Introduction
Urinary tract infections (UTIs) are an extensive and
pervasive health concern, ranking among the most common and
recurring bacterial infections, impacting a vast number of
individuals worldwide each passing year (1-4).
From 1990 to 2019, the total cases of UTIs surged by 60.40%, rising
from 252.25 million to 404.61 million (2). These infections affect people of all
ages, backgrounds, and geographical locations, representing a
substantial burden on healthcare systems and impacting the overall
well-being of affected populations (5,6).
Given the wide-ranging prevalence of UTIs, a
comprehensive exploration of their epidemiology and underlying
factors becomes of paramount necessity (2,7).
Investigating the seasonal distribution of UTIs can shed light on
potential environmental and climatic influences, helping identify
periods of heightened risk and enabling more proactive public
health interventions (8,9). Furthermore, understanding the
sex-specific patterns of UTI occurrence can provide valuable
insights into the complex interplay between anatomical differences,
hormonal factors and behavioral aspects that may contribute to
susceptibility (10,11).
Age-related distribution of bacterial pathogens in
UTIs is another crucial area of investigation (12). Age has been associated with the
incidence of UTIs and with treatment failure of UTIs in multiple
observational studies, sometimes yielding conflicting results
(13-15).
Therefore, recognizing how UTI incidence varies across different
age groups can aid in the development of targeted prevention
strategies and tailor-made treatment approaches, considering the
unique physiological and immunological characteristics of specific
populations (16,17).
A heightened understanding of the factors
contributing to the development and spread of UTIs can pave the way
for the implementation of effective public health measures,
including educational campaigns to raise awareness about preventive
practices and hygiene habits (18). Community-wide efforts can thus
foster a culture of proactive health management, ultimately
reducing the incidence of UTIs and improving the overall quality of
life for individuals worldwide (2,19).
However, the distribution of bacterial pathogens in
UTIs is not well understood, especially regarding seasonal,
sex-specific and age-related patterns, creating a crucial knowledge
gap. The present study aimed to address this gap by investigating
the prevalence and distribution of UTI bacterial pathogens in
hospitalized cases, and exploring their complex interaction with
various demographic characteristics. The valuable insights gained
from this research are expected to result in more effective
diagnosis, treatment and prevention strategies. With this
knowledge, the impact of UTIs can be proactively mitigated,
ultimately promoting better health outcomes and well-being for
individuals.
Patients and methods
Study design
This study employed a retrospective observational
design to investigate the seasonal, sex-specific and age-related
distribution of bacterial pathogens in UTIs. A total of 926
consecutive patients diagnosed with UTIs in Fuding Hospital (Fujian
University of Traditional Chinese Medicine, Fuding, China) were
enrolled. The patients included 343 men and 583 women diagnosed
with UTIs. The data collection period spanned from July 1, 2022, to
June 30, 2023. Data from electronic medical records and laboratory
reports were collected and analyzed to identify patterns of
bacterial prevalence.
Inclusion and exclusion criteria
Inclusion criteria were as follows: Participants
with three urine samples exhibiting >105
colony-forming units (CFU)/ml were included. Clean catch midstream
urine samples were obtained following proper instructions.
Exclusion criteria included participants with inflammatory
conditions unrelated to UTIs, those with cancer and pregnant
patients.
Data collection and outcome
measures
Data were obtained from 926 patients diagnosed with
UTIs in Fuding Hospital between July 1, 2022, and June 30, 2023.
Demographic information, including age and sex, was extracted from
electronic health records. Additionally, information on the date of
UTI diagnosis was recorded to categorize cases according to
seasonal variations.
Urine samples were obtained through either bladder
catheterization or the midstream clean catch method. The urine
specimens for routine urinalysis were tested using a Fully
Automated Urine Chemistry Analyzer UC-3500 and a Partical Analyzer
UF-5000 (Sysmex Corporation). Pyuria was defined as the presence of
>5 white blood cells per high-power field in the urine sediment,
and significant bacteriuria was identified as the growth of a
single pathogenic microorganism >100,000 CFU per ml of urine
(20). Patients exhibiting pyuria
in the urine analysis underwent a urine culture to confirm the
presence of a UTI and detect the causative organism. Cultures were
performed using cysteine, lactose, and electrolyte-deficient agar.
The culture was considered positive when it detected the presence
of at ≥50,000 CFUs per ml of a single urinary pathogen (21). Bacterial isolates derived from
urine cultures underwent identification processes that integrated
conventional microbiological techniques with the Microbial
Analyzing System of VITEKR 2 COMPACT (BioMérieux, Inc.)
(22). This comprehensive approach
involved methodologies such as Gram staining and biochemical tests.
For Gram staining, a standard procedure was followed at room
temperature. Bacterial smears were first fixed to a slide by
passing through a flame. The slide was then stained with crystal
violet for 1 min, followed by the application of iodine solution
for 1 min. After a brief rinse with water, the slide was
decolorized with alcohol for 20 sec and then counterstained with
safranin for 30 sec. The slides were observed under a light
microscope at x1,000 magnification. The classification of bacterial
species into either Gram-positive or Gram-negative groups was
established based on their unique responses to Gram staining.
The reference strains (Escherichia coli, ATCC
25922; Staphylococcus aureus, ATCC 25923; Pseudomonas
aeruginosa, ATCC 27853; and Enterococcus faecalis, ATCC
29212) were utilized as positive controls and were all obtained
from Fujian Provincial Inspection Center of China.
Classification based on season, sex
and age
To examine the seasonal distribution of bacterial
pathogens, UTI cases were categorized into four seasons: Spring,
summer, autumn and winter, based on the date of UTI diagnosis and
corresponding calendar months.
Patients were classified into two sex groups: Male
and female, based on their recorded sex in the electronic health
records.
Age-related distribution of bacterial pathogens was
explored by categorizing patients into four groups: Age group 1,
0-49 years; age group 2, 50-69 years; age group 3, 70-79 years; and
age group 4, ≥80 years.
Ethics
The present study was conducted in accordance with
ethical guidelines and received approval from the Medical Ethics
Committee of Fuding Hospital, Fujian University of Traditional
Chinese Medicine (approval no. Fuding Hospital 2022325). The
requirement for written informed consent was waived by the Medical
Ethics Committee of Fuding Hospital, Fujian University of
Traditional Chinese Medicine, due to the retrospective nature of
the study.
Statistical analysis
Statistical analysis was conducted using SPSS
version 22.0 software (IBM Corp.) and GraphPad Prism 8.0
(Dotmatics). Count data were expressed as n (%). Data normality was
assessed using the Kolmogorov-Smirnov test. For non-normally
distributed measurements, medians and interquartile ranges were
reported. Non-parametric tests were analyzed with the
Kruskal-Wallis H test for multiple groups. χ2 tests or
Fisher's exact tests, as appropriate, were used to determine the
significance of differences in bacterial prevalence across
different seasons, sexes and age groups. P<0.05 was considered
to indicate a statistically significant difference.
To address the issue of multiple comparisons within
the four groups, the Bonferroni correction was applied as a
post-hoc analysis after initial tests (Kruskal-Wallis H test and
χ2 tests) to indicate significant differences. With six
possible pairwise comparisons (4 groups choose 2), the adjusted
significance level (ɑadjusted) was calculated as ɑ/6 ≈
0.0083(23).
Results
The age distribution of the subjects in the four
seasonal groups was tested using the Kolmogorov-Smirnov test,
yielding results of Z=0.118-0.140, all P<0.001. These results
indicated a non-normal distribution of age data, prompting the use
of the non-parametric Kruskal-Wallis H test for statistical
analysis. Additionally, a χ2 test was performed to
compare sex distribution among the four groups. The results of both
the sex and age tests are documented in Table I. The statistical analysis revealed
that there was no statistically significant difference in age or
sex distribution among the four seasonal groups (P>0.05).
 | Table IClinical parameters of the four
seasonal groups in the study. |
Table I
Clinical parameters of the four
seasonal groups in the study.
| Parameter | Spring | Summer | Autumn | Winter |
Kruskal-Wallis/χ2 test | P-value |
|---|
| Median age (IQR),
years | 70.0
(57.0-78.0) | 68.0
(58.3-76.8) | 69.0
(57.0-75.0) | 69.0
(57.0-79.0) | 2.526 | 0.471 |
| Sex, n | 246 | 212 | 273 | 195 | 1.751 | 0.626 |
| Male, n (%)
(n=343) | 88 (35.8) | 79 (37.3) | 109 (39.9) | 67 (34.4) | | |
| Female, n (%)
(n=583) | 158 (64.2) | 133 (62.7) | 164 (60.1) | 128 (65.6) | | |
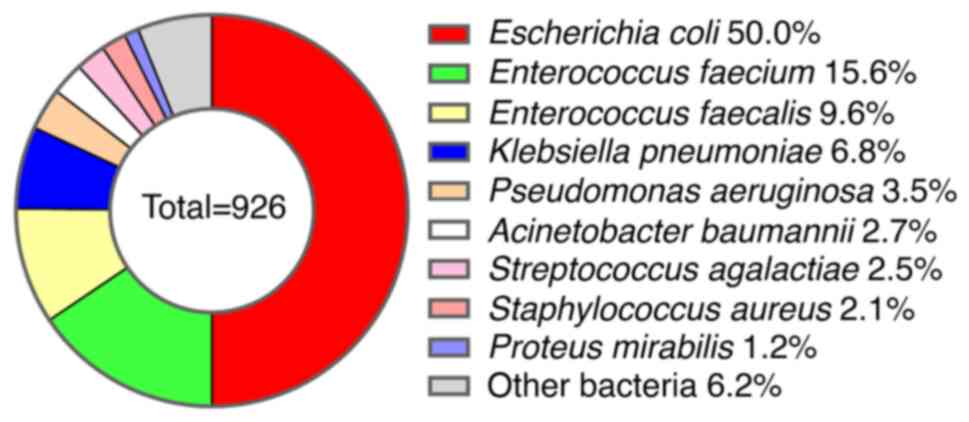
A total of 926 bacterial strains were isolated from
the studied patients, with 280 (30.2%) belonging to Gram-positive
bacteria and 646 (69.8%) to Gram-negative bacteria. Among these,
the top nine pathogenic bacteria were identified as follows: E.
coli (50.0%), Enterococcus faecium (15.6%), E.
faecalis (9.6%), Klebsiella pneumoniae (6.8%), P.
aeruginosa (3.5%), A. baumannii (2.7%), Streptococcus
agalactiae (2.5%), S. aureus (2.1%) and Proteus
mirabilis (1.2%). The remaining bacterial species accounted for
6.2% of the total isolates (Fig.
1).
Further analysis of specific bacterial species
revealed associations with the different seasons: E. faecium
demonstrated a substantial prevalence during the spring season,
with 22.0% of isolates (P=0.005); A. baumannii displayed a
notable association with the autumn season, accounting for 5.9% of
isolates (P=0.001) (Table
II).
 | Table IIAssociation between season and
distribution of bacteria (n=926) in urinary tract infections. |
Table II
Association between season and
distribution of bacteria (n=926) in urinary tract infections.
| Bacteria | Spring (n=246) | Summer (n=212) | Autumn (n=273) | Winter (n=195) | χ2
test | P-value |
|---|
| Gram-positive, n
(%) | 87 (35.4) | 70 (33.0) | 68 (24.9) | 50 (25.6) | 9.442 | 0.024 |
|
Enterococcus
faecium, n (%) | 54 (22.0) | 32 (15.1) | 29 (10.6) | 29 (14.9) | 12.824 | 0.005b |
|
Enterococcus
faecalis, n (%) | 20 (8.1) | 25 (11.8) | 29 (10.6) | 15 (7.7) | 2.930 | 0.403 |
|
Streptococcus
agalactiae, n (%) | 4 (1.6) | 10 (4.7) | 6 (2.2) | 3 (1.5) | 4.993 | 0.162a |
|
Staphylococcus
aureus, n (%) | 9 (3.7) | 3 (1.4) | 4 (1.5) | 3 (1.5) | 3.593 | 0.302a |
| Gram-negative, n
(%) | 159 (64.4) | 142 (67.0) | 205 (75.1) | 145 (74.4) | 9.442 | 0.024 |
|
Escherichia
coli, n (%) | 130 (52.8) | 99 (46.7) | 127 (46.5) | 107 (54.9) | 4.895 | 0.180 |
|
Klebsiella
pneumoniae, n (%) | 17 (6.9) | 8 (3.8) | 24 (8.8) | 14 (7.2) | 4.819 | 0.187 |
|
Pseudomonas
aeruginosa, n (%) | 7 (2.8) | 5 (2.4) | 14 (5.1) | 6 (3.1) | 3.412 | 0.342 |
|
Acinetobacter
baumannii, n (%) | 1 (0.4) | 5 (2.4) | 16 (5.9) | 3 (1.5) | 16.404 | 0.001b |
| Other bacteria n
(%) | 4 (1.6) | 25 (11.8) | 24 (8.8) | 15 (7.7) | 4.926 | 0.260 |
A total of 926 hospitalized subjects were enrolled
in the study, including 343 (37.0%) men and 583 (63.0%) women.
Gram-positive bacteria were found to be more prevalent in men
compared with women (35.9 vs. 26.9%; P=0.004). Particularly, E.
faecalis and S. aureus showed significant sex
differences, being more common in men (12.5%, P=0.021; and 4.4%,
P<0.001, respectively). Furthermore, among Gram-negative
bacteria, P. aeruginosa and A. baumannii demonstrated
notable sex disparities, with a higher prevalence in men compared
with women (7.4%, P=0.001; and 5.0%, P=0.001, respectively).
Conversely, E. coli exhibited a higher prevalence in women
compared with men (60.4%, P<0.001) (Table III).
 | Table IIIAssociation between sex and bacterial
distribution in urinary tract infections. |
Table III
Association between sex and bacterial
distribution in urinary tract infections.
| Microorganisms | Infection
Status | Male group, n (%)
(n=343) | Female group, n (%)
(n=583) | χ2
test |
P-valuea |
|---|
| Gram-positive
bacteria | Yes | 123 (35.9) | 157 (26.9) | 8.164 | 0.004b |
| | No | 220 (64.1) | 426 (73.1) | | |
|
Enterococcus
faecium | Yes | 56 (16.3) | 88 (15.1) | 0.250 | 0.617 |
| | No | 287 (83.7) | 495 (84.9) | | |
|
Enterococcus
faecalis | Yes | 43 (12.5) | 46 (7.9) | 5.366 | 0.021b |
| | No | 300 (87.5) | 537 (92.1) | | |
|
Staphylococcus
aureus | Yes | 15 (4.4) | 4 (0.7) | 14.607 |
<0.001b |
| | No | 328 (95.6) | 579 (99.3) | | |
|
Streptococcus
agalactiae | Yes | 7 (2.0) | 16 (2.7) | 0.441 | 0.506 |
| | No | 336 (98.0) | 567 (97.3) | | |
|
Enterococcus
psudoavium | Yes | 2 (0.6) | 3 (0.5) | 0.000 | 1.000c |
| | No | 341 (99.4) | 580 (99.5) | | |
| Gram-negative
bacteria | Yes | 220 (64.1) | 426 (73.1) | 8.164 | 0.004b |
| | No | 123 (35.9) | 157 (26.9) | | |
|
Escherichia
coli | Yes | 111 (32.4) | 352 (60.4) | 67.798 |
<0.001b |
| | No | 232 (67.6) | 231 (39.6) | | |
|
Klebsiella
pneumoniae | Yes | 30 (8.7) | 33 (5.7) | 3.243 | 0.072 |
| | No | 313 (91.3) | 550 (94.3) | | |
|
Pseudomonas
aeruginosa | Yes | 18 (5.2) | 14 (2.4) | 5.244 | 0.022b |
| | No | 325 (94.8) | 569 (97.6) | | |
|
Acinetobacter
baumannii | Yes | 17 (5.0) | 8(1.4) | 10.560 |
<0.001b |
| | No | 326 (95.0) | 575(98.6) | | |
|
Proteus
mirabilis | Yes | 6 (1.7) | 5 (0.9) | 1.463 | 0.227 |
| | No | 337 (98.3) | 578 (99.1) | | |
| Other bacteria | Yes | 38 (11.1) | 14 (2.4) | 30.678 |
<0.001b |
| | No | 305 (88.9) | 569 (97.6) | | |
The data in Table
IV revealed associations between age groups and the prevalence
of bacterial pathogens in UTIs. Gram-positive bacteria demonstrated
a significant increase in prevalence in the older age groups (age
groups 3 and 4) (≥70 years old) (P=0.005), indicating that these
bacterial species are more commonly found in elderly patients.
Additionally, E. faecium exhibited notable age-related
associations (P<0.001), being more prevalent in age groups 3 and
4 (≥70 years old) (18.4 and 23.8%) compared with that in the other
age groups.
 | Table IVAssociation between age and
distribution of bacteria (n=926) in urinary tract infections. |
Table IV
Association between age and
distribution of bacteria (n=926) in urinary tract infections.
| Bacteria | Age group 1 (≤50
years; n=128) | Age group 2 (50-69
years; n=351) | Age group 3 (70-80
years; n=283) | Age group 4 (>80
years; n=164) | χ2
test |
P-valuea |
|---|
| Gram-positive
(n=275), n (%) | 43 (33.6) | 80 (22.8) | 95 (33.6) | 57 (34.8) | 12.989 | 0.005b |
|
Enterococcus
faecium, n (%) | 18 (14.1) | 36 (10.3) | 52 (18.4) | 39 (23.8) | 17.775 |
<0.001b |
|
Enterococcus
faecalis, n (%) | 13 (10.2) | 33 (9.4) | 27 (9.5) | 15 (9.1) | 0.092 | 0.993 |
|
Streptococcus
agalactiae, n (%) | 5 (3.9) | 8 (2.3) | 8 (2.8) | 2 (1.2) | 2.350 | 0.503 |
| Gram-negative
(n=651), n (%) | 85 (66.4) | 271 (77.2) | 188 (66.4) | 107 (65.2) | 17.775 |
<0.001b |
|
Escherichia
coli, n (%) | 63(49.2) | 201 (57.3) | 126 (44.5) | 73 (44.5) | 12.813 | 0.005b |
|
Klebsiella
pneumoniae, n (%) | 7 (5.5) | 25 (7.1) | 16 (5.7) | 15 (9.1) | 2.426 | 0.489 |
|
Pseudomonas
aeruginosa, n (%) | 5 (3.9) | 11 (3.1) | 10 (3.5) | 6 (3.7) | 0.212 | 0.976 |
|
Acinetobacter
baumannii, n (%) | 5 (3.9) | 10 (2.8) | 10 (3.5) | 0 (0.0) | 6.038 | 0.110 |
|
Proteus
mirabilis, n (%) | 1 (0.8) | 6 (1.7) | 4 (1.4) | 0 (0.0) | 3.088 | 0.378 |
| Other bacteria,
n | 11 (8.6) | 21 (6.0) | 30 (10.6) | 14 (8.5) | 4.506 | 0.210 |
Among the Gram-negative bacteria, E. coli
showed a significant age association (P=0.005). Notably, a higher
prevalence was observed in younger age groups, with the highest
proportion in the 50-69 years age group (57.3%) and ≤50 years age
group (49.2%) (Table IV).
The findings in Table
IV suggested that the prevalence of certain bacterial pathogens
in UTIs was influenced by the age of the patients. Gram-positive
bacteria, E. faecium tended to be more prevalent in older
patients, while E. coli was more common in younger
patients.
Discussion
The present study sheds light on the distribution of
bacterial pathogens in UTIs concerning seasonality, sex, and age
factors. The predominant pathogenic bacteria identified were E.
coli (50.0%), E. faecium (15.6%), E. faecalis
(9.6%), K. pneumoniae (6.8%), P. aeruginosa (3.5%),
A. baumannii (2.7%), S. agalactiae (2.5%), S.
aureus (2.1%) and P. mirabilis (1.2%). These findings
align with those of previous studies (24-26).
Another similar study by Faine et al (27) showed that among the patients with
positive urine cultures, 84.7% had cultures that grew
Enterobacterales, with E. coli (63.2%) being the most common
pathogen isolated. Pathogenic E. coli was regarded as the
most common pathogen of UTIs (27-30).
The present analysis of seasonal distribution revealed a
significant association between specific bacterial species and
particular seasons. Notably, E. faecium showed higher
prevalence during the spring and A. baumannii during the
autumn. These findings aligned with a previous study by Alrashid
et al (31), which also
demonstrated a seasonal pattern, with the highest number of
confirmed UTIs in January and the lowest in April. These results
emphasized the seasonal variations in UTIs and suggested that the
prevalence of bacterial pathogens was influenced by seasonal
factors (9). This complexity adds
to our understanding of UTIs and highlights the need to consider
seasonal variations while devising diagnostic and preventive
strategies, as corroborated by the previous study by Simmering
et al (8).
The study enrolled a total of 926 hospitalized
subjects, with 343 (37.0%) men and 583 (63.0%) women, indicating a
higher incidence of UTIs in women, which is consistent with
previous studies (5,32,33).
UTIs were much more common in women than in men due to certain
anatomical and physiological characteristics, such as a shorter
urethra, the proximity of the urethral opening to the anus, changes
during menopause, pregnancy and other factors, with this difference
increasing with age (34,35). The analysis of sex distribution
revealed noteworthy differences in the prevalence of bacterial
pathogens between male and female groups. Men with UTIs exhibited a
greater susceptibility to Gram-positive bacterial infections
compared with their female counterparts (35.9 vs. 26.9%; P=0.004).
E. faecalis, S. aureus, P. aeruginosa and A.
baumannii showed a significant sex difference, being more
common in men. Silva et al (36) observed a higher prevalence of UTIs
caused by E. faecalis and P. aeruginosa in men
compared with women (8.8% for E. faecalis and 8.1% for P.
aeruginosa in men, compared with 1.8 and 1.6% in females,
respectively). A previous study conducted by Magliano et al
(37) demonstrated that P.
aeruginosa was found more frequently in men, whereas the
prevalence of E. faecalis aligned with the findings of the
current study. In the present study, the occurrence of E.
faecalis in men and women was 12.5 vs. 7.9% (P<0.05),
compared with 9.5 vs. 5.4% (P<0.05) as reported by Magliano
et al (37). E.
coli. demonstrated notable sex disparities, with a higher
prevalence in women compared with men (P<0.001). Amna et
al (38) determined that
non-E. coli bacteria were more prone to infect men, citing
the increased complexity of UTIs in men, often attributed to the
frequent use of catheters (39,40).
Specifically, Enterococcus and Pseudomonas have been
linked to infections associated with urinary tract catheters
(41,42). These findings shed light on the
intricate interplay between biological, anatomical and behavioral
factors in UTI prevalence.
The higher occurrence of Gram-positive bacteria in
men suggests the possibility of sex-related disparities in immunity
or physiological factors that may render men more susceptible to
UTIs (11,43). The finding of the present study,
showing a rise in S. aureus among men, aligns with the
research conducted by Stokes et al (44), which demonstrated a higher
prevalence of S. aureus in men, particularly in older age
groups with comorbidities. Understanding the sex-specific
variations in bacterial prevalence holds promise for targeted
interventions and tailored awareness campaigns for each sex group
(45). By addressing the unique
factors influencing UTIs in men and women, progress can be made
towards reducing UTI rates and enhancing overall health outcomes
(46).
The present investigation into the age distribution
of bacterial pathogens revealed significant associations between
certain species and different age groups. The study highlighted
that Gram-positive bacteria, especially E. faecium,
exhibited a marked increase in prevalence among elderly individuals
with UTIs. These findings were consistent with those of previous
studies (47,48). The notable age-related associations
observed for E. coli in the present study demonstrated
higher prevalence in younger individuals with UTIs. These
age-related patterns of bacterial pathogens add another layer of
complexity to our understanding of UTIs. The substantial changes in
bacteria prevalence of UTIs with age emphasize the importance of
age-specific management strategies leading to better healthcare
outcomes (49,50).
The retrospective design, the single tertiary
hospital setting, and the lack of detailed information on UTI types
are limitations of the present study. Future research with
prospective designs and multicenter collaborations through a
focused analysis of UTI types is a proactive way to address these
limitations and enhance the overall understanding of UTI
epidemiology.
In conclusion, the present study provided a
comprehensive exploration of UTIs, revealing intricate patterns in
bacterial pathogen distribution concerning seasonality, sex, and
age. E. coli was the predominant pathogen among UTIs.
Significant associations between bacterial species and seasons were
observed, with E. faecium prevalent in the spring and A.
baumannii in the autumn. Notable sex-specific differences were
found in bacterial prevalence, with S. aureus and E.
faecalis found more frequently in men. Additionally,
age-related associations revealed significant dynamics, with E.
faecium exhibiting a marked increase in prevalence with
advancing age, while E. coli had a higher prevalence in
younger individuals with UTIs. Future research with prospective
designs and multicenter collaborations aims to enhance our
understanding of UTI epidemiology.
Acknowledgements
Not applicable.
Funding
Funding: Financial support was provided by the Natural Science
Foundation Joint Project of Ningde, Ningde, China (grant no.
2022J54).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author upon reasonable
request.
Authors' contributions
ZSZhan and JS contributed equally to this work, and
were involved in the conception and design of the study, data
collection, analysis and interpretation of data, and drafting the
manuscript. ZSZheng contributed to the design of the study, data
analysis and critically revised the manuscript for important
intellectual content. XXZ contributed reagents, materials, analysis
tools and data, and analyzed and interpreted the data. JC and XYZ
played significant roles in data interpretation, and provided
substantial contributions to the manuscript revision. SYZ was
responsible for the study concept, analyzed and interpreted the
data, wrote and analyzed the methods, and reviewed and edited the
manuscript. XXZ and XYZ confirm the authenticity of all the raw
data. All the authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of Fuding Hospital, Fujian University of Traditional
Chinese Medicine (Fuding, China; approval no. Fuding Hospital
2022325). All methods were conducted according to relevant
guidelines and regulations. The requirement for informed consent
from participants was waived by the Medical Ethics Committee of
Fuding Hospital, Fujian University of Traditional Chinese Medicine,
due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim DS and Lee JW: Urinary tract infection
and microbiome. Diagnostics (Basel). 13(1921)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang X, Chen H, Zheng Y, Qu S, Wang H and
Yi F: Disease burden and long-term trends of urinary tract
infections: A worldwide report. Front Public Health.
10(888205)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duicu C, Cozea I, Delean D, Aldea AA and
Aldea C: Antibiotic resistance patterns of urinary tract pathogens
in children from Central Romania. Exp Ther Med.
22(748)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mancuso G, Midiri A, Gerace E, Marra M,
Zummo S and Biondo C: Urinary tract infections: The current
scenario and future prospects. Pathogens. 12(623)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chieng CCY, Kong Q, Liou NSY, Khasriya R
and Horsley H: The clinical implications of bacterial pathogenesis
and mucosal immunity in chronic urinary tract infection. Mucosal
Immunol. 16:61–71. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Medina M and Castillo-Pino E: An
introduction to the epidemiology and burden of urinary tract
infections. Ther Adv Urol. 11(1756287219832172)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Öztürk R and Murt A: Epidemiology of
urological infections: A global burden. World J Urol. 38:2669–2679.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Simmering JE, Polgreen LA, Cavanaugh JE,
Erickson BA, Suneja M and Polgreen PM: Warmer weather and the risk
of urinary tract infections in women. J Urol. 205:500–506.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Simmering JE, Cavanaugh JE, Polgreen LA
and Polgreen PM: Warmer weather as a risk factor for
hospitalisations due to urinary tract infections. Epidemiol Infect.
146:386–393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Soudais B, Ribeaucoup F and Schuers M:
Guidelines for the management of male urinary tract infections in
primary care: A lack of international consensus-a systematic review
of the literature. Fam Pract. 40:152–175. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deltourbe L, Lacerda Mariano L, Hreha TN,
Hunstad DA and Ingersoll MA: The impact of biological sex on
diseases of the urinary tract. Mucosal Immunol. 15:857–866.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cristina ML, Spagnolo AM, Giribone L,
Demartini A and Sartini M: Epidemiology and prevention of
healthcare-associated infections in geriatric patients: A narrative
review. Int J Environ Res Public Health. 18(5333)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Little P, Merriman R, Turner S, Rumsby K,
Warner G, Lowes JA, Smith H, Hawke C, Leydon G, Mullee M and Moore
MV: Presentation, pattern, and natural course of severe symptoms,
and role of antibiotics and antibiotic resistance among patients
presenting with suspected uncomplicated urinary tract infection in
primary care: Observational study. BMJ. 340(b5633)2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gharbi M, Drysdale JH, Lishman H, Goudie
R, Molokhia M, Johnson AP, Holmes AH and Aylin P: Antibiotic
management of urinary tract infection in elderly patients in
primary care and its association with bloodstream infections and
all cause mortality: Population based cohort study. BMJ.
364(l525)2019.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Goettsch WG, Janknegt R and Herings RMC:
Increased treatment failure after 3-days' courses of nitrofurantoin
and trimethoprim for urinary tract infections in women: A
population-based retrospective cohort study using the PHARMO
database. Br J Clin Pharmacol. 58:184–189. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mengistu DA, Alemu A, Abdukadir AA,
Mohammed Husen A, Ahmed F and Mohammed B: Incidence of urinary
tract infection among patients: Systematic review and
meta-analysis. Inquiry. 60(469580231168746)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang L, Huang C, Yan Y, Sun L and Li H:
Urinary tract infection etiological profiles and antibiotic
resistance patterns varied among different age categories: A
retrospective study from a tertiary general hospital during a
12-year period. Front Microbiol. 12(813145)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eslami V, Sany SBT, Tehrani H, Ghavami V
and Peyman N: Examining health literacy and self-efficacy levels
and their association with preventive behaviors of urinary tract
infection in Iranian pregnant women: Across sectional study. BMC
Womens Health. 23(258)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lean K, Nawaz RF, Jawad S and Vincent C:
Reducing urinary tract infections in care homes by improving
hydration. BMJ Open Qual. 8(e000563)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Prasada Rao CMM, Vennila T, Kosanam S,
Ponsudha P, Suriyakrishnaan K, Alarfaj AA, Hirad AH, Sundaram SR,
Surendhar PA and Selvam N: Assessment of Bacterial isolates from
the urine specimens of urinary tract infected patient. Biomed Res
Int. 2022(4088187)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Baz AMK, El-Agamy OAE and Ibrahim AM:
Incidence of urinary tract infection in neonates with significant
indirect Hyperbilirubinemia of unknown etiology: Case-control
study. Ital J Pediatr. 47(35)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gajdács M, Bátori Z, Ábrók M, Lázár A and
Burián K: Characterization of resistance in gram-negative urinary
isolates using existing and novel indicators of clinical relevance:
A 10-year data analysis. Life (Basel). 10(16)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee S and Lee DK: What is the proper way
to apply the multiple comparison test? Korean J Anesthesiol.
71:353–360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Islam MA, Islam MR, Khan R, Amin MB,
Rahman M, Hossain MI, Ahmed D, Asaduzzaman M and Riley LW:
Prevalence, etiology and antibiotic resistance patterns of
community-acquired urinary tract infections in Dhaka, Bangladesh.
PLoS One. 17(e0274423)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ullah H, Bashir K, Idrees M, Ullah A,
Hassan N, Khan S, Nasir B, Nadeem T, Ahsan H, Khan MI, et al:
Phylogenetic analysis and antimicrobial susceptibility profile of
uropathogens. PLoS One. 17(e0262952)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shi J, Zhan ZS, Zheng ZS, Zhu XX, Zhou XY
and Zhang SY: Correlation of procalcitonin and c-reactive protein
levels with pathogen distribution and infection localization in
urinary tract infections. Sci Rep. 13(17164)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Faine BA, Rech MA, Vakkalanka P, Gross A,
Brown C, Harding SJ, Slocum G, Zimmerman D, Zepeski A, Rewitzer S,
et al: High prevalence of fluoroquinolone-resistant UTI among US
emergency department patients diagnosed with urinary tract
infection, 2018-2020. Acad Emerg Med. 29:1096–1105. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
FarajzadehSheikh A, Veisi H, Shahin M,
Getso M and Farahani A: Frequency of quinolone resistance genes
among extended-spectrum β-lactamase (ESBL)-producing Escherichia
coli strains isolated from urinary tract infections. Trop Med
Health. 47(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Asadi Karam MR, Habibi M and Bouzari S:
Urinary tract infection: Pathogenicity, antibiotic resistance and
development of effective vaccines against Uropathogenic
Escherichia coli. Mol Immunol. 108:56–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zagaglia C, Ammendolia MG, Maurizi L,
Nicoletti M and Longhi C: Urinary tract infections caused by
Uropathogenic Escherichia coli strains-new strategies for an
old pathogen. Microorganisms. 10(1425)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Alrashid S, Ashoor R, Alruhaimi S, Hamed
A, Alzahrani S and Al Sayyari A: Urinary tract infection as the
diagnosis for admission through the emergency department: Its
prevalence, seasonality, diagnostic methods, and diagnostic
decisions. Cureus. 14(e27808)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Elser H, Rowland ST, Tartof SY, Parks RM,
Bruxvoort K, Morello-Frosch R, Robinson SC, Pressman AR, Wei RX and
Casey JA: Ambient temperature and risk of urinary tract infection
in California: A time-stratified case-crossover study using
electronic health records. Environ Int. 165(107303)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rădulescu D, David C, Turcu FL, Spătaru
DM, Popescu P and Văcăroiu IA: Combination of cranberry extract and
D-mannose-possible enhancer of uropathogen sensitivity to
antibiotics in acute therapy of urinary tract infections: Results
of a pilot study. Exp Ther Med. 20:3399–3406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Foxman B: Urinary tract infection
syndromes: Occurrence, recurrence, bacteriology, risk factors, and
disease burden. Infect Dis Clin North Am. 28:1–13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Czajkowski K, Broś-Konopielko M and
Teliga-Czajkowska J: Urinary tract infection in women. Prz
Menopauzalny. 20:40–47. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Silva A, Costa E, Freitas A and Almeida A:
Revisiting the frequency and antimicrobial resistance patterns of
bacteria implicated in community urinary tract infections.
Antibiotics (Basel). 11(768)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Magliano E, Grazioli V, Deflorio L, Leuci
AI, Mattina R, Romano P and Cocuzza CE: Gender and age-dependent
etiology of community-acquired urinary tract infections.
ScientificWorldJournal. 2012(349597)2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Amna MA, Chazan B, Raz R, Edelstein H and
Colodner R: Risk factors for non-Escherichia coli
community-acquired bacteriuria. Infection. 41:473–477.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Prieto J, Wilson J, Bak A, Denton A,
Flores A, Lusardi G, Reid M, Shepherd L, Whittome N and Loveday H:
A prevalence survey of patients with indwelling urinary catheters
on district nursing caseloads in the United Kingdom: The community
urinary catheter management (CCaMa) study. J Infect Prev.
21:129–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shackley DC, Whytock C, Parry G, Clarke L,
Vincent C, Harrison A, John A, Provost L and Power M: Variation in
the prevalence of urinary catheters: A profile of national health
service patients in England. BMJ Open. 7(e013842)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gaston JR, Andersen MJ, Johnson AO, Bair
KL, Sullivan CM, Guterman LB, White AN, Brauer AL, Learman BS,
Flores-Mireles AL and Armbruster CE: Enterococcus faecalis
polymicrobial interactions facilitate biofilm formation, antibiotic
recalcitrance, and persistent colonization of the catheterized
urinary tract. Pathogens. 9(835)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lara-Isla A, Medina-Polo J, Alonso-Isa M,
Benítez-Sala R, Sopeña-Sutil R, Justo-Quintas J, Gil-Moradillo J,
González-Padilla DA, García-Rojo E, Passas-Martínez JB and
Tejido-Sánchez Á: Urinary infections in patients with catheters in
the upper urinary tract: Microbiological study. Urol Int.
98:442–448. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gajdács M, Ábrók M, Lázár A and Burián K:
Increasing relevance of Gram-positive cocci in urinary tract
infections: A 10-year analysis of their prevalence and resistance
trends. Sci Rep. 10(17658)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Stokes W, Parkins MD, Parfitt ECT, Ruiz
JC, Mugford G and Gregson DB: Incidence and outcomes of
Staphylococcus aureus bacteriuria: A population-based study.
Clin Infect Dis. 69:963–969. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zychlinsky Scharff A, Rousseau M, Lacerda
Mariano L, Canton T, Consiglio CR, Albert ML, Fontes M, Duffy D and
Ingersoll MA: Sex differences in IL-17 contribute to chronicity in
male versus female urinary tract infection. JCI Insight.
5(e122998)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Storme O, Tirán Saucedo J, Garcia-Mora A,
Dehesa-Dávila M and Naber KG: Risk factors and predisposing
conditions for urinary tract infection. Ther Adv Urol.
11(1756287218814382)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
López-Cruz I, Esparcia A, Madrazo M,
Alberola J, Eiros JM and Artero A: Sex differences in aged 80 and
over hospitalized patients with community-acquired UTI: A
prospective observational study. Heliyon. 8(e11131)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kline KA and Lewis AL: Gram-Positive
uropathogens, polymicrobial urinary tract infection, and the
emerging microbiota of the urinary tract. Microbiol Spectr.
4(10)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ala-Jaakkola R, Laitila A, Ouwehand AC and
Lehtoranta L: Role of D-mannose in urinary tract infections-a
narrative review. Nutr J. 21(18)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Flores-Mireles AL, Walker JN, Caparon M
and Hultgren SJ: Urinary tract infections: Epidemiology, mechanisms
of infection and treatment options. Nat Rev Microbiol. 13:269–284.
2015.PubMed/NCBI View Article : Google Scholar
|