Introduction
Piperacillin-tazobactam is a broad-spectrum
antibacterial drug consisting of the penicillin antibacterial drug
piperacillin and the β-lactamase inhibitor tazobactam (1). Piperacillin-tazobactam is effective
against gram-positive (such as Staphylococcus aureus, etc.),
gram-negative aerobic (such as Escherichia coli, etc.) and
anaerobic bacteria (bacteroides fragilis, etc.). It is typically
used empirically for treating patients with pulmonary infections
(staphylococcus aureus, haemophilus influenzae, pseudomonas
aeruginosa, etc.) (2). As a
commonly used clinical antibiotic, piperacillin-tazobactam is
generally considered safe and well-tolerated (3).
Adverse reactions to piperacillin-tazobactam are
rarely reported, with the most common commonly observed adverse
reactions including gastrointestinal symptoms (0.9%) and skin
reactions (including rashes and itching) (1.3%) (3). Renal dysfunction may increase the
risk of adverse reactions. A previous case reported that a patient
treated with piperacillin-tazobactam developed an allergic reaction
(rash with pruritus) and drug-induced fever (despite negative
penicillin skin tests (4). In
addition, hematological adverse reactions associated with
piperacillin-tazobactam treatment mainly include hemolytic anemia
(<1%), thrombocytopenia (<1%) and neutropenia (<1%).
Notably, both anemia and thrombocytopenia are associated with
autoimmunity and neutropenia with bone marrow suppression (5). Another previous study reported that
piperacillin-tazobactam treatment caused DRESS syndrome (an adverse
reaction with eosinophilia and systemic symptoms, involving fever,
rash, eosinophilia and multi-organ failure). H1 receptor
antagonists and corticosteroids were used to treat the adverse
reaction (6).
The present article describes the case of an elderly
female patient with myocardial injury and heart failure induced by
piperacillin-tazobactam treatment. However, this patient
experienced a resolution of symptoms, return of blood indicators to
normal levels and improvements on electrocardiogram (ECG)
parameters following the discontinuation of
piperacillin-tazobactam.
Case report
In April 2023, a 75-year-old female patient
presented with an unexplained, irregular fever with a maximum
temperature of 40˚C that had lasted for 11 days, accompanied by a
generalized rash and joint pain. This patient possessed a history
of hypertension for >10 years and was treated accordingly with
irbesartan. The patient reported no history of coronary
atherosclerotic heart disease, diabetes mellitus or hyperlipidemia.
The patient was subsequently admitted to Shandong Provincial
General Hospital in Jinan, China.
On admission, the patient presented with a body
temperature of 38.5˚C, a heart rate (HR) of 78 beats/min and a
blood pressure of 123/82 mmHg. The patient was conscious but
fatigued, with a scattered rash on the skin of the chest and back.
Respiratory sounds were clear in both lungs with no dry or wet
rales. In addition, heart sounds were audible and rhythmical, with
no heart murmurs. There was no presence of edema in the lower
limbs. Results of laboratory tests demonstrated leukocyte levels of
15.58x109/l, neutrophil levels of
13.89x109/l, eosinophil levels of 0.10x109/l,
alanine aminotransferase levels of 57 U/l, glutamyl transpeptidase
levels of 144 U/l, albumin levels of 26.6 g/l, glucose levels of
8.37 mmol/l, C-reactive protein (CRP) levels of 144.54 mg/l,
D-dimer levels of 5.69 µg/ml, brain natriuretic peptide (BNP)
levels of 1,655.34 pg/ml, ferritin levels of 4,523.31 ng/ml and
positive anti-nuclear antibodies (Table I). The levels of leukocytes,
neutrophil, alanine aminotransferase, glutamyl transpeptidase,
glucose, CRP, D-dimer, BNP and ferritin were higher than the ref
range. These suggested the presence of underlying infection, liver
function impairment, hyperglycemia, heart failure, and blood
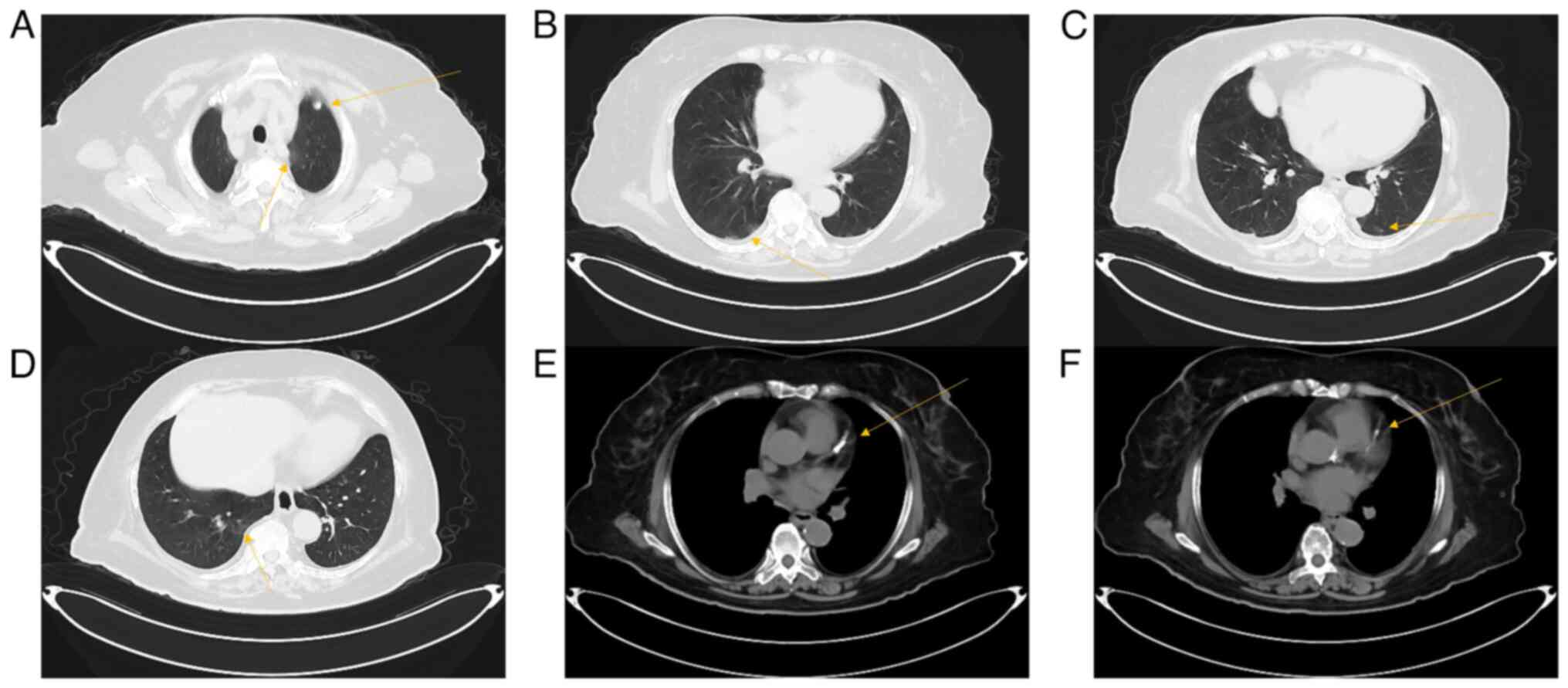
hypercoagulability. Results of a chest CT scan (supplier: Siemens,
imaging parameters: KV:120, ref.mAs:135, TI:0.5, SL
5.0/128.0x0.6/p1.5, W1200 C-600) demonstrated ground-glass opacity
in both lungs, where patchy and corded shadows were observed in the
middle and lower lobes of both right and left lungs (Fig. 1B-D). In addition, multiple small
ground glass nodular shadows were observed in the upper and lower
lobes of both lungs, where a large nodular shadow was observed in
the upper lobe of the left lung, with a diameter of ~0.7 cm
(Fig. 1A). There was calcification
of the aorta and coronary arteries (Fig. 1E and F). Results of a coronavirus-19 (COVID-19)
test, respiratory pathogen test, rheumatic series, Epstein-Barr
virus test and blood, sputum and urine cultures all returned
normal. Numerous clinical consultations and expert discussions led
to the potential diagnoses of adult Still disease, pulmonary
infection, hypoproteinemia, liver damage and hypertensive disease.
Anti-infective piperacillin-tazobactam (4.5 g q12h iv.) and
anti-inflammatory methylprednisolone sodium succinate (20 mg q12h
i.v.) were administered to the patient, alongside symptomatic
treatments, such as an anti-pyretic [ibuprofen (0.1 g prn po)] and
liver function protection [glutathione (0.4 g qd po)]. Following 3
days of hospitalization, the body temperature of the patient
returned to 37˚C, the rash cleared and the joint pain was
relieved.
 | Table ISpecific numbers of laboratory tests
during the entire treatment process of the patient. |
Table I
Specific numbers of laboratory tests
during the entire treatment process of the patient.
| Laboratory tests | Normal value
range | 11 days before
admission | 2 days before
admission | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 | Day 11 | Day 13 | Day 14 | Day 16 |
|---|
| Leukocyte,
x109/l | 3.5-9.5 | 15.58 | 11.84 | 16.47 | N | 21.27 | 15.49 | 11.94 | 7.62 | 11.1 | 21.95 | 7.53 |
| Neutrophil,
x109/l | 1.80-6.30 | 13.89 | 9.81 | 13.51 | N | 18.82 | 10.48 | 8.36 | 6.18 | 10.64 | 21.08 | 6.38 |
| Eosinophil,
x109/l | 0.02-0.52 | N | N | 0.38 | N | 0.15 | 0.32 | 0.10 | N | 0.05 | 0.12 | 0.02 |
| Albumin, g/l | 40-55 | 26.6 | 31.5 | N | 26.8 | N | N | 33.4 | N | N | N | N |
| Glucose, mmol/l | 3.9-6.1 | 8.37 | 3.82 | N | 7.73 | N | N | N | N | N | N | N |
| C-reactive protein,
mg/l | 0-10 | 144.54 | 16.95 | 43 | 43 | N | 26 | 8.6 | N | 4.7 | 4.1 | N |
| D-dimer, µg/ml | 0-0.5 | 5.69 | 6.71 | 14.37 | N | 4.91 | N | 2.67 | N | N | N | N |
| Brain natriuretic
peptide, pg/ml | 0-125 | 1655.34 | N | 730 | 7046 | N | N | 4922 | 2145 | 1621 | 6537 | 4888 |
| Ferritin, ng/ml | 13-150 | 4523.31 | 4286.03 | N | 4858 | N | N | N | N | N | N | 1145 |
| Troponin I,
ng/ml | 0-0.1 | N | N | 1.995 | 3.378 | 1.29 | 0.39 | 0.114 | N | N | 0.037 | N |
| Procalcitonin,
ng/ml | 0-0.1 | N | N | 2.13 | N | N | N | 1.81 | N | 0.35 | 15.28 | 3.41 |
| Creatine kinase
isoenzyme, ng/ml | 0-5 | N | N | 1.75 | 10.31 | 5.99 | N | 2.14 | N | 2.1 | 4.77 | 1.71 |
| Creatine kinase,
U/l | 50-310 | N | N | 195 | 366 | 174 | N | 10 | N | 19 | 355 | 50 |
| Lactate
dehydrogenase, U/l | 120-250 | N | N | 275 | 568 | 519 | N | 248 | N | 293 | 276 | 209 |
| α-hydroxybutyrate
dehydrogenase, U/l | 59-126.4 | N | N | 193 | 401 | 316 | N | 176 | N | 214 | 172 | 149 |
After nine of days, laboratory indicators were
assessed again and the results demonstrated leukocyte levels of
11.84x109/l, neutrophil levels of 9.81x109/l,
eosinophil levels of 0.15x109/l, glutamyl transpeptidase
levels of 97 U/l, albumin levels of 31.5 g/l, glucose levels of
3.82 mmol/l, CRP levels of 16.95 mg/l, sedimentation rate of 33
mm/h, D-dimer levels of 6.71 µg/ml and ferritin levels of 4,286.03
ng/ml (Table I). The levels of
leukocytes, neutrophil, glutamyl transpeptidase, CRP, sedimentation
rate, D-dimer, BNP and ferritin were higher than the ref range.
These suggested the presence of underlying infection, liver
function impairment, heart failure, and blood hypercoagulability.
In addition, levels of alanine aminotransferase returned to normal
(39 U/l, reference range: 9-50 U/l). However, the condition of the
patient worsened at night. Specifically, the temperature of the
patient increased to 40˚C, accompanied by chills, cold extremities
and a loss of mental activity. Furthermore, the cause of the fever
remained unknown and no improvements in the condition were
observed. Transferal to a different hospital for treatment was
recommended and the patient was discharged 2 days later and
presented to outpatient clinic of the affiliated hospital of
Shandong university of traditional Chinese medicine (Jinan,
China).
The patient was admitted following a review of their
medical history. On admission, her body temperature was 36.6˚C,
with an HR of 57 beats/min and blood pressure of 166/84 mmHg. The
patient's fever was paroxysmal. The patient would experience chills
when the body temperature was >37.5˚C. Once the fever had
subsided after ibuprofen (0.1 g prn po), the overall condition of
the patient improved, with the rash cleared, wrist and knee pain
being less severe compared with that reported 10 days prior. There
was also no cough or presence of sputum and a 93% oxygen saturation
recorded using a finger pulse. The ECG reading also appeared
normal. Results of laboratory tests demonstrated potassium levels
of 2.55 mmol/l, leukocyte levels of 16.47x109/l,
neutrophil levels of 13.51x109/l, eosinophil levels of
0.38x109/l, CRP levels of 43 mg/l, procalcitonin (PCT)
levels of 2.13 ng/ml and D-dimer levels of 14.37 µg/ml (Table I). In addition, myocardial enzymes
were increased, where results of further laboratory tests
demonstrated creatine kinase isoenzyme levels of 1.75 ng/ml,
creatine kinase levels of 195 U/l, lactate dehydrogenase levels of
275 U/l, α-hydroxybutyrate dehydrogenase levels of 193 U/l and BNP
levels of 730 pg/ml. It was determined that the patient had been
administered piperacillin-tazobactam at a previous hospital after a
negative penicillin skin test result, based on reports from the
patient family.
Although no bacterial infection was identified, the
patient had pneumonia and both leukocyte and neutrophil levels were
higher than the ref range. The patient was administered 4.5 g
piperacillin-tazobactam as the treatment of pneumonia every 12 h on
the day of admission, as recommended by American Thoracic
Society/Infectious Diseases Society of America guidelines (7). In addition, the patient was fitted
with a potassium chloride pump (30 mg st ivvp) and administered
potassium citrate granules (2 g tid po) for potassium
supplementation, ibuprofen (0.1 g prn po) for anti-inflammation and
antipyretic, valsartan (80 mg qd po) and nifedipine (30 mg qd po)
for blood pressure control, in addition to enoxaparin sodium (5000
U qd ih) for anticoagulation. At 7:00 p.m. on the first day of
admission (11 days after initial admission), the patient developed
chills, clenched hands, clenched teeth, fever with a temperature of
38˚C and irritability. The patient was subsequently intravenously
administered with 40 mg methylprednisolone sodium succinate and
low-flow oxygen. At 8:30 p.m, the chills subsided, though the
patient was still experiencing flushing and tachypnea with a body
temperature of 40˚C. Therefore, the patient was treated with
physical cooling, ibuprofen (0.1 g prn po), dexamethasone sodium
phosphate (5 mg st iv) and lansoprazole (30 mg st iv.). Blood
cultures were drawn and assessed, where ECG monitoring and finger
pulse oxygen monitoring were also performed. At 11:20 p.m, the
temperature of the patient had decreased to 37.5˚C and all other
symptoms were resolved.
The following morning (1 day post-admission), the
patient developed chills and a high fever on completion of a
piperacillin-tazobactam infusion. Myocardial enzymes were increased
and results of the laboratory tests demonstrated creatine kinase
isoenzyme levels of 10.31 ng/ml, creatine kinase levels of 366 U/l,
lactate dehydrogenase levels of 568 U/l, α-hydroxybutyrate
dehydrogenase levels of 401 U/l, troponin I levels of 3.378 ng/ml,
BNP levels of 7,046 pg/ml, ferritin levels of 4,858 ng/ml, albumin
levels of 26.8 g/l, glucose levels of 7.73 mmol/l, glycosylated
hemoglobin levels of 7% and CRP levels of 43 mg/l. In addition,
liver function, lymphocyte subsets, rheumatic series and
immunoglobulin levels were normal. ECG demonstrated mild ST-segment
elevation. The patient did not exhibit any chest tightness, chest
pain or dyspnea and had a blood pressure of 110/70 mmHg, an HR of
72 beats/min and no notable dry and wet rales or heart valve
murmurs on auscultation of both lungs. Subsequent consultation with
a cardiovascular specialist recommended that the patient should
undergo a coronary angiography. However, the patient's family
declined due to risks associated with old age and painful symptoms
experienced by the patient. Following review of the patient's
history of hypertension and other medical conditions, clinical
symptoms and examination results, the cardiovascular specialist
determined that the patient presented with myocardial injury with
heart failure. In addition, the potential for acute coronary
syndrome was suspected to be high. However, the cardiovascular
specialist did not exclude the possibility of myocarditis and
pericarditis. Subsequently, the patient was administered
spironolactone (20 mg qd po), sacubitril valsartan sodium (100 mg
qd po) and dapagliflozin (10 mg qd po) for the correction of heart
failure, aspirin (0.1 g qd po) for antiplatelet aggregation,
atorvastatin (20 mg qn po) and ezetimibe (10 mg qd po) to stabilize
plaque formation. Following expert advice, the advanced antibiotic
imipenem-cilastatin sodium hydrate was administered 0.5 g every 8 h
to control the lung infection. At 9:00 p.m, troponin I levels were
measured to be 2.003 ng/ml.
The next day (2 days post-admission, 13 days after
initial admission), the body temperature of the patient was
maintained at 37˚C. Results of a cardiac ultrasound suggested an
aortic valve calcification and reduced left ventricular systolic
function, with an ejection fraction of 49%. Results of the ECG
demonstrated a decrease in ST-segment elevation from the levels
previously observed. Results of the laboratory tests revealed
leukocyte levels of 21.27x109/l, neutrophil levels of
18.82x109/l, eosinophil levels of 0.15x109/l,
D-dimer levels of 4.91 µg/ml and troponin I levels of 1.29 ng/ml.
The levels of leukocytes, neutrophil, D-dimer, and troponin I were
higher than the ref range. These suggested the presence of
underlying infection, myocardial injury, and blood
hypercoagulability. In addition, myocardial enzymes were increased
and results of the laboratory tests demonstrated creatine kinase
isoenzyme levels of 5.99 ng/ml, creatine kinase levels of 174 U/l,
lactate dehydrogenase levels of 519 U/l and α-hydroxybutyrate
dehydrogenase levels of 316 U/l.
At 4 days (15 days after initial admission)
post-admission, results of laboratory tests demonstrated leukocyte
levels of 15.49x109/l, neutrophil levels of
10.48x109/l, eosinophil levels of 0.32x109/l,
CRP levels of 26 mg/l and troponin I levels of 0.39 ng/ml. The
levels of leukocyte, neutrophil, CRP, and troponin I were higher
than the ref range. These suggested the presence of underlying
infection, and myocardial injury. Results of the ECG demonstrated a
T-wave inversion as compared with the results previously described.
Results obtained following galactomannan and (1,3)-β-D-glucan detection, a COVID-19 test,
and an influenza virus test were all negative. In addition,
metagenome next-generation sequencing was performed externally
(Kindstar Global) using blood samples. This is based on
macro-genomics and high-throughput sequencing technology that
allows for the indiscriminate detection of all pathogens, including
bacteria, fungi, viruses, parasites, mycoplasma and chlamydia in a
variety of clinical specimens (8).
However, results of the metagenome next-generation sequencing
revealed that no pathogens were present. Since no definitive source
of infection was identified, an expert opinion was obtained from a
rheumatologist, who confirmed the potential for adult Still
disease. Therefore, the patient was administered methylprednisolone
sodium succinate (20 mg qd iv.) for anti-inflammation, methotrexate
(10 mg qw po) and folic acid (10 mg qw po) for immunosuppression,
calcium carbonate D3 (0.6 g qd po) for osteoporosis prevention and
metoprolol tartrate (12.5 mg qd po) for ventricular rate control.
Additional ECGs were performed the next day (5 days post-admission)
and the results demonstrated progressive decrease in the ST-segment
and an inverted T-wave. Subsequently, 1 day later (6 days
post-admission), results of laboratory tests demonstrated leukocyte
levels of 11.94x109/l, neutrophil levels of
8.36x109/l, eosinophil levels of 0.10x109/l,
CRP levels of 8.6 mg/l, D-dimer levels of 2.67 µg/ml, PCT levels of
1.81 ng/ml, troponin I levels of 0.114 ng/ml, BNP levels of 4,922
pg/ml and albumin levels of 33.4 g/l (reference range: 40-55 g/l).
In addition, myocardial enzymes were decreased and results of
laboratory tests demonstrated creatine kinase isoenzyme levels of
2.14 ng/ml, creatine kinase levels of 10 U/l, lactate dehydrogenase
levels of 248 U/l and α-hydroxybutyrate dehydrogenase levels of 176
U/l. Of note, improvements were observed in the results of all
laboratory tests. The condition of the patient was stable for the
following 5 days (7-11 days post-admission) and there was no change
in the treatment plan.
Since the condition of the patient stabilized,
further laboratory tests were performed on day 12 post-admission
(23 days after initial admission). Results of the laboratory tests
demonstrated leukocyte levels of 11.1x109/l, neutrophil
levels of 10.64x109/l, eosinophil levels of
0.05x109/l, CRP levels of 4.7 mg/l, PCT levels of 0.35
ng/ml and BNP levels of 1,621 pg/ml. In addition, myocardial
enzymes were stable; laboratory tests demonstrated creatine kinase
isoenzyme levels of 2.1 ng/ml, creatine kinase levels of 19 U/l,
lactate dehydrogenase levels of 293 U/l and α-hydroxybutyrate
dehydrogenase levels of 214 U/l. Results of the ECG demonstrated a
T-wave inversion. The patient's condition gradually stabilized, and
experts suggested that antibiotics should be downgraded. Following
expert advice, antibiotic step-down treatment was prescribed, where
4.5 g piperacillin-tazobactam was administered every 12 h to
control infection following a negative penicillin skin test.
Following infusion in the afternoon, the patient presented with a
sore throat, chills, flushing, tachypnea, an elevated temperature
of 38.8˚C and a 97% oxygen saturation. In addition, the blood
pressure of the patient was 110/80 mmHg, the HR was 100 beats/min
and the ECG demonstrated a mild ST-segment elevation. The patient
was treated with physical cooling, dexamethasone sodium phosphate
(5 mg st iv) and ibuprofen (0.1 g prn po), though the symptoms did
not improve. Therefore, the presence of adverse reactions following
treatment with piperacillin-tazobactam was considered. The patient
was administered anti-allergy treatment with promethazine
hydrochloride (25 mg st im) and calcium gluconate (1 g st iv), with
piperacillin-tazobactam discontinued. This resulted in the gradual
alleviation in the symptoms of the patient.
The following day (13 days post-admission), all
laboratory parameters were reviewed. Results of the laboratory
tests demonstrated leukocyte levels of 21.95x109/l,
neutrophil levels of 21.08x109/l, eosinophil levels of
0.12x109/l, CRP levels of 4.1 mg/l, PCT levels of 15.28
ng/ml, troponin I levels of 0.037 ng/ml and BNP levels of 6,537
pg/ml. In addition, myocardial enzymes were increased and results
of the laboratory tests demonstrated creatine kinase isoenzyme
levels of 4.77 ng/ml, creatine kinase levels of 355 U/l, lactate
dehydrogenase levels of 276 U/l and α-hydroxybutyrate dehydrogenase
levels of 172 U/l. Results of the ECG demonstrated ST-segment
recovery close to baseline compared with the results observed the
day prior, with T-wave inversion. Following the discontinuation of
piperacillin-tazobactam, no additional antibiotics were
administered.
All laboratory parameters were re-assessed 2 days
later (15 days post-admission). Results of the laboratory tests
demonstrated leukocyte levels of 7.53x109/l, neutrophil
levels of 6.38x109/l, eosinophil levels of
0.02x109/l, PCT levels of 3.41 ng/ml, BNP levels of
4,888 pg/ml and ferritin levels of 1,145 ng/ml. In addition,
myocardial enzymes were decreased, and results of the laboratory
tests demonstrated creatine kinase isoenzyme levels of 1.71 ng/ml,
creatine kinase levels of 50 U/l, lactate dehydrogenase levels of
209 U/l and α-hydroxybutyrate dehydrogenase levels of 149 U/l. The
next day (16 days post-admission), results of the ECG demonstrated
T-wave inversion and the patient demonstrated no signs of fever
since day 12 post-admission. By day 17 post-admission (28 days
after initial admission), the condition of the patient was stable
(joint pain and skin rash disappeared) and the patient was
discharged from the hospital following expert advice. One month
after discharge, myocardial injury and heart failure symptoms
gradually improved and she did not develop fever.
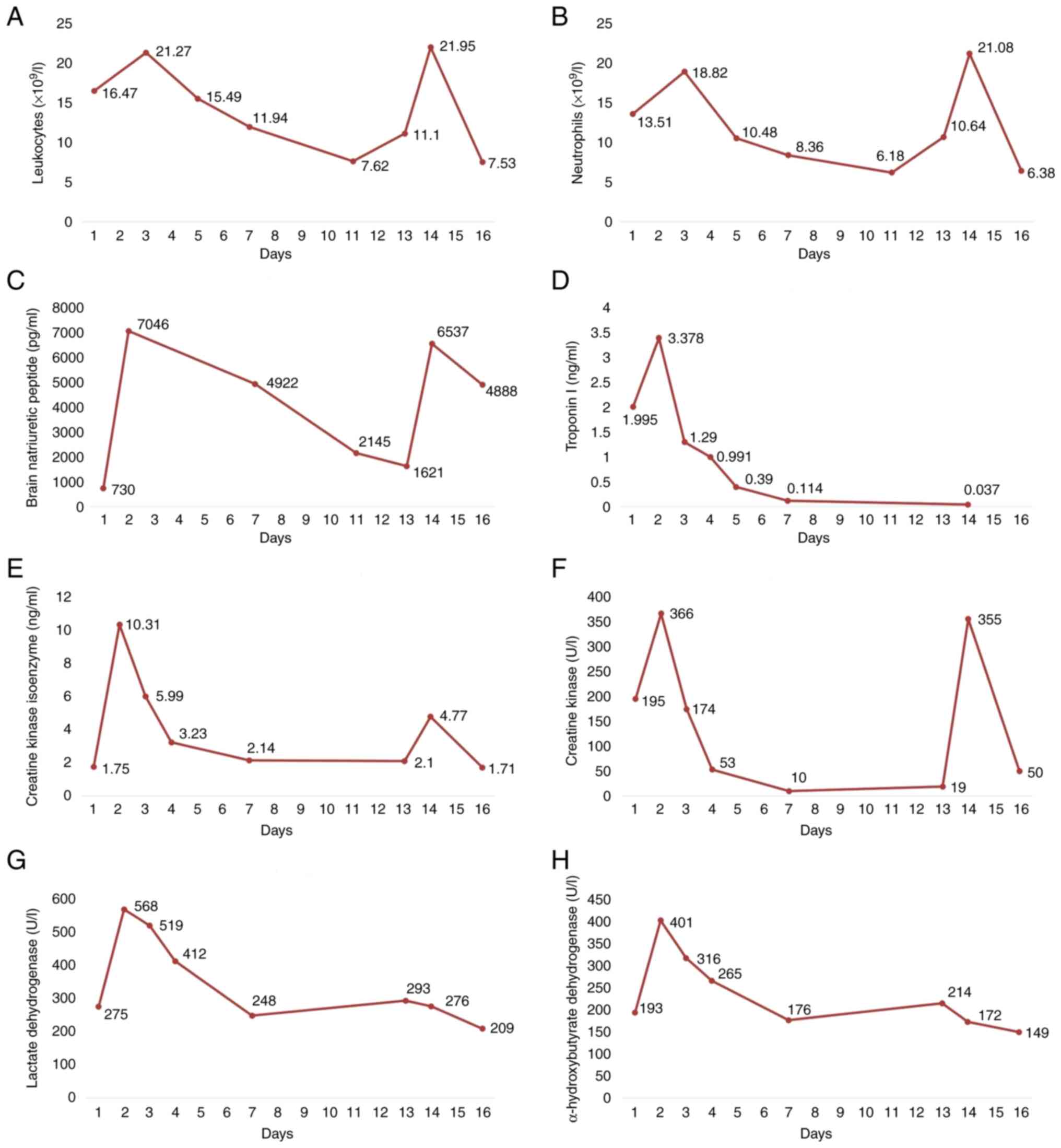
The change curves of laboratory examination results
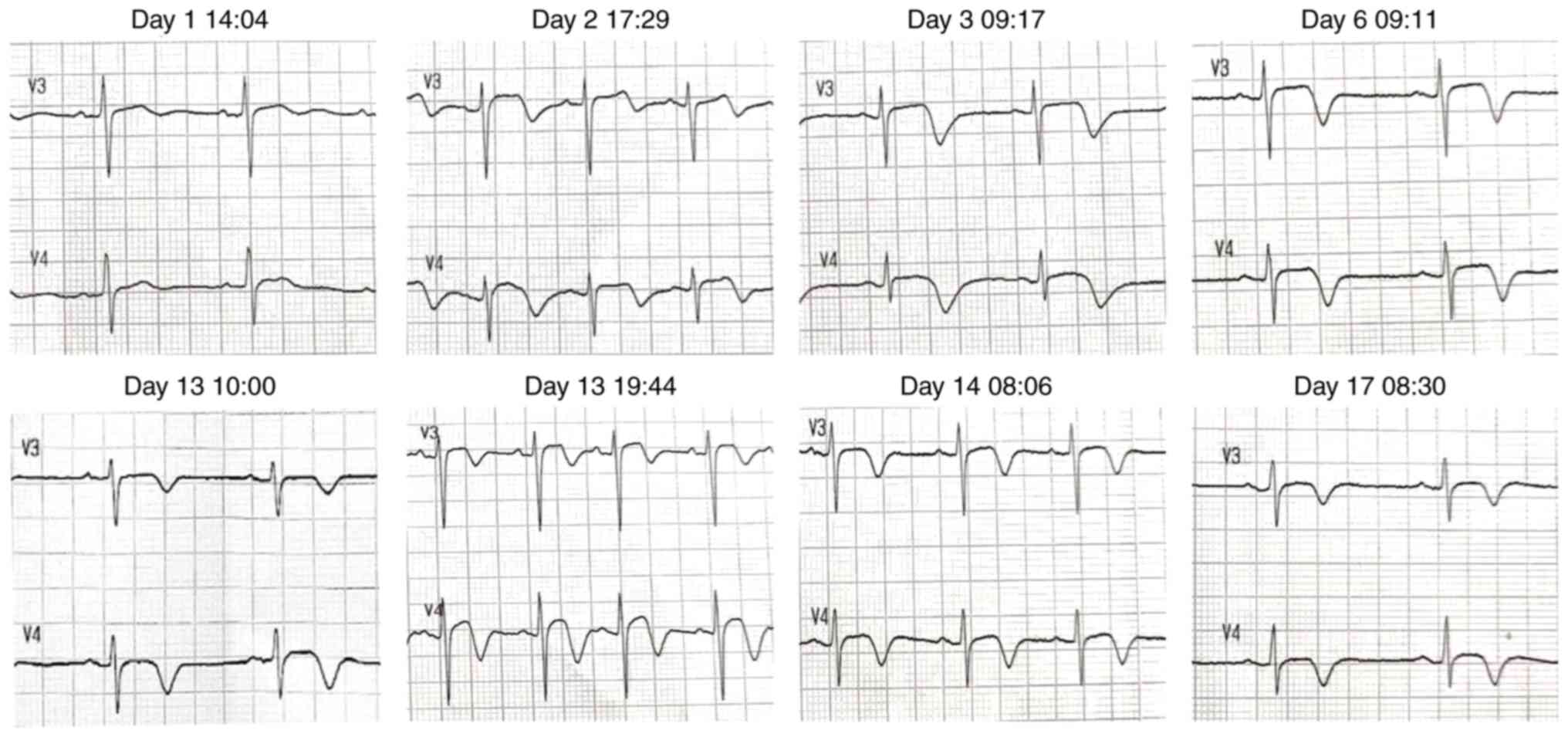
during hospitalization were shown in Fig. 2. The changes in electrocardiogram
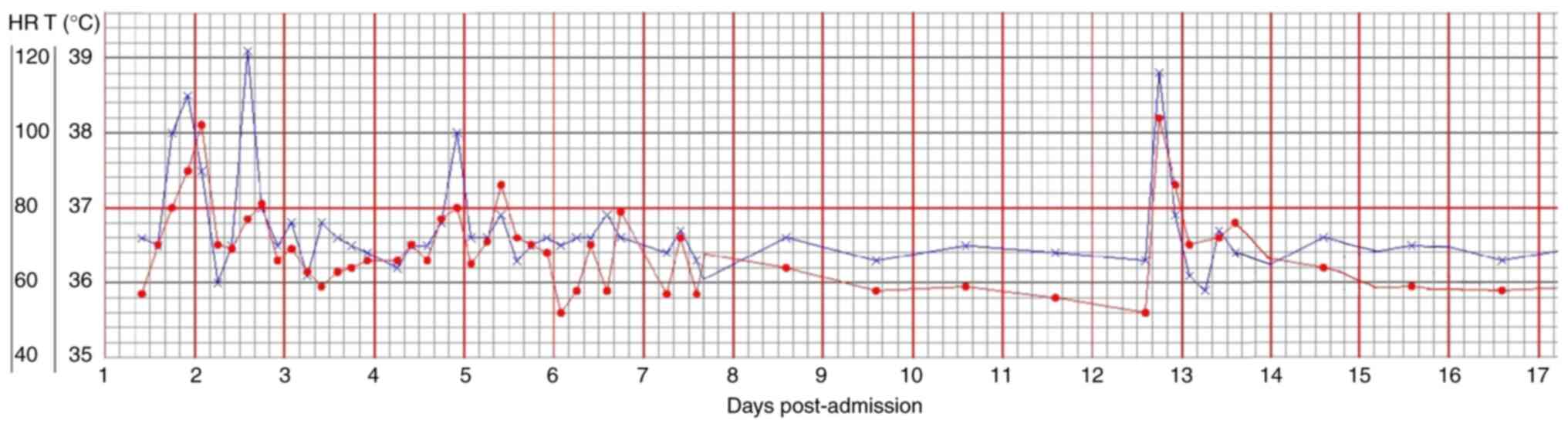
results during hospitalization were presented in Fig. 3. The changes in body temperature
and HR of the patient during hospitalization were presented in
Fig. 4. The patient and the family
of the patient approved the full treatment plan. Written informed
consent was obtained from the patient. The present case was
reported in accordance with the CARE guidelines (9).
Discussion
The present article reports the case of a rare
adverse reaction, specifically myocardial injury with heart
failure, caused by piperacillin-tazobactam. Previous studies
reported that the majority of adverse reactions associated with
piperacillin-tazobactam were gastrointestinal symptoms and skin
reactions (including rashes and itching). Neutropenia and
thrombocytopenia being the most common hematological adverse
reactions (10,11). However, the presence of fever
caused by an allergic reaction has also been reported (12), whereas a rare case of fever,
eosinophilia and liver injury caused by piperacillin-tazobactam
treatment has been documented in another case (13). Furthermore, Jafri et al
(14) reported a case of induced
hypersensitivity myocarditis, a rare complication of drug-related
myocardial injury, following the use of piperacillin-tazobactam.
Calogiuri et al (15)
reported a case of myocardial injury as a result of Kounis syndrome
following the intravenous administration of
piperacillin-tazobactam. Kounis syndrome is a rare allergic
reaction that manifests as coronary vasospasm, which may lead to
angina pectoris or anaphylactic myocardial infarction (16). Therefore, following the findings of
the aforementioned studies, the present case report may further the
current understanding of the association between
piperacillin-tazobactam use and myocardial injury, which provide
further evidence for guiding the use of piperacillin-tazobactam in
clinical practice.
The present article details the case of an adverse
reaction leading to myocardial injury with heart failure in a
patient treated with piperacillin-tazobactam for an unexplained
fever. The patient had a history of hypertension for >10 years.
Following admission to hospital, coronary artery calcification was
observed in the chest CT scan of the patient. Of note, it was
considered that the patient may have a history of sub-clinical
coronary atherosclerotic heart disease that the patient and family
were unaware of, due to a lack of clinical symptoms. Prior to
admission, fasting blood glucose levels were 8.37 mmol/l,
indicating that the patient had a history of hyperglycemia. As the
patient had recently been treated with glucocorticoids, it was
considered that the hyperglycemia may have been associated with the
use of hormones. The patient developed fever, chills, clenched
hands, clenched teeth, flushing and tachypnea following
piperacillin-tazobactam treatment. Blood indicators, including
leukocytes, neutrophils, creatine kinase isoenzyme, creatine
kinase, lactate dehydrogenase, α-hydroxybutyrate dehydrogenase and
BNP were all elevated, whereas the ECG demonstrated ST-segment
elevation in certain leads, with myocardial injury and heart
failure. Following the discontinuation of piperacillin-tazobactam,
the body temperature of the patient returned to normal, systemic
symptoms were resolved and all blood indicators returned to normal
levels after 4 days. Therefore, results of the present study
demonstrated a potential association between the aforementioned
adverse reactions and piperacillin-tazobactam treatment.
The present case was suspected with adult Still
disease before admission, which was also a suspected diagnosis in
the previous hospitals the patient was admitted to. The patient was
already prescribed methylprednisolone sodium succinate before
admission, which was continued following admission. Levels of CRP
and ferritin improved throughout the course of treatment without
notable fluctuations, which may be associated with improvements in
adult Still disease. The use of piperacillin-tazobactam again was
associated with the exacerbation of the disease and was not linked
to the dosage of steroids. Throughout the treatment course,
symptoms were closely associated with piperacillin-tazobactam. The
patient symptoms worsened and improved with the use and
discontinuation of piperacillin-tazobactam. In addition, eosinophil
levels remained within the normal range. PCT and troponin I levels
were transiently elevated, but the potential association of these
indicators with piperacillin-tazobactam treatment remains to be
fully elucidated. Following the discontinuation of
piperacillin-tazobactam treatment, PCT and troponin I levels
returned to normal.
The event of myocardial injury with heart failure
following piperacillin-tazobactam treatment is rare (14). The patient presented at Affiliated
hospital of Shandong university of traditional Chinese medicine
with an unexplained fever, where the underlying cause remained the
key focus. Whilst aiming to determine the underlying cause of the
fever and any potential infections, adverse effects associated with
piperacillin-tazobactam treatment were not acknowledged. Due to the
elevated levels of leukocytes accompanied by lung inflammation
observed on a CT scan, a penicillin skin test was performed.
Notably, results of the penicillin skin test were negative and the
patient had been administered piperacillin-tazobactam at a previous
hospital with no history of a penicillin allergy. Therefore,
piperacillin-tazobactam treatment was continued. However, following
antibiotic step-down treatment, the results of the present study
indicated that piperacillin-tazobactam was the direct cause of the
induction of myocardial injury and heart failure. Following
discontinuation of piperacillin-tazobactam, no additional
antibiotics were administered to the patient and the condition of
the patient improved. On admission to affiliated hospital of
Shandong university of traditional Chinese medicine, the patient
was only administered piperacillin-tazobactam twice, which did not
cause any further harm to the patient. The medical history of the
patient was then reviewed, where it was determined that underlying
cardiovascular risk factors, such as hypertension, hyperglycemia
and coronary artery calcification, may have resulted in
piperacillin-tazobactam-induced myocardial injury. Therefore,
clinicians should consider the use of piperacillin-tazobactam
treatment in patients with a history of cardiovascular risk
factors, such as hyperlipidemia, hyperglycemia and
hypertension.
In addition, the patient exhibited liver dysfunction
following piperacillin-tazobactam treatment at a previous hospital.
Following treatment, liver function had returned to normal before
discharge. Therefore, it was considered that liver dysfunction may
have been associated with the prolonged use of
piperacillin-tazobactam. Notably, a previous study reported the
case of a patient with liver dysfunction [serum alanine
aminotransferase levels exceeded the upper limit of reference range
(50 U/l)] resulting from piperacillin-tazobactam treatment
(16). However, the potential
association with mortality or disease progression remains unclear.
In a retrospective study, Saloojee et al (17) demonstrated that
piperacillin-tazobactam treatment was associated with liver
dysfunction in 225 critically ill patients. By contrast, McDonald
et al (18) previously
observed no significant difference in the levels of hepatotoxicity
between high and licensed doses of piperacillin-tazobactam
treatment. In the present case, the patient exhibited a normal ECG
with a mild elevation of cardiac enzymes on admission to the
affiliated hospital of Shandong University of traditional Chinese
medicine, rendering mild myocarditis initially considered. Since
the patient had been administered piperacillin-tazobactam several
times prior to admission, drug-induced myocarditis and pericarditis
could not be ruled out. It was considered that myocardial damage
may have been associated with the accumulation of
piperacillin-tazobactam in the body, specifically due to an
allergic reaction induced by repeated treatment. The patient
exhibited flushing, chills, tachypnea, tachycardia and decreased
blood pressure, which are all symptoms of allergy (19). In addition, clenched hands,
clenched teeth and muscle cramps may be signs of antibiotic
encephalopathy comparable with epilepsy (20). Therefore, drug fever, allergic
reactions and antibiotic encephalopathy, in addition to myocardial
injury and heart failure, were considered to be associated with
piperacillin-tazobactam treatment. However, the specific mechanisms
underlying myocardial injury caused by piperacillin-tazobactam
remain to be fully elucidated. It was hypothesized that myocardial
injury was induced by cardiovascular risk factors in the patient,
such as hypertension and hyperglycemia, whereby adverse reactions,
such as drug fever and allergic reactions, were not directly caused
by piperacillin-tazobactam treatment.
Adverse drug reactions may be considered if the
source of fever cannot be determined following radiography, blood
indicators or microbiological cultures. Notably, the patient in the
present case exhibited numerous underlying diseases, including
hypertension, hyperglycemia, coronary atherosclerotic heart
disease, a history of cerebral infarction, pneumonia and a
potential diagnosis of adult Still disease. Despite the involvement
of multidisciplinary experts, the condition of the patient was
complex and treatment designation was particularly difficult.
To conclude, the present article reports the case of
myocardial injury and heart failure caused by a
piperacillin-tazobactam-induced allergic reaction. Results of ECGs
and the levels of cardiac enzymes highlighted the potential for
myocardial injury due to drug-induced myocarditis. Therefore,
myocardial injury and heart failure, though rare, should be
considered adverse reactions of piperacillin-tazobactam treatment,
suggesting that piperacillin-tazobactam may be cardiotoxic.
Clinicians should cautiously consider the use of
piperacillin-tazobactam treatment in patients with a history of
cardiovascular risk factors, such as hyperlipidemia, hyperglycemia
and hypertension.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL contributed to conception and design. YL and LS
collected data and drafted the manuscript. YL analyzed and
interpreted data. CA and XA drew the figures and tables. YL and LS
obtained medical images (e.g. CT scans). YL, XZ, LS and QZ advised
on patient treatment. YL, CA, XA, XZ and LS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of any images or data included in the
present paper.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gin A, Dilay L, Karlowsky JA, Walkty A,
Rubinstein E and Zhanel GG: Piperacillin-tazobactam: A
beta-lactam/beta-lactamase inhibitor combination. Expert Rev Anti
Infect Ther. 5:365–383. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hayashi Y, Roberts JA, Paterson DL and
Lipman J: Pharmacokinetic evaluation of piperacillin-tazobactam.
Expert Opin Drug Metab Toxicol. 6:1017–1031. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Perry CM and Markham A:
Piperacillin/tazobactam: An updated review of its use in the
treatment of bacterial infections. Drugs. 57:805–843.
1999.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Linares T, Fernández A, Soto MT, Escudero
E and Gacías L: Drug fever caused by piperacillin-tazobactam. J
Investig Allergol Clin Immunol. 21:250–251. 2011.PubMed/NCBI
|
|
5
|
Wang Q, He Z, Wu X, Wei Y and Huang J:
Hematologic adverse effects induced by piperacillin-tazobactam: A
systematic review of case reports. Int J Clin Pharm. 42:1026–1035.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cabañas R, Calderon O, Ramirez E, Fiandor
A, Prior N, Caballero T, Herránz P, Bobolea I, López-Serrano MC,
Quirce S and Bellón T: Piperacillin-induced DRESS: distinguishing
features observed in a clinical and allergy study of 8 patients. J
Investig Allergol Clin Immunol. 24:425–430. 2014.PubMed/NCBI
|
|
7
|
Metlay JP, Waterer GW, Long AC, Anzueto A,
Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, et
al: Diagnosis and treatment of adults with community-acquired
pneumonia. An official clinical practice guideline of the american
thoracic society and infectious diseases society of America. Am J
Respir Crit Care Med. 200:e45–e67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gu W, Miller S and Chiu CY: Clinical
metagenomic next-generation sequencing for pathogen detection. Annu
Rev Pathol. 14:319–338. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gagnier JJ, Kienle G, Altman DG, Moher D,
Sox H and Riley D: CARE Group. The CARE guidelines: Consensus-based
clinical case reporting guideline development. BMJ Case Rep.
2013(bcr2013201554)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alzahrani M, Alrumaih I, Alhamad F and
Abdel Warith A: Rapid onset severe thrombocytopenia following
reexposure to piperacillin-tazobactam: Report of two cases and
review of the literature. Platelets. 29:628–631. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Beaulieu C, Kurczewski L and Yajnik V:
Cefepime challenge after piperacillin/tazobactam-induced
thrombocytopenia. J Thromb Thrombolysis. 48:167–170.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bai M, Govindaraj V, Kottaisamy R and
Vijayarangam N: Drug reaction with eosinophilia and systemic
symptoms syndrome related to piperacillin-tazobactam use. J
Postgrad Med. 68:102–105. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lv J, Wu G, Zhang F and Su X: An unusual
case of piperacillin-tazobactam-induced fever, eosinophilia,
thrombocytopenia and liver damage. Eur J Hosp Pharm Sci Pract.
29:e91–e94. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jafri F, Arif S and Ashraf U:
Hypersensitivity myocarditis associated with
piperacillin-tazobactam use. Chest. 158 (Suppl)(A226)2020.
|
|
15
|
Calogiuri GF, Nettis E, Di Leo E, Vacca A,
Ferrannini A and Kounis NG: Kounis syndrome induced by intravenous
administration of piperacillin/tazobactam: A case report. Int J
Cardiol. 155:e42–e44. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abdelghany M, Subedi R, Shah S and Kozman
H: Kounis syndrome: A review article on epidemiology, diagnostic
findings, management and complications of allergic acute coronary
syndrome. Int J Cardiol. 232:1–4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Saloojee A, Skinner DL, Loots E,
Hardcastle TC and Muckart DJJ: Hepatic dysfunction: A common
occurrence in severely injured patients. Injury. 48:127–132.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
McDonald C, Cotta MO, Little PJ, McWhinney
B, Ungerer JP, Lipman J and Roberts JA: Is high-dose β-lactam
therapy associated with excessive drug toxicity in critically ill
patients? Minerva Anestesiol. 82:957–965. 2016.PubMed/NCBI
|
|
19
|
Aun MV, Kalil J and Giavina-Bianchi P:
Drug-induced anaphylaxis. Immunol Allergy Clin North Am.
37:629–641. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bhattacharyya S, Darby RR, Raibagkar P,
Gonzalez Castro LN and Berkowitz AL: Antibiotic-associated
encephalopathy. Neurology. 86:963–971. 2016.PubMed/NCBI View Article : Google Scholar
|