In 2020, pancreatic cancer (PC) resulted in 496,000
new cases and 466,000 mortalities, with pancreatic ductal
adenocarcinoma (PDAC) as the predominant type (1,2). A
majority of patients with PC are diagnosed at an advanced stage,
therefore, only 20% are eligible for radical surgical resection
(3). However, despite surgery,
~90% of patients experience disease recurrence within 7-9 months,
leading to a 5-year overall survival (OS) rate <10% (4). The growing adoption of genetic
testing has enabled the implementation of precision medicine
(5). BRCA1, BRCA2 or both (BRCA)
mutations are present in 5-10% of familial patients with PDAC and
~3% of sporadic ones (6). Such
mutations can induce homologous recombination deficiency (HRD),
impeding the efficient repair of double-stranded DNA breaks
(7). The conventional treatment
for metastatic PDAC with germline BRCA mutations is platinum-based
chemotherapy coupled with poly (adenosine diphosphate-ribose)
polymerase (PARP) inhibitor maintenance therapy (5,8-10).
While this regimen can enhance survival metrics, a long-lasting
clinical response remains elusive, and its toxicity is noteworthy.
Exploring innovative therapeutic approaches is thus essential,
particularly for patients with PDAC carrying BRCA2 mutations.
Research suggests that BRCA2 mutations may boost
tumor cell immunogenicity and enhance responsiveness to immune
checkpoint inhibitors (ICIs) (11). Moreover, a retrospective study of
metastatic pancreatic or biliary cancer with HRD has demonstrated
significant clinical activity of ipilimumab/nivolumab with an
objective response rate (ORR) of 42% and a disease control rate
(DCR) of 58% (12). However, given
the limited sample size of the study, the efficacy of
ipilimumab/nivolumab for BRCA2-mutated metastatic PC requires
further validation. There's growing evidence that anti-angiogenic
drugs can modify the immune microenvironment of the tumor,
potentially amplifying immunotherapy benefits (13). Such synergy has been observed in
renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC) and
hepatocellular carcinoma (HCC) (14). Additionally, several instances
highlight the potential of integrating anti-angiogenic drugs with
ICIs in sequential treatments for PC (15,16).
Yet, the effectiveness of this combined approach as an initial
treatment for PDAC with germline BRCA2 mutations is still
uncertain.
The present study presented a case of a patient with
BRCA2-mutated PDAC who manifested metastasis in the posterior
cardia at 8 months after surgery. The combined therapy of
programmed cell death protein 1 (PD-1) inhibitor, tislelizumab,
with the anti-angiogenic agent, anlotinib, exhibited notable
efficacy in this patient. The patient provided written informed
consent for the publication of case information and accompanying
images.
A 68-year-old male with no family history presented
to the First Affiliated Hospital of Nanchang University (Jiangxi,
China) in August 2021, complaining of epigastric pain. An abdominal
computed tomography (CT) showed a space-occupying lesion in the
body of the pancreas. Enhanced CT scans of the chest and pelvis
showed no abnormalities. Preoperative carbohydrate antigen 19-9
(CA199) levels were significantly elevated at 798.7 U/ml (normal
range, 0-27 U/ml). Given the confined nature of the lesion and the
absence of distant metastasis, proceeding with surgical
intervention was deemed appropriate. Subsequently, the patient
underwent laparoscopic radical pancreatic body-tail resection and
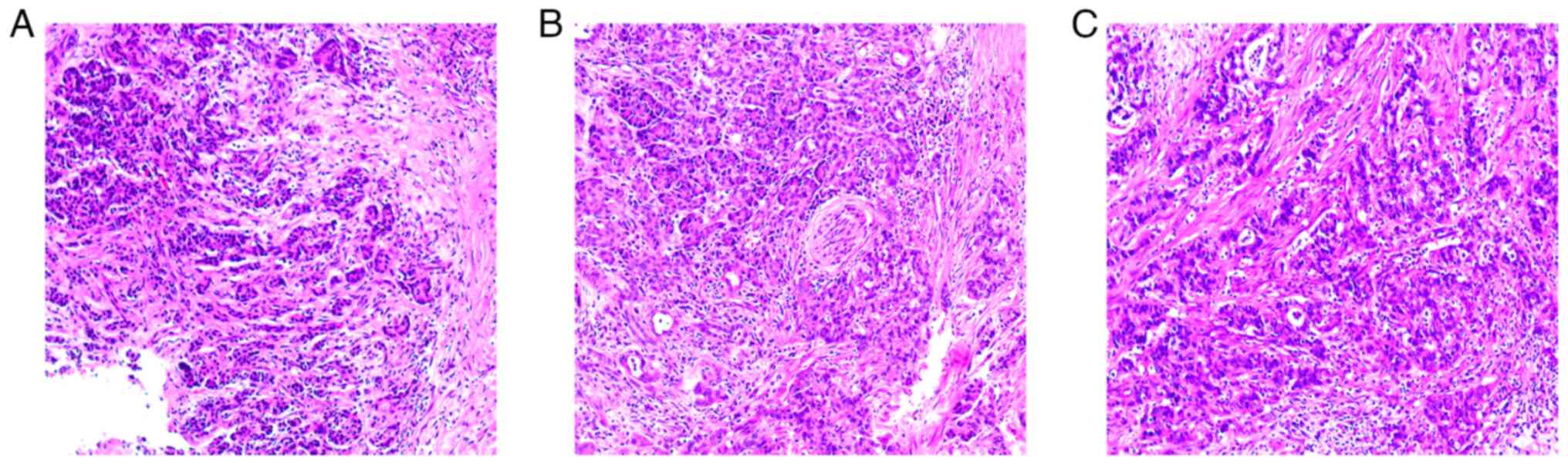
splenectomy. The postoperative pathology confirmed a diagnosis of
moderately differentiated ductal adenocarcinoma (pT3N0M0) (Fig. 1). The degrees of surgical resection
in patients are mainly divided into three parts: Complete resection
of the tumor (R0), microscopic residual (R1) and visual residual of
the tumor (R2), respectively. The present study mainly adopted the
presence or absence of tumor infiltration within 1 mm from the
cutting edge as the criterion for judging the R0 or R1 resection of
the tumor (17,18). Tumor cells are resected as R1 if
they are found within 1 mm of the tip of the tissue; if no tumor
cells are found, it is resected as R0. R2 indicates residual tumors
visible at the surgical margins (positive margins). The patient in
the present case had undergone laparoscopic radical pancreatic
body-tail resection and splenectomy, which was considered a R0
resection in conjunction with the postoperative pathological
results.
After surgery, the patient declined chemotherapy and
other treatments and asked for routine follow-up to monitor the
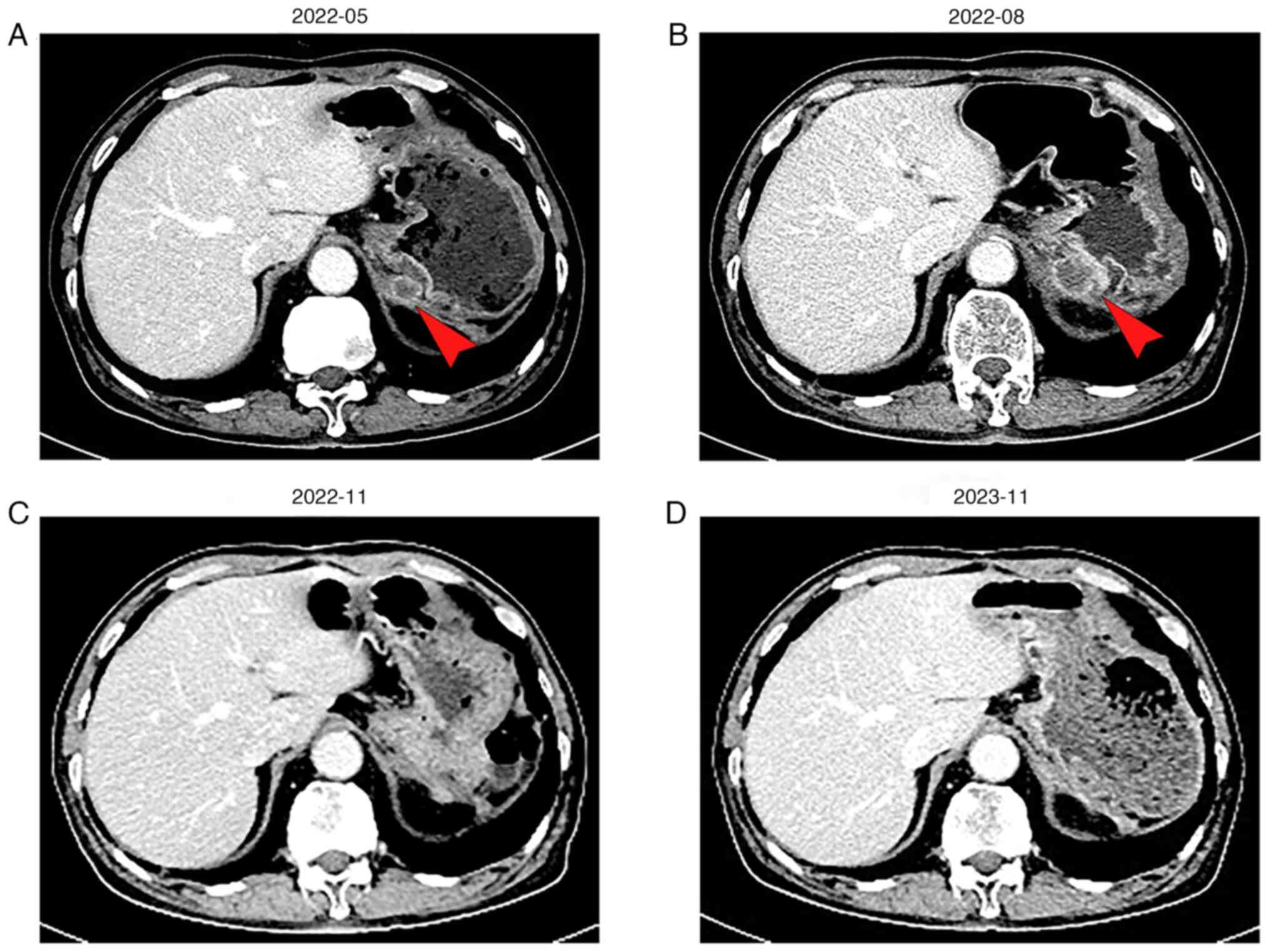
lesion. By May 2022, the patient progressed with the development of
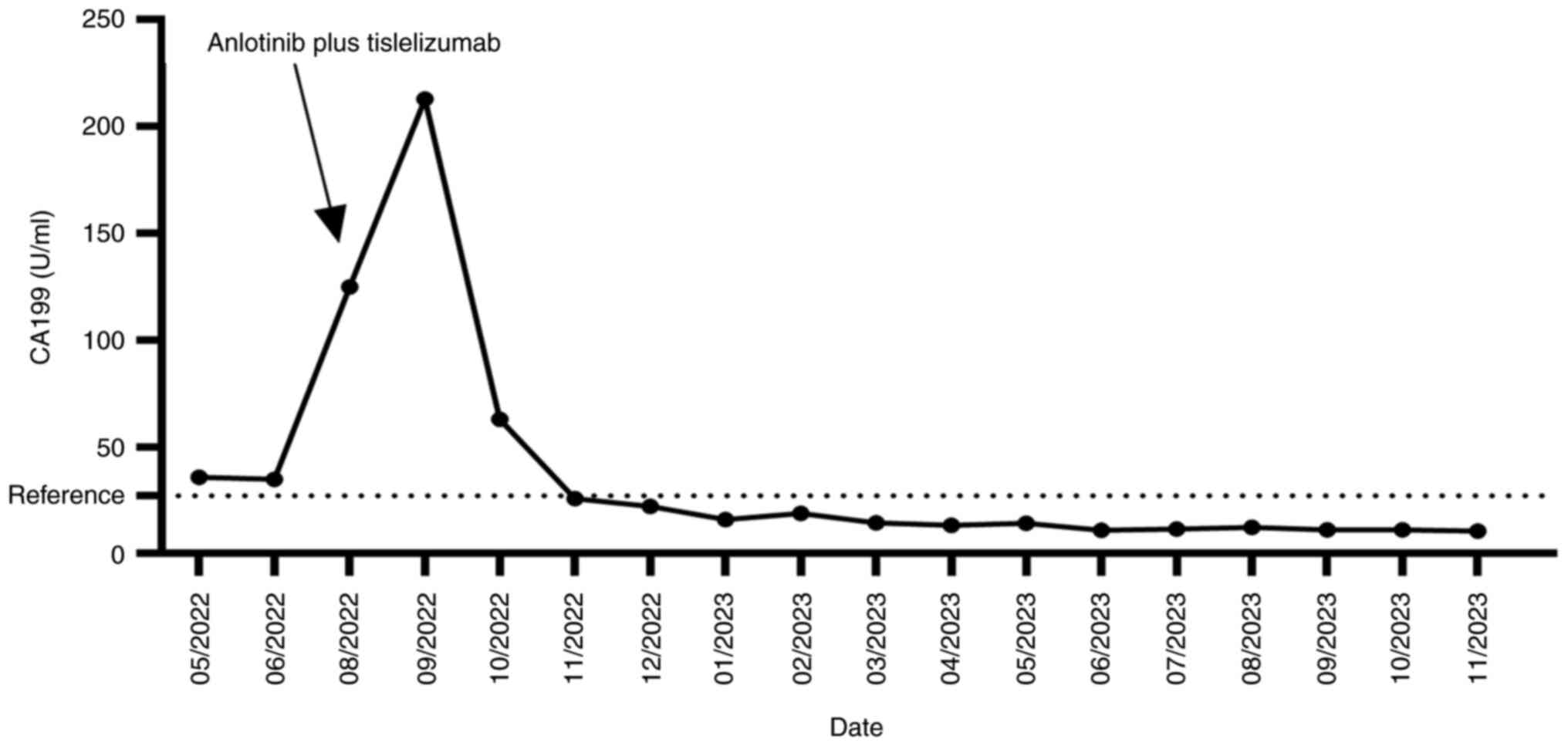
a posterior cardiac annular nodule (1.7x1.3 cm) (Fig. 2A) with CA199, which was 35.73 U/ml
(Fig. 3). Despite the presence of
new lesions, the patient was merely under observation without
receiving any treatment. A subsequent upper abdominal CT in August
2022 showed that the nodule had grown to 2.3x2.8 cm (Fig. 2B), and his CA199 level spiked to
124.8 U/ml (Fig. 3). Consequently,
the patient was diagnosed with recurrent metastatic PDAC and had an
Eastern Cooperative Oncology Group (ECOG) score of 1(19).
To uncover actionable mutations in the patient, the
present study conducted next-generation sequencing (NGS) on both
the surgical tissues and blood samples. The analysis identified a
BRCA2 mutation, microsatellite stability (MSS), a tumor mutational
burden (TMB) of 5.76 Muts/Mb and PD-L1 negativity. As per the 2022
Guidelines of the Chinese Society of Clinical Oncology (CSCO) for
Pancreatic Cancer and the NCCN Guidelines for Pancreatic
Adenocarcinoma, Version 2, 2023, the recommended course of action
was platinum-based chemotherapy (20). However, due to concerns about the
adverse effects of chemotherapy, the patient, in consultation with
his family, opted against this treatment.
In August 2022 the patient was initiated on a
treatment regimen comprising of anlotinib (10 mg, days 1-14; 7 days
off; 21-day cycle) and tislelizumab (200 mg, day 1; 20 days off;
21-day cycle). Following four cycles of combination therapy, the
patient achieved a complete response (CR), with total tumor
disappearance (Fig. 2C). To
prevent tumor recurrence, the patient continued this regimen
without any treatment-related adverse effects. At the latest
follow-up in November 2023, the patient remains on this regimen.
Abdominal CT scans continue to indicate CR with a progression-free
survival (PFS) duration of 14 months (Fig. 2D).
PDAC remains a highly aggressive and devastating
disease, typically characterized by a median OS of <13 months in
metastatic patients (21).
Posterior cardiac invasion is considered a manifestation of
peritoneal metastasis, rendering surgical resection not
recommended. Consequently, systemic chemotherapy is the established
first-line approach for metastatic PDAC (22).
In the present case, the application of NGS testing
on the blood and archived specimens of the patient revealed the
presence of a BRCA2 mutation, along with negative PD-L1 expression
and a low TMB and MSS. The BRCA gene is responsible for encoding
proteins involved in homologous recombination repair of
double-stranded DNA breaks (23).
Consequently, PDAC featuring a germline BRCA mutation exhibits
sensitivity to platinum and PARP inhibitors (24,25).
The POLO study investigated the efficacy of maintenance olaparib in
patients with platinum-sensitive metastatic PC who carried germline
BRCA1 or BRCA2 mutations (10).
The results showed that maintenance olaparib significantly extends
PFS compared with the placebo (median, 7.4 months vs. 3.8 months;
P=0.004) (10). However, there was
no significant difference in median OS between the two groups (18.9
months vs. 18.1 months; P=0.68) (10). Moreover, in this current case, the
patient and his family adamantly declined chemotherapy due to the
prohibitive cost of the procedure. Hence, it is imperative to
explore alternative chemotherapy-free treatment modalities, aiming
to provide enhanced survival prospects for individuals with
PDAC.
In recent years, significant attention has been
dedicated to the research and development of anti-angiogenic drugs
for managing PC. A phase III clinical trial has demonstrated the
superiority of combining erlotinib with gemcitabine compared with
gemcitabine alone, as it leads to notable improvements in both OS
and PFS in patients with locally advanced or metastatic PC
(26). However, it is noteworthy
that various other anti-angiogenic drug combinations with
gemcitabine have not yielded favorable outcomes concerning OS in PC
patients (27-34).
These findings suggest that the utilization of anti-angiogenic
drugs in conjunction with gemcitabine may have some inherent
limitations in extending the OS of PC patients. Therefore, there is
a pressing need to explore a new anti-angiogenic drug to improve
the efficacy of PC treatment.
Anlotinib is a novel multi-targeted tyrosine kinase
inhibitor renowned for its capacity to impede both tumor
angiogenesis and cell proliferation (35). This therapeutic agent exhibits a
broad spectrum of inhibitory effects on various critical targets,
encompassing receptor tyrosine kinases such as vascular endothelial
growth factor receptor 1 to 3, epidermal growth factor receptor
(EGFR), fibroblast growth factor receptor 1 to 4, platelet-derived
growth factor receptors α and β, stem cell factor receptors and
c-kit (36). Clinical studies have
shown that anlotinib has promising efficacy in the treatment of
advanced NSCLC, small cell lung cancer, soft tissue sarcoma (STS),
advanced thyroid carcinoma and metastatic RCC (37,38).
Moreover, its potential role in the context of PC has garnered
substantial attention. Yang et al found that anlotinib can
induce the apoptosis of pancreatic cells both in vitro and
in vivo by generating reactive oxygen species (39). Additionally, Zhang et al
have demonstrated that anlotinib possesses the capacity to inhibit
ribosomes within pancreatic cancer cells, thereby modulating
various cellular functions, including the cell cycle, RNA
metabolism and lysosome (40). In
a retrospective study, the combination of anlotinib and
nab-paclitaxel/gemcitabine showed a significant improvement in both
PFS (5 months vs. 2.7 months, P=0.0220) and OS (9 months vs. 6
months, P=0.0060) in patients with unresectable or metastatic PDAC
when compared with albumin-bound paclitaxel/gemcitabine (37). This treatment also maintains
favorable tolerability profiles (37). Therefore, anlotinib is expected to
be a feasible treatment modality for patients with locally advanced
and metastatic PC.
Emerging research has shed light on the potential
immunostimulatory effects of anti-angiogenic drugs and their
capacity for synergistic antitumor action when combined with ICIs
(51-56).
One notable example is the IMPOWER-150 study, which has
demonstrated that the addition of atezolizumab to bevacizumab, in
combination with chemotherapy as a first-line treatment for
metastatic NSCLC, leads to improved clinical outcomes for patients,
irrespective of their PD-L1 expression status or the presence of
EGFR or ALK mutations (52). In
addition, Kang et al reported a case in which the
combination of pembrolizumab and anlotinib exhibits substantial
antitumor activity in PC (16).
Similarly, Wang et al documented a long-term partial
response and favorable tolerability in a 41-year-old patient with
PDAC with KRAS G12V mutation and liver metastasis who received the
combination of anlotinib with PD-1 inhibitor plus chemotherapy
(15). Building upon these
insights, the present study sought to explore the potential
benefits of combining tislelizumab with anlotinib in the treatment
of PC, specifically focusing on patients with metastatic BRCA2
mutated PDAC. Following the administration of four treatment
cycles, the response of the patient to therapy was evaluated as CR,
and this efficacy persisted for a duration exceeding 14 months.
Clinical studies related to PC with BRCA2 or BRCA1/2 mutations are
summarized in Table I.
In conclusion, the current study presented a novel
and efficacious combination therapy involving immuno- and
anti-angiogenic drugs for the treatment of patients with recurrent
and metastatic PDAC and BRCA2 mutations. This innovative treatment
approach holds promise for diversifying the therapeutic options
available to patients with recurrent and metastatic PDAC and BRCA2
mutations, particularly for individuals who either decline or are
unable to tolerate conventional chemotherapy. Nonetheless, it is
imperative to emphasize that rigorous and extensive clinical trials
are still indispensable to substantiate its efficacy and safety,
thereby advancing its adoption in clinical practice.
Not applicable.
Funding: This study was funded by the National Natural Science
Foundation for Regional Fund (grant no. 82360507).
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
SP and ZL contributed to the conceptualization and
design of the study, the collection of clinical information and the
drafting of the manuscript. HH, XZ, JC, XD and FW obtained CT and
hematoxylin and eosin staining images, and analyzed patient data.
LC was responsible for formulating the patient's treatment plan. LC
and HH contributed to critical revisions of the intellectual
content. SP and ZL confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
The study was conducted according to the guidelines
of the Declaration of Helsinki. Ethical review and approval were
waived due to the type of the study. Written informed consent was
obtained from the patient.
Written informed consent was obtained from the
patient for publication of this paper and any accompanying
images.
The authors declare that they have no competing
interests.
|
1
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Versteijne E, Suker M, Groothuis K,
Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch
OR, Creemers GM, van Dam RM, et al: Preoperative chemoradiotherapy
versus immediate surgery for resectable and borderline resectable
pancreatic cancer: Results of the dutch randomized phase III
PREOPANC trial. J Clin Oncol. 38:1763–1773. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rojas LA, Sethna Z, Soares KC, Olcese C,
Pang N, Patterson E, Lihm J, Ceglia N, Guasp P, Chu A, et al:
Personalized RNA neoantigen vaccines stimulate T cells in
pancreatic cancer. Nature. 618:144–150. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
O'Reilly EM, Lee JW, Zalupski M, Capanu M,
Park J, Golan T, Tahover E, Lowery MA, Chou JF, Sahai V, et al:
Randomized, multicenter, phase II trial of gemcitabine and
cisplatin with or without veliparib in patients with pancreas
adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol.
38:1378–1388. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lai E, Ziranu P, Spanu D, Dubois M, Pretta
A, Tolu S, Camera S, Liscia N, Mariani S, Persano M, et al:
BRCA-mutant pancreatic ductal adenocarcinoma. Br J Cancer.
125:1321–1332. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wattenberg MM, Asch D, Yu S, O'Dwyer PJ,
Domchek SM, Nathanson KL, Rosen MA, Beatty GL, Siegelman ES and
Reiss KA: Platinum response characteristics of patients with
pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or
PALB2 mutation. Br J Cancer. 122:333–339. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Reiss KA, Mick R, O'Hara MH, Teitelbaum U,
Karasic TB, Schneider C, Cowden S, Southwell T, Romeo J, Izgur N,
et al: Phase II study of maintenance rucaparib in patients with
platinum-sensitive advanced pancreatic cancer and a pathogenic
germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin
Oncol. 39:2497–2505. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance olaparib for germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Samstein RM, Krishna C, Ma X, Pei X, Lee
KW, Makarov V, Kuo F, Chung J, Srivastava RM, Purohit TA, et al:
Mutations in BRCA1 and BRCA2 differentially affect the tumor
microenvironment and response to checkpoint blockade immunotherapy.
Nat Cancer. 1:1188–1203. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Terrero G, Datta J, Dennison J, Sussman
DA, Lohse I, Merchant NB and Hosein PJ: Ipilimumab/nivolumab
therapy in patients with metastatic pancreatic or biliary cancer
with homologous recombination deficiency pathogenic germline
variants. JAMA Oncol. 8:1–3. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang H, Zhong P, Zhu X, Fu S, Li S, Peng
S, Liu Y, Lu Z and Chen L: Immunotherapy combined with
rh-endostatin improved clinical outcomes over immunotherapy plus
chemotherapy for second-line treatment of advanced NSCLC. Front
Oncol. 13(1137224)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hack SP, Zhu AX and Wang Y: Augmenting
anticancer immunity through combined targeting of angiogenic and
PD-1/PD-L1 pathways: Challenges and opportunities. Front Immunol.
11(598877)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Y, Wang B, Xiang L, Deng J, Xu B, He
P, Pu W, Wang H, Fan Y and Chen H: Case report: Anlotinib combined
with PD-1 inhibitor and sequential GA regimen or FOLFIRINOX
chemotherapy in treatment of KRAS G12V mutated pancreatic ductal
adenocarcinoma with liver metastasis: A case and literature review.
Front Immunol. 13(1016647)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mafei K, Shengyuan X and Jieqiong S:
Pembrolizumab enhances the anti-pancreatic cancer activity of
anlotinib. Asian J Surg. 45:881–882. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ghaneh P, Kleeff J, Halloran CM, Raraty M,
Jackson R, Melling J, Jones O, Palmer DH, Cox TF, Smith CJ, et al:
The Impact of positive resection margins on survival and recurrence
following resection and adjuvant chemotherapy for pancreatic ductal
adenocarcinoma. Ann Surg. 269:520–529. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Verbeke CS, Leitch D, Menon KV, McMahon
MJ, Guillou PJ and Anthoney A: Redefining the R1 resection in
pancreatic cancer. Br J Surg. 93:1232–1237. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
20
|
NCCN Guidelines Panel Disclosures. NCCN
Clinical Practice Guidelines in Oncology-Pancreatic Adenocarcinoma
(Version 2.2023). [June 19, 2023]. https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients.
|
|
21
|
Middleton G, Silcocks P, Cox T, Valle J,
Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, et
al: Gemcitabine and capecitabine with or without telomerase peptide
vaccine GV1001 in patients with locally advanced or metastatic
pancreatic cancer (TeloVac): An open-label, randomised, phase 3
trial. Lancet Oncol. 15:829–840. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lellouche L, Palmieri LJ, Dermine S,
Brezault C, Chaussade S and Coriat R: Systemic therapy in
metastatic pancreatic adenocarcinoma: Current practice and
perspectives. Ther Adv Med Oncol.
13(17588359211018539)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Walsh CS: Two decades beyond BRCA1/2:
Homologous recombination, hereditary cancer risk and a target for
ovarian cancer therapy. Gynecol Oncol. 137:343–350. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Andrei AZ, Hall A, Smith AL, Bascuñana C,
Malina A, Connor A, Altinel-Omeroglu G, Huang S, Pelletier J,
Huntsman D, et al: Increased in vitro and in vivo sensitivity of
BRCA2-associated pancreatic cancer to the poly(ADP-ribose)
polymerase-1/2 inhibitor BMN 673. Cancer Lett. 364:8–16.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Y, Park JYP, Pacis A, Denroche RE,
Jang GH, Zhang A, Cuggia A, Domecq C, Monlong J, Raitses-Gurevich
M, et al: A preclinical trial and molecularly annotated patient
cohort identify predictive biomarkers in homologous
recombination-deficient pancreatic cancer. Clin Cancer Res.
26:5462–5476. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
national cancer institute of canada clinical trials group. J Clin
Oncol. 25:1960–1966. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Van Cutsem E, van de Velde H, Karasek P,
Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype
C, Neumann H, et al: Phase III trial of gemcitabine plus tipifarnib
compared with gemcitabine plus placebo in advanced pancreatic
cancer. J Clin Oncol. 22:1430–1438. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Van Cutsem E, Vervenne WL, Bennouna J,
Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang
A, Cosaert J and Moore MJ: Phase III trial of bevacizumab in
combination with gemcitabine and erlotinib in patients with
metastatic pancreatic cancer. J Clin Oncol. 27:2231–2237.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kindler HL, Niedzwiecki D, Hollis D,
Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF,
O'Reilly E, Wozniak TF, et al: Gemcitabine plus bevacizumab
compared with gemcitabine plus placebo in patients with advanced
pancreatic cancer: Phase III trial of the cancer and leukemia group
B (CALGB 80303). J Clin Oncol. 28:3617–3622. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kindler HL, Ioka T, Richel DJ, Bennouna J,
Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S,
et al: Axitinib plus gemcitabine versus placebo plus gemcitabine in
patients with advanced pancreatic adenocarcinoma: A double-blind
randomised phase 3 study. Lancet Oncol. 12:256–262. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gonçalves A, Gilabert M, François E, Dahan
L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X,
et al: BAYPAN study: A double-blind phase III randomized trial
comparing gemcitabine plus sorafenib and gemcitabine plus placebo
in patients with advanced pancreatic cancer. Ann Oncol.
23:2799–2805. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rougier P, Riess H, Manges R, Karasek P,
Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L and
Philip PA: Randomised, placebo-controlled, double-blind,
parallel-group phase III study evaluating aflibercept in patients
receiving first-line treatment with gemcitabine for metastatic
pancreatic cancer. Eur J Cancer. 49:2633–2642. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamaue H, Tsunoda T, Tani M, Miyazawa M,
Yamao K, Mizuno N, Okusaka T, Ueno H, Boku N, Fukutomi A, et al:
Randomized phase II/III clinical trial of elpamotide for patients
with advanced pancreatic cancer: PEGASUS-PC study. Cancer Sci.
106:883–890. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sinn M, Bahra M, Liersch T, Gellert K,
Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau
BM, et al: CONKO-005: Adjuvant chemotherapy with gemcitabine plus
erlotinib versus gemcitabine alone in patients after R0 resection
of pancreatic cancer: A multicenter randomized phase III trial. J
Clin Oncol. 35:3330–3337. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li
L, Wang F, Hao Y, Li C and Chi Y: Safety, pharmacokinetics, and
antitumor properties of anlotinib, an oral multi-target tyrosine
kinase inhibitor, in patients with advanced refractory solid
tumors. J Hematol Oncol. 9(105)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu H, Huang N, Zhao C, Hu X, Da L, Huang
W, Shen Y, Xiong F and Zhang C: Anlotinib plus
nab-paclitaxel/gemcitabine as first-line treatment prolongs
survival in patients with unresectable or metastatic pancreatic
adenocarcinoma: A retrospective cohort. Ann Transl Med.
10(294)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang
Z, Zhao F, Ahmad R and Zhao J: Anlotinib: A novel multi-targeting
tyrosine kinase inhibitor in clinical development. J Hematol Oncol.
11(120)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang L, Zhou X, Sun J, Lei Q, Wang Q, Pan
D, Ding M and Ding Y: Reactive oxygen species mediate
anlotinib-induced apoptosis via activation of endoplasmic reticulum
stress in pancreatic cancer. Cell Death Dis. 11(766)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang X, Liu Y, Zhang Z, Tan J, Zhang J,
Ou H, Li J and Song Z: Multi-omics analysis of anlotinib in
pancreatic cancer and development of an anlotinib-related
prognostic signature. Front Cell Dev Biol. 9(649265)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Timmer FEF, Geboers B, Nieuwenhuizen S,
Dijkstra M, Schouten EAC, Puijk RS, de Vries JJJ, van den Tol MP,
Bruynzeel AME, Streppel MM, et al: Pancreatic cancer and
immunotherapy: A clinical overview. Cancers (Basel).
13(4138)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang F, Wang Y, Yang F, Zhang Y, Jiang M
and Zhang X: The efficacy and safety of PD-1 inhibitors combined
with nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus
gemcitabine in the first-line treatment of advanced pancreatic
cancer: A retrospective monocentric study. Cancer Manag Res.
14:535–546. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shui L, Cheng K, Li X, Shui P, Zhou X, Li
J, Yi C and Cao D: Study protocol for an open-label, single-arm,
phase Ib/II study of combination of toripalimab, nab-paclitaxel,
and gemcitabine as the first-line treatment for patients with
unresectable pancreatic ductal adenocarcinoma. BMC Cancer.
20(636)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Q, Zhao G, Zhang X, Jiang N, Zhao Z,
Wang Y, Xu S, Zhu L, Lau WY, Dai G and Liu R: Nab-paclitaxel plus
S-1 with or without PD-1 inhibitor in pancreatic ductal
adenocarcinoma with only hepatic metastases: A retrospective cohort
study. Langenbecks Arch Surg. 407:633–643. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Padrón LJ, Maurer DM, O'Hara MH, O'Reilly
EM, Wolff RA, Wainberg ZA, Ko AH, Fisher G, Rahma O, Lyman JP, et
al: Sotigalimab and/or nivolumab with chemotherapy in first-line
metastatic pancreatic cancer: Clinical and immunologic analyses
from the randomized phase 2 PRINCE trial. Nat Med. 28:1167–1177.
2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Feng M, Xiong G, Cao Z, Yang G, Zheng S,
Song X, You L, Zheng L, Zhang T and Zhao Y: PD-1/PD-L1 and
immunotherapy for pancreatic cancer. Cancer Lett. 407:57–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Royal RE, Levy C, Turner K, Mathur A,
Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I and
Rosenberg SA: Phase 2 trial of single agent ipilimumab
(anti-CTLA-4) for locally advanced or metastatic pancreatic
adenocarcinoma. J Immunother. 33:828–833. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
O'Reilly EM, Oh DY, Dhani N, Renouf DJ,
Lee MA, Sun W, Fisher G, Hezel A, Chang SC, Vlahovic G, et al:
Durvalumab with or without tremelimumab for patients with
metastatic pancreatic ductal adenocarcinoma: A phase 2 randomized
clinical trial. JAMA Oncol. 5:1431–1438. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus axitinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Makker V, Rasco D, Vogelzang NJ, Brose MS,
Cohn AL, Mier J, Di Simone C, Hyman DM, Stepan DE, Dutcus CE, et
al: Lenvatinib plus pembrolizumab in patients with advanced
endometrial cancer: An interim analysis of a multicentre,
open-label, single-arm, phase 2 trial. Lancet Oncol. 20:711–718.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li SQ, Yang Y and Ye LS: Angiogenesis and
immune checkpoint dual blockade: Opportunities and challenges for
hepatocellular carcinoma therapy. World J Gastroenterol.
28:6034–6044. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Fogelman DR, Wolff RA, Kopetz S, Javle M,
Bradley C, Mok I, Cabanillas F and Abbruzzese JL: Evidence for the
efficacy of iniparib, a PARP-1 inhibitor, in BRCA2-associated
pancreatic cancer. Anticancer Res. 31:1417–1420. 2011.PubMed/NCBI
|
|
58
|
Kaufman B, Shapira-Frommer R, Schmutzler
RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G,
Stemmer SM, Hubert A, et al: Olaparib monotherapy in patients with
advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol.
33:244–250. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sehdev A, Gbolahan O, Hancock BA, Stanley
M, Shahda S, Wan J, Wu HH, Radovich M and O'Neil BH: Germline and
somatic DNA damage repair gene mutations and overall survival in
metastatic pancreatic adenocarcinoma patients treated with
FOLFIRINOX. Clin Cancer Res. 24:6204–6211. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Fumet JD, Limagne E, Thibaudin M, Truntzer
C, Bertaut A, Rederstorff E and Ghiringhelli F: Precision medicine
phase II study evaluating the efficacy of a double immunotherapy by
durvalumab and tremelimumab combined with olaparib in patients with
solid cancers and carriers of homologous recombination repair genes
mutation in response or stable after olaparib treatment. BMC
Cancer. 20(748)2020.PubMed/NCBI View Article : Google Scholar
|