Introduction
The central nervous system is mainly composed of
neurons and glial cells; the latter includes microglia which are
immune system-associated cells accounting for 5-10% of all brain
cells (1). Microglia in the brain
have scavenger functions and participate in the protective
mechanisms of the brain; however, under uncontrolled conditions,
they can produce various inflammatory factors, including nitric
oxide (NO), cytokines and chemokines, and lead to neuronal damage
and brain cell death (2). A number
of neurological diseases, including Alzheimer's disease (AD),
stroke and other brain disorders, are associated with the
activation of microglia. The secretion of inflammatory factors and
reactive oxygen species (ROS) by microglia upon activation exerts a
marked impact on the progression of these diseases (3,4).
During normal brain aging, age-associated
inflammatory markers are highly expressed in the majority of
elderly individuals, and these markers are associated with the loss
of mental, cognitive and other complex behaviors that are
characteristic of AD and Parkinson's disease (PD) (5). As the population ages, numerous
countries around the world are gradually acquiring an increasingly
elderly demographic. In the future, a marked proportion of elderly
individuals are likely to experience a notable decline in
neurological function and become more susceptible to neurological
disorders and injuries. Therefore, it is urgently necessary to
develop therapeutic approaches for the development of
anti-neuroinflammatory medications.
Lipofundin is a lipid emulsion used in parenteral
nutrition and vehicles. In a recent study, it was found that when
neutrophils were stimulated with phorbol 12-myristate 13-acetate
(PMA), lipofundin inhibited intracellular hypochlorous acid
production and ERK activation to reduce PMA-stimulated neutrophil
extracellular traps (NETs). It was also observed that lipofundin
reduced E. coli-induced NETs in neutrophils through a
ROS-independent pathway (6). In
another study, in which Staphylococcus aureus-infected mouse
RAW264.7 macrophages were tested, it was observed that lipofundin
reduced ROS production and phagocytosis via the inhibition of JNK
activation, thereby increasing bacterial survival (7). In the same macrophage model,
lipofundin was also observed to reduce interleukin (IL)-1β
secretion, ROS production and phagocytosis (8). These studies have shown that
lipofundin has excellent anti-inflammatory properties. However,
whether lipofundin also has anti-neuroinflammatory properties
remains largely unknown.
Secreted substances are important in intercellular
communication and cell signaling, and play a critical role in the
regulation of immune responses such as inflammation and defense
against microbial pathogens (9).
Abnormalities in secreted substances are associated with a wide
variety of inflammatory diseases (10). Luminex biomarker multi-analyte
profiling (xMAP®) technology was developed in the late
1990s as a high-throughput bioassay platform for the rapid,
cost-effective and simultaneous analysis of multiple soluble
analytes in a single small-volume sample (11). This technology enables the
determination of soluble analyte profiles in biological samples
derived from cell culture, animals or patients. This technology was
used to determine the effect of lipofundin on secreted substances,
and the results may serve as a reference for future translational
medicine.
Materials and methods
Experimental design. Murine microglial
cell experiment
The toxicity of lipofundin to murine microglial
cells and its impact on the inflammatory response were
investigated, via the examination of murine microglial cells in
inactivated and lipopolysaccharide (LPS)-induced activated
states.
Experimental strategies. In the preventive
approach, murine microglial cells were pre-treated with lipofundin,
followed by induction with LPS to activate the cells. In the
therapeutic approach, murine microglial cells were treated with LPS
to activate them, and the cells were subsequently treated with
lipofundin.
Measurement methods. MTT and lactate
dehydrogenase (LDH) assays were utilized to analyze the toxicity of
lipofundin to murine microglial cells, and the impact of lipofundin
on the inflammatory response was evaluated by the measurement of
nitric oxide (NO) secretion and NO synthase 2 (NOS2) expression,
categorized into preventive and therapeutic anti-inflammatory
aspects.
Human microglial cell experiment
The results obtained from the murine microglial cell
experiments were validated using human microglial cells.
Experimental strategies. Considering that
human microglial cells do not secrete NO upon activation, a
suitable marker was identified by the analysis of secreted proteins
using a Luminex multiplex cytokine assay. The results suggested
IL-1β as a suitable molecule. Preventive and therapeutic approaches
were used for the human microglial cells as described for the
murine cells.
Measurement methods. MTT and LDH assays were
employed to analyze the toxicity of lipofundin to human microglial
cells, and reverse transcription-quantitative PCR (RT-qPCR) and
enzyme-linked immunosorbent assay (ELISA) were used to measure the
expression of IL-1β and assess the impact of lipofundin on the
inflammatory response. This impact was categorized into preventive
and therapeutic anti-inflammatory aspects.
This comprehensive experimental strategy was
designed to explore the effects of lipofundin on microglia in mice
and human cells, addressing toxic and anti-inflammatory responses
in a variety of settings.
Cell culture and drug treatment
BV2 mouse microglia cells were kindly provided by Dr
Yuh-Chiang Shen from the National Research Institute of Chinese
Medicine (Taipei, Taiwan). HMC3 human microglia cells were
purchased from the American Type Culture Collection (CRL-3304). The
two cell lines were cultured in high glucose Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
and 100 U/ml penicillin/streptomycin at 37˚C in a humidified
chamber with 5% CO2. The treatment of BV2 and HMC3 cells
with lipofundin was carried out using two different conditions: i)
Preventive approach, in which BV2 cells underwent pretreatment with
specified concentrations of lipofundin (0-1,000 µg/ml) for 1 h
followed by co-treatment with 250 ng/ml LPS for 24 h, and HMC3
cells were pretreated with 62.5 µg/ml lipofundin for 1 h, followed
by co-stimulation with 250 ng/ml LPS for 24 h; and ii) therapeutic
approach, in which BV2 cells were pre-stimulated with 250 ng/ml LPS
for 1 h, followed by co-treatment with increasing doses of
lipofundin (0-1,000 µg/ml) for 24 h, and HMC3 cells were
pre-stimulated with 250 ng/ml of LPS for 1 h, followed by
co-treatment with 62.5 µg/ml lipofundin for 24 h at 37˚C.
MTT assay
Cell viability was determined by MTT assay. Briefly,
cells were treated with MTT reagent (5 mg/ml in PBS) and incubated
for 4 h at 37˚C. Then, the supernatant was aspirated and 100 µl
DMSO was add to each well to dissolve the formazan crystals. The
absorbance was measured at a wavelength of 550 nm using a
microplate reader, and the background value at a wavelength of 750
nm was subtracted. Cell viability was determined as the percentage
relative to that of untreated cells.
Griess assay
NO production was determined by the measurement of
its stable end-product, nitrite, using a Griess reagent. Briefly,
100 µl cell supernatant was added to a 96-well plate and mixed with
100 µl Griess reagent (1% sulfanilamide and 0.1% naphthyl
ethylenediamine dihydrochloride in 5% phosphoric acid). The mixture
was incubated at room temperature for 10 min to allow for color
development. The absorbance at 540 nm was measured using a
microplate reader and nitrite concentrations were determined with
reference to a calibration curve prepared with sodium nitrite
standards.
LDH assay
The potential cytotoxic effect of lipofundin on
cells was assessed by quantifying the leakage of LDH into the
extracellular fluid. The supernatant from each sample was collected
for the analysis of LDH using a Cytotoxicity Detection Kit (cat.
no. 11644793001, Sigma-Aldrich; Merck KGaA). The absorbance of each
sample was measured at 490 nm using a microplate reader. LDH
release was calculated as a percentage according to the following
equation: LDH release (%)=(LDH activity in the medium / total LDH
activity) x100, where total LDH activity is the sum of LDH in the
medium and LDH in the cells.
Western blot analysis
Cell lysis was performed using RIPA buffer (25 mM
Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and
0.1% sodium dodecyl sulfate) supplemented with a protease inhibitor
cocktail (cat. no. P8340), all purchased from Sigma-Aldrich (Merck
KGaA). The protein concentration was determined using a Pierce BCA
Protein Assay kit (cat. no. 23235; Thermo Fisher Scientific, Inc.).
Equal amounts of total protein (35 µg/lane) were separated by
SDS-PAGE with an 8% gel. The gels were subsequently transferred
onto PVDF membranes (MilliporeSigma). Following transfer, the
membranes were blocked with 5% skimmed milk in Tris-buffered saline
with 0.1% Tween-20 (TBST) at room temperature for 1 h. Next, the
membranes were incubated overnight at 4˚C with NOS2 (1:1,000; cat.
no. 22226-1-AP; Proteintech) and α-tubulin (1:10,000; cat. no.
05-829; MilliporeSigma) primary antibodies according to the
manufacturer's instructions. After washing with TBST, the membranes
were incubated with appropriate HRP-conjugated secondary
antibodies: Goat anti-rabbit IgG (cat. no. 20202; Leadgene Co.,
Ltd.) and goat anti-mouse IgG (cat. no. 115-035-003; Jackson
ImmunoResearch Laboratories, Inc.), both diluted 1:5,000, at room
temperature for 1 h. Finally, the membranes were washed with TBST
and the bound antibodies were visualized using an Immobilon Western
Chemiluminescent HRP Substrate (MilliporeSigma) and
autoradiography.
Collection of conditioned media
To understand the secretion of cytokines, chemokines
and growth factors, HMC3 cells were cultured in the presence of 250
ng/ml LPS for 24 h. Additionally, HMC3 cells were pretreated with
62.5 µg/ml lipofundin for 1 h and then co-stimulated with 250 ng/ml
LPS for 24 h. The supernatants were subsequently collected and
concentrated for analysis using multiplex immunoassays. The
conditioned media were concentrated to a volume of 150-200 µl using
Amicon Ultra-15 centrifugal filters with a 3-kDa cut-off
(MilliporeSigma) at 4,000 x g and 4˚C for ~12 h.
Luminex multiplex cytokine assay
To analyze the levels of cytokines, chemokines and
growth factors in the HMC3 cell supernatants, Luminex-based
Milliplex xMAP technology with a 48-plex Human Cytokine/Chemokine
Magnetic Bead Panel (cat. no. HCYTA-60K; MilliporeSigma) was
employed according to the manufacturer's instructions. The cells
were divided into three groups, namely the untreated, LPS-treated
and lipofundin + LPS-treated groups.
RT-qPCR
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., following the manufacturer's instructions. Subsequently, cDNA
synthesis was performed using M-MLV reverse transcriptase, Oligo
and dNTP Mix (Takara Bio, Inc.) at 37˚C for 30 min, followed by
heating to 85˚C for 5 sec. qPCR was then conducted with specific
gene primers using the MyGo PCR Detection System (IT-IS Life
Science, Ltd.), according to the manufacturer's guidelines. The
qPCR reactions were performed using KAPA SYBR Fast qPCR Master Mix
kit (cat. no. KR0389; KAPA Biosystems, Inc.), following the
recommended protocol. The qPCR reaction included an initial
denaturation step at 95˚C for 30 sec, followed by 40 cycles of
denaturation at 95˚C for 2 min and annealing/extension at 60˚C for
30 sec. The comparative Cq (2-IICq) method was utilized
to calculate relative gene expression, with the normalization of
the target gene to GAPDH (12).
The primer sequences used were as follows: IL-1β, forward:
5'-ATGATGGCTTATTACAGTGGCAA-3' and reverse:
5'-GTCGGAGATTCGTAGCTGGA-3'; GAPDH, forward:
5'-CACCCATGGCAAATTCCATGGCA-3'; and reverse:
5'-TCTAGACGGCAGGTCAGGTCCACC-3'.
ELISA
The concentrations of IL-1β in the cell supernatants
were detected using an ELISA kit (cat. no. KTE6013; Abbkine
Scientific Co., Ltd.) according to the manufacturer's protocol.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean and were obtained from a minimum of three independent
experiments. Statistical analysis was conducted using GraphPad
Prism 6.0 software (Dotmatics). One-way analysis of variance with
Tukey's post hoc test for multiple comparisons was used for the
comparison of differences among groups. P<0.05 was considered to
indicate a statistically significant result.
Results
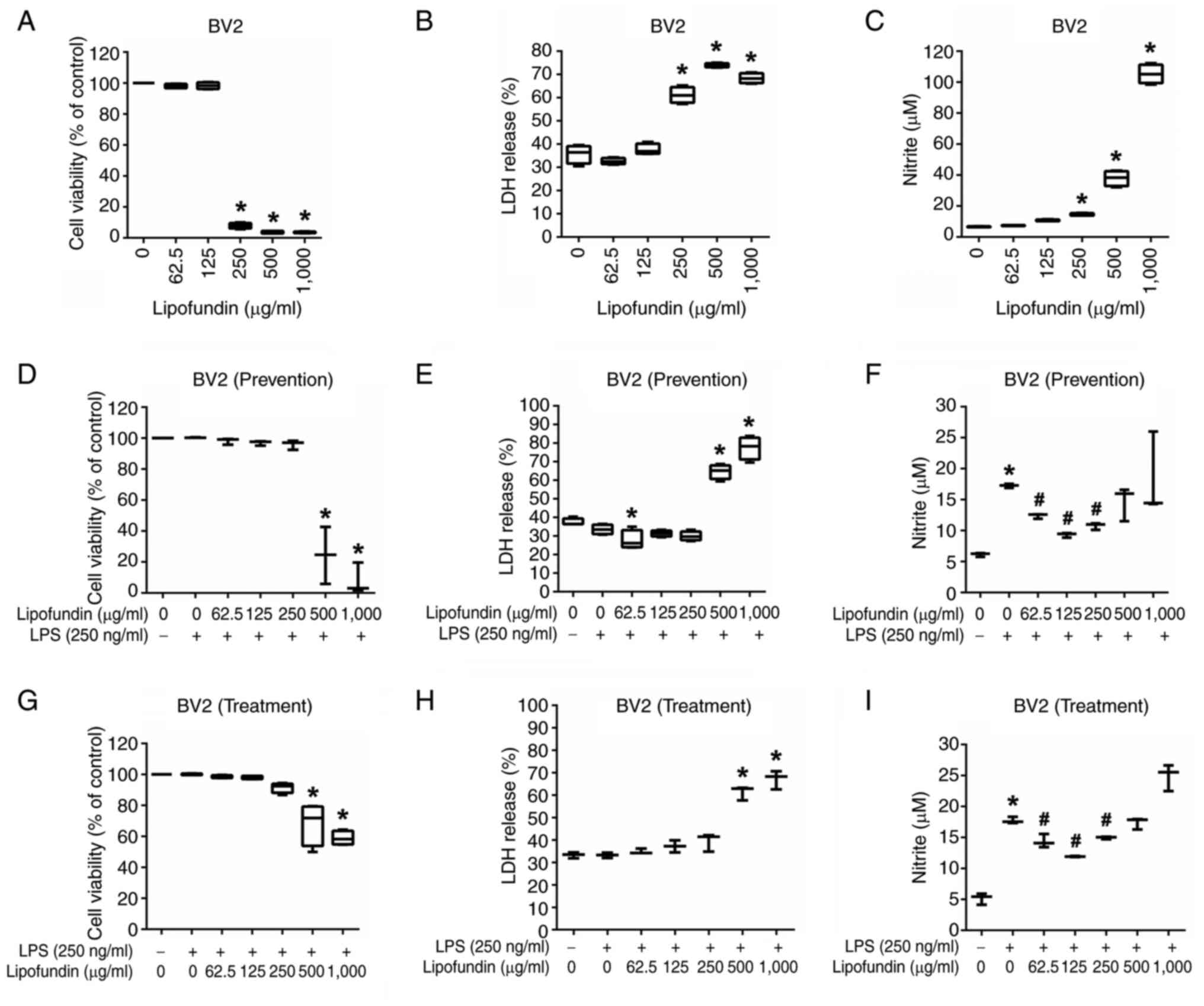
Cytotoxicity and activating effects of
lipofundin on BV2 cells in the absence of LPS stimulation
Firstly, the cytotoxicity of lipofundin and its
activating effects in BV2 cells were analyzed without LPS
stimulation. BV2 cells were treated with lipofundin at different
concentrations (0-1,000 µg/ml) for 24 h, followed by analysis using
an MTT assay. As shown in Fig. 1A,
significant cell death was observed only at lipofundin
concentrations >125 µg/ml. These results were validated using an
LDH assay, as shown in Fig. 1B.
Treatment with lipofundin at concentrations ≤125 µg/ml did not
induce cytotoxic effects in the BV2 cells, consistent with the
results of the MTT assay. Additionally, the Griess assay was
performed to investigate whether lipofundin has the potential to
activate inflammation. As shown in Fig. 1C, lipofundin induced a significant
inflammatory response in BV2 cells only at concentrations ≥250
µg/ml.
Preventive effects of lipofundin
against LPS-induced inflammation
Next, the ability of lipofundin pre-treatment to
attenuate LPS-induced inflammatory responses in BV2 cells was
analyzed. BV2 cells were pre-treated with lipofundin (0-1,000
µg/ml) for 1 h and then co-stimulated with LPS (250 ng/ml) for 24
h. The assessment of cell viability using the MTT assay showed that
high concentrations of lipofundin (500 and 1,000 µg/ml)
significantly decreased BV2 cell viability (Fig. 1D). Notably, during LPS activation,
BV2 cells exhibited heightened tolerance to lipofundin toxicity,
with significant cytotoxic effects observed only at concentrations
≥500 µg/ml. These results were validated using the LDH assay, the
results of which were consistent with the MTT findings. In
addition, treatment with 62.5 µg/ml lipofundin was observed to
reduce LDH release, indicating that it inhibited cell death in
LPS-activated BV2 cells (Fig. 1E).
The Griess assay was also utilized to investigate whether
lipofundin was able to prevent the inflammation induced by LPS in
BV2 cells. As shown in Fig. 1F,
treatment with lipofundin in the concentration range of 62.5-250
µg/ml significantly reduced NO production compared with that in
cells treated with LPS alone, with the maximal anti-inflammatory
effect observed at a concentration of 125 µg/ml. During the
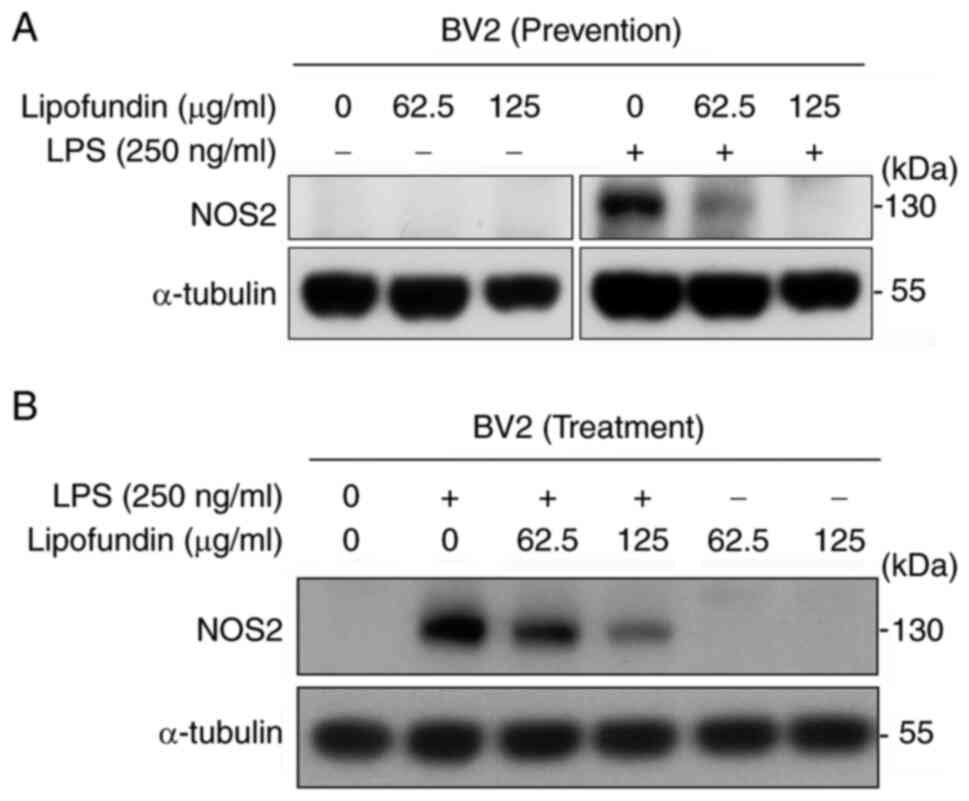
activation of BV2 cells, NOS2 expression is highly upregulated,
leading to the generation of a large amount of NO (13). To evaluate the activation status of
BV2 cells, the expression of NOS2 was assessed using western blot
analysis. As shown in Fig. 2A,
pre-treatment with lipofundin for 1 h caused a dose-dependent
reduction in NOS2 protein expression following LPS stimulation.
Therapeutic effects of lipofundin on
LPS-induced inflammation
The therapeutic effects of lipofundin were also
investigated, when used to treat BV2 cells following LPS
activation. The results of MTT and LDH assays (Fig. 1G and H) revealed that LPS-pre-stimulated BV2
cells exhibited cytotoxicity when treated with lipofundin at
concentrations of 500 and 1,000 µg/ml. Due to prior LPS activation,
the cells exhibited increased tolerance to lipofundin toxicity.
Even in LPS-primed BV2 cells, lipofundin effectively reduced
inflammation, with a maximal anti-inflammatory effect at a
concentration of 125 µg/ml (Fig.
1I). To further confirm the therapeutic effects of lipofundin
on LPS-induced activation, western blot analysis was performed to
examine the expression of NOS2. The results revealed a
dose-dependent reduction in NOS2 expression in LPS-induced cells
with increasing concentrations of lipofundin treatment (Fig. 2B). These results suggest that
lipofundin has the potential to prevent the occurrence of
inflammation and exhibits therapeutic effects on existing
inflammatory responses.
Exploring the cytotoxicity of
lipofundin and its influence on the secreted protein profile of
HMC3 cells under LPS stimulation
To better understand the effects of lipofundin on
human cells, the human microglial cell line HMC3 was analyzed. The
cytotoxicity of lipofundin to HMC3 cells was evaluated using MTT
and LDH assays. The results demonstrate that treatment with
lipofundin at a concentration of 62.5 µg/ml had no significant
impact on the viability of HMC3 cells (Fig. 3A and B). To understand the effects of LPS and
lipofundin on the secretion of various factors associated with
inflammation, including cytokines, chemokines and growth factors in
HMC3 cells, a Luminex multiplex cytokine assay was conducted to
analyze HMC3 cell culture supernatants. The results showed a
>5-fold increase in the secretion levels of various factors
under LPS treatment in HMC3 cells, including: granulocyte
colony-stimulating factor (G-CSF; 11.02-fold),
granulocyte-macrophage colony-stimulating factor (13.63-fold),
growth related oncogene α (9.23-fold), IL-1β (16.60-fold), IL-17A
(9.83-fold) and interferon-γ-inducible protein 10 (IP-10;
7.41-fold). However, pretreatment of the HMC3 cells with lipofundin
effectively reduced LPS-induced secretion by >3-fold for factors
including IL-1β and IL-17A, the secretion levels of which were
0.25- and 0.29-fold of those in cells treated with LPS alone. Also,
when the HMC3 cells were pretreated with lipofundin, certain
secretion levels were increased >3-fold compared with LPS
stimulation alone, including G-CSF (3.39-fold), IL-12 (p70)
(3.35-fold) and IP-10 (4.20-fold) as shown in Fig. 3C and Table I. These results suggest that
lipofundin inhibits neuroinflammation in human microglia cells and
promotes the secretion of substances such as growth factors that
may have a neuroprotective effect.
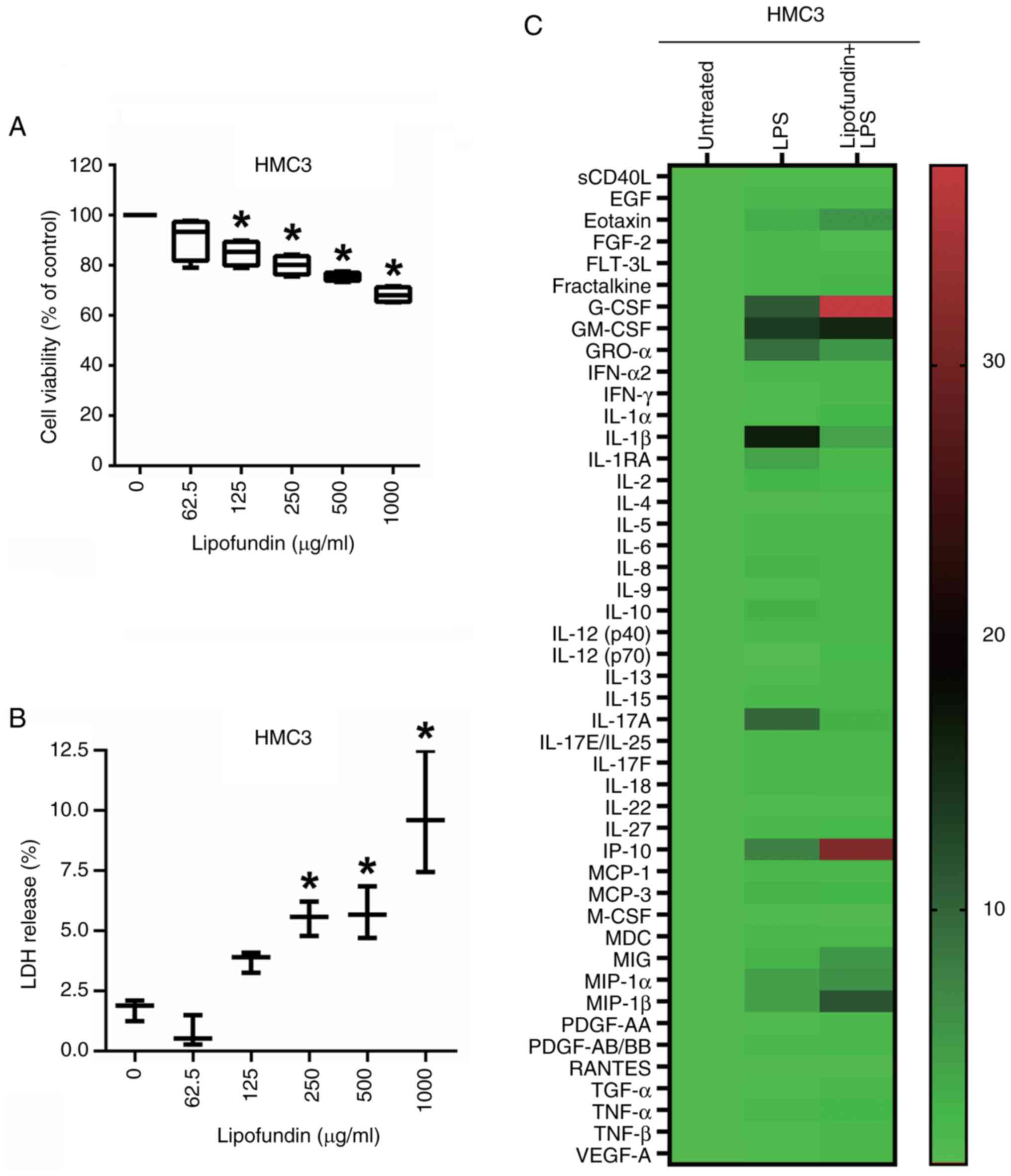 | Figure 3Cytotoxic effect of lipofundin on HMC3
cells and its secretion profile upon LPS stimulation. (A and B)
Cells were treated with increasing concentrations of lipofundin for
24 h. The cytotoxicity of lipofundin was determined by (A) MTT and
(B) LDH assays. *P<0.05 compared with the untreated
control group. (C) Heatmap depicting the impact of lipofundin on
factors associated with inflammation in HMC3 cells pretreated with
62.5 µg/ml lipofundin for 1 h and then co-stimulated with 250 ng/ml
LPS for 24 h. Conditioned media from these cells and untreated or
LPS-stimulated control cells were analyzed using a Luminex
multiplex assay. The heatmap displays the relative expression
levels, with color intensity indicating the magnitude. LPS,
lipopolysaccharide; LDH, lactate dehydrogenase; sCD40L, soluble
CD40 ligand; EGF, epidermal growth factor; FGF, fibroblast growth
factor; FLT-3L, Fms-related tyrosine kinase 3 ligand; G-CSF,
granulocyte colony-stimulating factor; GM-CSF,
granulocyte-macrophage colony-stimulating factor; GROα, growth
related oncogene α; IFN, interferon; IL, interleukin; IP,
IFN-γ-inducible protein; MCP, monocyte chemoattractant protein;
M-CSF, macrophage colony-stimulating factor; MDC,
macrophage-derived cytokine; MIG, monokine induced by IFN-γ; MIP,
macrophage inflammatory protein; PDGF, platelet-derived growth
factor; RANTES, regulated on activation, normal T cell expressed
and secreted; TGF, transforming growth factor; TNF, tumor necrosis
factor; VEGF, vascular endothelial growth factor. |
 | Table IEffect of lipofundin on the
concentrations of cytokines, chemokines and growth factors in
LPS-activated HMC3 cells as revealed by Luminex multiplex cytokine
assay. |
Table I
Effect of lipofundin on the
concentrations of cytokines, chemokines and growth factors in
LPS-activated HMC3 cells as revealed by Luminex multiplex cytokine
assay.
| | Concentration,
pg/ml |
|---|
| Analyte | Untreated | LPS | Lipofundin + LPS |
|---|
| sCD40L | 401.62 | 481.14 | 498.44 |
| EGF | 8.39 | 13.16 | 13.85 |
| Eotaxin | 2,059.03 | 6,504.67 | 11,509.91 |
| FGF-2 | 555.94 | 992.80 | 782.12 |
| FLT-3L | 122.11 | 226.03 | 236.42 |
| Fractalkine | 617.33 | 1003.70 | 1626.70 |
| G-CSF | 11.90 | 131.12 | 444.31 |
| GM-CSF | 116.33 | 1,585.81 | 1,825.56 |
| GROα | 1037.37 | 9573.20 | 5519.04 |
| IFN-α2 | 28.72 | 43.30 | 52.39 |
| IFN-γ | 7.86 | 9.58 | 11.81 |
| IL-1α | 12.06 | 16.73 | 31.29 |
| IL-1β | 10.59 | 175.85 | 44.22 |
| IL-1RA | 3.37 | 14.09 | 5.18 |
| IL-2 | 2.80 | 7.00 | 6.12 |
| IL-3 | ND | ND | ND |
| IL-4 | 19.69 | 23.10 | 24.23 |
| IL-5 | 1.09 | 1.96 | 1.62 |
| IL-6 | 7,348.58 | 14,338.89 | 13,782.77 |
| IL-7 | ND | ND | ND |
| IL-8 | 3,914.12 | 10,558.17 | 7,993.79 |
| IL-9 | 7.69 | 9.35 | 13.22 |
| IL-10 | 3.56 | 10.38 | 7.54 |
| IL-12 (p40) | 31.07 | 48.22 | 56.14 |
| IL-12 (p70) | 10.69 | 6.93 | 23.22 |
| IL-13 | 127.36 | 157.96 | 214.64 |
| IL-15 | 366.98 | 652.46 | 740.22 |
| IL-17A | 7.75 | 76.20 | 22.14 |
| IL-17E/IL-25 | 49.12 | 86.27 | 104.82 |
| IL-17F | 9.08 | 14.92 | 18.18 |
| IL-18 | 1.44 | 3.10 | 2.32 |
| IL-22 | 137.18 | 164.92 | 163.81 |
| IL-27 | 1865.87 | 3035.15 | 4210.36 |
| IP-10 | 71.22 | 527.81 | 2216.41 |
| MCP-1 | 12031.75 | 21478.41 | 20896.11 |
| MCP-3 | 45.72 | 124.21 | 119.16 |
| M-CSF | 2,132.59 | 2,591.80 | 2,055.54 |
| MDC | 2.42 | 4.65 | 4.58 |
| MIG | 36.21 | 97.73 | 194.07 |
| MIP-1α | 98.91 | 451.09 | 602.45 |
| MIP-1β | 43.83 | 192.54 | 494.33 |
| PDGF-AA | 3,660.79 | 4,607.03 | 5,844.00 |
| PDGF-AB/BB | 763.71 | 1,435.92 | 1,435.05 |
| RANTES | 4,956.10 | 5,385.24 | 4,140.58 |
| TGF-α | 100.24 | 141.58 | 166.93 |
| TNF-α | 56.12 | 114.07 | 129.30 |
| TNF-β | 140.10 | 184.50 | 209.64 |
| VEGF-A | 17,102.51 | 21,631.76 | 29,547.76 |
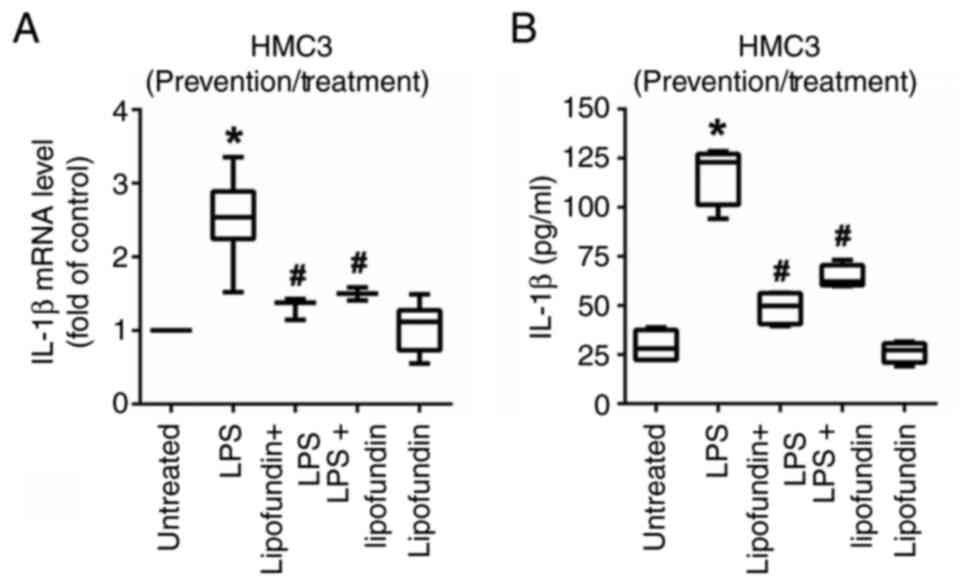
Lipofundin effectively suppresses the
pro-inflammatory cytokine IL-1β when used for prevention or
treatment
The results from the Luminex multiplex cytokine
assay demonstrated that the secretion of IL-1β by HMC3 cells was
significantly increased upon LPS treatment. Therefore, the
expression and secretion of IL-1β were analyzed to evaluate the
anti-inflammatory effect of lipofundin on HMC3 cells. As shown in
Fig. 4A, RT-qPCR analysis revealed
that lipofundin significantly downregulated the expression of IL-1β
mRNA in both preventive and therapeutic cell models. These findings
were further validated via ELISA, which demonstrated that
lipofundin effectively and significantly reduced the secretion of
IL-1β in both the preventive and therapeutic models (Fig. 4B).
Discussion
In the present study, the selection of doses was
carefully considered to ensure an appropriate range of lipofundin
concentrations, as an excessive dose may induce cell toxicity while
an insufficient dose may fail to elicit an effective response.
Non-activated mouse BV2 cells were first treated with different
concentrations of lipofundin to examine its impact on cell
toxicity. The results indicated that 250 µg/ml lipofundin exhibited
cytotoxic effects. This concentration also triggered the activation
of BV2 cells, suggesting that, for inflammation prevention, the
dose of lipofundin should be <250 µg/ml. In BV2 cells with
LPS-induced activation, the cytotoxic concentration of lipofundin
increased to 500 µg/ml. At this concentration, the effective
reduction of LPS-induced BV2 cell activation was observed,
indicating that for anti-inflammatory treatment the concentration
of lipofundin should be <500 µg/ml. In non-activated human HMC3
cells, treatment with 62.5 µg/ml lipofundin showed no cytotoxicity.
To ensure a safer dosage, 62.5 µg/ml lipofundin was selected for
preventive and therapeutic inflammation experiments. The findings
for these concentrations provide a foundation for cellular research
and offer crucial references for translational medical
applications.
Lipofundin is a widely used lipid emulsion for
intravenous injection, primarily employed for drug solubilization
and the provision of nutritional support through infusion.
Generally, when used at the correct dosage and in appropriate
contexts, lipofundin is considered relatively safe. However, the
results of the cell experiments in the present study reveal a dual
effect of lipofundin according to its concentration. At lower
concentrations, therapeutic effects were observed, which manifested
as significant anti-inflammatory properties. This suggests that
within this lower dosage range, lipofundin may modulate or inhibit
pathways associated with neuroinflammation, thereby demonstrating
therapeutic potential. Conversely, at higher concentrations,
lipofundin exhibited toxic effects. This toxicity may be associated
with the triggering of alternative cellular responses or toxic
pathways at elevated dosages, resulting in adverse effects.
Additionally, it was observed that higher doses led to marked
turbidity in the culture medium, which may have interfered with
subsequent analyses. These observations highlight the
dose-dependence of the effects of lipofundin, emphasizing that
careful consideration is necessary when selecting an appropriate
dose to ensure therapeutic efficacy without adverse effects.
Further research focusing on elucidation of the molecular
mechanisms of lipofundin at different concentrations is necessary,
to gain a deeper understanding of its impact at the cellular and
tissue levels, and ensure its safety in clinical applications.
The main clinical uses of lipid emulsions are in
nutritional support, detoxification and as solvents for
anesthetics. Clinical nutritional support may be administered
enterally or parenterally. In patients whose gastrointestinal
function is severely impaired, lipid emulsions can provide the
necessary nutrition via parenteral administration to promote
recovery and improve the prognosis of the patient (14). Lipid emulsions can cross the
blood-brain barrier due to their lipid solubility. A number of
studies have reported that lipid emulsions have neuroprotective
effects in mouse models of ischemic stroke (15-17).
It has also been reported that ω-3 polyunsaturated fatty acids are
able to regulate the functions of astrocyte and microglia, improve
neuronal survival and attenuate ischemic stroke injury in a rat
model of stroke using transient middle cerebral artery occlusion,
suggesting that they have potential in neuroprotective and
anti-inflammatory applications (18). In addition, ω-3 polyunsaturated
fatty acids were observed to attenuate microglia-induced
inflammation through the high mobility group box 1/Toll-like
receptor 4/NF-κB pathway in a traumatic brain injury-induced rat
brain injury model (19).
Furthermore, in a rat model of spinal cord injury, it was found
that the intravenous injection of ω-3 polyunsaturated fatty acids
immediately after injury regulated neuropathic inflammation and
reduced neuronal damage (20).
A recent study investigated LPS-induced
neuroinflammation and loss of learning and memory in C57BL/6J mice,
and found that supplementing the diet of the mice with an
astaxanthin-containing emulsion effectively ameliorated the
cognitive, learning and memory impairment caused by inflammation,
suggesting the potential use of the emulsion as a food or medicine
for the treatment of clinical neuroinflammation (21). Another study used cecal ligation
and perforation to induce sepsis in rats, which led to
neurocognitive impairment. It was found that the administration of
a fish oil-enriched lipid emulsion to the rats modulated
neuroinflammation and effectively prevented long-term cognitive
impairment after sepsis (22). In
addition, previous studies have shown that ω-3 polyunsaturated
fatty acids have a beneficial effect on PD via the inhibition of
proinflammatory cytokine release, promotion of neurotrophic factor
expression, recovery of mitochondrial function and membrane
fluidity, reduction of oxidant production levels, maintenance of
α-synuclein proteostasis, calcium homeostasis and axonal transport,
and reduction of endoplasmic reticulum stress (23).
The present study revealed that lipofundin increases
the secretion of G-CSF, and previous studies have indicated that
G-CSF has a role in the promotion of M2 macrophage polarization
(24,25). This suggests that the elevated
secretion of G-CSF may have anti-inflammatory effects. A previous
study has shown that intravenous lipid emulsion and G-CSF each have
neuroprotective effects and can effectively improve memory and
learning in an ischemic rat model, with a combination of the lipid
emulsion and G-CSF being more effective than either alone (26). G-CSF is a glycoprotein produced by
macrophages and endothelial cells, which stimulates the bone marrow
to produce granulocytes and stem cells. A number of previous
studies suggest that G-CSF reduces infarct size and improves brain
function after ischemia (27-29).
The present study has certain limitations. Firstly,
in vitro models comprising BV2 and HMC3 cell lines were
utilized, which may not comprehensively reflect the complex
processes of neuroinflammation in vivo. Therefore, further
validation in animal models and clinical trials is necessary for
translating the findings of the present study into practical
therapeutic applications. Secondly, using the data derived from the
Luminex multiplex cytokine assay, a more thorough exploration and
validation is required to elucidate how lipofundin regulates
secreted factors and its involvement in associated functions.
Finally, despite this initial exploration into the impact of
lipofundin on the modulation of neuroinflammation, further in-depth
research is imperative to elucidate the precise molecular
mechanisms and evaluate the potential therapeutic effects of
lipofundin in neuroinflammatory diseases.
In conclusion, the present study investigated the
preventive and therapeutic potential of lipofundin in inflammation.
The results demonstrate that lipofundin not only prevented
LPS-induced inflammatory responses in microglial cells but also
exhibited therapeutic effects in cells that were already activated.
These research findings highlight the potential efficacy of
lipofundin as a modulator of neuroinflammatory responses,
particularly its significant inhibitory effect on IL-1β. This
provides a beneficial theoretical foundation for the treatment of
neurological disorders.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Ditmanson Medical
Foundation Chia-Yi Christian Hospital (grant no. R111-69).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MSC and JCC conceived and designed the study. ZYC
performed the experiments. CLH and SKJ were responsible for data
analysis and interpretation. MSC and JCC drafted the manuscript.
All authors read and approved the final version of the manuscript.
JCC and MSC confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaur G, Han SJ, Yang I and Crane C:
Microglia and central nervous system immunity. Neurosurg Clin N Am.
21:43–51. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Spiteri AG, Wishart CL, Pamphlett R,
Locatelli G and King NJC: Microglia and monocytes in inflammatory
CNS disease: Integrating phenotype and function. Acta Neuropathol.
143:179–224. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Muzio L, Viotti A and Martino G: Microglia
in neuroinflammation and neurodegeneration: From understanding to
therapy. Front Neurosci. 15(742065)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Qiu M, Xu E and Zhan L: Epigenetic
regulations of microglia/macrophage polarization in ischemic
stroke. Front Mol Neurosci. 14(697416)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Scheiblich H, Trombly M, Ramirez A and
Heneka MT: Neuroimmune Connections in Aging and Neurodegenerative
Diseases. Trends Immunol. 41:300–312. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen MS, Yang KS, Lin WC, Fang CL, Chen HF
and Sheu SM: Lipofundin mediates major inhibition of intravenous
propofol on phorbol myristate acetate and Escherichia coli-induced
neutrophil extracellular traps. Mol Biol Rep. 9:6517–6529.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen MS, Tung YW, Hu CL, Chang HJ, Lin W
and Sheu SM: Three lipid emulsions reduce staphylococcus
aureus-stimulated phagocytosis in mouse RAW264.7 cells.
Microorganisms. 9(2479)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen MS, Lu PK, Lin WC, Shin HC, Sie SR
and Sheu SM: Lipofundin mediates the major inhibition of
intravenous propofol in IL-1β secretion and phagocytosis of
staphylococcus aureus-infected macrophages. Lipids. 55:45–52.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Daniels MJ and Brough D: Unconventional
pathways of secretion contribute to inflammation. Int J Mol Sci.
18(102)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Turner MD, Nedjai B, Hurst T and
Pennington DJ: Cytokines and chemokines: At the crossroads of cell
signalling and inflammatory disease. Biochim Biophys Acta.
1843:2563–2582. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baker HN, Murphy R, Lopez E and Garcia C:
Conversion of a capture ELISA to a Luminex xMAP assay using a
multiplex antibody screening method. J Vis Exp.
4084:2012.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lo J, Liu CC, Li YS, Lee PY, Liu PL, Wu
PC, Lin TC, Chen CS, Chiu CC, Lai YH, et al: Punicalagin Attenuates
LPS-Induced Inflammation and ROS Production in Microglia by
Inhibiting the MAPK/NF-κB Signaling Pathway and NLRP3 Inflammasome
Activation. J Inflamm Res. 15:5347–5359. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vinnars E and Wilmore D: Jonathan roads
symposium papers. History of parenteral nutrition. JPEN J Parenter
Enteral Nutr. 27:225–231. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Williams JJ, Mayurasakorn K, Vannucci SJ,
Mastropietro C, Bazan NG, Ten VS and Deckelbaum RJ: N-3 fatty acid
rich triglyceride emulsions are neuroprotective after cerebral
hypoxic-ischemic injury in neonatal mice. PLoS One.
8(e56233)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Berressem D, Koch K, Franke N, Klein J and
Eckert GP: Intravenous treatment with a long-chain omega-3 lipid
emulsion provides neuroprotection in a murine model of ischemic
stroke - a pilot study. PLoS One. 11(e0167329)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tanioka M, Park WK, Park J, Lee JE and Lee
BH: Lipid emulsion improves functional recovery in an animal model
of stroke. Int J Mol Sci. 21(7373)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zendedel A, Habib P, Dang J, Lammerding L,
Hoffmann S, Beyer C and Slowik A: Omega-3 polyunsaturated fatty
acids ameliorate neuroinflammation and mitigate ischemic stroke
damage through interactions with astrocytes and microglia. J
Neuroimmunol. 278:200–211. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W,
Chen J, Fu H and He H: Omega-3 polyunsaturated fatty acid
supplementation attenuates microglial-induced inflammation by
inhibiting the HMGB1/TLR4/NF-κB pathway following experimental
traumatic brain injury. J Neuroinflammation. 14(143)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Baazm M, Behrens V, Beyer C, Nikoubashman
O and Zendedel A: Regulation of inflammasomes by application of
omega-3 polyunsaturated fatty acids in a spinal cord injury model.
Cells. 10(3147)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao T, Ma D, Mulati A, Zhao B, Liu F and
Liu X: Development of astaxanthin-loaded layer-by-layer emulsions:
physicochemical properties and improvement of LPS-induced
neuroinflammation in mice. Food Funct. 12:5333–5350.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Della Giustina A, Goldim MP, Danielski LG,
Florentino D, Garbossa L, Joaquim L, Oliveira Junior AN, Mathias K,
Fileti ME, Zarbato GF, et al: Fish oil-rich lipid emulsion
modulates neuroinflammation and prevents long-term cognitive
dysfunction after sepsis. Nutrition. 70(110417)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li P and Song C: Potential treatment of
Parkinson's disease with omega-3 polyunsaturated fatty acids. Nutr
Neurosci. 25:180–191. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wen YT, Huang TL, Huang SP, Chang CH and
Tsai RK: Early applications of granulocyte colony-stimulating
factor (G-CSF) can stabilize the blood-optic-nerve barrier and
ameliorate inflammation in a rat model of anterior ischemic optic
neuropathy (rAION). Dis Model Mech. 9:1193–1202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wen Q, Kong Y, Zhao HY, Zhang YY, Han TT,
Wang Y, Xu LP, Zhang XH and Huang XJ: G-CSF-induced macrophage
polarization and mobilization may prevent acute graft-versus-host
disease after allogeneic hematopoietic stem cell transplantation.
Bone Marrow Transplant. 54:1419–1433. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rasouli B, Ghahari L, Safari M,
Shahroozian E and Naeimi S: Combination therapy of the granulocyte
colony stimulating factor and intravenous lipid emulsion protect
the hippocampus after global ischemia in rat: focusing on CA1
region. Metab Brain Dis. 35:991–997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schäbitz WR, Kollmar R, Schwaninger M,
Juettler E, Bardutzky J, Schölzke MN, Sommer C and Schwab S:
Neuroprotective effect of granulocyte colony-stimulating factor
after focal cerebral ischemia. Stroke. 34:745–751. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
England TJ, Gibson CL and Bath PM:
Granulocyte-colony stimulating factor in experimental stroke and
its effects on infarct size and functional outcome: A systematic
review. Brain Res Rev. 62:71–82. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Popa-Wagner A, Stöcker K, Balseanu AT,
Rogalewski A, Diederich K, Minnerup J, Margaritescu C and Schäbitz
WR: Effects of granulocyte-colony stimulating factor after stroke
in aged rats. Stroke. 41:1027–1031. 2010.PubMed/NCBI View Article : Google Scholar
|