Introduction
Acute myeloid leukemia (AML) is a malignant tumor of
the hematopoietic system characterized by abnormal differentiation
and excessive proliferation of hematopoietic stem cells, which may
also be accompanied by invasion of the bone marrow, peripheral
blood and extramedullary tissue (1). AML is the most common type of
leukemia in adults, with a median age of 68 years at diagnosis, and
its incidence increases with age (2). Compared with younger patients, older
patients are more likely to have adverse cytogenetic risks,
secondary AML, monosomal karyotypes and multidrug-resistant
phenotypes, as well as more comorbidities and impaired organ
function, thereby reducing their tolerance to intensive induction
therapy and leading to higher rates of treatment-related mortality
(3-6).
At present, the clinical treatment of AML mainly
follows ‘3+7’ induction chemotherapy and high-dose cytarabine-based
consolidation chemotherapy or allogeneic hematopoietic stem cell
transplantation. However, in the actual treatment, the space to
improve the clinical efficacy of the single application of
conventional chemotherapy for AML is limited (7). In recent years, with the rise of
molecular-targeted drugs for the treatment of leukemia,
molecular-targeted therapy with novel targeted drugs, the
combination of targeted drugs and their combination with intensive
chemotherapy have attracted increasing attention. The BCL-2 protein
is a key factor that regulates the mitochondrial apoptotic pathway,
and the survival of leukemia stem cells depends on oxidative
phosphorylation and BCL-2 upregulation (8). Notably, BCL-2 has recently become a
target for leukemia treatment. Venetoclax is a powerful oral BCL-2
inhibitor, the efficacy and safety of which have been confirmed.
The combination regimen of venetoclax with demethylated drugs
(decitabine or azacytidine) was approved by the U.S. Food and Drug
Administration in November 2018 for the clinical treatment of older
adult patients (≥65 years old) with AML (9,10).
Azacitidine, a recently developed demethylation drug, is a
nucleoside metabolic inhibitor that can exert the dual effect of
RNA and DNA demethylation and effectively inhibit the synthesis of
proteins in tumor cells (11,12).
Notably, it has been reported that venetoclax combined with
azacytidine has good clinical value in patients with AML (13). Although the long-term benefits were
not maintained in some patients, this regimen can significantly
improve survival in patients who are not candidates for intensive
chemotherapy. Most published studies are on a generally small size
(14-17);
therefore, the evidence for these findings is limited. To further
optimize the formulation of chemotherapy regimens for patients with
AML, exploration of the predictors of efficacy of combination
regimens is necessary to guide clinical decision-making. Therefore,
the present study focused on the efficacy of venetoclax combined
with azacitidine and azacitidine monotherapy in patients with
AML.
Materials and methods
Retrieval strategy
The Web of Science (https://www.webofscience.com/wos), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase
(https://www.embase.com), Cochrane Library
(https://www.cochranelibrary.com/), Weipu
Database (http://www.cqvip.com), Wanfang Digital
Periodicals (https://www.wanfangdata.com.cn), Sinomed (http://www.sinomed.ac.cn), China National Knowledge
Infrastructure (https://www.cnki.net/), ProQuest
Dissertations and Theses (http://pqdtopen.proquest.com/) and Cumulative Index to
Nursing and Allied Health Literature (https://www.ebsco.com/products/research-databases/cinahl-database)
were searched for relevant literature. The search was carried out
from the establishment of the database to May 2023, with two
researchers independently conducting literature searches. The
search keywords were (‘Venetoclax’ OR ‘ABT-199’ OR ‘Venclexta’ OR
‘RG7601’ OR ‘RG-7601’ OR ‘GDC-0199’, ‘leukemia, myeloid, acute’ OR
‘acute myeloid leukemia’ OR ‘AML’ OR ‘acute nonlymphocytic
leukemia’).
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Research
participants: Adult patients diagnosed with AML; ii) intervention
measure: Experimental group (venetoclax combined with azacitidine),
control group (azacitidine monotherapy); iii) outcome index:
Complete remission (CR), partial remission (PR), no remission (NR)
and adverse events (AEs); iv) Study design types: Controlled
clinical trial. The exclusion criteria were as follows: i) Age
<18 years, patients with non-AML; ii) reviews, systematic
reviews, case reports, letters and republished studies; iii)
non-case control studies; iv) incomplete or irrelevant treatment
outcome reports.
Data extraction
Two independent researchers extracted data
separately according to Cochrane systematic review methodology, and
when there was a disagreement, it was resolved through discussion
or joint evaluation with more senior researchers until a consensus
was reached. The literature was scored according to the
Newcastle-Ottawa scale (18). In
the outcome measurement items, the follow-up time was defined as ≥1
year, the loss rate was ≤15%, and the scores were divided into low,
medium and high as follows: <5, 5-8 and 8-9 points,
respectively.
Statistical analysis
All extracted data were analyzed using Review
Manager 5.4 (https://tech.cochrane.org/revman). Binary variables
were represented according to the odds ratio (OR) and 95%
confidence interval (CI) of the results. A random-effects model was
used for summary analysis when I² was ≤50% between the study
groups. When heterogeneity could not be completely eliminated, a
random-effects model was adopted. A funnel plot was constructed to
assess publication bias by removing studies with high heterogeneity
for the sensitivity analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Search result
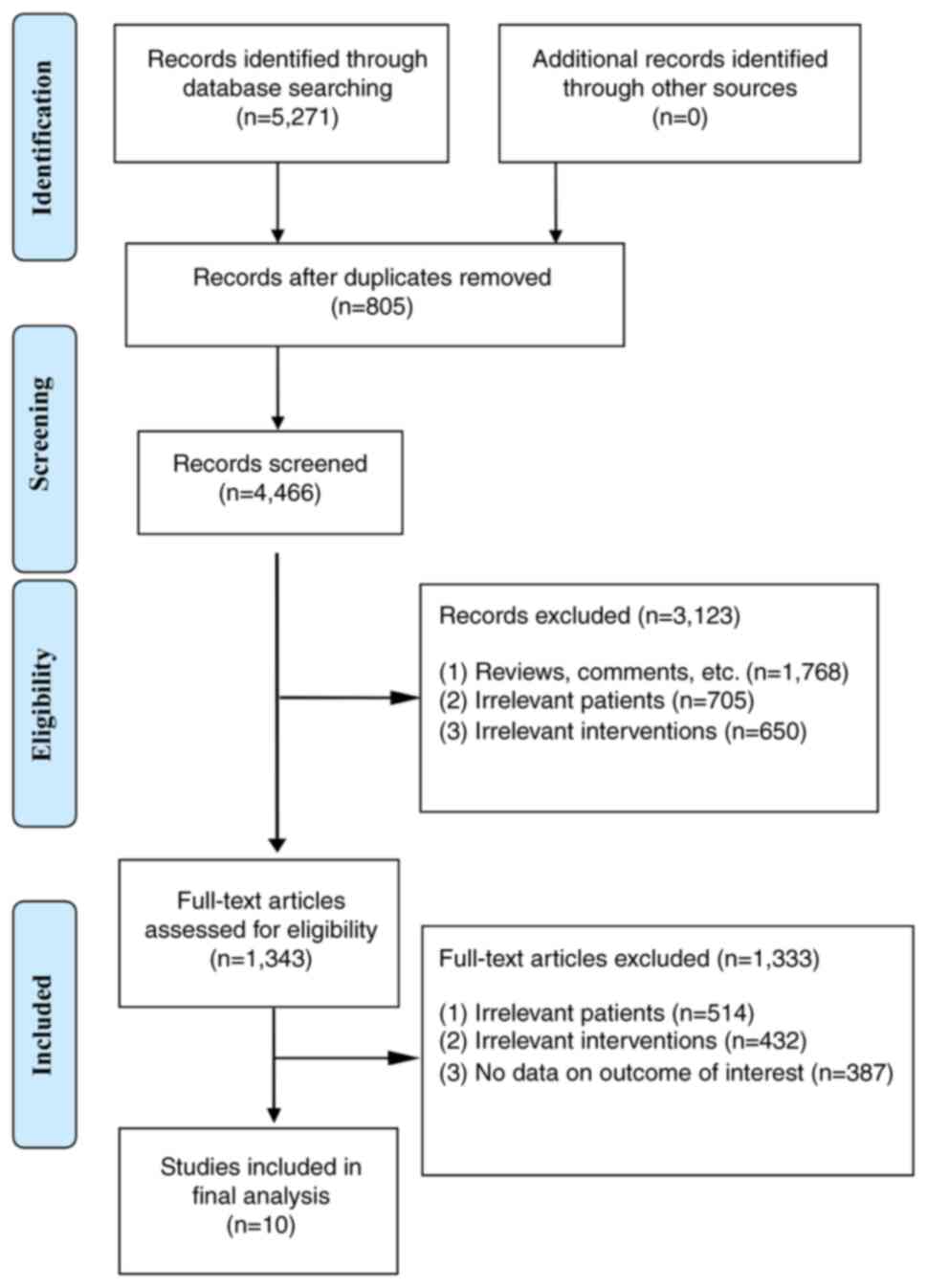
According to the search strategy of the present
study, 5,271 relevant articles were retrieved from major databases.
The inclusion and exclusion criteria were strictly implemented and
10 studies (14-17,19-24)
were included.
Information included from the
literature
A total of 1,988 patients were included, with 1,323
treated with venetoclax combined with azacitidine and 665 treated
with azacitidine monotherapy. The included studies reported six
hematological, five gastrointestinal, nine infectious and four
serious AEs, as well as two studies each of hypokalemia, decreased
appetite and hepatic insufficiency. CR was observed in eight
studies, PR in seven studies and NR in eight studies. The
literature screening process and results are shown in Fig. 1, and the basic characteristics of
the included studies are listed in Table I.
 | Table IBasic characteristics of the included
studies. |
Table I
Basic characteristics of the included
studies.
| First author,
year | Research type | Nation | Year | Intervention | Case, n | Age, years (±
SD) | Sex,
Male/Female | Outcome index | Quality evaluation,
NOS score | (Refs.) |
|---|
| Cui, 2022 | RCT | China | 2022 | VEN + AZA | 15 | 40.23±10.58 | 10/5 | (1-4) | 8 | (14) |
| | | | | AZA | 15 | 39.82±10.17 | 9/6 | | | |
| DiNardo, | RCT | Multinational | 2020 | VEN + AZA | 286 | Median age: 76 | 112/174 | (1) | 5 | (19) |
| | | | | AZA | 145 | Median age: 76 | 58/87 | | | |
| Fu, 2022 | RCT | China | 2022 | VEN + AZA | 30 | 77.9±4.9 | 13/17 | (1-4) | 8 | (20) |
| | | | | AZA | 30 | 78.6±4.6 | 12/18 | | | |
| Jonas, 2020 | Retrospective
cohort study | America | 2020 | VEN + AZA | 293 | - | - | (1-4) | 8 | (21) |
| | | | | AZA | 146 | - | - | | | |
| Pollyea, 2020 | Retrospective
cohort study | America | 2020 | VEN + AZA | 306 | - | - | (1,2) | 6 | (22) |
| | | | | AZA | 127 | - | - | | | |
| Pollyea, 2022 | Retrospective
cohort study | America | 2022 | VEN + AZA | 308 | - | 182/126 | (1,4) | 6 | (23) |
| | | | | AZA | 127 | - | 76/51 | | | |
| Wang, 2022 | RCT | China | 2022 | VEN + AZA | 10 | 70.21±8.39 | 5/5 | (1-4) | 9 | (15) |
| | | | | AZA | 10 | 70.44±7.61 | 6/4 | | | |
| Xia, 2023 | RCT | China | 2023 | VEN + AZA | 21 | - | 14/7 | (1-4) | 8 | (16) |
| | | | | AZA | 14 | - | 7/7 | | | |
| Yamamoto, 2021 | RCT | Japan | 2021 | VEN + AZA | 24 | - | 14/10 | (1-4) | 8 | (17) |
| | | | | AZA | 13 | - | 9/4 | | | |
| Yang, 2022 | Retrospective
cohort study | China | 2022 | VEN + AZA | 30 | 68.13±7.42 | 18/12 | (1-4) | 9 | (24) |
| | | | | AZA | 38 | 67.82±5.55 | 24/14 | | | |
Quality evaluation of the included
literature
A total of 10 studies, including six prospective and
four retrospective studies, were included. The Newcastle-Ottawa
scale was used for quality evaluation, among which two studies
scored 9 points, five studies scored 8 points, two studies scored 6
points, and one study scored 5 points. Seven studies were of high
quality and three were of medium quality.
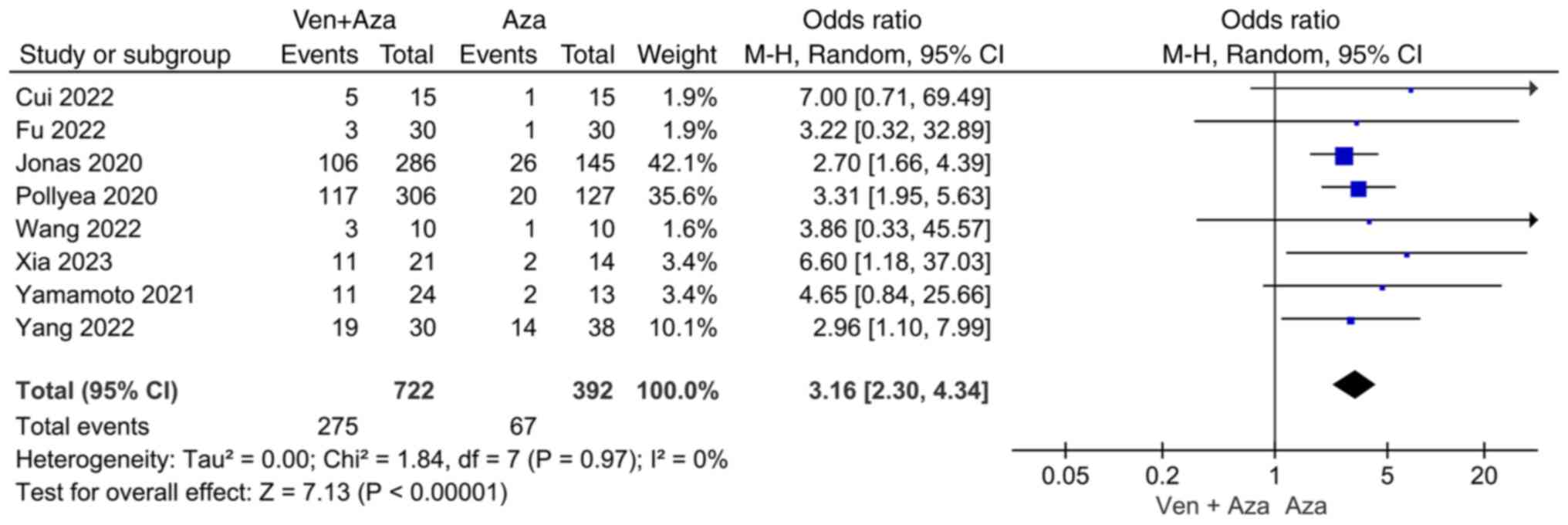
Meta-analysis results. Comparison of
CR
Eight studies compared CR events between venetoclax
combined with azacitidine and azacitidine monotherapy. The
heterogeneity test (I2=0%) indicated no significant
heterogeneity among the studies, and a random-effects model was
used for classification. The results showed that CR events in
patients with AML treated with azacitidine monotherapy were
significantly lower than those in patients with AML treated with
venetoclax combined with azacitidine (95% CI=2.30, 4.34;
P<0.00001; Fig. 2).
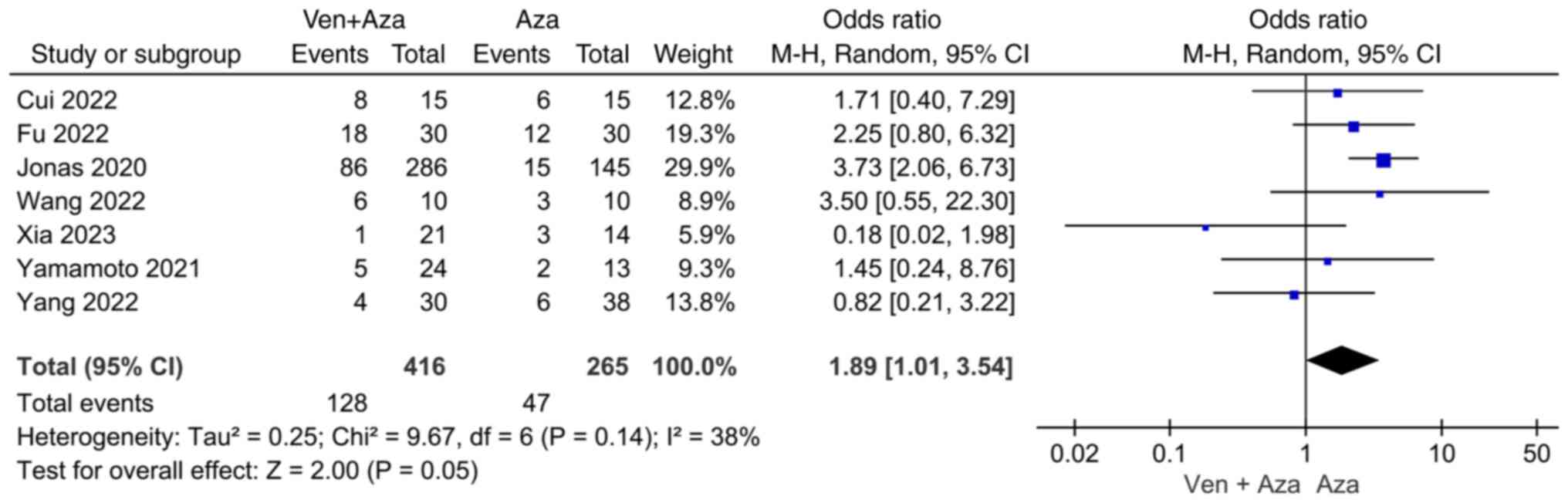
Comparison of PR. Seven studies compared PR
events between venetoclax combined with azacitidine and azacitidine
monotherapy. Heterogeneity was observed among the studies
(I2=38%); therefore, a random-effects model was used for
classification. The results revealed no significant difference in
PR events between the venetoclax combined with azacitidine group
and the azacitidine monotherapy group (95% CI=1.01, 3.54; P=0.05;
Fig. 3).
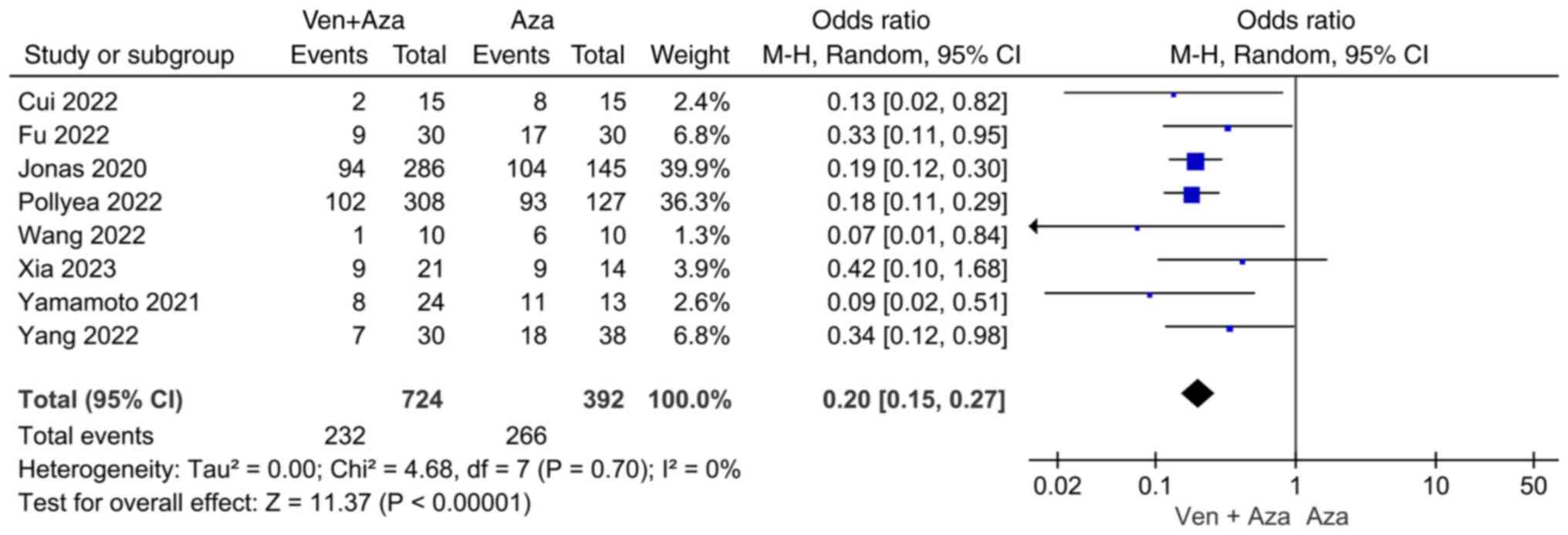
Comparison of NR. Eight articles compared NR
events between venetoclax combined with azacitidine and azacitidine
monotherapy. The heterogeneity test (I2=0%) indicated no
significant heterogeneity among the studies, and the random-effects
model was used for classification. The results showed that NR
events in patients with AML treated with venetoclax combined with
azacitidine were significantly lower than in patients with AML
treated with azacitidine monotherapy (95% CI=0.15, 0.27;
P<0.00001; Fig. 4).
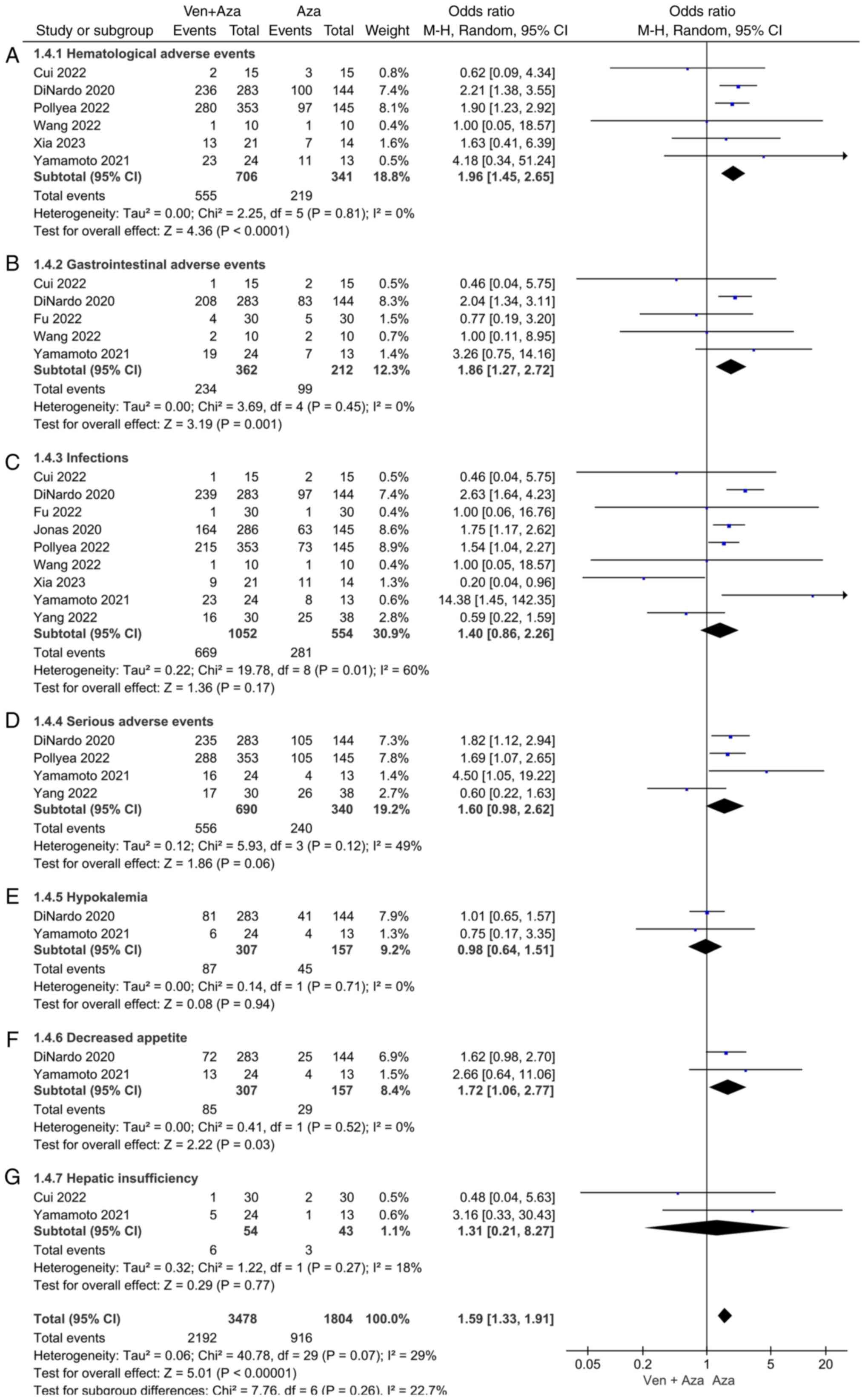
Comparison of AEs. Hematological,
gastrointestinal, infectious and serious AEs, as well as
hypokalemia, decreased appetite and hepatic insufficiency, were
included (Fig. 5). Six studies
compared hematological AEs between venetoclax combined with
azacitidine and azacitidine monotherapy for AML. The heterogeneity
test (I2=0%) indicated no significant heterogeneity
among the studies and a random-effects model was used for
classification. The results showed that hematological AEs in the
treatment of AML were significantly lower in patients treated with
azacitidine monotherapy than those treated with venetoclax combined
with azacitidine (95% CI=1.45, 2.65; P<0.0001).
Five studies compared gastrointestinal AEs in the
treatment of AML between venetoclax combined with azacitidine and
azacitidine monotherapy. The heterogeneity test (I2=0%)
indicated no significant heterogeneity among the studies and a
random-effects model was used for classification. The results
showed that in the treatment of AML, the incidence of
gastrointestinal AEs in response to azacitidine alone was lower
than that in response to venetoclax combined with azacitidine, and
the difference was statistically significant (95% CI=1.27, 2.72;
P=0.001).
Two studies compared the decreased appetite events
between venetoclax combined with azacitidine and azacitidine
monotherapy in patients with AML. The heterogeneity test
(I2=0%) indicated no significant heterogeneity among the
studies and a random-effects model was used for classification. The
results showed that in the treatment of AML, the incidence of
decreased appetite in response to azacitidine alone was lower than
that in response to venetoclax combined with azacitidine, and the
difference was statistically significant (95% CI=1.06, 2.77;
P=0.03).
In addition, no significant differences were
observed regarding infectious AEs, serious AEs, hypokalemia or
hepatic insufficiency between the two groups [(95% CI=0.86, 2.26;
P=0.17), (95% CI=0.98, 2.62; P=0.06), (95% CI=0.64, 1.51; P=0.94),
(95% CI=0.21, 8.27; P=0.77), respectively].
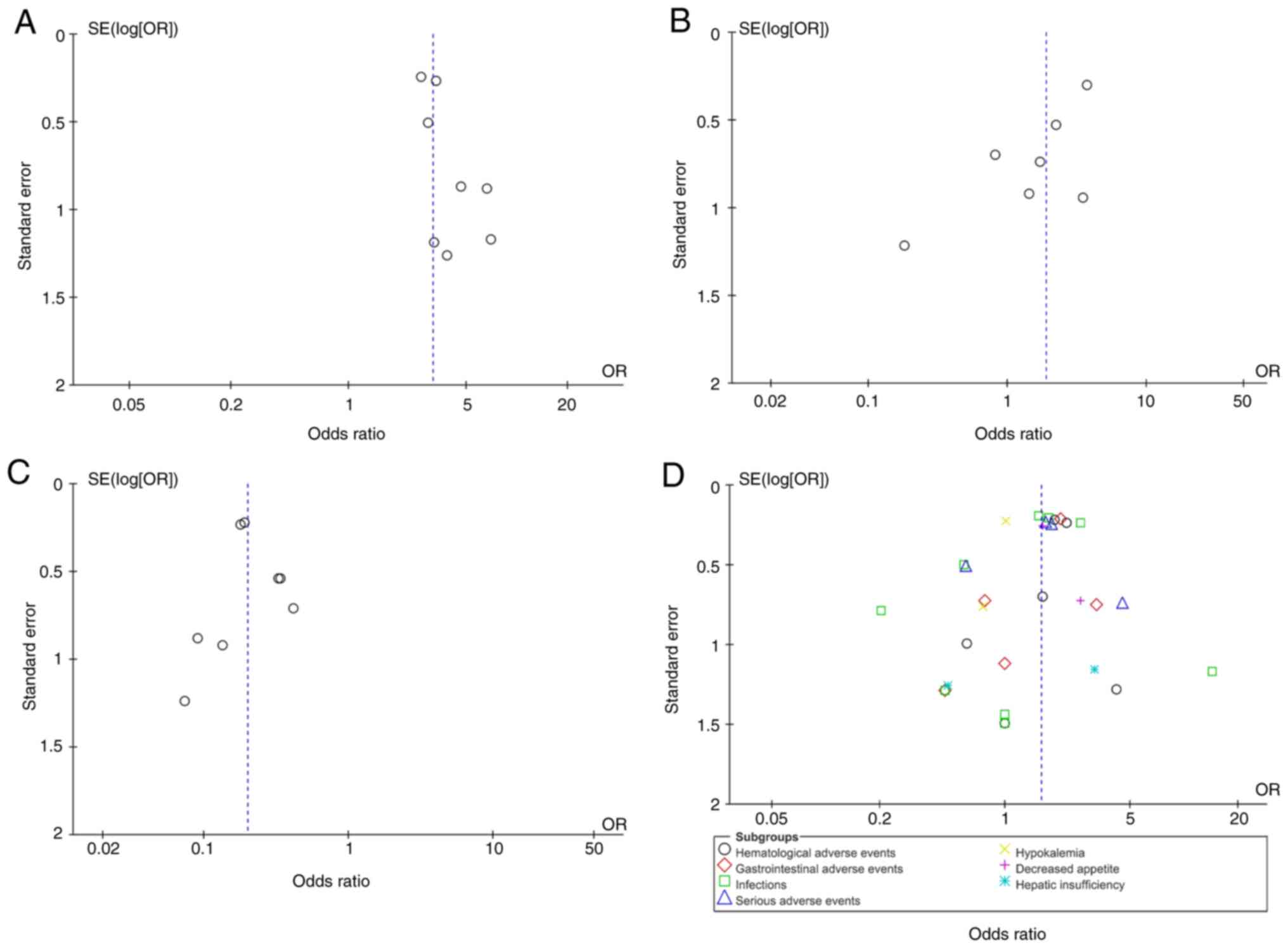
Publication bias and sensitivity analysis.
Review Manager 5.4 statistical software was used to analyze
publication bias for four outcome indicators: CR, PR, NR and AEs.
References were individually excluded for sensitivity analysis, and
the results were stable. The data were also considered stable and
reliable after the sensitivity analysis. The results showed that
the funnel plots were symmetric, suggesting no significant
publication bias (Fig. 6).
Discussion
AML is a hematological disease with a relatively
high incidence, which is characterized by rapid onset and
progression. Most patients have several notable symptoms after the
onset of the disease, which can have a serious impact on the life
and health of patients if not treated in a timely manner (25). AML is characterized by clonal
proliferation of malignant bone marrow stem cells, and is often
accompanied by infection, anemia and bleeding (26). Currently, combination chemotherapy
is the preferred treatment for clinically naïve patients with AML.
However, conventional chemotherapy regimens may lead to drug
resistance, whereas high-dose chemotherapy leads to severe
myelosuppression (27). In recent
years, the emergence of novel targeted drugs has provided
innovative options for patients with AML not eligible for intensive
induction chemotherapy, effectively improving the response and
survival rates.
BCL-2 is a key molecule in the regulation of
apoptosis of tumor cells and is a novel target for the treatment of
leukemia (28). The BCL-2 protein
family is an important regulator of endogenous apoptotic pathways.
Notably, BCL-2 is upregulated in AML and its stem cells, thereby
mediating the survival of AML cells, and their resistance to
chemotherapy and targeted therapies (29,30).
Venetoclax was the first marketed BCL-2 inhibitor (1); this drug induces the apoptosis of
tumor cells and improves AML treatment sensitivity by targeting
BCL-2. It has previously been shown that a combination of
venetoclax and hypomethylating agents can delay the development of
drug resistance, and improve the remission and survival rates of
patients with AML (31).
Azacitidine, a recently developed demethylation drug, is a
nucleoside metabolic inhibitor that can exert the dual effect of
RNA and DNA demethylation and effectively inhibit the synthesis of
proteins in tumor cells (31,32).
Relevant clinical trials have shown that compared with the
traditional treatment regimen, azacitidine can effectively optimize
the treatment effect and prolong the survival of patients with AML
(33). Moreover, azacitidine is an
effective and low-toxicity alternative for patients with AML who
have lost the opportunity for transplantation and have difficulty
tolerating traditional chemotherapy regimens (33,34).
However, in untreated patients with AML aged ≥65 years, azacitidine
monotherapy has a response rate of ≤30% and results in survival
time of <1 year (35).
Preclinical studies (11,36,37)
have shown that azacitidine enhances the antitumor effect of
venetoclax by activating the transcription of the pro-apoptotic
protein NOXA, and that the combination of azacitidine and
venetoclax induces deep and long-lasting anti-leukemia effects by
blocking the energy metabolism of leukemia stem cells.
To evaluate the therapeutic effect of venetoclax
combined with azacitidine, and provide more evidence for the
selection of clinical treatment plans, the present study conducted
a meta-analysis using venetoclax combined with azacitidine as the
observation group and azacitidine alone as the control group. The
aim was to observe the effects of these two treatment regimens on
clinical efficacy and the AEs of patients with AML. Through data
analysis, the present meta-analysis confirmed that azacitidine +
venetoclax combination therapy exhibited a significant advantage in
improving the CR rate of patients with AML (95% CI=2.30, 4.34;
P<0.00001). Significant heterogeneity was not observed
(I2=0%). DiNardo et al (19) reported that the composite CR rate
of patients in the azacitidine + venetoclax group was 66.4%, which
was significantly higher than that of patients in the azacitidine
monotherapy group. The results of Cui et al (14) also showed that the total effective
rate of the azacitidine + venetoclax group (86.67%) was
significantly higher than that of the azacitidine group (46.67%)
(P<0.05).
In the present study, no statistically significant
difference was observed between the azacitidine + venetoclax
combination treatment and azacitidine monotherapy groups regarding
the occurrence of PR events in patients with AML (95% CI=1.01,
3.54; P=0.05). However, there was a statistically significant
difference between the venetoclax + azacitidine combination
treatment and azacitidine monotherapy groups regarding the
occurrence of NR events (95% CI=0.15, 0.27; P<0.00001), with no
significant heterogeneity (I2=0%), suggesting that
venetoclax combined with azacitidine resulted in a lower incidence
of NR events than azacitidine monotherapy in the treatment of AML.
Therefore, it was concluded that azacitidine + venetoclax
combination therapy may be superior to azacitidine monotherapy, and
that combination therapy can significantly improve the incidence of
CR in patients with AML. The present results are consistent with
those of previous clinical studies (14-17,20-22,24)
and practical experience, supporting the clinical feasibility and
effectiveness of this treatment regimen. In addition, it is worth
noting that the present meta-analysis observed that the overall
incidence of AEs in patients with AML treated with venetoclax
combined with azacitidine was significantly higher than those in
patients treated with azacitidine monotherapy (95% CI=1.33, 1.91;
P<0.000001); however, there was heterogeneity
(I2=29%). The studies by Xia et al (16) and Yang et al (24) showed a significant impact on the
incidence of AEs. Moreover, the most common AEs in both groups were
hematological (pooled OR=1.96; 95% Cl=1.45, 2.65; P<0.0001) and
gastrointestinal (pooled OR=1.86; 95% Cl=1.27, 2.72; P=0.001).
These findings are consistent with those of previous studies
(17,23).
Compared with previous similar studies, such as
those by Du et al (38) and
Bewersdorf et al (39), the
present study has several advantages. First, few randomized
controlled trials were included in the previous studies, and there
was a lack of prospective studies that could affect the reliability
of the results. The present meta-analysis included six randomized
controlled trials, including prospective studies, which increased
the reliability of the results. Second, more than half of the
participants in previous studies were from the U.S.; therefore,
there is insufficient evidence on whether the results of previous
studies can be generalized to other populations. The present study
included research on Chinese patients and patients from other
countries, further demonstrating the efficacy and safety of
azacitidine + venetoclax for treating patients with AML from
different countries. Third, the data in previous meta-analyses were
highly heterogeneous, and it was difficult to determine the cause
of the heterogeneity. However, the current study showed low
heterogeneity in the statistical data, demonstrating the
reliability of the results.
Although the results of the present meta-analysis
showed the advantages of azacitidine + venetoclax in AML treatment,
some limitations should be noted. First, because the data sources
were mainly clinical trials and literature reports, there may have
been selective reporting and publication bias. Second, the dose and
course of treatment used in the different studies may have produced
some heterogeneity, affecting the reliability of the results. Based
on the findings of the present study, we recommend that azacitidine
+ venetoclax combination therapy for AML be further promoted in
clinical practice. However, more large-scale multicenter clinical
studies are needed to better evaluate the efficacy and safety of
this treatment regimen. We encourage further exploration of other
potential combination treatment options to improve the survival and
quality of life of patients with AML.
In conclusion, the present study observed that,
despite some adverse reactions, the combination regimen of
azacitidine and venetoclax did not lead to a deterioration in the
prognosis of patients with AML, which is of great significance for
the long-term treatment and quality of life of patients. Notably,
azacitidine monotherapy is often associated with relapse and a
series of side effects and AEs, such as bone marrow suppression and
liver function abnormalities (35). Therefore, the addition of
venetoclax serves a positive role in delaying disease recurrence
and alleviating adverse reactions in patients. In summary,
azacitidine + venetoclax has significant efficacy in AML treatment
and can improve the overall response rate of patients with high
safety.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Scientific and
Technological Innovation Programs of Higher Education Institutions
in Shanxi (grant no. 2021L353) and the Natural Science Foundation
for Young Scientists of Shanxi Province (grant no.
20210302124089).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YQX and XQW were responsible for the design of the
current study, and both performed the statistical analysis. YQX and
XQW confirm the authenticity of all the raw data. WWW and CSL were
responsible for the acquisition and sorting of data. PFH and YHY
performed the interpretation of the data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Konopleva M, Pollyea DA, Potluri J, Chyla
B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, et
al: Efficacy and biological correlates of response in a phase II
study of venetoclax monotherapy in patients with acute myelogenous
leukemia. Cancer Discov. 6:1106–1117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kadia TM, Reville PK, Wang X, Rausch CR,
Borthakur G, Pemmaraju N, Daver NG, DiNardo CD, Sasaki K, Issa GC,
et al: Phase II study of venetoclax added to cladribine plus
low-dose cytarabine alternating with 5-azacitidine in older
patients with newly diagnosed acute myeloid leukemia. J Clin Oncol.
40:3848–3857. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Richard-Carpentier G and DiNardo CD:
Venetoclax for the treatment of newly diagnosed acute myeloid
leukemia in patients who are ineligible for intensive chemotherapy.
Ther Adv Hematol. 10(2040620719882822)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pollyea DA, Amaya M, Strati P and
Konopleva MY: Venetoclax for AML: Changing the treatment paradigm.
Blood Adv. 3:4326–4335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wei AH, Strickland SA Jr, Hou JZ, Fiedler
W, Lin TL, Walter RB, Enjeti A, Tiong IS, Savona M, Lee S, et al:
Venetoclax combined with low-dose cytarabine for previously
untreated patients with acute myeloid leukemia: Results from a
phase Ib/II study. J Clin Oncol. 37:1277–1284. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
DiNardo CD, Pratz KW, Letai A, Jonas BA,
Wei AH, Thirman M, Arellano M, Frattini MG, Kantarjian H, Popovic
R, et al: Safety and preliminary efficacy of venetoclax with
decitabine or azacitidine in elderly patients with previously
untreated acute myeloid leukaemia: A non-randomised, open-label,
phase 1b study. Lancet Oncol. 19:216–228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vasu S, Kohlschmidt J, Mrózek K, Eisfeld
AK, Nicolet D, Sterling LJ, Becker H, Metzeler KH, Papaioannou D,
Powell BL, et al: Ten-year outcome of patients with acute myeloid
leukemia not treated with allogeneic transplantation in first
complete remission. Blood Adv. 2:1645–1650. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
DiNardo CD, Pratz K, Pullarkat V, Jonas
BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH,
Kantarjian HM, et al: Venetoclax combined with decitabine or
azacitidine in treatment-naive, elderly patients with acute myeloid
leukemia. Blood. 133:7–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choi JH, Bogenberger JM and Tibes R:
Targeting apoptosis in acute myeloid leukemia: Current status and
future directions of BCL-2 inhibition with venetoclax and beyond.
Target Oncol. 15:147–162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pollyea DA, Stevens BM, Jones CL, Winters
A, Pei S, Minhajuddin M, D'Alessandro A, Culp-Hill R, Riemondy KA,
Gillen AE, et al: Venetoclax with azacitidine disrupts energy
metabolism and targets leukemia stem cells in patients with acute
myeloid leukemia. Nat Med. 24:1859–1866. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yue X, Chen Q and He J: Combination
strategies to overcome resistance to the BCL2 inhibitor venetoclax
in hematologic malignancies. Cancer Cell Int.
20(524)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Garciaz S, Hospital MA, Alary AS, Saillard
C, Hicheri Y, Mohty B, Rey J, D'Incan E, Charbonnier A, Villetard
F, et al: Azacitidine plus venetoclax for the treatment of relapsed
and newly diagnosed acute myeloid leukemia patients. Cancers
(Basel). 14(2025)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cui H, Liu Z, Jin M, Wang D and Liu L:
Efficacy and safety of azacytidine and BCL-2 inhibitors in the
treatment of acute myeloid leukemia. Syst Med. 7:4–7. 2022.
|
|
15
|
Wang W, Luo Q, Chen Q, Pang A and Fang K:
Analysis of the clinical efficacy of azacytidine + venetoclax in
the treatment of elderly patients with relapsed refractory acute
myeloid leukemia. Evid Based Complement Alternat Med.
2022(8691835)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xia L, Tian W, Zhao Y, Jiang L, Qian W,
Jiang L, Ge L, Li J, Jin F and Yang M: Venetoclax and azacitidine
in Chinese patients with untreated acute myeloid leukemia
ineligible for intensive chemotherapy. Signal Transduct Target
Ther. 8(176)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamamoto K, Shinagawa A, DiNardo CD, Pratz
KW, Ishizawa K, Miyamoto T, Komatsu N, Nakashima Y, Yoshida C,
Fukuhara N, et al: Venetoclax plus azacitidine in Japanese patients
with untreated acute myeloid leukemia ineligible for intensive
chemotherapy. Jpn J Clin Oncol. 52:29–38. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman
MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et
al: Azacitidine and venetoclax in previously untreated acute
myeloid leukemia. N Engl J Med. 383:617–629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fu L, Liao X and Xiong M: Efficacy of
venetoclax combined with azacytidine in the treatment of elderly
patients with acute myeloid leukemia and its effect on immune
function. Gerontol Health Care. 28:125–128, 134. 2022.
|
|
21
|
Jonas BA, Dinardo CD, Fracchiolla N,
Pristupa A, Ishizawa K, Jin J, Konopleva M, Ofran Y, Montesinos P,
Kovacsovics T, et al: CYP3A inhibitors and impact of these agents
on outcomes in patients with acute myeloid leukemia treated with
venetoclax plus azacitidine on the VIALE-A study. Blood. 136 (Suppl
1):S50–S52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pollyea DA, Dinardo CD, Arellano ML,
Pigneux A, Fiedler W, Konopleva M, Rizzieri DA, Smith BD, Shinagawa
A, Lemoli RM, et al: Results of venetoclax and azacitidine
combination in chemotherapy ineligible untreated patients with
acute myeloid leukemia with IDH 1/2 mutations. Blood. 136 (Suppl
1):S5–S7. 2020.
|
|
23
|
Pollyea DA, DiNardo CD, Arellano ML,
Pigneux A, Fiedler W, Konopleva M, Rizzieri DA, Smith BD, Shinagawa
A, Lemoli RM, et al: Impact of venetoclax and azacitidine in
treatment-naïve patients with acute myeloid leukemia and IDH1/2
mutations. Clin Cancer Res. 28:2753–2761. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang L, Wang S, Hu W and Zhang W: Clinical
efficacy of azacytidine combined with Veneckla in the treatment of
elderly patients with acute myeloid leukemia. J Clin Hematol.
35:512–516, 521. 2022.
|
|
25
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shimony S, Stahl M and Stone RM: Acute
myeloid leukemia: 2023 Update on diagnosis, risk-stratification,
and management. Am J Hematol. 98:502–526. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Naqvi K, Konopleva M and Ravandi F:
Targeted therapies in acute myeloid leukemia: A focus on FLT-3
inhibitors and ABT199. Expert Rev Hematol. 10:863–874.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kwag D, Cho BS, Bang SY, Lee JH, Min GJ,
Park SS, Park S, Yoon JH, Lee SE, Eom KS, et al: Venetoclax with
decitabine versus decitabine monotherapy in elderly acute myeloid
leukemia: A propensity score-matched analysis. Blood Cancer J.
12(169)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lagadinou ED, Sach A, Callahan K, Rossi
RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer
KM, et al: BCL-2 inhibition targets oxidative phosphorylation and
selectively eradicates quiescent human leukemia stem cells. Cell
Stem Cell. 12:329–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ball S and Borthakur G: Apoptosis targeted
therapies in acute myeloid leukemia: An update. Expert Rev Hematol.
13:1373–1386. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y and Zhou H: B-cell
lymphoma/leukemia-2 inhibitor combined with azacytidine in the
treatment of acute myeloid leukemia after allogeneic hematopoietic
stem cell transplantation in two cases. Chin J Transplant (Electron
Ed). 15:45–48. 2021.
|
|
32
|
Wang WM, Wang J, Zhu MX, Wang YF, Liu YY
and Jing HM: Inductive effect of 5-azacitidine on apoptosis of
multiple myeloma cell lines and its mechanism. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 24:110–116. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
33
|
Zheng Y, Zheng H and Hu J: Clinical
observation on reinduction of azacytidine combined with CAG regimen
in the treatment of recurrent refractory acute myeloid leukemia in
children. Leuk Lymphoma. 30:470–474. 2019.
|
|
34
|
Miu W, Sha X and Liu Y: Treatment of four
cases of newly diagnosed aged acute myeloid leukemia with
azactidine and review of literature. Chin J Prim Med. 28:291–293.
2019.
|
|
35
|
Dombret H, Seymour JF, Butrym A,
Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC,
Candoni A, et al: International phase 3 study of azacitidine vs
conventional care regimens in older patients with newly diagnosed
AML with >30% blasts. Blood. 126:291–299. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jin S, Cojocari D, Purkal JJ, Popovic R,
Talaty NN, Xiao Y, Solomon LR, Boghaert ER, Leverson JD and
Phillips DC: 5-azacitidine induces NOXA to prime AML cells for
venetoclax-mediated apoptosis. Clin Cancer Res. 26:3371–3383.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cojocari D, Smith BN, Purkal JJ, Arrate
MP, Huska JD, Xiao Y, Gorska A, Hogdal LJ, Ramsey HE, Boghaert ER,
et al: Pevonedistat and azacitidine upregulate NOXA (PMAIP1) to
increase sensitivity to venetoclax in preclinical models of acute
myeloid leukemia. Haematologica. 107:825–835. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Du Y, Li C and Yan J: The efficacy and
safety of venetoclax and azacytidine combination treatment in
patients with acute myeloid leukemia and myelodysplastic syndrome:
Systematic review and meta-analysis. Hematology.
28(2198098)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bewersdorf JP, Giri S, Wang R, Williams
RT, Tallman MS, Zeidan AM and Stahl M: Venetoclax as monotherapy
and in combination with hypomethylating agents or low dose
cytarabine in relapsed and treatment refractory acute myeloid
leukemia: A systematic review and meta-analysis. Haematologica.
105:2659–2663. 2020.PubMed/NCBI View Article : Google Scholar
|