Introduction
Clonorchis sinensis is a trematode with a
high prevalence in East Asia that can cause hepatobiliary infection
(1). Clonorchis sinensis is
mainly prevalent in China, South Korea, northern Vietnam and parts
of Russia (2,3). Clonorchis sinensis (also
referred to as liver fluke) generally parasitizes the liver and
hepatic duct, and causes a serious foodborne parasitic disease
(4). Furthermore, ~15 million
humans have been estimated to be infected worldwide, with 85% of
worldwide infections occurring in China (5). Much research in recent years has
focused on endemic areas, and patients with Clonorchis
sinensis infections are usually asymptomatic or only mildly
symptomatic (2-4).
Therefore, the infection is easy to overlook in non-endemic areas,
and patients have little awareness of taking the initiative to seek
medical attention. Even when they do seek medical attention, the
probability of a missed diagnosis or misdiagnosis is high.
Clonorchiasis generally appears as jaundice, indigestion, biliary
inflammation and bile duct obstruction, and even liver cirrhosis,
cholangiocarcinoma and hepatic carcinoma (2). The lack of obvious clinical symptoms
in the early stages of Clonorchis sinensis infection often
leads to underdiagnosis (2).
Clonorchis sinensis infection is often misdiagnosed as
gastroenteritis and liver abscesses due to its non-specific
symptoms, such as inappetence, nausea, bellyache, jaundice and
hepatosplenomegaly (2). The
present report describes a case of Clonorchis sinensis
infection in a non-endemic area with a history of raw fish
consumption, as well as a characteristic presentation on ancillary
examination, which provides some basis for the diagnosis of
Clonorchis sinensis infection.
Case report
A 40-year-old woman was admitted to the Department
of Hepatobiliary and Pancreatic Surgery of the First Affiliated
Hospital of Zunyi Medical University (Zunyi, China) in December
2021 due to upper abdominal pain that had persisted for 2 weeks and
been aggravated for 2 days. There was no evident cause for the
abdominal pain, which had been intermittent and varied in intensity
for 2 weeks. Over the last 2 days, the pain had intensified,
extending to the back, with no chills or fever. In order to seek
further diagnosis and treatment, the patient was admitted to the
Outpatient Clinic with a liver space-occupying lesion, which
required further examination and preliminary diagnosis after
admission because the cause could not be determined prior to
hospitalization. The patient reported being generally healthy in
the past and consumed raw fish slices approximately once a
month.
A physical examination indicated the following: i)
No jaundice observed in the skin or sclera; ii) no signs of liver
palms or spider nevi; iii) no significant abnormalities detected in
the cardiac and pulmonary examinations; and iv) slight pain upon
applying pressure in the right upper abdomen, but no rebound
tenderness.
Routine blood laboratory examinations indicated the
following: i) White blood cell count, 5.6x109/l
(reference range, 3.5-9.5x109/l); ii) eosinophil
percentage, 16% (reference range, 0.4-8%); iii) absolute eosinophil
count, 0.9x109/l (reference range,
0.02-0.52x109/l); iv) alanine aminotransferase (ALT), 29
U/l (reference range, 9-50 U/l); and v) alkaline phosphatase (ALP),
136 U/l (reference range, 35-100 U/l).
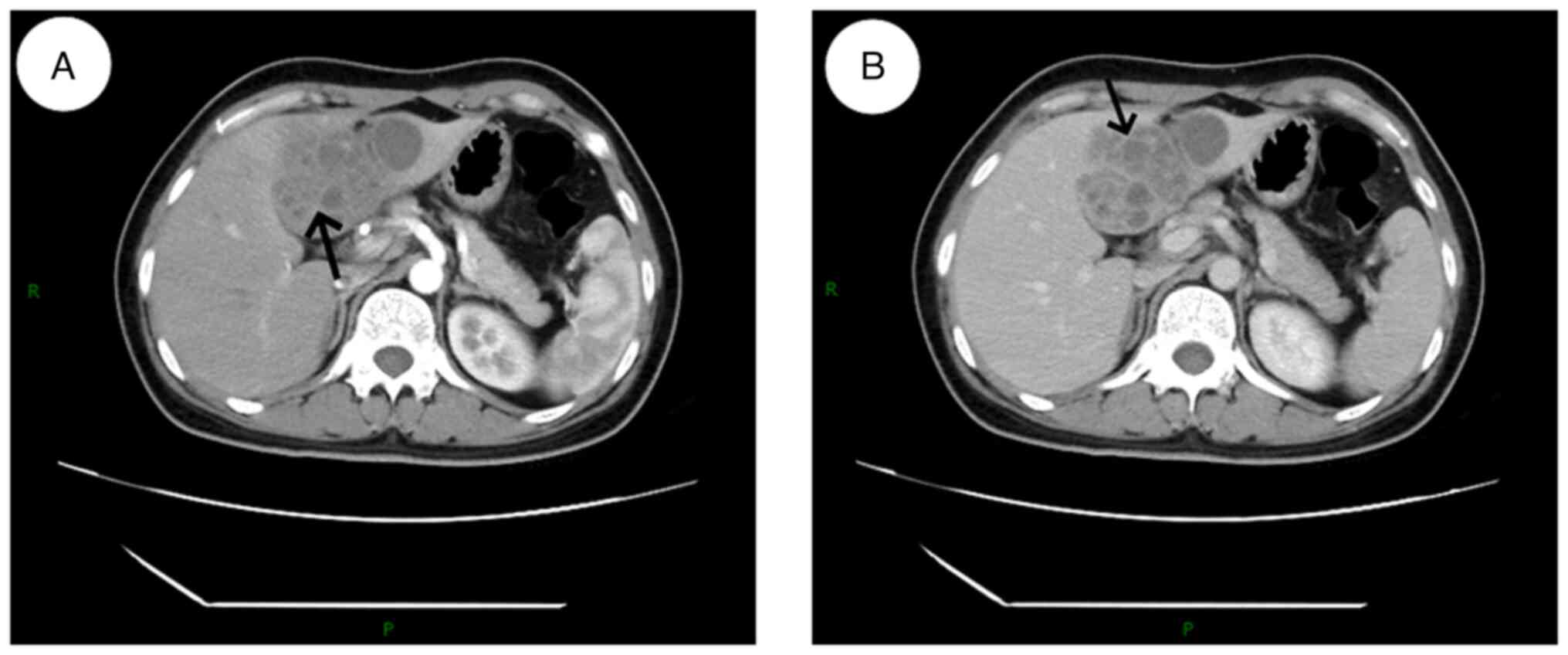
An upper abdominal computed tomography (CT)
examination revealed multiple rounded, small flaky hypodense
lesions in the left lobe of the liver, some of which were fused,
with a larger cross-section of ~58x54 mm. Enhanced scanning with
edge and segregation enhancement and marked inhomogeneous
enhancement of the hepatic parenchyma in the arterial phase around
the lesions were observed (Fig.
1). The diagnosis indicated a lesion in the left lobe of the
liver, considered to be of an infectious origin. A liver abscess
was highly likely, although a tumor could not be ruled out.
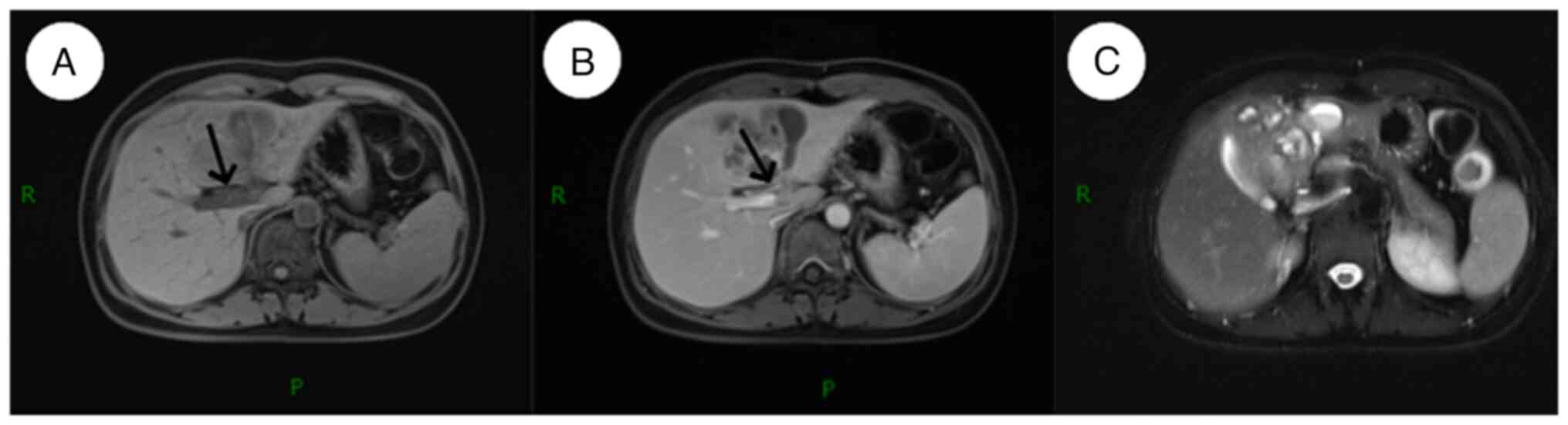
On hepatobiliary and pancreatic magnetic resonance
imaging (MRI), 78x7-mm mixed long T1 and T2 signals were observed
in the left lobe of the liver on a plain scan. The signal was
uneven with a poorly defined edge, and a small patch of short T1
signal was visible. T1-weighted imaging (T1WI) indicated a low
signal, while T2WI indicated a high signal. Enhanced scanning
revealed progressive mild to moderate uneven enhancement in the
lesion area. The central necrotic area was not enhanced, and a
small patch of edema was observed around it (Fig. 2). The diagnosis was of a suspected
liver abscess, and a neoplastic lesion needed to be excluded based
on a combination of the clinical symptoms and examination findings.
The postoperative pathology did not reveal tumor cells.
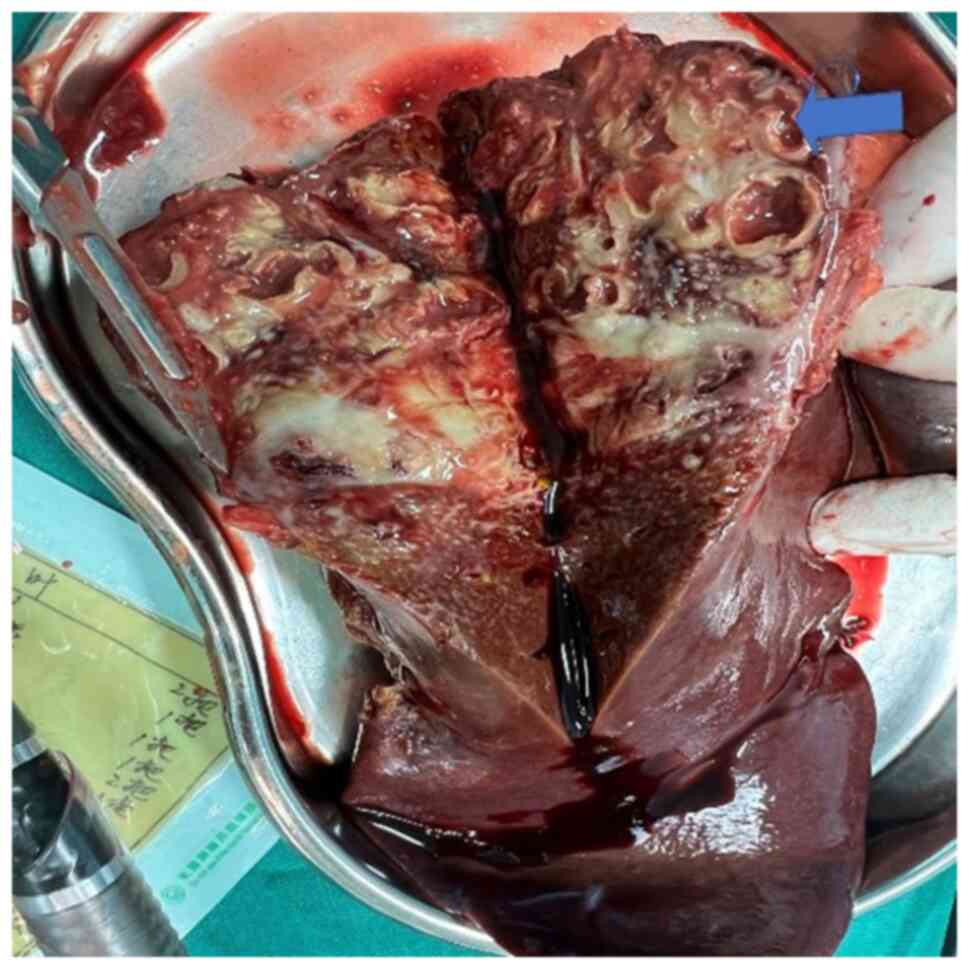
A laparoscopic left hepatic lobectomy and
laparoscopic cholecystectomy was performed under general anesthesia
in December 2021, 6 days after admission. Gross pathology during
the surgery revealed a partly cystic-solid grayish-white liver
tissue, and grayish-red tunnel-like lesions were observed in the
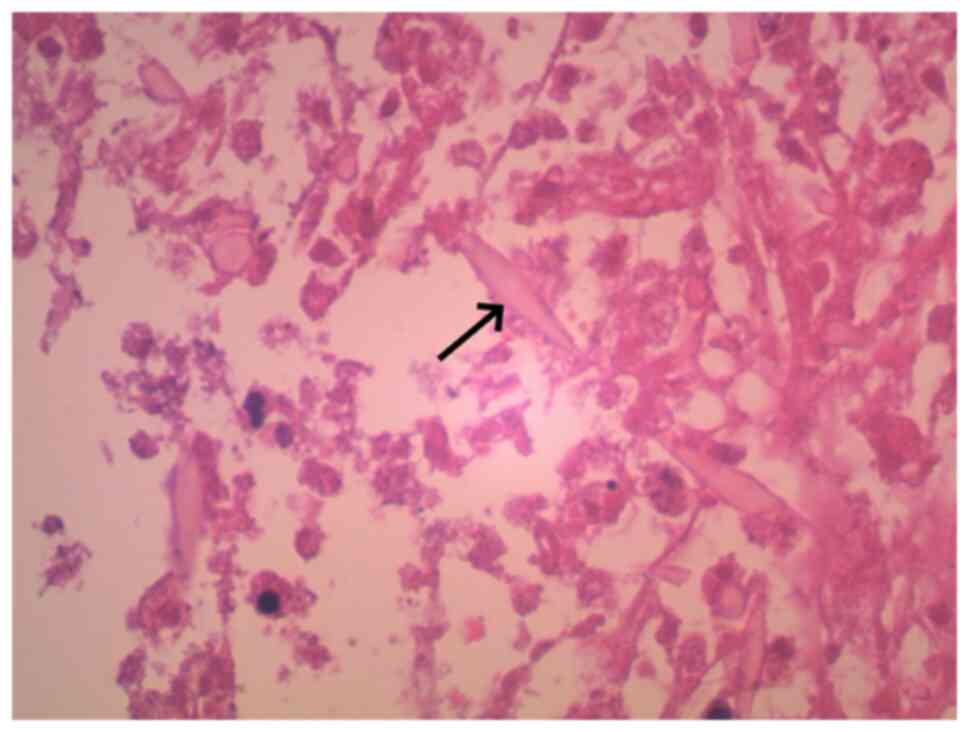
section of the remaining liver tissue (Fig. 3). Postoperative pathology results
indicated that the examined liver tissue (left half) exhibited
chronic inflammation in the bile duct area. Local fibrous
connective tissue proliferation, small bile duct hyperplasia and
eosinophilic granuloma formation were observed. Charcot-Leyden
crystals were found in the necrotic area of the granuloma (Fig. 4). The tissues were fixed with 10%
neutral formalin fix solution for 36 h at room temperature, and
stained with hematoxylin stain at a ratio of 1:6 for 4 h at room
temperature. Tissues were rehydrated in a descending ethanol series
(95, 80 and 70%) for 1 min each, the tissues were stained with
eosin stain at a ratio of 1:6 for 2 h at room temperature. The
tissues were then subjected to alcohol dehydration (acidic ethanol
at 1% for 1.5 h, followed by 100% ethanol dehydration for 1 h),
clearing with xylene in order to remove impurities from the tissue
and paraffin tissue embedding, and finally cut into 6 µm sections.
Observation was performed with a light microscope. Clonorchiasis
was suspected, and confirmation with clinical and laboratory
examinations was recommended. CT, MRI and routine blood laboratory
examination results were combined with the epidemiologic history
(consumption of raw fish). The postoperative diagnosis was
clonorchiasis (caused by Clonorchis sinensis infection) with
abscess formation. Postoperatively, the patient received
symptomatic supportive treatments, including anti-infection, liver
protection and maintenance of water and electrolyte balance
treatments (1 g ceftriaxone to 100 ml sodium chloride injection
twice daily for 3 days for a course of treatment, 0.1 g magnesium
isoglycyrrhizinate to 250 ml sodium chloride injection once daily
and 500 ml sodium potassium magnesium calcium injection twice daily
for 1 week for a course of treatment).
On the second postoperative day, a laboratory
examination revealed the following: i) White blood cell count,
11.6x109/l; ii) eosinophil percentage, 0%; iii) absolute
eosinophil count, 0; iv) ALT, 55 U/l; and v) ALP, 101 U/l.
The patient was cured and discharged from the
hospital 9 days postoperatively, and was re-examined in the
Department of Hepatobiliary and Pancreatic Surgery of the First
Affiliated Hospital of Zunyi Medical University at 1, 6 and 12
months after discharge. The disease lesions did not recur. The
patient was advised to undergo an abdominal CT every 6 months to
ensure that there was no recurrence.
Discussion
Clonorchiasis is a zoonotic parasitic disease caused
by the parasitism of Clonorchis sinensis in the human
intrahepatic bile ducts (6). In
2009, the International Agency for Research on Cancer listed
Clonorchis sinensis as a Group I carcinogen (7). This disease primarily transmits to
humans through the consumption of raw fish slices or undercooked
freshwater shrimp contaminated with the parasite (8). During its lifecycle, the liver fluke
undergoes eight morphological stages across various hosts. The
cercariae (larval stage) latch onto the fish or shrimp upon
entering freshwater, penetrating their flesh and maturing into
metacercariae. Humans and other definitive hosts, when consuming
contaminated raw or undercooked freshwater fish or shrimp, become
infected. Inside the duodenum, these cysts release larvae, which
migrate into the bile ducts, maturing into adult worms (2). In China, ~140 species of freshwater
fish and four species of shrimp have been recognized as
complementary intermediate hosts for Clonorchis sinensis
(9). Small fish such as
Pseudorasbora parva and Parapelecus argenteus are
more susceptible to infection with the metacercariae, in terms of
infection rates and metacercarial burden, than large fish such as
Cyprinus carpio and Parabramis pekinensis (10). It is difficult to eradicate
Clonorchis sinensis from the environment due to the wide
distribution and host range of this parasite in China. However,
human infections can be avoided or minimized by blocking the
transmission route of the parasite. The easiest way to do this is
to convince citizens in epidemic areas not to eat raw or
undercooked freshwater fish. The difficulty with prevention is that
most people have limited or no knowledge of the parasite and are
unaware of the dangers of consuming it (11). If people are not aware of the
threat of a raw fish diet, it is unrealistic to change their habits
(11). Therefore, one of the most
effective measures is to spread the knowledge that Clonorchis
sinensis is a biocarcinogen for bile duct cancer and to
encourage individuals to give up the habit of eating raw fish
(11). Subsequent to infection,
the main clinical manifestations encompass cholangitis,
cholecystitis and gallbladder stones and complications, such as
biliary obstruction, abscesses and even cholangiocarcinoma
(12). A predominant laboratory
finding associated with Clonorchis sinensis infection is an
elevated eosinophil count or percentage (13). In the present case, the
preoperative blood tests indicated an eosinophil percentage of 16%
and an absolute eosinophil count of 0.9x109/l.
In previous years, with the progress of medical
imaging technology, CT and MRI have been widely used in the
diagnosis of parasitic infections of the liver and biliary tract.
Due to the non-specific clinical symptoms of Clonorchis
sinensis infection, solely relying on the clinical presentation
for diagnosis is challenging, emphasizing the importance of
characteristic radiological findings (14). MRI findings typical of
Clonorchis sinensis infection include diffuse dilation of
the intrahepatic peripheral bile ducts, with larger and
extrahepatic bile ducts remaining undilated (14,15).
In the present case, high signal was observed on T2WI, and diffuse
mildly dilated terminal bile ducts were observed both in the center
and periphery of the lesion. This characteristic is attributed to
Clonorchis sinensis predominantly infesting the terminal
bile ducts, causing obstructions in the smaller peripheral ducts
(13). The most common finding on
MRI of Clonorchis sinensis is a diffuse mild dilatation of
the small intrahepatic bile ducts without dilatation of the
extrahepatic bile ducts (15). The
patient in the present report exhibited small patchy low-density
lesions within the liver on the preoperative abdominal CT, with
enhanced margins and septation upon contrast scanning. The
hepatobiliary and pancreatic MRI findings included mixed long T1
and T2 signals, inconsistent signal intensities with indistinct
borders, small patches of short T1 signals, low signal intensity on
T1WI and high signal intensity on T2WI. The enhanced scans revealed
a progressive, mild to moderate, uneven enhancement within the
lesion area.
Emphasizing early diagnosis and treatment can
prevent complications, such as recurrent suppurative cholangitis
and cholangiocarcinoma (16).
However, prevention is more pivotal than timely post-diagnosis
intervention. Recognizing the epidemiological characteristics of
clonorchiasis, the primary preventive strategy revolves around
amplifying public awareness, especially regarding the risks
associated with consuming raw fish slices and undercooked fish and
shrimp, and this is potentially the most effective method to
counteract Clonorchis sinensis infection (17). Preventing eggs from entering the
water by appealing to the public for improved fecal management is
another method of control. The easiest way to do this is to advise
the public not to dump or discharge fecal matter directly into the
water (18). After liver fluke
infection, most infected individuals do not have any symptoms; only
some of them may have epigastric pain, pain on applying pressure,
fever, jaundice, diarrhea and other clinical manifestations
(19). As a result, the condition
can easily be unnoticed, leading to a general lack of urgency in
seeking medical care. Given the non-specific nature of the
symptoms, there is potential for delays in both diagnosis and
treatment, which can culminate in a chronic infection (20). Therefore, even in non-endemic areas
of clonorchiasis, patients presenting with appropriate symptoms at
the time of medical consultation need to be made aware of the
differential diagnosis of clonorchiasis, focusing on the
investigation of its epidemiological history, to minimize the
occurrence of underdiagnosis and misdiagnosis.
In China, the Clonorchis sinensis epidemic is
mainly due to the habit of eating raw fish and shrimp (2). The current patient also had a history
of consuming raw fish slices, the imaging manifestations were
consistent with the characteristics of the disease and
characteristic Charcot-Leyden crystals were found on postoperative
pathological examination. In conclusion, the present report
describes a case of Clonorchis sinensis infection in a
non-endemic area and provides a basis for its diagnosis. It is
important to educate the public not to consume raw fish and shrimp,
even in non-endemic areas.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant no. 81960125) and the Guizhou
Science and Technology Planning Project [grant no. Guizhou Kehe
Support (2020) 1Y302].
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XC and JH wrote the manuscript and analyzed patient
data. XC, YX and CT collected the literature and obtained medical
images (including pathology, MRI and CT scans). YX collected the
patient files and signed the informed consent for case publication,
and improved the article for language and style. LZ advised on
patient treatment and gave final approval of the version to be
published. XC and LZ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Biomedical Research
Ethics Committee of the Affiliated Hospital of Zunyi Medical
University (Zunyi, China; approval no. KLL-2022-763).
Patient consent for publication
Written informed consent was obtained for the
publication of the present case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reddy AK, Chakrabarty M, Liu Y, Cohen SH
and Maniar AH: Case report: Clonorchis sinensis infection
associated with eosinophilic pneumonia. Am J Trop Med Hyg.
104:2065–2068. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tang ZL, Huang Y and Yu XB: Current status
and perspectives of Clonorchis sinensis and clonorchiasis:
Epidemiology, pathogenesis, omics, prevention and control. Infect
Dis Poverty. 5(71)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang Y, Gong QL, Lv QB, Qiu YY, Wang YC,
Qiu HY, Guo XR, Gao JF, Chang QC and Wang CR: Prevalence of
clonorchis sinensis infection in fish in South-East Asia: A
systematic review and meta-analysis. J Fish Dis. 43:1409–1418.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Correia da Costa JM, Vale N, Gouveia MJ,
Botelho MC, Sripa B, Santos LL, Santos JH, Rinaldi G and Brindley
PJ: Schistosome and liver fluke derived catechol-estrogens and
helminth associated cancers. Front Genet. 5(444)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Palmer LJ, Celedón JC, Weiss ST, Wang B,
Fang Z and Xu X: Ascaris lumbricoides infection is associated with
increased risk of childhood asthma and atopy in rural China. Am J
Respir Crit Care Med. 165:1489–1493. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oh JK, Lim MK, Yun EH, Cho H, Park EY,
Choi MH, Shin HR and Hong ST: Control of clonorchiasis in Korea:
Effectiveness of health education for community leaders and
individuals in an endemic area. Trop Med Int Health. 19:1096–1104.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chang JI, Lee K, Kim D, Yang JI, Park JK,
Choi K, Kang SH, Lee KH, Lee KT, Lee JK, et al: Clinical
characteristics of clonorchis sinensis-associated
cholangiocarcinoma: A large-scale, single-center study. Front Med
(Lausanne). 8(675207)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu M, Jiang Y, Yin J, Cao S, Shen Y and
Cao J: Risk factors for clonorchis sinensis infection in residents
of binyang, guangxi: A cross-sectional and logistic analysis study.
Front Public Health. 9(588325)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou P, Chen N, Zhang RL, Lin RQ and Zhu
XQ: Food-borne parasitic zoonoses in China: Perspective for
control. Trends Parasitol. 24:190–196. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ,
Yu XB and Fang YY: Clonorchiasis: A key foodborne zoonosis in
China. Lancet Infect Dis. 5:31–41. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lai DH, Hong XK, Su BX, Liang C, Hide G,
Zhang X, Yu X and Lun ZR: Current status of Clonorchis sinensis and
clonorchiasis in China. Trans R Soc Trop Med Hyg. 110:21–27.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim TS, Pak JH, Kim JB and Bahk YY:
Clonorchis sinensis, an oriental liver fluke, as a human biological
agent of cholangiocarcinoma: A brief review. BMB Rep. 49:590–597.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang XH, Huang D, Li YL and Chang B:
Novel mechanism of hepatobiliary system damage and immunoglobulin
G4 elevation caused by Clonorchis sinensis infection. World J Clin
Cases. 9:6639–6653. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choi D and Hong ST: Imaging diagnosis of
clonorchiasis. Korean J Parasitol. 45:77–85. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jeong YY, Kang HK, Kim JW, Yoon W, Chung
TW and Ko SW: MR Imaging Findings of Clonorchiasis. Korean J
Radiol. 5:25–30. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lo WK, Yu SM and Chan JC: Characteristic
imaging features of clonorchiasis. Hong Kong Med J. 24:206.e3–e4.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Y, Wen T, Lai DH, Wen YZ, Wu ZD, Yang
TB, Yu XB, Hide G and Lun ZR: Development and evaluation of
loop-mediated isothermal amplification (LAMP) for rapid detection
of Clonorchis sinensis from its first intermediate hosts,
freshwater snails. Parasitology. 140:1377–1383. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Na BK, Pak JH and Hong SJ: Clonorchis
sinensis and clonorchiasis. Acta Tropica.
203(105309)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hong ST and Fang Y: Clonorchis sinensis
and clonorchiasis, an update. Parasitol Int. 61:17–24.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qian MB, Utzinger J, Keiser J and Zhou XN:
Clonorchiasis. Lancet. 387:800–810. 2016.PubMed/NCBI View Article : Google Scholar
|