Introduction
In recent years, breast cancer (BC) has become the
most commonly diagnosed malignant tumor in women (1), and the mortality rate ranks first
among all types of female cancer worldwide (2). The current treatment methods for BC
mainly comprise chemotherapy, radiotherapy, immunotherapy, targeted
therapy and endocrine therapy (3).
Although various assistive devices and novel targeted drugs have
entered clinical practice, BC continues to be associated with a
high rate of recurrence and mortality due to its distant metastasis
to other organs (4), and the
underlying metastatic mechanism has yet to be fully elucidated.
Therefore, it is critical to identify both novel biomarkers and
therapeutic targets for the treatment of BC.
Chemotaxis fulfills an important role in tumor
metastasis, and is mediated by the chemoattractant epidermal growth
factor (EGF) and its receptor (EGFR), which is expressed on the
surface of tumor cells (5).
Protein kinase Cζ (PKCζ) belongs to the atypical class of PKCs and
fulfills multiple roles in different types of physiological
processes and diseases (6). A
previous study demonstrated that PKCζ is involved in the signaling
of insulin-regulated glucose uptake (7), and it also has a role in glucose
transport through regulating motor proteins (8). In addition, PKCζ was identified as a
pro-inflammatory factor in the pathogenesis of steatohepatitis
(9). The activation of PKCζ has
also been shown to have a crucial role in respiratory syncytial
virus replication and pathology (10). Moreover, PKCζ has continued to
attract attention in various tumor fields. For example, it has been
reported that PKCζ aggravates doxorubicin-induced cardiotoxicity
via inhibiting Wnt/β-catenin signaling (11). Knockdown of PKCζ was shown to
decrease tumor growth and lymphatic metastasis of prostate cancer
in a mice xenograft model (12).
In addition, PKCζ/ADAR2 (the RNA-editing enzyme adenosine deaminase
acting on RNA 2) axis is a critical regulator of colorectal cancer
metastasis, acting through modulation of the level of
miR-200(13). Moreover, PKCζ has
also been identified as a downstream signaling molecule of AKT,
which was shown to be required for the EGF-stimulated chemotaxis
and migration of human BC cells (14).
Pentraxin 3 (PTX3), also known as TNFAIP5 and
tsg-14, is a member of the n-pentameter protein superfamily, which
functions as a soluble recognition receptor protein produced by
phagocytes and non-immune cells at the site of inflammation
(15). Studies have shown that
PTX3 exerts a central role in the process of mineralization
(16), the natural immune response
and inflammation (17,18), and also functions as a
pro-angiogenic factor (19).
Beyond the multifunctional roles of PTX3, its overexpression has
been observed in several types of tumors, and its expression level
has been shown to be associated with the degree of malignancy of
tumors (20-22).
Moreover, it has been shown that PTX3 is associated with stem-like
features (23), and the
epithelial-mesenchymal transition (EMT), migration and invasion of
cancer cells (24). In addition,
previous studies reported that activation of the PI3K/Akt signaling
pathway led to an increase in the expression of PTX3 in
head-and-neck squamous cell carcinomas (25,26).
However, the specific mechanism through which PTX3 exerts a role in
BC cell proliferation and metastasis remains contentious. The aim
of the present study was therefore to verify whether PTX3 is an
important molecule modulating BC cell proliferation and metastasis,
and whether its primary role in BC cells may be exerted via the
activation of PKCζ.
Materials and methods
Patient samples
The present study was approved by The Clinical
Medical Research Ethical Committee of Tianjin Third Central
Hospital (Tianjin, China; approval no. IRB2023-039-01) for the use
of clinical biopsy specimens, and informed written consent was
obtained from the patients. Tissues from a total of 60 cases of
primary BC (including invasive ductal carcinoma and invasive
lobular carcinoma), and 22 cases of benign breast tissues were
collected from Tianjin Third Central Hospital (Tianjin, China). The
inclusion criteria were as follows: i) Patients with pathological
diagnosis of BC; and ii) patients who underwent radical mastectomy
with no adjuvant treatment prior to and after surgery. Patients
with serious heart, liver and kidney injuries, serious infections,
endocrine and blood system diseases were excluded. In addition, the
clinicopathological parameters of patients were all collected.
Cell culture
The human normal mammary epithelial cell line
MCF-10A, the BC cell lines MDA-MB-231 and MCF7, and the embryonic
kidney cell line 293T were purchased from the American Type Culture
Collection. Cells were grown in Dulbecco's modified Eagle medium
(DMEM; Biological Industries) supplemented with 10% Hyclone fetal
bovine serum (FBS; Cytiva) at 37˚C in an atmosphere of 5%
CO2.
Establishment of stably transfected
cell lines
293/293T cells (American Type Culture Collection)
were transfected with the lentiviral packaging Lenti-Mix vector
(pMDL, pVSVG or pRSV-Rev, 5:3:2; Thermo Fisher Scientific, Inc.)
carrying specific small hairpin RNA (shRNA) targeting nucleotides
to PTX3 (PTX3 shRNA) to generate stable cell lines. The sequence of
the selected PTX3 shRNA oligomer was 5'-GCATCCTGTGAGACCAATGAA-3'.
The plasmid backbone was pLVX-shRNA (Thermo Fisher Scientific,
Inc.) and 12 µg lentiviral plasmid plus 12 µg Lenti-Mix were used
for transfection. A third generation system was used and a MOI of
~40 was used to infect cells. Briefly, lentiviral supernatants were
collected, centrifuged at 3,000 x g at 4˚C for 10 min and filtered
through a 0.45-µm filter. MDA-MB-231 and MCF7 cells were
subsequently infected with lentiviral supernatant, incubated at
37˚C overnight and selected with 1.5 µg/ml puromycin (Biosharp Life
Sciences) for 14 days and maintained with 0.625 µg/ml puromycin for
7 days for use for subsequent experiments. Cells transfected with
an unrelated sequence (scrambled siRNA) were used as the negative
control. The sequence of the negative control siRNA is listed in
Table SI.
Cell viability assay
Cell viability was estimated by Cell Counting Kit-8
(CCK-8) assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's instructions. Briefly, cells
from the control and shPTX3 groups were seeded in 96-well plates
and cultured for different time periods (0, 1, 2, 3, 4 or 5 days).
Aliquots (15 µl) of CCK-8 were subsequently added to each well and
incubated at 37˚C for 2 h. Finally, the absorbance was measured at
450 nm.
Wound healing assay
Briefly, cells were inoculated into a 6-well plate,
and after the cells had reached 80-90% confluency, the cells were
starved for 12 h and scratched evenly at the bottom of the orifice
plate with a 10 µl tip. The distances of cell migration calculated
by changes in the widths of the scratches were recorded at
different time points (0, 3, 6, 9 or 12 h). A total of three
measurements at different positions along each scratch-line were
recorded using an Olympus BX53 light microscope (Olympus
Corporation).
Chemotaxis assay
Briefly, the chemoattractant (EGF; PeproTech, Inc.)
was loaded into the lower chamber, and cells (5x105/ml)
suspended in binding medium (DMEM containing 0.1% bovine serum
albumin and 25 mM Hepes) were subsequently loaded into the upper
chamber (Neuro Probe Inc.). The two chambers were segregated with a
10 µm membrane (Neuro Probe Inc.) that had been pretreated with 10
µg/ml fibronectin (MilliporeSigma) at 4˚C overnight. Subsequently,
the chambers were incubated in an atmosphere containing 5%
CO2 at 37˚C for 3 h. The filter membrane was then
washed, and the attached cells were fixed and stained with trypan
blue for 10 min at room temperature (RT). The number of migrating
cells was counted in three separate fields under an Olympus BX53
microscope, and the chemotaxis index was calculated according to
the following formula: Number of migrating cells in a
chemoattractant gradient/the number of migrating cells in the
control group.
Adhesion assay
After having starved the cells with serum-free
medium containing 50 ng/ml EGF, the cells were first plated in 35
mm dishes containing a glass coverslip that had been coated with 10
µg/ml fibronectin overnight at 4˚C, and then air-dried. Cells were
allowed to attach to the coverslips for time periods of 5, 15 or 30
min, and subsequently were washed gently twice with cold PBS, prior
to being fixed with 4% paraformaldehyde for 10 min at 4˚C. Finally,
all cells attached to each coverslip were counted with a
hemocytometer under a light microscope with five random fields
(magnification, x200).
Invasion assay
The upper surfaces of Transwell chambers containing
polycarbonate membranes with 8-µm pores were coated with 20 µl
diluted Matrigel™ (BD Biosciences) for 1 h at 37˚C. A total of
lx105 cells were starved in serum-free medium for 3 h
and subsequently added to the upper chamber, whereas the lower
chamber was filled with the same medium as in the upper chamber,
but with 10% FBS. After an incubation for 20 h at 37˚C, the cells
remaining on the upper layer of the chambers were wiped off,
whereas the cells attached to the submembrane surface were fixed
with neutral formaldehyde for 10 min at 4˚C and stained with Giemsa
for 10 min at 4˚C. Finally, the average numbers of invaded cells
per field of view were obtained from five random fields under a
light microscope.
F-actin polymerization assay
Briefly, cells were planted in 35 mm dishes
overnight, pretreated with serum-free medium for 3 h and
subsequently stimulated by adding 50 ng/ml EGF at 37˚C for time
periods of 0, 15 sec, 30 sec, 1, 2 or 5 min. Thereafter, cells were
fixed, permeabilized and stained in the dark with Molecular Probes™
Oregon Green 568 phalloidin (Thermo Fisher Scientific, Inc.)
diluted in F-buffer (10 mM Hepes containing 20 mM
KH2PO4, 5 mM EGTA and 2 mM MgCl2
in Dulbecco's PBSl pH 6.8) at RT for 1 h. The bound phalloidin was
extracted with methanol for 1 h, and the F-actin content was
subsequently measured with a fluorescence reader at an excitation
wavelength of 578 nm and an emission wavelength of 600 nm. The
results were expressed as the relative F-actin content, where
F-actinΔt/F-actin0=(fluorescenceΔt
mg/ml)/(fluorescence 0/mg/ml).
Western blotting assay
Cells were starved with serum-free medium for 3 h
and stimulated by 50 ng/ml EGF for various time periods. The
reactions were terminated and the cells washed twice with cold PBS,
before subsequently being lysed in 1X SDS lysis buffer [Tris-HCl;
pH 6.8; containing 62.5 mM (2%) SDS and 10% glycerol]. The protein
determination method was via bicinchoninic acid assay. Equal
amounts of protein (20 µg/lane) were separated by SDS-PAGE (10%
gels) and blotted onto PVDF membranes (MilliporeSigma). The
membranes were blocked with 5% non-fat milk for 1 h at RT and
incubated overnight at 4˚C with the primary antibodies raised
against β-actin (cat. no. 4967S; 1:3,000; Cell Signaling
Technology), PTX3 (cat. no. ab190838; 1:3,000; Abcam), PKCζ (cat.
no. 9372S; 1:3,000; Cell Signaling Technology), Akt (cat. no.
9272S; 1:3,000; Cell Signaling Technology), integrin β1 (cat. no.
4706S; 1:3,000; Cell Signaling Technology), cofilin (cat. no.
5175S; 1:3,000; Cell Signaling Technology) and their phosphorylated
proteins (Cell Signaling Technology), followed by an incubation at
RT with HRP-conjugated secondary antibody (cat. no. 7074S; 1:1,000;
Cell Signaling Technology) for 1 h. Finally, the western blots were
visualized using enhanced chemiluminescence reagents (Pierce;
Thermo Fisher Scientific, Inc.) and analyzed with ImageJ 1.53a
(National Institutes of Health).
Co-immunoprecipitation (IP) assay
After washing the MDA-MB-231 cells three times with
cold PBS, the cells were lysed in 1 ml ice-cold cell lysis buffer
(40 mM Hepes containing 120 mM NaCl, 1% Triton X-100, 10 mM
pyrophosphate, 10 mM glycerophosphate, 50 mM NaF, 1.5 mM
Na3VO4, 1 mM EDTA, 1X cocktail; pH 7.5) on
ice for 30 min. Following centrifugation at 12,000 x g for 15 min
at 4˚C, the supernatants were removed and precleared through the
addition of 50 µl Invitrogen® protein A (Thermo Fisher
Scientific, Inc.) for 1 h. Supernatants (50 µl) were
immunoprecipitated using anti-PKCζ antibody (cat. no. ab59364;
1:3,000; Abcam). Finally, the immunocomplexes were captured by
adding 50 ml protein A, and the immunocomplexes were then subjected
to western blotting analysis, which was performed as
aforementioned.
IP-mass spectrometry (IP-MS)
Briefly, 293T cells were transfected with exogenous
PKCζ and lysed with 1 ml ice cold cell lysis buffer (40 mM Hepes
containing 120 mM NaCl, 1% Triton X 100, 10 mM pyrophosphate, 10 mM
glycerophosphate, 50 mM NaF, 1.5 mM Na3VO4, 1 mM EDTA, 1X cocktail;
pH 7.5) after 24 h. The supernatants were removed after
centrifugation at 12,000 x g for 15 min at 4˚C and the PKCζ
antibody was subsequently added to pull down the PKCζ-bound
proteins. The protein determination method was use of the
bicinchoninic acid assay, and 10% SDS gel electrophoresis was
performed. Gels stained with Coomassie brilliant blue were
decolorized with glacial acetic acid for 1 h at RT. The bands on
the gel were then visualized and finally, proteins interacting with
PKCζ were identified by LTQ-orbitrap XL MS (Thermo Fisher
Scientific, Inc.). The LTQ Orbitrap XL MS was set for continuous
monitoring of positive ions, and data were collected over 15 min in
centroid mode over the mass range 50-1,000 m/z. The nitrogen gas
temperature was set 350˚C, and cone voltage was 150 V, 30 arb of
flow rate.
Immunofluorescence confocal
microscopy
Briefly, MDA-MB-231 cells were plated in 12-well
plates containing sterile coverslips and incubated for 24 h at
37˚C. Cells were subsequently fixed with 4% paraformaldehyde,
quenched with 50 mM NH4Cl, permeabilized with 0.2%
Triton X-100 in PBS and blocked with 3% bovine serum albumin
(AbMole Bioscience, Inc.). Cells were incubated with anti-PTX3
(cat. no. ab190838; 1:3,000; Abcam) and anti-PKCζ (cat. no.
AB59364; 1:3,000; Abcam) antibodies overnight and stained with
Invitrogen™ Alexa-Fluor 488 (cat. no. S11223; 1:3,000) and 594
(cat. no. S11227; 1:3,000) conjugated secondary antibodies for 1 h
at 4˚C (Thermo Fisher Scientific, Inc.). Finally, coverslips were
mounted and visualized using a confocal microscope (Nikon A1R; x400
magnification).
Immunohistochemical analysis
Paraffin tissue sections (5 µm in thickness) were
firstly fixed with 4% paraformaldehyde for 12 h at 4˚C, then
deparaffinized, rehydrated through graded alcohol solution and
subsequently boiled (100±5˚C) in 10 mM sodium citrate for 3 min in
an autoclave, followed by subsequent cooling to RT for antigen
retrieval. After that, the tissues were treated with 3% hydrogen
peroxide and blocked with 2% BSA for non-specific antigenic sites
for 1 h at RT. The sections were subsequently incubated with the
primary antibody against PTX3 (cat. no. ab190838; 1:500; Abcam)
overnight at 4˚C. After washing three times with PBS, sections were
incubated with the peroxidase-labeled secondary antibody (cat. no.
TA373083L; OriGene Technologies, Inc.) for 30 min at RT. The
reactions were visualized using an HRP DAB Detection Kit (OriGene
Technologies, Inc.), with subsequent counterstaining with
hematoxylin for 5 min at RT. The images of stained sections were
captured under an optical microscope, and subsequently evaluated by
three experienced pathologists. The results of staining were scored
as follows: 0 points, unstained; 1 point, light brown; 2 points,
brown; and 3 points, dark brown. Scores ≥1 point were considered to
represent positive staining.
Xenograft tumor growth and metastasis
assay
All animal experiments were performed in accordance
with the Guidelines for the Care and Use of Laboratory Animals of
Nankai University (Tianjin, China; approval no.
2023-SYDWLL-000545). Briefly, 4-6-week-old female severe combined
immunodeficient (SCID) mice (weight, 15-20 g) from Wei Tong Li Hua
Experimental Animal Co., Ltd. were randomly divided into two groups
(n=10) with one group acting as the control group (transfected with
pLKO.1-scrambled siRNA-luc), and another acting as the PTX3
knockdown group (transfected with pLKO.1-shPTX3-luc). The mice were
bred and housed singly using a 12:12 reverse light-dark cycle in
the SPF level Animal Experimental Laboratory of Nankai University,
with the temperature of 22˚C and 50% atmospheric humidity. Water
and food were available ad libitum. Luciferase labelling of
control cells and PTX3 knockdown of MDA-MB-231 cells were first
performed (giving rise to MDA-MB-231-pLKO.1-scrambled siRNA-luc and
MDA-MB-231-pLKO.1-shPTX3-luc cells, respectively), and
3x106 cells were subsequently injected into the mammary
fat pads of the SCID mice. The body weight of the mice was measured
individually every 3 days, and the survival times were monitored.
After 8 weeks, mice were intraperitoneally injected with luciferin
(Amresco, Inc.) and live IVIS imaging was performed to detect the
bioluminescence intensity of the tumors. The mice were sacrificed
by cervical dislocation, after which the tumors were excised, and
tumor volumes were measured. To examine spontaneous metastasis, the
lungs were fixed with formalin for 12 h at 4˚C, embedded in
paraffin, then cut into 4- to 6-µm thick sections and stained with
H&E stain for 5 min at RT. Finally, the metastatic tumor
nodules were counted under a Zeiss inverted microscope.
Statistical analysis
SPSS version 20.0 software (IBM Corp.) was used for
data analysis. Semi-quantification of the western blotting data was
performed using ImageJ software (version 1.8; National Institutes
of Health). Unpaired student's t-test and one-way ANOVA were used
to determine statistical significance for comparisons of 2 and ≥2
groups, respectively. Data are shown as the mean ± SD and P<0.05
was considered to indicate a statistically significant difference.
Tukey's test was applied for post-hoc comparisons.
Results
Overexpression of PTX3 is positively
associated with the metastasis of clinical BC
First, the expression level of PTX3 was examined in
60 cases of invasive BC tissues (including invasive ductal
carcinoma and invasive lobular carcinoma) and 22 cases of benign
breast tissues by immunohistochemical analysis in order to assess
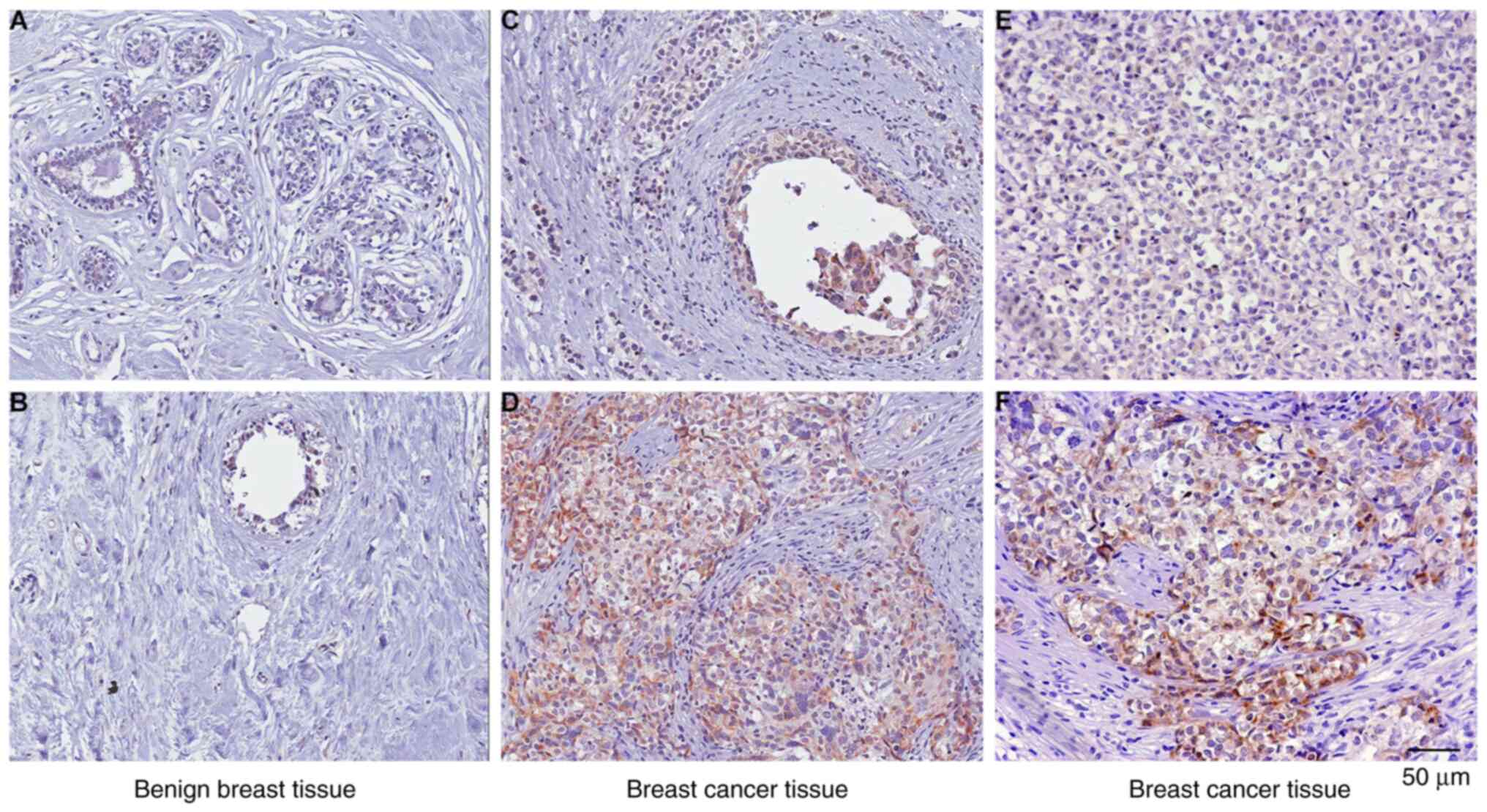
the importance of PTX3 in BC. The results obtained showed that
positive staining of PTX3 was detected in 52 tumor samples, but
only in two benign breast tissue samples (Table I), suggesting that the expression
level of PTX3 is increased during tumorigenesis (Fig. 1). It is noteworthy that PKCζ is a
cancer-promoting factor that is also highly expressed in BC
tissues. In addition, the statistical analysis revealed that PTX3
expression was detected in 51 of 52 lymph node metastasis cases
(P=0.003; Table II). However,
only one tumor tissue was identified as being positive in eight
cases of non-lymph node metastasis. Taken together, these results
strongly suggested that upregulation of PTX3 is associated with
lymph node metastasis of BC.
 | Table IExpression of PTX3 in benign breast
and BC tissues using immunohistochemical staining. |
Table I
Expression of PTX3 in benign breast
and BC tissues using immunohistochemical staining.
| Group | Total, n | Positive, n |
|---|
| BC tissues | 60 | 52 |
| Benign breast
tissues | 22 | 2a |
 | Table IIAssociation of PTX3 expression with
clinicopathological parameters in patients with breast cancer. |
Table II
Association of PTX3 expression with
clinicopathological parameters in patients with breast cancer.
| Parameters | Total, n | Positive, n | % | P-value |
|---|
| Menopausal | | | | |
|
Premenopausal | 33 | 21 | 63.6 | 0.228 |
|
Postmenopausal | 27 | 13 | 48.1 | |
| Tumor size, cm | | | | |
|
<2 | 25 | 17 | 68.0 | 0.853 |
|
≥2 | 35 | 23 | 65.7 | |
| Lymph node
status | | | | |
|
Negative | 8 | 1 | 12.5 | 0.003 |
|
Positive | 52 | 51 | 98.1 | |
| T stage | | | | |
|
1-2 | 40 | 23 | 57.5 | 0.348 |
|
3-4 | 20 | 14 | 70.0 | |
| p53 status | | | | |
|
Negative | 47 | 29 | 61.7 | 0.862 |
|
Positive | 13 | 9 | 69.2 | |
| ER status | | | | |
|
Negative | 15 | 8 | 53.3 | 0.274 |
|
Positive | 45 | 31 | 68.9 | |
| PR status | | | | |
|
Negative | 28 | 17 | 60.7 | 0.142 |
|
Positive | 32 | 25 | 78.1 | |
Knockdown of PTX3 inhibits the
proliferation and migration of BC cells
The initial assumption was made that PTX3 fulfills
an important role in the proliferation and migration of BC cells.
First, the expression levels of PTX3 in the human normal mammary
epithelial cell line (MCF-10A) and two BC cell lines (MDA-MB-231
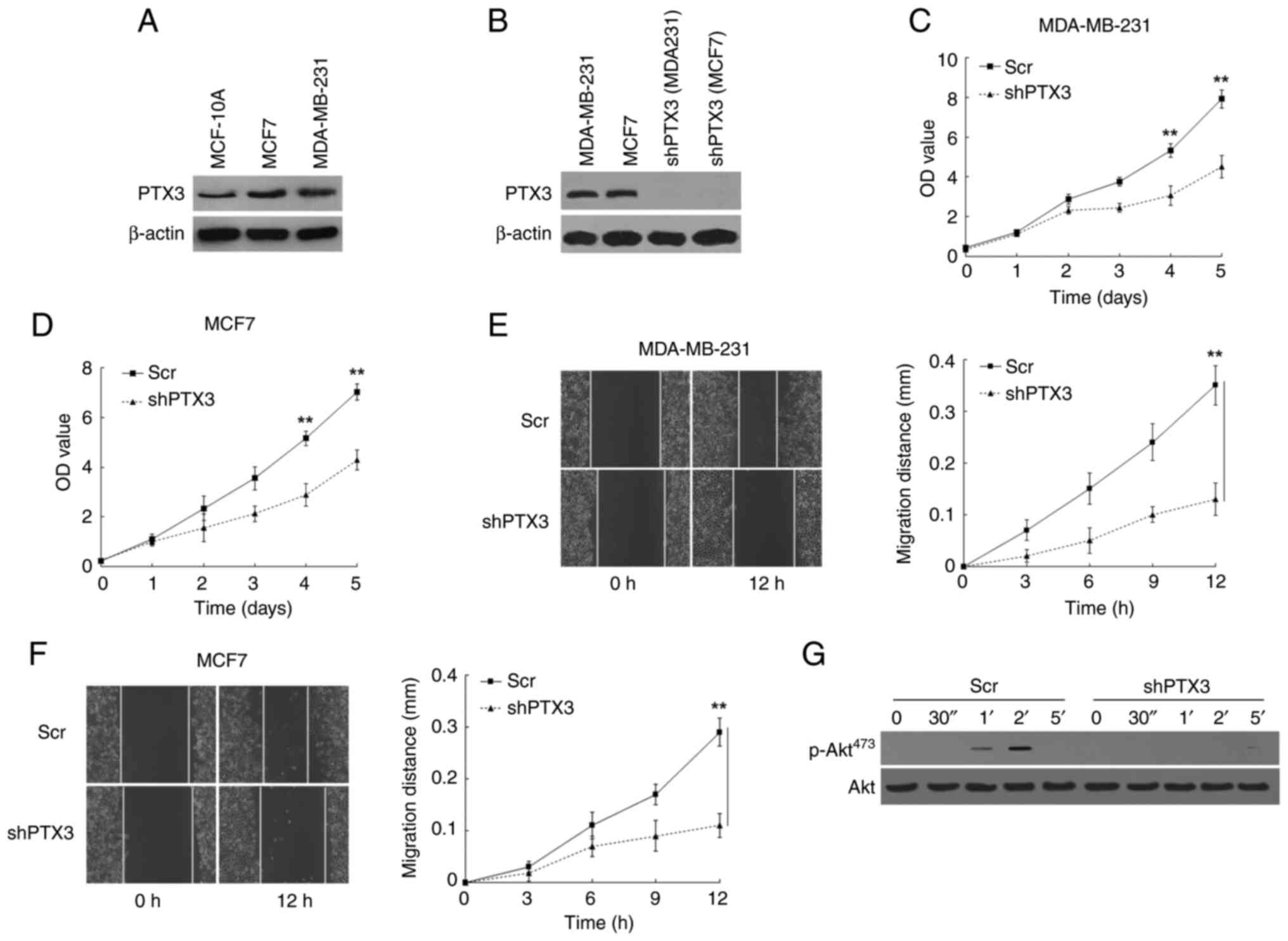
and MCF7) were evaluated by western blotting assay. The results
obtained showed that the expression levels of PTX3 in the BC
MDA-MB-231 and MCF7 cell lines were higher compared with that in
MCF-10A cells (Fig. 2A), and this
difference may have been associated with the malignant behavior of
BC cells. Subsequently, stable PTX3 downregulation of MDA-MB-231
and MCF7 cells was performed using lentiviral packaging vectors,
and the respectively transformed cells were used for the following
experiments (Fig. 2B). Cell
viability assay revealed that the proliferation rate of the shPTX3
cells was decreased compared with control BC cells (Fig. 2C and D). Subsequently, the migratory capability
of the BC cells was examined using a wound healing assay, and
changes in the scratch widths were recorded at different times. As
shown in Fig. 2E and F, shPTX3-transformed cells exhibited much
lower directional migratory rates compared with the control BC
cells, suggesting that knockdown of PTX3 led to a marked inhibition
of the migratory rate of BC cells. In addition, western blotting
analysis showed a blockade at EGF-induced Akt phosphorylation at
Ser473, indicating that the function of Akt was inhibited in shPTX3
cells (Fig. 2G). Taken together,
the aforementioned findings revealed that knockdown of PTX3
inhibited the proliferative and migratory capabilities of the BC
cells through the suppression of p-Akt expression.
Knockdown of PTX3 severely impairs the
metastasis of BC cells
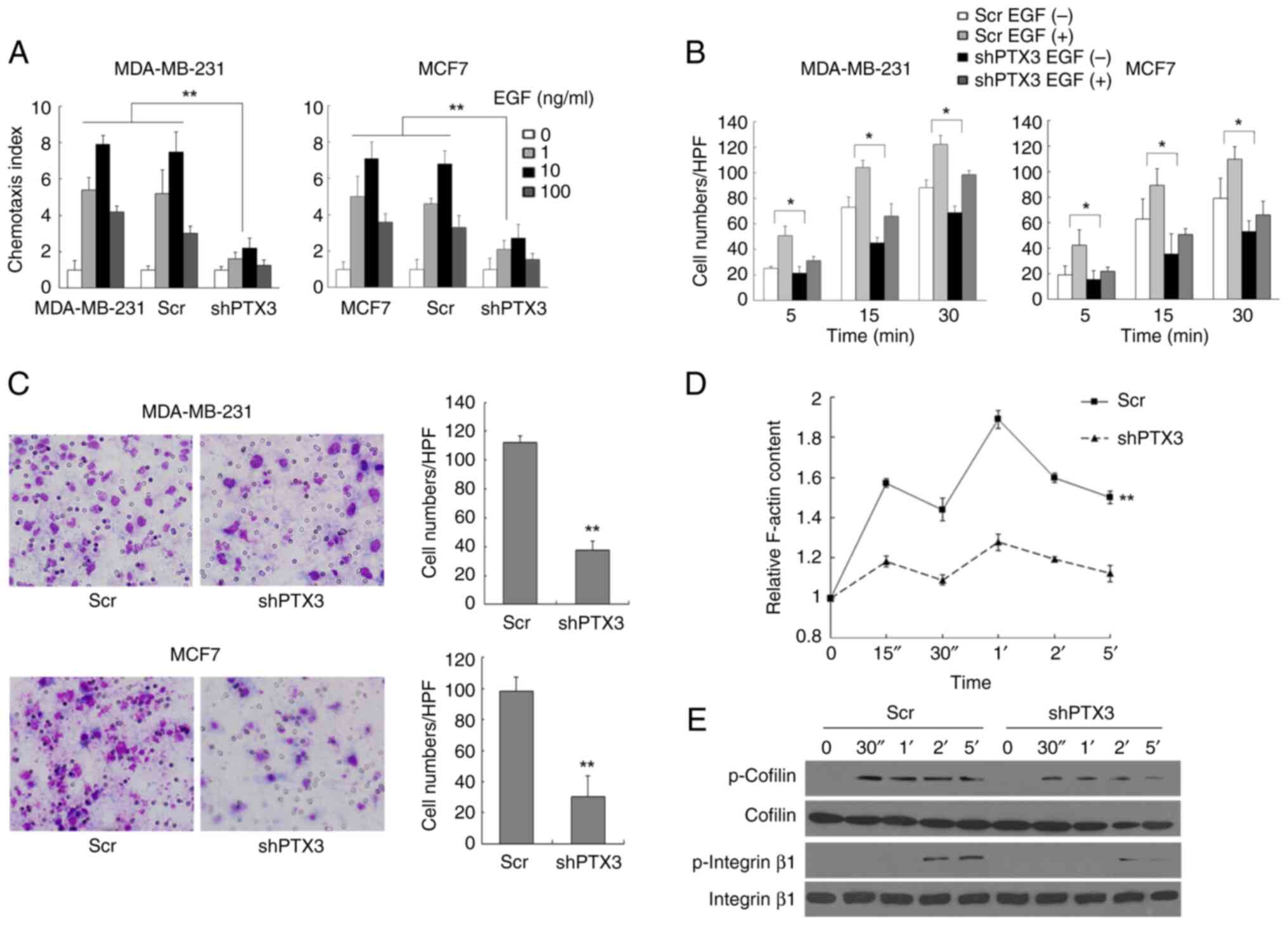
Subsequently, the chemotaxis of BC cells was
investigated to evaluate whether PTX3 exerts a pivotal role in the
metastasis of BC cells, and the results of these experiments are
shown in Fig. 3A. EGF-induced
chemotaxis of MDA-MB-231 and MCF7 cells was found to be markedly
inhibited following PTX3 knockdown compared with the control groups
(Fig. S1). As shown in Fig. 3B and D, both EGF-induced cell attachment and
actin polymerization were decreased to a statistically significant
extent (P<0.05) in shPTX3 cells compared with the control BC
cells. Furthermore, PTX3 downregulation also led to a marked
attenuation of the invasive capability of MDA-MB-231 and MCF7 cells
compared with control cells (Fig.
3C). As shown in Fig. 3E,
EGF-induced phosphorylation of both cofilin and integrin β1 was
severely impaired in shPTX3 cells. Taken together, these findings
suggested that downregulation of PTX3 inhibits EGF-induced BC cell
metastasis through inhibiting cofilin and integrin β1, which are
two downstream effectors of PKCζ.
PTX3 knockdown inhibits the metastasis
of BC cells through regulating PKCζ phosphorylation
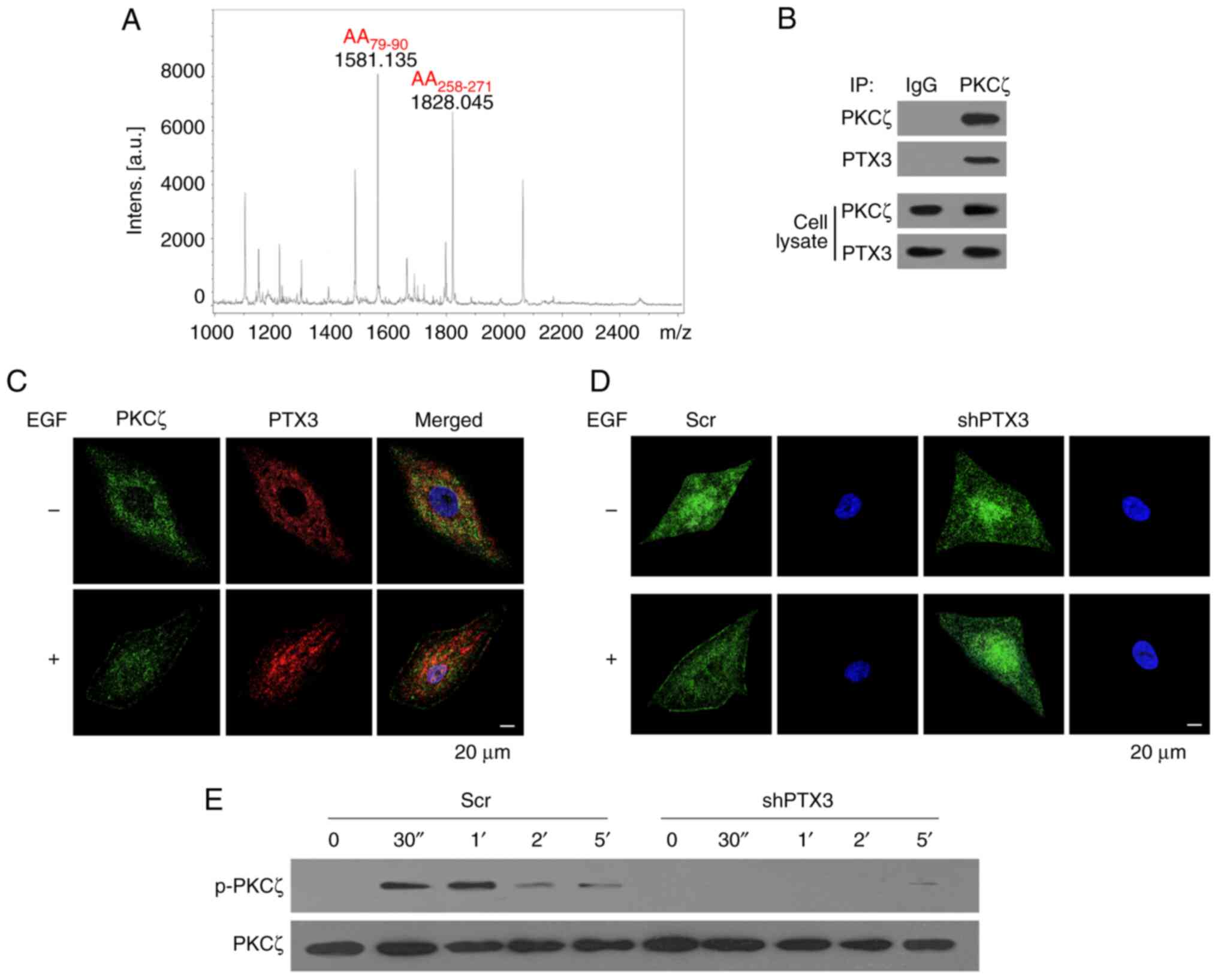
The results from the MS experiments first identified
PTX3 as a PKCζ-interacting protein (Fig. 4A). Therefore, it was surmised that
PTX3 could regulate EGF-induced BC cell chemotaxis and metastasis
through regulating the activation of PKCζ. As shown in Fig. 4B, co-IP assay confirmed that there
was an interaction between PTX3 and PKCζ in MDA-MB-231 cells.
Confocal microscopy analysis further revealed that both PTX3 and
PKCζ were distributed in the cytosol of resting cells. Upon
stimulation with 50 ng/ml EGF, however, both PTX3 and PKCζ were
co-translocated to the cell membrane, suggesting the activation of
proteins (Fig. 4C). The
translocation of PKCζ was also analyzed in experiments that
involved PTX3 knockdown of the MDA-MB-231 cells. Compared with the
Scr cells, EGF-induced translocation of PKCζ was markedly inhibited
in shPTX3 cells (Fig. 4D).
Furthermore, according to the findings of western blotting analysis
experiments, the phosphorylation of PKCζ was also reduced in shPTX3
cells upon knocking down PTX3 (Fig.
4E). Taken together, these results suggested that PTX3
co-localizes with PKCζ and regulates its activation.
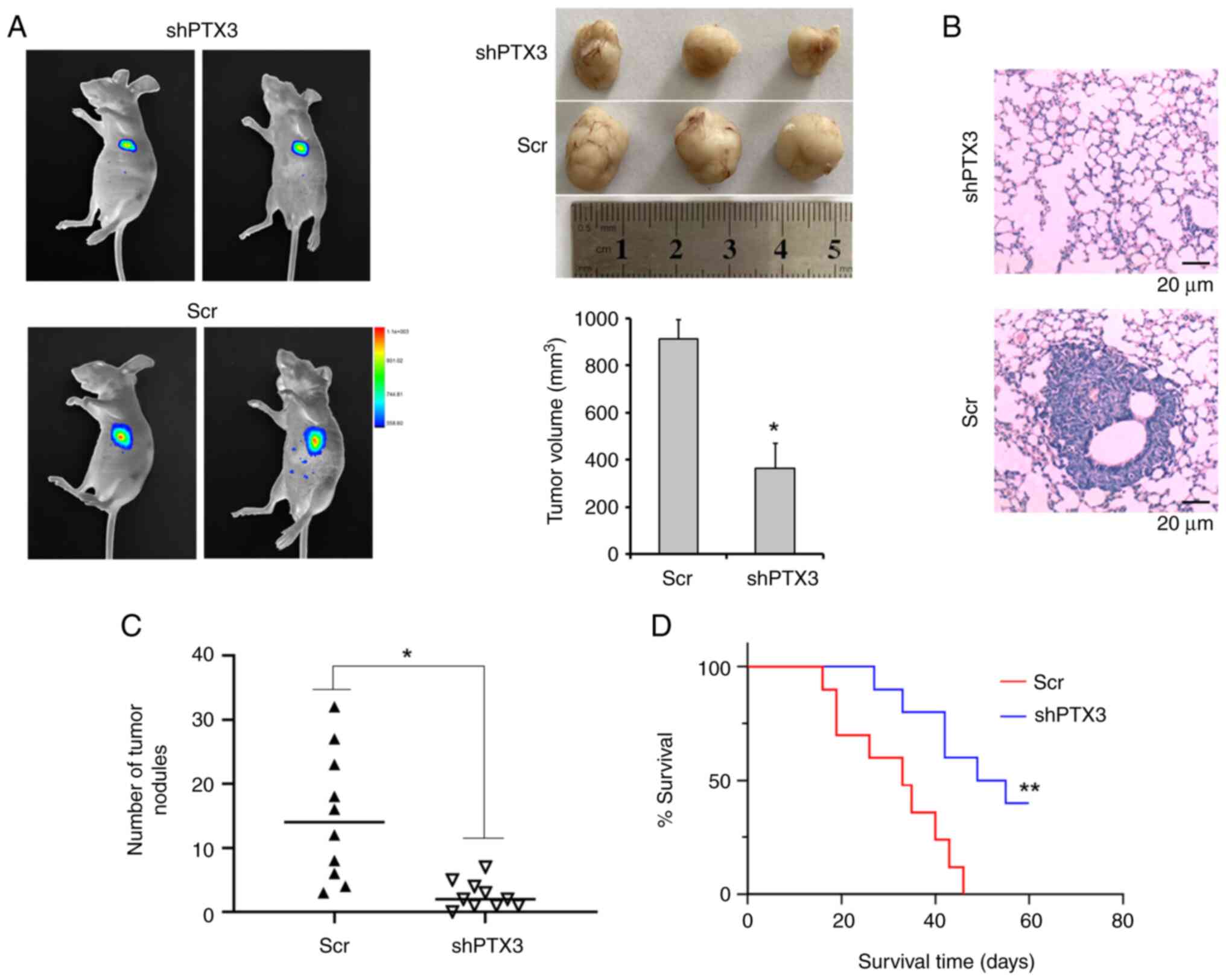
Knockdown of PTX3 inhibited the growth
and metastasis of breast tumors
Finally, the present study aimed to explore the
metastatic capabilities of shPTX3 cells in a BC xenograft model.
shPTX3 and control cells were implanted into the mammary fat pads
of SCID mice. After 8 weeks, the tumors were measured, and the
maximum tumor size was found to be ~990 mm3. The tumor
growth in mice with transplanted shPTX3 cells was found to be
markedly inhibited compared with the control group, suggesting that
PTX3 exerts an important role in tumor growth in vivo
(Fig. 5A). Subsequently, lung
metastasis of the human BC cells was evaluated using H&E
staining (Fig. 5B). A clear and
dramatic decrease in the number of pulmonary metastatic nodules in
shPTX3-implanted mice was detected compared with the control groups
(Fig. 5C). Moreover, the mice with
implanted shPTX3 cells also exhibited enhanced survival rates
(Fig. 5D) compared with the
control mice. Therefore, these results demonstrated that PTX3 is
required for the metastasis of human BCs in vivo, and PTX3
knockdown led to an inhibition of the growth and metastasis of
breast tumors.
Discussion
BC is the second most common cause of death
resulting from cancer in women (1). Both the incidence of BC and deaths
resulting from BC continue to rise, and its associated mortality is
mainly due to disease progression or metastasis (27). The lack of early-stage diagnostic
techniques and effective treatment methods continues to present
major problems for the administration of BC therapy worldwide
(28). Moreover, ~6-10% of newly
diagnosed cases of BC are identified at stage IV of the disease, or
the BC is already metastatic (27). Therefore, there is an urgent need
to explore the driving force and molecular mechanisms associated
with tumor metastasis to provide novel therapeutic targets for
BC.
Previous studies have shown both that PTX3 is
overexpressed in various tumors, and that it is involved in cancer
progression through multiple signaling pathways (28,29).
A recently published study (30)
demonstrated that the transcription factor SOX9 directly regulates
the expression of PTX3, whereas human leukocyte antigen
system-associated genes are significantly upregulated when PTX3 is
lacking in esophageal carcinoma (ESCA). Knockout of PTX3 in ESCA
was also demonstrated to lead to increases in cell apoptosis and
sensitivity to chemotherapy and radiotherapy (30). In addition, PTX3 expression was
shown to be markedly increased in prostate cancer tissues, and PTX3
exerts an important pathogenic role in the development of prostate
cancer through its ability to function as a modulator of complement
activation, promoting cellular proliferation, angiogenesis and
insensitivity to apoptosis, thereby leading to cancer cell invasion
and migration (31). It has also
been preliminarily shown that serum PTX3 may be a potential
biomarker to predict the risk of prostate cancer in patients
scheduled for prostate biopsy (32).
It has been shown that PTX3 is associated with tumor
metastasis (33). In
hepatocellular carcinoma (HCC), PTX3 expression is markedly
upregulated, and its ectopic expression has been shown to enhance
the migratory and invasive capabilities of HCC cells, thereby
inducing an EMT phenotype (33).
PTX3 has also been identified as a potential predictive factor of
poor prognosis for HCC (33). In
addition, PTX3 has also been shown to be overexpressed in ovarian
epithelial cancer and is strongly associated with the poor
prognosis of patients with ovarian epithelial cancer (34). Moreover, it has reported that the
expression level of PTX3 is higher in pancreatic ductal
adenocarcinoma, and PTX3 organizes hyaluronan in conjunction with
tumor necrosis factor-stimulated gene 6, thereby facilitating
stellate and cancer cell invasion (35). In patients with glioma, a higher
expression level of PTX3 was found to be associated with aggressive
tumor behavior and shorter survival times (36). In the present study, it was
observed that the expression level of PTX3 was increased both in
patients with BC and in BC cells. Pathological investigation of the
patient tissues revealed that a high expression of PTX3 was
associated with lymph node metastasis of BC. The data obtained
in vitro also showed that the downregulation of PTX3 led to
decreased rates of BC cell proliferation, migration, chemotaxis,
adhesion and invasion, and actin polymerization. In the in
vivo animal experiments, downregulation of PTX3 was found to
inhibit the metastasis of BC cells to the lungs of SCID mice,
thereby providing molecular evidence that PTX3 exerts a critical
role in BC cell metastasis.
As an atypical PKC isoform, PKCζ has been implicated
in various types of cancer, including BC, bladder cancer, colon
cancer and ovarian cancer (37,38).
It has been suggested that PKCζ participates in regulation of the
proliferation and invasion of different types of ovarian cancer
(39). Additionally, PKCζ has been
shown to have an important role in the cell viability, migration
and progression of bladder cancer (40). Cell adhesion and cytoskeleton
rearrangement are both key responses that are closely associated
with chemotaxis, and which are often regulated by PKCζ activity
(41). Cofilin, as a protein
associated with actin, is one of the core regulatory factors of
actin polymerization (42), and
integrin β1 has also been demonstrated to be involved in regulating
cytoskeleton rearrangement (43).
Previous studies by the research group of the present study have
also indicated that PKCζ is a downstream protein of AKT that is
required for the EGF-induced chemotaxis of BC cells. It has been
shown that p-Akt serves an important role in the migratory and
survival signaling pathways of cancer cells (44). Interestingly, activation of the
NF-κB-PI3K/Akt-signaling pathway was shown to promote PTX3
expression (25), as well as
having a role in mediating PTX3 transcriptional responses to EGF
(26). The present study has
demonstrated that endogenous PTX3 both interacts with PKCζ and is
co-localized with PKCζ in BC cells. Upon EGF stimulation, PTX3 and
PKCζ were co-translocated to the cell membrane, whereas knockdown
of PTX3 inhibited the processes of membrane translocation and full
activation of PKCζ. In addition, EGF-induced phosphorylation of
integrin β1 and cofilin, which may act as the downstream effectors
of PKCζ-mediated chemotaxis in BC cells, was also found to be
impaired in shPTX3-transfected cells. Therefore, taken together,
the findings of the present study have suggested that PTX3 is an
upstream regulatory molecule of PKCζ in BC cells. The mechanisms by
which PTX3 contributes to BC metastasis by regulating activation as
an upstream factor of PKCζ is novel and to the best of our
knowledge, has never been reported on, and the relationship between
PTX3 and PKCζ has been preliminarily studied in our research and
requires further investigation into their underlying mechanisms and
will be the direction of future work.
In conclusion, the present study has demonstrated
that PTX3 promotes BC cell proliferation and metastasis through
regulating the activation of PKCζ. A high expression level of PTX3
is associated with metastasis in patients with BC, and knockdown of
PTX3 was shown to effectively inhibit the proliferation, invasion
and metastasis of BC cells, thereby suggesting that PTX3 may serve
as a potential therapeutic target of BC. Further studies will be
directed towards uncovering the underlying molecular mechanisms of
PTX3 in terms of regulating PKCζ activation in BC.
Supplementary Material
Knockdown of PTX3 severely impairs
EGF-induced chemotaxis of breast cancer cells. Chemotaxis assay of
MDA-MB-231 and MCF7 cells (magnification, x200). PTX3, pentraxin 3;
EGF, epidermal growth factor; shPTX3, PTX3 stable knockdown; Scr,
scrambled siRNA.
Sequences of the negative control
siRNA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by Tianjin Key Medical
Discipline (Specialty) Construction Project and National Natural
Science Foundation Incubation Project of Tianjin Third Central
Hospital (grant no. 2017YNR3) and Tianjin Health Research Project
(grant no. TJWJ2023XK021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JW and RY conceived and designed the study, and SL
analyzed and interpreted the data. JW and SL wrote and edited the
manuscript. JW, RY, HG and YZ performed the experiments. JW and SL
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in line with the
principles of the Declaration of Helsinki. All animal experiments
were performed in accordance with the Guidelines for the Care and
Use of Laboratory Animals of Nankai University (Tianjin, China;
approval no. 2023-SYDWLL-000545). Ethics approval was obtained from
The Clinical Medical Research Ethical Committee of Tianjin Third
Central Hospital (Tianjin, China; approval no. IRB2023-039-01) for
the use of clinical biopsy specimens and informed written consent
was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lei S, Zheng R, Zhang S, Wang S, Chen R,
Sun K, Zeng H, Zhou J and Wei W: Global patterns of breast cancer
incidence and mortality: A population-based cancer registry data
analysis from 2000 to 2020. Cancer Commun (Lond). 41:1183–1194.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cardoso F, Paluch-Shimon S, Senkus E,
Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya
GS, Biganzoli L, et al: 5th ESO-ESMO international consensus
guidelines for advanced breast cancer (ABC 5). Ann Oncol.
31:1623–1649. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Biswenger V, Baumann N, Jürschick J, Häckl
M, Battle C, Schwarz J, Horn E and Zantl R: Characterization of
EGF-guided MDA-MB-231 cell chemotaxis in vitro using a
physiological and highly sensitive assay system. PLoS One.
13(e0203040)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dey A, Islam SMA, Patel R and
Acevedo-Duncan M: The interruption of atypical PKC signaling and
Temozolomide combination therapy against glioblastoma. Cell Signal.
77(109819)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosales-Soto G, Diaz-Vegas A, Casas M,
Contreras-Ferrat A and Jaimovich E: Fibroblast growth factor-21
potentiates glucose transport in skeletal muscle fibers. J Mol
Endocrinol. 1:JME190210.R2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Imamura T: The mechanisms of glucose
transporter type 4 translocation regulated by insulin receptor
signaling. Nihon Yakurigaku Zasshi. 158:169–172. 2023.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
9
|
Peng Y, Li JZ, You M and Murr MM:
Roux-en-Y gastric bypass improves glucose homeostasis, reduces
oxidative stress and inflammation in livers of obese rats and in
Kupffer cells via an AMPK-dependent pathway. Surgery. 162:59–67.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Griffiths CD, Bilawchuk LM, McDonough JE,
Jamieson KC, Elawar F, Cen Y, Duan W, Lin C, Song H, Casanova JL,
et al: IGF1R is an entry receptor for respiratory syncytial virus.
Nature. 583:615–619. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cao YJ, Li JY, Wang PX, Lin ZR, Yu WJ,
Zhang JG, Lu J and Liu PQ: PKC-ζ aggravates doxorubicin-induced
cardiotoxicity by inhibiting Wnt/β-catenin signaling. Front
Pharmacol. 13(798436)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zang G, Mu Y, Gao L, Bergh A and Landström
M: PKCζ facilitates lymphatic metastatic spread of prostate cancer
cells in a mice xenograft model. Oncogene. 38:4215–4231.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shelton PM, Duran A, Nakanishi Y,
Reina-Campos M, Kasashima H, Llado V, Ma L, Campos A, García-Olmo
D, García-Arranz M, et al: The secretion of miR-200s by a
PKCζ/ADAR2 signaling axis promotes liver metastasis in colorectal
cancer. Cell Rep. 23:1178–1191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu J, Liu S, Fan Z, Zhang L, Tian Y and
Yang R: A novel and selective inhibitor of PKC ζ potently inhibits
human breast cancer metastasis in vitro and in mice. Tumor Biol.
37:8391–8401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nome ME, Euceda LR, Jabeen S, Debik J,
Bathen TF, Giskeødegård GF, Taskén KA, Maelandsmo GM, Halvorsen B,
Yndestad A, et al: Serum levels of inflammation-related markers and
metabolites predict response to neoadjuvant chemotherapy with and
without bevacizumab in breast cancers. Int J Cancer. 146:223–235.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tarantino U, Greggi C, Cariati I, Visconti
VV, Gasparini M, Cateni M, Gasbarra E, Botta A, Salustri A and
Scimeca M: The role of PTX3 in mineralization processes and
aging-related bone diseases. Front Immunol.
11(622772)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Netti GS, Lucarelli G, Spadaccino F,
Castellano G, Gigante M, Divella C, Rocchetti MT, Rascio F, Mancini
V, Stallone G, et al: PTX3 modulates the immunoflogosis in tumor
microenvironment and is a prognostic factor for patients with clear
cell renal cell carcinoma. Aging (Albany NY). 12:7585–7602.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Firouzjahi A, Eris S, Jalali SF, Bijani A
and Ranaee M: Evaluation of serum pentraxin-3 level in patients
with acute myocardial infarction compared with control group. Iran
J Pathol. 16:243–247. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yadav NVS, Barcikowski A, Uehana Y, Jacobs
AT and Connelly L: Breast adipocyte co-culture increases the
expression of pro-angiogenic factors in macrophages. Front Oncol.
10(454)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Scimeca M, Antonacci C, Toschi N, Giannini
E, Bonfiglio R, Buonomo CO, Pistolese CA, Tarantino U and Bonanno
E: Breast osteoblast-like cells: A reliable early marker for bone
metastases from breast cancer. Clin Breast Cancer. 18:e659–e669.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nirgude S, Desai S, Mahadeva R, Ravindran
F and Choudhary B: ST08 altered NF-κB pathway in breast cancer
cells in vitro as revealed by miRNA-mRNA analysis and enhanced the
effect of cisplatin on tumour reduction in EAC mouse model. Front
Oncol. 112(835027)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chivot J, Ferrand N, Fert A, Van Dreden P,
Morichon R and Sabbah M: PARP inhibitor inhibits the vasculogenic
mimicry through a NF-κB-PTX3 axis signaling in breast cancer cells.
Int J Mol Sci. 23(16171)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang P, Liu Y, Lian C, Cao X, Wang Y, Li
X, Cong M, Tian P, Zhang X, Wei G, et al: SH3RF3 promotes breast
cancer stem-like properties via JNK activation and PTX3
upregulation. Nat Commun. 11(2487)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kampo S, Ahmmed B, Zhou T, Owusu L, Anabah
TW, Doudou NR, Kuugbee ED, Cui Y, Lu Z, Yan Q and Wen QP: Scorpion
venom analgesic peptide, BMK AGAP inhibits stemness, and
epithelial-mesenchymal transition by down-regulating PTX3 in breast
cancer. Front Oncol. 9(21)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chan SH, Tsai JP, Shen CJ, Liao YH and
Chen BK: Oleate-induced PTX3 promotes head and neck squamous cell
carcinoma metastasis through the up-regulation of vimentin.
Oncotarget. 8:41364–41378. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chang WC, Wu SL, Huang WC, Hsu JY, Chan
SH, Wang JM, Tsai JP and Chen BK: PTX3 gene activation in
EGF-induced head and neck cancer cell metastasis. Oncotarget.
6:7741–7757. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang X, Wang C, Guan J, Chen B, Xu L and
Chen C: Progress of breast cancer basic research in China. Int J
Biol Sci. 17:2069–2079. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiang J, Jiang S, Ahumada-Canale A, Chen
Z, Si L, Jiang Y, Yang L and Gu Y: Breast cancer screening should
embrace precision medicine: Evidence by reviewing economic
evaluations in China. Adv Ther. 40:1393–1417. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hu T, Qiao L, Li H, Ren H, Ning Q, Zhou H,
Chen X, Sun Z and Shen L: Pentraxin 3 (PTX-3) levels in
bronchoalveolar lavage fluid as a lung cancer biomarker. Dis
Markers. 2020(4652483)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fan Z, Zheng Y, Li X, Deng X, Ba Y, Feng
K, Su J, Wang H, Suo Z and Li L: Promoting role of pentraxin-3 in
esophageal squamous cell carcinoma. Mol Ther Oncolytics.
24:772–787. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Stallone G, Netti GS, Cormio L, Castellano
G, Infante B, Pontrelli P, Divella C, Selvaggio O, Spadaccino F,
Ranieri E, et al: Modulation of complement activation by
pentraxin-3 in prostate cancer. Sci Rep. 10(18400)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Falagario UG, Busetto GM, Netti GS,
Sanguedolce F, Selvaggio O, Infante B, Ranieri E, Stallone G,
Carrieri G and Cormio L: Prospective validation of pentraxin-3 as a
novel serum biomarker to predict the risk of prostate cancer in
patients scheduled for prostate biopsy. Cancers (Basel).
13(1611)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Song T, Wang C, Guo C, Liu Q and Zheng X:
Pentraxin 3 overexpression accelerated tumor metastasis and
indicated poor prognosis in hepatocellular carcinoma via driving
epithelial-mesenchymal transition. J Cancer. 9:2650–2658.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chang X, Li D, Liu C, Zhang Z and Wang T:
Pentraxin 3 is a diagnostic and prognostic marker for ovarian
epithelial cancer patients based on comprehensive bioinformatics
and experiments. Cancer Cell Int. 21(193)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Goulart MR, Watt J, Siddiqui I, Lawlor RT,
Imrali A, Hughes C, Saad A, ChinAleong J, Hurt C, Cox C, et al:
Pentraxin 3 is a stromally-derived biomarker for detection of
pancreatic ductal adenocarcinoma. NPJ Precis Oncol.
5(61)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ke HH, Hueng DY and Tsai WC: Low
expression of pentraxin 3 and nuclear factor-like 2 implying a
relatively longer overall survival time in Gliomas. Chin J Physiol.
62:35–43. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Islam SMA, Patel R, Bommareddy RR, Khalid
KM and Acevedo-Duncan M: The modulation of actin dynamics via
atypical Protein Kinase-C activated Cofilin regulates metastasis of
colorectal cancer cells. Cell Adh Migr. 13:106–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Smalley T, Islam SMA, Apostolatos C,
Apostolatos A and Acevedo-Duncan M: Analysis of PKC-ζ protein
levels in normal and malignant breast tissue subtypes. Oncol Lett.
17:1537–1546. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Smalley T, Metcalf R, Patel R, Islam SMA,
Bommareddy RR and Acevedo-Duncan M: The atypical protein kinase C
small molecule inhibitor ζ-Stat, and its effects on invasion
through decreases in PKC-ζ protein expression. Front Oncol.
10(209)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Patel R, Islam SA, Bommareddy RR, Smalley
T and Acevedo-Duncan M: Simultaneous inhibition of atypical protein
kinase-C and mTOR impedes bladder cancer cell progression. Int J
Oncol. 56:1373–1386. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kay RR, Langridge P, Traynor D and Hoeller
O: Changing directions in the study of chemotaxis. Nat Rev Mol Cell
Biol. 9:455–463. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sun R, Gao P, Chen L, Ma D, Wang J,
Oppenheim JJ and Zhang N: Protein kinase C ζ is required for
epidermal growth factor-induced chemotaxis of human breast cancer
cells. Cancer Res. 65:1433–1441. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ghatak S, Misra S, Moreno-Rodrigue RA,
Hascall VC, Leone GW and Markwald RR: Periostin/β1integrin
interaction regulates p21-activated kinases in valvular
interstitial cell survival and in actin cytoskeleton
reorganization. Biochim Biophys Acta Gen Subj. 1863:813–829.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu YQ, Ju CL, Wang BJ and Wang RG: PABPC1L
depletion inhibits proliferation and migration via blockage of AKT
pathway in human colorectal cancer cells. Oncol Lett. 17:3439–3445.
2019.PubMed/NCBI View Article : Google Scholar
|