is associated with long COVID
syndrome: A prospective cohort study. Clin Microbiol Infect.
28:611.e9–611.e16. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Torjesen I: Covid-19: Middle aged women
face greater risk of debilitating long term symptoms. BMJ.
372(n829)2021.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Opsteen S, Files JK, Fram T and Erdmann N:
The role of immune activation and antigen persistence in acute and
long COVID. J Investig Med. 71:545–562. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Phetsouphanh C, Darley DR, Wilson DB, Howe
A, Munier CML, Patel SK, Juno JA, Burrell LM, Kent SJ, Dore GJ, et
al: Immunological dysfunction persists for 8 months following
initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol.
23:210–216. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Souyris M, Mejía JE, Chaumeil J and Guéry
JC: Female predisposition to TLR7-driven autoimmunity: Gene dosage
and the escape from X chromosome inactivation. Semin Immunopathol.
41:153–164. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hanidziar D and Robson SC: Hyperoxia and
modulation of pulmonary vascular and immune responses in COVID-19.
Am J Physiol Lung Cell Mol Physiol. 320:L12–L16. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Patel BV, Arachchillage DJ, Ridge CA,
Bianchi P, Doyle JF, Garfield B, Ledot S, Morgan C, Passariello M,
Price S, et al: Pulmonary angiopathy in severe COVID-19:
Physiologic, imaging, and hematologic observations. Am J Respir
Crit Care Med. 202:690–699. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Silveyra P, Fuentes N and Rodriguez Bauza
DE: Sex and gender differences in lung disease. Adv Exp Med Biol.
1304:227–258. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Trakas N, Georgakopoulou VE, Melemeni D,
Damaskos C, Mantzouranis K, Garmpis N, Gkoufa A, Papalexis P,
Chlapoutakis S, Sklapani P, et al: Association between smoking
cessation and alterations in forced expiratory volume in one second
(FEV1). A follow-up study from a Greek tobacco cessation clinic.
Addict Health. 14:87–95. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Barthélémy H, Mougenot E, Duracinsky M,
Salmon-Ceron D, Bonini J, Péretz F, Chassany O and Carrieri P:
Smoking increases the risk of post-acute COVID-19 syndrome: Results
from a French community-based survey. Tob Induc Dis.
20(59)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Martinez-Pitre PJ, Sabbula BR and Cascella
M: Restrictive lung disease. In: StatPearls. StatPearls Publishing,
Treasure Island, FL, 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560880/.
|