Introduction
Colles fractures, which are usually caused by
low-energy injuries, refer to fractures that occur within 3 cm of
the articular surface of the distal radius. As one of the most
commonly occurring fractures, Colles fractures account for 90% of
distal radius fractures (1). They
are characterized by the displacement of the distal radius and
dorsal, as well as the proximal volar displacement (2). There are several causes of a Colles
fracture; the most common is a metacarpus landing after an
accidental fall and most occur in elderly individuals aged >60
years (3). At present, most
treatments for Colles fractures are conservative and include manual
reduction followed by splint fixation, which can achieve
satisfactory results for simple and stable extra-articular
fractures and some intra-articular fractures (1). Anatomical splints are an effective
method for the fixation and healing of Colles fractures (4).
In recent years, computational biomechanics analysis
technology has gradually been integrated into the clinical
application and basic research of medicine. Notably, finite element
analysis (FEA) is the most widely applied tool in the field of
orthopedics (5). FEA was first
proposed by Professor Clough in 1960 as an effective method to
calculate the discrete value (6)
and analyze the structural stress and deformation. Specifically,
FEA can reduce complex problems into simple ones, thereby solving
complex clinical or experimental problems using simple methods
(7). In 1972, Rybicki et al
(8) and Brekelmans et al
(9) applied FEA in the field of
orthopedic biomechanics research. Currently, advances in computer
technology have facilitated the rapid development of FEA.
Specifically, FEA modeling has developed from the two-dimensional
(2D) to the three-dimensional (3D) and the material properties have
progressed from linear to nonlinear. In addition, the methods to
construct the finite element mesh models have evolved from
simplified modeling by computer alone to the introduction of
computed tomography (CT) or magnetic resonance imaging (MRI).
With the increasing development of digital research
in clinical orthopedics, FEA has been applied in plastic surgery,
spinal limbs, articular cartilage and bone microstructure research.
In addition, FEA has also played a role in high-simulation data
models associated with ligament muscles, joint capsules and other
soft tissues (10). 3D finite
element analysis, with its inherent advantages for biomechanical
research (11), surpasses the
limitations of traditional experiments and is gradually being used
in the research of knee joint problems. The efficacy and safety of
splint fixation can be better evaluated through biomechanical
analysis based on a 3D finite element model. Therefore, the present
study explored the biomechanical distribution in various positions
with stable efficiency to maintain the Colles fractures for
anatomical splint fixation through the establishment of a 3D finite
element model of Colles fractures.
Materials and methods
Ethics statement
The present study was conducted in accordance with
the Declaration of Helsinki (as revised in 2013). It was approved
by ethics the committee of The Third Affiliated Hospital of
Guangzhou University of Chinese Medicine and oral informed consent
was obtained from the patient.
Materials
The Toshiba CT machine was obtained from Canon
Medical Systems Corporation. The bones were defined as having
isotropic homogeneous elastic material properties, ligaments served
as the shell elements and the various components are presented in
Table I. Mimics 15.0 software
(Materialise NV) was used to construct the 3D models. ANSYS
Workbench software (ANSYS Inc.) was used to create the 3D finite
element model. Imageware 5.0 (Siemens AG) was used for smoothing,
denoising, paving and other modifications to refine and optimize
the model.
 | Table ITissue parameters of various parts of
the human body. |
Table I
Tissue parameters of various parts of
the human body.
| Material | Elastic modulus | Poisson's ratio |
|---|
| Compact bone | 13.3 GPa | 0.30 |
| Cancellous bone | 0.69 GPa | 0.30 |
| Cartilage | 10 MPa | 0.45 |
| Ligament | 0.3 GPa | 0.40 |
| Interosseous
membrane | 0.95 GPa | 0.45 |
| Soft tissue | 0.15 MPa | 0.49 |
| Fracture line | 50 kPa | 0.05 |
Establishment of a 3D finite element
model of the forearm
Clinical CT data was obtained from a patient with a
Colles fracture [male; aged 34 years; Arbeitsgemeinschaftfür
Osteosynthesefragen (AO) classification (12) of fracture: A2] and without other
soft tissue or bone disorders. In addition to the fracture end, the
anatomy of the patient, except for the fractured region, was
anatomically consistent with the uninjured side. To acquire
detailed anatomical information, a 64-slice spiral CT scanning
procedure was performed using the Toshiba CT machine. The patient
assumed a supine position with spontaneously drooping arms during
the scan. The scanning range included the proximal forearm to the
distal finger, with the parameters set at a slice thickness of 0.6
mm with a total of 1,000 slices. Subsequently, the obtained CT data
were saved in DICOM3.0 format (13).
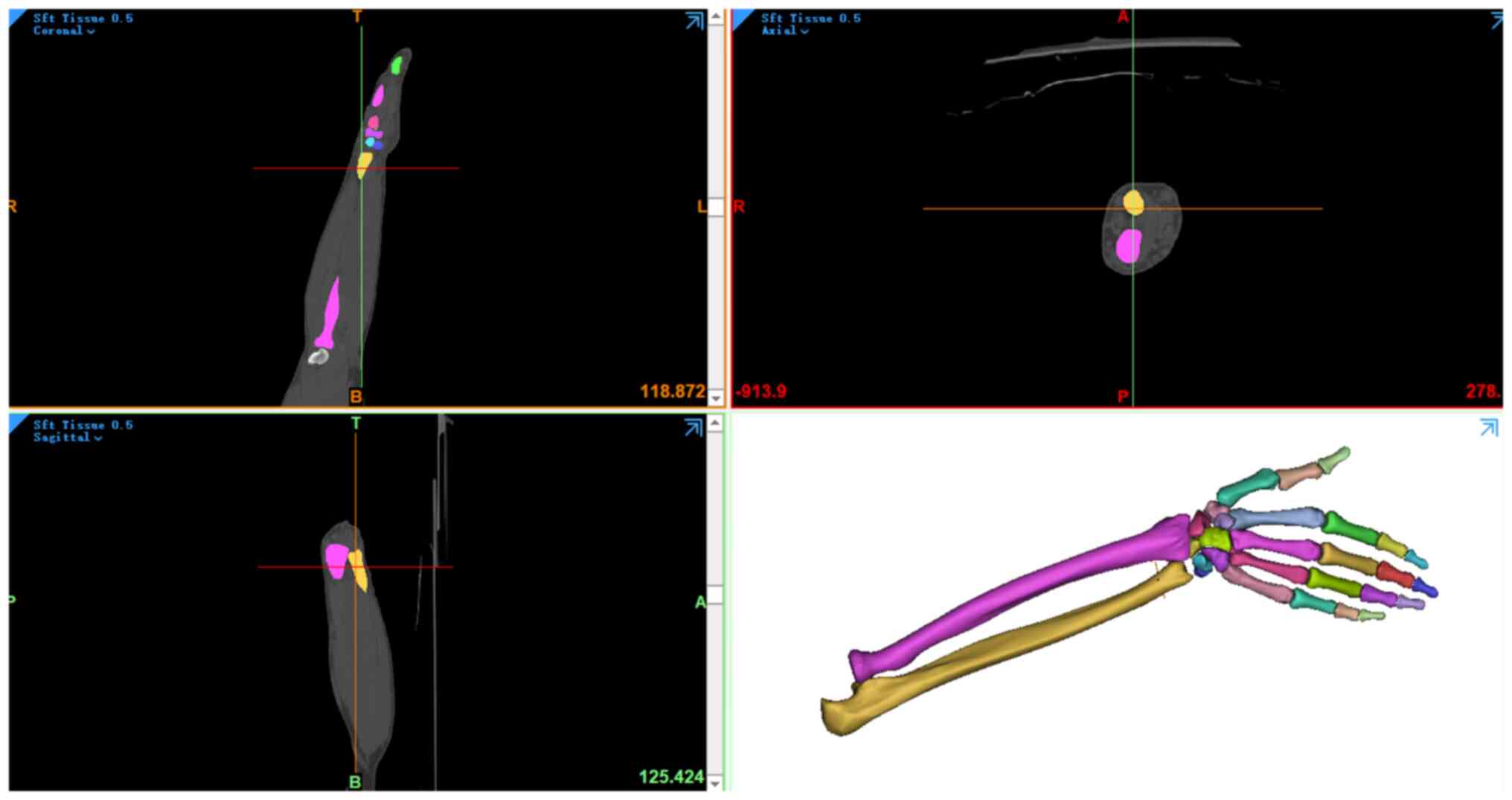
The collected data were then processed using Mimics
15.0 software (Materialise NV) to construct the 3D models (Fig. 1). The segmentation and
reconstruction of the bone structure in the image and the
adjustment of the connection between the joints, such as between
the radius and wrist, were performed so that they were in a fusion
state. At the same time, digital models of soft tissues in the
different parts were established using the aforementioned methods.
The bone structure and soft tissue model were imported into
Imageware 5.0 (Siemens AG) for smoothing, denoising, paving and
other modifications to refine and optimize the model. The optimized
model was inputted into PRO/E software (version 5.0; Parametric
Technology Corporation) for assembly. Based on the anatomical
locations described in the literature (14-16),
the appropriate components were selected from the parts library of
the software and structures such as ligaments, cartilage and
fibrocartilage were added to obtain a 3D model of the forearm and
hand. This model was inputted into ANSYS Workbench software (ANSYS
Inc.) to construct the 3D finite element model of the forearm and
hand (Fig. 2).
Validation of the 3D finite element
model
Contact relationships within the model were
established in accordance with current literature. Specifically,
the bone-to-bone and interior of the bones were designated as
‘Contact’, while bone-to-soft tissue interactions were defined as
‘Tie’. The friction coefficient within joints was intentionally
disregarded. To simulate realistic conditions, the radius and
proximal ulna were constrained using full degree of freedom
fixation. Subsequently, a 100 N axial compression load was applied
to the second and third metacarpals, according to established
protocols (17-22).
To validate the model, it was subjected to loading in different
directions to ensure that the generated stress patterns were
consistent with the expected biomechanical responses. The material
properties of the model were fine-tuned to simulate the conditions
of a Colles fracture. Reduction functions and mechanical changes
around the simulated fracture site were incorporated into the model
to enhance realistic conditions. This validation approach ensured
the fidelity of the 3D finite element model and its suitability for
biomechanical analysis and it adhered to the established
methodologies in the field.
Finite element analysis. Load
configuration and boundary constraint
The degrees of freedom of all nodes (displacement
and rotation in three directions) for the upper radioulnar notch of
the proximal section of the 3D finite element model were defined as
0. For the loading under a dynamic state, the compressive force of
the body was assumed to occur from the proximal to the distal
forearm; the vertical upward component was received by the surface
of the wrist, radius and ulna joints was set as 100 N; and the
tensile component as 100 N. When simulating the internal rotation
of the forearm, the stress on the radius arose from the internal
rotation; the loaded torsional moment was 1 Nm; and vertical
moments were applied to the medial and lateral distal radius to
simulate the working condition of the distal radius under torsional
stress.
The boundary constraints for the finite element
model of a Colles fracture are the restrictions on the radius and
ulna. The full degree of freedom fixation method was applied to
constrain the radius and ulna, thus simulating their basic
positions to keep them relatively stationary in the model. For the
conditions for the ligaments and soft tissues, the material
properties and geometric shapes of the ligaments and soft tissues
were considered to simulate their behavior under physiological
conditions. This involved treating the ligaments as shell elements
and assigning appropriate properties based on previously reported
data (Table I).
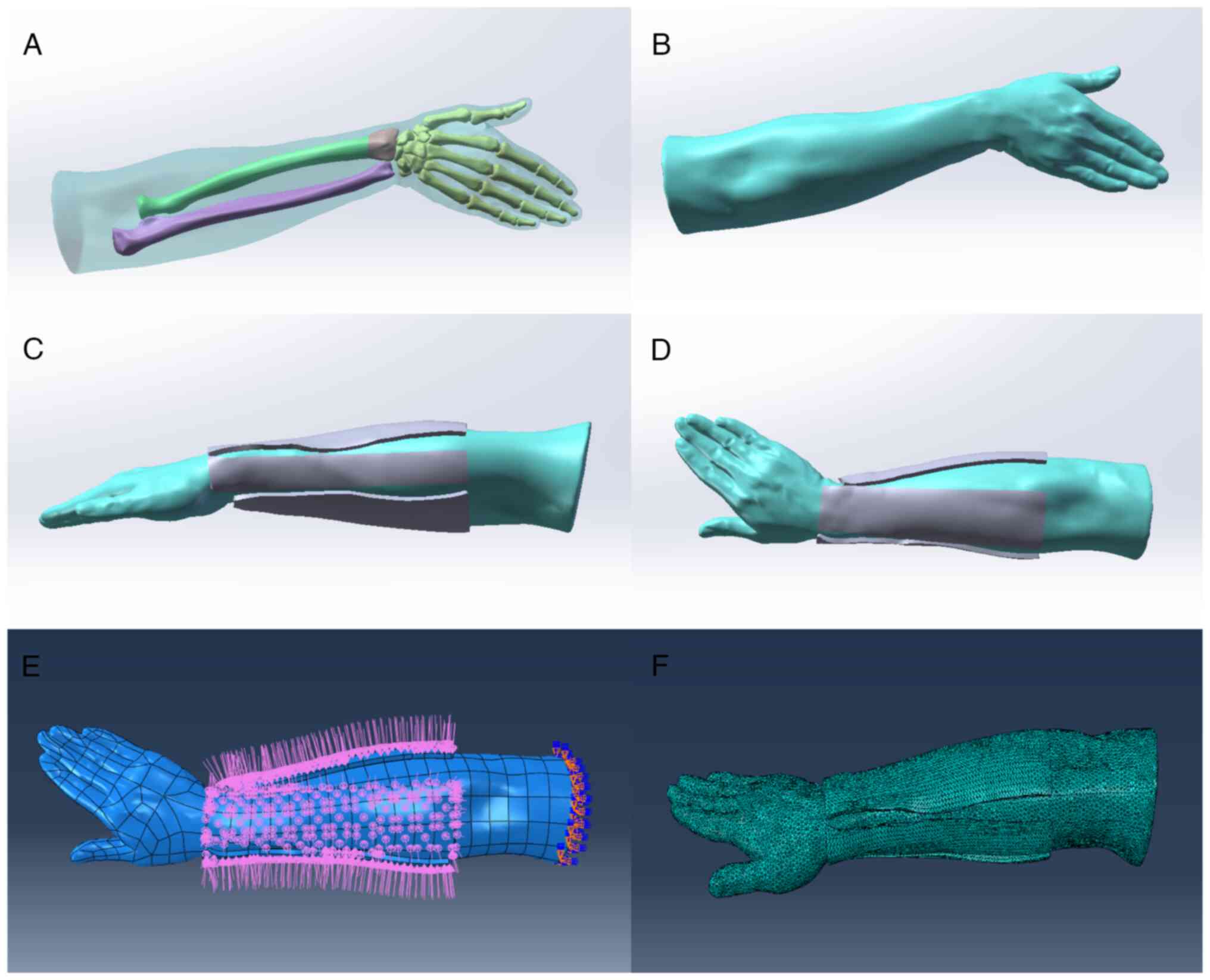
Mesh convergence. Mesh convergence was
performed for all the obtained bone structure and soft tissue model
using 3-matic (v5.1, Materialise company, Belgium). To generate the
finite element model of the volume meshes, the models were imported
into ANSYS.13.0 (ANSYS Inc.) and meshed using the SOLID72 element
type. SOLID72 is an element that is defined by four nodes, each of
which has six degrees of freedom: displacement in the axis x, y and
z directions, as well as rotation around the axis x, y and z
directions. The finite element mesh model is shown in Fig. 2F.
Simulation and calculation of anatomical splint
fixation state. The models were inputted into the ANSYS
Workbench software (ANSYS Inc.) and through the automatic block
function of the software, higher-order tetrahedron models were
selected for the model segmentation. In the segmented model,
fixation constraints for the full degrees of freedom for the
proximal radius and ulna were determined. A load of 28 N was
applied uniformly to each splint, the surface perpendicular to the
splint was considered the center and the calculation started from
the moment of contact with the skin and ended when mechanical
stability was achieved between the splint and skin (23). Under a 28 N load, the body surface
stress cloud diagrams and peak values of the anatomical splints in
the three different positions (pronation, median rotation and
supination) were measured. The measurement indicators mainly
included stress distributions in the different parts (e.g.,
tendons, ulnar head and thenar muscles) as well as the bone surface
stress diagrams and peaks. As for the simulation for lateral
rotation, the stress on the radius was from lateral rotation; the
loaded torsional moment was defined as 1 Nm; and vertical moments
were imposed on the medial and lateral distal radius to simulate
the working conditions of the distal radius under torsional stress.
Finally, the aforementioned boundary conditions and loading were
imported into the ANSYS Workbench software (ANSYS, Inc.) and the
stress distribution was then analyzed via a 3D finite element
model.
Statistical analysis
By using computational tools such as Mimics 15.0
(Materialise NV), Imageware 5.0 (Siemens AG), PRO/E (Parametric
Technology Corporation) and ANSYS Workbench (ANSYS Inc.), the
present study ensured the accuracy and validity of the 3D models.
The loaded 3D finite element model was then assessed in various
directions and its validity was confirmed through stress analysis.
Data of stress and displacement were expressed as the mean ±
standard deviation. Student's t test (unpaired) was used to compare
stress and displacement between the anatomical splints and soft
tissue. All tests were two-tailed and P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS 26.0 (IBM Corp.).
Results
Verification of the forearm model
The radiocarpal tissue stress was calculated
according to the present finite element model. The results of the
present study indicated that the stress distribution of the
radiocarpal tissue was not uniform; the maximum stress was located
in the scaphoid and lunate fossae of the articular surface of the
distal radius. Furthermore, the maximum contact stress at the wrist
joint surface was 9.57 MPa. According to a previous report
(4), the maximum contact stress
was ~9 MPa and the stress was mainly distributed in the scaphoid
and lunate fossae of the joint surface of the distal radius, which
is consistent with the results of this study. Similar simulation
results were reported in other literature (24,25).
Therefore, the forearm model was determined to be accurate and
effective.
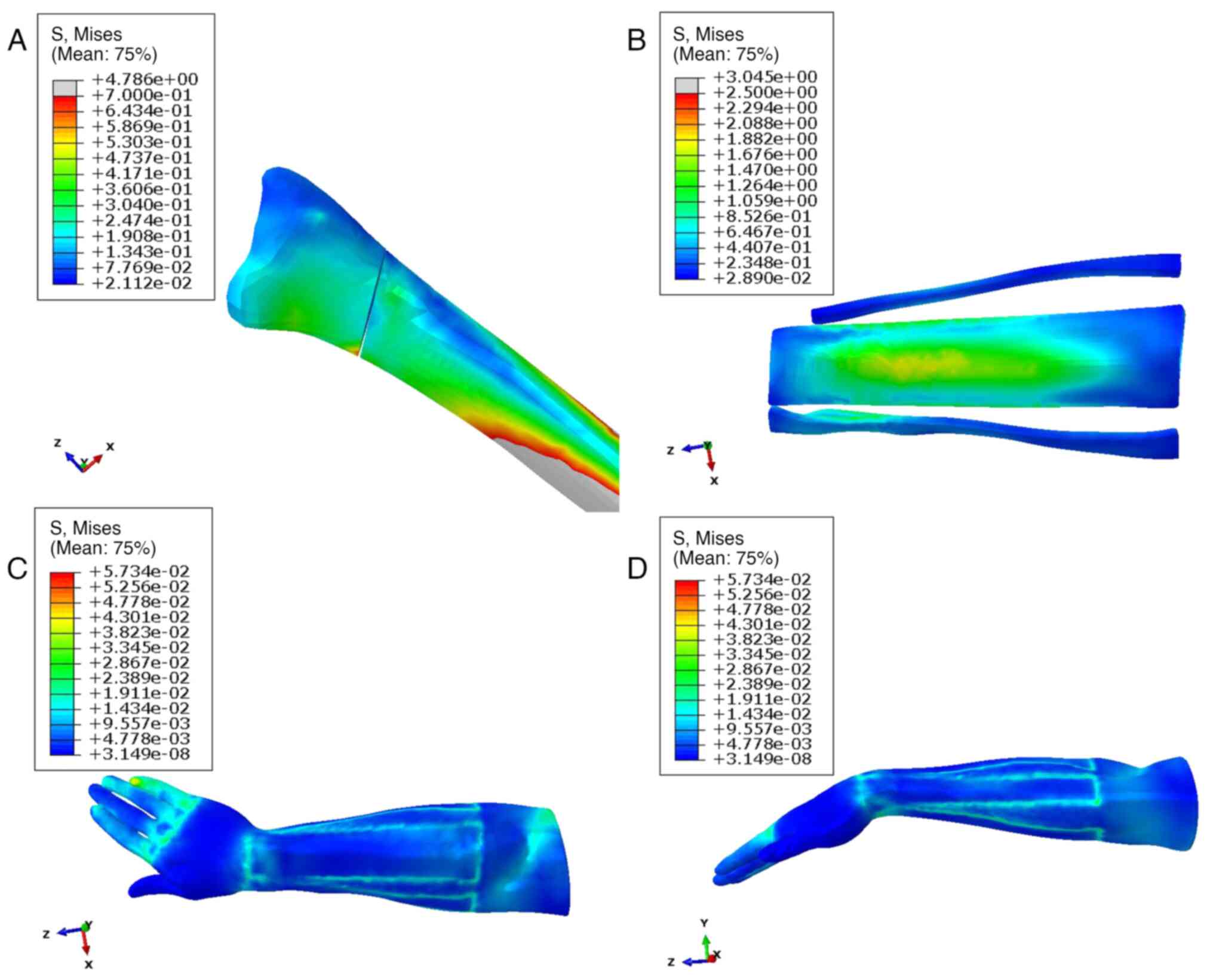
Distribution of soft tissue stress and
displacement in different body positions. Soft tissue stress
distribution in pronation
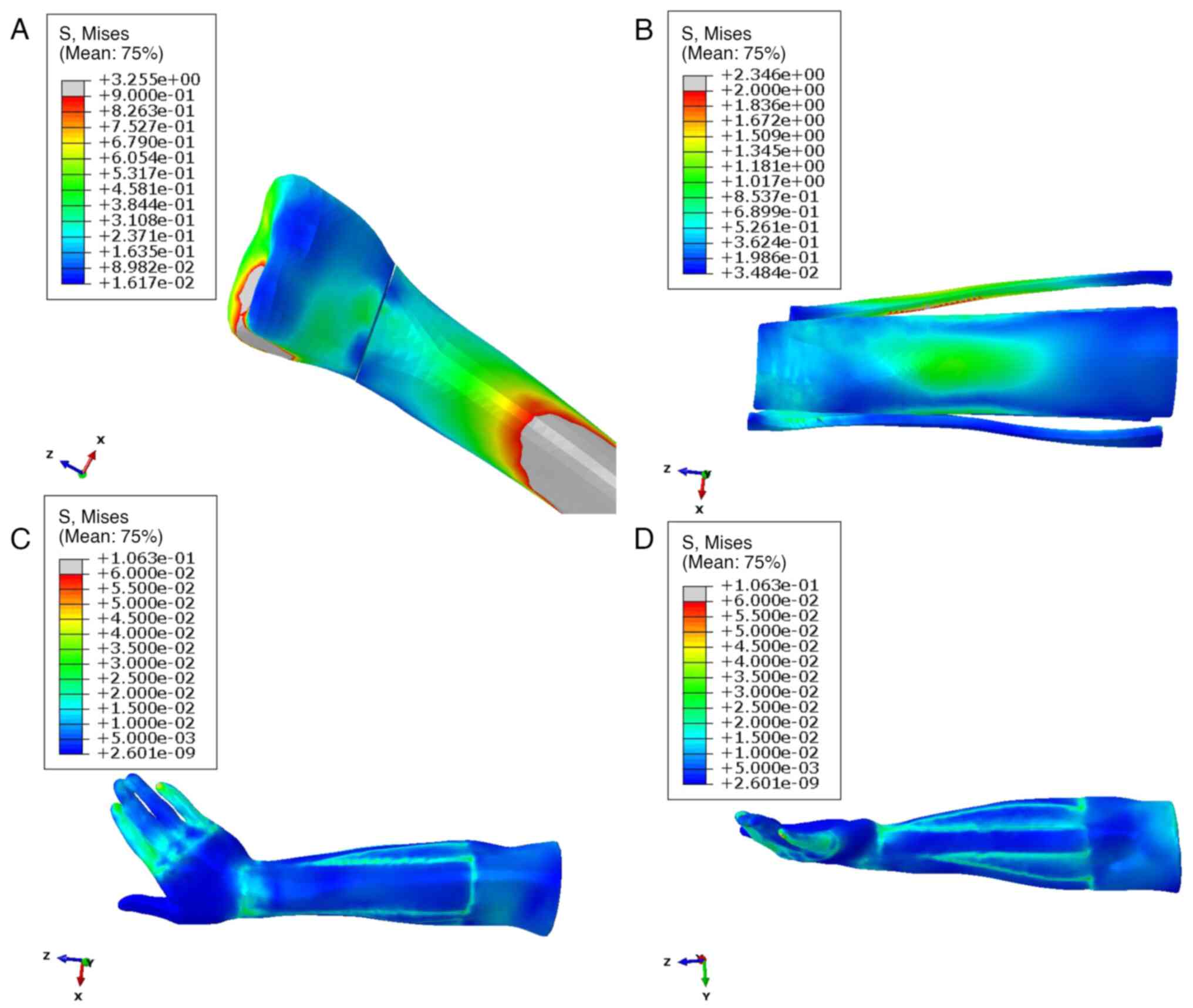
The stress distribution was consistently uniform
across the various radial fracture positions and encompassed
critical anatomical structures such as the thenar muscles, radial
styloid process and ulnar head. During pronation, the absolute
stress of anatomical splints was 0.491±0.346 MPa, reaching a
maximum of 2.346 MPa, whereas soft tissues exhibited significantly
lower values with 0.012±0.006 MPa and a maximum of 0.106 MPa
(P<0.001; Table II). In
addition, the anatomical splints and soft tissue exhibited the
similar absolute displacement (4.676±1.220 vs. 4.210±1.531,
P>0.05), but the soft tissues had bigger displacement in x-axis
than the anatomical splints (-2.450±1.462 vs. -3.490±2.929;
P=0.007; Table II). The
distribution pattern of the anatomical splints and soft tissue
stress during the pronation position is visually presented in
Fig. 3, which provides detailed
insight into the biomechanical response in this specific
configuration.
 | Table IIDistribution of anatomical splints and
soft tissue stress and displacement in different body
positions. |
Table II
Distribution of anatomical splints and
soft tissue stress and displacement in different body
positions.
| | Pronation | Median rotation | Supination |
|---|
| Variables | Anatomical
splints | Soft tissues | t-value | P-value | Anatomical
splints | Soft tissues | t-value | P-value | Anatomical
splints | Soft tissues | t-value | P-value |
|---|
| Stress (x-axis) | -0.0003±0.145 | -0.020±0.026 | 0.93 | 0.354 | -0.006±0.077 | -0.014±0.022 | 1.766 | 0.081 | -0.009±0.144 | -0.026±0.026 | 0.822 | 0.413 |
| Stress
(y-axis) | -0.006±0.041 | -0.025±0.028 | 2.684 | 0.009 | 0.007±0.117 | -0.013±0.021 | 1.190 | 0.237 | -0.013±0.066 | -0.029±0.026 | 1.595 | 0.114 |
| Stress
(z-axis) | 0.076±0.517 | -0.022±0.025 | 1.335 | 0.185 | 0.076±0.322 | -0.013±0.021 | 1.950 | 0.054 | 0.020±0.699 | -0.027±0.024 | 0.475 | 0.636 |
| Stress
(absolute) | 0.491±0.346 | 0.012±0.006 | 9.707 | <0.001 | 0.409±0.211 | 0.012±0.007 | 13.30 | <0.001 | 0.562±0.456 | 0.013±0.007 | 8.512 | <0.001 |
| Displacement
(x-axis) | -3.490±2.929 | -2.450±1.462 | -2.226 | 0.007 | 1.234±1.153 | 0.855±0.868 | 1.857 | 0.066 | -2.151±0.627 | -2.162±0.815 | 0.076 | 0.940 |
| Displacement
(y-axis) | -2.456±1.632 | -2.567±1.388 | 0.361 | 0.719 | -7.464±2.719 | -7.452±3.311 | 0.020 | 0.984 | -3.189±0.878 | -3.250±0.893 | 0.344 | 0.731 |
| Displacement
(z-axis) | -1.874±0.487 | -1.677±0.778 | -1.505 | 0.136 | -2.329±0.986 | -1.883±0.943 | 2.312 | 0.023 | -2.356±0.225 | -2.154±0.602 | 2.223 | 0.029 |
| Displacement
(absolute) | 4.676±1.220 | 4.210±1.531 | 1.667 | 0.099 | 8.117±2.542 | 7.908±3.143 | 0.366 | 0.716 | 4.595±0.672 | 4.532±1.078 | 0.351 | 0.727 |
Soft tissue stress distribution in median
rotation. In Fig. 4, the
stress distribution within the radial fracture displayed a
relatively dispersed pattern, with a notable concentration observed
in the dorsal wrist, hypothenar, radial styloid process and other
relevant anatomical regions. During median rotation, the absolute
stress of anatomical splints was 0.409±0.211 MPa, reaching a
maximum of 1.780 MPa, whereas soft tissues exhibited significantly
lower values with 0.012±0.007 MPa and a maximum of 0.069 MPa
(P<0.001; Table II). in
addition, the anatomical splints and soft tissue exhibited a
similar absolute displacement (8.117±2.542 vs. 7.908±3.143;
P>0.05), but the soft tissues had bigger displacement in z-axis
compared with the anatomical splints (-1.883±0.943 vs.
-2.329±0.986, P=0.023; Table
II).
Soft tissue stress distribution in
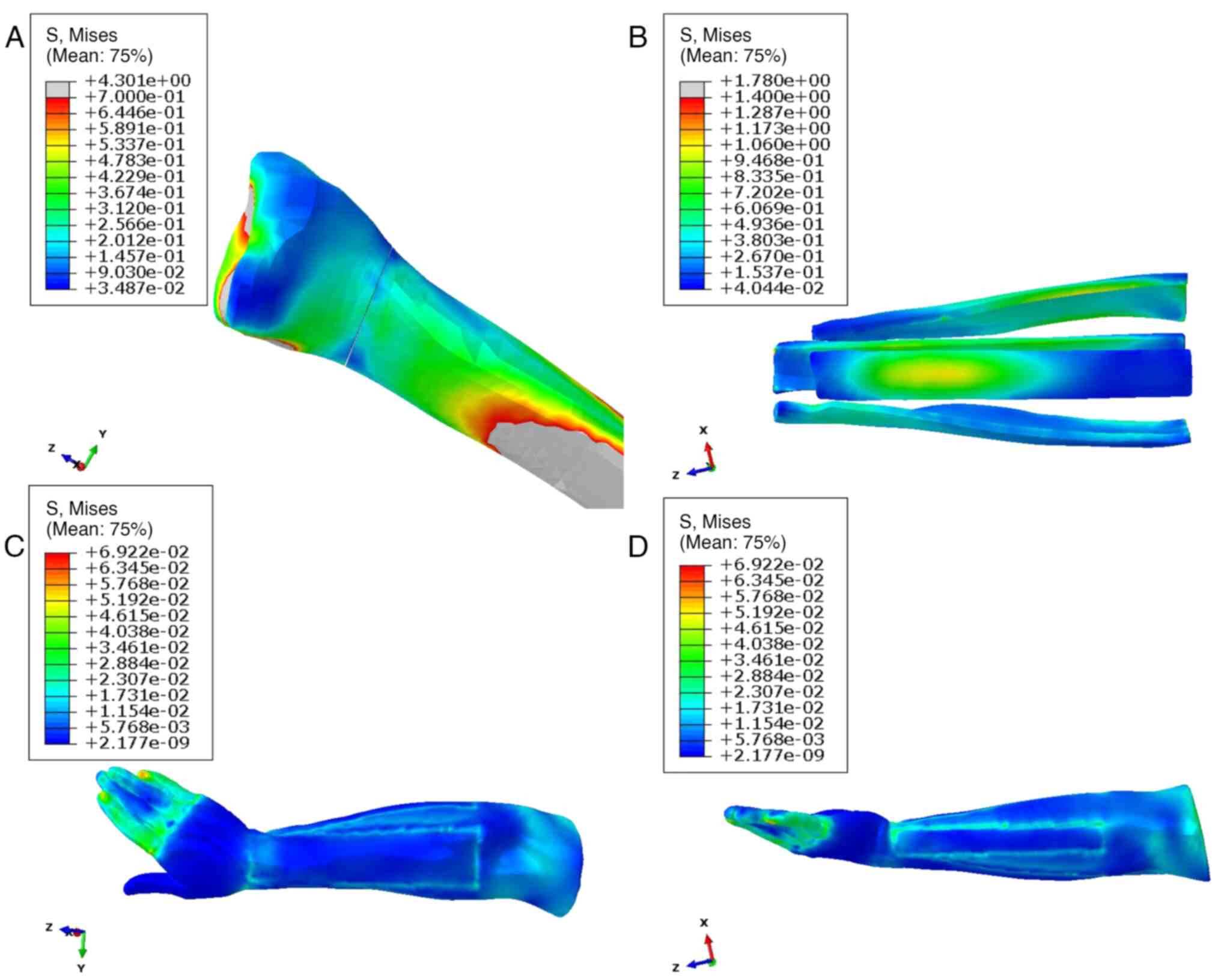
supination. The stress distribution within a radial fracture
exhibited a systematic pattern, with the stress concentration
notably centered around the key anatomical landmarks, including the
wrist joint, radial styloid process, ulnar head and thenar region.
During supination, the absolute stress of anatomical splints was
0.562±0.456 MPa, reaching a maximum of 3.045 MPa, whereas soft
tissues exhibited significantly lower values with 0.013±0.007 MPa
and a maximum of 0.057 MPa (P<0.001; Table II). In addition, the anatomical
splints and soft tissue exhibited a similar absolute displacement
(4.595±0.672 vs. 4.532±1.078, P>0.05), but soft tissues had
bigger displacement in z-axis than the anatomical splints
(-2.154±0.602 vs. -2.356±0.225, P=0.029; Table II). A comprehensive visualization
of this stress distribution and intensity is presented in Fig. 5, offering a detailed depiction of
the biomechanical response in the context of radial fracture
conditions.
Discussion
Biomechanical research is usually not widely
conducted because of technical and ethical restrictions. The tissue
attributes of human specimens are unstable and the replication of
their physiological environment is challenging; however,
biomechanical research relies almost exclusively on the
instrumental testing, thus incurring extremely high costs (26). Biomechanical FEA is generally
performed via a 3D model that is similar to the human body.
Specifically, the 3D model is constructed based on, for example
imaging data, material properties, loads, and boundary conditions
and the local regional information can be acquired after the
simulation of the structure, parameters and loads of the 3D model
(4). In general, biomechanical FEA
is distinguished by its cost-effectiveness, swift calculation
cycle, high simulation accuracy and excellent reproducibility. In
recent years, FEA has been increasingly applied to bone and joint
stress analysis, fracture risk prediction, internal fixator design
and surgical treatment guidance. A previous study established a 3D
finite element model of Colles fractures for the first time, thus
providing a reference for clinical work (27).
Colles fractures are a relatively common disorder
that are mainly caused by indirect violence and can result in joint
deformity, limited mobility, pain and swelling and are usually
treated conservatively (28,29).
Presently, the outcomes of fixation differ based on the materials
employed in clinical practice. Compared with traditional fixation
with a gypsum plate, fixation with small splints is more flexible
and conducive to blood reflux, movement and distraction of soft
tissues around the fracture (30).
Under actual physiological conditions, tissues such as bone,
cartilage, ligaments and interosseous membranes do not fully
exhibit elastic, linear and isotropic behavior. This is because
biomaterials may exhibit complex nonlinear responses under external
effects, which are influenced by various factors, including
deformation rate, loading history and humidity (31). In the present finite element model,
elastic, linear and isotropic properties were chosen mainly to
simplify the model, to improve computational efficiency and make it
easier to interpret. However, in biomechanical research,
understanding the true performance of biological tissues is
crucial. In reality, materials such as bones, cartilage and
ligaments may exhibit nonlinear, time-varying, or anisotropic
behavior under different load conditions. This may be influenced by
the complex structure and diversity of biological tissues, as well
as their interactions with the surrounding environment (32). To simulate these nonlinear
behaviors more accurately, it is necessary to use more complex
material models, such as hyperelastic models and progressive
failure models, for an improved reflection of the true performance
of biomaterials (33). However,
such a model can also increase computational complexity and
resource requirements. The present study made compromises in model
selection to ensure a preliminary simulation of the fracture
situation, but it was also realized that this is a simplification
that requires further exploration in actual biomechanical
research.
There are a few reports on FEA for fractures fixed
by splints. Hua et al (4)
explored the biomechanics of splint fixation of distal radius
fractures using three different materials by 3D FEA and analyzed
the cloud diagrams of the stress distribution in the soft tissue
and bone joints from the forearm to the wrist joint. CT scans were
used to obtain the models in that study. As the constitutive
equation of various tissues of the human body cannot be provided
through relevant mechanical experiments or basic research, the
heterogeneity and anisotropy of the tissue material of the human
body were assumed to be homogeneous and isotropic linear elastic
materials in the present study. According to the model and
calculation results, the stress distribution of the limb soft
tissue was uniform and the stress of easy entrapment sites such as
the apophysis and thenar region was small. Furthermore, maximum
stress of the anatomical splints and soft tissues was 2.346 and
0.106 MPa in pronation, 1.780 and 0.069 MPa in median rotation and
3.045 and 0.057 MPa in supination, respectively. Therefore, the
stress on the median rotation was minimal, providing a reliable
biomechanical basis for the clinical application of anatomical
splints. A notable departure is the examination of positions such
as pronation, median rotation and supination, shedding light on
diverse stress patterns. However, as in Hua et al (4), the present study assumed the tissue
material of the human body to be homogeneous and isotropic linear
elastic materials due to the unavailability of constitutive
equations through relevant mechanical experiments or basic
research. In contrast to the emphasis on distal radius fractures in
Hua et al (4), the present
study focused on Colles fractures, revealing distinct stress
distribution characteristics in anatomical splints and soft tissues
across different positions.
According to the FEA results, it can be observed
that the stress at the fracture site is minimal in the neutral
position, indicating the highest stability of the fracture. This
conclusion is drawn based on experimental findings. The forearm is
composed of two bones, the ulna and radius, along with the
interosseous membrane between them. The interosseous membrane is a
dense connective tissue that not only serves to connect and
transmit forces between the two bones but also provides stability,
thus limiting the maximum rotational movement of the forearm.
Previous research, including anatomical studies, suggests that the
interosseous membrane is most relaxed in the neutral position,
where it exerts the least traction on the bones and contributes to
the overall stability of the fracture site (34-36).
Additionally, using a splint for external fixation after a
fracture, with the forearm fixed in the neutral position, promotes
functional recovery (37). This
approach is advantageous because fixing the forearm in pronation or
supination positions is less stable than fixing it in the neutral
position. In addition, after removing the splint, restoring the
supination position is more challenging than restoring the neutral
position if it was initially fixed in pronation and vice versa. The
3D FEA of the biomechanics revealed that anatomical splints
maintained the palmar tilt and ulnar deviation fixation in Colles
fractures. As a rotating limb, different positions of the forearm
could cause changes to the mechanical structure (38). Additionally, the stress analysis of
Colles fractures fixed with anatomical splints indicated that the
maximum soft tissue stress was in pronation and the maximum splint
stress was in supination. Taken together, the application of
anatomical splints to fix the limb in neutral position can
effectively avoid local soft tissue compression and facilitate the
healing of the fracture.
In the present study, a finite element model of
Colles fractures was precisely designed, considering the various
contact interactions crucial to simulate the realistic
biomechanical responses. The model incorporated distinct contact
types: ‘Bone-to-Bone Contact’ for interactions between different
bone structures such as the radius and ulna; ‘Interior of Bones’ to
represent the internal bone interactions; and ‘Bone-to-Soft Tissue
Contact’ which specified connections between the bones and soft
tissues, such as ligaments. The decision to set the friction
coefficient within the joints as negligible aimed to simplify the
model while focusing on the primary factors influencing the
stability and stress distribution in Colles fractures. This
approach aligns with the established biomechanical models, which
ensures a comprehensive understanding of the intricate interactions
within the finite element model and enhances the reliability of the
simulation results.
However, there were still some limitations. The
finite element model used in this study was relatively simple. In
addition, the model in this study neglects the intrinsic influence
of dynamic structures such as muscles and tendons and is unable to
simulate the stress of various materials in functional training. In
addition, FEA also has some limitations. For instance, the finite
element model can only approach the real situation but cannot fully
replicate the actual environment. Therefore, the authenticity and
validity of the above results need to be verified through
additional experiments. Further study can be focus on conducting a
long-term follow-up study is imperative to assess the sustained
efficacy and potential complications associated with anatomical
splint fixation for Colles fractures. This longitudinal
investigation will provide crucial insights into the enduring
effectiveness of the treatment and its impact on key clinical
indicators. Furthermore, comparative studies should be undertaken
to juxtapose anatomical splint fixation with alternative treatment
modalities such as traditional fixation methods or surgical
interventions. Through a comprehensive comparison of the
biomechanical effects and clinical outcomes of different
therapeutic approaches, a clearer understanding of the advantages
and disadvantages of anatomical splint fixation can be elucidated.
In addition, patient stratification studies are essential to
evaluate the effects of anatomical splint fixation across diverse
patient subgroups, considering varying characteristics of Colles
fractures or pertinent medical histories. This stratified approach
will facilitate the assessment of treatment efficacy in different
patient cohorts, thereby contributing to personalized treatment
strategies and the optimization of therapeutic interventions.
The present study revealed that the apex of splint
stress occurred during supination, which contrasts with the peak of
soft tissue stress observed in pronation. When scrutinizing the
tissue and splint stress through a 3D finite element model of
Colles fracture during pronation, median rotation and supination,
notable findings emerged. Consequently, it is deduced that
anatomical splint fixation during median rotation proves
efficacious in averting the localized compression of soft
tissue.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Scientific
Research Project of Traditional Chinese Medicine Bureau of
Guangdong Province (grant no. 20201170).
Availability of data and materials
The data generated in the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
Conception and design was by FH. RT and MWW provided
administrative support. Provision of study materials or patients
was by RT, LCH, MW and ZW. Collection and assembly of data was by
SDS and JWH. Data analysis and interpretation was by YWL. FH and
YWL confirm the authenticity of all the raw data. All authors
participated in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Third Affiliated Hospital of Guangzhou University
of Chinese Medicine and oral informed consent was obtained from the
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blakeney WG: Stabilization and treatment
of Colles' fractures in elderly patients. Clin Interv Aging.
5:337–344. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Uppal G, Thakur A, Chauhan A and Bala S:
Magnesium based implants for functional bone tissue regeneration-A
review. J Magn Alloys. 10:356–386. 2022.
|
|
3
|
Mellstrand-Navarro C, Pettersson HJ,
Tornqvist H, Tornqvist H and Ponzer S: The operative treatment of
fractures of the distal radius is increasing: Results from a
nationwide Swedish study. Bone Joint J. 96-B:963–969.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hua Z, Wang JW, Lu ZF, Ma JW and Yin H:
The biomechanical analysis of three-dimensional distal radius
fracture model with different fixed splints. Technol Health Care.
26:329–341. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Taylor M and Prendergast PJ: Four decades
of finite element analysis of orthopaedic devices: Where are we now
and what are the opportunities? J Biomech. 48:767–778.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yu XF: The Analysis of the curative effect
of different surgical methods on distal radius fracture and
biomechanical analysis of associated three-dimensional finite
element analysis model. Shijiazhuang: Hebei Medical University,
2021. Available from: https://d.wanfangdata.com.cn/thesis/D02511085.
|
|
7
|
Clough RW: The Finite Element Method in
Plane Stress Analysis. 1960. Available from: https://www.mendeley.com/catalogue/3dce7055-cde9-3d07-b2a8-712b203dd6a2/.
|
|
8
|
Rybicki EF, Simonen FA and Weis EB Jr: On
the mathematical analysis of stress in the human femur. J Biomech.
5:203–215. 1972.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brekelmans WA, Poort HW and Slooff TJ: A
new method to analyse the mechanical behaviour of skeletal parts.
Acta Orthop Scand. 43:301–317. 1972.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiong H and Nie W: Accurate simulation of
stress state in bone joint and related soft tissue injury by
three-dimensional finite element analysis. Chi J Tissue Eng Res.
26:5875–5880. 2022.
|
|
11
|
Yang X, Li Y L and Liu DJ: Progress in the
application of three-dimensional finite element analysis in
anterior cruciate ligament reconstruction. Chi J Sports Med.
39:742–745. 2020.
|
|
12
|
Marongiu G, Leinardi L, Congia S, Frigau
L, Mola F and Capone A: Reliability and reproducibility of the new
AO/OTA 2018 classification system for proximal humeral fractures: A
comparison of three different classification systems. J Orthop
Traumatol. 21(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fishman EK: CT scanning and data
post-processing with 3D and 4D reconstruction: Are we there yet?
Diagn Interv Imaging. 101:691–692. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
White NJ and Rollick NC: Injuries of the
scapholunate interosseous ligament: An update. J Am Acad Orthop
Surg. 23:691–703. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang HJ: Atlas of Human Anatomy. Beijing:
People's Medical Publishing House Co., Ltd., 2005:411. Available
from: https://book.douban.com/subject/1448343/.
|
|
16
|
Xie RG, Tang JB and Tang TS: Morphological
and arthroscopical observation of the triangular fibrocartilage
complex of wrist. Chi J Joint Surg (Electronic Edition). 1:41–45.
2011.
|
|
17
|
Çelik A, Kovacı H, Saka G and Kaymaz İ:
Numerical investigation of mechanical effects caused by various
fixation positions on a new radius intramedullary nail. Comput
Methods Biomech Biomed Engin. 18:316–324. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mouzakis DE, Rachiotis G, Zaoutsos S,
Eleftheriou A and Malizos KN: Finite element simulation of the
mechanical impact of computer work on the carpal tunnel syndrome. J
Biomech. 47:2989–2994. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wei B, Xu ZC and Chang S: Biomechanical
properties of the lumbar pedicle screws by finite element analysis.
Chin J Tissue Eng Res. 22:3091–3096. 2018.
|
|
20
|
Xia CJ, Yuan ZF and Fang N: Biomechanical
characteristics of the distal radius fracture based on
three-dimensional finite element model of ulna and radius. Chi J
Tissue Eng Res. 24:893–897. 2020.
|
|
21
|
Matsuura Y, Kuniyoshi K, Suzuki T, Ogawa
Y, Sukegawa K, Rokkaku T and Takahashi K: Accuracy of
specimen-specific nonlinear finite element analysis for evaluation
of distal radius strength in cadaver material. J Orthop Sci.
19:1012–1018. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qin B, Huang YH and Ouyang Y: Finite
element analysis in scaphoid bone under action of axial stress. J
Army Med University. 32:1213–1315. 2010.
|
|
23
|
Li X: Splint binding force of small splint
for distal radius fracture on the elders: A clinical and
experimental quantitative research. Guangzhou: Guangzhou University
of Chinese Medicine, 2011. Available from: https://cdmd.cnki.com.cn/Article/CDMD-10572-1011132640.htm.
|
|
24
|
Wu ZP, Gao WY and Wu LJ: Progress of
finite element analysis on biomechanics of the wrist joint. Int J
Orthop. 29:307–309. 2008.
|
|
25
|
Zhou XN: Establishment of
three-dimensional finite element model of wrist joint and
biomechanical analysis of distal radius fracture. Beijing: Beijing
University of Chinese Medicine, 2014. Available from: https://cdmd.cnki.com.cn/Article/CDMD-10026-1014242530.htm.
|
|
26
|
Shapiro JA, Feinstein SD, Jewell E, Taylor
RR, Weinhold P and Draeger RW: A biomechanical comparison of
modified radioscapholunate fusion constructs for radiocarpal
arthritis. J Hand Surg Am. 45:983.e1–e7. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang D, Li Y, Yin H, Li J, Qu J, Jiang M
and Tian J: Three-dimensional finite element analysis of optimal
distribution model of vertebroplasty. Ann Palliat Med. 9:1062–1072.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grafstein E, Stenstrom R, Christenson J,
Innes G, MacCormack R, Jackson C, Stothers K and Goetz T: A
prospective randomized controlled trial comparing circumferential
casting and splinting in displaced Colles fractures. CJEM.
12:192–200. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li DL and Shi JM: Efficacy analysis of
different treatment on distal radius comminuted fracture in elderly
patients. Chi J Trad Med Traumatol Orthop. 41–43. 2012.
|
|
30
|
Huang AY, Li GQ and Cao LB: Treatment of
270 cases of colles fracture with Li's Manual reduction and willow
splint fixation. J Ext Ther Trad Chi Med. 64–66. 2022.
|
|
31
|
Salmon P: Loss of chaotic trabecular
structure in OPG-deficient juvenile Paget's disease patients
indicates a chaogenic role for OPG in nonlinear pattern formation
of trabecular bone. J Bone Miner Res. 19:695–702. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wear KA: Mechanisms of interaction of
ultrasound with cancellous bone: A review. IEEE Trans Ultrason
Ferroelectr Freq Control. 67:454–482. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Steiner JA, Ferguson SJ and van Lenthe GH:
Computational analysis of primary implant stability in trabecular
bone. J Biomech. 48:807–815. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Knothe Tate ML, Tami AE, Netrebko P, Milz
S and Docheva D: Multiscale computational and experimental
approaches to elucidate bone and ligament mechanobiology using the
ulna-radius-interosseous membrane construct as a model system.
Technol Health Care. 20:363–378. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Oliva F, Buharaja R, Iundusi R and
Tarantino U: Leg fracture associated with synostosis of
interosseous membrane during running in a soccer player. Transl Med
UniSa. 17:1–5. 2018.PubMed/NCBI
|
|
36
|
He J, Ma X, Hu Y, Wang S, Cao H, Li N,
Wang G, Guo L and Zhao B: Investigation of the characteristics and
mechanism of interosseous membrane injuries in typical maisonneuve
fracture. Orthop Surg. 15:777–784. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Adams JE, Culp RW and Osterman AL:
Interosseous membrane reconstruction for the Essex-Lopresti injury.
J Hand Surg Am. 35:129–136. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Thangavel M and Elsen Selvam R: Review of
physical, mechanical, and biological characteristics of 3D-Printed
bioceramic scaffolds for bone tissue engineering applications. ACS
Biomater Sci Eng. 8:5060–5093. 2022.PubMed/NCBI View Article : Google Scholar
|