Introduction
Acute obstructive suppurative cholangitis (AOSC) is
a severe infectious disease characterized by acute suppurative
infection and pus in the biliary system that is caused by biliary
obstruction, which results in hepatobiliary system damage. This
condition leads to the release of a large quantity of bacteria and
toxins into the bloodstream, causing biliary hypertension and
systemic damage to multiple organs. AOSC is considered to be a
serious form of acute cholangitis (AC) (1). It is the predominant cause of
mortality in patients with benign biliary disease. The clinical
presentation of AOSC commonly includes abdominal pain, fever,
jaundice and in severe cases it may be accompanied by septic shock
characterized by shock and symptoms of nervous system suppression.
AOSC can lead to multiple organ failure if not promptly detected
and treated, resulting in a morbidity and mortality rate ranging
from 13-88% (2). There are
numerous factors associated with biliary tract infections,
including bacterial infections, gallstones and trauma. When there
are gallstones in the biliary tract, these can lead to biliary
obstruction and, subsequently, enteric pathogens can retrogradely
migrate into the bile duct, causing biliary tract infections.
Systemic infections derived from bacteria in the bloodstream may
also enter the bile duct. Moreover, in cases of severe trauma,
burns or shock, the biliary system is prone to local vascular
disturbances, which can also result in biliary tract infections
(3).
In recent years, significant and rapid advances have
been made in terms of the development of minimally invasive
endoscopic intervention techniques. Endoscopic retrograde
cholangiopancreatography (ERCP) is used more and more often in the
clinic for the treatment of AOSC, due to its advantages of simple
operation and high safety. Especially in Europe and the United
States, with the development of endoscopic technology, the safety
and effectiveness of ERCP have been widely studied and established
in the treatment of biliary tract diseases (4,5).
However, current clinical studies on ERCP in China mainly focus on
patients with AC and there is still no conclusion on the efficacy
and safety of ERCP in the treatment of AOSC patients, especially
elderly patients (2,6). The present study therefore
investigated an effective minimally invasive treatment approach
that holds promise for improving treatment outcomes for patients
with AOSC, with the goals of alleviating suffering and reducing
complications, thereby offering a beneficial therapeutic option for
both physicians and patients. These findings should prove to be
beneficial for enhancing quality of life of patients and optimizing
the utilization of medical resources.
Materials and methods
AOSC diagnosis
The clinical data of 47 patients with AOSC treated
in Hangzhou First People's Hospital between March 2021 and March
2022 were retrospectively analyzed. The diagnosis of AOSC met with
the diagnostic criteria of the 2018 Tokyo Guidelines (TG 18) for
severe cholangitis (grade III) (1). The inclusion criteria included the
following: i) Age above 18 years; and ii) meeting the diagnosis of
AOSC. The exclusion criteria included the following: i) Complicated
blood system disease and severe renal failure; ii) cardiovascular
system dysfunction, consciousness disorder, respiratory disorder,
liver dysfunction, blood system dysfunction and renal dysfunction
caused by non-AOSC; iii) non-AOSC septic shock; and iv) incomplete
clinical data.
In accordance with TG 18, the diagnosis of AC was
determined on the basis of systemic inflammation, cholestasis and
imaging evidence, as shown in Table
I. In addition, once the diagnosis was confirmed the AC was
categorized further according to its grade, i.e., grade I
represented mild AC, grade II was moderate AC, and grade III was
severe AC, as shown in Table II.
All patients in this group met the inclusion criteria for AC grade
III.
 | Table IDiagnostic criteria for AC. |
Table I
Diagnostic criteria for AC.
| Diagnostic
criterion | Content |
|---|
| A. Systemic
inflammation | 1. T >38˚C and/or
chills |
| | 2. WBC
<4x109/l or >10x109/l, and CRP ≥1
mg/dl |
| B. Cholestasis | 1. TB ≥34
µmol/l |
| | 2. ALP, GGT, AST,
ALT >1.5x upper limit of normal |
| C. Imaging
evidence | 1. Biliary
dilation |
| | 2. Imaging findings
(stenosis, stones, stents) |
 | Table IIClassification criteria of AC. |
Table II
Classification criteria of AC.
| Grading
standard | Content |
|---|
| Mild AC (grade
I) | No diagnostic
criteria for either grade II or grade III |
| Moderate AC (grade
II; at least two items were met) | 1. WBC
>1.2x1010/l or <4x109/l |
| | 2. T ≥39˚C |
| | 3. Age ≥75 years
old |
| | 4. TB ≥5 mg/dl |
| | 5. ALB <0.7x
lower limit of normal |
| Severe grade (grade
III; (at least one item) | 1. Cardiovascular
system dysfunction: The need for dopamine 5 µg/kg1
min-1, or use of hypotension maintained by any dose of
noradrenaline |
| | 2. Nervous system
dysfunction: A disorder of consciousness |
| | 3. Respiratory
system dysfunction: PaO2/FiO2 <300
mmHg |
| | 4. Liver
dysfunction: PT-INR >1.5 |
| | 5. Renal
dysfunction: Oliguria, blood Cr >2 mg/dl |
| | 6. Hematological
system dysfunction: PLT <100x109/l |
Of the 47 included AOSC patients, 26 were males and
21 females. Their ages ranged from 31-93 years, with a mean age of
70±14 years. Among them, 14 patients had Charcot triad, shock and
nervous central system inhibition, whereas the remaining 33 had 2-3
manifestations of Charcot triad. Among the 14 patients with Charcot
triad, one patient developed coma, whereas 13 patients developed
shock and were treated with vasoactive drugs [liver dysfunction:
Prothrombin time-international normalized ratio (PT-INR) >1.5 in
nine cases; renal dysfunction: Oliguria, blood creatine (Cr) >2
mg/dl in two cases; blood system dysfunction: Platelets (PLT)
<100x109/l in two cases (see Tables III and IV)]. Of the 47 patients with AOSC, 26
had common bile duct stones. A total of 43 patients had various
underlying diseases, including five with bile duct malignant
tumors, 13 with pancreatic malignant tumors, nine with sepsis,
three with gastric malignant tumors and five with a personal
history of subtotal gastrectomy (three were Billroth II, one was
Billroth I and one was Roux-en-Y). There were a total of 16
patients diagnosed with hypertension, 17 patients diagnosed with
hypoproteinemia, eight patients diagnosed with diabetes, six
patients diagnosed with coronary heart disease, and two patients
with a history of cerebral infarction. Additionally, there was one
patient who was 16 weeks pregnant at the time of diagnosis. Further
information is provided in Table
V.
 | Table IIIOrgan or system dysfunction occurred
in 47 patients with AOSC. |
Table III
Organ or system dysfunction occurred
in 47 patients with AOSC.
| Organ or system
dysfunction | n (%) |
|---|
| Cardiovascular
system dysfunction | 15 (31.9) |
| Nervous system
dysfunction | 21 (44.7) |
| Respiratory system
dysfunction | 14 (29.8) |
| Dysfunction of
renal function | 2 (4.3) |
| Hepatic
dysfunction | 9 (19.1) |
| Dysfunction of the
blood system | 2 (4.3) |
 | Table IVMain clinical manifestations of 47
patients with AOSC. |
Table IV
Main clinical manifestations of 47
patients with AOSC.
| Clinical
manifestation | n (%) |
|---|
| Celialgia | 31 (66.0) |
| Fever | 32 (68.1) |
| Jaundice | 29 (61.7) |
| A disorder of
consciousnessa | 14 (29.8) |
| Shock | 13 (27.7) |
| Charcot's
triad | 33 (70.2) |
| Renault's
pentalogy | 14 (29.8) |
 | Table VConditions and complications of the
47 patients. |
Table V
Conditions and complications of the
47 patients.
| Condition or
characteristic | No. of patients (%,
if applicable) |
|---|
| Sex | |
|
Male | 26 (55.3) |
|
Female | 21 (44.7) |
| Age (years) | 70±14 |
|
Choledocholithiasis | 26 (55.3) |
| History of bile
duct malignancy | 5 (10.6) |
| History of
pancreatic malignancy | 13 (27.7) |
| History of gastric
malignancy | 3 (6.4) |
| History of major
gastrectomy | |
|
Billroth II
surgery | 3 (6.4) |
|
Billroth I
surgery | 1 (2.1) |
|
Roux-en-Y
surgery | 1 (2.1) |
| Hypertension | 16(34) |
| Coronary heart
disease | 6 (12.8) |
| Diabetes
mellitus | 8(17) |
|
Hypoproteinemia | 17 (36.2) |
| Sepsis | 9 (19.1) |
| Cerebral
infarction | 2 (4.3) |
| Pregnant | 1 (2.1) |
Treatment
All patients were given a comprehensive treatment,
including control of inflammation and infection, improvement of
microcirculation and rehydration capacity, maintenance of the
internal environment stability and systemic support therapy. After
admission, various antibiotics tailored to the patient's individual
condition were empirically administered. The most commonly used
antibiotic regimens were as follows: Piperacillin/Tazobactam
administered intravenously at 4.5 g every 8 h for 7 days; Meropenem
given intravenously at 1 g every 8 h for 7 days; Cefoperazone
administered intravenously at 2 g every 12 h for 7 days; Vancomycin
given intravenously at 1 g every 12 h for 7 days. Adjustments in
dosage and timing were made under specific circumstances.
Simultaneously, somatostatin was administered to prevent post-ERCP
pancreatitis. The primary method involved a pre-ERCP intravenous
injection of 250 µg followed by a post-ERCP intravenous infusion of
250 µg/h for at least 11 h. For patients with septic shock,
isotonic balanced crystalloid fluids were administered for fluid
resuscitation. In fasting patients, fluid supplementation was at 40
ml/kg, comprising daily physiological requirements of NaCl at 4.5
g, KCl at 3 g, along with necessary vitamins and trace elements to
maintain water-electrolyte balance. Fluid replacement amounts were
adjusted based on individual patient needs. Corticosteroids for
anti-inflammatory effects, vasopressors for blood pressure
elevation, oxygen therapy, gastric protection and other symptomatic
supportive treatments were provided when necessary. ERCP treatment
was performed for the patients within 24-48 h. The patients were
routinely administered 10 mg nalbuphl hydrochloride 40 mg
drotaverine and 2 mg midazolam (injected intravenously via a
needle), accompanied by electrocardiogram monitoring, inhalation of
oxygen and adopted intensive care. The duodenal papilla is found at
the descending part of the duodenum. The guide wire was inserted
into the common bile duct using an incision knife. After performing
cholangiography, the purulent bile and contrast agent were
extracted in time to reduce the pressure of the biliary tract.
Endoscopic sphincterotomy (EST), endoscopic nasobiliary drainage
(ENBD) and/or endoscopic retrograde biliary drainage (ERBD) were
performed according to the results of angiography. All patients
were first treated with ENBD or EST + ENBD/ERBD to relieve biliary
obstruction. EST plus basket removal was performed for patients
with simple common bile duct stones, or stones with duodenal
papilla and ampulla; alternatively, after having detected systemic
symptoms for patients with large or multiple stones, who were
unable to tolerate stone removal surgery for an extended period,
mechanical lithotripsy and basket removal was performed; if neither
of these treatments were feasible, stent implantation was performed
instead for patients for whom stone removal was impossible, or who
had bile duct cancer, including surgery or long-term drainage
treatment of ERBD following remission (Table VI).
 | Table VIThe different types of treatment for
surgery performed in the present study. |
Table VI
The different types of treatment for
surgery performed in the present study.
| Treatment
operation | [Number/percentages
of cases (%)] |
|---|
| ERCP + ENBD | 11 (23.4) |
| ERCP + ERBD | 3 (6.4) |
| ERCP + ENBD +
ERBD | 6 (12.8) |
| ERCP + EST +
ENBD | 18 (38.3) |
| ERCP + EST +
ERBD | 1 (2.1) |
| ERCP + EST + ERBD +
ENBD | 4 (8.5) |
| ERCP + EST + ERPD +
ENBD | 3 (6.4) |
| ERCP + EST + ERPD +
ERBD | 1 (2.1) |
| ERCP + stone
removal | 28 (59.6) |
| Secondary line of
ERCP | 3 (6.4) |
Statistical analysis
All the data were analyzed using SPSS26.0
statistical software (IBM Corp.). Measurement data are expressed as
mean ± standard deviation and a paired t-test was used for making
comparisons before and after the various treatments. P<0.05 was
considered to indicate a statistically significant difference.
Results
Charcot's triad, shock and central nervous system
depression were observed in 14 of 47 patients with AOSC. A total of
33 patients were found to have between two and four features of
Charcot's pentalogy, including one patient with coma. After having
performed clinical and endoscopic minimally invasive treatments,
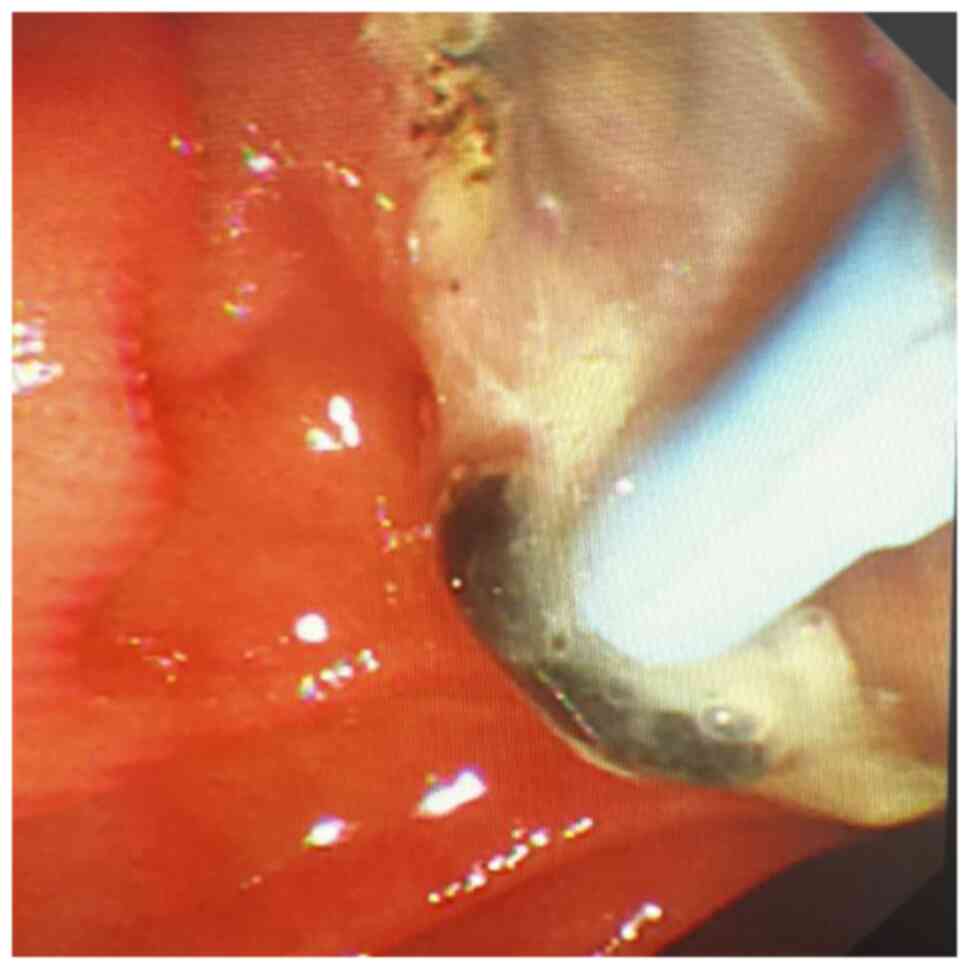
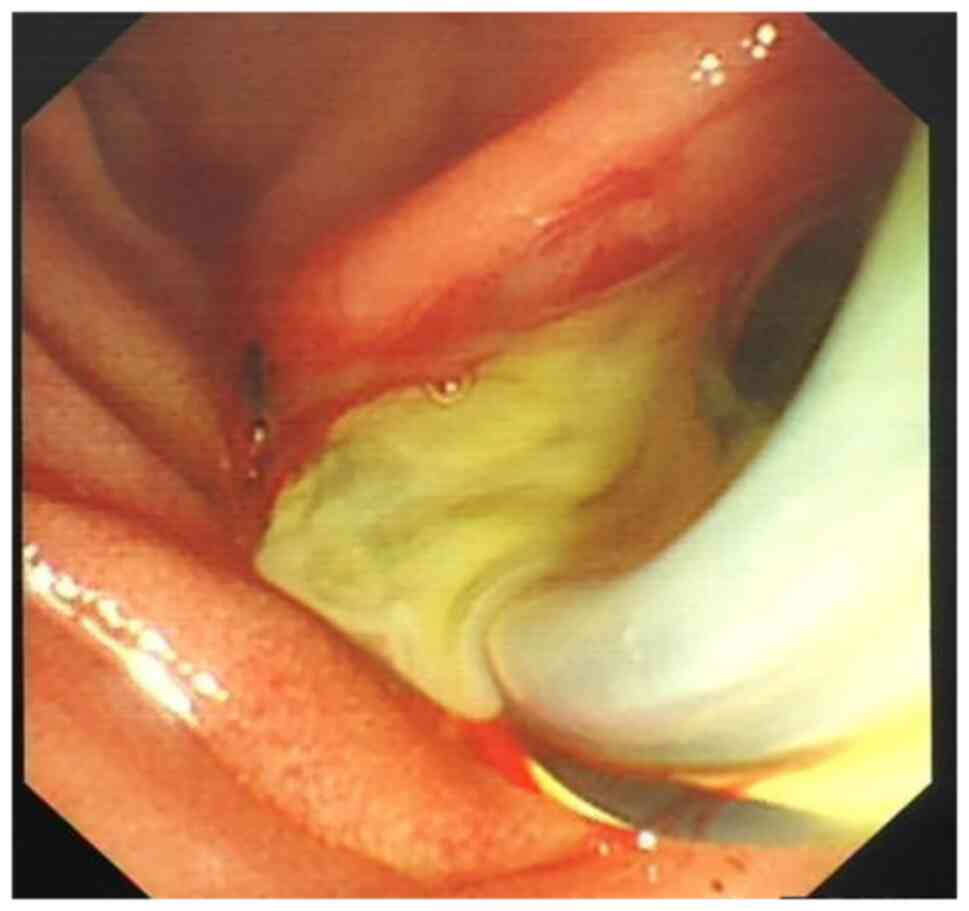
purulent bile outflow was observed in 47 patients with AOSC
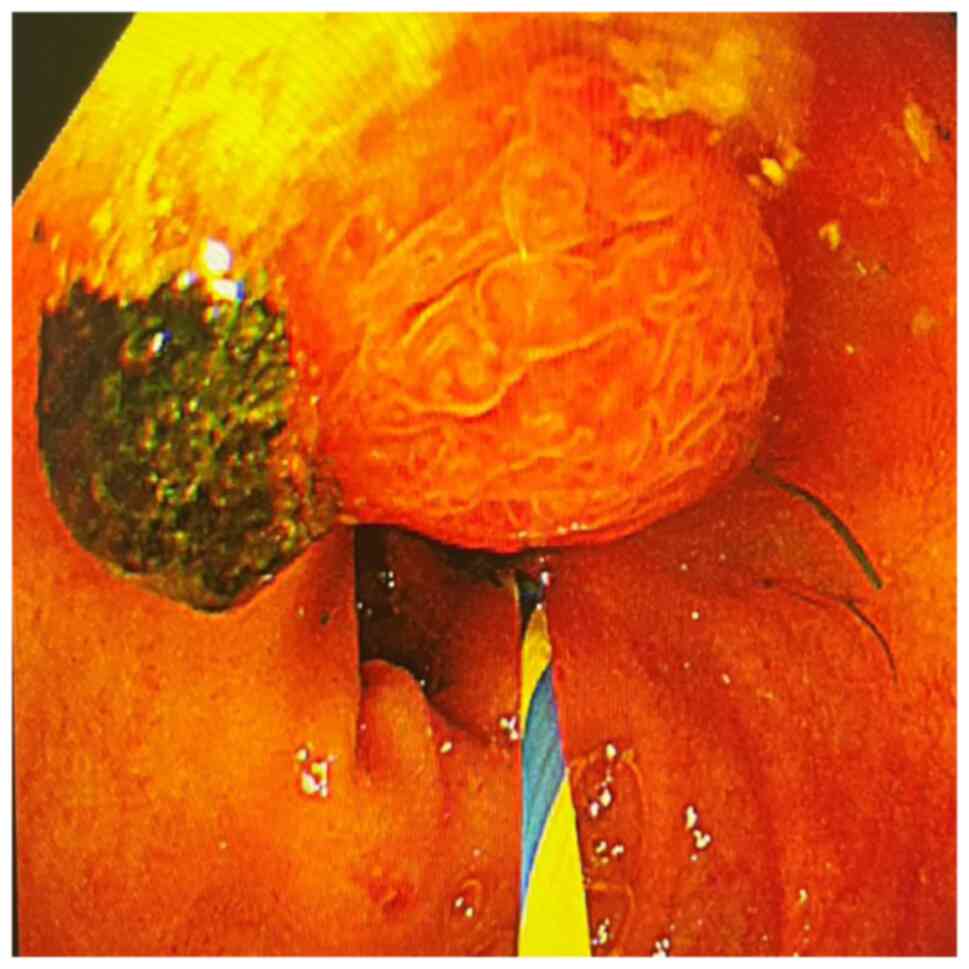
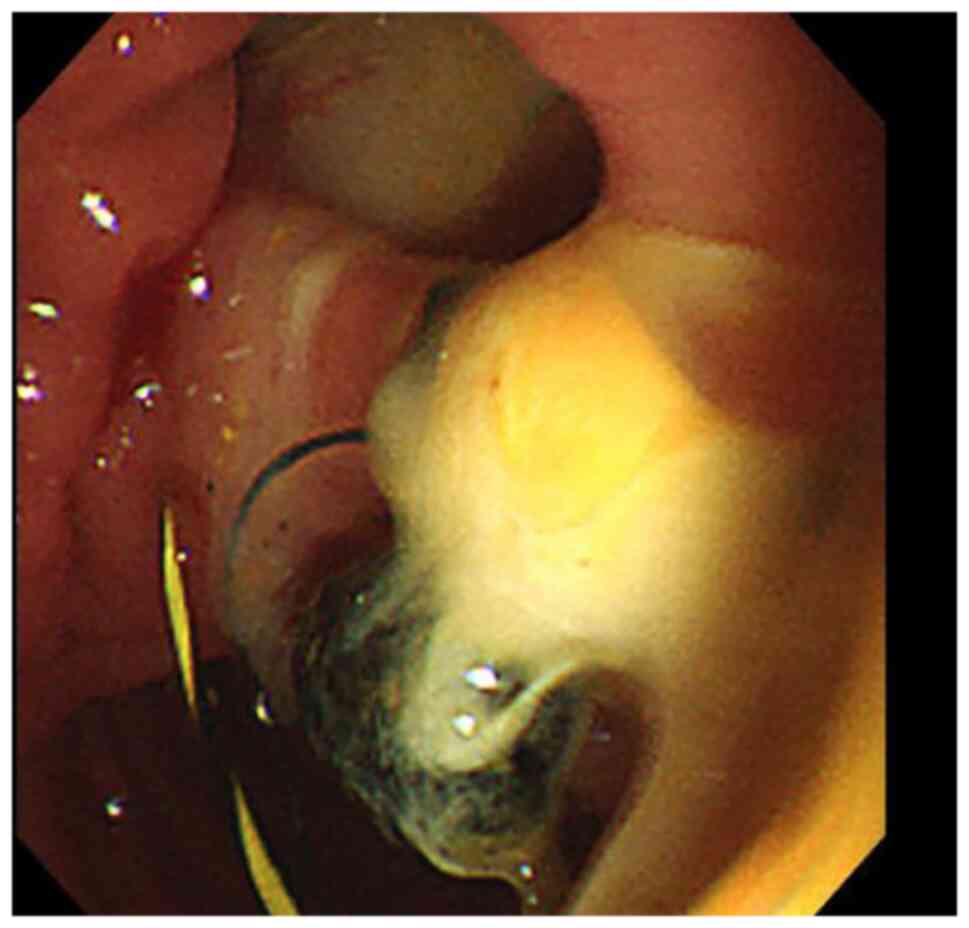
(Figs. 1 and 2), including 41 cases of common bile duct
stones (Figs. 3 and 4). The end-stage tumors included bile
duct malignancy in four cases and pancreatic cancer in two cases
and the clinical symptoms were significantly improved. In this
group, on day 7 after endoscopic treatment, blood inflammatory
indexes and liver function were found to be significantly improved
the most of the group compared with the measured values at
admission: The level of PCT decreased from 8.95±15.26 to 0.42±0.37
ng/ml, the level of CA-199 was reduced from 635.04±907.76 to
164.68±475.78 kU/l and that of ALT was decreased from 190.96±105.91
to 50.56±20.33 µmol/l. The value of the direct bilirubin test was
decreased from 62.21±36.23 to 30.64±15.40 µmol/l and these
differences were found to be statistically significant (P<0.05
or P<0.01), as shown in Table
VII. Out of the total of 47 patients, 16 cases involved the use
of ERCP and ENBD, 17 cases included the use of ERCP, EST and ENBD,
14 cases included the use of ERCP and ERBD, and 28 cases included
the use of ERCP for stone extraction. During ERCP examination,
purulent bile was discharged from the bile duct. A total of three
patients underwent secondary ERCP (Table VI). Out of the total cases, 35
(74.5%) showed positive results in the bile culture. The
predominant bacterium species identified were Gram-negative
bacteria, with Escherichia coli being the most frequently
observed in 11 instances (31.4%). Klebsiella pneumoniae was
detected in seven instances, accounting for 20% of the cases.
Pseudomonas faecium was identified in five cases (14.3%),
Enterococcus faecium in four cases (11.4%), Pseudomonas
aeruginosa in four cases (11.4%), Staphylococcus aureus
in three cases (8.6%) and Enterococcus casselifavus in two
cases (5.7%). There was one case of both E. faecium and
E. coli (2.9%), two cases of K. pneumoniae and
Candida albicans (5.7%), one case of E. faecalis,
K. pneumoniae and Enterococcus casselifavus (2.9%),
one case of Aeromonas hydrophila (strain found in guinea
pigs) and E. faecalis (2.9%), and two cases of E.
faecalis and E. coli (5.7%). There were two cases of
Citrobacter freundii and Stenotrophomonas
maltophilia, accounting for 5.7% of the cases. Additionally,
there was one case of E. faecium and C. albicans,
which made up 2.9% of the cases. Within 1 to 7 days after
treatment, the high fever of 45 of the patients disappeared and
their body temperature returned to normal. It was difficult to
completely control the infection in the remaining two patients
after 7 days of ERCP treatment. One of these patients was an
elderly patient (≥80 years old) with a secondary tumor and stenosis
of bilioenteric anastomosis complicated with AOSC. Even though the
infection was not entirely treated while the patient was in the
hospital, the family members requested that the patient be
discharged. Another patient with multiple common bile duct stones
complicated with sepsis underwent some improvements after a second
line of ERCP was performed and was discharged. Within 7 days of
treatment, the abdominal pain of 27 patients, and jaundice
experienced by 29 patients, were resolved. Hyperamylasemia or acute
pancreatitis (mild) occurred in three patients following ERCP,
which itself improved after conservative treatments. The average
hospital stay per patient was 11.9±4.6 days. Finally, no severe
complications, including perforation, bleeding, severe pancreatitis
or mortality, were identified for any of the patients.
 | Table VIIComparative analyses of hemograms,
blood biochemistry, PCT and CA199 results prior to and following
ERCP (n=47; mean ± standard deviation). |
Table VII
Comparative analyses of hemograms,
blood biochemistry, PCT and CA199 results prior to and following
ERCP (n=47; mean ± standard deviation).
| Time of
assessment | Blood WBC
(x109/l) | ALT (µmol/l) | AST (µmol/l) | TBil (µmol/l) | DBil (µmol/l) | ALP (U/l) | r-GT (U/l) | CA199 (kU/l) | PCT (ng/ml) |
|---|
| Before ERCP | 16.47±4.71 | 190.96±105.91 | 135.35±80.83 | 73.57±39.13 | 62.21±36.23 | 253.48±150.77 | 312.32±178.34 | 635.04±907.76 | 8.95±15.26 |
| One week after
ERCP |
6.35±2.94a |
50.56±20.33a |
47.19±16.11b |
32.85±15.54a |
30.64±15.40b |
183.10±90.61a |
192.44±91.36b |
164.68±475.78b |
0.42±0.37a |
Discussion
AOSC is one of the most common biliary tract
infections encountered in clinical practice and has the
characteristics of rapid onset and rapid progress. If it is not
diagnosed and treated in time, it may pose a serious threat to the
lives of patients (7), especially
those patients who have multiple comorbidities, including coronary
heart disease, hypertension, diabetes, pulmonary heart disease,
liver cirrhosis, renal insufficiency and a personal history of
cerebral infarction. AOSC is characterized by severe biliary tract
infection and empyema on the basis of biliary obstruction,
resulting in biliary hypertension, the leakage of bacterial
endotoxins into the blood and severe infectious diseases, including
multiple organ damage. The initial clinical manifestations of AOSC
are mild. Subsequently, as the pressure in the bile duct increases,
the septic substances in the bile duct flow back into the hepatic
bloodstream, leading to hyperbilirubinemia and sepsis. This may
result in symptoms such as chilliness, high fever, abdominal pain,
jaundice, shock and mental changes (all symptoms of Reynolds
pentalogy).
Biliary obstruction and bile infection are the two
major factors leading to the onset of AC. There are numerous causes
of biliary obstruction, the most common one being biliary stone
obstruction, as mediated by bile duct stones and gallstones.
Compression by malignant tumors of the biliary system, liver and
pancreatic head can also cause biliary obstruction and, if
accompanied by severe infection, this may also lead to cholangitis
(7). In a healthy state, there are
no bacteria present in the bile. The biliary tract, with its
tubular structure, is not conducive to bacterial presence. However,
due to its anatomical location, structure and physiological
characteristics, there is a high risk of infection when stone
diseases such as choledocholithiasis, cholelithiasis and biliary
ascariasis occur. These conditions can also disrupt the circulatory
system of the body, affect bile excretion and lead to bacterial
growth, resulting in infections (8). The biliary tract is connected to the
intestinal tract, and retrograde infection from intestinal bacteria
is the primary pathogenic cause of biliary tract infections.
Therefore, the isolated pathogens from bile are generally
consistent with the intestinal bacterial profile. The Gram-positive
bacteria we identified to be present were mainly
Enterococcus, whereas Gram-negative bacterial rods mainly
included Escherichia coli, Klebsiella pneumoniae and
Pseudomonas aeruginosa, findings that were in agreement with
the those of a similar study (9).
In some patients, the bacteria that remain in the gallbladder due
to recurrent gallstone attacks are able to alter both the
gallbladder's environment and the physicochemical properties of
bile. This alteration makes it conducive for bacteria to
proliferate within the biliary tract (10). Certain independent risk factors,
including diabetes, lead to deficiencies in the mechanisms of
immune defense in patients. In addition, high levels of blood sugar
negatively affect the functions of neutrophils and T-lymphocytes,
including their impaired migration, phagocytosis, intracellular
killing and chemotaxis (11,12).
This, in turn, leads to microvascular damage, compromising local
biliary circulation, leading to a reduction in blood supply,
worsening metabolic disorders and delaying the response to
infections, allowing the infection to spread. Additionally, it
causes autonomic nervous system dysregulation, resulting in
dysfunction of the Oddi sphincter and gallbladder contraction and
relaxation, leading to increased resting tension in the fasting
state, the promotion of bile stasis, and increasing the risk of
biliary tract infection (13). In
recent years, some researchers have also proposed that the
development of AC may be associated with an increase in vagal nerve
excitability (11-13).
However, due to the relatively limited research in this area,
further investigations are required for an improved understanding
of the role of the vagal nerve in AC.
Previous studies have also shown that the initial
basic factor for its occurrence is bile duct obstruction, which, as
explained above, causes an increase in intraluminal pressure, leads
to retrograde bacteria from the biliary tract into the blood and
biliary drainage disorders lead to infection. If there is no
biliary obstruction, even simple injection of bacteria into the
gallbladder or biliary tract will not cause biliary tract
infection. Ligation of the cystic duct or common bile duct at the
same time, resulting in cholestasis or obstruction of the blood
supply of the biliary tract, will induce severe infection, and this
has been borne out in the clinical results of a couple of
previously published studies (14,15).
In the present group of cases, the clinical manifestations of
patients were not completely consistent with bile duct empyema
(i.e., the most severe complication of acute cholangitis). Certain
patients exhibited atypical clinical symptoms, although their blood
tests revealed significantly elevated inflammatory markers,
indicating the presence of severe sepsis. ERCP aspiration of bile
revealed a large amount of pus in the bile duct, which is mainly
associated with the delayed response of elderly patients.
Collectively, the results suggested that the culture of aspirated
bile is helpful to guide clinical medication according to the
drug-sensitivity test, and to promote the recovery of patients.
Elderly patients, especially, are subjected to
weakened levels of immunity; moreover, they are often afflicted
with multiple diseases, and their condition occasionally
deteriorates rapidly, leading to mortality. Qin et al
(16) found that advanced age is
an independent risk factor for AOSC, and that the occurrence and
development of sepsis was positively correlated with an advanced
age. One of the explanations that may account for this is that
early manifestations of patients are not typically identified, and
Charcot's triad is rarely encountered in the early stage (17). It is often necessary to combine the
patient's medical history, imaging examination and various
laboratory indicators to evaluate the severity of AC in
patients.
Previous studies have also demonstrated that early
intervention with AOSC is crucial in terms of alleviating biliary
obstruction and facilitating the drainage of infected bile within a
24-h timeframe. Failure to do so can result in the development of
severe infections resulting from biliary obstruction and
cholestasis. An elevated pressure in the bile duct can cause the
contaminated bile to flow back into the bloodstream, leading to the
onset of sepsis, shock, and even mortality (18,19).
It has also been reported that hypoproteinemia is one of the
factors that affects the poor prognosis of patients with sepsis
(20). In severe infection, due to
the increases in body catabolism and capillary permeability, the
level of albumin (ALB) may be decreased. In addition, ischemia,
hypoxia and oxidative damage may occur in severe infection and ALB
is the main target of extracellular oxidative stress (21). Schneider et al (22) and Gravito-Soares et al
(23) found that ALB at a
concentration of 30 g/dl was an important risk predictor of
in-hospital mortality in patients with AC. Cozma et al
(13) showed that ALB is a
protective factor for severe cholangitis, and the lower the level
of ALB, the higher the incidence of severe cholangitis. There were
19 very elderly patients (≥80 years old) in the present group of
cases, and 15 patients had hypoproteinemia. Among the 15 patients
with hypoproteinemia, nine patients developed sepsis and three
patients exhibited poor therapeutic effects following the first
ERCP, although their condition improved significantly after the
second ERCP. The explanation for this was closely associated with
obstruction drainage after ERCP. Therefore, the key to the
treatment of AOSC is to relieve the biliary obstruction in a timely
manner, effectively reducing the biliary pressure, and then to
drain the purulent bile, block the liver-intestinal circulation of
bilirubin so as to reduce the level of jaundice and relieve its
injurious symptoms and gradually restore the function of the
damaged bilirubin transporters and microvilli. Conjugated bilirubin
in the blood is secreted into the biliary system and, as a
consequence, the jaundice is gradually decreased and its symptoms
are improved (24). Implementing
this strategy should enable the body's recovery. In addition to the
improvements in symptoms, the parameters of blood routine, TBil,
PCT and CA199 were found to be significantly decreased at 3 days
after operation. Previous studies (25,26)
demonstrated that the success rate of ENBD treatment for AOSC was
97%, which marks a significant improvement compared with that of
surgical drainage. ERCP minimally invasive endoscopic treatment can
therefore be used as the first-choice treatment for AOSC (27-29).
PCT is a type of glycoprotein that lacks hormonal
activity. In recent years, it has been found that PCT is closely
associated with deep infections, such as liver abscess and sepsis.
Bacterial infection can induce the production of inflammatory
factors throughout the body, and initiate the body's neuroendocrine
cells, macrophages, monocytes and other cells to secrete PCT in
large quantities, such that the serum PCT level is increased
(30). In addition, a previous
study found that PCT detection is not affected by steroid drugs or
autoimmune status (including immune diseases, HIV and liver
cirrhosis) and PCT is a specific indicator for the diagnosis of
bacterial infection (30). An
increased level of PCT detection is often indicative of a deep
infection and it is also used to distinguish bacterial infection
from viral infection in clinical practice. The results obtained in
the present study showed that the serum PCT level in patients with
AOSC was increased, although this was decreased significantly one
week after ERCP in the majority of the patients. A previous study
reported that the serum PCT level in patients with AOSC changed
with the severity of cholangitis, although this tended to return to
normal after the inflammation had been controlled (31). Following ERCP, the peripheral blood
white blood cells, ALT, bilirubin and other indicators of the
patients were found to be significantly decreased, the inflammatory
reaction in the body was significantly controlled and so the liver
function was restored and the PCT level was also significantly
improved. These findings were consistent with those reported in a
previous study (32). In that
study, with patients with severe infection caused by bile duct
obstruction, their levels of white blood cells, liver function
indexes and PCT were often increased significantly, although the
majority of these indexes improved significantly after the
obstruction had been relieved.
CA199 is a carbohydrate antigen that has been
confirmed to be associated with tumors. A previous study confirmed
that CA199 is able to regulate the migration and adhesion of white
blood cells in the inflammatory area, resulting in a leukocyte
aggregation reaction (33).
Although CA199 is a tumor marker, its expression is closely
associated with biliopancreatic duct obstruction, which has also
been confirmed by clinical observations. Zeng et al
(34) found that the levels of
CA199 and PCT were increased in patients with severe AC. Although
abnormal changes in the level of CA199 do occur in malignant
biliopancreatic tumors, Zhang et al (35) demonstrated that an increase in
CA199 may be associated with biliopancreatic duct obstruction. When
AC occurs, the serum concentration of CA199 is significantly
increased, and this increase is positively correlated with the
severity of cholangitis, which can therefore be used as a predictor
of AC (35); a finding that was
consistent with the results of the present study. It has also been
shown that CA199 has certain reference value for the diagnosis of
AC when it reaches a level of 52.50 kU/l (36). In the present study, there were two
cases where the CA199 values were >12,000 kU/l. Following
examination, it was found that there were no tumor lesions, and the
level of CA199 returned to normal after ERCP. It was recently
reported by Chen et al (37) that CA199 is associated with
calculous cholecystitis. The level of CA199 was found to be
elevated in certain patients with calculous cholecystitis but
without tumor disease, whereas the level of CA199 in these patients
returned to normal following cholecystectomy. Therefore, an
elevated level of CA199 is not completely representative of
neoplastic diseases, and it needs to be differentiated from other
types of obstructive inflammatory disease.
In conclusion, the present study showed that,
compared with traditional surgical drainage methods such as common
bile duct incision, decompression and drainage of pus or
cholecystostomy, endoscopic minimally invasive interventional
therapy has a high success rate and a low incidence of
postoperative bleeding, cholangitis and bile leakage (38). It can effectively relieve bile duct
obstruction, reduce the difficulty of surgical clinical treatment
to a certain extent, and improve the quality of life of patients.
With the continuing development of endoscopic minimally invasive
treatment technologies, as a treatment method, the endoscopic
treatment of AOSC has the advantages of being rapid, effective,
associated with lower levels of trauma, and it enables the rapid
prognosis of patients, aspects that are in line with the
developmental direction of modern medical therapies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Hangzhou Medical
and Health Science and Technology Project, China (grant no.
ZD20220041).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author upon reasonable
request.
Authors' contributions
ZF and JL confirm the authenticity of all the raw
data. ZF was the guarantor on the present study, and designed,
planned and implemented the study. JL collected data, analyzed the
data and interpreted patient data concerning AOSC, completed data
statistics and was a major contributor to the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
As a retrospective study, the present study was
approved by the ethics committee of Hangzhou First People's
Hospital (Hangzhou, China; approval no. 2021174-01). The study was
performed in accordance with the 1964 Declaration of Helsinki and
later amendments. Written informed consent was obtained from all
the participants prior to their enrollment of the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kiriyama S, Kozaka K, Takada T, Strasberg
SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, et
al: Tokyo guidelines 2018: Diagnostic criteria and severity grading
of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci.
25:17–30. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Meng M, Feng H, Tang S and Peng X:
Efficacy of ultrasound-guided percutaneous transhepatic biliary
drainage for acute obstructive suppurative cholangitis combined
with septic shock. Clinics (Sao Paulo). 78(100258)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sung JY, Costerton JW and Shaffer EA:
Defense system in the biliary tract against bacterial infection.
Dig Dis Sci. 37:689–696. 1992.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lam SK: A study of endoscopic
sphincterotomy in recurrent pyogenic cholangitis. Br J Surg.
71:262–266. 1984.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Navaneethan U, Gutierrez NG, Jegadeesan R,
Venkatesh PG, Sanaka MR, Vargo JJ and Parsi MA: Factors predicting
adverse short-term outcomes in patients with acute cholangitis
undergoing ERCP: A single center experience. World J Gastrointest
Endosc. 6:74–81. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma CL, Wang LP, Qiao S, Wang XF, Zhang X,
Sun RJ, Liu JG and Li YC: Risk factors for death of elderly
patients with acute obstructive suppurative cholangitis. West
Indian Med J. 65:316–319. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Biliary Surgery Group, Surgery Branch of
Chinese Medical Association. Guidelines for diagnosis and treatment
of acute biliary tract infections (2021). Chin J Surg. 59:422–429.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hu Z, Ni P, Fan X, Men R and Yang L: Past
hepatitis B virus infection was not associated with poorer response
or the UK-PBC risk score in ursodeoxycholic acid-treated patients
with primary biliary cirrhosis. Eur J Gastroenterol Hepatol.
31(277)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao J, Wang Q and Zhang J: Changes in
microbial profiles and antibiotic resistance patterns in patients
with biliary tract infection over a six-year period. Surg Infect
(Larchmt). 20:480–485. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen M, Wang L, Wang Y, Wei W, Yao YL,
Ling TS, Shen YH and Zou XP: Risk factor analysis of post-ERCP
cholangitis: A single-center experience. Hepatobiliary Pancreat Dis
Int. 17:55–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu Y, Zhang C, Song M, Han X and Jiao D:
Predicting early biliary infection after stenting of malignant
biliary obstruction: Model development and internal validation.
Abdom Radiol (NY). 48:2456–2465. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Turk Wensveen T, Gašparini D, Rahelić D
and Wensveen FM: Type 2 diabetes and viral infection; cause and
effect of disease. Diabetes Res Clin Pract.
172(108637)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cozma MA, Dobrică EC, Shah P, Shellah D,
Găman MA and Diaconu CC: Implications of type 2 diabetes mellitus
in patients with acute cholangitis: A systematic review of current
literature. Healthcare (Basel). 10(2196)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsujino T, Sugita R, Yoshida H, Yagioka H,
Kogure H, Sasaki T, Nakai Y, Sasahira N, Hirano K, Isayama H, et
al: Risk factors for acute suppurative cholangitis caused by bile
duct stones. Eur J Gastroenterol Hepatol. 19:585–588.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khozhibaev AM, Atadzhanov SK, Khakimov BB
and Khoshimov MA: Minimally invasive interventions for acute
cholecystitis complicated by mechanical jaundice in elderly and
senile patients. Vestn Khir Im I I Grek. 166:66–69. 2007.PubMed/NCBI(In Russian).
|
|
16
|
Qin YS, Li QY, Yang FC and Zheng SS: Risk
factors and incidence of acute pyogenic cholangitis. Hepatobiliar
Pancreat Dis Int. 11:650–654. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kiriyama S, Takada T, Hwang TL, Akazawa K,
Miura F, Gomi H, Mori R, Endo I, Itoi T, Yokoe M, et al: Clinical
application and verification of the TG13 diagnostic and severity
grading criteria for acute cholangitis: An international
multicenter observational study. J Hepatobiliary Pancreat Sci.
24:329–337. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Kogure H, Tsujino T, Yamamoto K, Mizuno S,
Yashima Y, Yagioka H, Kawakubo K, Sasaki T, Nakai Y, Hirano K, et
al: Fever-based antibiotic therapy for acute cholangitis following
successful endoscopic biliary drainage. J Gastroenterol.
46:1411–1417. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yıldız BD, Özden S, Saylam B, Martlı F and
Tez M: Simplified scoring system for prediction of mortality in
acute suppurative cholangitis. Kaohsiung J Med Sci. 34:415–419.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mohan R, Goh SWL, Tan G, Junnarkar SP,
Huey C and Shelat VG: Validation of TG07 and TG13/TG18 criteria for
acute cholangitis and predictors of in-hospital mortality in
patients over 80 years old. Clin Exp Hepatol. 7:396–405.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin M, Si L, Qin W, Li C, Zhang J, Yang H,
Han H, Zhang F, Ding S, Zhou M, et al: Predictive value of serum
albumin level for the prognosis of severe sepsis without exogenous
human albumin administration: A prospective cohort study. J
Intensive Care Med. 33:687–694. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schneider J, Hapfelmeier A, Thöres S,
Obermeier A, Schulz C, Pförringer D, Nennstiel S, Spinner C, Schmid
RM, Algül H, et al: Mortality risk for acute cholangitis (MAC): A
risk prediction model for in-hospital mortality in patients with
acute cholangitis. BMC Gastroenterol. 16(15)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gravito-Soares E, Gravito-Soares M, Gomes
D, Almeida N and Tomé L: Clinical applicability of Tokyo guidelines
2018/2013 in diagnosis and severity evaluation of acute cholangitis
and determination of a new severity model. Scand J Gastroenterol.
53:329–334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaur M, Chandel K, Reddy P, Gupta P,
Samanta J, Mandavdhare H, Sharma V, Singh H, Naseem S, Sinha SK, et
al: Neutrophil-lymphocyte ratio predicts clinical response to
percutaneous transhepatic biliary drainage in acute cholangitis. J
Clin Exp Hepatol. 13:390–396. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Y, Sun J, Zhang Q, Jin B, Zhu M and
Zhang Z: Identification of bile survivin and carbohydrate antigen
199 in distinguishing cholangiocarcinoma from benign obstructive
jaundice. Biomark Med. 11:11–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Minaga K, Kitano M, Imai H, Yamao K,
Kamata K, Miyata T, Omoto S, Kadosaka K, Yoshikawa T and Kudo M:
Urgent endoscopic ultrasound-guided choledochoduodenostomy for
acute obstructive suppurative cholangitis-induced sepsis. World J
Gastroenterol. 22:4264–4269. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kinney TP: Management of ascending
cholangitis. Gastrointest Endosc Clin N Am. 17:289–306.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Leung JW: Does the addition of endoscopic
sphincterotomy to stent insertion improve drainage of the bile duct
in acute suppurative cholangitis? Gastrointest Endosc. 58:570–572.
2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kajbafzadeh AM, Keihani S, Kameli SM and
Hojjat A: Maternal urinary carbohydrate antigen 19-9 as a novel
biomarker for evaluating fetal hydronephrosis: A pilot study.
Urology. 101:90–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shinya S, Sasaki T, Yamashita Y, Kato D,
Yamashita K, Nakashima R, Yamauchi Y and Noritomi T and Noritomi T:
Procalcitonin as a useful biomarker for determining the need to
perform emergency biliary drainage in cases of acute cholangitis. J
Hepatobiliary Pancreat Sci. 21:777–785. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Umefune G, Kogure H, Hamada T, Isayama H,
Ishigaki K, Takagi K, Akiyama D, Watanabe T, Takahara N, Mizuno S,
et al: Procalcitonin is a useful biomarker to predict severe acute
cholangitis: A single-center prospective study. J Gastroenterol.
52:734–745. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Song J, Park DW, Moon S, Cho HJ, Park JH,
Seok H and Choi WS: Diagnostic and prognostic value of
interleukin-6, pentraxin 3, and procalcitonin levels among sepsis
and septic shock patients: A prospective controlled study according
to the Sepsis-3 definitions. BMC Infect Dis. 19(968)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zheng BH, Yang LX, Sun QM, Fan HK, Duan M,
Shi JY, Wang XY, Zhou J, Fan J, Ma ZY and Gao Q: A new preoperative
prognostic system combining CRP and CA199 for patients with
intrahepatic cholangiocarcinoma. Clin Transl Gastroenterol.
8(e118)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zeng PJ, Li H, Chen YL, Pei H and Zhang L:
Serum CA199 levels are significantly increased in patients
suffering from liver, lung, and other diseases. Prog Mol Biol
Transl Sci. 162:253–264. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang K, Zhang L and Zheng L: Clinical
predictive value of different serological indicators for acute
cholangitis secondary to common bile duct stones. J Local Surg
Surg. 31:512–515. 2021.
|
|
36
|
Mei Y, Chen L, Peng CJ, Wang J, Zeng PF,
Wang GX, Li WP, Luo YQ, Du C, Liu K, et al: Diagnostic value of
elevated serum carbohydrate antigen 199 level in acute cholangitis
secondary to choledocholithiasis. World J Clin Cases. 6:441–466.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen W, Wang S, Zhao H, Wang G, Qin R,
Huang F, Geng W, Liu Z, Wang W, Wu R, et al: High level of tumor
marker CA19-9 returned to normal after cholecystectomy in calculous
cholecystitis patients. Scand J Gastroenterol. 58:643–648.
2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Khizar H, Zhicheng H, Chenyu L, Yanhua W
and Jianfeng Y: Efficacy and safety of endoscopic drainage versus
percutaneous drainage for pancreatic fluid collection; a systematic
review and meta-analysis. Ann Med. 55(2213898)2023.PubMed/NCBI View Article : Google Scholar
|