Introduction
Heart failure (HF), a critical endpoint of cardiac
dysfunction due to significant structural abnormalities, stands as
a primary contributor to cardiac mortality and recurrent hospital
admissions worldwide, imposing substantial healthcare and
socioeconomic costs (1,2). Over the past years, HF classification
has evolved into four stages (A-D), highlighting the progression of
the disease and the varying phenotypes discernible via
echocardiography (3). Unlike HF
with reduced ejection fraction (HFrEF), HF with preserved ejection
fraction (HFpEF) exhibits normal systolic function but impaired
ventricular filling and relaxation (4). This distinction underscores the need
for tailored treatment approaches for the diverse HF categories.
Despite significant advancements in medical treatments and cardiac
support devices reducing the incidence of hospitalization and
improving survival rates for HFrEF, the overall outlook for heart
failure patients remains suboptimal (5). There is also a pressing need to
refine the selection process for various treatments, including
standard medications and newer oral agents such as sodium-glucose
cotransporter 2 inhibitors (SGLT2is) and vericiguat, to suit
individual patient groups (6,7). A
deeper comprehension of the underlying mechanisms of HF is crucial
for the creation of more effective therapeutic strategies.
Mitochondria are the powerhouse of the cell and
their well-being is crucial for maintaining normal cellular
metabolism and shielding cells from oxidative damage caused by
reactive oxygen species (ROS) (8).
Dynamin-related protein 1 (Drp1) is present in the cytoplasm and
initiates mitochondrial division, effectively isolating impaired
mitochondrial parts within a depolarized offspring organelle marked
for mitophagy, which is pivotal in managing mitochondrial integrity
(9). The heart is the most
metabolically active organ in the body, and some previous studies
have demonstrated that impaired mitochondrial energetics could
greatly contribute toward the onset and progression of maladaptive
cardiac hypertrophy and HF (10,11).
Our previous research also suggested that regulating Drp1
expression might normalize mitochondrial fission and enhanced
cardiac metabolism, which in turn could decrease apoptosis in
cardiomyocytes within the infarcted myocardium, thereby improving
heart function (12). However,
there is a notable scarcity of studies investigating the
relationship between serum Drp1 levels and HF prognosis. As a
result, the present study was initiated to uncover independent risk
predictors for patients with HF.
Materials and methods
Study design and patient
selection
The present study was an observational,
single-center, prospective analysis aimed of investigating the
association between serum Drp1 concentrations and the clinical
outcomes in a broad spectrum of HF patients. Between 1 June, 2021
and 31 March, 2022, patients hospitalized at Zhongda Hospital
(Nanjing, China) were consecutively assessed for eligibility. A
comprehensive cohort of HF patients (aged 18-85 years) were
included, encompassing those with major risk factors such as
ischemic heart disease (IHD), hypertension, cardiomyopathy,
diabetes mellitus (DM) and obesity, as well as individuals who had
received a definitive HF diagnosis confirmed by at least two
experienced cardiologists. Patient characteristics are given in
Table I. The exclusion criteria
were: i) Primary diagnosis of macrovascular conditions such as
aortic dissection or arteritis, congenital heart defects, pulmonary
diseases, peripheral vascular disorders, pericardial diseases,
myocarditis, cardiophobia, costochondritis and shock; endocrine
disorders including thyroid diseases; malignancies, or severe
infections; ii) presence of significant hepatic impairment
(aspartate aminotransferase levels >140 U/l, alanine
aminotransferase levels >140 U/l) or renal impairment (estimated
glomerular filtration rate <30 ml/min/1.73 m2); and
iii) non-compliance with, or refusal to participate in, the present
study. For the classification and progression of HF, the diagnostic
criteria adhered to the current guidelines (3,5).
Additionally, HF was categorized into HFrEF (EF <50%) and HFpEF
(EF ≥50%) based on echocardiographic results. The ethics committee
of Zhongda Hospital (Nanjing, China) approved the present study
protocol (approval no. 2020ZDSYLL306-P01).
 | Table IBaseline characteristics between the
low and high Drp1 groups. |
Table I
Baseline characteristics between the
low and high Drp1 groups.
| A, Demographics |
|---|
| Variables | Total | Drp1 ≤2.66 ng/ml
(n=101) | Drp1 >2.66 ng/ml
(n=155) | P-value |
|---|
| Age, years | 68.7±10.8 | 68.6±12.3 | 68.8±9.7 | 0.87 |
| Sex (male), n
(%) | 152 (59.4) | 67 (66.3) | 85 (54.8) | 0.07 |
| BMI,
kg/m2 | 25.5±4.3 | 25.5±4.8 | 25.5±3.9 | 0.916 |
| Heart rate, bpm | 80.3±17.8 | 83.9±16.2 | 81.3±18.8 | 0.247 |
| SBP, mmHg | 131.8±21.9 | 129.7±22.5 | 133.2±21.5 | 0.219 |
| DBP, mmHg | 77.5±13.7 | 77.0±14.4 | 77.8±13.2 | 0.627 |
| B, Risk
factors |
| Smoking, n (%) | 64 (25.0) | 30 (29.7) | 34 (21.9) | 0.185 |
| Coronary artery
diseases, n (%) | 162 (63.3) | 81 (80.2) | 81 (52.3) | <0.001 |
| Prior MI, n
(%) | 91 (35.5) | 68 (67.3) | 23 (14.8) | <0.001 |
| Hypertension, n
(%) | 181 (70.7) | 75 (74.3) | 106 (68.4) | 0.329 |
| Diabetes, n
(%) | 85 (33.2) | 39 (38.6) | 46 (29.6) | 0.174 |
| Stroke, n (%) | 81 (31.6) | 39 (38.6) | 42 (27.1) | 0.056 |
|
Hypercholesterolemia, n (%) | 24 (9.4) | 8 (7.9) | 16 (10.3) | 0.662 |
| C, Laboratory
results |
| NT-proBNP,
pg/mla | 1015.0 (22.0,
35000.0) | 1970.0 (77.0,
35000) | 182.5 (22.0,
20400.0) | <0.001 |
| WBC,
x109/l | 6.9±3.1 | 7.1±2.3 | 6.8±3.6 | 0.495 |
| Hb, g/l | 132.1±19.5 | 130.5±20.8 | 133.2±18.6 | 0.27 |
| Plt,
x109/l | 202.2±74.6 | 189.7±68.2 | 210.4±77.7 | 0.03 |
| FPG, mmol/l | 6.7±2.8 | 6.9±3.0 | 6.5±2.5 | 0.223 |
| HbA1C, % | 6.6±1.6 | 6.8±1.6 | 6.5±1.5 | 0.151 |
| ALT, U/l | 24.7±22.0 | 27.1±28.6 | 23.1±16.4 | 0.209 |
| eGFR, ml/(min*1.73
m2) | 79.4±21.1 | 75.5±22.7 | 81.9±19.6 | 0.018 |
| Urea nitrogen,
mmol/l | 7.0±3.4 | 7.9±4.5 | 6.4±2.3 | 0.002 |
| Total-cholesterol,
mmol/l | 3.9±1.2 | 3.7±1.0 | 4.1±1.3 | 0.004 |
| Triglycerides,
mmol/l | 1.3±0.9 | 1.3±1.0 | 1.4±0.9 | 0.652 |
| LDL-C, mmol/l | 2.2±0.8 | 2.1±0.7 | 2.3±0.9 | 0.02 |
| HDL-C, mmol/l | 1.2±0.3 | 1.1±0.3 | 1.2±0.3 | 0.004 |
| D, NYHA
classification |
| I, n (%) | 81 (31.6) | 1 (1.0) | 80 (51.6) | <0.001 |
| II, n (%) | 136 (53.1) | 77 (76.2) | 59 (38.1) | <0.001 |
| III, n (%) | 33 (12.9) | 19 (18.8) | 14 (9.0) | 0.034 |
| IV, n (%) | 6 (2.3) | 4 (4.0) | 2 (1.3) | 0.216 |
| E, Clinical
presentationb |
| HFrEF, n (%) | 86 (50.3) | 65 (64.4) | 21 (13.5) | <0.001 |
| HFpEF, n (%) | 85 (49.7) | 35 (34.7) | 50 (32.3) | 0.786 |
| DAPA, n (%) | 56 (21.9) | 23 (22.8) | 33 (21.3) | 0.877 |
Biochemical analyses, procedure and
medications
On the next morning following admission, fasting
blood samples were collected from patients. (3-5 ml) and
temporarily maintained at 4˚C, before being processed within 2 h.
After centrifugating at 1,500 x g for 30 min at 4˚C, the serum was
collected for further measurements of Drp1 concentrations using an
ELISA kit (cat. no. EH14381) following manufacturer's instructions
(Wuhan Fine Biotech Co., Ltd.). All ELISA data were analyzed
according to the standard curve and each sample was measured twice
to acquire a mean value. In addition, three experienced primary
interventionists were in charge of all the potential interventional
procedures according to the current standards and the common
perioperative antithrombotic therapies were applied following the
current guidelines (13). Routine
therapies, including β-blockers, angiotensin-converting enzyme
inhibitors/angiotensin receptor blockers, aldosterone antagonists,
or sacubitril valsartan sodium were applied as appropriate, while
possible use of sodium-glucose co-transporter 2 inhibitions or
ivabradine were also recommended as adjunctive therapies for
secondary prevention if necessary (14).
Study endpoints and relevant
definitions
Patients were evaluated at 1 and 6 months after
discharge, mainly through telephone call or clinical office visits.
An independent cardiologist blinded to the present study assessed
and recorded the relevant clinical events. The primary outcome was
the risk of composite major adverse cardiac events (MACEs),
incuding cardiac mortality and rehospitalization for HF. Cardiac
mortality was defined as mortality without a clear non-cardiac
cause as confirmed in clinic or autopsy. Rehospitalization for HF
was defined following the criteria described in Cardiovascular
Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart
Failure Patients with Functional Mitral Regurgitation trial
(15).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 23.0; IBM Corp.). The present study summarized
baseline characteristics and clinical outcomes using frequencies
with percentages or means with standard deviation as appropriate.
Continuous variables were first assessed for normality using the
Shapiro-Wilk test. For data following a normal distribution,
comparisons between two groups were conducted with the Student's
t-test. For non-normally distributed data, the Mann-Whitney U test
was applied instead. Categorical variables were compared using the
chi-square test or Fisher's exact test, depending on the expected
frequencies. To evaluate the predictive value of serum Drp1 levels
for the absence of MACEs, a receiver operating characteristic (ROC)
curve was constructed and the optimal threshold determined using
the Youden index. This threshold was then used to categorize the
subjects. In addition, binary logistic regression was performed to
control for confounding factors and pinpoint independent predictors
of the primary endpoint. Time-to-event data were illustrated using
Kaplan-Meier curves, with group differences assessed through the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient selection and baseline
characteristics
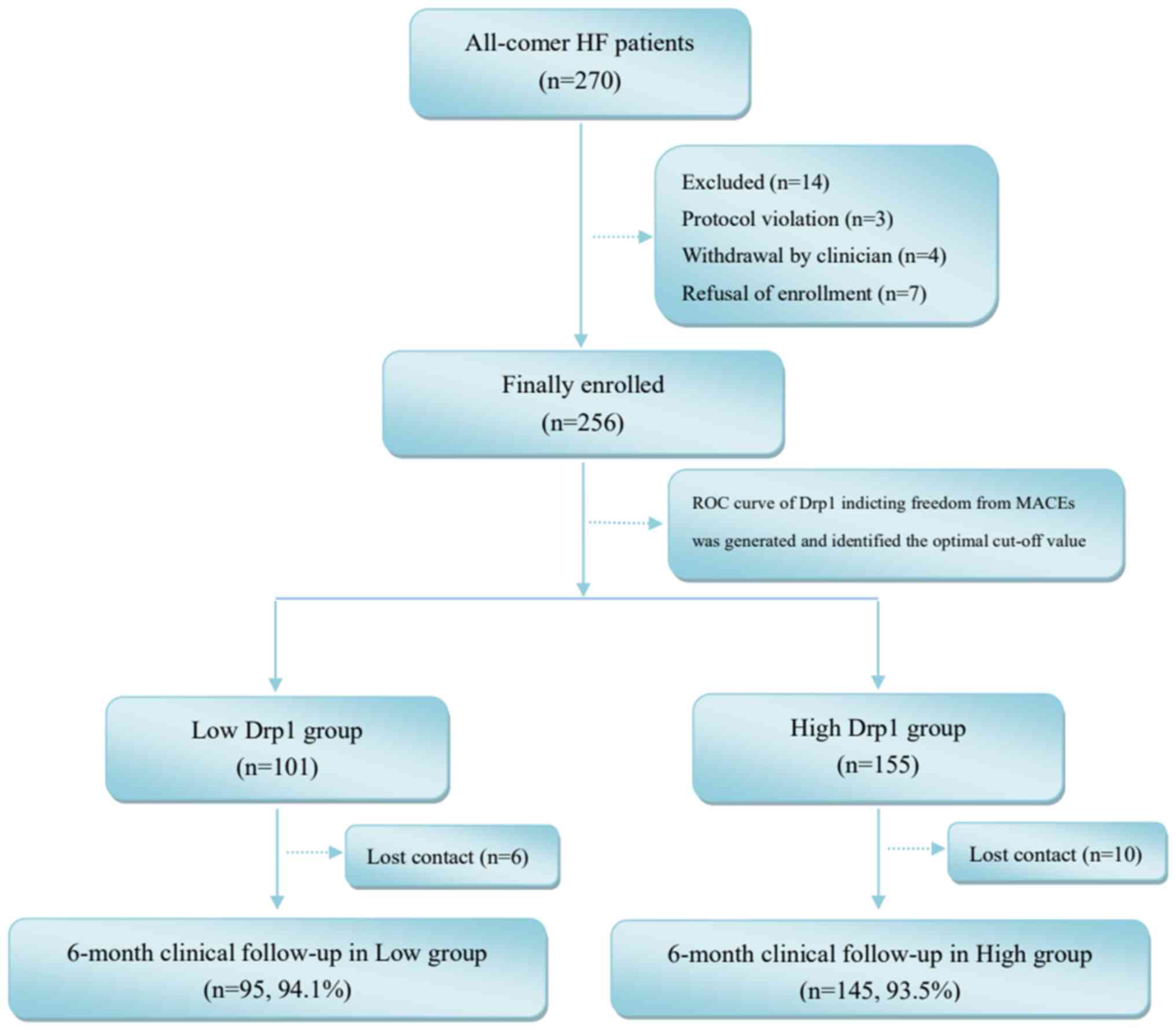
A total of 256 patients from Zhongda Hospital
(Nanjing, China) were finally enrolled in the present study
(Fig. 1). A 6-month follow-up was
accomplished in most of the enrolled patients and contact was lost
in only 6.25% of participants. The serum Drp1 concentrations were
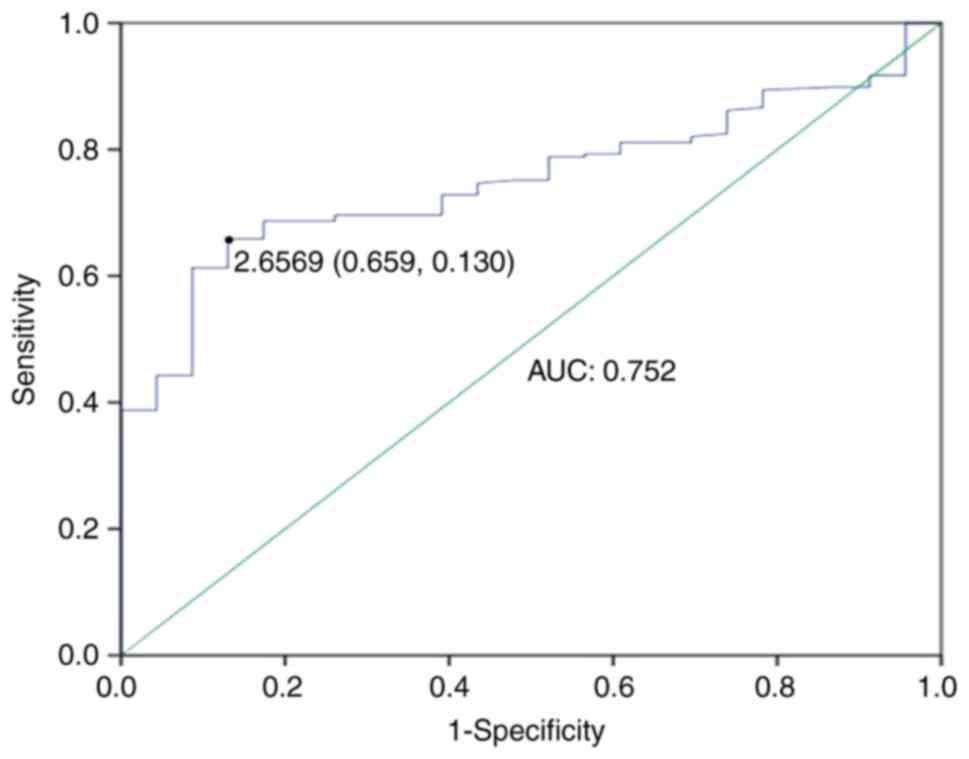
measured in all participants (5.2±4.5 ng/ml). Upon analyzing the
ROC curve for serum Drp1 levels in assessing the absence of MACE
risk, a threshold of 2.66 ng/ml was established as the optimal
cutoff point (Youden index=0.529). This value forecasts the
likelihood of evading MACEs with a sensitivity of 65.9% and a
specificity of 87% [area under the curve: 0.752; 95% confidence
interval (CI): 0.679-0.824, P<0.001; Fig. 2]. Subsequently, the participants of
the present study were categorized into two cohorts: Low Drp1 group
(Drp1 ≤2.66 ng/ml; n=101) and high Drp1 group (Drp1 >2.66 ng/ml;
n=155). An overview of the baseline traits of these individuals
revealed comparable demographics across both groups, with the
exception of higher incidences of coronary artery disease (80.2%
vs. 52.3%, P<0.001) and previous myocardial infarction (MI;
67.3% vs. 14.8%; P<0.001) in the high Drp1 group (Table I).
Association between the serum Drp1
level and cardiac function and clinical outcomes
Fig. 3 illustrates
that the serum Drp1 levels were significantly reduced in the low
Drp1 group, with a mean concentration of 1.8 ng/ml, in contrast to
7.4 ng/ml in the high Drp1 group (P<0.001; Fig. 3A). By contrast, N-terminal
pro-B-type natriuretic peptide (NT-proBNP) levels were
substantially elevated in the low Drp1 group, with a median of
1,970.0 pg/ml compared with 182.5 pg/ml in the high Drp1 group
(P<0.001; Fig. 3B).
Additionally, patients with diminished serum Drp1 concentrations
were associated with a more severe New York Heart Association
(NYHA) functional classification, averaging 2.3, as opposed to 1.6
for those with elevated concentrations (P<0.001; Fig. 3C).
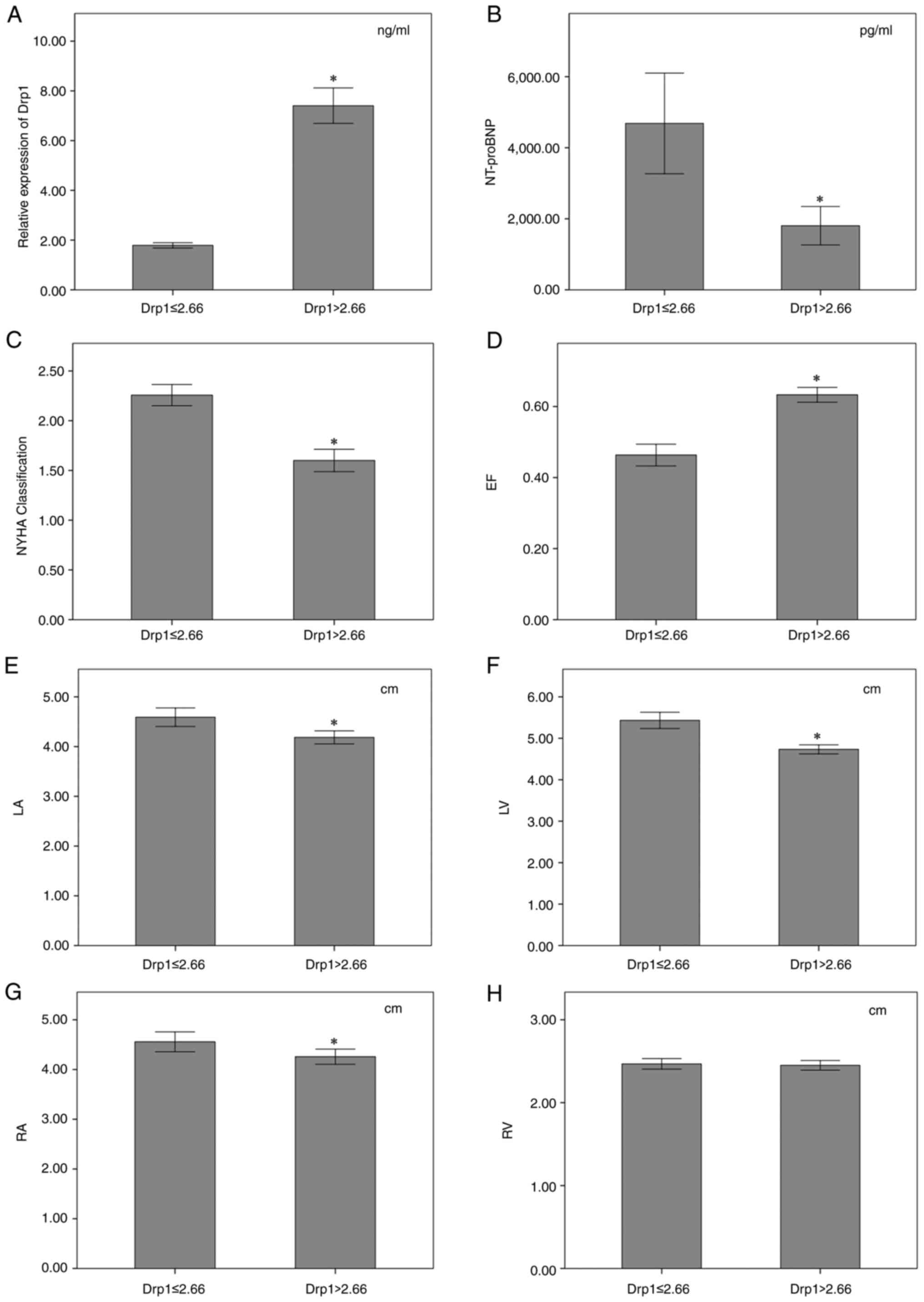 | Figure 3Characteristics of clinical
manifestation, cardiac structure and function in the low Drp1 group
(Drp1 ≤2.66 ng/ml) and the high Drp1 group (Drp1 >2.66 ng/ml).
Comparisons of (A) serum Drp1 level, (B) NT-proBNP level, (C) NYHA
classification, (D) left ventricular EF, (E) internal diameter of
LA, (F) internal diameter of LV, (G) internal diameter of RA and
(H) internal diameter of RV in low Drp1 group vs. the high Drp1
group. Error bars indicate ± SD. *P<0.05. Drp1,
dynamin-related protein 1; NT-proBNP, N-terminal pro-B-type
natriuretic peptide; NYHA, New York Heart Association; EF, ejection
fraction; LA, left atrium; LV, left ventricle; RA, right atrium;
RV, right ventricle. |
As shown in Fig. 3,
the serum Drp1 concentrations were markedly decreased in the low
Drp1 group (1.8±0.5 vs. 7.4±4.5 ng/ml, P<0.001; Fig. 3A) while the NT-proBNP levels were
much higher (1970.0 pg/ml vs. 182.5 pg/ml, P<0.001; Fig. 3B). Patients with low serum Drp1
concentrations showed higher grade of NYHA functional
classification than those with high concentrations (2.3±0.5 vs.
1.6±0.7, P<0.001; Fig. 3C).
Simultaneously, the echocardiography data revealed a clear
association between low serum Drp1 levels and compromised heart
structure and function. This was especially evident in the reduced
ejection fraction (EF), which was significantly lower in the group
with diminished Drp1 (46.3±15.5% vs. 63.3±13.0%; P<0.001;
Fig. 3D). Additionally, there was
an increase in the internal diameter measurements of the left
atrium (LA; 4.6±0.9 cm vs. 4.2±0.8 cm; P<0.001; Fig. 3E), left ventricle (LV; 5.4±1.0 cm
vs. 4.7±0.7 cm; P<0.001; Fig.
3F), and right atrium (RA; 4.6±1.0 vs. 4.3±1.0 cm; P=0.018;
Fig. 3G), indicating significant
dilatation of heat. By contrast, the right ventricular internal
diameter (RV) showed no significant difference between groups
(2.5±0.3 cm vs. 2.5±0.4 cm; P=0.703; Fig. 3H), suggesting that specific areas
of the heart were more affected by Drp1 levels.
The clinical outcomes are listed in Table II. After 6-month follow-up, a
significant association was observed between low serum Drp1 levels
and an elevated risk of MACEs, with incidences of 21.1% in the low
Drp1 group compared with 2.8% in the counterpart group (P<0.001;
Fig. 4A). This association appears
to be primarily driven by increased rates of rehospitalization in
the low Drp1 group (21.1% vs. 2.1%; P<0.001; Fig. 4B). Although not statistically
conclusive, patients with higher Drp1 concentrations exhibited a
notable decrease in cardiac death risk (4.2% for the low Drp1 group
vs. 0.7% for the high Drp1 group; P=0.082). However, the comparison
of all-cause mortality between the two groups did not yield
statistically significant results (4.2% vs. 1.4%; P=0.217).
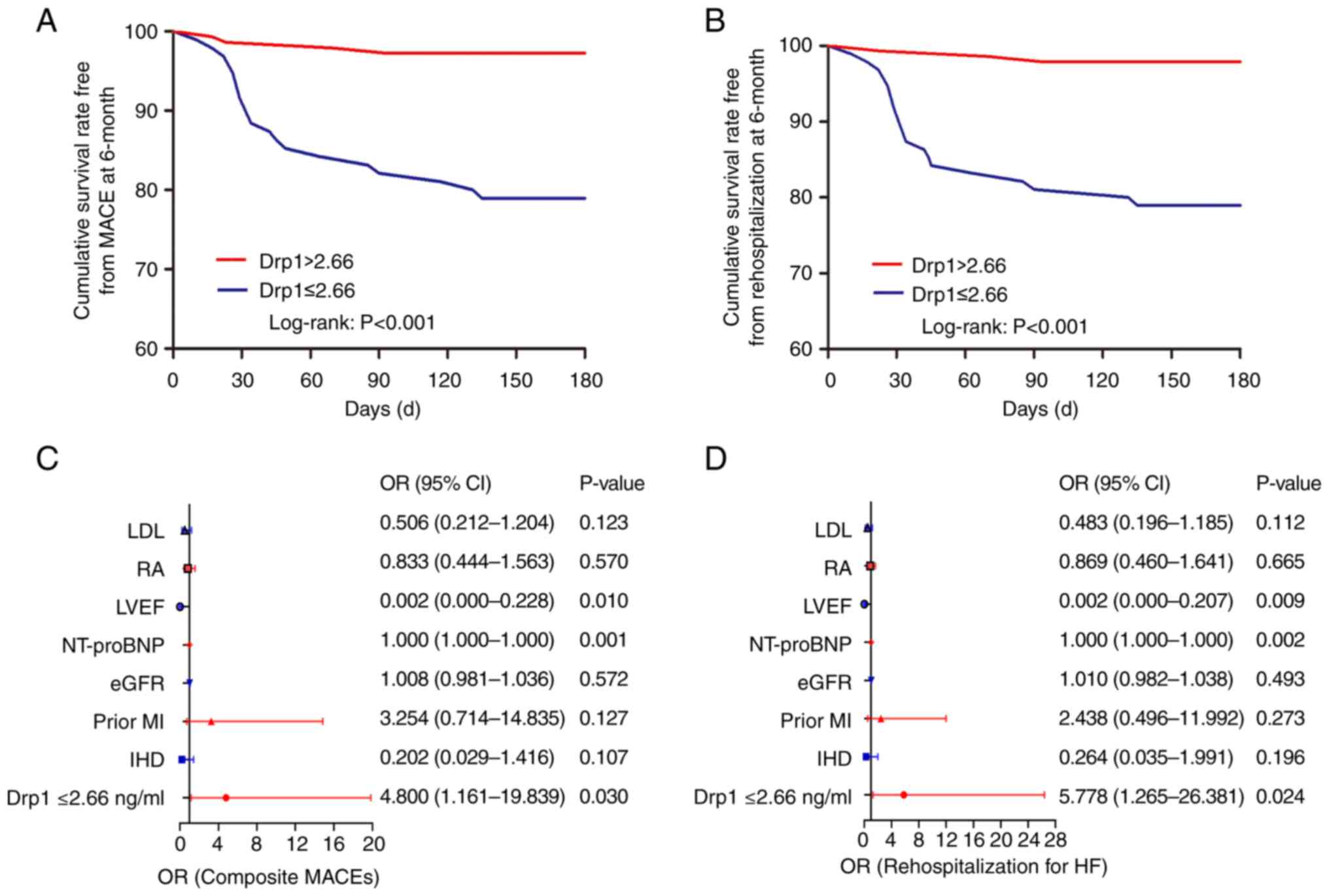 | Figure 4Survival curves and forest plots. (A)
Kaplan-Meier curves for MACEs and (B) rehospitalization for HF in
the low Drp1 group (Drp1 ≤2.66 ng/ml, red line) vs. the high Drp1
group (Drp1 >2.66 ng/ml; blue line) at 6-month follow-up. Forest
plots revealing the association between Drp1 at the threshold of
>2.66 ng/ml and (C) a composited MACE and (D) rehospitalization
for HF. MACEs, major adverse cardiac events; HF, heart failure; OR,
odds ratio; LDL, lipoprotein; RA, right atrium; LVEF, left
ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type
natriuretic peptide; eGFR, estimated glomerular filtration rate;
MI, myocardial infarction; IHD, ischemic heart disease. |
 | Table IIClinical follow-up in the low and
high Drp1 groups. |
Table II
Clinical follow-up in the low and
high Drp1 groups.
| | 1-month, n (%) | 6-month, n (%) |
|---|
| Parameter | Drp1 ≤2.66
(n=101) | Drp1 >2.66
(n=155) | P-value | Drp1 ≤2.66
(n=101) | Drp1 >2.66
(n=155) | P-value |
|---|
| MACEs | 11 (10.9) | 2 (1.3) | 0.001 | 20 (19.8) | 4 (1.8) | <0.001 |
| Rehospitalization
for HF | 11 (10.9) | 1 (0.6) | <0.001 | 20 (19.8) | 3 (1.9) | <0.001 |
| Cardiac
mortality | 1 (1.0) | 1 (0.6) | 1.000 | 4 (4.0) | 1 (0.6) | 0.082 |
| All-cause
mortality | 1 (1.0) | 1 (0.6) | 1.000 | 4 (4.0) | 2 (1.3) | 0.217 |
For further confirmation of the associations,
logistic regression analysis was performed to identify the
potential independent risk predictors of MACEs in these patients
(Fig. 4C). Low serum Drp1
concentration [odds ratio (OR): 4.800, 95% CI: 1.161-19.839;
P=0.03)], NT-proBNP levels (OR: 1.000, 95% CI: 1.000-1.000;
P=0.001) and left ventricular ejection fraction (OR: 0.002, 95% CI:
0.000-0.228; P=0.01) were identified as the independent factors for
predicting the occurrence of MACEs after regressing in a
multivariate model. These three predictors were also confirmed to
be associated with an increased risk of rehospitalization for HF
after adjusting for confounding factors, including IHD, prior MI,
estimated glomerular filtration rate, internal diameter of right
atrium and lipoprotein (Fig.
4D).
Discussion
The present study unveiled a novel link between
serum Drp1 levels and the outcomes of patients with HF across the
board. The pivotal discovery revealed that lower serum Drp1
concentrations were associated with significant cardiac structural
abnormalities and impaired heart function. This association
significantly heighted the risk of MACEs, positioning serum Drp1 as
a standalone predictor of risk.
HF is typically a consequence of abnormalities in
cardiac structure and function. Despite advancements in medical
treatment and rehabilitation efforts, HF remains linked to a
significant rate of readmissions and cardiac incidents globally.
This persistent challenge may be attributed to the predominant
clinical focus on symptom alleviation rather than addressing
underlying causes (16,17). Notably, a number of standard
medical treatments, which have been rigorously evaluated in large
clinical trials, have yielded underwhelming results in terms of
efficacy. For instance, the use of ß-blockers in the OPTIMIZE-HF
study (18), spironolactone in the
TOPCAT trial (19) and Ibersartan
in the I-Preserve study (20), all
reported outcomes that fell short of expectations. Therefore, it
should be crucial to tailor medical interventions to the specific
progressive stages and phenotypes of HF to avoid varying clinical
outcomes (21). Moreover, while
novel oral medications such as SGLT2 inhibitors and vericiguat have
demonstrated significant advantages for HF patients (7,22,23),
their application in clinical practice continues to be a subject of
discussion. As a result, there has been a surge in research efforts
aimed at deciphering the intricate mechanisms driving HF
progression, with the goal of creating more targeted and effective
treatments.
A recent study by Zhuang et al (24). showed a direct association between
the inhibition of mitochondrial bioenergetics and the advancement
of cardiac hypertrophy and HF. Drp1, a member of the dynamin family
of GTPases, exhibits several splice variants and is predominantly
expressed in vital tissues such as the heart, skeletal muscle,
brain and kidneys (25,26). It is recognized for its pivotal
role in regulating mitochondrial fission, a process integral to
maintaining mitochondrial integrity (9). This regulation is critically
important as it substantially influences cardiac metabolism and the
mechanisms of programmed cell death (27). The key pathophysiological factors
contributing to HF progression include both the reduction in
contractile units and the diminished mitochondrial bioenergetics
within surviving cardiomyocytes after myocardial injuries (28,29).
Building on our previous research, restoring balance to
mitochondrial fission by regulating Drp1 expression in the affected
myocardial tissue could enhance cardiac metabolism and then
mitigate apoptosis in MI (12).
The current study indicated an association between lower serum
levels of Drp1 and more pronounced cardiac structural anomalies,
alongside deteriorated cardiac function. The findings correspond
with earlier fundamental research that demonstrated the conditional
suppression of Drp1 in mice leads to gradual LV enlargement
followed by a notable decrease in EF (8). However, it is important to note that
a majority of subjects in the Low Drp1 group (67.3%) had a history
of MI, predisposing them to a high likelihood of developing HFrEF
as a consequence of severe ROS-induced cellular damage. This might
mainly account for the significantly decreased serum Drp1
concentrations and the deteriorated cardiac function observed in
these participants. Moreover, low serum Drp1 concentrations were
observed to have a positive association with a heightened risk of
MACEs, predominantly due to a surge in the rate of
rehospitalization. Indeed, epidemiological studies have previously
reported comparable rates of mortality and morbidity in cases of
HFpEF compared with HFrEF (30).
Notably, despite a substantially higher prevalence of HFrEF in the
group with low Drp1 levels (64.4% vs. 13.5%; P<0.001), only the
rehospitalization rates increased correspondingly, whereas
mortality rates remained unchanged.
By contrast, Chen et al (31) indicated that Drp1 could act as a
regulator of hyperlipidemia, inflammation and myocardial injuries
in rats with hyperlipidemia-MI by affecting mitochondrial
dysfunction and NLRP3 expression. The low Drp1 group exhibited
substantially reduced serum levels of total cholesterol,
high-density lipoprotein-cholesterol (HDL-C), and low-density
lipoprotein-cholesterol (LDL-C). These markers, integral to lipid
metabolism, have a strong association with the fibrous cap
thickness in lipid-rich plaques. Thinner caps can precipitate
sudden cardiac events through the rupture of coronary
atherosclerotic plaques (32).
This association could further explain the lack of a significant
difference in cardiac mortality rates between the two study groups.
To the best of the authors' knowledge, sudden mortality is
frequently a consequence of cardiovascular diseases, particularly
due to mechanical complications or severe arrhythmias following a
MI (33). Additionally, research
had shown that Drp1-dependent mitochondrial autophagy is initially
triggered but subsequently suppressed in mouse hearts under
pressure overload, a process that is critical in the progression of
mitochondrial dysfunction and HF (34). Thus, in light of the logistic
regression analysis in the present study, lower serum levels of
Drp1 might serve as an independent prognostic indicator for MACEs
in patients with HF.
There are several limitations in the design and
conduct of the current study. Initially, despite incorporating data
from 256 patients, the research was characterized by its
single-center, prospective, observational nature, coupled with a
relatively limited sample size. To enhance the robustness of the
findings, future research would benefit from a larger-scale,
multi-center, randomized trial with greater statistical power.
Furthermore, the potential influence of oral medications on Drp1
expression cannot be entirely ruled out. This is particularly
relevant concerning the use of Dapagliflozin (DAPA), which has been
shown to modulate Drp1 expression in infarcted heart tissue
(12). Despite no substantial
disparity in the baseline utilization of DAPA between the low and
high Drp1 groups, the origin of plasma Drp1 is yet to be
determined, underscoring the need for further research.
Additionally, a longer follow-up period is recommended to reinforce
the established association. Finally, while white blood cell counts
did not differ significantly between the groups, the absence of
data on hypersensitive C-reactive protein and procalcitonin hinders
a comprehensive assessment of these inflammatory markers and the
prevention of measurement bias.
The findings of the present study revealed that
individuals exhibiting diminished levels of serum Drp1 were more
likely to experience pronounced cardiac structural irregularities
and impaired heart functionality. Furthermore, a low serum Drp1
concentration was validated as an autonomous prognostic indicator,
heightening the likelihood of MACE and recurrent hospital
admissions in patients with HF irrespective of the underlying
cause. Consequently, serum Drp1 could potentially act as a
predictive biomarker for the clinical outcome of such all-comer HF
cases and may also represent an innovative therapeutic avenue
within the disease's pathological trajectory.
Acknowledgements
The authors would like to thank the Biobank of
Zhongda Hospital Affiliated to Southeast University for technical
assistance. The authors also would like to thank Dr Wan-Xin Wang of
the Department of Cardiology, Zhongda Hospital, School of Medicine,
Southeast University, for helping assessing patients for
eligibility.
Funding
Funding: The present study was supported by National Natural
Science Foundation of China (grant no. 82070295) and Jiangsu
Provincial Key Medical Discipline (grant no. ZDXKA2016023).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
GSM conceived the project and designed the present
study. ZGF and YX assessed the patients for eligibility in terms of
inclusion and exclusion criteria. ZGF, YX and MYJ performed the
ELISA. SHH evaluated and recorded all clinical events. ZGF and CC
performed the statistical analyses. MYJ, CC and YX constructed the
figures. All authors analyzed and discussed the data. ZGF wrote the
manuscript, and revision was by CC, GSM and SHH. All authors
contributed to drafting the manuscript. GSM and SHH confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Zhongda Hospital (Nanjing,
China) approved the present study protocol (approval no.
2020ZDSYLL306-P01). Informed consent was obtained from all
participants in the present study.
Patient consent for publication
All patients provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the American heart association. Circulation. 135:e146–e603.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Normand C, Kaye DM, Povsic TJ and
Dickstein K: Beyond pharmacological treatment: An insight into
therapies that target specific aspects of heart failure
pathophysiology. Lancet. 393:1045–1055. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hunt SA, Baker DW, Chin MH, Cinquegrani
MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G,
Jessup ML, et al: ACC/AHA guidelines for the evaluation and
management of chronic heart failure in the adult: Executive
summary. A report of the American college of cardiology/American
heart association task force on practice guidelines (committee to
revise the 1995 guidelines for the evaluation and management of
heart failure). J Am Coll Cardiol. 38:2101–2113. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
O'Connor CM: HFpEF: From early
observations to worldwide awareness. JACC. Heart Fail. 6:718–719.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure: The task force for
the diagnosis and treatment of acute and chronic heart failure of
the european society of cardiology (ESC). Developed with the
special contribution of the heart failure association (HFA) of the
ESC. Eur J Heart Fail. 18:891–975. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Verma S, McGuire DK and Kosiborod MN: Two
tales: One story: EMPEROR-reduced and DAPA-HF. Circulation.
142:2201–2204. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Aimo A, Pateras K, Stamatelopoulos K,
Bayes-Genis A, Lombardi CM, Passino C, Emdin M and Georgiopoulos G:
Relative efficacy of sacubitril-valsartan, vericiguat, and SGLT2
inhibitors in heart failure with reduced ejection fraction: A
systematic review and network meta-analysis. Cardiovasc Drugs Ther.
35:1067–1076. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Song M, Mihara K, Chen Y, Scorrano L and
Dorn GW II: Mitochondrial fission and fusion factors reciprocally
orchestrate mitophagic culling in mouse hearts and cultured
fibroblasts. Cell Metab. 21:273–286. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Twig G, Elorza A, Molina AJ, Mohamed H,
Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al:
Fission and selective fusion govern mitochondrial segregation and
elimination by autophagy. EMBO J. 27:433–446. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tham YK, Bernardo BC, Ooi JY, Weeks KL and
McMullen JR: Pathophysiology of cardiac hypertrophy and heart
failure: Signaling pathways and novel therapeutic targets. Arch
Toxicol. 89:1401–1438. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brown DA, Perry JB, Allen ME, Sabbah HN,
Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA,
et al: Expert consensus document: Mitochondrial function as a
therapeutic target in heart failure. Nat Rev Cardiol. 14:238–250.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fan ZG, Xu Y, Chen X, Ji MY and Ma GS:
Appropriate dose of dapagliflozin improves cardiac outcomes by
normalizing mitochondrial fission and reducing cardiomyocyte
apoptosis after acute myocardial infarction. Drug Des Devel Ther.
16:2017–2030. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Writing Committee Members. Lawton JS,
Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM,
Bittl JA, Cohen MG, DiMaio JM, et al: 2021 ACC/AHA/SCAI guideline
for coronary artery revascularization: A report of the American
college of cardiology/American heart association joint committee on
clinical practice guidelines. J Am Coll Cardiol. 79:e21–e129.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heidenreich PA, Bozkurt B, Aguilar D,
Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM,
Evers LR, et al: 2022 AHA/ACC/HFSA guideline for the management of
heart failure: A report of the American college of
cardiology/American heart association joint committee on clinical
practice guidelines. Circulation. 145:e895–e1032. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stone GW, Lindenfeld J, Abraham WT, Kar S,
Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia
SR, et al: Transcatheter mitral-valve repair in patients with heart
failure. N Engl J Med. 379:2307–2318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American college of cardiology
foundation/American heart association task force on practice
guidelines. J Am Coll Cardiol. 62:e147–e239. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wilcox JE, Fonarow GC, Ardehali H, Bonow
RO, Butler J, Sauer AJ, Epstein SE, Khan SS, Kim RJ, Sabbah HN, et
al: ‘Targeting the heart’ in heart failure: Myocardial recovery in
heart failure with reduced ejection fraction. JACC Heart Fail.
3:661–669. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hernandez AF, Hammill BG, O'Connor CM,
Schulman KA, Curtis LH and Fonarow GC: Clinical effectiveness of
beta-blockers in heart failure: Findings from the OPTIMIZE-HF
(organized program to initiate lifesaving treatment in hospitalized
patients with heart failure) registry. J Am Coll Cardiol.
53:184–192. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pitt B, Pfeffer MA, Assmann SF, Boineau R,
Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al:
Spironolactone for heart failure with preserved ejection fraction.
N Engl J Med. 370:1383–1392. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Massie BM, Carson PE, McMurray JJ, Komajda
M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger
C, et al: Irbesartan in patients with heart failure and preserved
ejection fraction. N Engl J Med. 359:2456–2467. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bhagat AA, Greene SJ, Vaduganathan M,
Fonarow GC and Butler J: Initiation, continuation, switching, and
withdrawal of heart failure medical therapies during
hospitalization. JACC Heart Fail. 7:1–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McMurray JJV, Solomon SD, Inzucchi SE,
Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS,
Anand IS, Bělohlávek J, et al: Dapagliflozin in patients with heart
failure and reduced ejection fraction. N Engl J Med. 381:1995–2008.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Anker SD, Butler J, Filippatos G, Ferreira
JP, Bocchi E, Böhm M, Brunner–La Rocca HP, Choi DJ, Chopra V,
Chuquiure-Valenzuela E, et al: Empagliflozin in heart failure with
a preserved ejection fraction. N Engl J Med. 385:1451–1461.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhuang L, Jia K, Chen C, et al:
DYRK1B-STAT3 Drives Cardiac Hypertrophy and Heart Failure by
Impairing Mitochondrial Bioenergetics. Circulation. Mar 15
2022;145(11):829-846.
|
|
25
|
Imoto M, Tachibana I and Urrutia R:
Identification and functional characterization of a novel human
protein highly related to the yeast dynamin-like GTPase Vps1p.
Journal of cell science. May 1998;111 (Pt 10):1341-1349.
|
|
26
|
Yoon Y, Pitts KR, Dahan S and McNiven MA:
A novel dynamin-like protein associates with cytoplasmic vesicles
and tubules of the endoplasmic reticulum in mammalian cells. J Cell
Biol. 140:779–793. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tong M, Zablocki D and Sadoshima J: The
role of Drp1 in mitophagy and cell death in the heart. J Mol Cell
Cardiol. 142:138–145. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ferrannini E, Mark M and Mayoux E: CV
protection in the EMPA-REG OUTCOME trial: A ‘thrifty substrate’
hypothesis. Diabetes Care. 39:1108–1114. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jose Corbalan J, Vatner DE and Vatner SF:
Myocardial apoptosis in heart disease: does the emperor have
clothes? Basic Res Cardiol. 111(31)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lam CS, Donal E, Kraigher-Krainer E and
Vasan RS: Epidemiology and clinical course of heart failure with
preserved ejection fraction. Eur J Heart Fail. 13:18–28.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen X, Liang J, Bin W, Luo H and Yang X:
Anti-hyperlipidemic, anti-inflammatory, and ameliorative effects of
DRP1 inhibition in rats with experimentally induced myocardial
infarction. Cardiovasc Toxicol. 21:1000–1011. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Virmani R, Kolodgie FD, Burke AP, Farb A
and Schwartz SM: Lessons from sudden coronary death: A
comprehensive morphological classification scheme for
atherosclerotic lesions. Arterioscler Thromb Vasc Biol.
20:1262–1275. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kumar A, Avishay DM, Jones CR, Shaikh JD,
Kaur R, Aljadah M, Kichloo A, Shiwalkar N and Keshavamurthy S:
Sudden cardiac death: epidemiology, pathogenesis and management.
Rev Cardiovasc Med. 22:147–158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shirakabe A, Zhai P, Ikeda Y, Saito T,
Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B and Sadoshima J:
Drp1-dependent mitochondrial autophagy plays a protective role
against pressure overload-induced mitochondrial dysfunction and
heart failure. Circulation. 133:1249–1263. 2016.PubMed/NCBI View Article : Google Scholar
|