Introduction
Corneal ectasia is a group of eye disorders
characterized by bilateral thinning and distortion of the central,
paracentral or peripheral cornea. The primary forms include
keratoconus and ectasia after refractive surgery (1). Previously, crosslinking (CXL) with
ultraviolet-A and riboflavin (UVA/RF) was introduced as an
effective, minimally invasive means to treat these progressive
corneal diseases (2). By promoting
the CXL reaction, the corneal collagenous fiber is stiffened and
the deformation-resistant performance of the corneal tissue is
improved (3). After the CXL
procedure, astigmatism, the best corrected visual acuity, and the
maximum simulated keratometry values markedly improved in patients
with keratoconus (4). Clinical
results showed that a flattening of ≤2 diopter within 2 years after
CXL was observed and the effect of flattening was demonstrated for
a period >10 years (5,6). Despite its promising results, some
sequelae and complications of UVA/RF CXL, mostly related to the
toxic nature of UV, have also been reported. Keratocyte loss of
anterior corneal layers is inevitable (7), and endothelial cell density loss has
been reported as an outcome of the thinning of the cornea after UVA
CXL (8). Corneal haze has been
reported in 10-90% of patients (9). Additionally, in vivo confocal
microscopy has shown that there is an elevation in stromal
reflectivity, which is indicative of edema and activation of
keratocytes. This typically occurs within a timeframe of 3-6 months
following treatment (10). The
aforementioned issues render numerous patients unsuitable for this
type of treatment.
Enzyme induced CXL, which is commonly used in the
food and agriculture industry, may be a better alternative
procedure. Biochemical CXL uses enzymes to induce additional
peptide bonds between collagen molecules, which differs from free
radical-induced CXL using photochemical CXL, such as UVA/RF CXL.
Recently, the mRNA and protein levels of fibronectin and
transglutaminases (Tgases) were reported to be higher in human
corneal keratocytes treated with UVA/RF CXL, so the induction of
Tgases in the cornea has been proposed as a new mechanism for
inducing CXL (11). In the present
study, the biomechanical effects were measured and the histological
changes were observed after in vitro Tgase-induced CXL in
the porcine cornea, and the potential of this as a novel and
effective method to stiffen corneal collagen was evaluated.
Materials and methods
Cornea preparations
A total of 60 fresh porcine eyes with intact and
transparent corneas were purchased from the local abattoir (Beijing
Shunxin Agricultural Co., Ltd., Pengcheng Food Branch; Beijing,
China) <4 h postmortem. All porcine eyes were obtained from
animals slaughtered for the food industry after routine slaughter.
Corneoscleral rings with a diameter of 16 mm were harvested and
corneal strip pairs were obtained from each cornea using a
self-designed triple-blade scalpel through the corneal center,
including a length of 10-mm cornea tissue and 3-mm sclera on the
bilateral ends. A pair of corneal strips from the same cornea was
distributed randomly into two groups: One was incubated in 1,500
U/g Tgase solution (CAS no. 80146-85-6; Hefei Bomei Biotechnology
Co., Ltd.), and the other one was incubated in PBS and used as a
control.
CXL procedure
A total of 60 corneal pairs were divided into four
groups, Groups A-D. CXL strips in Groups A-D were incubated in
Tgase solution, with physiological saline solution was used as the
solvent. After full dissolution, the CXL agent solution was formed.
The tissue material was immersed at 37˚C for 30 min in 2 U/ml Tgase
solution (Group A; n=15), 1 U/ml Tgase solution (Group B; n=15),
0.5 U/ml Tgase solution (Group C; n=15) or 0.25 U/ml Tgase solution
(Group D; n=15). A total of 60 untreated corneal strips (15 per
group), which were paired with CXL-treated strips, were placed in
PBS at 37˚C for 30 min. The temperature of a normal human body
varies between 36.1 and 37.2˚C (12). Incubation was carried out at 37˚C,
as this temperature is close to the temperature of the human body.
Additionally, as the enzyme is found naturally and is functional in
the human body, a temperature of 37˚C was considered appropriate
for optimal performance. The corneal materials were then separately
stored in PBS at room temperature (25˚C). All corneal strips were
used for subsequent biomechanical measurements 0-3 h after CXL.
Corneal morphology and thickness
After CXL, the transparency and morphology of a
hemisphere of corneal material were observed and recorded. The
thickness and width of every corneal strip were measured using a
micron-thickness gauge (Yiwu Exploit Hardware Co., Ltd.) and a
Vernier caliper.
Biomechanical assessment
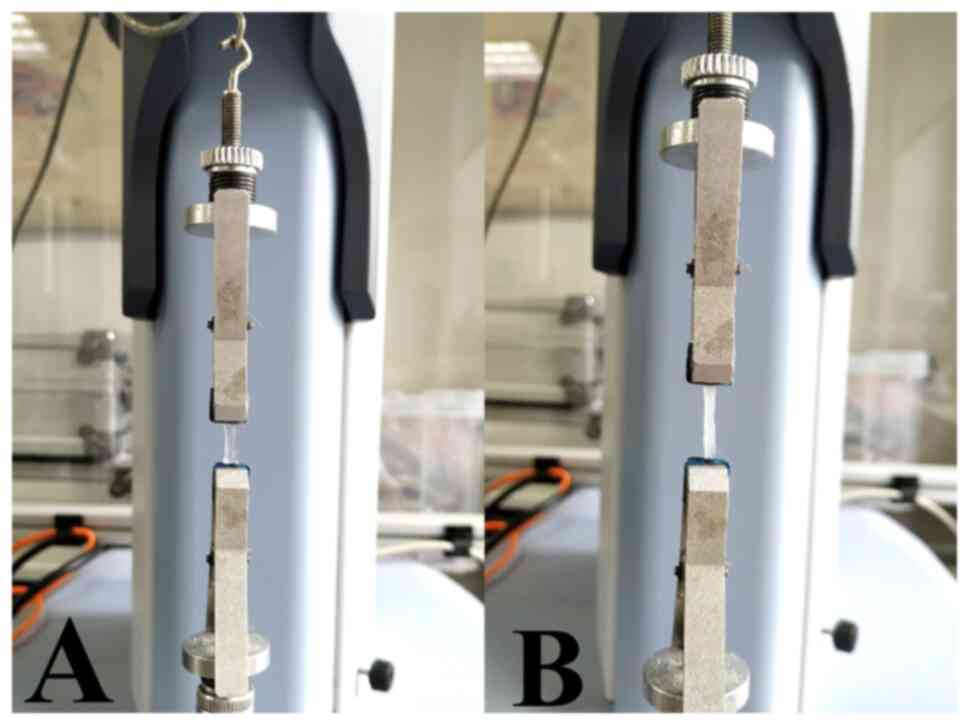
The corneal strips were clamped vertically in the
tongs of an electronic universal testing machine (Shimadzu
Corporation) with the sclera and corneoscleral tissue both held in
the tongs at both ends. The tensile strain test was run at 2 mm/min
the initial strain loading to 0.05 N, with the length of the
corneal trips between both tongs recorded as the initial length
(Fig. 1). The elastic modulus was
calculated using the software included with the testing machine
(Trapezium; version 1.5.1; Shimadzu Corporation).
Histological staining
Three samples were randomly selected from each group
for morphological observations. These samples were fixed in 4%
paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd.) at room
temperature for 24 h. The main body of the three corneal materials
was used to create corneal test strips, while the remaining parts
of the cornea were subjected to hematoxylin and eosin (H&E) and
Masson staining. For H&E staining, the prepared sections were
immersed in a series of graded ethanol baths followed by xylene to
dehydrate the tissues. The thickness of the sections were 4-µm.
After dehydration, the tissues were immersed in paraffin. They were
then stained with H&E (hematoxylin 2 min; eosin 1 min) before
examination. For Masson trichrome staining, the prepared sections
were immersed in hematoxylin (5 min), scarlet-acid (5 min) and
aniline blue (1 min) solutions successively, with 70% ethanol used
for dehydration between the stains. The thickness of the sections
were 4-µm. All staining steps were performed at room temperature
(25˚C). All images were captured using a light microscope at x200
magnification with a high-resolution charge-coupled device camera.
The images were analyzed using NIS-Element imaging software
(version 3.2; Nikon Corporation). Changes in histological
morphology between the CXL and control group were assessed.
Statistical analysis
The exponential fitting of the stress-strain curves
was produced using OriginPro (version 2018; OriginLab Corporation).
Data for treated and control cornea materials were compared using
an independent sample t-test in SPSS (version 24; IBM Corp.). The
distribution of data between the different groups was investigated
using the Kruskal-Wallis test. Data are presented as mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
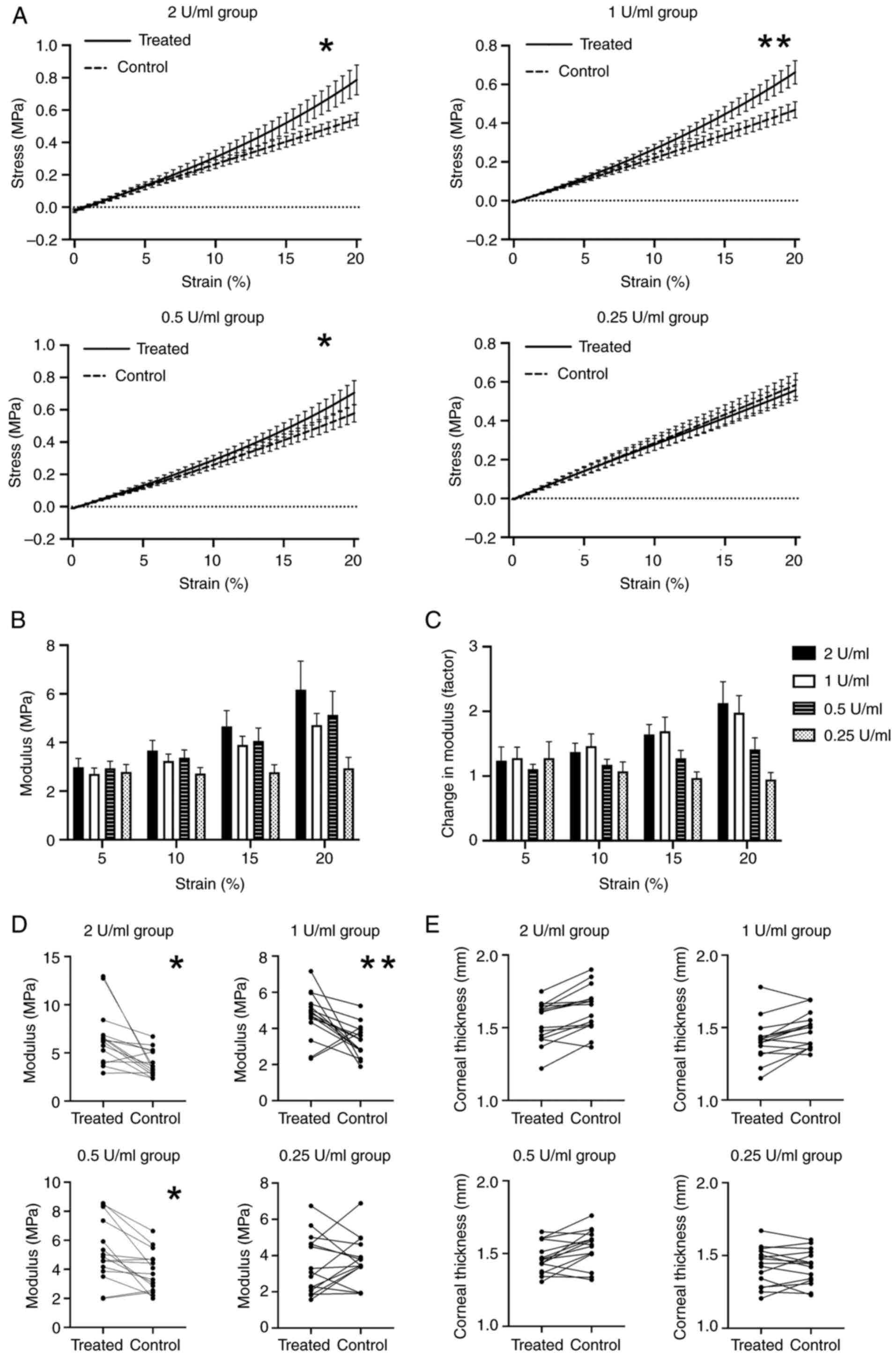
Tangent gradients in the stress-strain
curves become steeper after CXL
The stress-strain curves showed a marked increase in
the biomechanical properties (elasticity modulus) of treated
corneas in a concentration-dependent pattern. Compared with the
control groups, the tangent gradients in the stress-strain curve of
the treated group were steeper at higher concentrations of Tgase
(Group A, B and C). These differences were statistically
significant in Group A, B and C. Although the stress-strain curve
of the control group in the lower Tgase concentration group (Group
D) seemed to be steeper than that of the experimental group, there
was no significant statistical difference between the two groups
(Fig. 2A and Table I).
 | Table IEffect of different enzyme
concentrations on elastic modulus in porcine corneas. |
Table I
Effect of different enzyme
concentrations on elastic modulus in porcine corneas.
| | Elastic modulus,
MPa | |
|---|
| Groups | Treated groups | Control groups | t-value | P-value |
|---|
| A | 6.56±2.93 | 3.94±1.35 | 2.557 | 0.016 |
| B | 4.72±1.29 | 3.30±0.91 | 3.449 | 0.002 |
| C | 5.24±2.13 | 3.85±1.40 | 1.852 | 0.049 |
| D | 3.48±1.60 | 3.72±1.31 | -0.447 | 0.658 |
Elastic modulus of the cornea
increases after CXL
As a nonlinear material, the elastic modulus of the
cornea strips was altered under different strains and the changes
in elastic modulus were different in the four groups. Differences
were particularly notable in the 10-20% strain measurements
(Fig. 2B and C). The elastic modulus of the CXL-treated
corneas and their corresponding controls were calculated and listed
at 20% strain (Table I). The
average increase in elastic modulus in the different concentration
CXL subgroups (2, 1 and 0.5 U/ml) was 166, 143 and 136%,
respectively, compared with those in the control group. These
differences were found to be statistically significant (Fig. 2D). There was no significant
difference in the distribution of elastic modulus among the control
subgroups at different concentrations (P=0.591).
Thickness of the cornea remains
unchanged
The central corneal thickness of the CXL strips and
controls were assessed (Fig. 2E).
The corneal thickness of the experimental group (Tgase-treated
cornea strips) showed a trend of thinning compared with the control
group at the following Tgase concentrations: 2, 1 and 0.5 U/ml, but
the difference was not statistically significant (Table II).
 | Table IICentral corneal thickness after
enzyme-induced crosslinking in porcine cornea. |
Table II
Central corneal thickness after
enzyme-induced crosslinking in porcine cornea.
| | Mean corneal
thickness, µm | |
|---|
| Groups | Treated groups | Control groups | t-value | P-value |
|---|
| A | 1.54±0.14 | 1.63±0.16 | -1.883 | 0.069 |
| B | 1.41±0.15 | 1.48±0.12 | -1.412 | 0.169 |
| C | 1.47±0.11 | 1.54±0.13 | -1.547 | 0.136 |
| D | 1.43±0.13 | 1.42±0.12 | 0.064 | 0.950 |
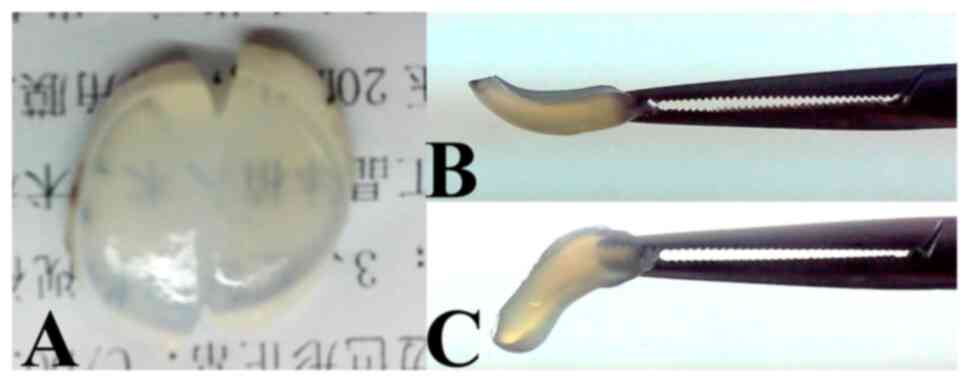
Transparency of the cornea remains
unchanged
Transparency was similar in the CXL cornea strips
(Fig. 3A, left) and control strips
(Fig. 3A, right).
Stiffness of the cornea becomes
stronger after CXL
The stiffness and deformation resistance of corneal
strips in the CXL groups (Fig. 3B)
were better than those in the control groups (Fig. 3C).
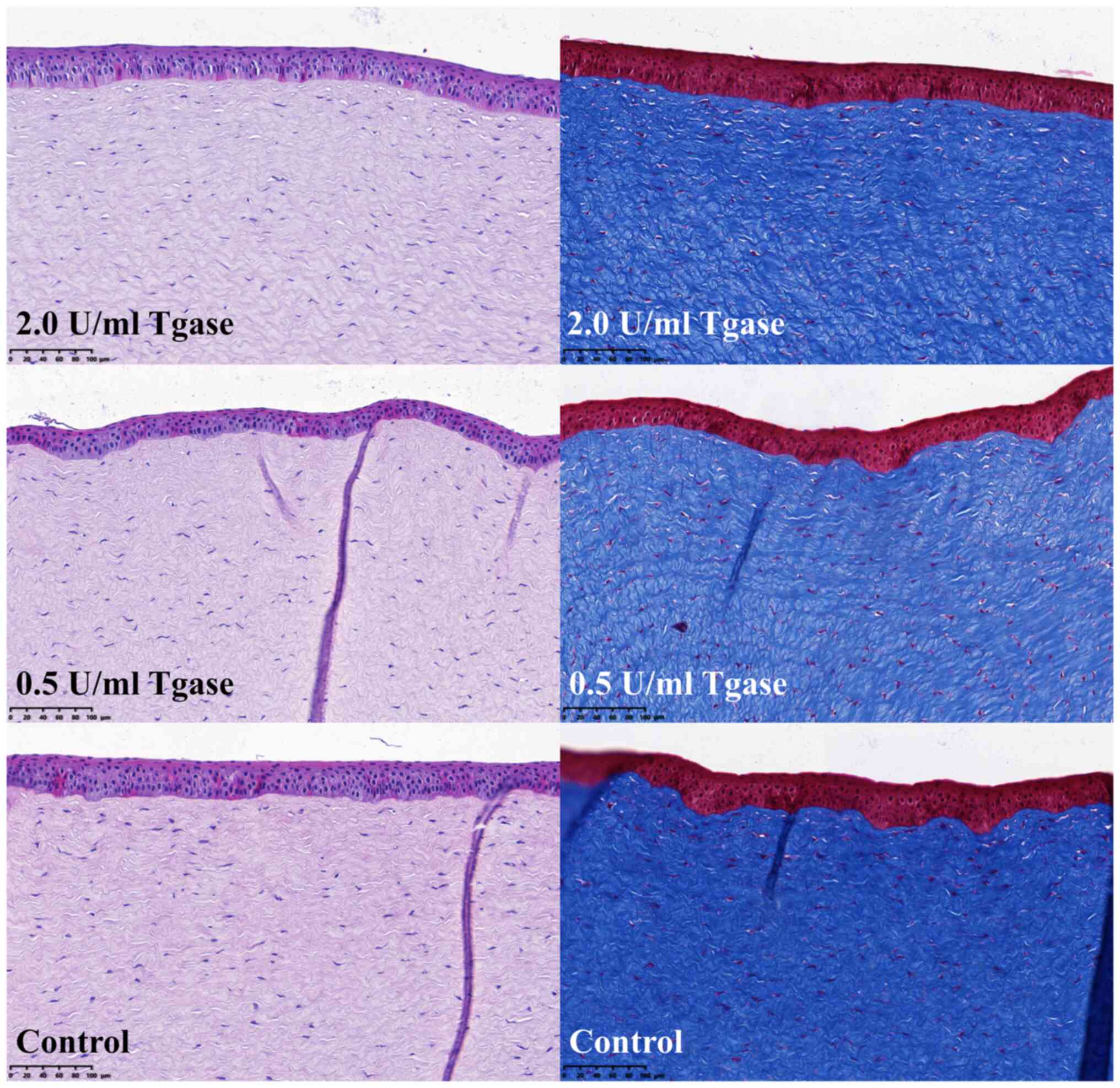
Histological structure of the cornea
is not damaged
H&E staining showed that the overall structure
of the cornea was not damaged after Tgase-induced CXL treatment.
Masson trichrome staining showed that the arrangement of fibrils in
the CXL groups was as ordered as that in the control group. The
interfibrillar space was similar in both the CXL and control groups
(Fig. 4).
Discussion
The present study used elasticity modulus to
investigate the changes in the corneal biomechanical properties
after enzyme-induced CXL. The results of the present study
indicated that the corneal biomechanical property (elasticity
modulus) was significantly enhanced by enzyme-induced CXL and that
the increase was dose-dependent. CXL solutions concentrations of 2,
1 and 0.5 U/ml resulted in increases in the elastic modulus of 166,
143 and 136%, respectively. It was shown that the increase in the
Young's modulus produced by Tgase was between 1.36 and 1.66x,
depending on the concentration. In the current study, a decrease
was observed in the elastic modulus of the materials when using the
lowest concentration of Tgase (0.25 U/ml) for CXL. However, these
observed differences could likely be attributed to measurement
errors. Initially, the elastic modulus was expected to remain
relatively stable. Measurements were performed using an electronic
universal testing machine and the research outcomes were sensitive
to environmental conditions. Factors such as room humidity during
material testing, the moisture content of the tissue and the
duration of tissue detachment all serve a marked role in
influencing the measurement results (13). Therefore, if the changes are
statistically insignificant, it can be concluded that the material
properties remain unaffected. Due to the lack of statistical
differences observed in the low concentration group (0.25 U/ml), we
hypothesize that enzyme-induced CXL therapy may require the enzyme
to reach a certain concentration in order to achieve therapeutic
effects.
Cornea, as a nonlinear material, had no directly
proportional relationship of stress to strain. The relationship of
stress vs. strain is often used to reflect the biomechanical
characteristics of the material. At a moderate-to-high enzyme
concentration, the tangent gradients in the stress-strain curve of
the treated group were steeper compared with the control groups.
The modulus-strain plots also showed that the biomechanical nature
(elasticity modulus) of the treated corneal strips in the 2, 1 and
0.5 U/ml concentration groups were significantly increased compared
with the controls at a high strain (10-20%) after CXL. All the
curves and elastic moduli showed no significant difference at a low
Tgase concentration (0.25 U/ml). This finding indicated that the
effective concentration should not be <0.5 U/ml in future
clinical practice. However, increases in values might be lower
in vivo because the enzyme solution may not be able to
penetrate the cornea and only the anterior half of the cornea could
be crosslinked. In the present study, the whole cornea including
both epithelial and endothelial surfaces was crosslinked, which
should increase the CXL effectiveness. Compared with the 2 U/ml
group, the 1 U/ml group reduced the dosage of the drug by half,
while the effective value only decreased slightly. Therefore, 1
U/ml may be a more appropriate dose in vivo.
Compared with that in the control group, the
thickness of the cornea in the treatment group demonstrated a trend
of decrease after CXL at a moderate-to-high concentration; however,
there was no statistically significant difference. It has been
reported that thickness was reduced after UVA/RF CXL (14-16).
The decrease may occur at the end of the CXL operation (11) and continue for ≥1 year (15,16).
Anatomical and microstructural changes in corneal collagen fibrils,
such as the compression of collagen fibrils, may change corneal
hydration and edema (15). Further
research is required to confirm this. In the present study, the
transparency of corneas treated with Tgase-induced CXL was found to
be comparable to that in the control group when observed with the
naked eye. Additionally, there were no observed changes in the
structure of the corneal collagen fibers when examined under a
light microscope. Histological observations of the fibrils showed
no noticeable alterations after Tgase-induced CXL treatment, and
the orientation and separation of collagen lamellae were consistent
across the different concentration groups. These findings suggested
that Tgase-induced CXL may be safe for future in vivo
applications. However, further investigation is needed to determine
if there are any changes at a smaller scale, such as changes at the
molecular level. All scleral tissues were fixed in formaldehyde
before H&E and Masson's staining; however, formaldehyde is also
a chemical CXL agent and it may present false-negative results in
the morphological analysis (17).
Additional studies are needed to evaluate the changes in the
diameter of collagen fibers and the interfibrillar spacing of
stromal collagen fibrils using electron microscopy.
Tgases are considered a widespread enzyme family and
are present in numerous different cell types and tissues with
diverse functions, such as programmed cell death, cell adhesion,
and interaction between the cell and the extracellular matrix
through the CXL of proteins (18).
The mechanism underlying CXL induction by Tgases is the formation
of isopeptide bonds between the γ-carboxamides of glutamine
residues (donor) and the first-order e-amine groups of different
compounds (19). In the past,
commercial Tgases could be obtained only from animal tissues, such
as guinea pig livers, with the low yields and high price preventing
Tgases from being more widely applied. Tgases may now be obtained
from microorganisms at increased yields and this allowed a number
of novel potential applications to be developed using Tgases
(20). In the food industry, it
can be used for protein modification, while in the biotechnology
and pharmaceuticals, it can be used to mediate bioconjugation
(21). Although Tgases are enzymes
that are widely distributed throughout the human body, they are
scarce in the cornea because of their lack of blood and lymphatic
vessels. The cornea consists almost exclusively of type I collagen
and is rich in glutamic acid and lysine. In theory, corneal
collagen fibers could be crosslinked by Tgases, potentially
resulting in more resistant mechanical properties (22). Previously, the mRNA and protein
expression levels of fibronectin and Tgases were reported to be
increased in human corneal keratocytes following UVA/RF CXL
treatment, and the induction of Tgase in the cornea was proposed as
a novel mechanism for inducing CXL (11).
Tgases are widely used in various fields, including
the food and manufacturing industries. The induction of Tgases has
been suggested to have a low toxic potential and be safe for the
human body (23). At the same
time, enzyme-induced CXL works as a direct CXL method and does not
require UV irradiation. The cytotoxicity of UVA may produce a
highly active oxygen species that causes oxidative stress and
induces necrosis or apoptosis in keratocytes (7). Currently, UVA/RF CXL is the only
clinically approved CXL technique, but it is unsuitable for corneas
<400-µm thick due to cytotoxicity (24). The disadvantages of cytotoxicity in
UVA/RF CXL may be avoided by Tgase-induced CXL. Previous studies
have shown that Tgase-induced CXL does not cause damage to the
endothelium and keratocytes in the cornea (25). Therefore, Tgase-induced CXL is
expected to become a new generation of CXL used in corneal ectasia.
Nevertheless, direct administration of Tgases as eye drops into the
conjunctival sac may not be feasible. To the best of our knowledge,
there are currently no studies that have directly administered
Tgases as eye drops into the conjunctival sac. The dilution by
tears and its potential toxic side effects on nearby tissues may
need to be considered. Possible routes of administration required
further investigation in future studies.
To the best of our knowledge, no studies on the
effect of Tgase-induced CXL on the cornea have been previously
reported. The findings of the present study indicate that Tgases
markedly increase the stiffness of the cornea.
Enzymatic/biochemical CXL has a large number of unknown and
technical points to be investigated. However, it is possible that
in the future, Tgases could be developed as pharmaceutical agents
to modify the biomechanical properties of the cornea, which could
potentially slow down or treat keratectasia and provide an optimal
treatment approach for conditions such as keratoconus. Furthermore,
the ocular surface typically has a temperature of ~34˚C. In future
clinical use, the temperature range is anticipated to be 34-37˚C.
Further research is required to investigate the safety, enzyme
concentration, enzymatic reaction time, long-term efficacy and
other aspects of enzyme-induced corneal CXL in vivo and
in vitro.
In conclusion, enzyme-induced CXL should be
investigated further and is expected to be a new generation CXL
method which could be used in corneal ectasia.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by National High Level
Hospital Clinical Research Funding: Interdepartmental Clinical
Research Project of Peking University First Hospital (grant no.
2022CR24).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW conceived and designed the study. SZ, WZ, SX, YZ,
DC and XL performed the experiments and collected the data. DC and
XL finished the uniaxial tensile test and calculated the Young's
modulus of materials. YW contributed to the data analysis. SZ and
WZ contributed equally to the manuscript writing. YW and SZ confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved
(approval no. J2022103) by the Laboratory Animal Ethics Committee
of the First Hospital of Peking University (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ziaei M, Barsam A, Shamie N, Vroman D, Kim
T, Donnenfeld ED, Holland EJ, Kanellopoulos J, Mah FS, Randleman
JB, et al: Reshaping procedures for the surgical management of
corneal ectasia. J Cataract Refract Surg. 41:842–872.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Spoerl E, Huhle M and Seiler T: Induction
of cross-links in corneal tissue. Exp Eye Res. 66:97–103.
1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gkika M, Labiris G and Kozobolis V:
Corneal collagen cross-linking using riboflavin and ultraviolet-A
irradiation: A review of clinical and experimental studies. Int
Ophthalmol. 31:309–319. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Raiskup-Wolf F, Hoyer A, Spoerl E and
Pillunat LE: Collagen crosslinking with riboflavin and
ultraviolet-A light in keratoconus: Long-term results. J Cataract
Refract Surg. 34:796–801. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wollensak G, Spoerl E and Seiler T:
Riboflavin/ultraviolet-a-induced collagen crosslinking for the
treatment of keratoconus. Am J Ophthalmol. 135:620–627.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Noor IH, Seiler TG, Noor K and Seiler T:
Continued long-term flattening after corneal crosslinking for
keratoconus. J Refract Surg. 34:567–570. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wollensak G, Spoerl E, Reber F and Seiler
T: Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro.
Eye (Lond). 18:718–722. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kymionis GD, Portaliou DM, Diakonis VF,
Kounis GA, Panagopoulou SI and Grentzelos MA: Corneal collagen
crosslinking with riboflavin and ultraviolet-A irradiation in
patients with thin corneas. Am J Ophthalmol. 153:24–28.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Greenstein SA, Fry KL, Bhatt J and Hersh
PS: Natural history of corneal haze after collagen crosslinking for
keratoconus and corneal ectasia: Scheimpflug and biomicroscopic
analysis. J Cataract Refract Surg. 36:2105–2114. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mastropasqua L, Nubile M, Lanzini M,
Calienno R, Mastropasqua R, Agnifili L and Toto L: Morphological
modification of the cornea after standard and transepithelial
corneal cross-linking as imaged by anterior segment optical
coherence tomography and laser scanning in vivo confocal
microscopy. Cornea. 32:855–861. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kopsachilis N, Tsaousis KT, Tsinopoulos
IT, Kruse FE and Welge-Luessen U: A novel mechanism of UV-A and
riboflavin-mediated corneal cross-linking through induction of
tissue transglutaminases. Cornea. 32:1034–1039. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dakappa PH and Mahabala C: Analysis of
long-term temperature variations in the human body. Crit Rev Biomed
Eng. 43:385–399. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Seiler TG, Shao P, Frueh BE, Yun SH and
Seiler T: The influence of hydration on different mechanical moduli
of the cornea. Graefes Arch Clin Exp Ophthalmol. 256:1653–1660.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mazzotta C and Caragiuli S: Intraoperative
corneal thickness measurement by optical coherence tomography in
keratoconic patients undergoing corneal collagen cross-linking. Am
J Ophthalmol. 157:1156–1162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Greenstein SA, Shah VP, Fry KL and Hersh
PS: Corneal thickness changes after corneal collagen crosslinking
for keratoconus and corneal ectasia: One-year results. J Cataract
Refract Surg. 37:691–700. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moghadam RS, Akbari M, Alizadeh Y,
Medghalchi A and Dalvandi R: The outcome of corneal collagen
cross-linking in patients with advanced progressive keratoconus: A
2-year follow-up study. Middle East Afr J Ophthalmol. 26:11–16.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hoffman EA, Frey BL, Smith LM and Auble
DT: Formaldehyde crosslinking: A tool for the study of chromatin
complexes. J Biol Chem. 290:26404–26411. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cai D, Ben T and De Luca LM: Retinoids
induce tissue transglutaminase in NIH-3T3 cells. Biochem Biophys
Res Commun. 175:1119–1124. 1991.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kieliszek M and Misiewicz A: Microbial
transglutaminase and its application in the food industry. A
review. Folia Microbiol (Praha). 59:241–250. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhu Y and Tramper J: Novel applications
for microbial transglutaminase beyond food processing. Trends
Biotechnol. 26:559–565. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vasić K, Knez Ž and Leitgeb M:
Transglutaminase in foods and biotechnology. Int J Mol Sci.
24(12402)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Meek KM: Corneal collagen-its role in
maintaining corneal shape and transparency. Biophys Rev. 1:83–93.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bernard BK, Tsubuku S and Shioya S: Acute
toxicity and genotoxicity studies of a microbial transglutaminase.
Int J Toxicol. 17:703–721. 1998.
|
|
24
|
Zhang ZY and Zhang XR: Corneal collagen
cross-linking with riboflavin and ultraviolet-A irradiation in
patients with thin corneas. Am J Ophthalmol. 153:1002–1003.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu Y, Song W, Tang Y, Elsheikh A, Shao Y
and Yan X: Efficacy and safety of transglutaminase-induced corneal
stiffening in rabbits. Transl Vis Sci Technol. 8(27)2019.PubMed/NCBI View Article : Google Scholar
|