Introduction
The Caco-2 cell line (colon adenocarcinoma cell
line) cultured under certain conditions may resemble enterocytes
present in a normal intestine and previously have been used as a
model system in research (1). The
intestinal epithelium has the ability to function as a barrier
between the external and internal environment. This ability is
essential for human health, and disorders in this functioning due
to increased intestinal permeability are associated with diseases
such as: inflammatory bowel disease (IBD), irritable bowel syndrome
(IBS) or celiac disease (1). There
are reports that tofacitinib may improve the functioning of the
cellular barrier in the form of the intestinal epithelium in
patients with enteritis (2). Most
of the in vitro studies that have been conducted to date
have used the Caco-2 and HT-29 cell lines to follow these reports.
Therefore, an experiment was conducted using the Caco-2 cell line
to verify the effect of tofacitinib on intestinal epithelial cells
after inducing inflammation in them. The cell line used is not a
reflection of the healthy intestinal epithelium found in the human
body, as it is a cancer cell line, but the model is similar to that
found in the human body.
The TNFα used in the experiment was aimed at
inducing an inflammatory phenotype in the tested cell line, the
markers of which were: SOD, NADPH, CAT as an assessment of
oxidative stress, NF-κB and Bcl-2 as an assessment of the
percentage of cells in apoptosis and CLD-1 representing assessment
of the state of intercellular connections. TNFα is also mentioned
in the literature as being involved in causing inflammation
associated with UC (3,4).
Ulcerative colitis (UC) belongs to the group of
inflammatory bowel diseases (IBD). It is a disease with a complex
and so far, not fully understood etiology. Histopathological
features of inflammation include hyperemia of the mucosa
accompanied by edema, inflammatory infiltration covering only the
mucosa, as well as the presence of desquamated epithelial cells, or
the presence of granulocytes on the surface of the mucosa, but also
in between epithelial cells (2).
More and more research results indicate the important role of
enterocytes in IBD. The intestinal epithelium is the physical,
protective barrier of the intestinal microflora and actively
contributes to the protection of the immune system of the
intestinal mucosa. This barrier is mainly formed by a single layer
of specialized intestinal epithelial cells that are crucial in
maintaining intestinal homeostasis. Therefore, damage to the
epithelium may increase intestinal permeability, lead to
disturbances in the interactions between intestinal epithelial
cells and immune cells, and thus disturb the homeostasis of the
intestinal immune system (3).
Functional defects occurring on the intestinal epithelial barrier
in the course of UC, which result in the lack of expression of
P-glycoprotein, may be one of the causes of the development of
inflammation. Some of the drugs commonly used in the treatment of
IBD are substrates of P-glycoprotein and seem to have a potential
influence in regards to the treatment of patients with UC (4). The above issues seem to be crucial
for the course of IBD. It remains an open question whether new
drugs used in inflammatory bowel diseases affect the phenotype and
function of the intestinal epithelium.
A plethora of novel drugs affecting intracellular
signaling pathways have recently been approved for therapy or are
under development (5). Moving the
site of blockage of the inflammatory signal inside the cell has
numerous advantages. It allows for even more precise modification
of the effects of the inflammatory stimulus, and moreover, these
compounds usually have a low-molecular weight (i.e. they are
relatively simple chemical entities), which may significantly
reduce the costs of therapy in the future. An example of a drug
with such properties is tofacitinib (TBF), which has been used,
among others, in the treatment of ulcerative colitis. It is an
orally administered inhibitor of Janus kinases (JAK) which, by
modifying the JAK/STAT signaling pathway, reduces the effect of
inflammatory cytokines in the gut. However, its effect on
intestinal epithelial cells is not fully understood (6).
In our work, we tested the effect of TBF on Caco-2
cells by evaluating its effect on oxidative stress observed by gene
analysis-superoxide dismutase (SOD-1), catalase (CAT), nicotinamide
adenine dinucleotide phosphate oxidase (NADPHox), cell apoptosis
and inflammatory signaling assessed by nuclear factor κB (NF-κB)
and a family of regulatory proteins involved in apoptosis (Bcl-2)
and the condition of intercellular junctions, which included the
expression of claudin 1 (CLD-1) were observed. These parameters
were assessed in the cell after inducing inflammation with TNF
alpha and after using TBF.
It seems that with the existing state of knowledge,
the search for information on the effect of TBF on the intestinal
epithelium and new biological ways of UC therapy may contribute to
a reduction in the risk of exacerbation of the disease.
Materials and methods
Cell culture
Human colon adenocarcinoma cells-Caco2 (colon
adenocarcinoma) from the European Collection of Authenticated Cell
Cultures (ECACC) cell line collection, purchased from Sigma-Aldrich
(Poznań, Poland), were used to conduct the experiment. Cells were
cultured in Eagle's Minimum Essential Medium (EMEM, Lonza, USA)
supplemented with 10% Fetal Bovine Serum (FBS, Gibco, USA), 1% MEM
Non-essential Amino Acid Solution 100x (Sigma-Aldrich, Poznań,
Poland) and 1% antibiotics/antifungal compounds
(Antibiotic-Antimycotic Solution 100x) (Sigma-Aldrich, Poznań,
Poland). The culture was carried out at 37˚C and in a
CO2-enriched atmosphere (5.3%) in a 24-well culture
plate (Nunc™, Sigma-Aldrich, Poznań, Poland) in
duplicate. The cells were cultured for 24-48 h in a medium free of
inflammatory substances. The medium was replaced with fresh and
TNFα 10 ng/ml (Sigma-Aldrich, Poznań, Poland) was added to induce
inflammation in the cells, the next step was to add TBF
(Sigma-Aldrich, Poznań, Poland) at a concentration of 100 nM.
Confirmation of the presence of Caco-2
cells in the culture
The cell phenotype, normal culture and lack of
contamination with other cell populations were confirmed by
immunofluorescence staining showing the presence of retinol binding
protein 2 RBP-2 in Caco-2 cells, which is commonly used for their
detection (4). The cultured cells
were flooded with cold methanol and incubated in the freezer for 10
min, then double permeabilization was performed with 0.1% Triton
x100 (Sigma-Aldrich, Poznań, Poland). The next step was to block
the fixed preparation in 3% BSA (Sigma-Aldrich, Poznań, Poland) and
treat it with the primary antibody 1:500 (Anti-CRABP2,
Sigma-Aldrich, Poznań, Poland) overnight at 4 degrees C, after
which the secondary fluorescent antibody 1:300 (Alexa
Flour™ 546 goat anti mouse IgG (H+L), Invitrogen, Thermo
Fisher Scientific, Massachusetts, USA) was added and rinsed with
PBS, observations were made using a Delta Optical IB-100 inverted
microscope (Delta Optical, Mińsk Mazowiecki, Poland) with a
fluorescent attachment. We compared the obtained images with images
of Normal Human Astrocyte (NHA) primary cells from the ECACC cell
line collection, purchased from Lonza (Lonza, USA), to show
differences in the detection of a specific marker for Caco-2 cells.
NHA cells were cultured in astrocyte basal medium (Lonza, USA)
supplemented with 10% FBS (Gibco, USA), supplements for a complete
growth medium developed especially for NHA (AGM
SingleQuots™; Lonza, USA) and 1% antibiotics/antifungal
compounds (Antibiotic-Antimycotic Solution 100X; Sigma-Aldrich,
Poznań, Poland). The culture was carried out at 37˚C in a
CO2-enriched atmosphere (5.3%) in a 24-well culture
plate (Nunc™; Sigma-Aldrich, Poznań, Poland) in
duplicate. The cell phenotype, normal culture and lack of
contamination with other cell populations were confirmed by
immunofluorescence staining showing the presence of RBP-2 in Caco-2
cells, which is commonly used for their detection. We performed the
same experiment to confirm the phenotype of Caco-2 cells, using the
correct culture as aforementioned. We subjected the NHA cell
culture to immunofluorescence staining to show the absence of the
RBP-2 in NHA cells; this staining is characteristic only of Caco-2
cells.
Cell viability assessment-Trypan
Blue
Cell viability was assessed using a
Bio-rad® TC20 (Bio-Rad Poland, Warszawa, Poland)
automated cell counter. Samples from various stages of the
experiments were taken from the cell culture. 10 µl of trypan blue
0,4% was added to 10 µl of the cell suspension after
trypsinization, waited 5 min and 10 µl of the sample was collected
and placed on a special cell viability plate. The next step was to
insert the plate into the device and measure the number of live and
dead cells in the sample (7).
Assessment of proliferation-MTT
method
Cell proliferation was examined by the MTT method
(8). It is a colorimetric test
that evaluates the metabolic activity of cells. For this purpose,
the enzyme NADPH-dependent cellular oxidoreductase is used, which
in active mammalian cells reduces MTT to a colored formazan
product. After dissolution, the formazan absorbance was measured
with a Bio-rad® (Bio-Rad Poland, Warszawa, Poland)
microplate absorbance reader. Cells were grown in a 96-well plate
and exposed to reagents for 24 h. The absorbance was then read at
750 nm.
Cell lysis with RIPA buffer and
protein isolation
After completion of the cell culture, supernatant
was collected and afterwards, using 150 µl of RIPA Buffer
(Sigma-Aldrich, Poznań, Poland) supplemented with protease
inhibitors [Thermo Scientific Halt™ Protease Inhibitor
Cocktail (100X) (Thermo Fisher Scientific, Massachusetts, USA)]
adherent cells lysis was obtained (9). The samples were subjected to
vortexing, sonication, and finally centrifugation at 4˚C at 12,000
x g for 5 min. The protein concentration was determined using the
BCA method, i.e. the quantitative determination of protein with
bicinchoninic acid. This method uses the binding of the protein to
the Brilliant Blue G dye. The XMark microplate Spectrophotometer
(Bio-Rad Poland, Warszawa, Poland) detector was used for the
measurement, which uses the maximum absorbance of the dye at 562
nm. The absorbance value is proportional to the protein
concentration of the standard samples (bovine serum albumin-BSA,
Sigma-Aldrich, Poznań, Poland), which allows the drawing of a
standard curve.
Western blot technique
The western blot technique is used to detect and
identify proteins. The first step is protein electrophoresis in a
10% polyacrylamide gel. Electrophoresis is the separation of
proteins (20 µl protein and 5 µl Sample Buffer, Laemmli 2X
Concentrate, Sigma-Aldrich, Poznań, Poland) on a polyacrylamide gel
carried out at a constant voltage of 175-180 V.
The next step of this technique is the transfer of
proteins onto the membrane. The gel and membrane are placed in the
transfer buffer and transferred overnight.
After transfer we block the membrane in 10X casein
solution (Vector Laboratories Inc., Newark, USA). Blocking is a
step to prevent non-specific binding of the antibodies. On the
following step of procedure binding of unlabeled antibodies
(primary antibody in concentration 1:1,000: Anti-ACTB, Anti-SOD-1,
Anti-CAT, Anti-NADPH, Invitrogen, Thermo Fisher Scientific,
Massachusetts, USA; Anti-CLD1, Millipore Corp. Merck,
Sigma-Aldrich, Poznań Poland; Anti-NF-κB, Sigma-Aldrich, Poznań,
Poland; Anti-Bcl-2, Cell Signaling Technology, Lab-JOT, Warszawa,
Poland) to the antigens present on the membrane takes place. Next a
secondary antibody in concentration 1:2,000 (Anti-Rabbit IgG in
goat, Sigma-Aldrich, Poznań, Poland) is added to mark the unlabeled
primary antibody used (10).
The last step is protein detection using the
horseradish peroxidase reaction (SuperSignal™ Pierce™
Thermo Fisher Scientific, Massachusetts, USA). Visualization is
done by chemiluminescence using a LI-COR C-DiGit Chemiluminescence
Western Blot Scanner (Polygen, Wrocław, Poland). The results were
developed using the ImageJ program (11). Beta actin (ACTB) was chosen as the
reference gene for analysis.
Cell lysis with TRI Reagent (TRIzol)
and RNA isolation
Cells from the culture plate intended for RNA
analysis are purified from the supernatant, and then 1 ml of
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Massachusetts, USA) is added to the well. This reagent is used to
isolate total RNA from a sample.
The next step was to add 200 µl of chloroform to the
samples and incubate them at room temperature for 2-3 min. The
material was then centrifuged and two layers were obtained. The
upper layer, called the DNA-containing aqueous phase, was
transferred to a new tube, 500 µl of isopropanol was added and
incubated for 10 min at room temperature. The next step was to
centrifuge the samples to obtain a visible precipitate at the
bottom of the tubes, which was dissolved in 1 ml of cold 75%
ethanol by vortexing followed by centrifugation. The resulting
supernatant was removed and the resulting samples were dried at
room temperature for 5-10 min. 50 µl of highly purified analytical
water Molecular Biology Grande Water Eppendorf® (Thermo
Fisher Scientific, Massachusetts, USA) was added to the prepared
sediment and dissolved in a Dry Block Thermostat Bio-TDB (Biosan,
Biogenet, Józefów, Poland) at 55˚C for 10 min. BioPhotometer Model
#6131 (Eppendorf® Marshall Scientific, Cambridge, United
Kingdom) spectrophotometer was used to measure the concentration of
the obtained RNA at the absorbance of 260-280 nm.
Reverse transcription-quantitative PCT
(RT-qPCR)
The qPCR technique is used to quantitatively analyze
the mRNA expression of the studied genes. The analysis was carried
out using the Roche LightCycler 480 (Roche Polska, Warszawa)
apparatus. The first step was to perform a reverse transcription
reaction using the Applied Biosystem protocol (Thermo Fisher
Scientific, Massachusetts, USA). This process consists in
transcribing the RNA template into cDNA while creating appropriate
reaction conditions. The reaction is performed using an MJ Mini
Personal Thermal Cycler (Bio-Rad® Poland, Warszawa
Poland).
The second step was to perform qPCR analysis with
the use of cDNA obtained in the process of reverse transcription.
The method using the dye SYBR Green I (Roche Poland, Warszawa,
Poland) was used. This mixture is placed in tubes suitable for the
Roche LightCycler 480 (Roche Poland, Warszawa, Poland) instrument.
he sequence of the primers for the tested genes was obtained from
PrimerBank (https://pga.mgh.harvard.edu/primerbank/GenBank
Accession NM_000207): SOD1: forward-GAAGGTGTGGGGAAGCATTA;
reverse-CCACCGTGTTTTCTGGATAGA. CAT: forward-TCAGGCAGAAACTTTTCCATT;
reverse-TGGGTCGAAGGCTATCTGTT. NADPHox:
forward-GAAGAAGATGTGGGAACGGG; reverse-GTATGTCTTTGCCTCCCACC. CLD1:
forward-CCTATGACCCCAGTCAATGC; reverse-TCCCAGAAGGCAGAGAGAAG. NF-κB:
forward-TTGCTGGTCCCACATAGTTG; reverse-ATGTATGTGAAGGCCCATCC. Bcl-2:
forward-CGGAGGCTGGGATGCCTTTG; reverse-TTTGGGGCAGGCATGTTGAC.
This stage of the research was carried out with the
following profile: 95˚C/5 min, 45 repetitions (95˚C/30 sec, 63˚C/50
sec, 58˚C/30 sec, 72˚C/40 sec) the profile was as follows: 95˚C/5
min, 35 repetitions (95˚C/30 sec, 63˚C/50 sec, 58˚C/30 sec, 72˚C/40
sec) with single signal effect at the end each stage of primer
annealing (12).
ACTB was used as the reference gene (forward:
TCATGAAGTGTGACGTGGACC, revers: CAGGAGGAGCAATGATCTTGATCT). The IICq
method was used to determine the mRNA expression of the studied
genes (13).
Statistical analysis
All experiments were repeated three times. All data
were analyzed using Statistica TIBCO, version 13. The normality of
variable distribution was confirmed by the Shapiro-Wilks test and
visual analysis of data distribution. The one-way ANOVA and
post-hoc Tukey test were used to analyze the data. Data are
represented by means and SEM. P<0.05 was considered to indicate
a statistically significant difference.
Results
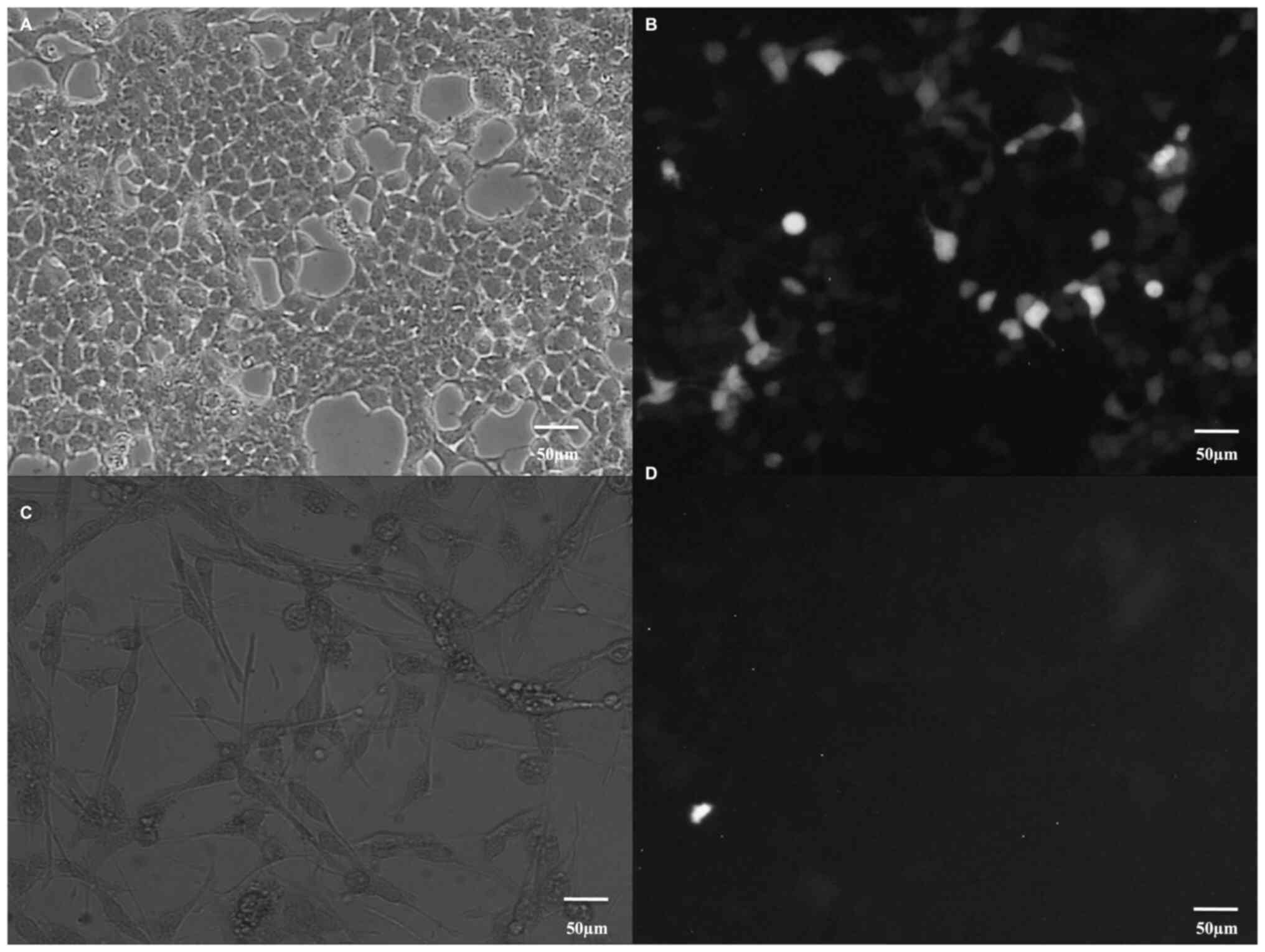
Retinol binding protein 2-RBP-2
At the first stage of the experiments, the
correctness of the culture was confirmed by showing the specific
marker of Caco-2 cells, which is RBP-2. In Fig. 1 (Fig.
1A-D), protein expression in Caco-2 cells is shown and compared
to the low expression of RBP-2 in astrocytes-NHA, cells that do not
produce retinol binding protein.
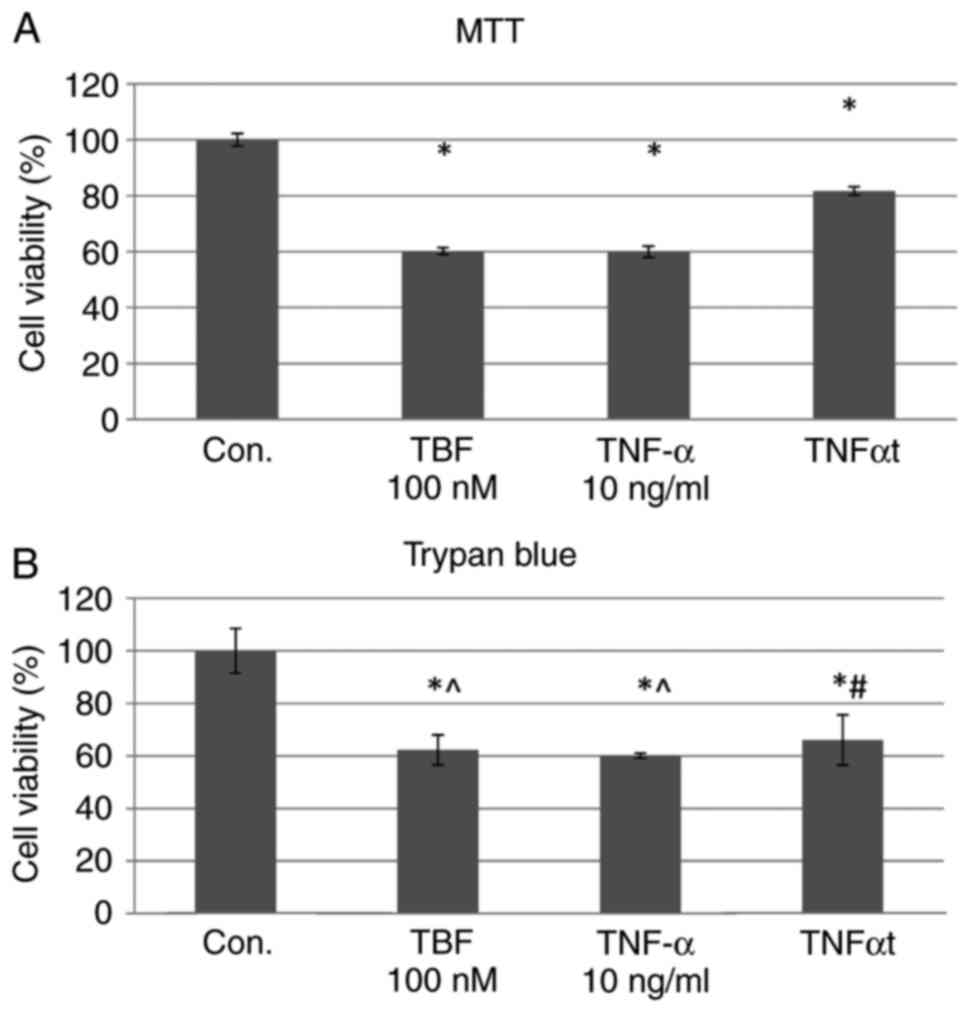
Evaluation of cell viability-trypan
blue
The use of TNFα and TBF resulted in changes compared
with in the control group, but we didn't observe differences
between used reagents (Fig.
2B).
Evaluation of cell proliferation-MTT
method
Analyzing the results of the experiment, we found
that the proliferation was higher after the addition of TBF
relative to TNFα, although still lower than the control [TNFα with
TBF (TNFαt) vs. TNFα 36.28%; P=0.001] (Fig. 2A).
Expression of proteins involved in the
maintenance of redox balance (SOD1, CAT, NADPH) SOD1
In the RT-qPCR study, after added TBF to the
culture, an increase in SOD expression was observed in cells
exposed to TNFα (TNFαt vs. TNFα 123.92%; P=0.0456), as well as
compared to the control sample (Con.) (TNFαt vs. Con. 24.5%;
P=0.0001) (Fig. 3A). This is
confirmed by the result of the western blot analysis, where
increased in the expression of the tested gene in relation to TNFα
(TNFαt vs. TNFα 62.56%; P=0.0001) and control (TNFαt vs. Con.
111.44%; P=0.0001) were noted (Fig.
3A).
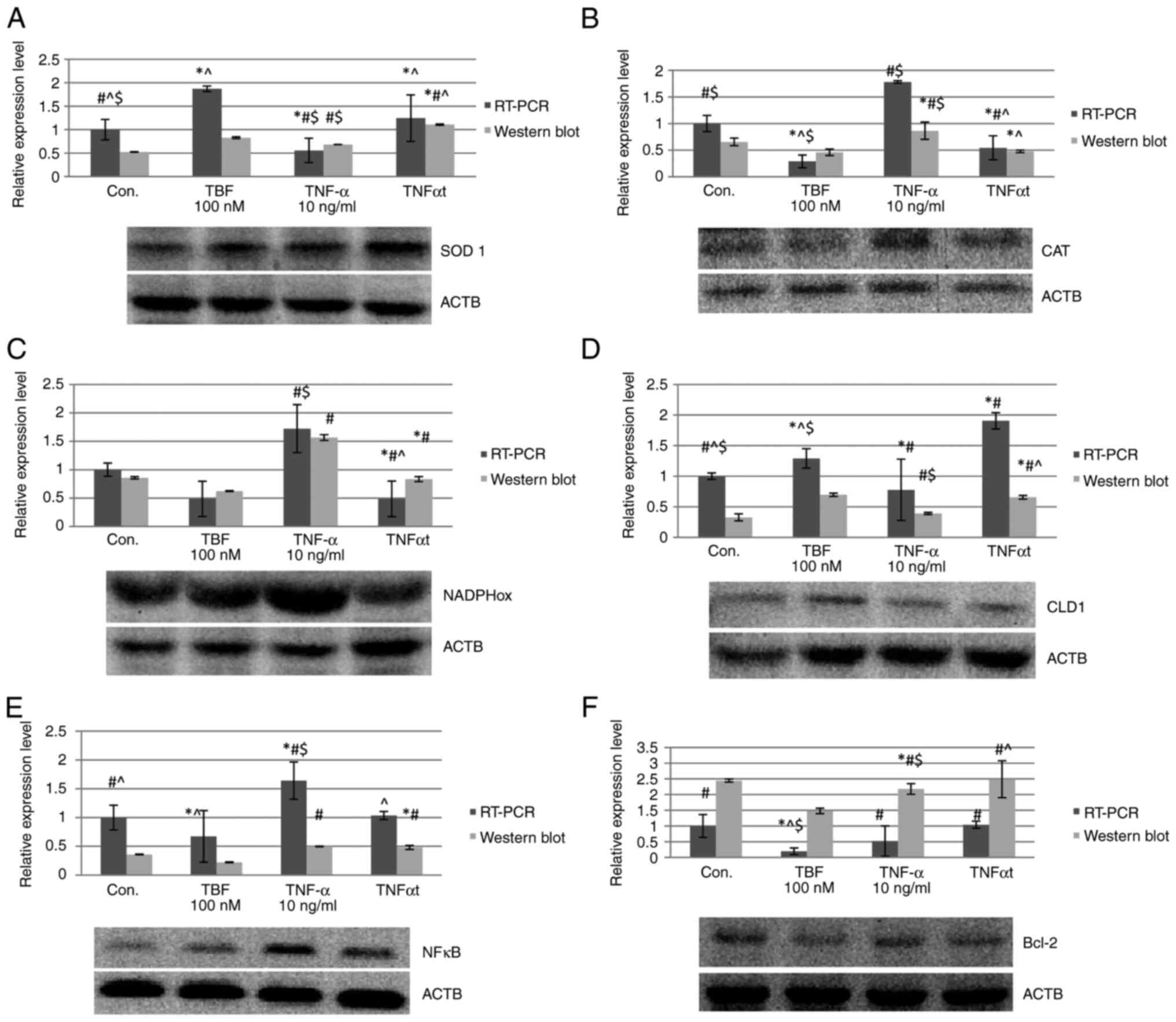 | Figure 3Expression results of the assessed
genes by RT-qPCR and western blotting. (A) SOD-1, (B) CAT, (C)
NADPHox, (D) CLD1, (E) NF-κB (nuclear factor κB) and (F) Bcl-2
(family of regulatory proteins involved in apoptosis) (F). Data
expressed as the mean ± SEM. *P<0.05 vs. Con.;
#P<0.05 vs. TBF; ^P<0.05 vs. TNFα;
$P<0.05 vs. TNFαt. ACTB, β-actin; Con-control; TBF,
tofacitinib; TNFα, tumor necrosis factor α; TNFαt, TNFα with TBF;
SOD-1, superoxide dismutase 1; CAT, catalase; NADPHox, nicotinamide
adenine dinucleotide phosphate oxidase; CLD1, claudin 1; NF-κB,
nuclear factor κB. |
CAT. The results of the RT-qPCR analysis
shown that medium containing TNFα with TBF decreased the expression
relative to medium containing only TNF (TNFαt vs. TNFα 69.41%;
P=0.0322). This decrease is also observed compared to controls
(TNFαt vs. Con. 45.5%; P=0.0001) (Fig.
3B). The results are consistent with western blot analysis
(TNFαt vs. TNFα 45.05%; P=0.0001; TNFαt vs. Con. 27.47%; P=0.0001)
(Fig. 3B).
NADPH. RT-qPCR analysis in the case of TNFα
together with TBF showed that the expression of the tested gene in
relation to TNFα was reduced (TNFαt vs. TNFα 71.80%; P=0.0015),
which was also observed in relation to the control culture (TNFαt
vs. Con. 57.41%; P=0.0033) (Fig.
3C). Western blot results show a decrease in NADPH expression
for TNFα with TBF relative to TNFα (TNFαt vs. TNFα 46.77%;
P=0.0001), but an increase relative to controls (TNFαt vs. Con.
2.76%; P=0.0001) (Fig. 3C).
Evaluation of cell phenotype-CLD1
expression
TNFα increased CLD-1 expression. The addition of TBF
to the medium containing TNF by RT-qPCR (TNFαt vs. TBF 47.44%;
P=0.0001) (Fig. 3D). This was
confirmed by western blot for TNFα (TNFαt vs. TNFα 67.48%;
P=0.0005) (Fig. 3D).
NF-κB expression
TBF reduces gene expression in the TNF experiment
(TNFαt vs. TNFα 36.97%; P=0.0001) when analyzed by RT-qPCR
(Fig. 3E). Western blot analysis
did not reach statistical significance for TNF with TBF (Fig. 3E).
Apoptosis assessment-Bcl-2
expression
For this analysis, a statistically significant
result was obtained only when TBF was added to culture medium
containing TNFα, where a slight increase in Bcl-2 expression was
observed (TNFαt vs. TNFα 110.57%; P=0.0068) (Fig. 3F). Statistical significance was not
obtained in the control case by western blot analysis and
confirmation of this result by RT-qPCR analysis (Fig. 3F).
Discussion
In our study, we assessed the effect of TBF on
Caco-2 cells after prior induction of inflammation by the
pro-inflammatory cytokine-TNFα, based on the analysis of parameters
such as anti-inflammatory, anti-apoptotic activity and markers of
oxidative stress. It was observed that the NADPHox (nicotinamide
adenine dinucleotide phosphate oxidase) expression was higher after
exposure to TNFα than after the use of TBF, which reduced the
expression of the studied gene. In addition, it was observed that
in the case of TNFα, there was an increase in the expression of
CLD-1, which may indicate an improvement in the tightness of the
cell barrier, and thus a protective effect on the epithelium.
Similar to observations in another study that showed a positive
effect of TBF on the cell barrier of IBD patients (3). In the first case, the authors induced
inflammation using IFNγ, which was responsible for increasing
permeability of the cell barrier and that effect was reduced after
the use of TBF (3). In the second
publication, the authors showed that TBF corrected defects in cell
barrier permeability both in vitro and in vivo
(14). Therefore, it can be
assumed that TBF has a beneficial effect on the regeneration of the
intestinal epithelium in people suffering from UC, and this
contributes to the remission of intestinal inflammation associated
with this disease.
In our experimental model, we observed an increase
in NADPHox and CAT expression and a decrease in SOD expression in
the case of TNFα. TBF reduced the increase in NADPHox and CAT
expression. In order for the oxygen explosion to occur, NADPHox
must be activated, which is associated with the appearance of ROS,
hence it can be assumed that ROS appeared in the experiment, which
secondarily triggered the cascade of cell protection against
oxidative stress. A decrease in SOD expression and an increase in
NADPH expression may indicate a direct SOD stimulation or an
indirect one involving p22 NADPHox oxidase. The increase in CAT
expression is caused by the action of reactive oxygen species
derived from ROS activation, which under the influence of SOD form
a hydrogen peroxide compound affecting CAT activation, and this
increases its expression in tests, which is a response to oxidative
stress caused by this situation. Similar to the observations of
Yang and Xie, it can be observed that TBF has an effect on the
mitigation of oxidative stress (15), which also confirms our
observations.
The increase in NF-κB expression with TNFα, followed
by a decrease in expression with the addition of TBF, illustrates
the involvement of NF-κB in cell protection processes. In addition,
SOD expression increases upon addition of TBF as observed by Zuo
et al in studies on the treatment of squamous cell carcinoma
of the esophagus, where it was observed that higher expression of
SOD-2 is associated with the expression of TNFα (16). It has also been shown that TNFα can
regulate SOD-2 and increase its level through the NF-κB pathway,
contributing to cell proliferation (16). ROS stimulate cells to produce
cytokines during inflammation, which leads to their damage and
consequently, to the aggravation of inflammation through TNFα,
which induces the production of intracellular mitochondrial ROS
(17). Similar to the observations
of Zhao et al, the present study observed an increase in
NADPHOx expression in the case of TNFα, which may indicate the
appearance of ROS in Caco-2 cells (17).
Studies have shown that administration of TBF
increases the expression of Bcl-2. This may indicate a protective
effect of TBF on intestinal epithelial cells by inhibiting
apoptotic processes induced by TNF-induced inflammation. This was
also demonstrated in a study by Yang and Xie, who observed similar
results in their study where they checked cell viability with Bcl-2
after inflammation and when fed into a TBF culture. It has been
shown that TBF can increase cell viability and at the same time
inhibit apoptosis (15). In
another study, TBF was also observed to inhibit the expression of
anti-apoptotic BCL-A1 and BCL-XL and induce apoptosis in human
dendritic plasmacytoids (PDC). Stimulation of Toll-like 7 receptors
increased the level of anti-apoptotic Bcl-2 family members and
initiated the induction of PDC apoptosis by TBF (18).
The increase in CLD-1 expression after the addition
of TBF may suggest a positive effect of TBF on the intestinal
epithelium, stimulating it to regenerate by increasing
intercellular connections after TNFα stimulation. The activation of
cells by an inflammatory factor followed by inhibition by TBF in
the case of CLD-1 appears to be related to the JAK-STAT pathway,
which is involved in cell repair processes. Similarly to the
observations obtained by Sayoc-Becerra et al, the use of
IFNγ as an inflammatory factor and then TBF confirmed its positive
effect on intestinal epithelial cells (19). As observed in another study on the
epithelial-mesenchymal transition (EMT) in cancer progression and
malignancies, including colorectal cancer, TNF also increased the
expression of claudin-1, accompanied by an increase in
proliferation and wound healing rate (20). Zhang et al reported that
tight junction dysfunction plays an important role in some chronic
inflammatory diseases. TNFα acts as a disruptor of the tight
junctions of the intestinal epithelium in inflammatory conditions.
It was observed that the expression of claudin-2 was induced by
TNFα (21) which is confirmed by
our observations.
The increase in NF-κB TNFα expression may result
from the stimulation of the immune system through contact of the
intestinal microbiome with the pathogen. As observed by Mahalanobis
et al, the immune system maintains a balance with the gut
microbiome and recognizes molecular patterns associated with
pathogens that activate transcription factors, including NF-κB and
related inflammatory pathways (22). Immune cells of the intestinal
mucosa are also involved in the inflammatory process, producing
pro-inflammatory factors such as TNFα and IFNγ. This activates
pro-apoptotic signaling involving proteins such as
caspase-1(23).
The increase in CLD-1 expression relative to the
control in the case of TNFα with TBF may be related to the
phenomenon of autophagy. Foerster et al in their work
described the phenomenon of macroautophagy/autophagy, the role of
which in maintaining the intestinal epithelium seems to be an
interesting process (24). Several
autophagy-related genes have been linked to gut diseases. It is a
catabolic cellular process responsible for the destruction of
cellular pathogens and the processing of organelles and protein
aggregates involving lysosomes. It has been observed that the
autophagy process is involved in intestinal repair, but also
supports the function of the intestinal barrier by regulating tight
junctions and protecting against cell death, and is responsible for
maintaining proper homeostasis (24). The phenomenon of autophagy may
explain the increase in CLD-1 expression for TNFα from TBF observed
in my study. Autophagy is of great importance for intestinal stem
cells (IECs), taking part in their metabolic processes and
affecting their proliferative and regenerative abilities, and is
also of great importance for reducing oxidative stress caused by
ROS (24). Therefore, we can
assume that the phenomenon of autophagy occurred in our experiment,
which seems to be confirmed by the obtained results.
In conclusion, studies using TBF as a molecule to
mitigate inflammation caused in Caco-2 cells contribute to a
positive effect on cells and cellular connections. In our studies,
we showed that TBF positively affected the mitigation of oxidative
stress associated with inflammation, had an anti-apoptotic effect
by reducing Bcl-2 expression, and also affected the reconstruction
of cellular connections lost as a result of the irritating effect
of burning cytokines. Many of our studies have been cited in
publications supporting the hypotheses.
Due to of the use of only the Caco-2 cell line as a
model of the intestinal epithelium in the study, this led to
limitations in the results. These are cancerous cells and
incompletely represent healthy, non-cancerous intestinal
epithelium. In addition, in vitro conditions do not fully
reflect the complexity of pathways between cells occurring in
living organisms. The reaction of tumor cells to inflammatory
factors after the use of classical inducers and inhibitors of
inflammation may differ from the reaction of normal enterocyte
cells.
Due to the described limitations, we plan to conduct
similar in vivo studies using laboratory animals in the
future to confirm the obtained results.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a research grant
from Medical University of Silesia (grant no. KNW-2B32/D/8/N).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ES conceived the study, performed the data analysis
and wrote the manuscript. LB designed the study, conducted the data
analysis and reviewed the manuscript. GM made substantial
contributions to study conception and design, or acquisition of
data, reviewed the experiments and laboratory studies, and reviewed
the manuscript. BO was responsible for data interpretation, and
critically revised the manuscript for important intellectual
contents. LB and GM confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Estera Skudrzyk ORCID ID 0000-0003-2536-3927. Łukasz
Bułdak ORCID ID 0000-0002-2017-5516. Grzegorz Machnik ORCID ID
0000-0002-9081-0984. Bogusław Okopień ORCID ID
0000-0001-7228-2906.
References
|
1
|
Kaniewska M, Eder P, Gąsiorowska A,
Gonciarz M, Kierkuś J, Małecka-Panas E and Rydzewska G: Biosimilar
biological drugs in the treatment of inflammatory bowel diseases.
Prz Gastroenterol. 14:223–227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Greenwood-Van Meerveld B: Intestinal
barrier function in health and gastrointestinal disease: Review
Article. Neurogastroenterol Motil. 24(889)2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spalinger MR, Sayoc-Becerra A,
Ordookhanian C, Canale V, Santos AN, King SJ, Krishnan M, Nair MG,
Scharl M and McCole DF: The JAK inhibitor tofacitinib rescues
intestinal barrier defects caused by disrupted
epithelial-macrophage interactions. J Crohns Colitis. 15:471–484.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Du L and Ha C: Epidemiology and
pathogenesis of ulcerative colitis. Gastroenterol Clin North Am.
49:643–654. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pilarczyk-Zurek M, Strus M, Adamski P and
Heczko PB: The dual role of Escherichia coli in the course of
ulcerative colitis. BMC Gastroenterol. 16(128)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ordás I, Eckmann L, Talamini M, Baumgart
DC and Sandborn WJ: Ulcerative colitis. Lancet. 380:1606–1619.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol Appendix 3: Appendix 3B,
2001.
|
|
8
|
Buranaamnuay K: The MTT assay application
to measure the viability of spermatozoa: A variety of the assay
protocols. Open Vet J. 11:251–269. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kurien BT and Hal Scofield R: Western
blotting: Methods and protocols. West Blotting Methods Protoc.
1312:1–509. 2015.
|
|
10
|
Bułdak Ł, Skudrzyk E, Machnik G, Bołdys A,
Bułdak RJ and Okopień B: Exenatide improves antioxidant capacity
and reduces the expression of LDL receptors and PCSK9 in human
insulin-secreting 1.1E7 cell line subjected to hyperglycemia and
oxidative stress. Postepy Hig Med Dosw. 76:16–23. 2022.
|
|
11
|
Bourne R and Bourne R: ImageJ. Fundam
Digit Imaging Med. 9:185–188. 2010.
|
|
12
|
Machnik G, Łabuzek K, Skudrzyk E, Rekowski
P, Ruczyński J, Wojciechowska M, Mucha P, Giri S and Okopień B: A
peptide nucleic acid (PNA)-mediated polymerase chain reaction
clamping allows the selective inhibition of the ERVWE1 gene
amplification. Mol Cell Probes. 28:237–241. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zihni C, Mills C, Matter K and Balda MS:
Tight junctions: From simple barriers to multifunctional molecular
gates. Nat Rev Mol Cell Biol. 17:564–580. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang J and Xie X: Tofacitinib protects
intestinal epithelial cells against oxygen-glucose
deprivation/reoxygenation injury by inhibiting the JAK/STAT3
signaling pathway. Exp Ther Med. 22(1108)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zuo J, Zhao M, Liu B, Han X, Li Y, Wang W,
Zhang Q, Lv P, Xing L, Shen H and Zhang X: TNF-α-mediated
upregulation of SOD-2 contributes to cell proliferation and
cisplatin resistance in esophageal squamous cell carcinoma. Oncol
Rep. 42:1497–506. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao M, Tang S, Xin J, Wei Y and Liu D:
Reactive oxygen species induce injury of the intestinal epithelium
during hyperoxia. Int J Mol Med. 41:322–330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bhat AA, Ahmad R, Uppada SPB, Singh AB and
Dhawan P: Claudin-1 promotes TNF-α-induced epithelial-mesenchymal
transition and migration in colorectal adenocarcinoma cells. Exp
Cell Res. 349:119–127. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sayoc-Becerra A, Krishnan M, Fan S,
Jimenez J, Hernandez R, Gibson K, Preciado R, Butt G and McCole DF:
The JAK-Inhibitor tofacitinib rescues human intestinal epithelial
cells and colonoids from cytokine-induced barrier dysfunction.
Inflamm Bowel Dis. 26:407–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Catanzaro D, Rancan S, Orso G, Dall'Acqua
S, Brun P, Giron MC, Carrara M, Castagliuolo I, Ragazzi E,
Caparrotta L and Montopoli M: Boswellia serrata preserves
intestinal epithelial barrier from oxidative and inflammatory
damage. PLoS One. 10(e0125375)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang C, Yan J, Xiao Y, Shen Y, Wang J, Ge
W and Chen Y: Inhibition of Autophagic degradation process
contributes to claudin-2 expression increase and epithelial tight
junction dysfunction in TNF-α treated cell monolayers. Int J Mol
Sci. 18(157)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mahalanobish S, Dutta S, Saha S and Sil
PC: Melatonin induced suppression of ER stress and mitochondrial
dysfunction inhibited NLRP3 inflammasome activation in COPD mice.
Food Chem Toxicol. 144(111588)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Friedrich M, Pohin M and Powrie F:
Cytokine networks in the pathophysiology of inflammatory bowel
disease. Immunity. 50:992–1006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Foerster EG, Mukherjee T, Cabral-Fernandes
L, Rocha JDB, Girardin SE and Philpott DJ: How autophagy controls
the intestinal epithelial barrier. Autophagy. 18:86–103.
2022.PubMed/NCBI View Article : Google Scholar
|