Introduction
Perthes disease, which results in the ischemic
necrosis of the femoral epiphysis, mostly occurs in children aged
4-8 years with a worldwide incidence of 0.4-29.0/100,000
individuals <15 years of age. Notably, the incidence of this
disease in male children is 4-5 times higher than that in female
children (1,2). The most serious complication of
Perthes disease is premature hip osteoarthritis caused by
deformation of the femoral head (3). Conservative treatment using abduction
plaster casts or inclusive surgery, which prevents secondary
degenerative arthritis by maintaining the spherical shape of the
femoral head and consistency in the femoral acetabular
relationship, are adopted in clinical treatment; however, residual
femoral head deformity remains inevitable in some patients, and can
influence the normal development and physical and mental health of
children. Although some researchers have attempted to treat this
disease with drugs, such as zoledronic and ibandronate, it has
achieved little effect (4,5).
The molecular mechanism underlying femoral head
malformation in Perthes disease is still unclear, and may be
related to endothelial dysfunction and abnormal vascular structure
(6,7). IL-6 is a key pathogenic factor in
Perthes disease. Kamiya et al (8) demonstrated that the concentration of
the proinflammatory factor IL-6 in the hip synovial fluid of
patients with Perthes disease is significantly elevated. By
performing animal experiments, it was also revealed that the IL-6
receptor blocker can promote blood supply recovery to the femoral
head epiphysis in Perthes disease (9). Endothelial microparticles (EMPs) are
extracellular vesicles that are secreted by endothelial cells
(10). When the body is in a
pathological state, the secretion of EMPs increases, and the EMPs
released by activated or apoptotic endothelial cells can reflect
the degree of endothelial dysfunction (11). Our previous study (12) reported that the concentration of
CD31+/CD42b- EMPs is increased in the plasma
of patients with Perthes disease and that this is related to the
concentration of IL-6. Furthermore, CD31+ EMPs produced
by IL-6-stimulated human umbilical vein endothelial cells (HUVECs)
in vitro can also induce endothelial dysfunction (12). On the basis of these findings,
hypotheses have been made on whether drugs can inhibit this
phenotype and alleviate the deformation of the femoral head in
Perthes disease.
Biochanin A (BCA) is an oxygen-methylated isoflavone
that exists in various herbs, such as Spatholobi Caulis, red
clover, soy, chickpea and a number of other plants (13,14).
Numerous studies have shown that BCA has pharmacological
activities, such as anticancer (15), anti-inflammatory (16), neuroprotective (17), antioxidant (18), antimicrobial (19) and hepatoprotective (20) effects. Studies have also reported
that BCA can reduce the expression of the inflammatory factor IL-6
and inhibit inflammation (21,22).
Our previous study (23) revealed
that BCA inhibits the NFκB signaling pathway to alleviate
inflammatory responses in a murine calvaria model with osteolysis
induced by Ti particle. Kole et al (24) also found that BCA can inhibit
proinflammatory cytokines in mouse macrophage cell line. However,
whether BCA inhibits IL-6-EMP-mediated endothelial dysfunction in
Perthes disease via the NFκB signaling pathway remains to be
determined.
The present study explored the effect and mechanism
of BCA in IL-6-EMP-induced endothelial dysfunction through in
vitro and in vivo experiments such as ELISA,
immunofluorescence, reverse transcription-quantitative PCR, western
blot and construction of rat model of femoral head ischemic
necrosis.
Materials and methods
Main equipment
A multifunctional microplate reader and cell
incubator were purchased from Thermo Fisher Scientific, Inc.; a
cryogenic centrifuge was obtained from Eppendorf SE; an ultrahigh
rotational speed centrifuge was from Beckman Coulter, Inc.; at
-80˚C refrigerator was obtained from Haier Group; and the
fluorescence inverted microscope was from Leica Microsystems
GmbH.
Main reagents
RPMI-1640 medium, penicillin-streptomycin mixture
and trypsin were from Gibco; Thermo Fisher Scientific, Inc.; fetal
bovine serum (FBS) was purchased from Zhejiang Tianhang
Biotechnology Co., Ltd.; BCA was obtained from Chengdu Desite
Biotechnology Co., Ltd.; PBS, the Cell Counting Kit-8 (CCK-8), Dil
fluorescent dye, Actin-Tracker Green-488, BCEBF-AM, SDS-PAGE sample
loading buffer, BCA protein assay kit and IL-6 were purchased from
Beyotime Institute of Biotechnology; the E-Selectin ELISA kit (cat.
no. ml057603), vascular cell adhesion molecule-1 (VCAM-1) ELISA kit
(cat. no. ml060757) and intercellular cell adhesion molecule-1
(ICAM-1) ELISA kit (cat. no. ml023955) was from Shanghai
Enzyme-linked Biotechnology Co., Ltd.; DAPI was obtained from
Sangon Biotech Co., Ltd.; sodium citrate buffer, DAB Substrate kit
(20X), Hematoxylin-Eosin (HE) Stain kit, RIPA buffer (high),
phenylmethylsulfonyl fluoride (PMSF), protein phosphatase
inhibitor, aprotinin from bovine lung and DMSO used to dissolve BCA
was purchased from Beijing Solarbio Science & Technology Co.,
Ltd.; Bovine serum albumin (BSA) was purchased from Servicebio;
96-, 48- and 6-well cell culture plates and T75 and T25 cell
culture flasks were from Corning, Inc.; E.Z.N.A.® Total
RNA Kit I was purchased from Omega Bio-Tek, Inc.; RevertAid First
Strand cDNA Synthesis kit was purchased from Thermo Fisher
Scientific, Inc.; 2x SYBR Green PCR Mastermix was purchased from
Thermo Fisher Scientific, Inc.; primary antibodies against ICAM-1
(cat. no. #4915), zonula occludens-1 (ZO-1; cat. no. #5406), NFκB
(cat. no. #8242), IκB (cat. no. #4812) and VE-Cadherin (cat. no.
#2500) for western blotting were purchased from Cell Signaling
Technology, Inc; primary β-actin antibody (cat. no. GB15003) for
western blot was purchased from Wuhan Servicebio Technology Co.,
Ltd.; primary ICAM-1 antibody (cat. no. #380990) for
immunofluorescence and primary IL-6 antibody (cat. no. #500286) for
immunohistochemical was purchased from Chengdu Zhengneng
Biotechnology Co., Ltd.; primary ZO-1 antibody (cat. no. #AF5145)
for immunofluorescence was purchased from Affinity Biosciences;
horseradish peroxidase-labeled goat anti-rabbit IgG secondary
antibody (cat. no. A0208) for western blotting was purchased from
Beyotime Institute of Biotechnology; Fluor594-conjugated goat
anti-rabbit IgG secondary antibody (cat. no. #S0006) for
immunofluorescence was purchased from Affinity Biosciences; West
ECL was purchased from Hycezmbio; Universal two-step detection kit
(cat. no. PV-9000) was purchased from OriGene Technologies,
Inc.
Cell culture
The human umbilical vein endothelial cells (HUVECs)
used in the present study were an immortalized cell line (cat. no.
GDC0635), which was obtained from the China Center for Type Culture
Collection. The cells were cultured in RPMI-1640 medium,
supplemented with 10% FBS and 1% penicillin-streptomycin mixture,
and were placed in a cell incubator containing 5% CO2 at
37˚C. When the cells reached a confluence of 80-90% on the bottom
of the T25 or T75 culture flasks, they were routinely digested and
cultured in different culture flasks or plates in accordance with
the experimental plan. The human monocyte cell line THP-1 was
obtained from Procell Life Science & Technology Co., Ltd. THP-1
was cultured in RPMI-1640 complete medium obtained from Procell
Life Science & Technology Co., Ltd., which contained 0.05 mM
β-mercaptoethanol, 1% penicillin-streptomycin mixture and 10% FBS.
The cells were placed in a cell incubator containing 5%
CO2 at 37˚C. The cells were passaged for 10 times when
they had grown to a cell density of 1x106 cells/ml.
Extraction of EMPs
HUVECs were cultured in T75 culture flasks. Briefly,
the FBS used for HUVEC culture was centrifuged at 120,000 x g and
4˚C overnight to remove the extracellular vesicles contained
therein. Various concentrations of IL-6 (0, 1, 10, 100 and 1,000
pg/ml) were added at room temperature after cell adherence and
HUVECs were cultured at 37˚C for 24 h. When the cells had grown to
~90% confluence, the supernatant was gathered, centrifuged at 4˚C
and 1,000 x g for 10 min, collected and centrifuged at 4˚C and
16,000 x g for 5 min, and collected and centrifuged again at 4˚C
and 16,000 x g for 60 min. The supernatant was then discarded, and
the EMPs were resuspended in 500 µl PBS and stored at -80˚C until
further use (10).
Endocytosis assay
EMPs were incubated with the fluorescent dye Dil at
37˚C for 1 h, then they were centrifuged at 16,000 x g and 4˚C for
60 min. After discarding the supernatant, Dil-labeled EMPs were
obtained. Dil-labeled EMPs were added to HUVECs for co-incubation
at 37˚C for 4 h. At the end of the co-incubation, HUVECs were fixed
with 4% paraformaldehyde at room temperature for 5 min and washed
with 0.2% BSA-PBS three times. Then HUVECs were incubated with 0.3%
Triton X-100 at room temperature for 5 min, incubated with 3%
BSA-PBS at room temperature for 30 min and washed again with 0.2%
BSA-PBS three times. HUVECs were stained with Actin-Tracker
Green-488 at room temperature for 1 h and DAPI at room temperature
for 10 min. At last, the cells were washed with PBS three times,
observed under a fluorescence microscope and images were
captured.
Adhesion assay
After routine digestion, HUVECs were cultivated in
96-well culture plates at a density of 3x103 cells/well.
After cell adhesion, HUVECs were treated with various
concentrations of IL-6-EMPs (0, 1, 10, 100 or 1,000 pg/ml) or 100
pg/ml IL-6-EMPs plus different concentrations of BCA (0, 5 or 10
µM) at 37˚C for 24 h. The human monocytic cell line THP-1 was
stained with BCECF-AM at 37˚C at a final concentration of 10 µM for
2 h. Subsequently, 1.5x104 THP-1 cells/well were added
to 96-well culture plates for 6-8 h of co-culture with HUVECs. At
the end of the co-culture, the cells were washed with 1X PBS three
times, observed under a fluorescence microscope and images were
captured.
CCK8
After routine digestion, HUVECs were cultivated in
96-well culture plates at the density of 3x103
cells/well. After cell adhesion, different concentrations of
IL-6-EMPs (0, 1, 10, 100 and 1,000 pg/ml) and/or different
concentrations of BCA (0, 2.5, 5, 10, 20 and 40 µM) were added to
the plates. The plates were then incubated for 24 h in a 37˚C
incubator containing 5% CO2, after which, 10 µl CCK8
reagent/well was added and the cells were incubated at 37˚C for 2 h
in the dark. The optical density (OD) of each well was measured at
a wavelength of 450 nm, which represents the viability of the
cells.
ELISA
After routine digestion, HUVECs were cultivated in
6-well culture plates at a density of 1x105 cells/well.
Different concentrations of IL-6-EMPs (0, 1, 10, 100 and 1,000
pg/ml) were added, and the supernatant was collected after 24 h of
intervention at 37˚C. Subsequently, 10 µl supernatant and 40 µl
diluent in the kit were added per well of a 96-well ELISA plate and
the plate was incubated for 1 h at 37˚C in the dark. A total of 100
µl HRP-conjugated reagent was added to each well, with the
exception of the blank well, and the plates were incubated at 37˚C
for 60 min for antibody capture. Each well was then washed with
diluted detergent five times. After washing, 50 µl chromogenic
agent A and 50 µl chromogenic agent B were added to each well
successively, and the plates were incubated at 37˚C for 15 min.
Finally, 50 µl stop solution was added to each well to terminate
the reaction, and the OD of each well was measured at a wavelength
of 450 nm.
Reverse transcription-quantitative PCR
(RT-PCR)
After routine digestion, HUVECs were cultivated in
6-well culture plates at a density of 1x105 cells/well,
and intervention was performed in accordance with the following
groups: i) 1X PBS; ii) 100 pg/ml IL-6-EMPs; iii) 100 pg/ml
lL-6-EMPs + 5 µM BCA; and iv) 100 pg/ml IL-6-EMPs + 10 µM BCA.
After 24 h of intervention at 37˚C, total RNA was extracted using
E.Z.N.A.® Total RNA Kit I and reverse transcribed into
cDNA using RevertAid First Strand cDNA Synthesis Kit. The RT
temperature protocol was according to manufacturer's protocol. The
fluorophore used in qPCR was 2x SYBR Green PCR Mastermix. The
thermocycling conditions were as follows: Initial denaturation at
95˚C for 5 min, followed by 40 cycles of denaturation (95˚C for 10
sec), annealing (60˚C for 15 sec) and 40 cycles of elongation
(single fluorescence measurement at 72˚C for 15 sec), with a
melting curve program of 60-95˚C, 0.11˚C/sec temperature rise
(continuous fluorescence measurement), and cooling at 40˚C. Data
were analyzed with the 2-IICq method (25), using GAPDH as the normalization
gene. The primers used in the present study are listed in Table I.
 | Table IPrimers for reverse
transcription-quantitative PCR analysis. |
Table I
Primers for reverse
transcription-quantitative PCR analysis.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| ICAM-1 |
GTCACCTATGGCAACGACTCCTTC |
AGTGTCTCCTGGCTCTGGTTCC |
| E-Selectin |
GCACATCTCAGGGACAATGGACAG |
CATCCTTCAGGACAGGCGAACTTG |
| GAPDH |
GAAGGTCGGAGTCAACGGAT |
CCTGGAAGATGGTGATGGG |
Immunofluorescence (IF) staining
After routine digestion, HUVECs were cultivated in
48-well culture plates at a density of 1x104 cells/well.
The intervention for group 1 was: i) 0 pg/ml IL-6-EMPs; and ii) 100
pg/ml IL-6-EMPs. The intervention for group 2 was: i) 1X PBS; ii)
100 pg/ml IL-6-EMPs; iii) 100 pg/ml IL-6-EMPs + 5 µM BCA; and iv)
100 pg/ml IL-6-EMPs + 10 µM BCA. After 24 h of intervention at
37˚C, the medium was discarded and the cells were washed three
times with PBS. The cells were then fixed with 4% paraformaldehyde
for 10 min at room temperature, washed with PBS three times and
permeabilized for 5 min at room temperature with 0.1% Triton-X-100,
which was then discarded. Subsequently, 3% BSA-PBS was added to the
cells for 1 h at room temperature of blocking and was discarded.
Washing was performed with 0.2% BSA-PBS three times. The primary
antibodies of ICAM-1 (1:200) and ZO-1 (1:500) were then added into
each well, respectively, and were incubated with the cells for 12 h
at 4˚C in the dark. Subsequently, the primary antibodies were
discarded, the cells were washed with 0.2% BSA-PBS three times, and
the Fluor594-conjugated goat anti-rabbit IgG secondary antibody
(1:500) was used to incubate the cells at room temperature for 2 h
in the dark. After the secondary antibody was discarded, the cells
were washed with 0.2% BSA-PBS three times, and were stained with
DAPI reagent for 10 min at room temperature. Finally, the DAPI
reagent was discarded, the cells were washed with 0.2% BSA-PBS
three times, and an anti-fluorescence quenching agent was added.
The cells were observed under a fluorescence microscope and images
were captured.
Western blot analysis
After routine digestion, HUVECs were cultivated in
6-well culture plates at a density of 1x105 cells/well.
Group 1 was treated with: i) 1X PBS; ii) 0 pg/ml IL-6-EMPs; iii)
100 pg/ml IL-6-EMPs; and iv) 1,000 pg/ml IL-6-EMPs. Group 2 was
treated with: i) 1X PBS; ii) 100 pg/ml IL-6-EMPs; iii) 100 pg/ml
IL-6-EMPs + 5 µM BCA; and iv) 100 pg/ml IL-6-EMPs + 10 µM BCA.
After 24 h of intervention at 37˚C, proteins were extracted from
each group as following protocol: The supernatant was discarded and
the cells were washed with PBS once. Subsequently, 116.4 µl RIPA
buffer, 1.2 µl protease aprotinin, 1.2 µl protein phosphatase
inhibitor and 1.2 µl PMSF were added to each well. After the
culture plates were incubated on ice for 20 min, the lysis solution
was collected in 1.5 ml EP tubes respectively. EP tubes were
centrifuged at 4˚C and 12,000 rpm for 20 min. The protein
concentration of the supernatant was detected according to the
instructions of the BCA protein assay kit. Then, the loading buffer
was added into the EP tubes and they were incubated at 100˚C for 15
min. The concentration of proteins was 20 µg per lane. Proteins
were separated by SDS-PAGE on 8% gels and were subsequently
transferred onto PVDF membranes. The membranes were washed with
TBS-0.05%Tween (TBST) three times and blocked with 5% BSA-PBS with
agitation for 1 h at room temperature. After blocking, the
membranes were horizontally cut to probe proteins with different
molecular weights, and incubated with different corresponding
primary antibodies for 12-14 h at 4˚C. The primary antibodies used
included anti-β-actin (1:1,000), anti-ICAM (1:500), anti-ZO-1
(1:500), anti-NFκB (1:500), anti-IκB (1:500) and anti-VE-Cadherin
(1:500). The membrane was then washed three times with 1X TBST and
incubated with the corresponding horseradish peroxidase-labeled
goat anti-rabbit IgG secondary antibodies (1:10,000) with agitation
at room temperature for 1 h. Images of the membrane were acquired
using West ECL and the Image Quant LAS 4000 system (Cytiva) and
analyzed using ImageJ software V1.8.0 (National Institutes of
Health).
Animal model of femoral head
necrosis
Since Perthes disease is more likely to occur in
male children, male Sprague-Dawley (SD) rats were selected for
modeling. A total of 18 6-week-old male SD rats (weight,
194.0±9.068 g) were purchased from the Experimental Animal Center
of Guangxi Medical University (Nanning, China). The animal
experiment was approved by the Animal Care & Welfare Committee
of Guangxi Medical University (approval no. 202111011; Nanning,
China). The animal ethics review followed the guiding opinions on
the treatment of laboratory animals (https://www.most.gov.cn/) issued by the Ministry of
Science and Technology of the People's Republic of China and the
guidelines for the ethical review of laboratory animal welfare
issued by the National Standard GB/T35892-2018 of the People's
Republic of China (26). All the
animal experiments were carried out according to the ARRIVE
guidelines (27).
The rats were reared at a room temperature of 25˚C
under 60% relative humidity and a 12-h light/dark cycle, with free
access to water and food. The rats were randomly divided into the
following three groups: The sham surgery group (n=6), the femoral
head necrosis group (n=6) and the BCA group (drug group; n=6). The
present study used the femoral neck girdling method, which is a
common method of modeling femoral head necrosis in rats (6,28).
The rats in the sham operation group underwent exposure of the
femoral head through surgery without femoral neck girdling, whereas
the femoral head necrosis group and the BCA group underwent femoral
neck girdling. The surgery protocol was as follows: Following
intraperitoneal injection of 200 mg/kg tribromoethanol for
anesthesia and subcutaneous injection of 5 mg/kg carprofen for pain
relief, the hip joint of the right lower limb was prepared and
disinfected. The rats were fixed on the operating table in a prone
position, deep anesthesia was ensured and the surgical area was
disinfected with iodophor. The hip joint was checked to confirm
that it was located under the gluteus and abductor muscles, to
determine the position of the hip joint. Subsequently, an incision
was made into the skin, the gluteus and abductor muscles were
separated directly along the muscle fibers, and the hip capsule was
exposed. The joint capsule was cut longitudinally along the femoral
neck axis, and the hip joint was pulled and bent longitudinally,
causing the femoral head to be semi-dislocated, thus exposing the
round ligament, which was cut off. Two No. 1-sized non-absorbable
sutures were placed around the femoral neck and tightly crossed to
block the ascending branch of the circumflex femoral artery
supplying the epiphysis of the femoral head. Finally, the joint
capsule and gluteal muscle were repaired in turn, and the skin was
closed in layers (28,29).
After modeling, the rats were observed every day,
paying particular attention to fur color, wound sutures, wound
healing, any hunched-back behavior and food and drink intake, to
monitor the health of the animals. A total of 2 days after surgery,
each rat received intramuscular injection of 18 mg/kg penicillin
daily to prevent infection. In addition, the BCA group was injected
with BCA (2.5 mg/kg) intraperitoneally every 2 days for 4 weeks,
whereas the sham operation group and the femoral head necrosis
group were injected with 1X PBS every 2 days at the same dose as
the BCA group for 4 weeks. In the present study, excessive fur
grooming, hunched behavior and 20% body weight loss were used as
evaluation indexes of humane endpoints. When the three abnormal
behaviors occurred at the same time, the humane endpoint was
considered to be reached. The weight of the rats in the sham group,
femoral head necrosis group and BCA group at the beginning of the
study was 195.8±7.627, 192.7±7.992 and 193.5±12.29 g respectively;
the weight of the rats in the sham group, femoral head necrosis
group and BCA group at the end of the study was 278.7±10.25,
276.8±5.776 and 280.7±13.16 g, respectively. No rats reached the
humane endpoint before the end of the study (30,31).
After 4 weeks, referring to the animal euthanasia methods
recommended by the AVMA Guidelines for the Euthanasia of Animals
(https://www.avma.org/), excessive anesthesia with
pentobarbital sodium (200 mg/kg; intraperitoneal injection) was
used to euthanize the animals. The death of the rats was confirmed
as follows: By verifying a lack of respiration or pulse;
confirmation of a lack of heartbeat for >5 min, as determined
using a stethoscope or by touching the chest cavity; disappearance
of the corneal and nerve reflexes; and pupil dilation.
After euthanasia, the femurs were removed and fixed
with 4% paraformaldehyde for 48 h at room temperature. After
fixation, 10% ethylenediaminetetraacetic acid was utilized to
decalcify the fixed rat femoral head specimens for 3 weeks at
25-30˚C. Subsequently, the specimens were embedded in paraffin and
sectioned with a thickness of 4 µm, and the sections were processed
for IL-6 immunohistochemical staining. Firstly, the sections were
immersed in fresh xylene for 10 min for three times. The sections
were subsequently soaked in gradient ethanol: 100% for 3 min for
three times, 95% for 3 min for two times, 75% for 3 min for two
times. The sections were washed with sterilized pure water for 1
min for three times. Then, the sections were immersed in the sodium
citrate buffer and heated in the microwave until the buffer boiled,
and the microwave was immediately maintained at low heat for 10
min. After that, the sections were removed from the microwave and
cooled naturally to room temperature. We washed the sections with
PBS for 3 min for three times and added an appropriate amount of
endogenous peroxidase blocker from the Universal two-step detection
kit and incubated at room temperature for 10 min. An appropriate
amount of IL-6 primary antibody (1:100) was added to the sections,
and they were placed in a wet box and incubated at 4˚C overnight.
At the end of the incubation, the sections were washed with PBS for
3 min for three times. An appropriate amount of reaction enhancing
solution from the Universal two-step detection kit was added to the
sections, which were incubated at 37˚C for 20 min. After
incubation, the sections were washed with PBS for 3 min for three
times. An appropriate amount of enhanced enzyme-linked sheep anti
mouse/rabbit IgG polymer from the Universal two-step detection kit
was added to the sections and the sections were incubated at 37˚C
for 20 min. An appropriate amount of DAB colorimetric solution was
added to the sections and the sections were incubated for 5-8 min
at room temperature. And the sections were washed with tap-water to
terminate staining. Finally, the sections were dyed with
hematoxylin solution for 3-5 min at room temperature and washed
with tap-water to terminate staining. The sections were observed
under microscope (BX53F; Olympus Corporation) and images were
captured. ImageJ Software V1.8.0 (National Institutes of Health)
was used to count the number of IL-6-positive cells. In addition,
rat liver and kidney samples were collected. To observe the
hepatorenal toxicity of BCA, hematoxylin and eosin (H&E)
staining was performed as the following protocol: The specimens
were embedded in paraffin and sectioned with a thickness of 3 µm.
After dewaxing, hydration and pretreatment, the sections were dyed
with hematoxylin solution for 3-5 min at room temperature and with
eosin solution for 15 sec at room temperature. After staining, all
sections were observed under a microscope (BX53F; Olympus
Corporation) and images were captured.
Statistical analysis
All the experiments were repeated at least three
times. All data analyses were carried out using SPSS 26.0 (IBM
Corp.) and presented as mean ± standard deviation. The charts were
generated using GraphPad 7.0 (Dotmatics) and grouped in Adobe
Illustrator 2019 (Adobe Systems, Inc.). Differences between two
groups were analyzed using unpaired Student's t-test. Differences
between three or more groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
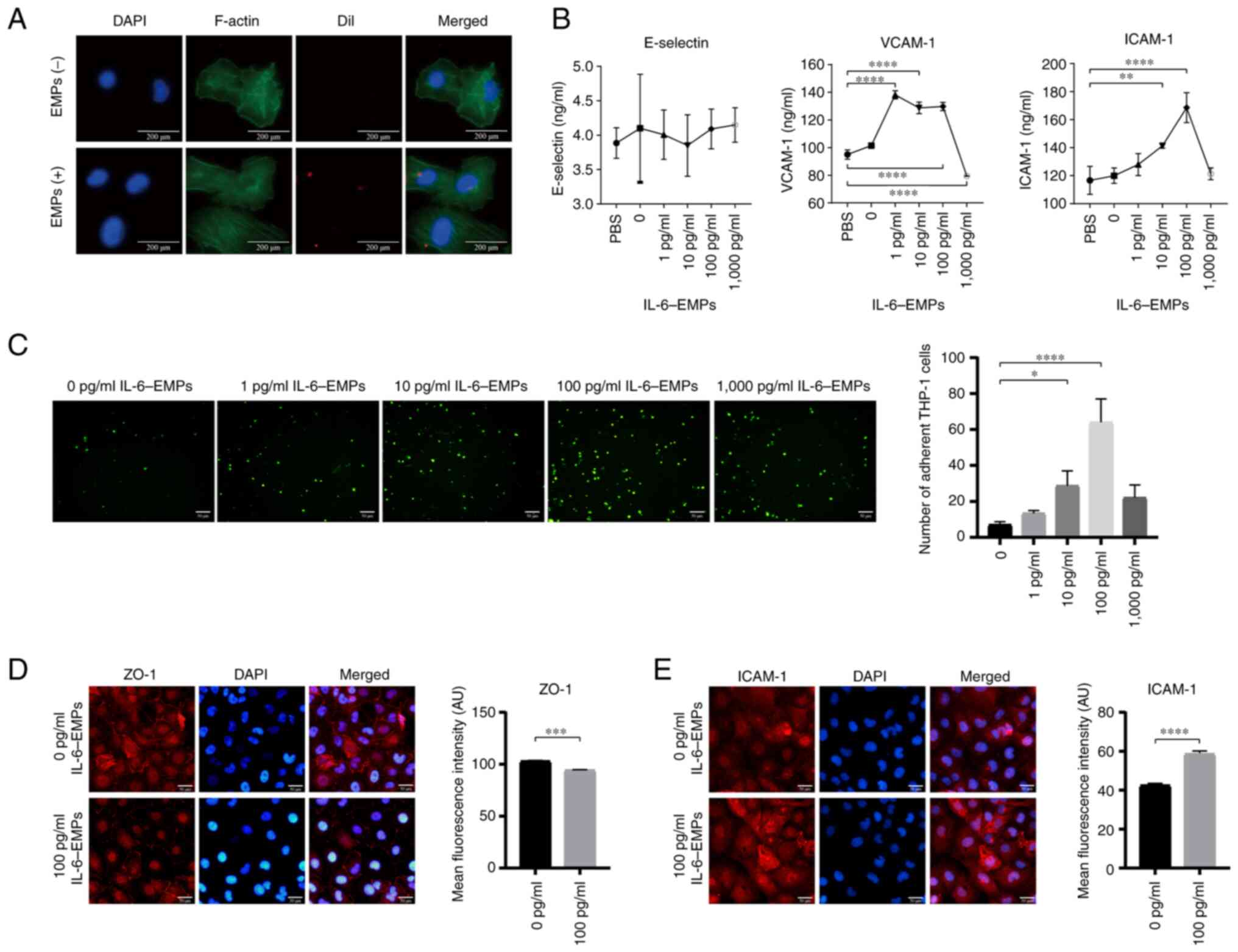
EMPs secreted by IL-6-stimulated
HUVECs can induce endothelial dysfunction
HUVECs were treated with different concentrations of
IL-6 (0, 1, 10, 100 and 1,000 pg/ml) to obtain IL-6-EMPs. The
endocytosis experiment revealed that IL-6-EMPs were absorbed into
HUVECs and played a role in affecting the function of HUVECs
(Fig. 1A). In addition, ELISAs
were performed to explore the effect of IL-6-EMPs on the markers of
endothelial cell dysfunction. The results showed that treatment
with 100 pg/ml IL-6-EMPs promoted the expression of VCAM-1 and
ICAM-1 in HUVECs compared with the PBS group; however, they did not
affect the expression of E-Selectin (Fig. 1B). Notably, peak ICAM-1 levels were
detected following treatment with 100 pg/ml IL-6-EMPs. By contrast,
1,000 pg/ml IL-6-EMPs inhibited the levels of ICAM-1 and VCAM-1
compared with the 100 pg/ml IL-6-EMPs group. It has been
demonstrated that damaged endothelial cells can promote monocyte
adhesion (32). After the HUVECs
were treated with different concentrations of IL-6-EMPs (0, 1, 10,
100 and 1,000 pg/ml), they were co-cultured with
fluorescent-labeled monocytes. The results showed that IL-6-EMPs
can promote monocyte adhesion to HUVECs in a
concentration-dependent manner within the concentration range of
0-100 pg/ml IL-6 (Fig. 1C).
Similar to the ELISA results, monocyte adhesion was also inhibited
by 1,000 pg/ml IL-6-EMPs compared with the 100 pg/ml IL-6-EMPs
group. In addition, IF-staining suggested that IL-6-EMPs reduced
the expression of ZO-1, but promoted the expression of ICAM-1 in
HUVECs, indicating that 100 pg/ml IL-6-EMPs could induce
endothelial dysfunction (Fig. 1D
and E).
BCA can inhibit IL-6-EMP-induced
endothelial dysfunction
BCA is an isoflavone and its chemical structure is
shown in Fig. 2A. HUVECs were
treated with different concentrations of BCA (0, 2.5, 5, 10, 20 and
40 µM) and IL-6-EMPs (0, 1, 10, 100 and 1,000 pg/ml) to determine
whether BCA and IL-6-EMPs had toxic effects on HUVECs. The results
of the CCK8 assay showed that 0-20 µM BCA and IL-6-EMPs did not
affect the viability of HUVECs; however, co-treatment with 100
pg/ml IL-6-EMPs and 20 or 40 µM BCA reduced the viability of HUVECs
compared with the control group (Fig.
2B). Furthermore, based on the results of ELISA (Fig. 1B), adhesion experiment (Fig. 1C) and CCK8, HUVECs were treated
with 100 pg/ml IL-6-EMPs and 0, 5 and 10 µM BCA, and RT-qPCR,
adhesion assay and IF staining were performed. RT-qPCR results
revealed that BCA could reduce the expression levels of ICAM-1 and
E-selectin in HUVECs in a concentration-dependent manner compared
with the increase induced by 100 pg/ml IL-6-EMPs (Fig. 2C). The results of the adhesion
assay demonstrated that BCA could inhibit IL-6-EMPs-induced
endothelial dysfunction in a concentration-dependent manner,
thereby reducing the number of adherent monocytes (Fig. 2D). Furthermore, IF staining results
revealed that BCA increased the expression of ZO-1 compared with
the increase induced by 100 pg/ml IL-6-EMPs, and thus inhibited the
endothelial dysfunction induced by IL-6-EMPs (Fig. 2E).
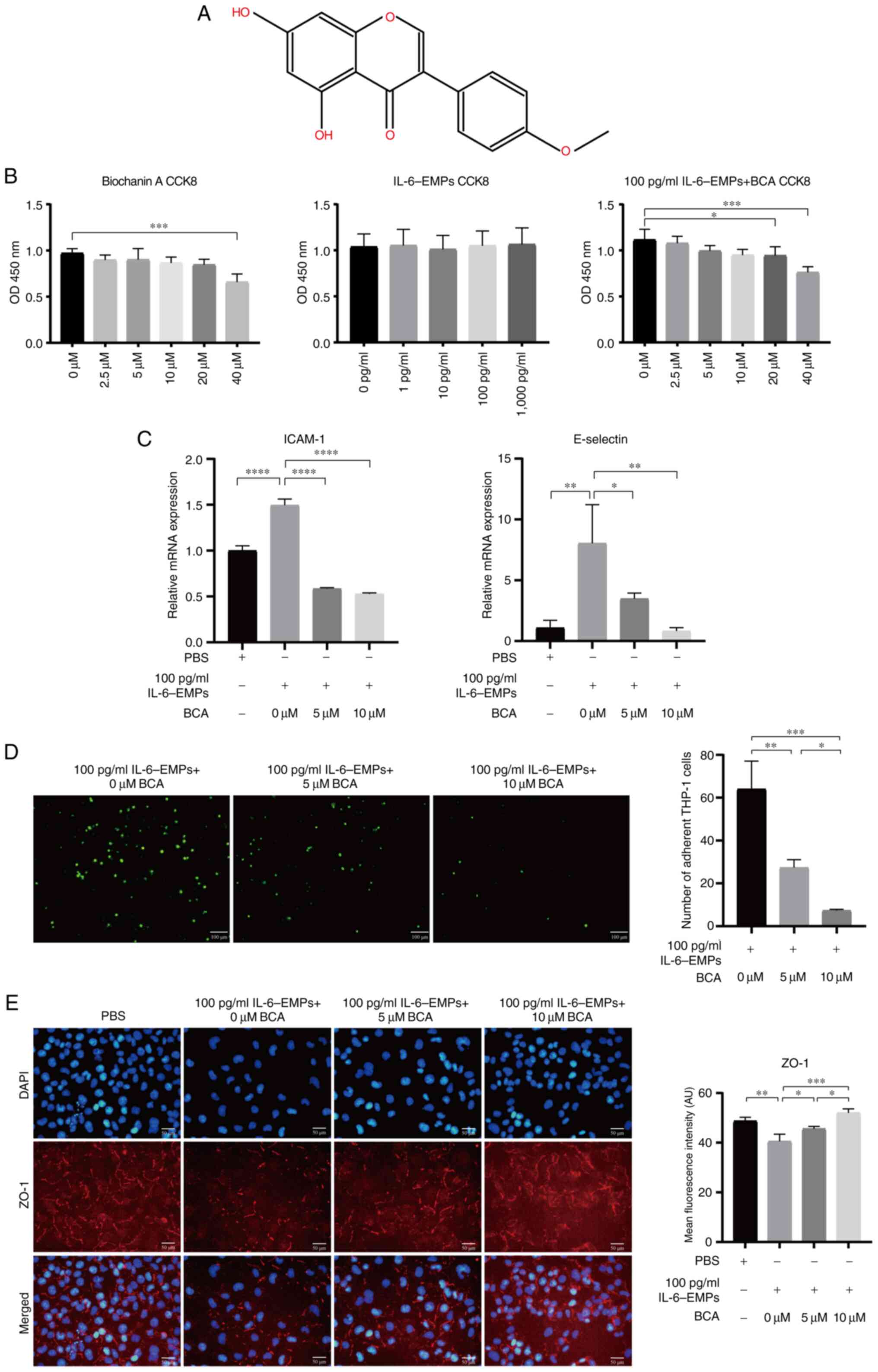 | Figure 2BCA inhibits endothelial dysfunction
induced by IL-6-EMPs in the non-cytotoxic concentration range. (A)
Chemical structure of BCA. (B) CCK8 assay results showed that BCA
had no toxic effect on cells in the concentration range of 0-20 µM,
IL-6-EMPs had no toxic effect on cells in the concentration range
of 0-1,000 pg/ml, and 100 pg/ml IL-6-EMPs + 0-10 µM BCA had no
toxic effect on cells (n=3). (C) After treatment with BCA, the
indexes of endothelial dysfunction ICAM-1 and E-selectin were
decreased with the increase in BCA concentration, indicating that
BCA can effectively reduce the endothelial dysfunction induced by
IL-6-EMPs (n=3). (D) Adhesion assay showed that BCA could inhibit
the injury of endothelial cells induced by IL-6-EMPs and reduce the
adhesion of monocytes to human umbilical vein endothelial cells.
Green fluorescence represents monocytes (n=3). Scale bar=100 µm.
(E) IL-6-EMPs (100 pg/ml) decreased the expression of ZO-1. After
treatment with BCA, the endothelial dysfunction induced by
IL-6-EMPs was inhibited and the expression of ZO-1 was increased
(n=3). Scale bar=50 µm. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. BCA, biochanin A; CCK8, Cell Counting
Kit 8; EMPs, endothelial microparticles; ICAM-1, intercellular cell
adhesion molecule-1; ZO-1, zonula occludens-1. |
BCA inhibits endothelial dysfunction
induced by IL-6-EMPs via the NFκB signaling pathway
Western blot analysis was performed to investigate
the activation of the NFκB signaling pathway and the mechanism
underlying the inhibitory effect of BCA on IL-6-EMPs-induced
endothelial dysfunction. Following treatment of HUVECs with 100 and
1,000 pg/ml IL-6-EMPs, the expression levels of ICAM-1 were
elevated and those of ZO-1 were decreased, indicating that
IL-6-EMPs induced endothelial dysfunction (Fig. 3A and B). Subsequently, HUVECs were treated with
100 pg/ml IL-6-EMPs combined with 0, 5 and 10 µM BCA. The results
showed that BCA could reduce the expression levels of NFκB in a
dose-dependent manner, while increasing the expression levels of
IκB compared with the changes induced by 100 pg/ml IL-6-EMPs
(Fig. 3C and D). Moreover, after intervention with BCA,
the protein expression levels of the endothelial dysfunction marker
ICAM-1 was decreased, whereas those of ZO-1 and VE-cadherin were
increased compared with the changes induced by 100 pg/ml
IL-6-EMPs.
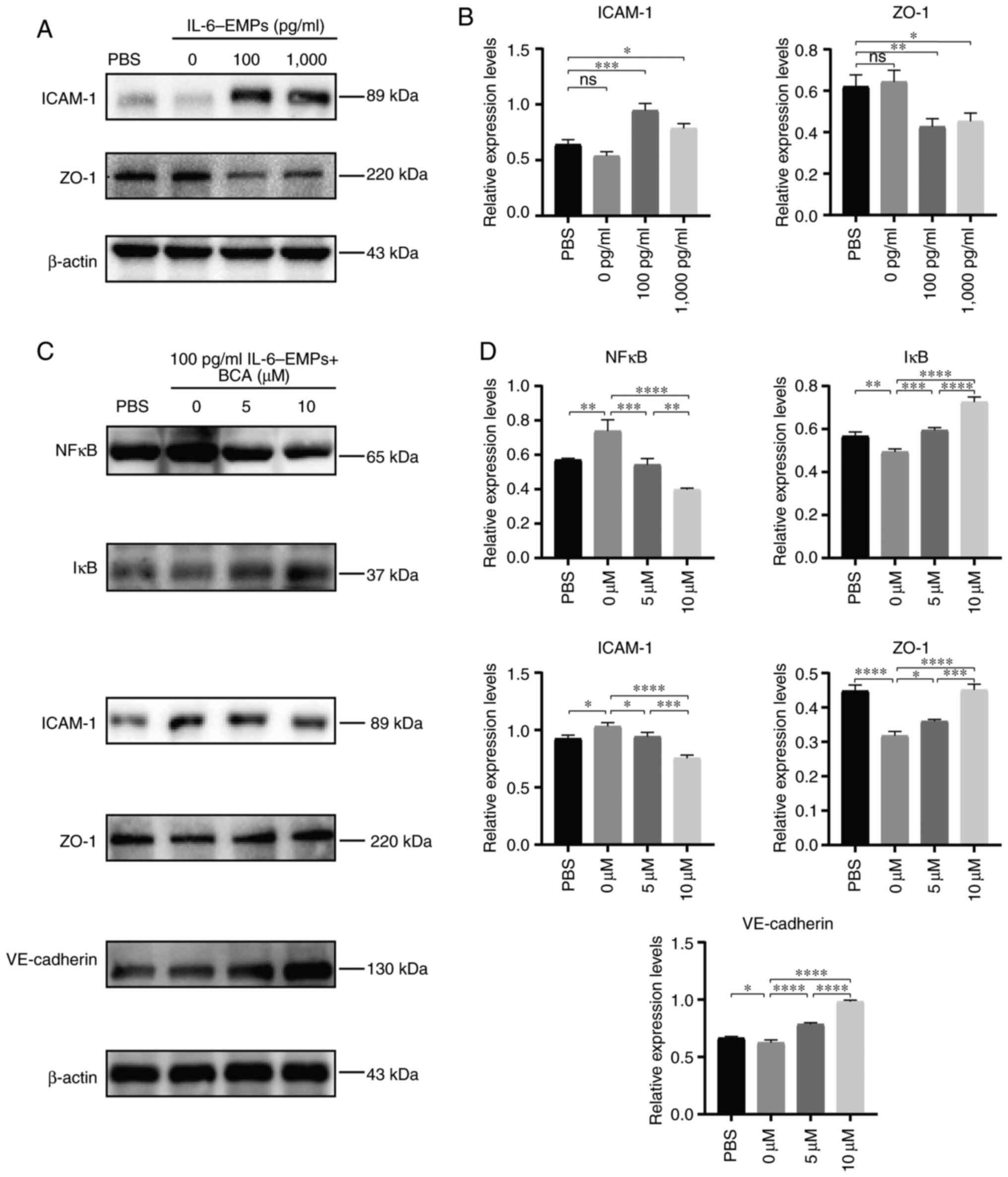 | Figure 3BCA inhibits endothelial dysfunction
via the NFκB signaling pathway. (A) After HUVECs were treated with
different concentrations of IL-6-EMPs (0, 100 and 1,000 pg/ml), the
expression levels of ICAM-1 and ZO-1 were analyzed by western
blotting. Representative images showed that the expression levels
of ICAM-1 were increased with the increase in IL-6-EMPs
concentration, whereas the expression levels of ZO-1 were decreased
with the increase in IL-6-EMPs concentration (n=3). (B) Relative
expression levels of ICAM-1 and ZO-1 were calculated. (C) HUVECs
were treated with 100 pg/ml IL-6-EMPs + BCA (0, 5 and 10 µM). After
treatment, the expression levels of NFκB, IκB, ICAM-1, ZO-1 and
VE-cadherin were analyzed by western blotting. Representative
images showed that the expression levels of NFκB and ICAM-1 were
decreased with the increase in BCA concentration, and the
expression levels of IκB, ZO-1 and VE-cadherin were increased with
the increase in BCA concentration (n=3). (D) Relative expression
levels of NFκB, IκB, ICAM-1, ZO-1 and VE-cadherin were calculated.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. BCA,
biochanin A; EMPs, endothelial microparticles; HUVECs, human
umbilical vein endothelial cells; ICAM-1, intercellular cell
adhesion molecule-1; ns, not significant; ZO-1, zonula
occludens-1. |
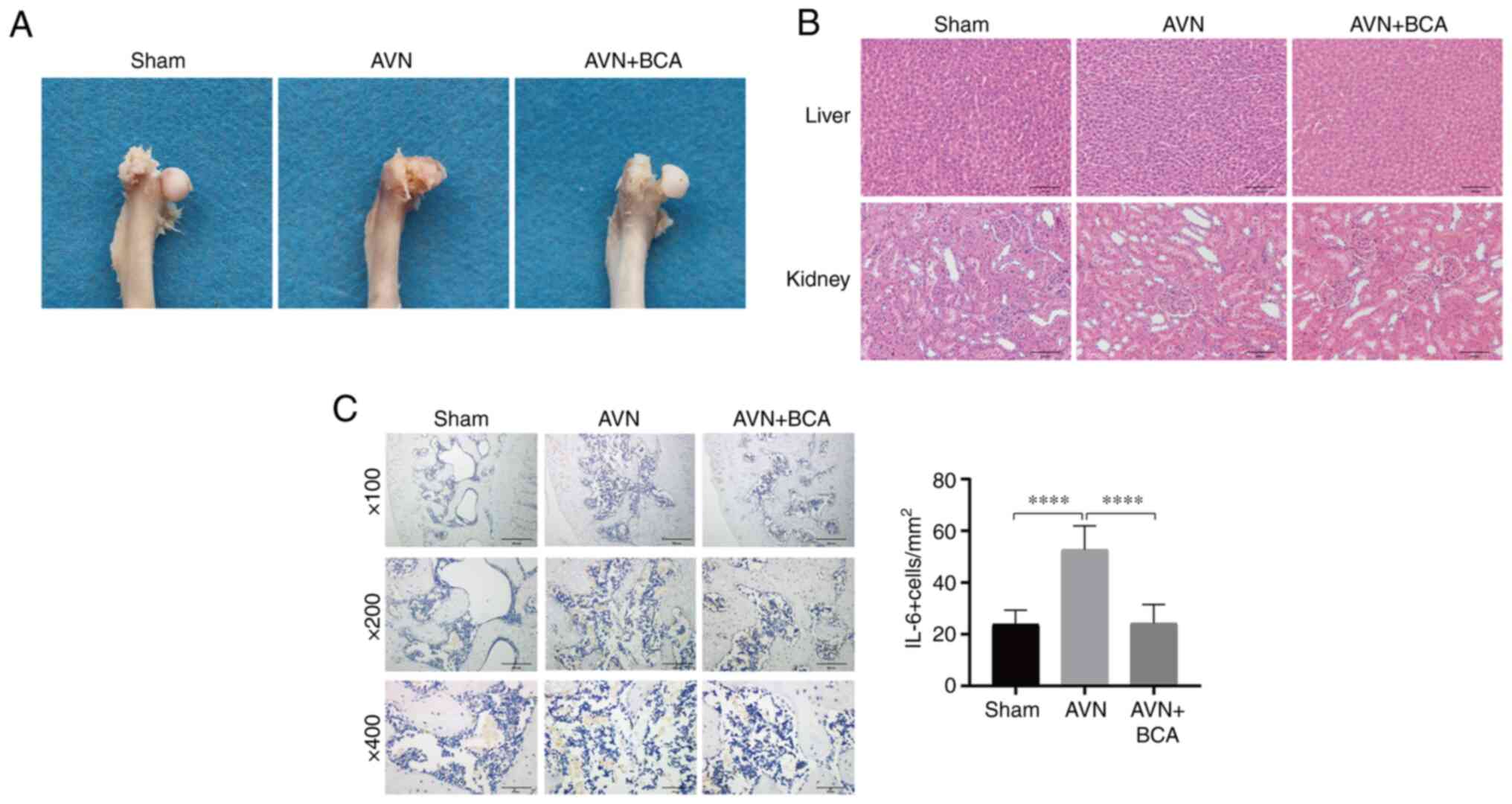
BCA inhibits the expression of IL-6 in
bone tissues after ischemic necrosis of the femoral head
A rat model of ischemic necrosis of the femoral head
was established to investigate the effect of BCA on the expression
levels of IL-6 in bone tissues. The femoral head in the sham
surgery group remained intact, whereas that in the femoral head
necrotic group collapsed and appeared flatter and more severely
damaged than that in the BCA group (Fig. 4A). H&E staining of liver and
kidney sections indicated that there was no marked difference in
histology between the three groups (Fig. 4B). Immunohistochemical analysis
suggested that the expression levels of IL-6 were significantly
elevated in the femoral head section of the femoral head necrotic
group compared with those in the other two groups, indicating that
BCA could reduce the expression of IL-6 after the ischemic necrosis
of the femoral head (Fig. 4C).
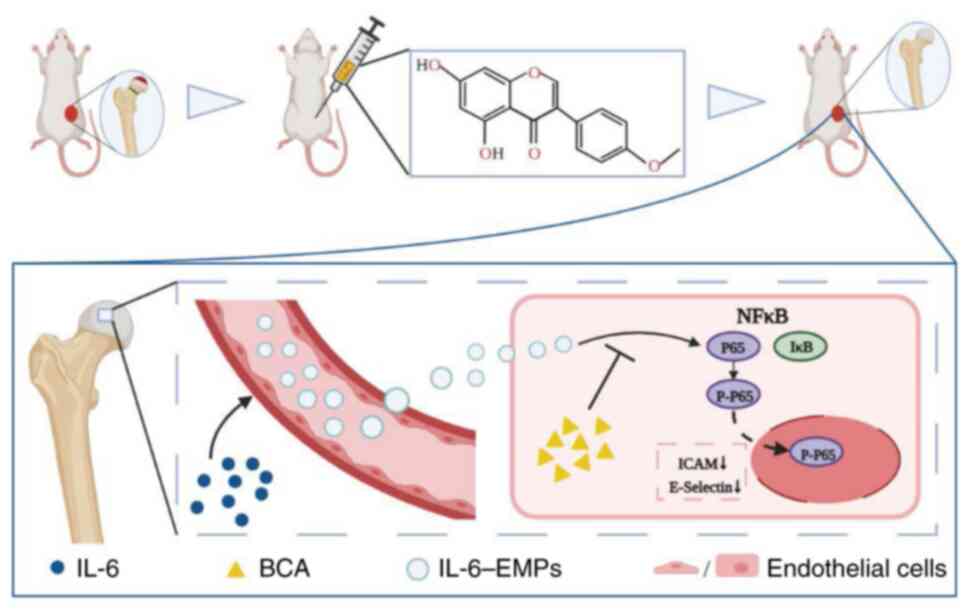
Based on the aforementioned in vitro and in vivo
experiments, the present study concluded that BCA could reduce the
expression levels of ICAM-1 and E-selectin, and improve endothelial
dysfunction induced by IL-6-EMPs through the NFκB pathway, thus
improving the collapse of femoral head in femoral head necrosis
rats. The potential mechanism underlying the effects of BCA in the
treatment of Perthes disease is shown in Fig. 5.
Discussion
Perthes disease is a disabling and teratogenic
disease in children, which is characterized by ischemic necrosis of
the femoral head and may cause the deformation of femoral head if
the diagnosis and treatment are not timely. In the absence of any
intervention, the collapse and deformation of the femoral head can
induce premature arthritis, which eventually causes different
degrees of disability in children (33). At present, the clinical treatment
of Perthes disease includes absolute weight-free bed rest (avoiding
trying to stand with affected lower limb) and surgical treatment
(34). However, these treatments
are not very efficient, and no effective drug treatment exists.
Therefore, it is necessary to study the etiology, pathogenesis and
possible therapeutic drugs for Perthes disease. Our previous study
(12) found that in patients with
Perthes disease, upregulation of IL-6 stimulated EMPs to induce
endothelial dysfunction. And the same result was found in a rat
model of ischemic necrosis of the femoral head in the present
study. Moreover, BCA could inhibit IL-6-EMPs-induced endothelial
dysfunction through the NFκB signaling pathway, and may thus be
considered a potential drug for Perthes disease.
The etiology of Perthes disease has not been
clarified, and numerous hypotheses have been proposed for its
pathogenesis. The hypothesized pathogenic factors include
microtrauma, tobacco exposure, prenatal factors, blood
hypercoagulation status and collagen mutations (1). However, these assumptions have not
been fully confirmed or are controversial. Notably, it has been
shown that endothelial dysfunction induced by IL-6-EMPs may be a
key pathological process in Perthes disease (7). Kuroyanagi et al (35) established an animal model of
Perthes disease and found that IL-6 is significantly increased in
the joint fluid after induction of ischemic necrosis of the femoral
head. In addition, IL-6 gene knockout can result in
revascularization in a mouse model of Perthes disease (35). Our previous study (12) demonstrated that the expression
levels of IL-6 are elevated in the plasma of patients with Perthes
disease and that IL-6 is associated with
CD31+/CD42b- EMPs. EMPs are exosomes with a
diameter of 100-1,000 nm, which are secreted into the plasma after
endothelial cell dysfunction (36), and this was characterized by flow
cytometry in our previous study (12). The antigen carried on the EMP
surface is the same as that found in endothelial cells; therefore,
the state of endothelial cells can be assessed by detecting the
cell-surface antigen of EMPs (37). The expression of CD31 is elevated
during endothelial apoptosis, and the expression of CD62E is
increased upon endothelial cell activation; therefore, the ratio of
CD31+CD42b-/CD62E+ is commonly
used to assess the extent of endothelial dysfunction (38). In our previous study (12), it was revealed that the proportion
of CD62E+ EMPs are not significantly elevated compared
with the control group, but the ratio and proportion of
CD31+CD42b- EMPs to CD62E+ EMPs
was higher in patients with Perthes disease compared with the
control group. This phenomenon suggested that the number of
apoptotic EMPs may be greater than that of activated EMPs in
patients with Perthes disease. These results from our previous
study (12) indicated that IL-6
may be the key inflammatory factor for the induction of endothelial
dysfunction in Perthes disease and that IL-6-EMPs-induced
endothelial dysfunction could be the key to the treatment of
Perthes disease.
Endothelial cells contain several characteristic
markers, such as VCAM-1, E-selectin, von Willebrand factor, ICAM-1
and endothelial nitric oxide synthase (NOS), which can be utilized
as indicators of endothelial dysfunction. ICAM-1 is a surface
glycoprotein and adhesion receptor that is involved in various
physiological processes, such as leukocyte adhesion (39), tumor cell transfer (40), barrier function (41), proliferation (42) and epithelial cell activation
(43). It is poorly expressed in
endothelial, immune and epithelial cells, but is upregulated when
stimulated by inflammation (44).
VCAM-1 is an adhesion factor that mediates the binding of
eosinophils, monocytes and other vascular endothelial cells. It is
mostly expressed in smooth muscle and vascular endothelial cells,
and serves a role in endothelial dysfunction by participating in
the development and progression of inflammation; reducing VCAM-1
gene expression can alleviate vascular inflammation and endothelial
dysfunction (45-47).
In Perthes disease, upregulation of the inflammatory factor IL-6
increases the expression levels of VCAM-1 and ICAM-1 in endothelial
cells, and impairs the permeability of endothelial cells, which
subsequently promotes endothelial dysfunction (48). ZO-1 has a marked effect on the
maintenance of the blood-brain barrier and the close connection
between endothelial cells (48).
It is also an important protein in the physiological process of
angiogenesis. The reduction in ZO-1 expression decreases the tight
connection of endothelial cells and increases their permeability.
In the present study, treating endothelial cells with IL-6-EMPs
promoted monocyte adhesion to HUVECs in a dose-dependent manner,
and significantly increased VCAM-1 and ICAM-1 production. IF
analysis also revealed that the expression levels of ZO-1 were
decreased, whereas those of ICAM-1 were increased in endothelial
cells. Therefore, it was indicated that IL-6-EMPs induced
endothelial dysfunction.
At present, no effective clinical drug exists for
Perthes disease. Certain researchers have attempted to use
antiosteoporosis drugs, such as bisphosphate and strontium ranelate
(4,5), to treat this disease. However,
bisphosphate may lead to side effects, such as jaw necrosis,
atypical femoral fractures and delayed bone healing in children
with osteogenesis imperfecta (49-51).
And strontium ranelate may have undesirable side effects including
allergy and increased risk for cardiovascular events in systemic
use (52). These side effects and
adverse reactions limit the clinical application of these two
drugs. Therefore, the active exploration of effective clinical
drugs for Perthes disease is of great importance. BCA can be
extracted from plants, such as Caulis Spatholobi (13), soybean, peanut, red clover,
chickpea and alfalfa (14). Among
its various biological characteristics, its anti-inflammatory
effects have attracted attention. It has been shown that the
anti-inflammatory characteristics of BCA are mainly manifested by
inhibiting the expression of VCAM-1, IL-8, ICAM-1 and tumor
necrosis factor α (TNF-α) in HUVECs, thereby reducing the
activation of NFκB (53).
Furthermore, BCA blocks the expression of NFκB in macrophages to
reduce the lipopolysaccharide-activated expression of NO and
inducible NOS (24), and can also
effectively inhibit the expression of TNF-α and IL-6. Our previous
study found that BCA can inhibit bone resorption and osteoclast
production via the MAPK and NFκB signaling pathways (23). The role of BCA in animals has also
been reported. BCA can inhibit the phosphorylation level of
prostaglandin E-2, NOS-2, NFκB and cyclooxygenase-2 to protect rat
chondrocytes from IL-induced inflammation (54). Notably, there are other Chinese
traditional medicine extracts or prescriptions that can resist
inflammation and improve endothelial dysfunction, such as
hederagenin (55), cycloastragenol
(56), epigallocatechin-3-gallate
(57), He xue ming mu tablet
(58), glycyrrhizic acid (59), tanshinone IIA sodium sulfonate
(60) and quercetin (61), but some of them exhibit clinical
toxicity and side effects, such as nausea, insomnia and
hepatotoxicity. At present, there is no relevant research comparing
BCA with the aforementioned drugs in terms of anti-inflammatory
activity and improving endothelial dysfunction, therefore it is not
possible to identify a clear advantage of BCA over the
aforementioned drugs. However, in the present animal experiments,
it was revealed that BCA had no obvious hepatorenal toxicity
through H&E staining of the liver and kidney specimens of the
three groups of rats, suggesting that BCA may exhibit low toxicity.
Therefore, the present study speculated on whether BCA could
inhibit IL-6-EMPs-induced endothelial dysfunction via the NFκB
pathway. It was revealed that the expression levels of ICAM-1 were
decreased after intervention with IL-6-EMPs and different
concentrations of BCA. The outcomes of IF staining demonstrated
that the expression of ZO-1 was increased by BCA, and western
blotting showed that the expression levels of NFκB were decreased
after BCA intervention compared with those before BCA intervention,
whereas the expression of IκB was increased. These findings
indicated that BCA may inhibit endothelial dysfunction induced by
IL-6-EMPs via the NFκB pathway.
In young animal models, Perthes disease may be
induced through the ischemic osteonecrosis of the femoral head by
ligation of the femoral neck. Yamaguchi et al (62) showed that in piglets, a model of
ischemic osteonecrosis of the femoral head can be constructed by
ligating the femoral neck, cutting off the round ligament, and
implementing supra knee amputation at the junction of the distal
epiphysis and epiphysis of the femur. In this model, the IL-6
receptor monoclonal antibody can reduce hip synovitis and
osteoclast bone resorption, and increase bone formation. In
addition, a mouse model of bone ischemic necrosis can be
successfully induced through the microscopic burning of four blood
vessels, including the popliteal artery branch at the distal end of
the femur and the medial, central and lateral blood vessels of the
knee (35,63). In the present study, a rat model of
ischemic necrosis of the femoral head was established in
consideration of the fact that we were unable to raise piglets and
complete the femoral head necrosis model with piglets and mice
under the conditions provided by our experimental animal center in
a short term. After successful modeling, the femoral heads in the
sham operation group were smooth and complete; those in the femoral
head necrosis group were flattened, collapsed and severely damaged;
and those in the BCA group were less damaged than those in the
femoral head necrosis group and showed improvements in deformities.
The results of the present in vivo study suggested that BCA
could alleviate deformation of the femoral head in Perthes disease.
In addition, the immunohistochemical staining of pathological
sections revealed that BCA reduced the expression levels of IL-6 in
femoral head tissue, indicating that BCA may reduce the secretion
of IL-6-EMP and inhibit the endothelial dysfunction induced by
IL-6-EMPs in vivo. In conclusion, BCA can effectively
inhibit endothelial dysfunction in Perthes disease and improve
deformation of the femoral head. Therefore, it may be considered as
a potential drug for the treatment of Perthes disease.
In Perthes disease, IL-6-EMPs can induce endothelial
dysfunction; however, via the NFκB pathway, BCA can inhibit
IL-6-EMPs-induced endothelial dysfunction and reduce the expression
levels of IL-6 in bone tissue following ischemic necrosis of the
femoral head. These findings suggested that BCA may be utilized as
a therapeutic drug for Perthes disease.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant nos. 82060396, 82160809 and 81960768),
the Natural Science Foundation of Guangxi Province (grant nos.
2017GXNSFAA198305, 2018GXNSFBA281090, 2018GXNSFBA138036 and
2020GXNSFAA259088), the ‘Medical Excellence Award’ Funded by the
Creative Research Development Grant from the First Affiliated
Hospital of Guangxi Medical University (grant no. 2022014) and the
Youth Talent Training Program of Guangxi-Collaborative Innovation
Center for Biomedicine (grant no. 0240622005C).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
XD and SL confirm the authenticity of all the raw
data. XD and SL were involved in conceptualization and design of
the methodology. JL and CL performed the experiments. ST, XL, RL
and YL contributed to data curation. BL, QH and XC analyzed the
data. JL wrote the original draft. SL, CL, RL and YL reviewed and
edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiment was approved by the Animal
Care & Welfare Committee of Guangxi Medical University
(approval no. 202111011; Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leroux J, Abu Amara S and Lechevallier J:
Legg-Calvé-Perthes disease. Orthop Traumatol Surg Res.
104:S107–S112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhao Y, Liao S, Lu R, Dang H, Zhao J and
Ding X: Endothelial nitric oxide synthase gene polymorphism is
associated with Legg-Calvé-Perthes disease. Exp Ther Med.
11:1913–1917. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim S, Oh H, Lim J, Cho S and Jung S:
Results of early proximal femoral osteotomy at skeletal maturity in
Legg-Calvé-Perthes disease: Implication for the bypass of
fragmentation stage. J Pediatr Orthop. 41:e768–e773.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Little D, McDonald M, Sharpe I, Peat R,
Williams P and McEvoy T: Zoledronic acid improves femoral head
sphericity in a rat model of perthes disease. J Orthop Res.
23:862–868. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim HKW, Randall TS, Bian H, Jenkins J,
Garces A and Bauss F: Ibandronate for prevention of femoral head
deformity after ischemic necrosis of the capital femoral epiphysis
in immature pigs. J Bone Joint Surg Am. 87:550–557. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Johnson CP, Wang L, Tóth F, Aruwajoye O,
Kirkham B, Carlson CS, Kim HKW and Ellermann JM: Quantitative
susceptibility mapping detects neovascularization of the epiphyseal
cartilage after ischemic injury in a piglet model of
Legg-Calvé-Perthes disease. J Magn Reson Imaging. 50:106–113.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Perry DC, Green DJ, Bruce CE, Pope D,
Dangerfield P, Platt MJ, Hall AJ and Jones H: Abnormalities of
vascular structure and function in children with Perthes disease.
Pediatrics. 130:e126–e131. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kamiya N, Yamaguchi R, Adapala N, Chen E,
Neal D, Jack O, Thoveson A, Gudmundsson P, Brabham C, Aruwajoye O,
et al: Legg-Calvé-Perthes disease produces chronic hip synovitis
and elevation of interleukin-6 in the synovial fluid. J Bone Miner
Res. 30:1009–1013. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kamiya N, Kuroyanagi G, Aruwajoye O and
Kim HKW: IL6 receptor blockade preserves articular cartilage and
increases bone volume following ischemic osteonecrosis in immature
mice. Osteoarthritis Cartilage. 27:326–335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kao CY and Papoutsakis ET: Extracellular
vesicles: Exosomes, microparticles, their parts, and their targets
to enable their biomanufacturing and clinical applications. Curr
Opin Biotechnol. 60:89–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deng F, Wang S and Zhang L: Endothelial
microparticles act as novel diagnostic and therapeutic biomarkers
of circulatory hypoxia-related diseases: A literature review. J
Cell Mol Med. 21:1698–1710. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li B, Huang Q, Lin C, Lu R, Wang T, Chen
X, Liu Z, Liu Y, Wu J, Wu Y, et al: Increased circulating
CD31+/CD42b-EMPs in Perthes disease and inhibit HUVECs angiogenesis
via endothelial dysfunction. Life Sci. 265(118749)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu XY, Zhang YB, Yang XW, Xu W, Liu L,
Zhang P, Gong Y, Liu NF and Peng KF: Simultaneous determination of
twenty-five compounds with anti-inflammatory activity in Spatholobi
Caulis by using an optimized UFLC-MS/MS method: An application to
pharmacokinetic study. J Pharm Biomed Anal.
204(114267)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sarfraz A, Javeed M, Shah M, Hussain G,
Shafiq N, Sarfraz I, Riaz A, Sadiqa A, Zara R, Zafar S, et al:
Biochanin A: A novel bioactive multifunctional compound from
nature. Sci Total Environ. 722(137907)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hsu YN, Shyu HW, Hu TW, Yeh JP, Lin YW,
Lee LY, Yeh YT, Dai HY, Perng DS, Su SH, et al: Anti-proliferative
activity of biochanin A in human osteosarcoma cells via
mitochondrial-involved apoptosis. Food Chem Toxicol. 112:194–204.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yu C, Zhang P, Lou L and Wang Y:
Perspectives regarding the role of biochanin A in humans. Front
Pharmacol. 10(793)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Khanna S, Stewart R, Gnyawali S, Harris H,
Balch M, Spieldenner J, Sen CK and Rink C: Phytoestrogen isoflavone
intervention to engage the neuroprotective effect of glutamate
oxaloacetate transaminase against stroke. FASEB J. 31:4533–4544.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liang F, Cao W, Huang Y, Fang Y, Cheng Y,
Pan S and Xu X: Isoflavone biochanin A, a novel nuclear factor
erythroid 2-related factor 2 (Nrf2)-antioxidant response element
activator, protects against oxidative damage in HepG2 cells.
Biofactors. 45:563–574. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hanski L, Genina N, Uvell H, Malinovskaja
K, Gylfe Å, Laaksonen T, Kolakovic R, Mäkilä E, Salonen J, Hirvonen
J, et al: Inhibitory activity of the isoflavone biochanin A on
intracellular bacteria of genus Chlamydia and initial development
of a buccal formulation. PLoS One. 9(e115115)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jalaludeen AM, Ha WT, Lee R, Kim JH, Do
JT, Park C, Heo YT, Lee WY and Song H: Biochanin A ameliorates
arsenic-induced hepato- and hematotoxicity in rats. Molecules.
21(69)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sangeethadevi G, V V SU, Jansy Isabella
RAR, Saravanan G, Ponmurugan P, Chandrasekaran P, Sengottuvelu S
and Vadivukkarasi S: Attenuation of lipid metabolic abnormalities,
proinflammatory cytokines, and matrix metalloproteinase expression
by biochanin-A in isoproterenol-induced myocardial infarction in
rats. Drug Chem Toxicol. 45:1951–1962. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xue Z, Li A, Zhang X, Yu W, Wang J, Li Y,
Chen K, Wang Z and Kou X: Amelioration of PM2.5-induced
lung toxicity in rats by nutritional supplementation with biochanin
A. Ecotoxicol Environ Saf. 202(110878)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liao S, Feng W, Liu Y, Wang Z, Ding X,
Song F, Lin X, Song H, Kc A, Su Y, et al: Inhibitory effects of
biochanin A on titanium particle-induced osteoclast activation and
inflammatory bone resorption via NF-κB and MAPK pathways. J Cell
Physiol. 236:1432–1444. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kole L, Giri B, Manna SK, Pal B and Ghosh
S: Biochanin-A, an isoflavon, showed anti-proliferative and
anti-inflammatory activities through the inhibition of iNOS
expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB
nuclear translocation. Eur J Pharmacol. 653:8–15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
MacArthur Clark JA and Sun D: Guidelines
for the ethical review of laboratory animal welfare People's
Republic of China National Standard GB/T 35892-2018 [Issued 6
February 2018 Effective from 1 September 2018]. Animal Model Exp
Med. 3:103–113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG: NC3Rs Reporting Guidelines Working Group: Animal
research: Reporting in vivo experiments: the ARRIVE guidelines. J
Gene Med. 12:561–563. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Norman D, Reis D, Zinman C, Misselevich I
and Boss JH: Vascular deprivation-induced necrosis of the femoral
head of the rat. An experimental model of avascular osteonecrosis
in the skeletally immature individual or Legg-Perthes disease. Int
J Exp Pathol. 79:173–181. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu R, Ma C, Li G, Xu J, Feng D and Lan X:
Inhibition of toll-like receptor 4 signaling pathway accelerates
the repair of avascular necrosis of femoral epiphysis through
regulating macrophage polarization in Perthes disease. Tissue Eng
Regen Med. 20:489–501. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hawkins P, Armstrong R, Boden T, Garside
P, Knight K, Lilley E, Seed M, Wilkinson M and Williams RO:
Applying refinement to the use of mice and rats in rheumatoid
arthritis research. Inflammopharmacology. 23:131–150.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Katri A, Dąbrowska A, Löfvall H, Ding M,
Karsdal MA, Andreassen KV, Thudium CS and Henriksen K: Combining
naproxen and a dual amylin and calcitonin receptor agonist improves
pain and structural outcomes in the collagen-induced arthritis rat
model. Arthritis Res Ther. 21(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun H, Zhang H, Li K, Wu H, Zhan X, Fang
F, Qin Y and Wei Y: ESM-1 promotes adhesion between monocytes and
endothelial cells under intermittent hypoxia. J Cell Physiol.
234:1512–1521. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Moreno Grangeiro P, Rodrigues JC, de
Angeli LRA, Leão Filho H, Montenegro NB, Guarniero R, Dempsey M and
Kim HKW: Feasibility of magnetic resonance angiography in patients
with Legg-Calvé-Perthes disease. J Pediatr Orthop. 41:e774–e779.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kumar V, Ali S, Verma V and Singh A: Do
bisphosphonates alter the clinico-radiological profile of children
with Perthes disease? A systematic review and meta-analysis. Eur
Rev Med Pharmacol Sci. 25:4875–4894. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kuroyanagi G, Adapala NS, Yamaguchi R,
Kamiya N, Deng Z, Aruwajoye O, Kutschke M, Chen E, Jo C, Ren Y and
Kim HKW: Interleukin-6 deletion stimulates revascularization and
new bone formation following ischemic osteonecrosis in a murine
model. Bone. 116:221–231. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lugo-Gavidia LM, Burger D, Matthews VB,
Nolde JM, Galindo Kiuchi M, Carnagarin R, Kannenkeril D, Chan J,
Joyson A, Herat LY, et al: Role of microparticles in cardiovascular
disease: Implications for endothelial dysfunction, thrombosis, and
inflammation. Hypertension. 77:1825–1844. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yuana Y, Sturk A and Nieuwland R:
Extracellular vesicles in physiological and pathological
conditions. Blood Rev. 27:31–39. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jimenez JJ, Jy W, Mauro LM, Soderland C,
Horstman LL and Ahn YS: Endothelial cells release phenotypically
and quantitatively distinct microparticles in activation and
apoptosis. Thromb Res. 109:175–180. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu X, Barnum SR, Wohler JE, Schoeb TR and
Bullard DC: Differential ICAM-1 isoform expression regulates the
development and progression of experimental autoimmune
encephalomyelitis. Mol Immunol. 47:1692–1700. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kesanakurti D, Chetty C, Rajasekhar
Maddirela D, Gujrati M and Rao JS: Essential role of cooperative
NF-κB and Stat3 recruitment to ICAM-1 intronic consensus elements
in the regulation of radiation-induced invasion and migration in
glioma. Oncogene. 32:5144–5155. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bonan S, Albrengues J, Grasset E, Kuzet S,
Nottet N, Bourget I, Bertero T, Mari B, Meneguzzi G and Gaggioli C:
Membrane-bound ICAM-1 contributes to the onset of proinvasive tumor
stroma by controlling acto-myosin contractility in
carcinoma-associated fibroblasts. Oncotarget. 8:1304–1320.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kim JY, Kim DH, Kim JH, Lee D, Jeon HB,
Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, et al: Soluble
intracellular adhesion molecule-1 secreted by human umbilical cord
blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell
Death Differ. 19:680–691. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li CH, Liao PL, Shyu MK, Liu CW, Kao CC,
Huang SH, Cheng YW and Kang JJ: Zinc oxide nanoparticles-induced
intercellular adhesion molecule 1 expression requires Rac1/Cdc42,
mixed lineage kinase 3, and c-Jun N-terminal kinase activation in
endothelial cells. Toxicol Sci. 126:162–172. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bui TM, Wiesolek HL and Sumagin R: ICAM-1:
A master regulator of cellular responses in inflammation, injury
resolution, and tumorigenesis. J Leukoc Biol. 108:787–799.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cook-Mills JM, Marchese ME and
Abdala-Valencia H: Vascular cell adhesion molecule-1 expression and
signaling during disease: Regulation by reactive oxygen species and
antioxidants. Antioxid Redox Signal. 15:1607–1638. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Meigs JB, Hu FB, Rifai N and Manson JE:
Biomarkers of endothelial dysfunction and risk of type 2 diabetes
mellitus. JAMA. 291:1978–1986. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dessein PH, Joffe BI and Singh S:
Biomarkers of endothelial dysfunction, cardiovascular risk factors
and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther.
7:R634–R643. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
48
|
Li C, Zhang Y, Liu R and Mai Y: Anagliptin
protected against hypoxia/reperfusion-induced brain vascular
endothelial permeability by increasing ZO-1. ACS Omega.
6:7771–7777. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Urquiza-Fornovi I, Redondo-Alamillos M,
García-Recuero I and Romance-García A: Mandible fracture in a child
with osteogenesis imperfecta on bisphosphonates. Open versus closed
treatment? A case report. Dent Traumatol. 36:692–696.
2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Malmgren B, Tsilingaridis G,
Monsef-Johansson N, Qahtani ZHA, Dahllöf G and Åström E:
Bisphosphonate therapy and tooth development in children and
adolescents with osteogenesis imperfecta. Calcif Tissue Int.
107:143–150. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nasomyont N, Hornung LN and Wasserman H:
Intravenous bisphosphonate therapy in children with spinal muscular
atrophy. Osteoporos Int. 31:995–1000. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen YP, Tan A, Ho WP, Chuang TY, Chen C
and Chen CH: Effectiveness of strontium ranelate in the treatment
of rat model of Legg-Calve-Perthes disease. Indian J Orthop.
52:380–386. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ming X, Ding M, Zhai B, Xiao L, Piao T and
Liu M: Biochanin A inhibits lipopolysaccharide-induced inflammation
in human umbilical vein endothelial cells. Life Sci. 136:36–41.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Oh JS, Cho IA, Kang KR, You JS, Yu SJ, Lee
GJ, Seo YS, Kim CS, Kim DK, Kim SG, et al: Biochanin-A antagonizes
the interleukin-1β-induced catabolic inflammation through the
modulation of NFκB cellular signaling in primary rat chondrocytes.
Biochem Biophys Res Commun. 477:723–730. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li Y, Dong J, Shang Y, Zhao Q, Li P and Wu
B: Anti-inflammatory effects of hederagenin on diabetic
cardiomyopathy via inhibiting NF-κB and Smads signaling pathways in
a type-2 diabetic mice model. RSC Adv. 9:26238–26247.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang J, Wu ML, Cao SP, Cai H, Zhao ZM and
Song YH: Cycloastragenol ameliorates experimental heart damage in
rats by promoting myocardial autophagy via inhibition of
AKT1-RPS6KB1 signaling. Biomed Pharmacother. 107:1074–1081.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li ZH, Shi Z, Tang S, Yao HP, Lin X and Wu
F: Epigallocatechin-3-gallate ameliorates LPS-induced inflammation
by inhibiting the phosphorylation of Akt and ERK signaling
molecules in rat H9c2 cells. Exp Ther Med. 20:1621–1629.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xi Y, Miao Y, Zhou R, Wang M, Zhang F, Li
Y, Zhang Y, Yang H and Guo F: Exploration of the specific pathology
of HXMM tablet against retinal injury based on drug attack model to
network robustness. Front Pharmacol. 13(826535)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB,
Song J, Ding SM, Jia XB and Hu SY: Protection of glycyrrhizic acid
against AGEs-induced endothelial dysfunction through inhibiting
RAGE/NF-κB pathway activation in human umbilical vein endothelial
cells. J Ethnopharmacol. 148:27–36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhu J, Xu Y, Ren G, Hu X, Wang C, Yang Z,
Li Z, Mao W and Lu D: Tanshinone IIA Sodium sulfonate regulates
antioxidant system, inflammation, and endothelial dysfunction in
atherosclerosis by downregulation of CLIC1. Eur J Pharmacol.
815:427–436. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Li MT, Ke J, Guo SF, Wu Y, Bian YF, Shan
LL, Liu QY, Huo YJ, Guo C, Liu MY, et al: The protective effect of
quercetin on endothelial cells injured by hypoxia and
reoxygenation. Front Pharmacol. 12(732874)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yamaguchi R, Kamiya N, Kuroyanagi G, Ren Y
and Kim HKW: Development of a murine model of ischemic
osteonecrosis to study the effects of aging on bone repair. J
Orthop Res. 39:2663–2670. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kamiya N, Yamaguchi R, Aruwajoye O,
Adapala NS and Kim HKW: Development of a mouse model of ischemic
osteonecrosis. Clin Orthop Relat Res. 473:1486–1498.
2015.PubMed/NCBI View Article : Google Scholar
|