Introduction
Osteoporotic vertebral compression fractures can
lead to lower back pain and long-term complications in elderly
individuals, including spinal deformity, thrombosis and other
issues associated with prolonged bed rest (1). In 2013, the International
Osteoporosis Foundation reported that an osteoporotic fracture
occurs every 3 sec worldwide (2).
Additionally, ~50% of women and 20% of men will have suffered an
osteoporotic fracture after the age of 50 years. Furthermore, 50%
of patients with osteoporotic fractures are likely to suffer a
re-fracture (3). In women, the
risk of re-fracture of an osteoporotic vertebral fracture is four
times higher than that of a non-vertebral fracture. Osteoporotic
fractures of the thoracolumbar spine account for >90% of all
spinal fractures, making it the most common site for such fractures
(4). Surgical interventions
typically involve unilateral or bilateral percutaneous
vertebroplasty (PVP), in which polymethylmethacrylate is injected
into the fractured vertebra to alleviate pain and correct kyphosis
(5-7).
While unilateral vertebroplasty has benefits such as a shorter
duration of surgery and reduced radiation exposure when compared
with bilateral PVP, it often results in uneven bone cement
distribution (8). To achieve a
more even cement spread, the abduction angle of the metal puncture
rod is typically increased during unilateral puncture, which could
theoretically elevate the risk of spinal cord injuries. In this
context, the present study introduces a refined unilateral PVP
method that is aimed enhance the safety and efficacy of the
procedure. It may serve as a comprehensive guide for those new to
the technique.
Materials and methods
Ethics approval and patient
consent
Prior to the procedure, each patient provided
written informed consent, granting permission for the use of
relevant clinical images for scientific research and online
publication. The research received approval from the Ethics
Committee of the People's Liberation Army Hospital No. 923 Support
Force (Nanning, China) and adhered to the ethical principles of the
Declaration of Helsinki 2013 revision (9). All patients underwent PVP using the
unilateral pedicle cement anchoring technique. The procedures were
consistently conducted by an experienced surgeon from the
Department of Spine Surgery at People's Liberation Army Hospital
No. 923 (Nanning, China) from March 2020 to January 2023.
Study patients
Prior to surgery, all patients received symptomatic
treatments, including bed rest and pain management. They underwent
routine physical evaluations, with laboratory tests, computed
tomography (CT) scans, X-rays, magnetic resonance imaging (MRI) and
other diagnostic imaging. Surgical tolerance was assessed for each
patient, and those with underlying conditions were given
appropriate symptomatic support treatments.
The inclusion criteria were as follows: i) X-ray, CT
and MRI showed a single- or double-level fresh lumbar vertebral
compression fracture; ii) with or without a history of low-energy
trauma; iii) the detection result of lumbar bone mineral density
indicated osteoporosis; iv) age >60 years; v) percussion pain of
the injured vertebra; and vi) lower back pain activity was limited,
and mainly manifested as obvious pain upon getting up and turning
over. The exclusion criteria comprised: i) Severe cardiovascular
and cerebrovascular diseases such that the patient could not
tolerate surgery; ii) mental disorders that prevented the patient
from cooperating with the surgery; iii) clearly abnormal
coagulation function; iv) evident scoliosis or kyphosis of the
spine; and v) spinal bones destroyed by infection or tumor. A total
of 68 cases were included, including 22 males and 46 females. The
age of the patients ranged from 60 to 86 years, with an average of
70.06±5.58 years. There were 92 vertebral bodies with fractures:
T12 in 28 vertebrae, L1 in 36 vertebrae, L2 in 16 vertebrae, L3 in
5 vertebrae, L4 in 5 vertebrae and L5 in 2 vertebrae. The detailed
characteristics of the patients are summarized in Table I.
 | Table ICharacteristics of the study
patients. |
Table I
Characteristics of the study
patients.
| Characteristics | Patients |
|---|
| Sex (males/females),
n | 22/46 |
| Age, years | 70.06±5.58 |
| Follow-up duration,
months | 15.41±3.74 |
| Vertebral segment,
n | |
|
Total | 92 |
|
T12 | 28 |
|
L1 | 36 |
|
L2 | 16 |
|
L3 | 5 |
|
L4 | 5 |
|
L5 | 2 |
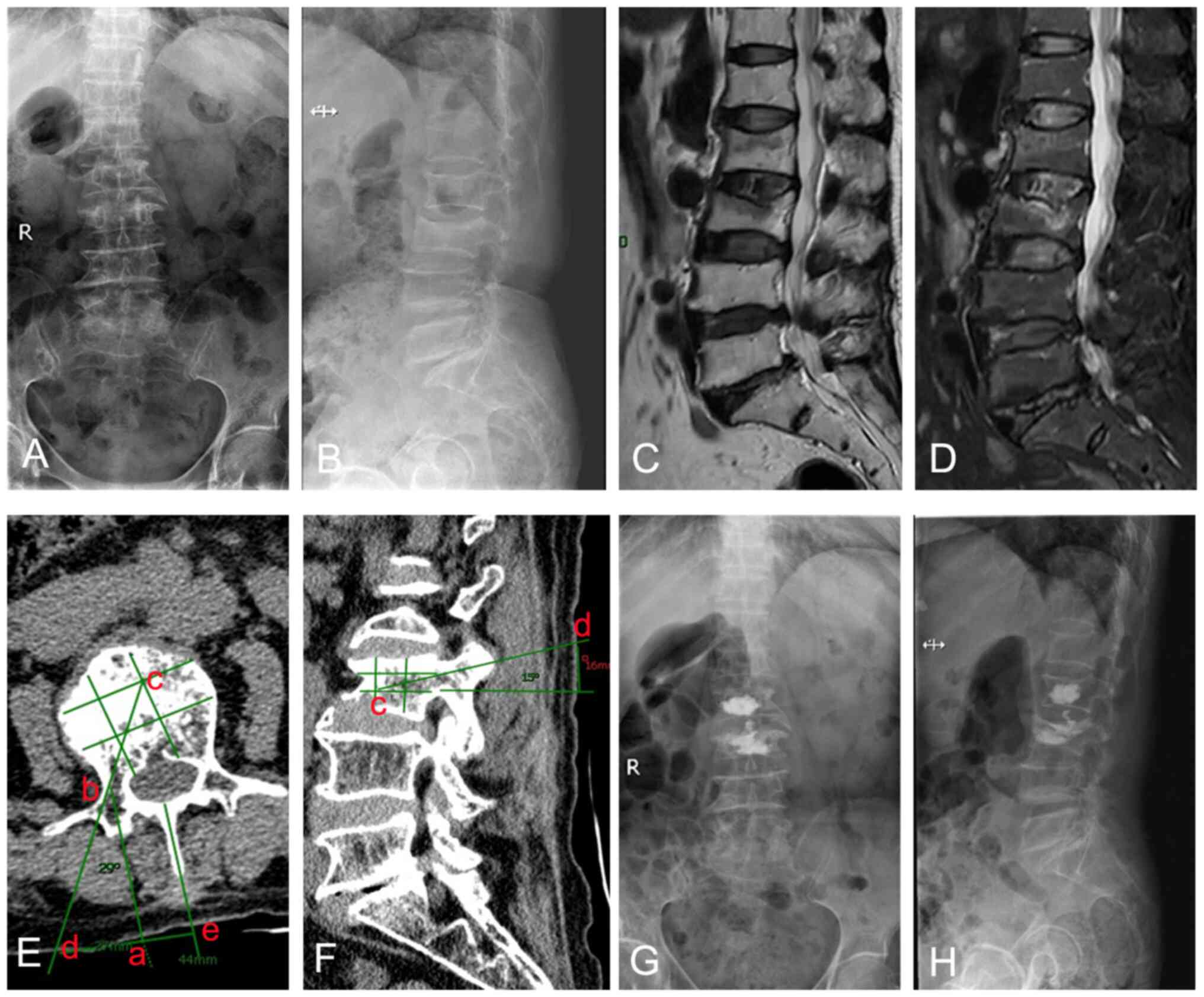
Surgical management. Preoperative CT
imaging preparation
The extent of vertebral compression could be
determined from preoperative radiographs taken in the orthostatic
(Fig. 1A) and lateral (Fig. 1B) positions. The injured vertebrae
exhibited a low signal in the T1 sequence (Fig. 1C) and a high signal in the T2
sequence (Fig. 1D) of the MR. It
is important to note that this evaluation was objective and based
solely on radiographic findings. It was critical for the puncture
to be performed according to the angle and approximate distance
measured on the preoperative CT film. On the CT section, the
cross-section of the fractured vertebral body was roughly divided
into nine equal parts, and various points were marked on the
section (Fig. 1E). Point c marked
the target puncture point, and point b marked the outer edge of the
pedicle. Point b was projected onto the surface skin as point a,
which was connected to line bc, which extended to the surface of
the body as point d. Point d was the point of puncture on the
surface skin, and the angle between cd and ab was the angle of
puncture. Similarly, the direction and angle of puncture in the
sagittal plane were also measured (Fig. 1F). In this case, point c on the
sagittal plane was the target of the puncture, and line dc was the
direction of the puncture. Point e was the projection of the
midpoint of the spinous process.
Anesthesia and body position. Surgery was
performed under local anesthesia combined with basic anesthesia,
which provide a very good analgesic effect and facilitated blood
pressure control. Local anesthesia was applied using 2% lidocaine
injection 5 ml + 1% ropivacaine injection 5 ml + 0.9% sodium
chloride injection 10 ml. In addition, a 0.02 mg/kg dose of
midazolam was administered intravenously before the procedure,
followed by a maintenance dose of dexmedetomidine hydrochloride at
0.6 µg/kg/h to enhance sedation and analgesia based on the
patient's weight. All patients were in the prone position during
surgery, and the abdomen was suspended by cushions beneath the
anterior superior iliac spine and the upper chest, to allow the
fracture position to be reset. The operating table was adjusted
appropriately to ensure that the muscles on both sides of the waist
were as level as possible to make it easier to determine the
correct inclination angle of the puncture.
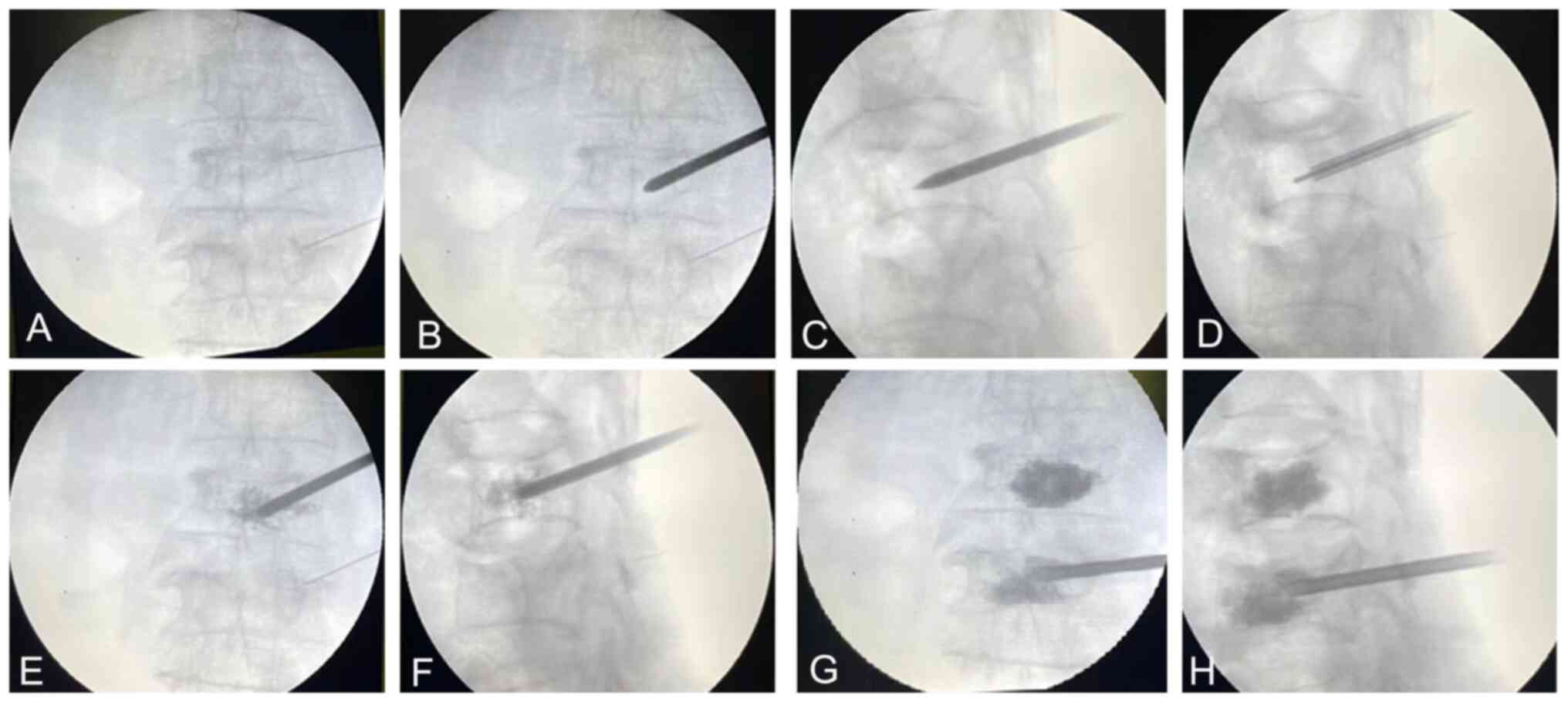
Surgical procedures. C-arm fluoroscopy was
used to identify the fractured vertebral body. Following routine
disinfection and towel laying, a syringe needle with a length of
~10 cm was inserted at the approximate needle entry point beside
the spinous process according to the preoperative measurements. The
needle tip was inserted until it reached the outer upper edge of
the unilateral pedicle, and the position of the injection needle
was adjusted under the perspective of a C-arm X-ray machine until
it was higher than the lateral level of the injured vertebral
pedicle. Then, a bone cement puncture cannula was used to puncture
the transverse process along the trajectory of the positioned
connection line. The puncture continued along the outer wall of the
pedicle, and extended to the opposite side of the midline of the
vertebral body using the bisection method. In order to ensure
safety during puncture, it was necessary to perform fluoroscopy 3-5
times. When observing the spine using fluoroscopy, it was possible
to see when the needle crossed the midline level of the vertebral
body, enabling movement of the needle to be stopped at the
appropriate position. Then, the puncture needle was taken out of
the cannula, and a Kirschner needle with a diameter of ~1 mm was
inserted into the cannula. Exploration revealed the presence of a
bony structure at the distal end of the channel. The bone cement
was then slowly injected. After each injection of 0.5-1 ml of bone
cement, it was necessary to check whether there was any bone cement
leakage. After each injection, the patient was asked whether any
numbness was evident in the lower limbs. Positive and lateral
perspectives were checked to confirm that the distribution of bone
cement was satisfactory. When the bone cement was solidified, the
working sleeve was rotated and pulled out. Before pulling out the
sleeve, it was necessary to check that there was no bone cement in
the sleeve in order to prevent any bone cement from being left
behind in the soft tissue (Fig.
2). The patients were required to stay in bed for 24 h after
the operation, and to carry out muscle contraction training and
joint activity training in bed to prevent deep vein thrombosis. At
6 months after the surgery, the patients were prescribed oral
risedronate sodium for anti-osteoporosis treatment.
Clinical and radiographic
assessments
The number of X-ray fluoroscopy examinations during
operation (the total times of fluoroscopy during surgery plus those
when puncturing with the positioning tube), the duration of surgery
(from positioning the syringe needle to pulling out the working
channel following solidification of the cement), complications
(vascular nerve injury, pulmonary embolism, cement insertion
syndrome and cement leakage) and the distribution of cement were
recorded. In addition, 24 h after the surgery, the curative effect
was evaluated using the lumbar MacNab standard (10). The visual analog scale (VAS) score
(11) and Oswestry disability
index (ODI) (12) of lumbar pain
were compared before the procedure, 24 h after surgery and at the
last follow-up after the surgery. The height and cobb angle of the
anterior edge of the injured vertebra were measured by the hospital
imaging system before and after surgery using X-ray lateral films
and compared.
Statistical analyses
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.). Data that adhered to a normal distribution
are presented as the mean ± standard deviation. Data were analyzed
using repeated measures of analysis of variance (ANOVA) followed by
Bonferroni's correction. P<0.05 was considered to indicate a
statistically significant difference.
Results
Surgical findings and follow-up
A total of 92 vertebral body operations in 68
patients were successfully completed. The average duration of
surgery was 37.69±6.91 min (range, 26-55 min). The total number of
X-ray fluoroscopy examinations performed during the surgery was
18.37±4.35 (range, 12-28). Bone cement leakage occurred in 23
vertebral bodies, including 15 cases where leakage was to the side
or front of the vertebral body, seven cases where leakage to the
upper intervertebral disc occurred, and 1 case of leakage to the
posterior wall of the vertebral body. The 68 patients were followed
up for a mean duration of 15.41±3.74 months (range, 8-23 months).
No complications, such as vascular nerve injury, pulmonary embolism
or bone cement implantation syndrome, occurred in any of the
patients. The anteroposterior X-ray images showed that the bone
cement was distributed in the form of a central aggregate mass in
60 vertebral bodies, a unilateral aggregate mass in 21 vertebral
bodies, and a diffuse honeycomb in 11 vertebral bodies. The mean
injected cement volume was 5.5±1.0 ml. Compared with preoperative
values, the average Cobb angle and anterior vertebral height were
significantly improved (P<0.05). The mean Cobb angle decreased
from 17.69±4.13˚ before surgery to 10.68±2.35˚ 24 h
post-operatively. At the final follow-up, the mean Cobb angle was
12.61±4.52˚. The anterior height of the vertebral body increased
from 19.05±4.62 mm before surgery to 22.34±2.57 mm 24 h after the
surgery. At the final follow-up, the height of the anterior
vertebral body was 20.91±2.07 mm. Statistically significant
differences in kyphosis correction and vertebral height recovery
were detected between preoperative and postoperative time points
(P<0.05; Table II).
 | Table IIComparisons of preoperative,
postoperative and final follow-up clinical parameters. |
Table II
Comparisons of preoperative,
postoperative and final follow-up clinical parameters.
| Parameters | Preoperative | 24 h after
surgery | Final follow-up | F-value | P-value |
|---|
| VAS (n1) | 8.08±0.79 |
2.25±0.71a |
1.58±0.51b | 1,875.36 | <0.001 |
| ODI, % (n1) | 67.75±7.91 |
19.74±2.90a |
28.00±4.89b | 1,417.33 | <0.001 |
| AVH, mm (n2) | 19.05±4.62 |
22.34±2.57a |
20.91±2.07b | 23.51 | <0.001 |
| Cobb angle, ˚
(n1) | 17.69±4.13 |
10.68±2.35a |
12.61±4.52b | 62.20 | <0.001 |
Therapeutic effect
The therapeutic effect was evaluated by the lumbar
MacNab standard 24 h after the operation, and was found to be
excellent in 54 cases, good in 10 cases and fair in 4 cases. The 4
cases whose curative effect was evaluated as fair all had a clear
history of trauma, and all had obvious lumbar fascia edema on
preoperative magnetic resonance T2-weighted images. After these 4
cases were given oral non-steroidal analgesic drugs to assist with
acupuncture and physiotherapy, their lower back pain was improved
and they were able to get out of bed freely.
Effect on VAS scores and ODI
values
The back pain of the patients was significantly
ameliorated after the surgery, and the self-care ability and
quality of life of the patients was also improved (Table II). The mean VAS score was
8.08±0.79 prior to surgery, and decreased to 2.25±0.71 24 h
post-operatively. At the final follow-up, the VAS score remained
low at 1.58±0.51. The differences in the VAS scores of lumbar pain
before and after surgery were statistically significant
(F=1,875.36, P<0.05), and the VAS score of lumbar pain 24 h
post-operatively was significantly lower than the average
pre-operative VAS score (t=45.15, P<0.05). In addition, the VAS
score of pain at the last follow-up after surgery was significantly
lower compared with that at 24 h after surgery (t=6.33, P<0.05).
The average pre-operative ODI was 67.75±7.91; at 24 h post-surgery,
it dropped to 19.74±2.90, and at the last follow-up it was
28.00±4.89. The ODI values before and after surgery were
statistically significantly different (F=1,417.33, P<0.05). The
ODI value 24 h post-surgery was significantly lower than that
before surgery (t=46.98, P<0.05), and the ODI index at the last
follow-up after surgery was higher than that 24 h after the
procedure (t=-11.92, P<0.05). The patients included 8 cases who
developed fresh compression fractures in other vertebral bodies
during the follow-up.
Discussion
The present study indicates that the unilateral PVP
technique is safe and effective in the treatment of osteoporotic
compression fractures. Traditional internal fixation surgery for
spinal fractures presents drawbacks such as extensive trauma and
prolonged recovery time (13).
However, the emergence of minimally invasive spinal surgery
technology has led to the widespread clinical use of PVP (14,15).
This technique has been shown to be effective in achieving improved
analgesia and increased vertebral body strength, as is widely
acknowledged in the field (16,17).
PVP stabilizes the fractured vertebral body and reduces the pain
caused by vertebral fracture edema. This promotes patient recovery
and reduces the incidence of chronic non-healing pain. Unilateral
or bilateral pedicle puncture PVP is a key procedure performed by
spinal surgeons (18). A previous
study demonstrated the safety and minimal invasiveness of
unilateral puncture PVP, which results in reduced soft-tissue
damage and good cement distribution compared with bilateral PVP
(19). It has been observed that
there is no significant difference in pain relief between
unilateral and bilateral puncture PVP (20). However, it has been suggested that
to diffuse the cement bilaterally during unilateral vertebroplasty,
the angle of puncture adduction must be increased so that the tip
of the puncture needle is positioned near to the contralateral side
of the vertebral body. This approach, however, also heightens the
risk of cement leakage and peripheral vascular and neurological
injuries (21). Li et al
(22) discovered that the
implementation of 3D-printed navigation templates during PVP
resulted in significantly lower intraoperative puncture positioning
times and reduced the requirement for X-ray fluoroscopies compared
with that in cases where freehand positioning was used. Although
the navigation templates were found to be effective, they were
prone to inaccuracies caused by skin deformation and changes in
body position. The use of modified surgical instruments with
directional pins for unilateral PVP has been shown to provide very
good results without increased clinical risk (23). However, the drawbacks include a
steeper learning curve and the intraoperative procedure being more
complex. Complications associated with PVP are mainly associated
with the surgical puncture technique and the injected bone cement.
Reported perioperative complications predominantly comprise
injuries to the spinal cord and nerve roots, pulmonary embolism,
cement leakage and cement implantation syndrome (24-28).
Spinal cord and nerve root injuries can be classified into two
categories. The first category is caused by puncture and may be
associated with the level of experience of the surgeon and
inadequate monitoring during surgery. The second category is
cement-related injury, which can result from the site of puncture
access being too close to the spinal cord, resulting in local
compression injuries and thermal damage. It is important to note
that these two categories of injury can have severe consequences
and should be prevented whenever possible. This may be achieved by
carefully studying the imaging data to gain a full understanding of
the clinical signs of the patient. Segments with lesions such as
partial destruction of the posterior edge of the vertebral body,
vertebral body collapse, endplate rupture, and pedicle erosion and
destruction should be carefully selected. Any abnormal sensations
in the lower extremities and changes in the patient's complaints
about pain and numbness that occur during the puncture should be
fully considered and carefully managed. It is also important for
the surgeon to master the surgical techniques correctly. In
particular, the puncture technique and the quality of imaging
surveillance should be optimized. In severely compressed and
deformed vertebrae, the puncture point and route are particularly
individualized, and the experience of the operator is very
important.
In the present study, it was found that thorough and
diffuse distribution of the cement was obtained by the use of CT
and the division of the vertebral body into nine equal parts, with
puncture along the superior articular process-vertebral pedicle or
the lateral aspect of the superior articular process-vertebral
pedicle only to the anterolateral zone close to point c or directly
to point c. This has the following advantages: i) Improved safety
of the procedure. The abduction angle of the puncture device in the
transverse plane and the cephalic tilt angle in the sagittal plane
can be determined prior to surgery. With regard to the method of
lumbar pedicle nailing during open surgery, if the lateral aspect
of the base of the upper articular process is chosen as the
insertion point during surgery, accurate puncture is generally
possible. Intraoperatively, it is usually possible for the lateral
aspect of the base of the superior articular process to be
accurately identified as the final entry point as long as it is
possible to detect a sense of ‘slippage’ from the lateral aspect of
the synchondrosis and to explore the transverse process. The 68
patients in the present study had no evidence of spinal cord or
nerve root injury and were safely treated. ii) High satisfaction
rate of bone cement morphology. When the puncture breaks through
the midline of the spinous process on the fluoroscopic
orthopantomogram and reaches the anterior contralateral third of
the vertebral body on the lateral X-ray, the cement is slowly
pushed into the vertebral body. When injecting bone cement, the
distribution of cement can be adjusted by varying the depth of the
channel cannula of the bone cement injection device. Postoperative
orthopantomograms of the 92 vertebrae showed the cement was present
as centrally aggregated masses in 60 vertebrae, unilaterally
aggregated masses in 21 vertebrae, and dispersed honeycombs in 11
vertebrae. iii) It can effectively reduce the pain felt by the
patient. The procedure creates only one 0.5-mm diameter puncture
wound, and uses a number of preoperatively planned puncture points
and puncture paths with the aim of achieving the correct needle
placement in order to avoid repeated multi-point puncturing, which
would destroy the integrity of the vertebral body. The mean VAS
score decreased from 8.08±0.79 preoperatively to 2.25±0.71
postoperatively and was reduced further to 1.58±0.51 at the final
follow-up appointment. The mean ODI improved from 67.75±7.91
preoperatively to 19.74±2.90 postoperatively, and remained low at
28.00±4.89 until the final follow-up. iv) It is easy to use. The
procedure can be performed by a single person, and involves the
one-sided injection of bone cement, which shortens the injection
time and reduces the waste of bone cement that hardens too quickly
to be used.
The goal of PVP is to increase vertebral stability
by restoring or increasing the strength of the vertebral body, and
overfilling results in suboptimal biomechanics (29,30).
In addition, the greater the injection volume, the greater the risk
of leakage (31). In the present
study, the mean volume of cement injected was 5.5±1.0 ml, which is
very small, but good efficacy was achieved in all cases. To prevent
leakage to the anterior side, the tissue anterior to the trocar can
be gently touched with a probe to determine whether it is a bony
structure or not; if it is a hollow or ductile structure, a small
gelatin sponge can be applied before the cement is injected. If a
leak is observed that is not posterior to the vertebral body, it
can be observed for 30 sec, and then re-injection of the cement can
be attempted while the first injected bone cement continues to
polymerize and solidify, sealing the leak, or the procedure can be
terminated. It is essential that the viscosity of the bone cement
is appropriate, the injection pressure is not too high, and the
whole process is monitored by X-ray. If it is found that the bone
cement is spreading rapidly with venous return or leaking into the
epidural or intervertebral foramen, injection of the cement must be
stopped immediately and the viscosity increased slightly prior to
continuing the injection. Intravertebral leakage often has terrible
consequences; therefore, if it occurs, the injection of cement
should be stopped completely, and a CT scan should be performed if
possible, to clarify the degree of loading. In addition, the
neurological function of the patient should be closely monitored.
For patients with severe destruction of vertebral cortical bone
confirmed by preoperative examination, it is preferable to use a
higher viscosity injection agent. The viscosity of the bone cement
is important, as if it is too thin it will easily leak, while if it
is too thick it will be difficult to inject.
In addressing the occurrence of new fractures
post-vertebroplasty, it is critical to explore preventative
measures and management strategies. The aim of preventative
approaches is to mitigate the risk factors associated with new
fractures. This includes optimizing bone density through the
management of osteoporosis, which is of utmost importance (32). Moreover, lifestyle modifications
such as weight-bearing exercises and fall prevention strategies are
essential components of a comprehensive prevention plan (33). In terms of management, the early
identification and treatment of new fractures are crucial. The
literature suggests a multidisciplinary approach that encompasses
pain management, physical therapy and, where appropriate, surgical
intervention (34). It is also
important to consider the role of the distribution of cement in the
vertebral body and the precision of fracture repositioning during
the initial treatment, as these factors can influence the
likelihood of subsequent fractures. Furthermore, for patients
presenting with mild symptoms post-re-fracture, non-surgical
management involving rest, medication and careful monitoring may be
sufficient, as suggested by Clark et al (35). By contrast, for cases with severe
complications, such as progressive kyphosis or neurological
deficit, revision surgery should be considered, as indicated in the
American Academy of Orthopedic Surgeons Clinical Practice
Guidelines reported by McGuire (36). Ultimately, a tailored approach
based on individual patient factors, including the severity of
osteoporosis, the extent of the initial fracture and the overall
health status of the patient is important to inform the prevention
and management strategies for post-treatment fractures.
However, the present study has certain limitations.
Firstly, it was a retrospective study and did not use bilateral
vertebroplasty as a control. Although good clinical results were
achieved, the sample size was small, and a prospective randomized
controlled trial with a large sample size is required to further
validate the clinical efficacy of the method. Secondly, there were
21 vertebral bodies with unilateral bone cement distribution in the
present study with a short follow-up period, and it is not clear
whether the uncemented side would become recompressed, leading to
worsening recurrent pain. While the current study effectively
demonstrates the short-term efficacy of unilateral PVP in
alleviating pain and disability, it is important to note as a
limitation the absence of long-term outcome data, specifically
regarding the risk of secondary fractures post-procedure. Future
research with extended follow-up is essential to elucidate the
potential long-term implications and to develop comprehensive
strategies for the prevention and management of new fractures in
this patient population.
In conclusion, the findings of the present study
support the use of unilateral PVP in the treatment of acute
osteoporotic vertebral compression fractures. By optimizing the
puncture angle in the unilateral puncture PVP procedure, the
potential harm to essential structures as well as cement leakage
and associated risks are reduced. The outcomes were positive,
irrespective of the cement distribution pattern, with no notable
variance in patient prognosis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a grant from People's
Liberation Army Hospital No. 923 (grant no. 2023YNKT-YX-03).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DS wrote the manuscript. XL, FH and GW analyzed and
interpreted the patient data. DS and ZL made substantial
contributions to the conception, design and intellectual content of
the study. DS and ZL confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki. All participants signed an informed
consent form. This study was approved by the Ethics Committee of
People's Liberation Army Hospital No. 923 Support Force (approval
no. 923LL-KY-2023LW-012-01; Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Firanescu CE, de Vries J, Lodder P,
Venmans A, Schoemaker MC, Smeets AJ, Donga E, Juttmann JR, Klazen
CAH, Elgersma OEH, et al: Vertebroplasty versus sham procedure for
painful acute osteoporotic vertebral compression fractures (VERTOS
IV): Randomised sham controlled clinical trial. BMJ.
361(k1551)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Akesson K, Marsh D, Mitchell PJ, McLellan
AR, Stenmark J, Pierroz DD, Kyer C and Cooper C: IOF Fracture
Working Group. Capture the fracture: A best practice framework and
global campaign to break the fragility fracture cycle. Osteoporos
Int. 24:2135–2152. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Si L, Winzenberg TM and Palmer AJ: A
systematic review of models used in cost-effectiveness analyses of
preventing osteoporotic fractures. Osteoporos Int. 25:51–60.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoo JH, Moon SH, Ha YC, Lee DY, Gong HS,
Park SY and Yang KH: Osteoporotic fracture: 2015 position statement
of the korean society for bone and mineral research. J Bone Metab.
22:175–181. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Voormolen MH, Lohle PN, Lampmann LE, van
den Wildenberg W, Juttmann JR, Diekerhof CH and de Waal Malefijt J:
Prospective clinical follow-up after percutaneous vertebroplasty in
patients with painful osteoporotic vertebral compression fractures.
J Vasc Interv Radiol. 17:1313–1320. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zou D, Wang H, Zhao Y, Sun X and Du WP:
Evaluation of the clinical efficacy of the bilateral pedicle cement
anchoring technique in percutaneous vertebroplasty for Kümmell
disease. Exp Ther Med. 26(391)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hammed A, Mahfoud M and Mohamad O:
Effectiveness of unilateral percutaneous vertebroplasty for acute
traumatic non-osteoporotic compression vertebral fractures.
Medicine (Baltimore). 102(e35177)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhuo Y, Liu L, Wang H, Li P, Zhou Q and
Liu Y: A modified transverse process-pedicle approach applied to
unilateral extrapedicular percutaneous vertebroplasty. Pain Res
Manag. 2021(6493712)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
World Medical Association. World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ahsan K, Najmus-Sakeb Hossain A, Khan SI
and Awwal MA: Discectomy for primary and recurrent prolapse of
lumbar intervertebral discs. J Orthop Surg (Hong Kong). 20:7–10.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jacobs WC, van der Gaag NA, Kruyt MC,
Tuschel A, de Kleuver M, Peul WC, Verbout AJ and Oner FC: Total
disc replacement for chronic discogenic low back pain: A cochrane
review. Spine (Phila Pa 1976). 38:24–36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fairbank JC and Pynsent PB: The oswestry
disability index. Spine (Phila Pa 1976). 25:2940–2952.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Nakshabandi NA: Percutaneous
vertebroplasty complications. Ann Saudi Med. 31:294–297.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Buchbinder R, Johnston RV, Rischin KJ,
Homik J, Jones CA, Golmohammadi K and Kallmes DF: Percutaneous
vertebroplasty for osteoporotic vertebral compression fracture.
Cochrane Database Syst Rev. 4(CD006349)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Filippiadis DK, Marcia S, Masala S,
Deschamps F and Kelekis A: Percutaneous vertebroplasty and
kyphoplasty: Current status, new developments and old
controversies. Cardiovasc Intervent Radiol. 40:1815–1823.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schnake KJ, Scheyerer MJ, Spiegl UJA, Perl
M, Ullrich BW, Grüninger S, Osterhoff G, Katscher S and Sprengel K:
Arbeitsgruppe Osteoporotische Frakturen der Sektion Wirbelsäule.
Minimally invasive stabilization of thoracolumbar osteoporotic
fractures. Unfallchirurg. 123:764–773. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
17
|
Noriega D, Marcia S, Theumann N, Blondel
B, Simon A, Hassel F, Maestretti G, Petit A, Weidle PA, Mandly AG,
et al: A prospective, international, randomized, noninferiority
study comparing an implantable titanium vertebral augmentation
device versus balloon kyphoplasty in the reduction of vertebral
compression fractures (SAKOS study). Spine J. 19:1782–1795.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kushchayev SV, Wiener PC, Teytelboym OM,
Arrington JA, Khan M and Preul MC: Percutaneous vertebroplasty: A
history of procedure, technology, culture, specialty, and
economics. Neuroimaging Clin N Am. 29:481–494. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun Y, Ma H, Yang F, Tang X, Yi P and Tan
M: Clinical efficacy and safety of zoledronic acid combined with
PVP/PKP in the treatment of osteoporotic vertebral compression
fracture: A systematic review and meta-analysis of randomized
controlled trials. Biomed Res Int. 2021(6650358)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pan D and Chen D: Comparison of
unipedicular and bipedicular percutaneous kyphoplasty for Kummell's
disease. Geriatr Orthop Surg Rehabil.
13(21514593221099264)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Q, Liu L and Liang G: Distribution
characteristics of bone cement used for unilateral puncture
percutaneous vertebroplasty in multiple planes. Orthopade.
47:585–559. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Z, Xu D, Li F, Liu M, Xu G and Yang M:
Design and application of a novel patient-specific 3D printed drill
navigational guiding template in percutaneous thoracolumbar pedicle
screw fixation: A cadaveric study. J Clin Neurosci. 73:294–298.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Soon WC, Mathew RK and Timothy J:
Comparison of vertebroplasty using directional versus straight
needle. Acta Radiol Open. 4(2047981615569268)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alsoof D, Anderson G, McDonald CL, Basques
B, Kuris E and Daniels AH: Diagnosis and management of vertebral
compression fracture. Am J Med. 135:815–821. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang JD, Poffyn B, Sys G and Uyttendaele
D: Comparison of vertebroplasty and kyphoplasty for complications.
Orthop Surg. 3:158–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Boss S, Srivastava V and Anitescu M:
Vertebroplasty and Kyphoplasty. Phys Med Rehabil Clin N Am.
33:425–453. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie LL, Chen XD, Yang CY, Yan ZL, Zhu J,
Quan KQ and Pu D: Efficacy and complications of (125)I seeds
combined with percutaneous vertebroplasty for metastatic spinal
tumors: A literature review. Asian J Surg. 43:29–35.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Imamudeen N, Basheer A, Iqbal AM, Manjila
N, Haroon NN and Manjila S: Management of osteoporosis and spinal
fractures: Contemporary guidelines and evolving paradigms. Clin Med
Res. 20:95–106. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wilcox RK: The biomechanics of
vertebroplasty: A review. Proc Inst Mech Eng H. 218:1–10.
2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Baroud G and Bohner M: Biomechanical
impact of vertebroplasty. Postoperative biomechanics of
vertebroplasty. Joint Bone Spine. 73:144–150. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cui Y, Pan Y, Lin Y, Mi C, Wang B and Shi
X: Risk factors for predicting cement leakage in percutaneous
vertebroplasty for spinal metastases. J Orthop Sci. 27:79–83.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hettchen M, von Stengel S, Kohl M, Murphy
MH, Shojaa M, Ghasemikaram M, Bragonzoni L, Benvenuti F, Ripamonti
C, Benedetti MG, et al: Changes in menopausal risk factors in early
postmenopausal osteopenic women after 13 months of high-intensity
exercise: The randomized controlled ACTLIFE-RCT. Clin Interv Aging.
16:83–96. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Franco MR, Pereira LS and Ferreira PH:
Exercise interventions for preventing falls in older people living
in the community. Br J Sports Med. 48:867–868. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen LH, Hsieh MK, Liao JC, Lai PL, Niu
CC, Fu TS, Tsai TT and Chen WJ: Repeated percutaneous
vertebroplasty for refracture of cemented vertebrae. Arch Orthop
Trauma Surg. 131:927–933. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Clark W, Bird P, Gonski P, Diamond TH,
Smerdely P, McNeil HP, Schlaphoff G, Bryant C, Barnes E and Gebski
V: Safety and efficacy of vertebroplasty for acute painful
osteoporotic fractures (VAPOUR): A multicentre, randomised,
double-blind, placebo-controlled trial. Lancet. 388:1408–1416.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
McGuire R: AAOS clinical practice
guideline: The treatment of symptomatic osteoporotic spinal
compression fractures. J Am Acad Orthop Surg. 19:183–184.
2011.PubMed/NCBI View Article : Google Scholar
|