Introduction
Chronic obstructive pulmonary disease (COPD) is a
prevalent and devastating global health issue, placing a
substantial strain on both population health and healthcare
resources (1). This disease is
characterized by persistent respiratory symptoms and irreversible
airflow limitation (2). COPD is
closely linked to chronic bronchitis and emphysema, which are the
primary underlying conditions leading to the development of COPD,
often presenting with overlapping features (3). Epidemiological data from 2015
estimated that ~299 million individuals worldwide were affected by
COPD, with >3 million deaths attributed to this chronic ailment
(4). COPD not only exacts a
significant economic toll on society but also poses a grave threat
to the physical and psychological well-being of individuals
(5).
In recent years, comorbidity has emerged as a global
concern, referring to the coexistence of two or more chronic
diseases. COPD is a systemic ailment commonly associated with
various chronic conditions, such as atrial fibrillation (AF),
cardiovascular disease, diabetes, lung cancer, osteoporosis and
depression (6-8).
AF, a significant complication of COPD, is known to worsen the
quality of life and increase all-cause mortality, thereby imposing
a substantial disease and economic burden on COPD patients
(9-11).
Currently, AF is the most prevalent supraventricular arrhythmia,
affecting an estimated 8 million patients in China alone (12). A meta-analysis involving 4.2
million COPD patients revealed that 13% of them had concurrent AF
(13). Furthermore, Goudis et
al (14) demonstrated that
COPD patients face a twofold increased risk of developing AF
compared to non-COPD patients, with severe COPD patients exhibiting
a fourfold higher incidence. Although the risk factors for COPD
combined with AF remain unclear, a study involving 2,352 AF
patients identified left atrial enlargement and decreased left
ventricular ejection fraction as potential risk factors for this
comorbidity (15). A meta-analysis
conducted in 2020 revealed that advanced age (>65), male gender
and Caucasian ethnicity are associated with an increased risk of AF
in patients with COPD (16).
Furthermore, independent risk factors for COPD-induced AF include
myocardial infarction, coronary artery disease, chronic heart
failure, pulmonary infections, acute respiratory failure,
mechanical ventilation, chronic kidney disease and the use of
ipratropium bromide. Notably, the administration of β-adrenergic
agonists and theophylline during acute exacerbation of COPD was
found to elevate cardiac instability, which is an independent risk
factor for COPD-induced AF (17).
However, no significant association was observed between
hypertension, hyperlipidemia, diabetes, liver failure and the risk
of new-onset AF in the present study. Conversely, literature
reports have identified diabetes, hypertension, peripheral vascular
disease and liver failure as independent risk factors for the
development of AF in non-COPD patients (16). Given the current controversy
surrounding the identification of risk factors for COPD combined
with AF, it is imperative to establish a diagnostic model for this
condition.
To identify COPD patients at high risk of developing
AF and with poor survival rates, the present study aimed to
construct a diagnostic model for predicting the risk of COPD
combined with AF. Additionally, a prognostic model was developed to
predict the prognosis of COPD combined with AF. This was achieved
by utilizing demographic and common hematological parameters of
COPD patients admitted to the Second Affiliated Hospital of Guilin
Medical University between January 2020 and May 2022. The ultimate
goal was to identify specific patient subgroups and tailor
personalized treatment strategies, leading to improved clinical
outcomes and enhanced quality of life.
Materials and methods
Study population
This retrospective study analyzed patients with COPD
who were admitted to the Second Affiliated Hospital of Guilin
Medical College (Guilin, Guangxi Zhuang Autonomous Region, P.R.
China) between January 2020 and May 2022. Based on the presence or
absence of AF, the study population was divided into the AF group
(COPD with AF) and the non-AF group (COPD without AF). The study
was approved by the Ethics Committee of The Second Affiliated
Hospital of Guilin Medical University (approval no.
NO.YJS-2021011). The inclusion criteria were as follows: i)
Patients aged 40 years or older, of any gender, admitted for the
treatment of dyspnea, cough, or exacerbation of sputum in COPD; ii)
COPD diagnosis in accordance with the 2021 Global Initiative for
Chronic Obstructive Lung Disease (GOLD) guidelines (1); iii) completion of electrocardiography
or 24-h Holter monitoring, echocardiography, pulmonary function
tests, blood gas analysis and renal function tests; iv) alert and
able to communicate effectively. The exclusion criteria were as
follows: i) Patients with concomitant valvular heart disease and
recurrent AF after catheter radiofrequency ablation; ii) presence
of active chronic respiratory diseases such as obstructive sleep
apnea syndrome, bronchial asthma, severe pneumonia, bronchiectasis,
tuberculosis, or pulmonary malignancies; iii) presence of other
systemic diseases such as rheumatic autoimmune diseases or severe
hepatic or renal failure; iv) need for tracheal intubation and
invasive mechanical ventilation, or concomitant multiple organ
failure; v) incomplete research data. The definition and diagnostic
criteria for COPD were based on the 2018 GOLD guidelines (1). The diagnostic criteria for AF were
the presence of AF on surface electrocardiography or single-lead
electrocardiographic recording lasting >30 sec (18). The diagnosis of pulmonary arterial
hypertension was based on the 2022 European Respiratory Society and
European Society of Cardiology guidelines for the diagnosis of
pulmonary hypertension (19).
Data collection
Clinical data were collected, including age, gender,
height, weight, systolic and diastolic blood pressure upon
admission, duration of COPD, smoking and alcohol history,
comorbidities and respiratory medication history. Laboratory test
results upon admission included complete blood count, renal
function and blood gas analysis (Table
I). Transthoracic echocardiography was performed to measure
relevant parameters such as left atrial diameter (LAD), right
atrial diameter, left ventricular ejection fraction and pulmonary
artery systolic pressure. Pulmonary function tests were conducted
using a Master Screen spirometer by trained technicians. Prior to
the tests, the height and weight of patients were measured in a
standing position and the tests were conducted according to the
guidelines established by the Pulmonary Function Group of the
Chinese Medical Association Respiratory Branch. Patients received
1-2 practice sessions before the tests and there were no absolute
contraindications to pulmonary function testing in any of the
patients. The main outcome measures collected were forced
expiratory volume in one second (FEV1), FEV1 as a percentage of
predicted (FEV1%Pred) and the FEV1/forced vital capacity (FVC)
ratio. The follow-up period for the study extended until April 31,
2023. Survival time was defined as the duration from the date of
the interview to the date of the participant succumbing or the end
of the follow-up period.
 | Table IComparison of baseline data between
AF group and non-AF group. |
Table I
Comparison of baseline data between
AF group and non-AF group.
| Characteristic | AF | non-AF | P-value | Statistic | Method |
|---|
| N | 87 | 199 | | | |
| Sex (Male/Female),
n (%) | | | 0.237 | 1.400 | χ2 |
|
Male | 65 (74.7) | 161 (80.9) | | | |
|
Female | 22 (25.3) | 38 (19.1) | | | |
| Age, years (median
IQR) | 77 (70-81) | 69 (63-76) | <0.001 | | Wilcoxon |
| Hypertension, n
(%) | 37 (42.5) | 63 (31.7) | 0.076 | 3.146 | χ2 |
| Chronic heart
failure, n (%) | 61 (70.1) | 58 (29.1) | <0.001 | 41.821 | χ2 |
| History of
myocardial infarction, n (%) | 0 (0) | 1 (0.5) | 1 | | Fisher test |
| Chronic kidney
disease, n (%) | 23 (26.4) | 15 (7.5) | <0.001 | 18.767 | χ2 |
| Respiratory
failure, n (%) | 22 (25.3) | 32 (16.1) | 0.067 | 3.350 | χ2 |
| Pulmonary
infection, n (%) | 87(100) | 196 (98.5) | 0.603 | 0.271 | Yates'
correction |
| Cerebrovascular
accident, n (%) | 16 (18.4) | 27 (13.6) | 0.294 | 1.102 | χ2 |
| Smoking, n (%) | 37 (42.5) | 105 (52.8) | 0.111 | 2.537 | χ2 |
| SBP, mmHg, (median
IQR) | 129 (114-143) | 130 (116-142) | 0.878 | | Wilcoxon |
| DBP, mmHg, mean ±
standard deviation | 78.977±14.487 | 80.477±12.73 | 0.380 | -0.879 | T test |
| Heart rate, beats
per minute, (median IQR) | 92 (78-101) | 93 (82.5-102) | 0.674 | | Wilcoxon |
| Respiratory rate,
breaths per minute, (median IQR) | 22 (20.5-23.5) | 22 (21-23) | 0.647 | | Wilcoxon |
| Duration of COPD,
(median IQR) | 10 (2-10) | 8 (3-10) | 0.750 | | Wilcoxon |
| Home oxygen
therapy, n (%) | 7(8) | 10(5) | 0.320 | 0.988 | χ2 |
| ICS, n (%) | 2 (2.3) | 9 (4.5) | 0.572 | 0.320 | Yates'
correction |
| Anticholinergic
drugs, n (%) | 33 (37.9) | 63 (31.7) | 0.301 | 1.068 | χ2 |
| LABA, n (%) | 28 (32.2) | 59 (29.6) | 0.668 | 0.184 | χ2 |
| Xanthine drugs, n
(%) | 45 (51.7) | 127 (63.8) | 0.055 | 3.694 | χ2 |
| ICS + LABA, n
(%) | 37 (42.5) | 93 (46.7) | 0.511 | 0.432 | χ2 |
| WBC, (median
IQR) | 7.6
(5.905-11.28) | 8.04
(6.26-10.125) | 0.893 | | Wilcoxon |
| RBC, (median
IQR) | 4.34
(3.75-4.63) | 4.43
(4.0625-4.8675) | 0.057 | | Wilcoxon |
| Hb, (median
IQR) | 128 (114-142) | 132 (122-144) | 0.099 | | Wilcoxon |
| Plt, (median
IQR) | 208 (162-275) | 230 (191-268) | 0.103 | | Wilcoxon |
| Lymphocyte count,
(median IQR) | 1.34
(0.76-1.45) | 1.305
(0.93-1.74) | 0.062 | | Wilcoxon |
| Monocyte count,
(median IQR) | 0.66
(0.51-0.9) | 0.65
(0.4925-0.8375) | 0.318 | | Wilcoxon |
| Neutrophil count,
(median IQR) | 5.89
(3.96-9.35) | 5.57
(3.885-7.9125) | 0.640 | | Wilcoxon |
| Hematocrit, median,
(median IQR) | 39.5 (35.7-44) | 40.7
(37.2-43.7) | 0.232 | | Wilcoxon |
| CRP, (median
IQR) | 6.965
(4-21.828) | 5.36
(4-28.265) | 0.588 | | Wilcoxon |
| pH, (median
IQR) | 7.41
(7.39-7.445) | 7.41
(7.3893-7.43) | 0.302 | | Wilcoxon |
| PCO2,
(median IQR) | 43
(37.3-51.25) | 44.85
(39.825-52.75) | 0.138 | | Wilcoxon |
| PO2,
(median IQR) | 76.1
(61-88.85) | 77.55
(67-89.8) | 0.570 | | Wilcoxon |
| HCO3,
(median IQR) | 27.1 (24.8-32) | 27.9 (25.5-32) | 0.276 | | Wilcoxon |
| FEV1, (median
IQR) | 1.05
(0.65-1.82) | 1.3
(0.825-1.815) | 0.201 | | Wilcoxon |
| Predicted FEV1,
(median IQR) | 48.3 (31-61) | 48.6
(34.2-69.8) | 0.329 | | Wilcoxon |
| FVC, (median
IQR) | 2.14
(1.62-2.875) | 2.23
(1.71-2.93) | 0.410 | | Wilcoxon |
| Predicted FVC,
(median IQR) | 56.7
(15.945-68.15) | 53.2
(1.99-66.9) | 0.108 | | Wilcoxon |
| FEV1/FVC, (median
IQR) | 57.04
(42.205-75.95) | 64.14
(48.69-76.85) | 0.233 | | Wilcoxon |
| Predicted FEV1/FVC,
mean ± standard deviation | 59.174±17.409 | 59.296±13.334 | 0.953 | -0.058 | Welch t' test |
| Pulmonary
hypertension, n (%) | 58 (75.3) | 75 (37.7) | <0.001 | 31.498 | χ2 |
| Pulmonary artery
pressure, (median IQR) | 48 (37.5-59) | 39 (31-46) | 0.002 | | Wilcoxon |
| LAD, (median
IQR) | 38 (31.5-45) | 28 (25-31) | <0.001 | | Wilcoxon |
| RAD, (median
IQR) | 38 (30-45.5) | 29 (26-32) | <0.001 | | Wilcoxon |
| UA, (median
IQR) | 413
(329.5-484.5) | 336.5
(274.25-396) | <0.001 | | Wilcoxon |
Construction of diagnostic and
prognostic models
A stepwise backward logistic regression analysis was
employed to identify key factors for constructing a diagnostic
model for the coexistence of COPD and AF and a visually appealing
nomogram was generated. Univariate and multivariate Cox regression
analyses were conducted to select factors influencing the prognosis
of COPD combined with AF and a prognostic nomogram was constructed
using the identified factors. The accuracy of the models was
evaluated using the concordance index (c-index), receiver operating
characteristic (ROC) curve and area under the curve (AUC), with
higher values indicating superior accuracy. The predictive ability
of the models was assessed using calibration curves, where a
well-calibrated model would align closely with the 45-degree
diagonal line.
Survival analysis
The Kaplan-Meier method was employed to plot the
survival curves of the COPD + AF group and the COPD group (20). The log-rank test was utilized to
compare the survival differences between these two patient
groups.
Statistical analysis
Continuous variables were described using mean and
standard deviation or median and interquartile range, depending on
the distribution of the data. For variable comparisons, the
two-sample t-test or Wilcoxon rank-sum test with continuous
correction based on data normality and homogeneity of variance were
employed. Categorical data were presented as absolute values and
percentages and the χ2 test was used to compare
categorical variables between the two groups. Data were organized
using Excel 16.0 (Microsoft Corporation) and analyzed using RStudio
version 4.1.2(21). In the R
software, several packages were employed, including ‘readxl’,
‘car’, ‘autoReg’, ‘dplyr’, ‘officer’, ‘foreign’, ‘moonBook’,
‘rrtable’, ‘survival’, ‘survivalROC’, ‘survminer’, ‘rms’,
‘foreign’, and ‘tableone’. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline data comparison
The present study enrolled a cohort of 286 patients,
with a mean age of 77.11±8.67 years, including 226 males. Among
them, 87 patients were classified in the AF group, with an average
age of 75.36±7.51 years, while the remaining 199 patients were
assigned to the non-AF group, with an average age of 69.25±8.50
years. Notably, the AF group exhibited significantly advanced age,
elevated levels of UA, pulmonary artery pressure, as well as a
higher prevalence of comorbidities such as chronic heart failure,
pulmonary hypertension and chronic kidney disease. Moreover, the AF
group displayed significantly enlarged LAD and right atrial
diameter, with statistically significant differences (P<0.05). A
comprehensive overview of the baseline demographic characteristics
for both groups is presented in Table
I.
Logistic regression analysis of risk
factors for COPD with AF
To identify potential risk factors associated with
COPD and AF, univariate and multivariate logistic regression
analyses were conducted for variables demonstrating significant
differences between the two groups (age, chronic heart failure,
chronic kidney disease, UA, pulmonary hypertension, pulmonary
artery pressure, LAD, right atrial diameter). Following stepwise
regression analysis, age, UA and LAD were identified as independent
risk factors (Table II).
 | Table IIStepwise logistic regression analysis
assessing the risk of AF development in individuals diagnosed with
chronic obstructive pulmonary disease COPD. |
Table II
Stepwise logistic regression analysis
assessing the risk of AF development in individuals diagnosed with
chronic obstructive pulmonary disease COPD.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | Total, n | Odds Ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| Age | 286 | 1.095
(1.058-1.133) | <0.001 | 1.072
(1.019-1.128) | 0.007 |
| Chronic heart
failure | 119 | 5.704
(3.286-9.901) | <0.001 | 2.122
(0.907-4.962) | 0.083 |
| Chronic kidney
disease | 38 | 4.408
(2.167-8.966) | <0.001 | 2.202
(0.704-6.889) | 0.175 |
| UA | 271 | 1.006
(1.003-1.008) | <0.001 | 1.004
(1.001-1.008) | 0.010 |
| Pulmonary
hypertension | 133 | 5.047
(2.792-9.124) | <0.001 | 2.065
(0.678-6.295) | 0.202 |
| Pulmonary artery
pressure | 200 | 1.018
(1.002-1.034) | 0.025 | 0.993
(0.964-1.023) | 0.649 |
| LAD | 286 | 1.198
(1.143-1.256) | <0.001 | 1.195
(1.098-1.301) | <0.001 |
| RAD | 286 | 1.118
(1.081-1.156) | <0.001 | 1.014
(0.945-1.089) | 0.691 |
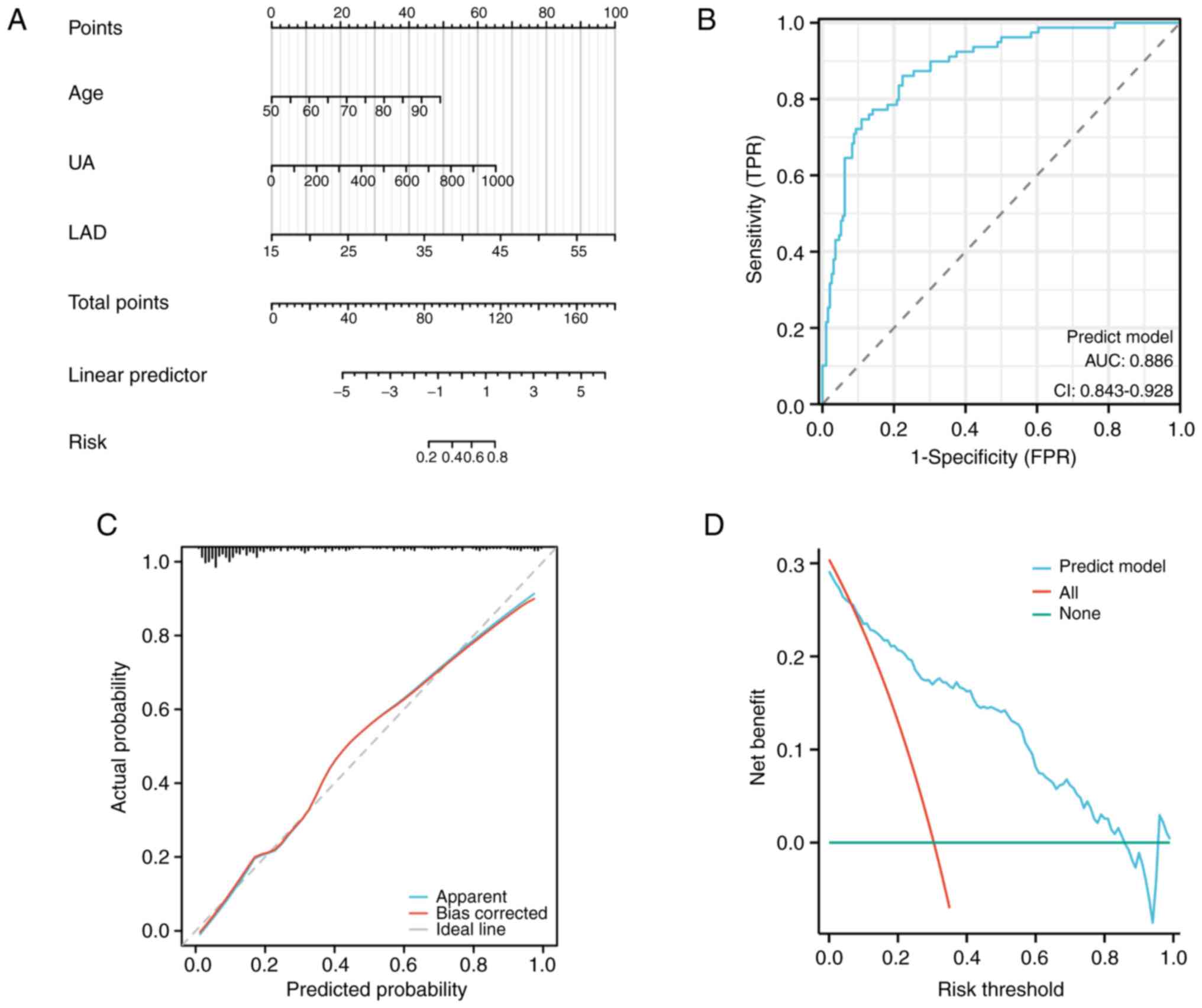
Nomogram model for predicting COPD
with AF
The present study developed a nomogram model to
predict the occurrence of COPD with AF, incorporating age, UA and
LAD as predictors (Fig. 1A). The
ROC curve analysis revealed an AUC of 0.886, indicating excellent
discriminative ability of the nomogram model (Fig. 1B). Furthermore, the calibration
plot demonstrated a close agreement between the predicted and
observed probabilities, indicating reliable calibration of the
nomogram model (Fig. 1C).
Additionally, the decision curve analysis (DCA) curve illustrated
the clinical utility of the nomogram model (Fig. 1D).
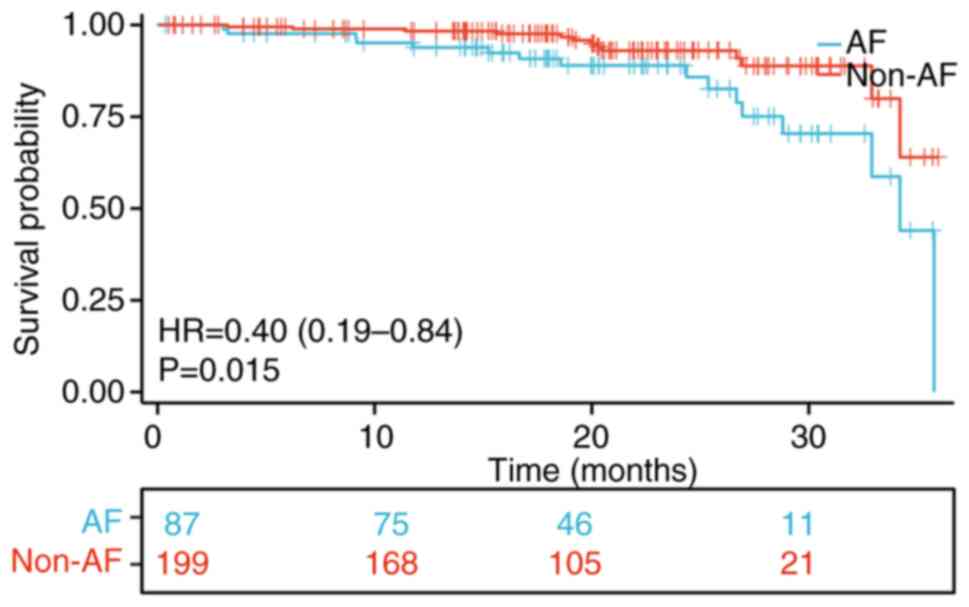
Survival analysis
To assess the effect of AF on the prognosis of COPD
patients, Kaplan-Meier analysis was performed to compare the
all-cause mortality rate between individuals with COPD alone and
those with COPD and AF. The findings revealed a significant
difference in the all-cause mortality rate between the two groups.
Notably, the COPD with AF group exhibited substantially lower
survival rates compared to the COPD group (P<0.05; Fig. 2).
Prognostic model and nomogram
Univariate and multivariate Cox regression analyses
were conducted to identify factors influencing the prognosis of
patients diagnosed with both COPD and AF. As presented in Table III, both univariate and
multivariate Cox regression analyses revealed that levels of uric
acid (UA) and LAD were independent prognostic factors for COPD
patients with concurrent AF. Based on these significant factors, a
prognostic nomogram for COPD with AF was constructed (Fig. 3A), yielding a c-index of 0.886 (95%
confidence interval: 0.842-0.930). The area under the receiver
operating characteristic curve (AUC) values for predicting 5-month,
10-month and 15-month survival rates using the nomogram were 0.952,
0.851 and 0.881, respectively (Fig.
3B). Furthermore, the calibration curve demonstrated excellent
agreement between the predicted and observed 5-month, 10-month and
15-month survival rates (Fig.
3C).
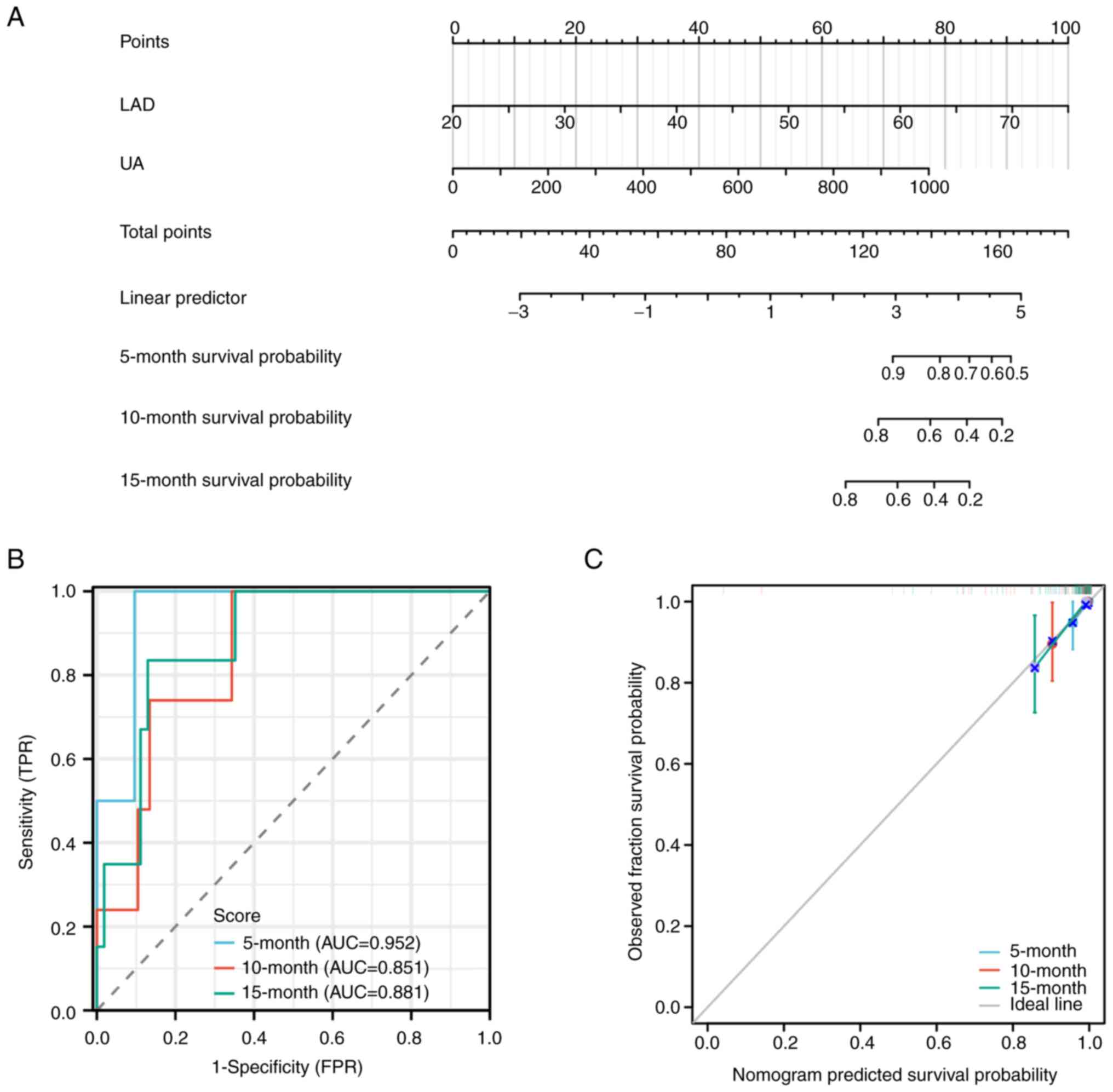 | Figure 3Construction and validation of the
prognostic nomogram model. (A) Nomogram results of the prognostic
model using UA and LAD. Each prognostic factor corresponds to a
score and the individual scores are summed to obtain the total
score for predicting the 5-, 10- and 15-month survival rates of
COPD patients with AF. A straight line is drawn on the axis of the
total score to predict the survival rates. (B) ROC curve of the
prognostic Nomogram for predicting the 5-month, 10-month and
15-month survival rates of COPD patients with AF. (C)
Re-calibration curve of the prognostic nomogram for predicting the
5-, 10- and 15-month survival rates of COPD patients with AF. UA,
uric acid; LAD, left atrial diameter; COPD, chronic obstructive
pulmonary disease; AF, atrial fibrillation; ROC, receiver operating
characteristic; AUC, area under the curve; TPR, true-positive rate;
FPR, false-positive rate. |
 | Table IIIUnivariate and multivariate Cox
regression analysis predicting the long-term mortality rate
associated with atrial fibrillation occurrence in patients
diagnosed with COPD. |
Table III
Univariate and multivariate Cox
regression analysis predicting the long-term mortality rate
associated with atrial fibrillation occurrence in patients
diagnosed with COPD.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | Total, n | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Pulmonary
hypertension | 77 | | | | |
|
1 | 58 | Reference | | | |
|
0 | 19 | 1.981
(0.597-6.571) | 0.264 | | |
| Pulmonary artery
pressure | 71 | 1.007
(0.971-1.044) | 0.711 | | |
| LAD | 87 | 1.120
(1.060-1.183) | <0.001 | 1.104
(1.046-1.165) | <0.001 |
| RAD | 87 | 0.989
(0.935-1.047) | 0.708 | | |
| UA | 79 | 1.006
(1.002-1.010) | 0.005 | 1.004
(1.000-1.008) | 0.042 |
| Age | 87 | 1.008
(0.940-1.082) | 0.818 | | |
| Chronic Heart
Failure | 87 | | | | |
|
0 | 26 | Reference | | | |
|
1 | 61 | 1.305
(0.413-4.126) | 0.650 | | |
| Chronic kidney
disease | 87 | | | | |
|
0 | 64 | Reference | | | |
|
1 | 23 | 0.571
(0.158-2.060) | 0.392 | | |
Discussion
The present study collected clinical data of
patients with COPD admitted to the Second Affiliated Hospital of
Guilin Medical College between January 2020 and May 2022. A
practical nomogram was constructed to predict the risk of AF in
COPD patients and predicted the 5-, 10- and 15-month survival rates
of COPD patients based on available demographic, clinical and
hematological parameters. The results showed that the model for
predicting AF risk in COPD patients had an AUC of 0.886 and the
models for predicting 5-, 10- and 15-month survival had AUCs of
0.952, 0.851 and 0.881, respectively. The present study, for the
first time to the best of the authors' knowledge, identified age,
UA levels and LAD as independent risk factors for the development
of AF in patients with COPD. These findings fill a knowledge gap in
the existing literature and provide new guidance for risk
assessment of AF in COPD patients.
AF is the most common supraventricular arrhythmia in
clinical practice and its prevalence is associated with age and
gender. A study (12) indicated
that the prevalence of AF in males under 60 years old was 0.43 and
0.44% in females, while in males >60 years old, the prevalence
increased to 1.83 and 1.92% in females. A recent survey in China
(22) showed that the prevalence
of AF reached 10% in individuals >75 years old. Prospective
studies from Japan and the United States have also confirmed the
relationship between age and the incidence of new-onset AF, with an
increased risk of AF with advancing age (23). As age increases, the lung function
of COPD patients gradually declines, leading to worsened hypoxia,
which is a key mechanism for AF development in COPD, causing atrial
structural changes and intimal thickening (24,25).
The present study found that COPD patients with AF were
significantly older than those without AF and logistic regression
analysis indicated age as an independent risk factor for COPD with
AF. This finding is consistent with a meta-analysis report in
2020(16), which showed that
clinical characteristics of COPD with AF have significant
demographic features such as age over 65, male gender and Caucasian
ethnicity, indicating a higher risk of AF in these populations.
Therefore, it was hypothesized that the risk of COPD with AF
increases with age and clinicians should be more vigilant about the
occurrence of AF in older COPD patients.
LAD, measured by echocardiography, plays an
important role in the occurrence and development of AF (26,27).
A cohort study by Vaziri et al (28) showed that for every 5 mm increase
in LAD, the risk ratio for AF was 1.39 and LAD enlargement was an
independent risk factor for AF. Furthermore, univariate and
multivariate Cox regression analysis results indicated that LAD
enlargement was an independent prognostic factor for AF. It has
been discovered that changes in the ultrastructure of atrial
myocytes and atrial fibrosis are the main forms of atrial
remodeling in AF patients (29,30).
Enlarged atrial myocardium not only affects atrial mechanical
function but also increases the pathological basis for the
formation of reentrant arrhythmias in AF, as the enlarged atrial
myocardium can accommodate more reentrant wavelets (31,32).
Research has found that inflammatory reactions are closely related
to pathological processes such as electrical remodeling, structural
remodeling and autonomic nervous system remodeling (33,34).
COPD patients experience increased pulmonary
vascular resistance due to the positive end-expiratory pressure,
which leads to the invasion of the interventricular septum into the
left ventricle, impairing left ventricular filling and resulting in
elevated left atrial and pulmonary vein pressures (35). This process of left atrial
remodeling is particularly pronounced in COPD patients with
comorbidities such as AF (36).
The inflammatory response triggered by chronic hypoxia in COPD
patients contributes to the process of atrial remodeling (37). Once AF occurs, the discordant
contraction of atrial muscles further exacerbates atrial
remodeling, creating a vicious cycle that promotes the development
of AF and ultimately leads to further deterioration of cardiac
structure and function (38). In
the present study, LAD was found to be an independent risk factor
for AF in COPD patients. Therefore, by controlling the pressure on
LAD, it may be possible to reduce the risk of AF in COPD patients.
Further research could explore interventions such as medication
treatment or other measures to reduce pulmonary arterial
hypertension or improve left ventricular function, thereby reducing
the pressure on LAD and lowering the likelihood of AF in COPD
patients.
UA plays an important role in the development and
prognosis of COPD. The level of UA is directly proportional to the
severity of tissue hypoxia. When tissue hypoxia occurs, ATP
synthesis decreases, leading to increased degradation of adenine
nucleotides and elevated UA levels (39,40).
Plasma UA mainly originates from the metabolism of intracellular
purine substances, with most of it being excreted by the kidneys
and the remainder being degraded in the digestive tract (41). Existing research has indicated a
close relationship between UA and oxidative stress (42). UA acts as a selective antioxidant
and plays an important role in the plasma antioxidant mechanism by
stabilizing serum vitamin C, preventing the oxidation inactivation
of endothelial enzymes and maintaining vascular dilation capacity
(43). Studies have found that
serum UA levels are associated with inflammatory factors such as
CRP, IL-1, IL-6 and TNF-α (44-46).
UA may induce atrial cell apoptosis and fibrosis through the
inflammatory pathway, leading to atrial remodeling and promoting
the occurrence of AF. Studies have reported a close relationship
between plasma UA levels and the occurrence and adverse prognosis
of cardiovascular diseases (47,48).
Furthermore, elevated plasma UA levels have been confirmed as an
independent risk factor for AF, increasing the risk of AF
occurrence (45,49). A study found that elevated plasma
UA levels were an independent risk factor for new-onset AF and the
plasma UA levels were even higher in patients with persistent AF,
suggesting a correlation between UA and the severity and duration
of AF (50). The present study
found that UA levels were an independent risk factor for AF in COPD
patients. Therefore, controlling UA levels may have significance in
preventing AF in COPD patients. Further research could explore
interventions to lower UA levels, such as medication or dietary
adjustments and their impact on the occurrence and prognosis of AF
in COPD patients.
The present study discovered that plasma UA levels
were significantly higher in COPD patients with AF compared to
those without AF and this difference was statistically significant.
Furthermore, both univariate and multivariate Cox regression
analyses revealed that plasma UA levels were an independent
prognostic factor for AF. UA is a routinely measured biochemical
marker, easily obtained through simple methods. Therefore, when
elevated UA levels are found in COPD patients, it should not be
simply interpreted as an increased risk of gout, but rather as a
potential indicator for cardiovascular events.
However, the present study had certain limitations.
First, the follow-up period was relatively short, only until May
2023, and it is necessary to ensure a sufficiently long follow-up
duration. Second, the present study employed a retrospective
clinical design with a small sample size, thus requiring
larger-scale, multicenter prospective studies to confirm the
diagnostic and prognostic efficacy of LAD and UA in COPD with AF.
Additionally, the model constructed only validated the predictive
performance using the data from the modeling itself and further
validation of the model's accuracy is needed using external data.
To improve the utility of the nomogram in predicting the risk and
long-term prognosis of COPD with AF in clinical practice, more
rigorous multicenter prospective studies are necessary to validate
the model developed in the present study.
In summary, the present study has developed a
nomogram to predict the risk of AF in COPD patients and predict the
5-, 10- and 15-month survival rates of COPD patients with AF. The
nomogram can assist clinicians and patients in early identification
of COPD with AF and predict their 5-, 10- and 15-month survival
rates, providing appropriate clinical information for personalized
treatment strategies and improving quality of life for
patients.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TH was responsible for conceptualization,
methodology, resources, writing the original draft and reviewing
and editing. XH was responsible for investigation, formal analysis,
writing the original draft, reviewing and editing and supervision.
XC was responsible for investigation, supervision and writing. QD
was responsible for investigation, supervision, resources and
writing. All authors read and approved the final manuscript. TH,
XH, XC and QD confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The Ethical Committee of The Second Affiliated
Hospital of Guilin Medical University approved the present research
(approval no. NO.YJS-2021011). The authors confirmed that all
methods conform to the provisions of Helsinki Declaration. All
participants signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Halpin DMG, Criner GJ, Papi A, Singh D,
Anzueto A, Martinez FJ, Agusti AA and Vogelmeier CF: Global
initiative for the diagnosis, management, and prevention of chronic
obstructive lung disease. The 2020 GOLD science committee report on
COVID-19 and chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 203:24–36. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Labaki WW and Rosenberg SR: Chronic
obstructive pulmonary disease. Ann Intern Med. 173:ITC17–ITC32.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim V and Criner GJ: Chronic bronchitis
and chronic obstructive pulmonary disease. Am J Respir Crit Care
Med. 187:228–237. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ruvuna L and Sood A: Epidemiology of
chronic obstructive pulmonary disease. Clin Chest Med. 41:315–327.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Iheanacho I, Zhang S, King D, Rizzo M and
Ismaila AS: Economic burden of chronic obstructive pulmonary
disease (COPD): A systematic literature review. Int J Chron
Obstruct Pulmon Dis. 15:439–460. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Negewo NA, Gibson PG and McDonald VM: COPD
and its comorbidities: Impact, measurement and mechanisms.
Respirology. 20:1160–1171. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Decramer M, Janssens W and Miravitlles M:
Chronic obstructive pulmonary disease. Lancet. 379:1341–1351.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lauder L, Mahfoud F, Azizi M, Bhatt DL,
Ewen S, Kario K, Parati G, Rossignol P, Schlaich MP, Teo KK, et al:
Hypertension management in patients with cardiovascular
comorbidities. Eur Heart J. 44:2066–2077. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Z, Zhao H and Wang J: Metabolism and
chronic inflammation: The links between chronic heart failure and
comorbidities. Front Cardiovasc Med. 8(650278)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Keshishian A, Xie L, Dembek C and Yuce H:
Reduction in hospital readmission rates among medicare
beneficiaries with chronic obstructive pulmonary disease: A
Real-world Outcomes Study of Nebulized Bronchodilators. Clin Ther.
41:2283–2296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou T, Liu P, Dhruva SS, Shah ND,
Ramachandran R, Berg KM and Ross JS: Assessment of hypothetical
out-of-pocket costs of guideline-recommended medications for the
treatment of older adults with multiple chronic conditions, 2009
and 2019. JAMA Intern Med. 182:185–195. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chugh SS, Havmoeller R, Narayanan K, Singh
D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr,
Zheng ZJ, et al: Worldwide epidemiology of atrial fibrillation: A
global burden of disease 2010 study. Circulation. 129:837–847.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Romiti GF, Corica B, Pipitone E, Vitolo M,
Raparelli V, Basili S, Boriani G, Harari S, Lip GYH and Proietti M:
AF-COMET International Collaborative Group. Prevalence, management
and impact of chronic obstructive pulmonary disease in atrial
fibrillation: A systematic review and meta-analysis of 4,200,000
patients. Eur Heart J. 42:3541–3554. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goudis CA, Konstantinidis AK, Ntalas IV
and Korantzopoulos P: Electrocardiographic abnormalities and
cardiac arrhythmias in chronic obstructive pulmonary disease. Int J
Cardiol. 199:264–273. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Naser N, Dilic M, Durak A, Kulic M, Pepic
E, Smajic E and Kusljugic Z: The impact of risk factors and
comorbidities on the incidence of atrial fibrillation. Mater
Sociomed. 29:231–236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang Q, Xiong H, Shuai T, Zhang M, Zhang
C, Wang Y, Zhu L, Lu J and Liu J: Risk factors for new-onset atrial
fibrillation in patients with chronic obstructive pulmonary
disease: A systematic review and meta-analysis. PeerJ.
8(e10376)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wood-Baker R, Cochrane B and Naughton MT:
Cardiovascular mortality and morbidity in chronic obstructive
pulmonary disease: The impact of bronchodilator treatment. Intern
Med J. 40:94–101. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tousoulis D: Biomarkers in atrial
fibrillation; From pathophysiology to diagnosis and treatment. Curr
Med Chem. 26:762–764. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 ESC/ERS Guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Respir
J. 61(2200879)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ranstam J and Cook JA: Kaplan-Meier curve.
Br J Surg. 104(442)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Abraham CR and Li A: Aging-suppressor
Klotho: Prospects in diagnostics and therapeutics. Ageing Res Rev.
82(101766)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo Y, Tian Y, Wang H, Si Q, Wang Y and
Lip GYH: Prevalence, incidence, and lifetime risk of atrial
fibrillation in China: New insights into the global burden of
atrial fibrillation. Chest. 147:109–119. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koshiyama M, Tamaki K and Ohsawa M:
Age-specific incidence rates of atrial fibrillation and risk
factors for the future development of atrial fibrillation in the
Japanese general population. J Cardiol. 77:88–92. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McDonough JE, Yuan R, Suzuki M, Seyednejad
N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson
HO, et al: Small-airway obstruction and emphysema in chronic
obstructive pulmonary disease. N Engl J Med. 365:1567–1575.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo J, Chen Y, Zhang W, Tong S and Dong J:
Moderate and severe exacerbations have a significant impact on
health-related quality of life, utility, and lung function in
patients with chronic obstructive pulmonary disease: A
meta-analysis. Int J Surg. 78:28–35. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Soeki T, Matsuura T, Tobiume T, Bando S,
Matsumoto K, Nagano H, Uematsu E, Kusunose K, Ise T, Yamaguchi K,
et al: Clinical, electrocardiographic, and echocardiographic
parameter combination predicts the onset of atrial fibrillation.
Circ J. 82:2253–2258. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Debonnaire P, Joyce E, Hiemstra Y, Mertens
BJ, Atsma DE, Schalij MJ, Bax JJ, Delgado V and Marsan NA: Left
atrial size and function in hypertrophic cardiomyopathy patients
and risk of new-onset atrial fibrillation. Circ Arrhythm
Electrophysiol. 10(e004052)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vaziri SM, Larson MG, Benjamin EJ and Levy
D: Echocardiographic predictors of nonrheumatic atrial
fibrillation. The Framingham Heart Study. Circulation. 89:724–730.
1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Alfadhel M, Nestelberger T, Samuel R,
McAlister C and Saw J: Left atrial appendage closure-Current status
and future directions. Prog Cardiovasc Dis. 69:101–109.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mo BF, Lian XM and Li YG: Current evidence
on the safety and efficacy of combined atrial fibrillation ablation
and left atrial appendage closure. Curr Opin Cardiol. 37:74–79.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang H, Tang Z, Han Z, Zeng L and Wang C:
Role of real time-three dimensional transesophageal
echocardiography in left atrial appendage closure with LACBES(®)
devices. Exp Ther Med. 17:1456–1462. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang Q, Wang JF, Dong QQ, Yan Q, Luo XH,
Wu XY, Liu J and Sun YP: Evaluation of left atrial volume and
function using single-beat real-time three-dimensional
echocardiography in atrial fibrillation patients. BMC Med Imaging.
17(44)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sagris M, Vardas EP, Theofilis P,
Antonopoulos AS, Oikonomou E and Tousoulis D: Atrial fibrillation:
Pathogenesis, predisposing factors, and genetics. Int J Mol Sci.
23(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hu YF, Chen YJ, Lin YJ and Chen SA:
Inflammation and the pathogenesis of atrial fibrillation. Nat Rev
Cardiol. 12:230–243. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Khalid K, Padda J, Komissarov A, Colaco
LB, Padda S, Khan AS, Campos VM and Jean-Charles G: The coexistence
of chronic obstructive pulmonary disease and heart failure. Cureus.
13(e17387)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Canepa M, Franssen FME, Olschewski H,
Lainscak M, Böhm M, Tavazzi L and Rosenkranz S: Diagnostic and
therapeutic gaps in patients with heart failure and chronic
obstructive pulmonary disease. JACC Heart Fail. 7:823–833.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Grymonprez M, Vakaet V, Kavousi M,
Stricker BH, Ikram MA, Heeringa J, Franco OH, Brusselle GG and
Lahousse L: Chronic obstructive pulmonary disease and the
development of atrial fibrillation. Int J Cardiol. 276:118–124.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jesel L, Abbas M, Toti F, Cohen A, Arentz
T and Morel O: Microparticles in atrial fibrillation: A link
between cell activation or apoptosis, tissue remodelling and
thrombogenicity. Int J Cardiol. 168:660–669. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu N, Xu H, Sun Q, Yu X, Chen W, Wei H,
Jiang J, Xu Y and Lu W: The role of oxidative stress in
hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid
Med Cell Longev. 2021(1470380)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Durmus Kocak N, Sasak G, Aka Akturk U,
Akgun M, Boga S, Sengul A, Gungor S and Arinc S: Serum uric acid
levels and uric acid/creatinine ratios in stable chronic
obstructive pulmonary disease (COPD) patients: Are these parameters
efficient predictors of patients at risk for exacerbation and/or
severity of disease? Med Sci Monit. 22:4169–4176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Park JH, Jo YI and Lee JH: Renal effects
of uric acid: Hyperuricemia and hypouricemia. Korean J Intern Med.
35:1291–1304. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hu L, Hu G, Xu BP, Zhu L, Zhou W, Wang T,
Bao H and Cheng X: U-Shaped association of serum uric acid with
all-cause and cause-specific mortality in US adults: A cohort
study. J Clin Endocrinol Metab. 105(dgz068)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Uk Kang T, Park KY, Kim HJ, Ahn HS, Yim SY
and Jun JB: Association of hyperuricemia and pulmonary
hypertension: A systematic review and meta-analysis. Mod Rheumatol.
29:1031–1041. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu ZD, Yang XK, He YS, Ni J, Wang J, Yin
KJ, Huang JX, Chen Y, Feng YT, Wang P and Pan HF: Environmental
factors and risk of gout. Environ Res. 212(Pt
C)(113377)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li S, Cheng J, Cui L, Gurol ME, Bhatt DL,
Fonarow GC, Benjamin EJ, Xing A, Xia Y, Wu S and Gao X: Cohort
study of repeated measurements of serum urate and risk of incident
atrial fibrillation. J Am Heart Assoc. 8(e012020)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Crawley WT, Jungels CG, Stenmark KR and
Fini MA: U-shaped association of uric acid to overall-cause
mortality and its impact on clinical management of hyperuricemia.
Redox Biol. 51(102271)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang Y, Tian J, Zeng C, Wei J, Li LJ, Xie
X, Yang T, Li H and Lei GH: Relationship between hyperuricemia and
risk of coronary heart disease in a middle-aged and elderly Chinese
population. J Int Med Res. 45:254–260. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Perez-Ruiz F and Becker MA: Inflammation:
A possible mechanism for a causative role of hyperuricemia/gout in
cardiovascular disease. Curr Med Res Opin. 31 (Suppl 2):S9–S14.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li N, Zhang S, Li W, Wang L, Liu H, Li W,
Zhang T, Liu G, Du Y and Leng J: Prevalence of hyperuricemia and
its related risk factors among preschool children from China. Sci
Rep. 7(9448)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chao TF, Hung CL, Chen SJ, Wang KL, Chen
TJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC and Chen SA: The
association between hyperuricemia, left atrial size and new-onset
atrial fibrillation. Int J Cardiol. 168:4027–4032. 2013.PubMed/NCBI View Article : Google Scholar
|