Introduction
Atopic dermatitis (AD) is a common chronic,
recurrent inflammatory skin disease with a complex pathogenesis.
Its main symptoms include dry skin, chronic eczematous lesions and
severe itching. Patients often experience allergic rhinitis, asthma
and other specific diseases, which significantly impact their
quality of life (1). One of the
key mechanisms involved in AD is the disruption of skin barrier
function. Keratin 17 (K17) is the main structural protein and
characteristic component of keratinocytes (KCs) and other
epithelial cells. As a marker of cell proliferation and
inflammation, K17 is rarely expressed in healthy skin, but is
highly expressed in skin with abnormal differentiation and
inflammatory injury (2). Loss of
function and mutation of the filaggrin (FLG) gene reduce the
accumulation of keratin filaments in the skin, thus affecting the
levels of natural moisturizing factor, causing dry skin and
creating conditions associated with the occurrence of AD (3,4).
Therefore, increasing the FLG content and controlling K17
expression may be a viable strategy for the treatment of AD.
The Janus kinase/signal transducer and activator of
transcription (JAK/STAT) pathway mediates the intracellular
signaling of various cytokines, including AD-related cytokines, in
particular, Th2 cytokines, including interleukin (IL)-4, IL-5,
IL-13 and IL-31, and thymic stromal lymphopoietin, contribute to
the symptoms of chronic inflammation and pruritus in AD.
Furthermore, the JAK/STAT is involved in the regulation of the
epidermal barrier and the modulation of peripheral nerves related
to the transduction of pruritus. The pathogenesis of AD is related
to activation of the downstream JAK/STAT pathway by its receptors
(5). When IL-4 and IL-13 bind to
their respective receptors, the JAK family is activated and
phosphorylates STAT3 (6-8).
STAT3, a versatile transcription factor, promotes excessive
proliferation of KCs and endothelial cells upon phosphorylation,
causing increased permeability of the skin barrier to pathogens
(9). In addition, IL-31 induces
STAT3 activation in KCs when it binds to its receptor, yielding the
production of β-endorphin, which may contribute to peripheral
pruritus in AD. IL-6, as a cytokine that activates STAT3, has been
shown to regulate autophagy through inhibitory or stimulatory
effects (10). Moreover, STAT3 can
influence autophagy regardless of its phosphorylation status
(11). Beclin 1 plays a crucial
role in the regulation of the autophagic process and serves as a
marker for the initiation of autophagy. Microtubule-associated
protein 1 light chain 3 (LC3) is involved in the formation of
autophagosomes and serves as specific protein marker of
autophagosomes. The combination of Beclin 1 and LC3 enables
accurate detection of changes in autophagic activity. Autophagy
plays a critical role in the differentiation of keratinocytes and
in their function as a skin barrier. Autophagy occurs continuously
and actively in the epidermal layer. Various stresses applied to
the epidermis can serve as triggers to activate autophagy, which
contributes to keratinocyte differentiation. For example,
mitochondrial reactive oxygen species produced during oxidative
phosphorylation and released into the cytoplasm in suprabasal
keratinocytes trigger autophagy, which is necessary for epidermal
differentiation (12). Therefore,
inhibiting cytokine, growth factor and hormone receptor signaling
pathways may prove to be an effective treatment for AD.
Proteolysis-targeting chimera (PROTAC) technology is
a novel approach in small-molecule drug development. Over the past
two decades, targeted protein degradation using PROTAC molecules to
harness the ubiquitin proteasome system has transitioned from
research to industry (13,14). Several companies have now disclosed
programs at the preclinical and early clinical development stages.
With clinical proof-of-concept demonstrated against two well-known
cancer targets (oestrogen receptor and androgen receptor), research
is now exploring previously considered ‘undruggable’ targets
(14). PJ-001 is a cereblon-based
degrader of JAK2 synthesized by our laboratory using PROTAC
technology. In our previous experiment, PJ-001 was used to induce
degradation of JAK protein in Jeko-1 cells and it was observed that
the half-maximal degradation concentration (DC50) was
<100 nM (unpublished data). The present study evaluated the
therapeutic effect of PJ-001 using the 2,4-dinitrofluorobenzene
(DNFB)-induced mouse model of AD, providing experimental and
theoretical evidence for the potential application of
small-molecule protein digesters in the treatment of AD.
Materials and methods
Reagents and instruments
DNFB was obtained from MilliporeSigma. Total RNA
Extractor (Trizol), Tween-20, TBS buffer, Premixed Powder (1X) and
TBE Buffer, Premixed Powder (1X) were purchased from Shanghai
Shenggong Biology Engineering Technology Service, Ltd. Bradford
protein concentration determination kit (detergent-compatible),
bovine serum albumin (BSA) and RIPA buffer (cat. no. 89900) were
purchased from Thermo Fisher Scientific Inc. BeyoRT™ III cDNA
first-strand synthesis premix (5X), Easy-Load™ PCR Master Mix
(Green), BeyoECL Moon, agarose, nucleic acid green, antibodies and
primers were purchased from Beyotime Institute of Biotechnology.
Crisaborole ointment was purchased from Pfizer, Inc. Compound
dexamethasone ointment was purchased from China Resources Sanjiu
Medical & Pharmaceutical Co., Ltd.
Drug formulation
A 0.5% DNFB solution was prepared by adding 51 µl
DNFB solution (purity: 98%) to 9.949 ml acetone/olive oil solution
(4:1 ratio by volume). The mixture was uniformly suspended by
vortex shaking, resulting in a final volume of 10 ml (15). For 4% PJ-001 solution preparation,
the aqueous phase was prepared by weighing 4 g PJ-001 sample and
adding to the following reagents: 15 g 70% ethanol, 10 g propylene
glycol, 10 g glycerol, 33.9 g water and 0.3 g nipagin. The oil
phase consisted of 8 g Vaseline, 2.5 g monoglyceride, 2.5 g
peregal, 5.5 g octadecyl alcohol and 8 g liquid paraffin. The
aqueous and oil phases were heated and melted, mixed at 80˚C and
stirred well. The mixture was then cooled to 30-40˚C to produce a
cream (100 g). According to the preparation method of crisaborole
ointment (2% concentration), 2% PJ-001 was also prepared (16).
Experimental animals
A total of 42 specific pathogen-free grade BABL/c
male mice (age, 6 weeks; weight, 18-22 g) were purchased from
Charles River Laboratories, Inc. The mice were housed in
individually ventilated cages maintained at constant temperature
(20-26˚C) and humidity (40-60%). The animals were housed in a 12 h
light/12 h dark cycle, with free access to water and food. Both the
feed and water provided to the mice were sterilized. The animal
license number was SYXK (Su) 2016-0045. At the end of the
experiment, the mice were euthanized with CO2 (17). The mice were placed in a container,
and the concentration of CO2 was gradually increased
using a flow rate of 5.5 l/min to achieve a CO2
displacement rate of 30% (18)
until their breathing stopped (3.0-3.5 min). Subsequently,
bilateral pneumothorax chest opening was performed on the mice to
prevent their recovery from asphyxia. It is crucial to allow an
adequate interval between euthanasia procedures, 1-2 min, to ensure
the dissipation of CO2 from the container. This
precautionary measure guarantees that subsequent groups of animals
are not suddenly exposed to high levels of CO2 gas. At
the end of euthanasia, mouse spleens are collected and weighed. The
skin was collected from the lesion site for further
experiments.
Establishment of a mouse model of
AD
All animal experiments were approved by the Ethics
Committee of Experimental Animal Management and Animal Welfare of
Jiangnan University [approval no. JN. No20210930b0801218(353)];
Wuxi, China). After 1 week of adaptive feeding, the mice acclimated
to the unfamiliar surroundings and were than randomly assigned to
the following six groups based on their body mass: Control group,
model group, dexamethasone group, crisaborole group, 4% PJ-001
group and 2% PJ-001 group (n=7 mice/group). The weight deviation of
each mouse was consistently around 20% of the average body weight.
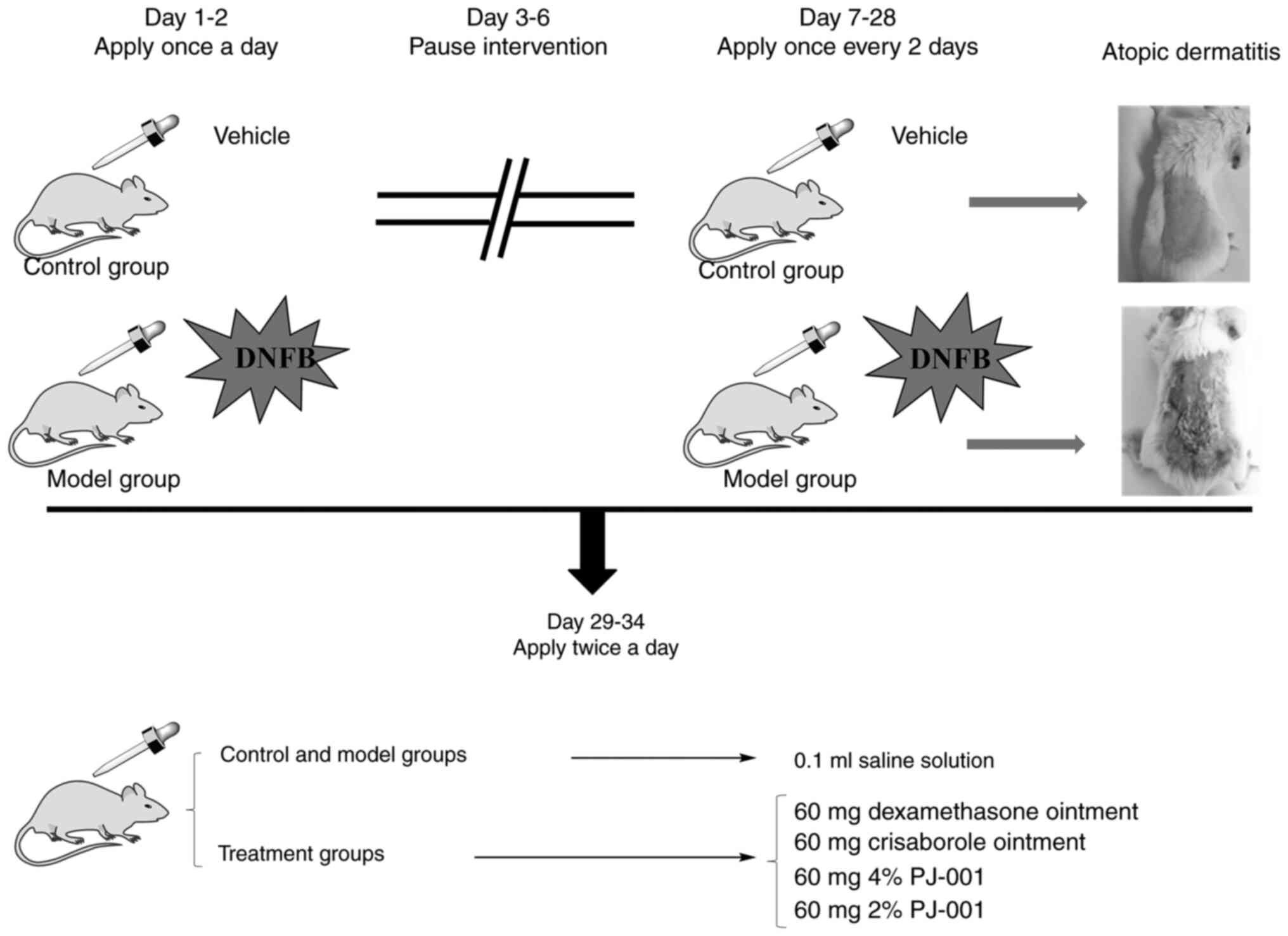
The experimental process is shown in Fig. 1. On the first and second days of
the experiment, 100 µl acetone/olive oil (4:1) solution without
DNFB was applied to the back of the control group mice once a day.
In all mice, with the exception of the control group, 100 µl
acetone/olive oil (4:1) solution containing 0.5% DNFB was applied
to the back once a day. On days 3-6 of the experiment, the
intervention was suspended. On days 7-28 of the experiment, the
control group was administered the vehicle [acetone/olive oil (4:1)
solution without DNFB], and the remaining mice were coated with 70
µl acetone olive oil (4:1) solution containing 0.5% DNFB every 2
days (15,19). At the end of the modeling process,
2 non-compliant mice (with skin broken during shaving or being
over/underweight) were excluded from each group, and the remaining
mice were subjected to 6 days of topical administration. Both the
control and model groups were administered 100 µl saline, twice a
day. The other groups were treated with 60 mg compound
dexamethasone ointment, crisaborole ointment (19), 4% PJ-001 or 2% PJ-001, twice a
day.
Changes in mouse skin lesions and
scratching frequency
On days 0, 2, 4 and 6 of administration, changes in
the specific dermatitis lesions were observed and the severity of
mouse skin lesions was scored using the scoring standard provided
in Table I (20). At 1 h after the last application
administration, scratching frequency in mice was recorded over a
duration of 10 min; for all the mice, scratching the back skin once
was counted as one effective scratching incident, and continuous
scratching for >3 sec was counted as two incidents.
 | Table ILesion severity score. |
Table I
Lesion severity score.
| Observation
index | Rating/Score |
|---|
| Skin level with
healthy skin | 0 |
| Skin slightly
elevated above normal skin surface | 1 |
| Skin is moderately
raised, and the edges of the plaques are circular or sloping | 2 |
| Skin hypertrophy
with obvious protrusions | 3 |
| Skin is highly
thickened and has extremely prominent protrusions | 4 |
Hematoxylin and eosin (H&E)
staining
Following euthanasia, skin from the back of the mice
was collected and immersed in 10% (v/v) neutral formalin at 4˚C for
24 h; then, the tissues were embedded in paraffin and sliced into
5-µm slices. The samples were then placed in xylene for 5 min at
room temperature for dewaxing. The xylene was then replaced with
fresh xylene, followed by another 5 min of dewaxing. The samples
were subsequently cleaned sequentially with different gradient
concentrations of ethanol and distilled water to rehydrate them.
Next, the sections were stained with hematoxylin solution for 5 min
at room temperature. The excess staining solution was washed off by
soaking the samples in water for ~10 min, followed by another wash
in distilled water. The samples were then stained with eosin
staining solution for 2 min and washed twice with water.
Dehydration and permeabilization were performed using absolute
ethanol and xylene sequentially, and the slices were sealed with
neutral gum. Finally, the slides were placed under an optical
microscope for image collection and analysis (15).
Western blotting
Mouse skin tissue was cut into small pieces and
mixed with precooled RIPA lysis buffer. The mixture was then
centrifuged at 4˚C and 9,500 x g for 10 min. The resulting
supernatant was collected as the protein lysate. Protein
quantification was performed using the Bradford protein
concentration assay kit, and the proteins were separated on 10%
gels using SDS-PAGE and transferred onto a polyvinylidene fluoride
membrane. The membranes were then blocked with 5% BSA at room
temperature for 1 h and incubated with the following primary
antibodies: Toll-like receptor 4 (TLR4; 1:1,000; cat. no. AF8187),
nuclear factor-κB (NF-κB; 1:1,000; cat. no. AF1234), JAK2 (1:1,000;
cat. no. AF1489), STAT3 (1:1,000; cat. no. AF1492), Beclin 1
(1:1,000; cat. no. AF5123), LC3 (1:1,000; cat. no. AL221) and GAPDH
(1:10,000; cat. no. AF1186) at 4˚C overnight. Subsequently, the
membrane was incubated with secondary antibodies (1:10,000; cat.
no. A0208) at room temperature for 1 h. The aforementioned primary
antibodies were all anti-rabbit polyclonal/monoclonal antibodies
from Beyotime Institute of Biotechnology. A 5% BSA solution and
antibodies were prepared using TBS buffer containing 1% Tween-20.
Beyo-ECL Moon Kit was used to develop the membrane and a Gel
Imaging System (Tanon Science and Technology Co., Ltd.) was used to
capture images. The gray value was analyzed using ImageJ (1.46r)
software (National Institutes of Health) (21). The following formula was used for
semi-quantification: Protein expression (% of
control)=(GA-target/GA-GAPDH)/(GC-target/GC-GAPDH) x100. G
indicates grayscale value; A-target indicates target protein of the
model and treatment group; A-GAPDH indicates GAPDH protein of the
model and treatment group; C-target indicates target protein of the
control group; C-GAPDH indicates GAPDH protein of the control
group.
Reverse transcription (RT)-PCR
Mouse skin tissue was cut into small pieces and
precooled total RNA extractor (TRIzol) was added, according to the
manufacturer's protocol. The extracted RNA sample was then placed
into a MK-20 dry thermostat and heated at 70˚C for 5 min. The
concentration and purity of the RNA sample were measured using a
micro nucleic acid protein analyzer. RNA was then subjected to RT
to obtain cDNA, and the reaction procedure was as follows: 42˚C for
60 min and 80˚C for 10 min. Then, primers were added to amplify the
target gene; PCR conditions were as follows: 94.0˚C for 3 min;
followed by 35 cycles at 94.0˚C for 30 sec, 56.0˚C for 30 sec and
72.0˚C for 1 min; and a final step at 72.0˚C for 1 min, finally
maintained at 4˚C. Primer sequences are shown in Table II. The amplified products were
subjected to 2% agarose gel electrophoresis, and the results were
analyzed by ImageJ software with GAPDH as an internal control. The
agarose gel was prepared using TBE buffer, then microwaved and
mixed with Nucleic Acid Green.
 | Table IIPrimer sequences used for PCR. |
Table II
Primer sequences used for PCR.
| Primer | Sequence
(5'-3') | Molecular weight,
bp |
|---|
| IL-10 | F:
AGGCGCTGTCATCGATTTCT | 489 |
| | R:
AGGAAGAACCCCTCCCATCA | |
| FLG | F:
AAAAGATGTCCGCTCTCCTGG | 252 |
| | R:
TTGCCAGCTTTAGCACCAGT | |
| K17 | F:
GACCACCCGTTAAGGACTCA | 161 |
| | R:
AGGCCACAGTTCACTTCAGGT | |
| GAPDH | F:
ATCAGCAATGCCTCCTGCAC | 242 |
| | R:
TTCCCGTTCAGCTCAGGGAT | |
Statistical analysis
Data were analyzed using GraphPad Prism 8.0.1
statistical software (Dotmatics), and the experimental results are
presented as the mean ± standard deviation and median. Statistical
analyses and comparisons among groups were performed using one-way
analysis of variance followed by Tukey's post hoc test for
parametric data, and the Kruskal-Wallis test followed by the
Dunn-Bonferroni post hoc test for non-parametric data. P<0.05
was considered to indicate a statistically significant
difference.
Results
Comparison of scratch frequency and
skin lesion severity scores in mice
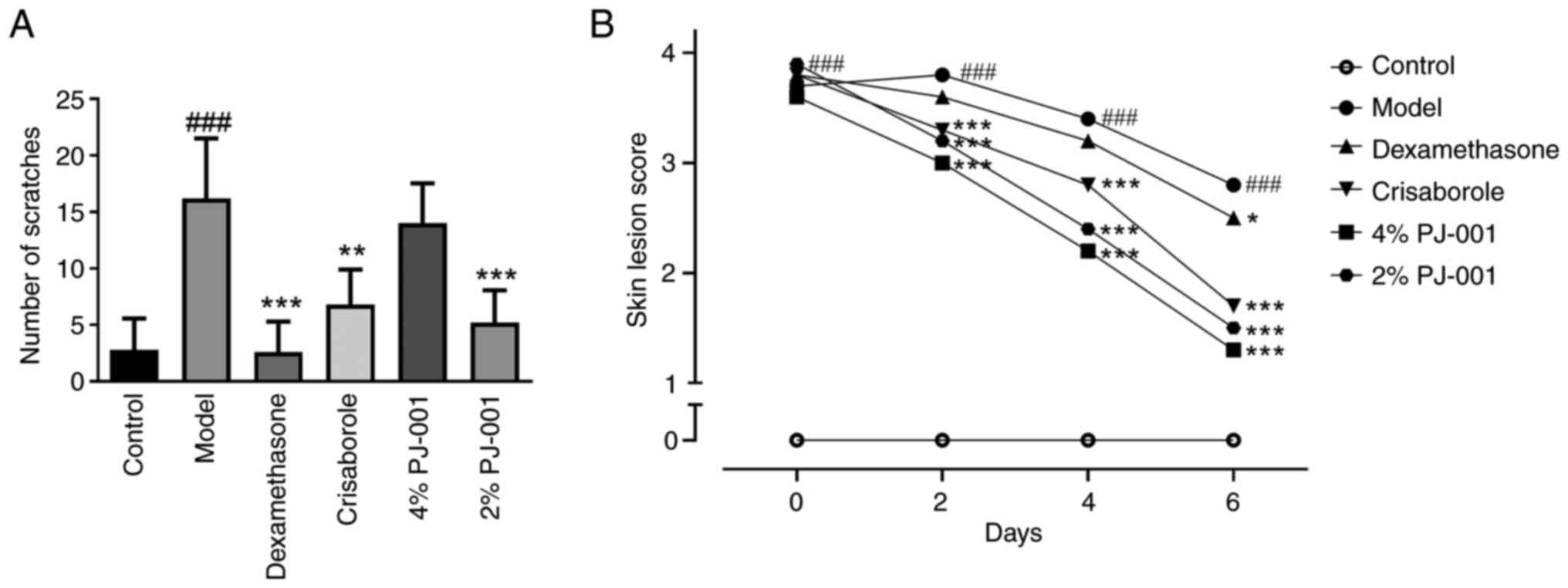
As shown in Fig.
2A, the number of scratches in the model group was
significantly higher than that in the control group. However, the
number of scratches in the compound dexamethasone group,
crisaborole group and 2% PJ-001 group was significantly reduced
compared with that in the model group (P<0.01). This finding
indicated that 2% PJ-001 had anti-pruritic properties. The skin
lesion score of the model group mice was higher than that of the
control group (Fig. 2B). As
expected, the crisaborole group, 4% PJ-001 group and 2% PJ-001
group exhibited a decrease in skin lesion scores.
Histopathological changes in mouse
skin
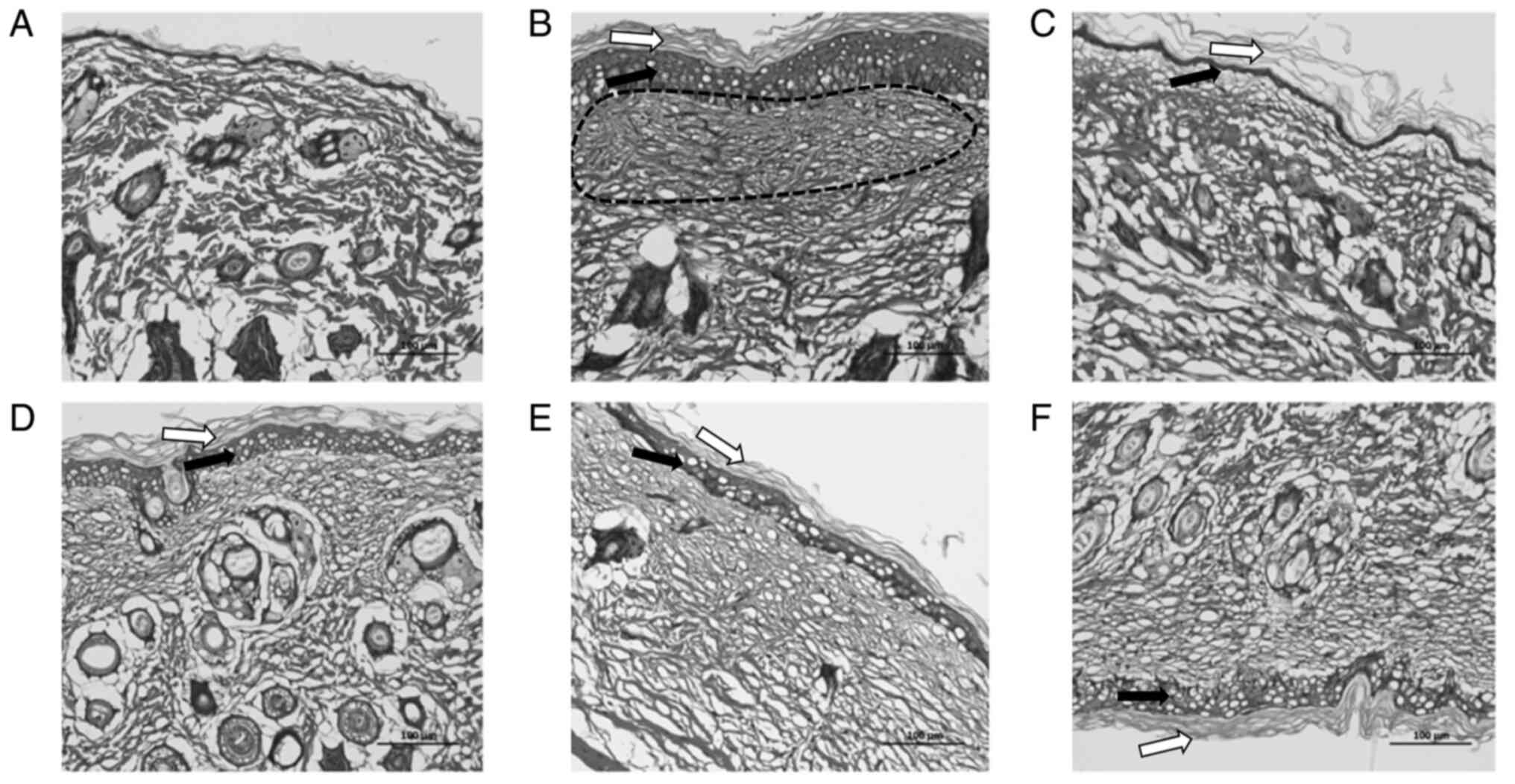
As shown in Fig.
3B, compared with the control (Fig. 3A), the model group exhibited
excessive and incomplete keratinization, thickening of the spinous
layer, mild sponge edema, decreased hair follicles, dilated
capillaries and a substantial presence of inflammatory cell
infiltration. In comparison to the model group, the crisaborole
group demonstrated a marked thinning of the epidermal layer and an
increase in hair follicles, although incomplete keratinization and
a small amount of inflammatory cell infiltration persisted
(Fig. 3D). The 2% PJ-001 group
exhibited a notable increase in the structure of hair follicles and
a slight decrease in the thickness of the spinous layer; however,
severe edema was observed (Fig.
3F). In the 4% PJ-001 group, the corneum and spinous layer
became thinner, cell edema decreased and inflammatory cell
infiltration decreased slightly (Fig.
3E). The compound dexamethasone group showed no marked
difference compared with the control group, with only a slightly
thicker epidermal layer and minimal infiltration of inflammatory
cells. Cellular edema could still be observed (Fig. 3C).
Effects of PJ-001 on the JAK2/STAT3
pathway in mouse skin tissue
As shown in Fig.
4A-C, the protein expression levels of JAK2 and STAT3 in the AD
model group were significantly increased compared with those in the
control group (P<0.001). However, in the compound dexamethasone
group, crisaborole group, 4% PJ-001 group and 2% PJ-001 group, the
expression levels of JAK2 were significantly decreased compared
with those in the model group (P<0.001). The expression levels
of JAK2 in the 4 and 2% PJ-001 groups decreased by 65.2 and 61.3%,
respectively. Moreover, PJ-001 also inhibited STAT3 expression. The
expression levels of STAT3 in the 4 and 2% PJ-001 groups were
significantly decreased by 53.4 and 29.8%, respectively
(P<0.001).
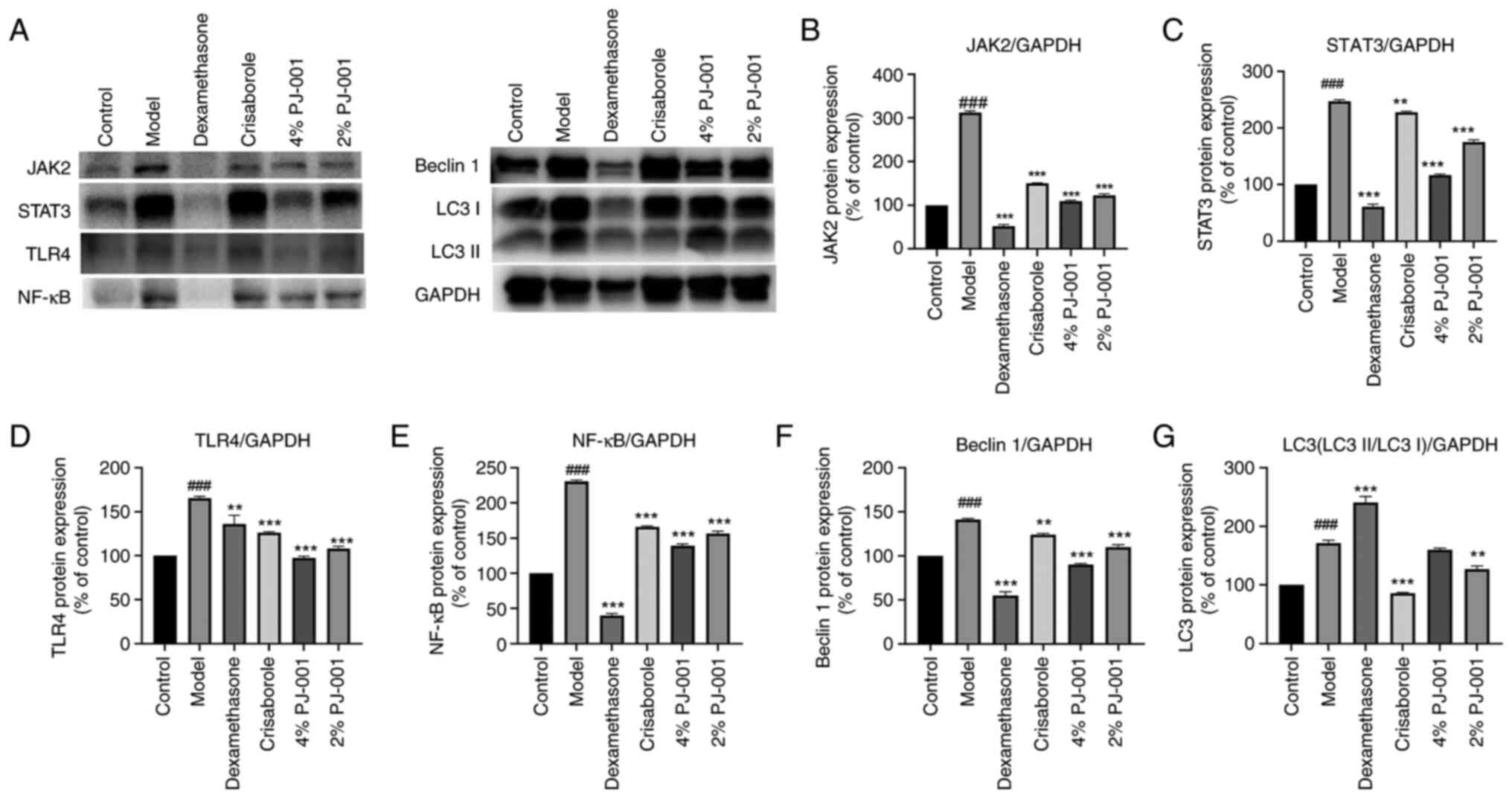 | Figure 4Effects of PJ-001 on protein
expression in the skin tissue of mice. (A) Western blotting of each
protein. PJ-001 effectively inhibited the JAK/STAT pathway,
mediated downstream TLR4/NF-KB pathway downregulation and reduced
excessive autophagy. Protein expression levels of (B) JAK2, (C)
STAT3, (D) TLR4, (E) NF-κB, (F) Beclin 1 and (G) LC3 were
semi-quantified. GAPDH was used as a loading control. Data are
presented as a percentage of the control. A minimum of three
independent experiments were carried out, and data are presented as
the mean ± SD. ###P<0.001 vs. Control;
**P<0.01, ***P<0.001 vs. Model. JAK2,
Janus kinase 2; LC3, microtubule-associated protein 1 light chain
3; NF-κB, nuclear factor-κB; STAT3, signal transducer and activator
of transcription 3; TLF4, Toll-like receptor 4. |
Effects of PJ-001 on TLR4/NF-κB in
mouse skin tissue
As shown in Fig.
4A, D and E, the protein expression levels of TLR4
and NF-κB were significantly higher in the AD model group than
those in the control group (P<0.001). Compared with those in the
model group, the expression levels of TLR4 were significantly
downregulated in the compound dexamethasone group, crisaborole
group and PJ-001 groups (P<0.01). Specifically, the protein
expression levels of TLR4 in the 4 and 2% PJ-001 groups were
decreased by 41.1 and 34.7%, respectively. In addition, the protein
expression levels of NF-κB were significantly decreased in the
compound dexamethasone group, crisaborole group, 4% PJ-001 group
and 2% PJ-001 group (P<0.001). Specifically, the protein
expression levels of NF-κB were downregulated by 46.6% in the 4%
PJ-001 group and 39.9% in the 2% PJ-001 group. These findings
indicated that the inflammatory pathway involving TLR4/NF-κB was
inhibited by PJ-001.
Effects of PJ-001 on Beclin 1 and LC3
in mouse skin tissue
As shown in Fig.
4A, F and G, compared with those in the control
group, the expression levels of Beclin 1 and LC3 (LC3II/LC3I) were
significantly increased in the model group (P<0.001). The
protein expression levels of Beclin 1 in the compound dexamethasone
group, crisaborole group, 4% PJ-001 group and 2% PJ-001 group were
significantly lower than those in the model group (P<0.01).
Specifically, the expression levels of Beclin 1 were decreased by
36.2% in the 4% PJ-001 group compared with that in the model group,
whereas they were decreased by 22.0% in the 2% PJ-001 group.
Furthermore, the expression levels of LC3 were significantly
decreased in the crisaborole group and 2% PJ-001 group compared
with those in the model group (P<0.01).
Effects of PJ-001 on the mRNA
expression levels of K17 and FLG in mouse skin tissue
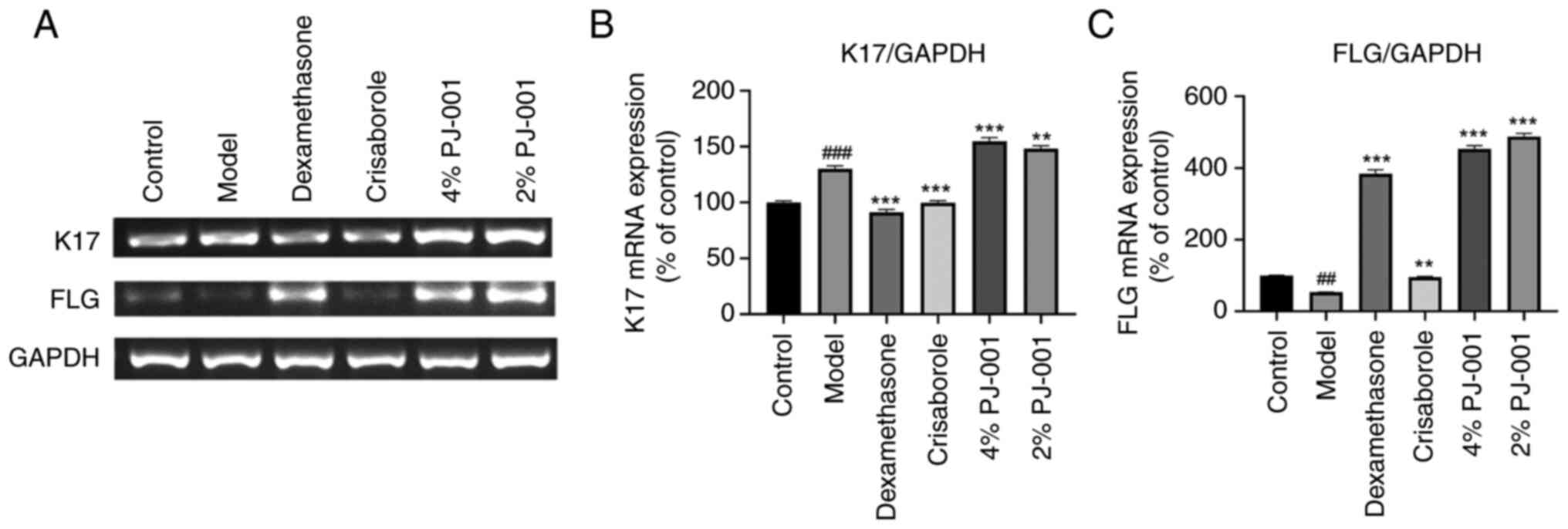
As shown in Fig. 5A
and B, the mRNA expression levels
of K17 in the AD model group exhibited a significant increase
(P<0.001) compared with those in the control group. The positive
controls, dexamethasone and crisaborole, demonstrated a notable
suppressive effect on K17 mRNA (P<0.001). However, the PJ-001
treatment was ineffective in suppressing the expression and
transcription of K17 (Figs. 4H and
5B). The mRNA expression levels of
FLG in the AD model group were significantly reduced by PJ-001
group (P<0.001; Fig. 5A and
C). By contrast, the groups
treated with compound dexamethasone, 4% PJ-001 and 2% PJ-001 showed
a significant increase in the expression levels of FLG
(P<0.001). Specifically, the 4% PJ-001 group exhibited an 847%
increase, whereas the 2% PJ-001 group showed a 911% increase
(Fig. 5C).
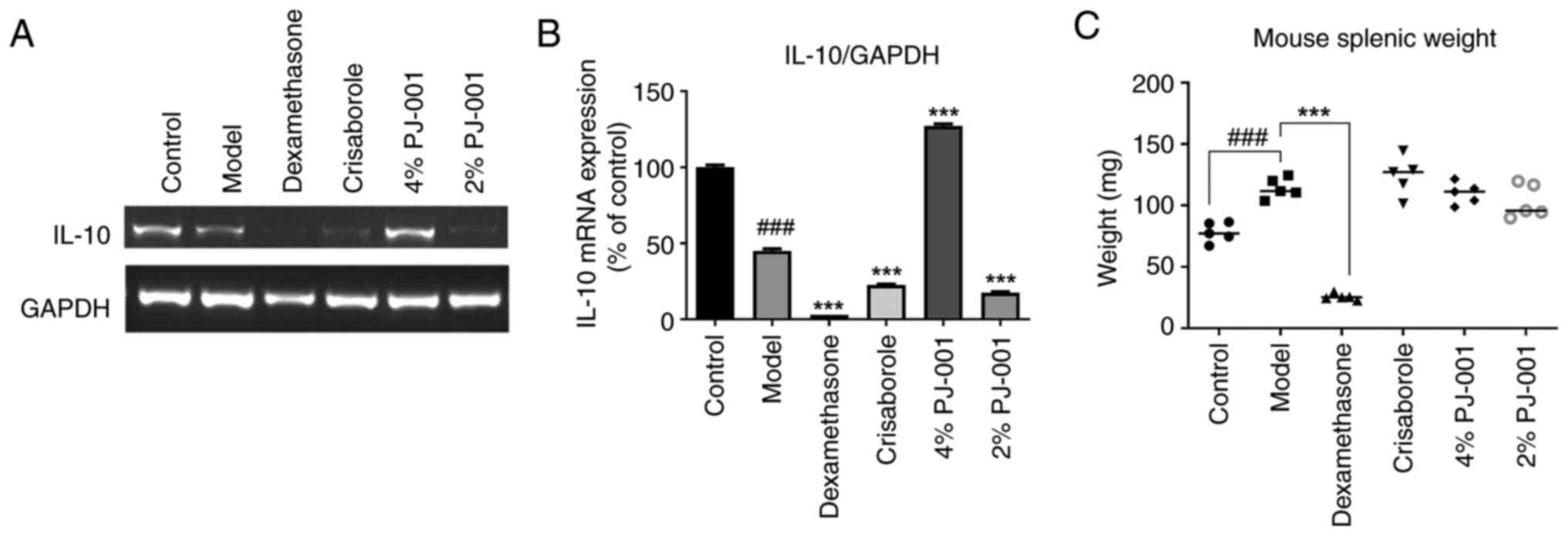
Mouse splenic weight and IL-10 mRNA
expression levels
As shown in Fig.
6C, splenic weight was significantly increased in the model
group compared with that in the control group (P<0.001).
However, the compound dexamethasone group exhibited a significant
decrease in splenic weight (P<0.001). By contrast, there was no
notable difference observed in the crisaborole group, 4% PJ-001
group and 2% PJ-001 group relative to the model group. Furthermore,
the mRNA expression levels of IL-10 in the splenic tissues of mice
in the model group were significantly lower than those in the
control group (P<0.001; Fig. 6A
and B). In the 4% PJ-001 group,
the mRNA expression levels of IL-10 were significantly increased by
282.2% (P<0.001) compared with those in the model group. The
compound dexamethasone group, crisaborole group and the 2% PJ-001
did not succeed in inducing IL-10 expression.
Discussion
The pathogenesis of AD is a complex process that
involves various factors, including the external environment,
genetic susceptibility, skin barrier dysfunction and immune
response (4). Currently, there are
several drugs available for treating AD, primarily systemic drugs
such as topical corticosteroids and oral antihistamines; however,
these drugs have limited efficacy and notable side effects.
Compound dexamethasone ointment, a long-term glucocorticoid, has
the ability to suppress the synthesis of inflammatory factors,
reduce exudation, relieve itching, and exhibit anti-inflammatory
and anti-allergic effects (22).
However, as a hormone-based drug, prolonged usage can lead to skin
atrophy, capillary dilation, pigmentation and secondary infections
(23). Crisaborole ointment is a
new small-molecule drug used to treat AD. Its main component,
crisaborole, functions as a phosphodiesterase 4 inhibitor. Although
this ointment has demonstrated high efficacy and safety, without
causing skin atrophy, it exhibits a slow onset and does not provide
immediate relief from itching, which may reduce medication
compliance among patients with AD (24). To enhance treatment options for
patients with AD, it is crucial to continue the development of
drugs that provide faster onset and effective relief from itching,
with fewer side effects.
In recent years, PROTAC drugs have advanced to the
clinical research stage, representing a significant development
from a conceptual standpoint. Unlike traditional small-molecule
inhibitor drugs, PROTAC drugs utilize the ubiquitin-proteasome
system to degrade target proteins, showing immense potential in
targeting ‘non-drug targets’ (25). KT-474 is a potential first-in-class
IRAK4 degrader that is being developed for the treatment of
TLR/IL-1R-driven immune inflammatory diseases, such as AD (26). The utilization of PROTAC technology
holds promise as a novel approach in drug development for the
treatment of inflammatory diseases. PJ-001 is a degrader of JAK
synthesized by our laboratory using PROTAC technology. The original
purpose of the present study was to develop a PROTAC molecule with
the potential to alleviate AD. To evaluate the effectiveness of the
molecule, a comparison with clinically recommended drugs, namely
dexamethasone and crisaborole, was performed. The severity of skin
lesions in AD mice was assessed by comparing the number of
scratches and severity scores among different groups. The results
demonstrated that PJ-001 can effectively reduce the severity of
skin lesions, particularly when used at a concentration of 2%.
However, the results did not conform to the expected dose-effect
relationship, as lower doses showed a more significant effect than
higher doses. The potential explanation for this is that each drug
has an upper limit, which is known as the ‘hook effect’ that PROTAC
drugs need to overcome. When the concentration is higher than the
DC50 value, PROTAC molecules can saturate binding sites
on either the target protein or the E3 ligase, inhibiting the
formation of the required ternary complex and resulting in the
generation of an unproductive binary complex. This results in loss
of efficacy at higher concentrations (27). Additionally, analysis of mouse
H&E pathological sections revealed that PJ-001 could reduce the
thickness of the spinous layer in AD mice and alleviate excessive
or incomplete epidermal keratinization. Notably, the application of
2% PJ-001 demonstrated a marked restoration of hair follicle
structure, whereas 4% PJ-001 exhibited a substantial reduction in
the infiltration of inflammatory cells. These findings provide
direct evidence that PJ-001 effectively mitigates inflammatory
infiltration, thereby improving skin itching and epidermal
keratinization.
FLG, an essential component of the outer membrane of
KCs, is produced during the process of KC differentiation. Its main
function is to maintain epidermal homeostasis by facilitating the
regular gathering of keratin filaments. Additionally, mutations or
abnormal expression of FLG-related genes can result in a decrease
in ceramides and antimicrobial peptides, while also causing an
increase in water loss through the epidermis (3,28).
Consequently, this leads to increased infiltration of environmental
stimuli, allergens and microorganisms, ultimately contributing to
the development of AD (29). The
expression of FLG has been observed to decrease in the epidermis of
patients diagnosed with AD (30).
In the present study, a significant decrease in FLG mRNA expression
was observed in the skin lesions of AD mice. However, following
treatment with PJ-001, FLG mRNA expression was upregulated ~9 times
compared with that in the model group. These findings suggested
that PJ-001 has the ability to promote the expression of FLG and to
enhance the resistance of the stratum corneum. As a result, it may
effectively prevent the penetration and invasion of harmful
substances and allergens.
The JAK/STAT pathway is a major signaling pathway
regulated by cytokines; it serves a crucial role in priming innate
immunity, coordinating adaptive immune mechanisms, and ultimately
suppressing inflammation and immune responses (31). NF-κB is present in almost all
animal cell types and participates in cell responses to various
stimuli, such as stress, cytokines, free radicals, heavy metals,
ultraviolet radiation, and bacterial or viral antigens. NF-κB is
essential in regulating the immune response to infection (32). Both JAK/STAT and NF-κB pathways are
connected through the phosphatidylinositol 3-kinase/protein kinase
B (PI3K/AKT) pathway. Receptors phosphorylated by JAKs recruit
PI3K, which activates the PI3K/Akt pathway. This leads to the
phosphorylation of inhibitor κB, enabling the activation of NF-κB.
Once activated, NF-κB enters the nucleus, interacts with DNA, and
initiates or inhibits the transcription of related genes (33). PJ-001 is a PROTAC molecule designed
to target the JAK protein. The present study indicated that PJ-001
can effectively decrease JAK/STAT3 and TLR4/NF-κB protein
expression to alleviate skin damage in AD mice. Moreover, PJ-001
induced a significant downregulation of the autophagy factors
Beclin 1 and LC3, thereby inhibiting excessive autophagy in the
inflammatory tissues.
IL-10, an important anti-inflammatory cytokine, has
a controversial role in the pathogenesis of AD. A previous study
detected high expression of IL-10 in the serum of patients with AD
(34). However, Hussein et
al (35) found no significant
abnormality in the serum of IL-10 in patients with atopy. By
contrast, Zhang et al (36)
demonstrated that IL-10 levels in the supernatants of peripheral
blood culture were lower in patients with severe AD compared with
those in normal controls. In the present study, it was observed
that 4% PJ-001 significantly increased the mRNA expression levels
of IL-10 in the spleens of AD mice. We hypothesize that PJ-001 can
inhibit JAK/STAT signaling, thereby modulating macrophage
activation to suppress inflammatory diseases. This modulation may
promote the repolarization of inflammatory (M1) macrophages into
anti-inflammatory (M2) macrophages, increasing the transcription of
IL-10(37). However, evidence has
suggested that IL-10 may have a dual role, as it can stimulate the
immune response and potentially trigger diseases instead of
suppressing them (38). In future
studies, we aim to evaluate the molecular mechanism of PJ-001 in
alleviating AD by inhibiting the JAK/STAT pathway using inhibitor
interference. Additionally, we will utilize mature PROTACs for
comparison to ensure a more comprehensive evaluation of our test
compound.
In conclusion, PJ-001 can reduce the inflammatory
response in a mouse model of AD by inhibiting the activation of
inflammatory pathways. In addition, it may alleviate excessive
autophagy in skin lesions and increase the expression of skin
barrier factors. These findings suggested that targeted inhibition
of the JAK2/STAT3 and TLR4/NF-κB signaling pathways represents a
potential therapeutic approach for AD.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82004027).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PL and ZC drafted the final manuscript. PL, ZC and
JL made substantial contributions to the conception and design of
the study. PL and JL critically revised the manuscript and provided
constructive feedback. YL and HS participated in animal experiments
and assisted in interpreting data. PL and JL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
guidelines laid out by the Ethics Committee for Animal Experiments
of Jiangnan University (Wuxi, China) and was approved by the Ethics
Committee of Experimental Animal Management and Animal Welfare of
Jiangnan University [approval no. JN. No20210930b0801218(353)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hadi HA, Tarmizi AI, Khalid KA, Gajdács M,
Aslam A and Jamshed S: The epidemiology and global burden of atopic
dermatitis: A narrative review. Life (Basel).
11(936)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cornaghi L, Gagliano N, Preis FWB,
Prignano F and Donetti E: Inside-out and outside-in organotypic
normal human skin culture: JAK-STAT pathway is activated after
pro-inflammatory psoriatic cytokine exposure. Tissue Cell.
74(101675)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moosbrugger-Martinz V, Leprince C, Méchin
MC, Simon M, Blunder S, Gruber R and Dubrac S: Revisiting the roles
of filaggrin in atopic dermatitis. Int J Mol Sci.
23(5318)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Vuyst E, Salmon M, Evrard C, Lambert de
Rouvroit C and Poumay Y: Atopic dermatitis studies through in vitro
models. Front Med (Lausanne). 4(119)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Welsch K, Holstein J, Laurence A and
Ghoreschi K: Targeting JAK/STAT signalling in inflammatory skin
diseases with small molecule inhibitors. Eur J Immunol.
47:1096–1107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bao L, Zhang H and Chan LS: The
involvement of the JAK-STAT signaling pathway in chronic
inflammatory skin disease atopic dermatitis. JAKSTAT.
2(e24137)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang L, Xian YF, Loo SKF, Ip SP, Yang W,
Chan WY, Lin ZX and Wu JCY: Baicalin ameliorates
2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin
lesions in mice through modulating skin barrier function, gut
microbiota and JAK/STAT pathway. Bioorg Chem.
119(105538)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goenka S and Kaplan MH: Transcriptional
regulation by STAT6. Immunol Res. 50:87–96. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang J, Liu C, Liu S, Zhou X, Lu J, Li M
and Zhu L: Inhibition of JAK1/STAT3 pathway by 2-methoxyestradiol
ameliorates psoriatic features in vitro and in an imiquimod-induced
psoriasis-like mouse model. Eur J Pharmacol.
933(175276)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kang R, Tang D, Lotze MT and Zeh HJ:
AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and
bioenergetics through the IL6-pSTAT3 pathway. Autophagy. 8:989–991.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li H, Chen L, Li JJ, Zhou Q, Huang A, Liu
WW, Wang K, Gao L, Qi ST and Lu YT: miR-519a enhances
chemosensitivity and promotes autophagy in glioblastoma by
targeting STAT3/Bcl2 signaling pathway. J Hematol Oncol.
11(70)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim HJ, Park J, Kim SK, Park H, Kim JE and
Lee S: Autophagy: Guardian of skin barrier. Biomedicines.
10(1817)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li X and Song Y: Proteolysis-targeting
chimera (PROTAC) for targeted protein degradation and cancer
therapy. J Hematol Oncol. 13(50)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Békés M, Langley DR and Crews CM: PROTAC
targeted protein degraders: The past is prologue. Nat Rev Drug
Discov. 21:181–200. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xiong X, Huang C, Wang F, Dong J, Zhang D,
Jiang J, Feng Y, Wu B, Xie T and Cheng L: Qingxue jiedu formulation
ameliorated DNFB-induced atopic dermatitis by inhibiting
STAT3/MAPK/NF-κB signaling pathways. J Ethnopharmacol.
270(113773)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Woo TE and Kuzel P: Crisaborole 2%
ointment (Eucrisa) for atopic dermatitis. Skin Therapy Lett.
24:4–6. 2019.PubMed/NCBI
|
|
17
|
Tadvalkar G, Pal-Ghosh S, Pajoohesh-Ganji
A and Stepp MA: The impact of euthanasia and enucleation on mouse
corneal epithelial axon density and nerve terminal morphology. Ocul
Surf. 18:821–828. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shomer NH, Allen-Worthington KH, Hickman
DL, Jonnalagadda M, Newsome JT, Slate AR, Valentine H, Williams AM
and Wilkinson M: Review of rodent euthanasia methods. J Am Assoc
Lab Anim Sci. 59:242–253. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zheng BW, Wang BY, Xiao WL, Sun YJ, Yang C
and Zhao BT: Different molecular weight hyaluronic acid alleviates
inflammation response in DNFB-induced mice atopic dermatitis and
LPS-induced RAW 264.7 cells. Life Sci. 301(120591)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang N, Shao H, Deng J, Yang Y, Tang Z, Wu
G and Liu Y: Dictamnine ameliorates chronic itch in DNFB-induced
atopic dermatitis mice via inhibiting MrgprA3. Biochem Pharmacol.
208(115368)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gallo-Oller G, Ordoñez R and Dotor J: A
new background subtraction method for Western blot densitometry
band quantification through image analysis software. J Immunol
Methods. 457:1–5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schuler CFt, Billi AC, Maverakis E, Tsoi
LC and Gudjonsson JE: Novel insights into atopic dermatitis. J
Allergy Clin Immunol. 151:1145–1154. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu T and Zhi-rong Y: Research and therapy
progress on the mechanisms of pruritus in atopic dermatitis. Med J
Peking Union Med. 13:473–479. 2022.
|
|
24
|
McDowell L and Olin B: Crisaborole: A
novel nonsteroidal topical treatment for atopic dermatitis. J Pharm
Technol. 35:172–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wen-xing L, Ming H and Yu R: Opportunities
and challenges for PROTACs. Chinese J Med Chem. 30:745–764.
2020.
|
|
26
|
Therapeutics K: Kymera announces positive
results from phase 1 clinical trial evaluating KT-474 in patients
with HS and AD and Sanofi's decision to advance KT-474 into phase 2
clinical trials. Kymera Therapeutics, Inc., Watertown MS, 2022.
|
|
27
|
Madan J, Ahuja VK, Dua K, Samajdar S,
Ramchandra M and Giri S: PROTACs: Current trends in protein
degradation by proteolysis-targeting chimeras. BioDrugs.
36:609–623. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Luger T, Amagai M, Dreno B, Dagnelie MA,
Liao W, Kabashima K, Schikowski T, Proksch E, Elias PM, Simon M, et
al: Atopic dermatitis: Role of the skin barrier, environment,
microbiome, and therapeutic agents. J Dermatol Sci. 102:142–157.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stander S: Atopic dermatitis. N Engl J
Med. 384:1136–1143. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao Y, Terron-Kwiatkowski A, Liao H, Lee
SP, Allen MH, Hull PR, Campbell LE, Trembath RC, Capon F, Griffiths
CE, et al: Filaggrin null alleles are not associated with
psoriasis. J Invest Dermatol. 127:1878–1882. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cha B, Lim JW and Kim H: Jak1/Stat3 is an
upstream signaling of NF-κB activation in Helicobacter
pylori-induced IL-8 production in gastric epithelial AGS cells.
Yonsei Med J. 56:862–866. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ahmad SF, Ansari MA, Zoheir KM, Bakheet
SA, Korashy HM, Nadeem A, Ashour AE and Attia SM: Regulation of
TNF-α and NF-κB activation through the JAK/STAT signaling pathway
downstream of histamine 4 receptor in a rat model of LPS-induced
joint inflammation. Immunobiology. 220:889–898. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ramadass V, Vaiyapuri T and Tergaonkar V:
Small molecule NF-κB pathway inhibitors in clinic. Int J Mol Sci.
21(5164)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lesiak A, Zakrzewski M, Przybyłowska K,
Rogowski-Tylman M, Wozniacka A and Narbutt J: Atopic dermatitis
patients carrying G allele in -1082 G/A IL-10 polymorphism are
predisposed to higher serum concentration of IL-10. Arch Med Sci.
10:1239–1243. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hussein PY, Zahran F, Ashour Wahba A,
Ahmad AS, Ibrahiem MM, Shalaby SM, El Tarhouny SA, El Sherbiny HM
and Bakr N: Interleukin 10 receptor alpha subunit (IL-10RA) gene
polymorphism and IL-10 serum levels in Egyptian atopic patients. J
Investig Allergol Clin Immunol. 20:20–26. 2010.PubMed/NCBI
|
|
36
|
Zhang YY, Wang AX, Xu L, Shen N, Zhu J and
Tu CX: Characteristics of peripheral blood CD4+CD25+ regulatory T
cells and related cytokines in severe atopic dermatitis. Eur J
Dermatol. 26:240–246. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Quero L, Tiaden AN, Hanser E, Roux J,
Laski A, Hall J and Kyburz D: miR-221-3p drives the shift of
M2-macrophages to a pro-inflammatory function by suppressing
JAK3/STAT3 activation. Front Immunol. 10(3087)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Amend A, Wickli N, Schäfer AL, Sprenger
DTL, Manz RA, Voll RE and Chevalier N: Dual role of interleukin-10
in murine NZB/W F1 lupus. Int J Mol Sci. 22(1347)2021.PubMed/NCBI View Article : Google Scholar
|