Introduction
Intrauterine growth restriction (IUGR) is defined as
a reduced rate of fetal growth compared with the growth potential
of a specific infant based on their ethnicity and sex (1). Genetic factors, fetal factors,
placental issues or maternal health issues can all lead to the
occurrence of IUGR (2,3). IUGR is one of the leading causes of
perinatal-neonatal morbidity and mortality (4), which also carries long-term
consequences (5). Notably, IUGR
can result in significant neurological deficits (6), which can affect the development of
the brain in offspring (7). In
clinical practice, to prioritize the development of the brain, a
catch-up growth pattern is recommended to facilitate a swift
recovery to a normal growth trajectory for infants with IUGR
(8-10).
Rapid catch-up growth promotes development but also
presents numerous potential problems. For individuals with IUGR,
postnatal catch-up growth can help them achieve a normal weight,
height, head circumference and neurodevelopment; however, adverse
consequences of catch-up growth have been identified, including
abnormal gut microbiota (8),
cardiovascular disease (11),
insulin resistance (12) and
obesity (13). Our previous
research showed that while postnatal rapid catch-up growth in IUGR
rats can increase pulmonary artery pressure (PAP) in adulthood,
postnatal delayed growth reduces the risk of pulmonary vascular
dysfunction but has long-term negative effects on cognitive
function (14). Determining
whether to provide adequate nutrition for infants with IUGR to
achieve catch-up growth, and to what extent catch-up growth should
be pursued remains challenging.
Offspring with IUGR have a higher susceptibility to
elevated PAP in adulthood compared with offspring that receive
adequate intrauterine nutrition (15,16).
A randomized controlled trial indicated that catch-up growth in
early postnatal life, rather than simply being born with IUGR,
poses a risk for developing cardiovascular diseases later in
adulthood (17). This result
emphasizes the significance of early postnatal growth patterns in
children with IUGR. A systematic review by Kelishadi et al
(18) also supported this
viewpoint, stating that rapid postnatal catch-up growth of low
birthweight neonates is a more important factor than low
birthweight alone in cardiovascular disease and its risk factors.
Furthermore, animal experiments have demonstrated that IUGR is
associated with the development of pulmonary arterial hypertension
(PAH) in adulthood (19-21).
PAH is characterized by excessive pulmonary vascular remodeling,
which involves dysregulated proliferation of cells in the intima,
media and adventitia (22). The
pathological mechanisms underlying PAH include increased
inflammation and proliferation, and reduced apoptosis of pulmonary
artery smooth muscle cells (PASMCs) (23), as well as excessive proliferation
and survival of pulmonary vascular endothelial cells (PVECs)
(22). Based on the fact that
postnatal rapid catch-up growth in IUGR rats may lead to elevated
PAP in adulthood (14), it is
worth exploring whether rapid catch-up growth in IUGR rats also
leads to PAH-like pathophysiological development of smooth muscle
cells and endothelial cells.
It is well known that excessive nutrition can lead
to cardiovascular diseases, while malnutrition can impact brain
development. In the case of infants with IUGR, there is currently
no consensus on the optimal nutritional intervention strategies
after birth, particularly regarding how to balance cardiovascular
function and brain function in adulthood. ‘Healthy catch-up growth’
is a future research direction (10); a reasonable strategy to achieve
this is to implement postnatal interventions with the aim of
avoiding rapid catch-up growth by promoting moderate prolonged
growth (24). In the present
study, an IUGR model was established by restricting maternal
nutrition during pregnancy and different postnatal growth patterns
were achieved by adjusting the litter size during lactation. It was
hypothesized that early moderate catch-up growth would preserve
mean PAP (mPAP) in adult IUGR rats while not affecting the
development of their memory function. The present study may provide
valuable insights into further exploration of the mechanisms and
effects of early postnatal nutritional interventions in human
infants with IUGR.
Materials and methods
Ethics statement
All animal care and experiments were performed in
strict accordance with the guidelines for the Care and Use of
Laboratory Animals (25) and were
approved by the Institutional Animal Care and Use Committee (IACUC)
of Zhejiang University (Hangzhou, China; approval no. ZJU20160215).
The extraction of primary PASMCs and primary PVECs from rat lungs
for research purposes was also approved and permitted by the IACUC
of Zhejiang University (approval no. ZJU20160215; Hangzhou, China).
Sprague Dawley (SD) rats were obtained from the Animal Center of
the Chinese Academy of Sciences (http://www.slaccas.com/; Shanghai, China). All
surgical procedures were performed under anesthesia using sodium
pentobarbital, and every effort was made to minimize pain and
discomfort.
In the present study, humane endpoints were
implemented to monitor the well-being of the animals and to ensure
ethical treatment. These endpoints included assessing vital signs,
body weight, behavior and other relevant indicators. In addition,
the rats were closely observed for any signs of distress, pain or
significant deterioration in health. If any animals reached the
predefined endpoint criteria, such as severe weight loss (weight
loss range, 20-25%), inability to eat or drink, or signs of severe
illness, they were euthanized to minimize suffering. Euthanasia was
performed by intraperitoneal injection of 200 mg/kg sodium
pentobarbital. These humane endpoints were established in
accordance with ethical guidelines and were approved by the
relevant animal welfare committee.
IUGR and catch-up growth animal
model
The SD rats (total of 198; 99 males and 99 females;
9 weeks old) were housed in a specific pathogen-free environment,
under an ambient temperature of 22±2˚C and ~60% humidity, with a
12-h light/dark cycle. As described previously, a rodent model of
nutrient restriction during pregnancy was used to induce IUGR in
the offspring (14,19,26).
When virgin female SD rats reached a weight of 250-300 g, they were
mated overnight with male rats in a 1:1 ratio. The following day,
vaginal smears were taken to confirm successful pregnancy by
examining the presence of sperm, which was considered the first day
of pregnancy. The pregnant rats (n=99) were randomly assigned to
either a normal diet group (n=63) or a restricted diet group
(n=36). The normal diet group was provided ad libitum access
to food, whereas the restricted diet group was provided with 50% of
the normal food amount. Both groups had free access to water. Each
pregnant rat was housed in a cage until delivery and the offspring
were weighed on the first day after birth. Each dam underwent only
one reproductive cycle. The offspring of the normal diet group were
labeled as the control pups, whereas those in the restricted diet
group with birth weights below the 10th percentile of the control
group (<6.665 g) were labeled as the IUGR pups. Rats with birth
weights ≥10th percentile of the control group were euthanized by
intraperitoneal injection of an overdose of 2% sodium pentobarbital
(200 mg/kg). After birth, the litter size was adjusted and the pups
were divided into four experimental groups. The control group was
fed with a litter size of eight control offspring/litter. The IUGR
pups were randomly assigned to three different feeding strategy
groups, with litter sizes of 5, 10 or 16 pups/litter; these groups
represented the rapid catch-up growth, moderate catch-up growth and
delayed growth groups, respectively. All pups from the four groups
were breastfed by dams with ad libitum access to food during
pregnancy (15 dams were used to breastfeed the control pups, while
the remaining 48 dams were used to breastfeed the IUGR pups).
During the lactation period, the male-to-female ratio of pups in
each litter was 1:1, except for the rapid catch-up growth group,
which had a ratio of 3:2. IUGR rats with excessively low birth
weights were prone to early postnatal mortality, and in this study,
the mortality rate during the lactation period for IUGR rats was
30.3%; this rate is lower than that reported in a previous study
(19), a difference that could be
attributed to the birth weight of the IUGR rats and the rearing
environment. If pup mortality occurred during lactation, an equal
number of female rats born during the same period were added to
maintain a consistent litter size.
Based on previous research, 3-week-old rats are
equivalent to 2-year-old human infants (27). Additionally, it has been observed
that most human SGA individuals exhibit catch-up growth within the
first 2 years (8,9). Therefore, rats that achieved a weight
≥10th percentile (≥P10) of the control group at weaning (3 weeks)
were deemed to have successfully achieved catch-up growth. After
excluding rats whose weight did not meet the requirement (rats with
a weight >90th percentile were considered overweight), rats were
divided into two groups: Moderate catch-up growth and rapid
catch-up growth. This division was based on the 50th percentile
(P50), with ~50% of the rats meeting the weight criteria. In
addition, rats with a weight <P10 of the normal group were
classified as the delayed growth group, and all rats in this group
met the weight criteria. When weaning the offspring at 3 weeks of
age, the percentiles (P10, P50 and P90) of the body weight in the
normal control group were calculated. Male IUGR rats that met the
weight requirements for follow-up studies were used, whereas those
that did not meet the weight requirements and female rats were
euthanized via intraperitoneal injection of 2% sodium pentobarbital
(200 mg/kg). Death was assessed when the rats exhibited a loss of
pain response, showing no reaction to the manual pressure applied
to their toes. Additionally, their pupils were fixed and dilated,
and the absence of heartbeat and respiration was confirmed, along
with a noticeable decrease in body temperature, conclusively
indicating successful sacrifice.
As the weights of the IUGR rats obtained through
different feeding methods did not all meet the experimental
requirements, further selection was necessary at 3 weeks old. IUGR
rats were divided into three subgroups based on their weight at the
time of weaning (3 weeks): IUGR with rapid catch-up growth in early
postnatal life (IUGR + RC, P50-P90), IUGR with moderate catch-up
growth in early postnatal life (IUGR + MC, P10-P50, including P10
and P50), and IUGR with delayed growth in early postnatal life
(IUGR + DG, <P10). Subsequently, all four groups of rats were
provided ad libitum access to food and water until they
reached 13 weeks of age, and their body weights were monitored on a
weekly basis. Each cage contained four rats to mitigate the
influence of housing density on body weight. The experiments were
performed at three time points: 3, 9 and 13 weeks. Each group at
each time point consisted of 20-22 eligible offspring rats, which
were used for subsequent experiments. To eliminate the variation
caused by the hormonal cycle of female rats, only male rats were
used in subsequent experiments.
During the lactation period, the health and behavior
of the animals were monitored daily, ensuring a consistent number
of pups per litter. After weaning at 3 weeks, the animal health and
behavior was assessed once a week and their weights were recorded.
In the present study, no rats reached any of the humane endpoints,
such as severe weight loss, inability to eat or drink, or signs of
severe illness.
Y-maze test for spontaneous
alternation
The Y-maze spontaneous alternation test is used to
assess spatial working memory in rats by allowing them to freely
explore the three arms of a maze (28,29).
This behavior is driven by the innate curiosity of rodents to
explore areas they have not previously visited. The apparatus
consists of three identical black arms, each at a 120˚ angle, 50 cm
in length, 35 cm in height and 18 cm in width. Three spontaneous
alternation tests at 3, 9 and 13 weeks of age were performed to
evaluate the working memory of rats at the different stages of
development (n=20-22/group). The rats were placed at the end of one
arm of the Y-maze, with their backs to the center, and allowed to
freely explore for 8 min. A camera positioned above the center of
the maze captured the movement trajectory of the rats throughout
the maze. The ANY-maze version 7.0 software (Stoelting Co.) was
used to analyze the percentage of alternation, total number of arm
entries and total distance traveled in the maze. The percentage of
alternation was calculated using the following formula (30,31):
Alternation (%)=(number of alternations/total arm entries-2)
x100.
Hemodynamic and right ventricular
hypertrophy assessment
An open-chest surgical procedure was used to assess
the right ventricular system in 3-week-old rats. Due to their small
body size, the mean abdominal aortic pressure (mAAP) of the rats
was not measured. For 9- and 13-week-old rats, closed-chest surgery
was used to measure their PAP.
For 3-week-old rats, as described in our previous
study (32), the rats were
anesthetized with 2% sodium pentobarbital (50 mg/kg,
intraperitoneal injection) and fixed on a temperature-controlled
surgical table (37˚C) after weaning. The fur on the chest and neck
was removed, and the chest and neck area was disinfected with 75%
alcohol. Subsequently, the skin was cut to expose the trachea. The
tracheal intubation catheter was connected to a small animal
ventilator, and the respiratory rate and tidal volume were
calculated and adjusted based on weight (33). After a few minutes of stable
breathing, the right third and fourth intercostal spaces were
carefully separated to expose the right ventricle (RV). A
heparinized saline infusion needle (0.45x13.5 mm) was inserted
vertically into the RV, and the other end was connected to a
pressure sensor. The physiological data acquisition system (Biopac
Systems, Inc.) recorded the hemodynamic parameters. The PAP value
was indirectly reflected by the right ventricular systolic
pressure. Additionally, the mean right ventricular pressure (mRVP)
was recorded. After measurement, the rats were euthanized by
exsanguination through the abdominal aorta, and the heart and lungs
were exposed. Subsequently, 0.9% cold saline was injected into the
left ventricle (LV) until the liver turned yellow; this is a
commonly used method to ensure thorough perfusion of the brain and
lungs. The perfused brain (white) and lungs (white) were collected
for subsequent morphometric analysis. The heart was completely
removed, including the left and right atria, as well as the free
large vessels. The right ventricular wall was cut along the
interventricular septum (S). To assess the extent of right
ventricular hypertrophy, the weights of the RV, LV and S were
measured. The right ventricular hypertrophy index (RVHI) was then
calculated using the following formula: RVHI=RV/(LV + S).
For the 9- and 13-week-old rats, a closed-chest
surgical method was performed to measure their PAP (19). After anesthesia using 2% sodium
pentobarbital (50 mg/kg, intraperitoneal injection), the rats were
immersed in 75% alcohol for disinfection, then the fur on their
neck and abdomen was removed. They were then placed on a
temperature-regulated surgical table set at 37˚C. A PE-50 catheter
filled with heparin saline was connected to a pressure sensor at
one end and inserted through the right jugular vein into the RV,
and finally into the pulmonary artery. The position of the catheter
was determined based on the waveform measured by the physiological
data acquisition system (Biopac Systems, Inc.). After the waveform
stabilized, hemodynamic values were measured for 2-3 min. Then, the
catheter was removed, and pressure was applied with a cotton ball
to stop bleeding. The abdominal cavity was then opened, and the
abdominal aorta was fully exposed. After flushing the catheter with
heparin saline, it was inserted into the abdominal aorta to measure
the mean mAAP. After measuring, the rats were euthanized by
exsanguination through the abdominal aorta, and the heart and lungs
were exposed. A total of 10 ml 0.9% cold physiological saline was
injected into the RV and perfused through the pulmonary artery to
the entire lung. The perfused lungs (appearing white) were
collected for subsequent extraction of PASMCs, PVECs, western
blotting and morphometric analysis. The same steps as
aforementioned were used to calculate RVHI. In addition, the ratio
of pulmonary and systemic circulation pressure was calculated as
Pp/Ps. No rats died during anesthesia or surgical procedures in the
present study.
Quantitative analysis of neural stem
cell numbers in the hippocampus
Double immunofluorescence staining of hippocampal
tissue was used to investigate neurogenesis in the dentate gyrus.
The isolated brain tissue was fixed overnight at 4˚C in 4%
paraformaldehyde, dehydrated through a graded series of alcohols,
and then embedded in paraffin to form paraffin blocks, and cut into
5-µm thick paraffin-embedded sections. Brain paraffin-embedded
sections were subjected to dewaxing, hydration, high-temperature
antigen retrieval using sodium citrate antigen retrieval solution
at 100˚C for 20 min (cat. no. P0081; Beyotime Institute of
Biotechnology) and blocking in 5% BSA containing 0.5% Triton X-100
at 37˚C for 30 min (cat. no. ST025-5g; Beyotime Institute of
Biotechnology). The sections were then incubated overnight at 4˚C
with the following primary antibodies: Anti-NeuN (1:200; cat. no.
ab104224; Abcam) and anti-Ki67 (1:100; cat. no. ab16667; Abcam).
Subsequently, fluorescent secondary antibodies were incubated with
the sections in the dark for 1 h at 37˚C: Goat anti-mouse Alexa
Fluor® 594 (1:1,000; cat. no. ab150116; Abcam) and goat
anti-rabbit Alexa Fluor 488 (1:1,000; cat. no. ab150077; Abcam).
The nuclei were counterstained with DAPI in the dark at room
temperature for 7 min and the sections were observed under a
fluorescence microscope (Carl Zeiss AG). The number of
Ki67-positive cells in the subgranular zone (SGZ) of the dentate
gyrus was counted, and considered the number of neural stem
cells.
Hematoxylin and eosin (H&E)
staining
H&E staining was used to evaluate pulmonary
vascular remodeling. The lung tissues perfused in each group were
fixed in 4% paraformaldehyde for 24 h. They were then subjected to
alcohol gradient dehydration, paraffin embedding and sectioning
into 5-µm thick slices. The sections were stained according to the
instructions provided in the H&E Staining Kit (cat. no. C0105S;
Beyotime Institute of Biotechnology), and finally sealed with a
neutral resin. The morphology of rat lung blood vessels was
observed using a light microscope. Small arteries (diameter, 20-100
µm) were selected for analysis. ImageJ (version 1.8.0; National
Institutes of Health) was used to analyze the percentage of
vascular medial wall thickness (WT%). This was calculated using the
following formula: WT%=(outer diameter-inner diameter)/outer
diameter x100.
Immunohistochemical staining
Rat lung tissues embedded in paraffin were sectioned
into 5-µm sections. Lung tissue sections were baked at 62˚C for 1
h, followed by deparaffinization in xylene and rehydration in a
descending ethanol series. Then, the sections were treated at 100˚C
for 20 min in citrate antigen retrieval solution (cat. no. P0081;
Beyotime Institute of Biotechnology). After cooling, the sections
were incubated at room temperature in 3% hydrogen peroxide for 20
min to block endogenous peroxidase activity. Subsequently, the
sections were incubated at 37˚C for 30 min in 5% BSA (cat. no.
ST025-5g; Beyotime Institute of Biotechnology) for blocking. After
blocking, the sections were incubated overnight at 4˚C with diluted
α-SMA antibody (1:400; cat. no. 19245s; Cell Signaling Technology,
Inc.). The next day, the sections were incubated for 2 h at 37˚C
with goat anti-rabbit IgG H&L (HRP) secondary antibody
(1:1,000; cat. no. ab6721; Abcam). Color development was performed
using the DAB Horseradish Peroxidase Color Development Kit (cat.
no. P0203; Beyotime Institute of Biotechnology) according to the
instruction manual. Images were captured under white light using an
optical microscope and analyzed with ImageJ (version 1.8.0
software; National Institutes of Health). Immunohistochemical
staining was conducted to detect the expression of α-SMA in the
pulmonary arterioles at three different time points, further
evaluating the degree of pulmonary vascular muscularization in the
13-week-old rats. The area and outer circumference of α-SMA
positive staining (yellow) in pulmonary arterioles (diameter,
20-100 µm) were measured and calculated using ImageJ. The ratio was
calculated to evaluate the thickness of the vascular medial layer,
with a higher ratio indicating a thicker pulmonary vascular medial
layer. Quantitative analysis of the number of alveolar ducts and
vascular alveolar walls in 13-week-old rats was performed using a
light microscope. Each vessel was categorized as muscular,
partially muscular or non-muscular. A total of 30 vessels were
counted per rat. The number and degree of muscularization of these
arteries were quantitatively analyzed as described previously
(34,35).
Primary PASMCs culture and
identification
PASMCs were extracted from the lungs of 9- and
13-week-old rats in a laminar flow hood, using sterile techniques.
As described previously (36), the
pulmonary artery was isolated, and the adventitia and endothelium
were carefully scraped off. The pulmonary blood vessels were washed
three times in sterile PBS then placed into a smooth muscle
digestion solution (papain and collagenase dissolved in PBS;
Sigma-Aldrich; Merch KGaA), cut into ~1 mm3 pieces using
ophthalmic scissors, and subsequently digested in a constant
temperature water bath at 37˚C for 30 min. After completing the
digestion, the samples were centrifuged at 1,000 x g for 5 min at
room temperature. The supernatant was discarded, the sedimented
tissues were combined with 3 ml high-glucose DMEM (cat. no.
CR-12800-S; Zhejiang Senrui Biotechnology Co., Ltd.) supplemented
with 15% FBS (cat. no. 10099-141C, Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin-streptomycin. The mixture was then evenly
distributed at the bottom of a 25-cm2 culture flask.
After 3 days of culturing, the medium was replaced with fresh
medium. After 5 days, cells began to grow out from the tissue
samples. The medium was replaced with fresh medium every 2 days
thereafter. When the cells reached 80% confluence, they were
passaged using 0.25% trypsin (Gibco; Thermo Fisher Scientific,
Inc.). Cell morphology was observed under a bright-field
microscope, and PASMCs were identified by immunofluorescent
staining using an α-SMA antibody (1:400; cat. no. 19245s; Cell
Signaling Technology, Inc.), with a cell purity of >90%.
Subsequent studies were conducted using PASMCs from passages
2-4.
Primary PVECs culture and
identification
According to our previous study (19), primary PVECs were extracted from
13-week-old rats. Briefly, the lungs were perfused and washed with
ice-cold sterile PBS. A 2 mm-wide strip was cut along the edge of
the lung and sliced into 3-5 mm pieces, and strips were evenly
spread in a 75 cm2 culture flask. The flask was filled
with endothelial cell-specific culture medium (5% FBS, 1%
endothelial cell growth supplement and 1% penicillin-streptomycin;
ScienCell Research Laboratories, Inc.). Additionally, the medium
was supplemented with 90 U/ml heparin (cat. no. H3149;
Sigma-Aldrich; Merch KGaA) to inhibit fibroblast growth and purify
endothelial cells. On day 2, the medium was replaced with fresh
endothelial cell culture medium, and on day 5, tissue blocks were
removed by tapping the culture flask. When the confluence of
endothelial cells reached ~80%, cell morphology was observed under
a light microscope. PVECs were then identified by immunofluorescent
staining using a CD31 antibody (1:100; cat. no. ab24590; Abcam),
with a cell purity of >90%. Subsequent studies were conducted
using PVECs from passages 1-2.
Immunofluorescence staining
Primary PASMCs or PVECs, cultured in 24-well plates,
were fixed with 4% paraformaldehyde on ice for 30 min. They were
then blocked with a solution of 3% BSA and 0.5% Triton X-100
dissolved in PBS for 1 h at 37˚C. The cells were then incubated
with α-SMA (1:400; cat. no. 19245s; Cell Signaling Technology,
Inc.) or CD31 (1:100; cat. no. ab222783; Abcam) primary antibodies
overnight at 4˚C. The following day, the cells were incubated with
the following secondary antibodies for 1 h at 37˚C in the dark:
Goat anti-rabbit Alexa Fluor 488 (1:1,000; cat. no. ab150077;
Abcam) or goat anti-mouse Alexa Fluor 488 (1:1,000; cat. no.
ab150113; Abcam). Then, the nuclei were labeled with DAPI for 7 min
at room temperature. All images were observed under a fluorescence
microscope (Carl Zeiss AG).
EdU cell proliferation assay
Cell proliferation was detected using an EdU Cell
Proliferation Assay Kit (Apollo®567; Guangzhou RiboBio
Co., Ltd.) according to the manufacturer's instructions. PASMCs or
PVECs (1x105 cells/ml) cultured in a 96-well plate were
incubated with 50 µM EdU for 2 h at 37˚C, and then the medium was
discarded. After fixing with 4% paraformaldehyde at room
temperature for 30 min, and permeabilizing with 0.5% Triton X-100
for 10 min at room temperature, 100 µl 1X Apollo staining reaction
solution was added to each well and incubated at room temperature
in the dark for 30 min. Cells were then stained with 1X Hoechst
33342 for 30 min at room temperature to visualize the cell nuclei,
and cell counting was performed using an inverted fluorescence
microscope (Carl Zeiss AG). To quantify the proliferation rate of
PASMCs or PVECs, the relative EdU-positive ratio was calculated by
randomly selecting six visual fields from each sample image.
Transwell assay
A Transwell assay was used to evaluate the migratory
ability of PASMCs. Transwell chambers (24-well plate; pore size,
8.0 µm; Corning, Inc.) coated with Matrigel Basement Membrane
Matrix (diluted 1:15 in DMEM; cat. no. 354234; Corning, Inc.) and
incubated for 2 h at 37˚C before use. After the Matrigel dried, the
chamber was placed in a 24-well plate. Primary PASMCs were
resuspended in serum-free DMEM and seeded in the upper chamber,
whereas DMEM containing 10% FBS was loaded into the lower chamber.
After incubating at 37˚C for 48 h, the cells on the membrane
surface were carefully removed. The PASMCs that had migrated to the
underside of the membrane were then fixed with 4% paraformaldehyde
at room temperature for 20 min and stained with 0.1% crystal violet
(Beyotime Institute of Biotechnology) at room temperature for 20
min. Images were obtained using an optical microscope (Carl Zeiss
AG), and the number of cells that had migrated through the membrane
was counted using ImageJ.
TUNEL staining
PASMCs were in a proliferative state in conventional
complete culture medium (high-glucose DMEM supplemented with 15%
FBS and 1% penicillin-streptomycin), with low levels of apoptosis.
To investigate the anti-apoptotic activity of PASMCs, the cells
were cultured in DMEM supplemented with 0.1% FBS for 48 h. This use
of a low serum concentration creates a ‘hunger’ condition that
induces apoptosis (37). According
to our previous study (19), the
one-step TUNEL Cell Apoptosis Detection Kit (cat. no. C1086;
Beyotime Institute of Biotechnology) was used to detect the
apoptotic rate of PASMCs. All images were observed under a
fluorescence microscope (Carl Zeiss AG). To quantify the apoptotic
rate of PASMCs, the relative ratio of FITC-labeled (green) positive
cells was calculated by randomly selecting six visual fields from
each sample image.
Western blotting
The perfused, clean lung tissues and collected
PASMCs were stored at -80˚C for future use. Total protein was
extracted from lung tissues and PASMCs for western blot analysis to
investigate differences in the protein expression levels between
the groups. The specific steps have been described previously
(38). After blocking the
membranes with 5% milk at room temperature for 1 h, they were
incubated with the following primary antibodies overnight at 4˚C:
α-SMA (1:1,000; cat. no. 19245s; Cell Signaling Technology, Inc.),
endothelial nitric oxide synthase (eNOS; 1:1,000; cat. no.
ab199956; Abcam), inducible NOS (iNOS; 1:1,000; cat. no.
18985-1-AP; ProteinTech Group, Inc.), Bcl-2 (1:1,000; cat. no.
26593-1-AP; ProteinTech Group, Inc.), Bax (1:1,000; cat. no
ET1603-34; Huabio), cleaved caspase-3 (1:1,000; cat. no. 9664s;
Cell Signaling Technology, Inc.), caspase-3 (1:1,000; cat. no.
9662s; Cell Signaling Technology, Inc.), Ki67 (1:100; cat. no.
ab16667; Abcam) or β-actin (1:10,000; cat. no. 8457s; Cell
Signaling Technology, Inc.). The following day, a HRP-linked
secondary antibody (goat anti-rabbit; 1:3,000; cat. no. 7074; Cell
Signaling Technology, Inc.) was used to incubate the membranes at
room temperature for 2 h. The ECL chemiluminescent developing
solution (cat. no. WBKLS0050; Sigma-Aldrich) was prepared at a 1:1
ratio and shielded from light. After the addition of the developing
solution, the bands were detected using the G: BOX gel doc system
(Syngene). All bands were generated on a single PVDF membrane
(IPVH00010/ISEQ00010; MilliporeSigma). For examination of proteins
with similar molecular weights, membranes were stripped using a
stripping buffer (cat. no. 46430; Thermo Fisher Scientific, Inc.),
and after blocking with 5% milk for 1 h, the membrane was incubated
with the next primary and secondary antibodies as aforementioned.
Protein expression was semi-quantified by normalizing to the
relative β-actin expression using ImageJ.
Statistical analysis
All experiments were repeated three times.
Statistical analyses were performed using SPSS version 20.0 (IBM
Corp.) and GraphPad Prism version 8.3.0 (Dotmatics). One-way ANOVA
followed by Tukey's post hoc test was used to analyze the
differences between more than two groups. Independent samples
between two groups were analyzed using an unpaired Student's
t-test. Data are presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of three different
growth patterns in early postnatal life and weight analysis of IUGR
rats
The IUGR model was established by restricting
maternal nutrition during pregnancy, and different postnatal growth
patterns were constructed by adjusting the number of pups in each
group during lactation (Fig. 1A).
The offspring of dams with restricted maternal nutrition had birth
weights <P10 (6.665 g) of the control group (4.70±0.06 g vs.
7.22±0.11 g; Fig. 1B), indicating
the successful establishment of the IUGR model. These IUGR rats
were randomly assigned to be nursed by dams on a normal diet during
lactation, with litter sizes of 5, 10 or 16 pups per dam. There
were no differences in the birth weight among the three IUGR groups
with different growth patterns (Fig.
1C). In addition, the weight curve indicated that there were no
significant differences in the weights between the IUGR + RC group
and the control group at weaning (3 weeks). The weight curve of the
IUGR + MC group remained between that of the IUGR + RC and the IUGR
+ DG groups throughout the study. Notably, the weight of the IUGR +
DG group was lower than that of the control group at all time
points (Fig. 1D). These results
indicated that, compared with the IUGR + DG group, the rats in the
two other catch-up growth groups demonstrated improved growth and
development.
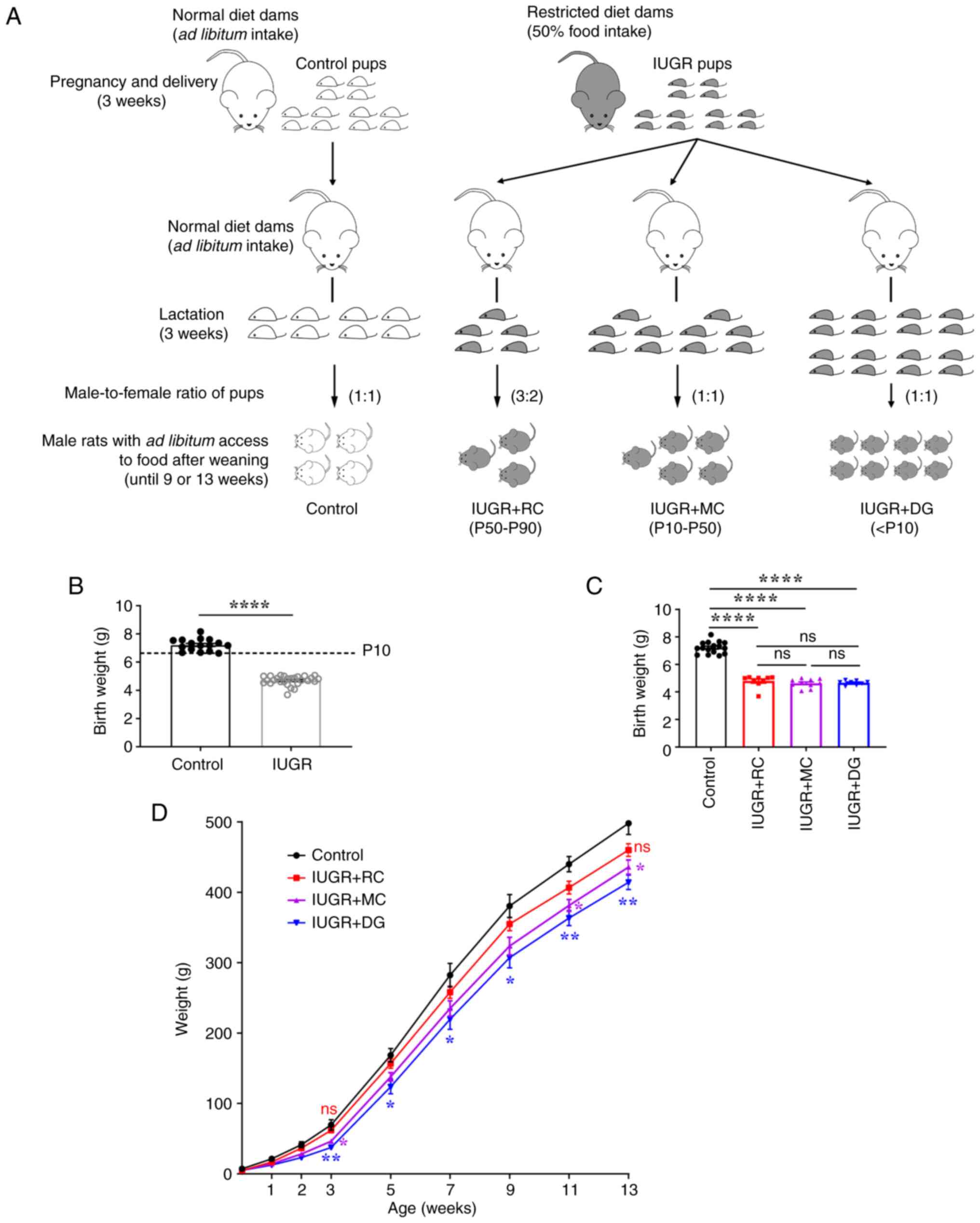 | Figure 1Preparation of the animal model and
body weight curve of rats in each group. (A) Schematic diagram of
the generation of IUGR and different postnatal growth pattern
models. (B) Birth weights of pups (Control, n=16; IUGR, n=27). Data
were analyzed using Student's two-tailed t-test. (C) Birth weight
of the four groups of rats (Control, n=16; IUGR + RC, n=9; IUGR +
MC, n=10; IUGR + DG, n=8). (D) Weight curve of rats from birth to
13 weeks of age (n=8-16/group). *P<0.05,
**P<0.01, and ns vs. the control group. Data were
analyzed using one-way ANOVA followed by Tukey's post hoc test.
Data are presented as the mean ± SEM. *P<0.05,
**P<0.01, ****P<0.0001. ns, not
significant; IUGR, intrauterine growth restriction; RC, rapid
postnatal catch-up growth; MC, moderate postnatal catch-up growth;
DG, delayed growth in early postnatal life; P, percentile. |
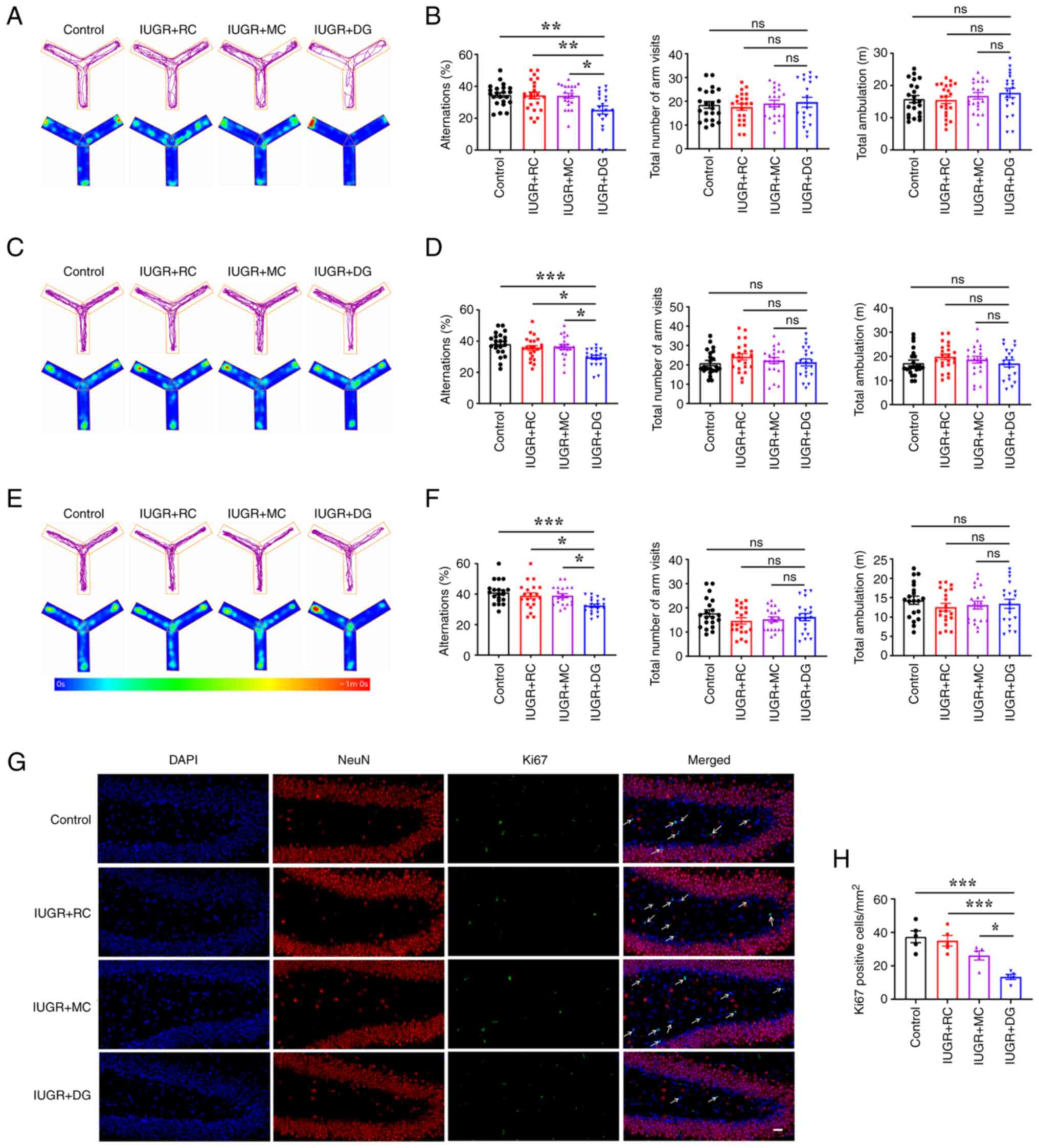
Postnatal catch-up growth in IUGR rats
can improve memory function deficits
The Y-maze was used to assess short-term memory in
rats. The spontaneous alternation test was performed on rats at 3,
9 and 13 weeks of age, and the movement trajectory of the rats in
the Y-maze was recorded (Fig. 2A,
C and E). Quantitative analysis showed that the
percentage of spontaneous alternations in the IUGR + DG group was
significantly lower than that in the control group at all three
time points (Fig. 2B, D and F).
In addition, there was no significant difference in the total
number of arm entries and total distance traveled in the maze among
the groups at the three time points.
Neurogenesis in the dentate gyrus of the hippocampus
was investigated in 3-week-old rats using double immunofluorescence
staining for NeuN and Ki67 (Fig.
2G). The number of Ki67-positive cells in the SGZ in the IUGR +
DG group was significantly lower than that in the other three
groups (Fig. 2H). These results
indicated that delayed growth after IUGR led to a decrease in the
number of proliferating neurons in the SGZ, resulting in a decrease
in spatial working memory in rats. However, postnatal catch-up
growth, whether it was moderate or rapid, could improve memory
function deficits in IUGR rats during both childhood and
adulthood.
Moderate postnatal catch-up growth in
IUGR rats preserves normal PAP in adulthood
Subsequently, the effects of the three different
growth patterns on PAP and RVP were investigated through invasive
hemodynamic analysis. The results showed that at 3 (Fig. 3A and B) and 9 weeks of age (Fig. 3C and D), there were no significant differences
in mPAP, mRVP and RVHI between the control and the IUGR + RC group.
However, at 13 weeks of age, the mPAP of the IUGR + RC group was
significantly higher than that of the control group (18.37±0.18
mmHg vs. 21.1±0.44 mmHg; Fig. 3E
and F). In addition, the mPAP of
the IUGR + MC group (19.25±0.20 mmHg) and the IUGR + DG group
(18.64±0.23 mmHg) were significantly lower than that of the IUGR +
RC group. There were no signficant differences in the mRVP and RVHI
among the groups at 13 weeks of age. Next, the systemic circulation
pressure of rats was evaluated by measuring the mAAP (Fig. 3G). At 9 weeks of age, no
significant differences were observed in mAAP and Pp/Ps among the
four groups of rats (Fig. 3H). At
13 weeks of age, although mAAP remained similar, the Pp/Ps of the
IUGR + RC group was higher than that of the control group (Fig. 3I). This further demonstrated that
rapid catch-up growth led to an increase in pulmonary circulation
pressure in adult IUGR rats, but did not affect systemic
circulation pressure. Moderate catch-up growth and delayed growth
did not have a marked impact on PAP and RVP in adult rats. These
results indicated that compared with the other two growth patterns,
early moderate catch-up growth following IUGR led to better memory
and pulmonary vascular function in adulthood.
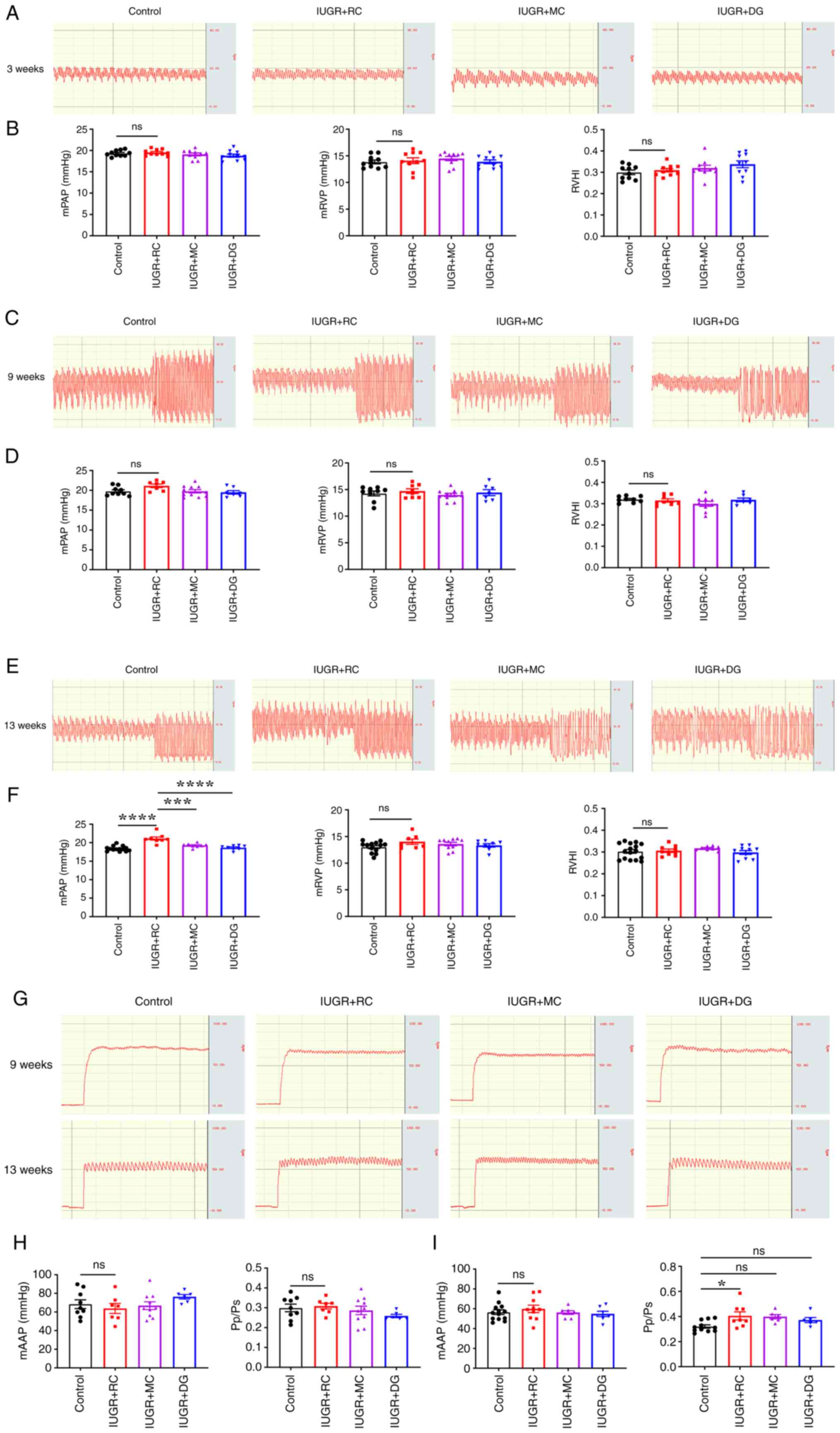 | Figure 3Moderate catch-up growth does not
lead to increased PAP in adult IUGR rats. (A) Representative graphs
of PAP in rats at 3 weeks of age. (B) Quantitative analysis of
mPAP, mRVP and RVHI in 3-week-old rats (n=10/group). (C)
Representative graphs of PAP in rats at 9 weeks of age. (D)
Quantitative analysis of mPAP (n=7-11/group), mRVP (n=7-11/group)
and RVHI (n=7-9/group) in 9-week-old rats. (E) Representative
graphs of PAP in rats at 13 weeks of age. (F) Quantitative analysis
of mPAP (n=8-14/group), mRVP (n=8-13/group) and RVHI (n=8-16/group)
in 13-week-old rats. (G) Representative graphs of abdominal aortic
pressure in rats at 9 and 13 weeks of age. (H) Quantitative
analysis of mAAP (n=6-10/group) and Pp/Ps (n=6-10/group) in
9-week-old rats. (I) Quantitative analysis of mAAP (n=7-13/group)
and Pp/Ps (n=7-13/group) in 13-week-old rats. Data were analyzed
using one-way ANOVA followed by Tukey's post hoc test. Data are
presented as the mean ± SEM. *P<0.05,
***P<0.001, ****P<0.0001. ns, not
significant; IUGR, intrauterine growth restriction; RC, rapid
postnatal catch-up growth; MC, moderate postnatal catch-up growth;
DG, delayed growth in early postnatal life; mPAP, mean pulmonary
artery pressure; mRVP, mean right ventricular pressure; RVHI, right
ventricular hypertrophy index; Pp/Ps, ratio of pulmonary and
systemic circulation pressure. |
Moderate postnatal catch-up growth
prevents pulmonary arteriolar remodeling compared with rapid
catch-up growth
Due to the observation that postnatal rapid catch-up
growth increased PAP in adulthood, whether it also affected
pulmonary arteriolar remodeling was assessed. To test this
hypothesis, H&E staining was used to observe lung small artery
structure in rats at 3, 9 and 13 weeks of age in the different
groups (Fig. 4A). No significant
differences were observed in WT% among the groups at 3 and 9 weeks
of age. At 13 weeks, the IUGR + RC group showed a significant
increase in medial WT% compared with that in the control group
(29.06±1.32% vs. 55.16±4.75%; Fig.
4B). The IUGR + MC and IUGR + DG groups showed a marked
decrease in WT% compared with that in the IUGR + RC group (Fig. 4B). Subsequently, the thickness of
the medial smooth muscle layer and muscularization of lung small
vessels were evaluated using α-SMA immunohistochemical staining
(Fig. 4C-F). The results showed
that at 13 weeks of age, compared with the control group, the IUGR
+ RC group exhibited significantly increased medial smooth muscle
layer thickness (Fig. 4D) and
percentage of muscular vessels (15.53±3.99% vs. 48.9±4.02%;
Fig. 4E and F). Expression levels of α-SMA were also
upregulated only in the IUGR + RC group compared with the control
group (Fig. 4G and H). However, compared with in the IUGR +
RC group, the IUGR + MC and the IUGR + DG groups exhibited a marked
decrease in the thickness of the medial smooth muscle layer, the
percentage of muscularized small blood vessels, and the expression
of α-SMA at 13 weeks of age (Fig.
4E-H). Additionally, there was an increase in the percentage of
non-muscularized blood vessels in the IUGR + MC and IUGR + DG
groups compared with that in the IUGR + RC group (Fig. 4E and F). These results suggested that moderate
catch-up growth had a minimal impact on pulmonary arteriolar
remodeling, thus not leading to a significant elevation of PAP in
adulthood.
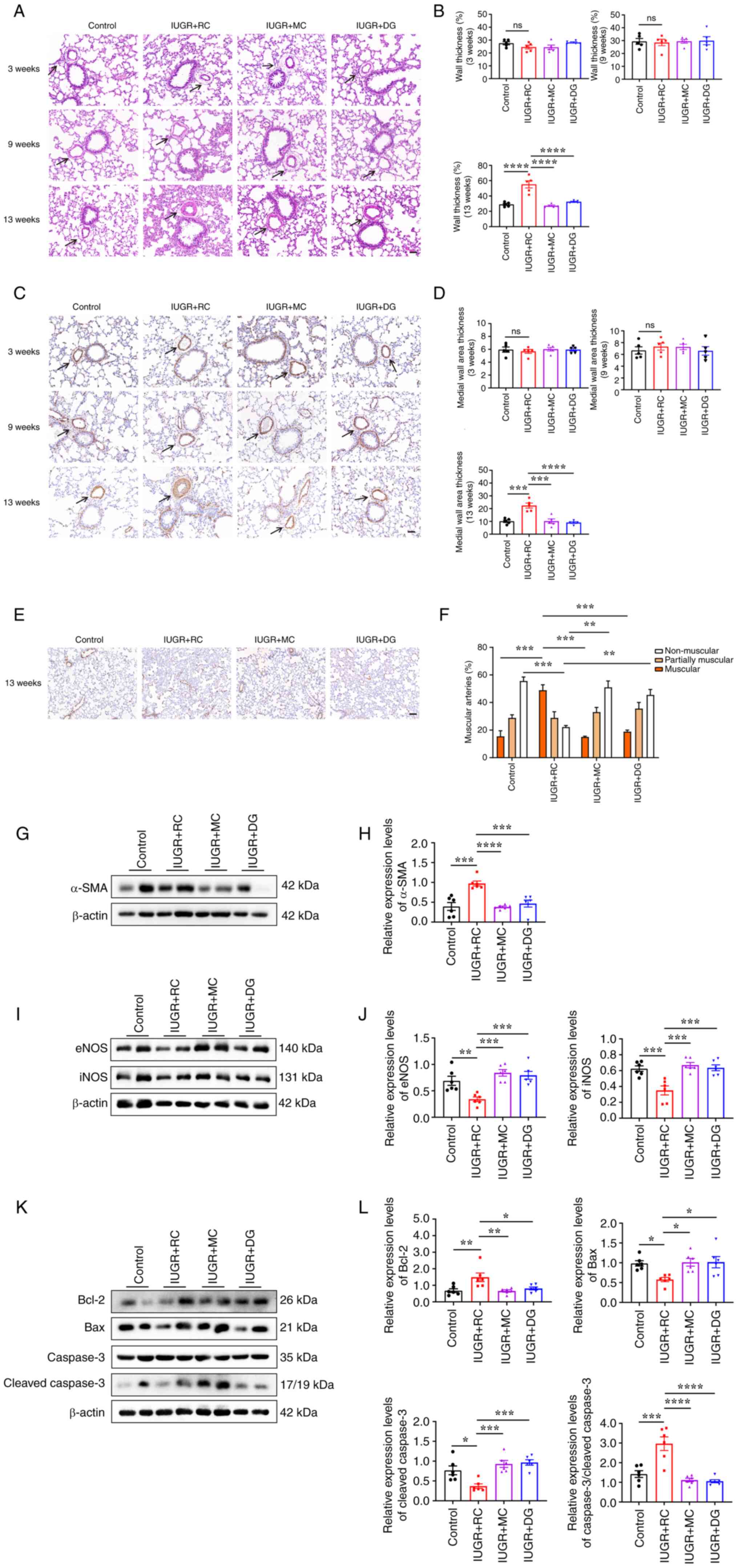 | Figure 4Rapid catch-up growth leads to
pulmonary artery remodeling, and downregulation of pulmonary NOS
and apoptotic proteins in adult IUGR rats. (A) Representative
images of hematoxylin and eosin-stained pulmonary arterioles at
three different time points. Scale bar, 50 µm. The black arrows
indicate the pulmonary arterioles (diameter, 20-100 µm). (B)
Quantitative analysis of medial thickness as a percentage of vessel
diameter (n=5/group). (C) Representative images of α-SMA
immunohistochemical staining of pulmonary arterioles at three
different time points. Scale bar, 50 µm. The black arrows indicate
the pulmonary arterioles (diameter range, 20-100 µm). (D)
Quantitative analysis of α-SMA-positive medial thickness
(n=5/group). (E) Representative images of small vessels around
alveolar walls or bronchioles in 13-week-old rats. Scale bar, 100
µm. (F) Quantitative analysis of the percentage of muscularized
vessels (n=3/group, with 30 vessels selected per rat). (G) Western
blotting and (H) semi-quantitative analysis of α-SMA protein
expression in the lung tissues of 13-week-old rats (n=6/group). (I)
Western blotting and (J) semi-quantitative analysis of eNOS and
iNOS protein expression in the lung tissues of 13-week-old rats
(n=6/group). (K) Western blotting and (L) semi-quantitative
analysis of Bcl-2, Bax, caspase-3 and cleaved caspase-3 protein
expression in the lung tissues of 13-week-old rats (n=6/group).
Data were analyzed using one-way ANOVA followed by Tukey's post hoc
test. Data are presented as the mean ± SEM. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. ns, not significant; IUGR, intrauterine
growth restriction; RC, rapid postnatal catch-up growth; MC,
moderate postnatal catch-up growth; DG, delayed growth in early
postnatal life; eNOS, endothelial nitric oxide synthase; iNOS,
inducible nitric oxide synthase. |
Rapid catch-up growth downregulates
the expression levels of eNOS, iNOS and apoptotic proteins in the
lungs of adult IUGR rats
To further explore the molecular mechanisms
underlying the increased PAP caused by rapid catch-up growth,
western blotting was performed to analyze the protein expression
levels in the lung tissue of adult rats from each group. The
protein expression levels of eNOS and iNOS were assessed, and it
was revealed that the IUGR + RC group exhibited decreased
expression levels compared with those in the control group, whereas
the IUGR + MC group exhibited expression levels similar to those in
the control group (Fig. 4I and
J).
The expression levels of apoptosis-related proteins
in the lungs were also assessed. The results showed that the IUGR +
RC group exhibited increased expression levels of the
anti-apoptotic protein Bcl-2, and decreased expression levels of
the pro-apoptotic proteins Bax and cleaved caspase-3, whereas the
IUGR + MC and the IUGR + DG groups did not exhibit significant
differences compared with the control group (Fig. 4K and L). It was hypothesized that the increase
in anti-apoptotic proteins in the IUGR + RC group may be associated
with resistance to apoptosis in PASMCs. This was further supported
by the increased ratio of total caspase-3 to cleaved caspase-3,
indicating a potential suppression of apoptosis (Fig. 4L). In summary, these results
indicated that postnatal rapid catch-up growth in IUGR may lead to
a decrease in levels of pulmonary NOS, potentially resulting in
reduced production of the vasodilator substance, NO. This, in turn,
could have contributed to the elevation of PAP in adulthood.
Moderate catch-up growth prevents
excessive proliferation, migration and anti-apoptotic effects of
PASMCs compared with rapid catch-up growth
To gain a deeper understanding of the cellular
mechanisms underlying pulmonary arteriolar remodeling in IUGR rats
with postnatal rapid catch-up growth, primary PASMCs were cultured
and cell identification assays were performed. Under an optical
microscope, most primary rat PASMCs appeared as elongated spindle
shapes with branching projections. Immunofluorescence staining for
α-SMA showed a cell purity of ~91.3% (Fig. 5A), indicating successful isolation
and cultivation of primary PASMCs. Next, the migration and
proliferation of the primary PASMCs extracted from 9- and
13-week-old rats were evaluated. The results showed that there was
no evident difference in the migratory and proliferative abilities
of PASMCs among the groups at 9 weeks of age (Fig. 5B-E). However, at 13 weeks of age,
the IUGR + RC group exhibited significantly increased migration
(Fig. 5F and H) and a greater percentage of
EdU-positive proliferating cells compared with those in the control
group (Fig. 5G and I). The expression levels of Ki67 in the
IUGR + RC group were also significantly higher than those in the
control group (Fig. 5J-M).
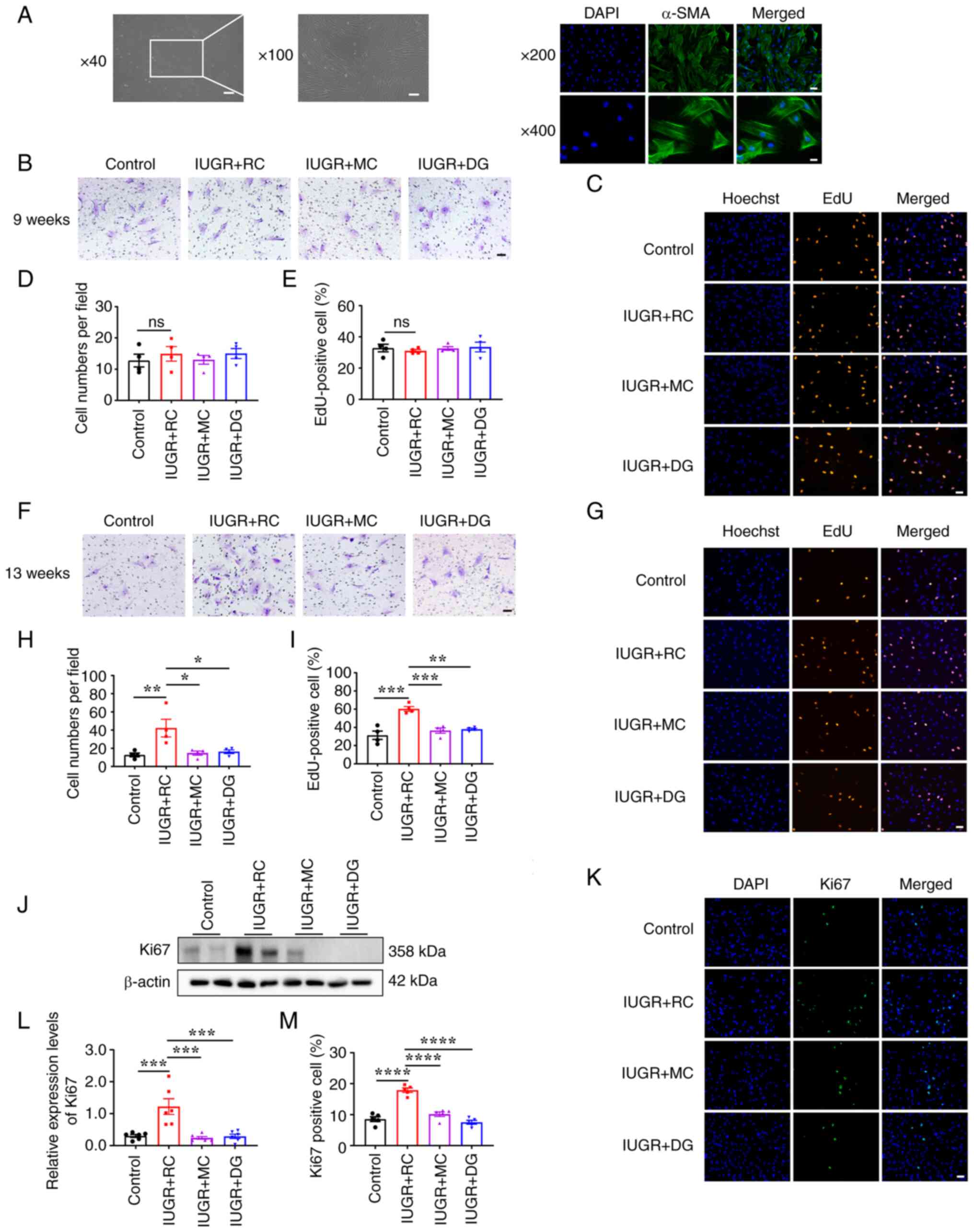 | Figure 5Rapid catch-up growth in IUGR rats
leads to excessive proliferation and migration of PASMCs in
adulthood. (A) Identification of primary PASMCs in rats. Light
microscopy image (scale bar, 250 µm at x40 magnification; scale
bar, 100 µm at x100 magnification) and α-SMA fluorescence image
(scale bar, 50 µm at x200 magnification; scale bar, 25 µm at x400
magnification). (B) Transwell assay of PASMCs extracted from
9-week-old rats (scale bar, 50 µm). (C) EdU proliferation assay of
PASMCs extracted from 9-week-old rats (scale bar, 50 µm). (D)
Quantitative analysis of the results of the Transwell assay using
the PASMCs from 9-week-old rats (n=4/group). (E) Quantitative
analysis of the percentage of EdU-positive PASMCs from 9-week-old
rats (n=4/group). (F) Transwell assay of PASMCs extracted from
13-week-old rats (scale bar, 50 µm). (G) EdU proliferation assay of
PASMCs extracted from 13-week-old rats (scale bar, 50 µm). (H)
Quantitative analysis of the Transwell assay results in 13-week-old
PASMCs (n=4/group). (I) Quantitative analysis of the percentage of
EdU-positive PASMCs from 13-week-old rats (n=4/group). (J)
Representative western blotting images of Ki67 protein expression
in primary PASMCs isolated from 13-week-old rats. (K)
Representative immunofluorescence staining images for Ki67 in
primary PASMCs. (L) Semi-quantitative analysis of Ki67 protein
levels based on the western blotting shown in J (n=6/group). (M)
Quantitative analysis of Ki67 immunofluorescence staining in
primary PASMCs (n=5/group). Data were analyzed using one-way ANOVA
followed by Tukey's post hoc test. Data are presented as the mean ±
SEM. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. ns, not
significant; IUGR, intrauterine growth restriction; RC, rapid
postnatal catch-up growth; MC, moderate postnatal catch-up growth;
DG, delayed growth in early postnatal life; PASMCs, pulmonary
artery smooth muscle cells. |
Next, changes in apoptosis-related proteins in
PASMCs extracted from the lungs of 13-week-old rats were evaluated
using western blotting. The results showed that the expression
levels of Bcl-2 were significantly upregulated, whereas the
expression levels of Bax and cleaved caspase-3 were significantly
downregulated in the IUGR + RC group compared with those in the
control group (Fig. 6A). The
increased ratio of total caspase-3 to cleaved caspase-3 further
confirmed the apoptotic resistance in the PASMCs of the IUGR + RC
group (Fig. 6A). TUNEL staining
showed that PASMCs in the IUGR + RC group were more resistant to
apoptosis induced by serum deprivation compared with that in the
control group. By contrast, PASMCs in the IUGR + MC and IUGR + DG
groups were more prone to undergo apoptosis in a low-serum
environment (Fig. 6B).
Collectively, these data suggested that the excessive
proliferation, migration and anti-apoptotic phenotype of PASMCs are
among the primary contributing factors to the medial thickening and
luminal narrowing observed in pulmonary arterioles during postnatal
rapid catch-up growth in adult IUGR. However, these changes in
PASMCs were not as pronounced in the group of rats with moderate
catch-up growth.
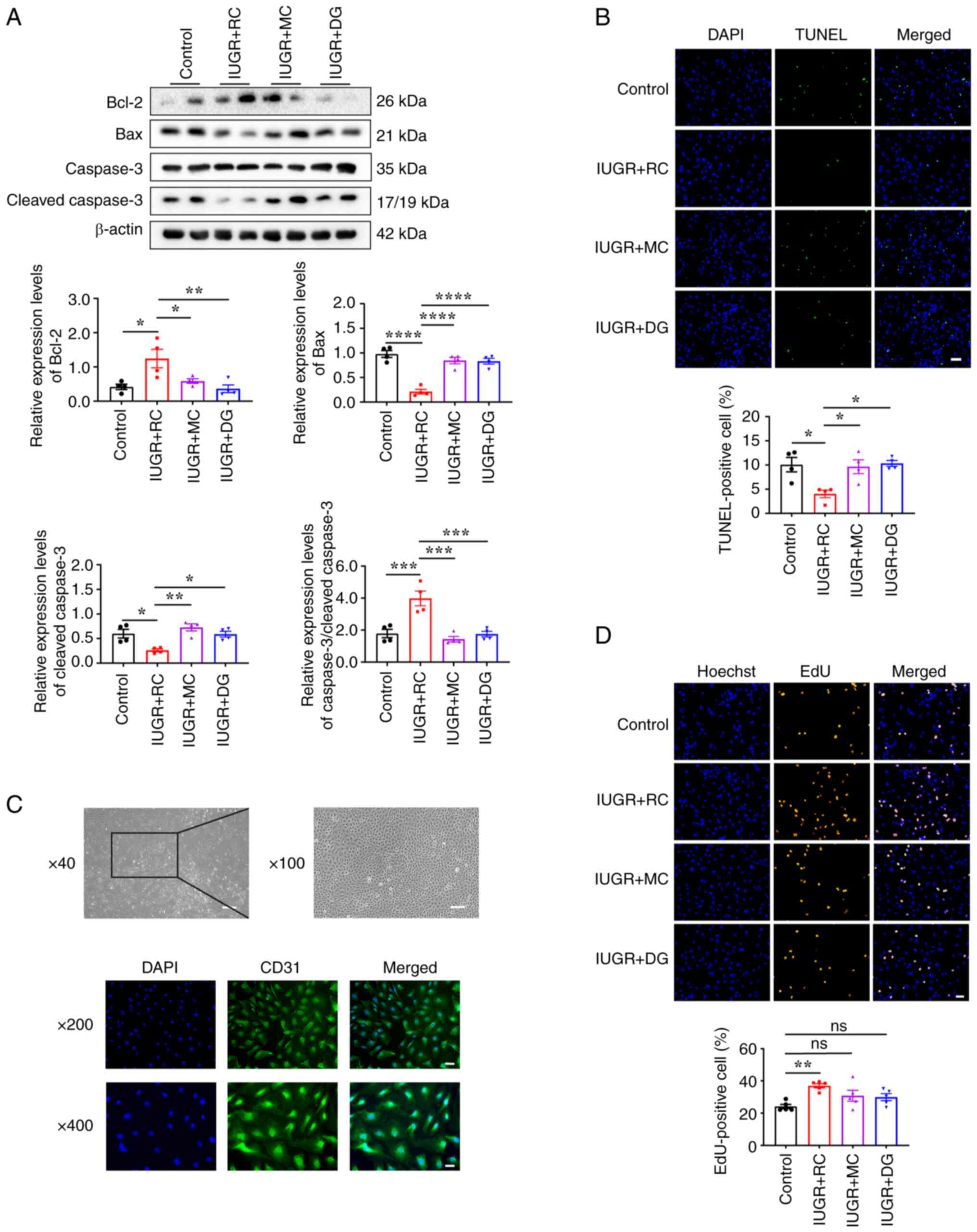 | Figure 6PASMCs from adult IUGR + RC rats
exhibit an enhanced anti-apoptotic capacity and PVECs exhibit
excessive proliferation. (A) Western blotting and semi-quantitative
analysis of apoptosis-related proteins Bcl-2, Bax, caspase-3 and
cleaved caspase-3 in PASMCs extracted from 13-week-old rats
(n=4/group). (B) TUNEL staining and quantitative analysis of PASMCs
treated with low serum for 48 h (n=4/group; scale bar, 50 µm). (C)
Identification of primary PVECs from rats using light microscopy
(scale bar, 250 µm at x40; scale bar, 100 µm at x100) and CD31
immunofluorescence staining (scale bar, 50 µm at x200; scale bar,
25 µm at x400). (D) Representative image and quantitative analysis
of EdU incorporation in PVECs extracted from 13-week-old rats
(n=5/group; scale bar, 50 µm). Data were analyzed using one-way
ANOVA followed by Tukey's post hoc test. Data are presented as the
mean ± SEM. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. ns, not
significant; IUGR, intrauterine growth restriction; RC, rapid
postnatal catch-up growth; MC, moderate postnatal catch-up growth;
DG, delayed growth in early postnatal life; PASMCs, pulmonary
artery smooth muscle cells; PVECs, pulmonary vascular endothelial
cells. |
Moderate catch-up growth prevents
excessive proliferation of PVECs compared with rapid catch-up
growth
Whether the remodeling of pulmonary arterioles is
associated with functional changes in endothelial cells was next
assessed by culturing and identifying primary PVECs. Under an
optical microscope, primary rat PVECs appeared as tightly connected
cobblestone-like cells. Immunofluorescence staining for CD31 showed
a cell purity of ~97.6% (Fig. 6C),
indicating successful isolation and cultivation of primary PVECs.
The results of the EdU cell proliferation assay showed that the
proliferative ability of PVECs in the IUGR + RC group was stronger
than that in the control group (Fig.
6D). The proliferative ability of cells in the IUGR + MC and
IUGR + DG groups showed a trend of upregulation compared with the
control group, although the difference was not significant.
Additionally, there were no significant differences in
proliferation between the IUGR + MC and IUGR + DG groups compared
to the IUGR + RC group (Fig. 6D).
Taken together, these results demonstrated that the excessive
proliferation of PVECs served an important role in the elevation of
PAP caused by rapid catch-up growth in adulthood.
Discussion
The present study demonstrated that both young and
adult IUGR rats performed better in the Y-maze test than the
delayed growth group, regardless of whether they experienced
moderate or rapid catch-up growth. Furthermore, it was revealed
that the proliferation of neural stem cells in the SGZ of the
hippocampus was reduced in the delayed growth group. Conversely,
experiencing rapid catch-up growth during lactation increased
susceptibility to cardiovascular disease in adulthood; this
included increased mPAP, medial thickening of pulmonary arterioles,
luminal narrowing and increased muscularization of pulmonary small
vessels. Furthermore, PASMCs exhibited excessive proliferation,
enhanced migratory ability and anti-apoptotic activity. Similarly,
PVECs exhibited excessive proliferation. By contrast, moderate
catch-up growth and delayed growth in IUGR rats did not result in
negative effects on cardiovascular function. It was also observed
that these adverse cardiovascular outcomes caused by rapid catch-up
growth only occurred during adulthood. Thus, the present study
provides experimental evidence that moderate catch-up growth
following IUGR is superior to rapid catch-up growth or delayed
growth, particularly in promoting optimal pulmonary vascular
function and memory function in adulthood. These findings provide a
research foundation for developing appropriate feeding patterns for
infants with IUGR.
Research has shown that providing sufficient
nutrition during the perinatal period is crucial for
neurodevelopment, which is necessary to support the growth and
development of nerve cells (39).
Throughout the life of adult mammals, neurogenesis occurs in the
brain, and neural stem cells are present in the subventricular zone
of the lateral ventricles and the SGZ of the dentate gyrus in the
hippocampus. These neural stem cells can continuously generate new
neurons and integrate them into local neural circuits (40,41).
Ki67 is a nuclear protein that can be used as a marker to determine
the state of a cell in the cell cycle, with its expression varying
in the different stages of the cell cycle. It also serves as an
indicator of neural stem cell proliferation status (42). In the development and repair of the
nervous system, Ki67 is widely used for the identification and
analysis of neural stem cells and neural progenitor cells (43). In our previous study, it was
demonstrated that IUGR accompanied by delayed postnatal growth
affects the expression of memory-related gene zif268 and
transcription factor recruitment factor p300 in the hippocampus and
leads to a decrease in synaptic plasticity in the CA1 region. This,
in turn, causes irreversible damage to the cognitive function of
adult rats (14). In the present
study, it was further revealed that delayed growth affected the
number of Ki67-positive neural stem cells in the SGZ region of IUGR
rats. Therefore, catch-up growth is beneficial for brain
development. This process can increase the proliferation of neural
stem cells in the hippocampus and improve memory deficits caused by
IUGR.
The ‘Developmental Origins of Health and Disease’
theory states that the degree of growth in the early stages of life
and nutritional exposure have an impact on chronic diseases in
adulthood (44). When it comes to
cardiovascular function, the results of the present study showed
that only the IUGR + RC group exhibited an increase in mPAP in
adulthood, whereas mRVP and RVHI did not change. These results
suggested that the increase in mPAP in the IUGR + RC group was
temporary and had not yet affected right ventricular function.
Therefore, there was no evidence of right ventricular hypertrophy.
However, this does not mean that catch-up growth does not lead to
right ventricular dysfunction, and longer-term experiments are
required to verify this. Research has shown that IUGR increases the
risk of hypertension in adults (45,46).
A clinical study by Leunissen et al (47) reported that childhood weight gain,
especially fat mass, not birth weight, determines blood pressure in
young adults. However, in the present study, neither catch-up
growth nor postnatal nutritional restriction led to an increase in
mAAP at all time points, which represents systemic circulation
pressure. A possible reason is that the subjects were still too
young to exhibit changes in systemic circulation pressure.
Endothelial cell dysfunction, characterized by an
imbalanced production of vasoconstrictors and vasodilators, and a
reduced availability of bioactive NO, is considered a crucial
underlying factor in various clinical and experimental forms of
pulmonary hypertension (48). In
the present study of the mechanism underlying adult PAH caused by
rapid catch-up growth, it was observed that the IUGR + RC group
exhibited an increase in smooth muscle layer thickness in the
pulmonary arteries, narrowed vascular lumens and a higher
proportion of muscularization in pulmonary small vessels. Notably,
these histopathological changes did not occur in the IUGR + MC
group during adulthood. In addition, the protein expression levels
of eNOS and iNOS in the lungs of the IUGR + RC group were decreased
during adulthood, potentially resulting in a decrease in NO
production in the lungs. NO is not only an effective pulmonary
vasodilator, but it also prevents hypoxia-induced pulmonary
vasoconstriction (49), inhibits
smooth muscle proliferation and platelet aggregation (50), and downregulates ET-1 production
(51). The decrease in the
expression of these NOSs may have an important role in the elevated
PAP.
Previous studies have shed light on the underlying
mechanism of PAH, revealing that the primary cause of its
persistent progression and resistance to treatment lies in
pulmonary vascular remodeling (52,53),
which is primarily caused by medial hypertrophy and the formation
of neointimal lesions. Apoptosis resistance, proliferation and
migration of PASMCs also serve a vital role in this process
(36). In the present study,
PASMCs extracted from the lungs of 13-week-old rats exhibited
enhanced proliferative and migratory abilities under normal oxygen
conditions. TUNEL results also revealed that PASMCs in the IUGR +
RC group exhibited anti-apoptotic properties in a low serum,
pro-apoptotic ‘starvation’ environment. In addition, disordered
proliferation of endothelial cells accompanied by
neovascularization is a common pathological feature of pulmonary
vessels in patients with PAH (54). In the present study, it was found
that PVECs extracted from the lungs of IUGR + RC adult rats
exhibited increased proliferation. Dysfunction of these PASMCs and
PVECs resulted in remodeling of the pulmonary arterioles,
ultimately leading to elevated mPAP in adult IUGR rats after rapid
catch-up growth. By contrast, the rats in the IUGR + MC and IUGR +
DG groups did not exhibit dysfunction in these cells. These
findings support the notion that a discrepancy between early life
experiences and the intrauterine environment can result in
cardiovascular dysfunction (44).
All of these results demonstrated that moderate catch-up growth
after birth is more beneficial for children with IUGR to achieve
appropriate cardiovascular and cognitive function in adulthood.
Based on our previous findings that delayed
postnatal growth leads to cognitive impairment but reverses the
elevations in mPAP induced by postnatal catch-up growth (14). Since no distinction was made
regarding the rate of catch-up growth, it is evident that this
result has limitations. Therefore, we aimed to assess new postnatal
nutritional strategies to maintain optimal pulmonary vascular
function in adult individuals with a history of IUGR without
compromising essential brain functions. Building upon this
preliminary research, an animal model of moderate catch-up growth
was established. The innovative aspect of the present study was the
investigation of the effects of various early-life nutritional
interventions on the short-term and long-term health outcomes of
IUGR rats, without being restricted to a single time period. To the
best of our knowledge, the present study is the first to
demonstrate the superiority of moderate catch-up growth as a
postnatal nutritional pattern in terms of both cognitive function
and pulmonary vascular function. The findings suggested that
adopting a strategy of moderate catch-up growth in nutritional
interventions for children with IUGR may result in improved health
outcomes. This discovery provides guidance for clinical practice
and postnatal intervention measures in managing IUGR. However, the
present study has certain limitations. There was a focus on the
effects of moderate catch-up growth on the pulmonary vascular and
memory functions of rats. However, the issues of glucose and lipid
metabolism caused by rapid catch-up growth cannot be ignored.
Further research is required to determine whether postnatal
moderate catch-up growth also leads to insulin resistance and
obesity. The mechanisms underlying the impact of IUGR on memory
function also require further investigation. Additionally, it is
crucial to investigate the mechanisms that contribute to the
dysfunction of rat PASMCs and PVECs in response to rapid catch-up
growth. Notably, the offspring of IUGR rats in the present study
were generated from dams that experienced prenatal nutritional
restriction. As aforementioned, the causes of IUGR include genetic
factors, fetal factors, placental factors and maternal health
factors (2,3). The conclusions in the present study
only apply to IUGR caused by maternal health issues, rather than
all types of IUGR.
Taken together, the present findings suggested that
different catch-up growth patterns after IUGR have significant
long-term health effects. Notably, moderate catch-up growth may be
a more favorable option. Moderate catch-up growth appears to be a
healthier approach to catch-up growth, which may enable children
with IUGR to achieve improved cognitive function and pulmonary
vascular function in adulthood. These results highlight the
importance of considering healthier catch-up growth patterns in the
postnatal management of IUGR and provide insights into potential
strategies to improve long-term outcomes in these individuals.
Acknowledgements
The authors would like to thank Dr Chao Chen
(Zhejiang University) for their assistance with measuring pulmonary
artery pressure.
Funding
Funding: The present study was funded by the National Natural
Science Foundation of China (grant nos. 81630037 and 82241017).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LY and LD conceived the present study. LY performed
data curation. Conception and design were the responsibility of LY
and YH. CH, KC, YY and LZ were responsible for the analysis and
interpretation of data. LY and LD were responsible for supervision
and project administration, and writing, reviewing and editing the
manuscript. Data visualization was the responsibility of XL. LY
wrote the original draft of the manuscript. LD was responsible for
funding acquisition. LY and YH confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal study protocol was approved by the Animal
Care and Use Committee of Zhejiang University (approval no.
ZJU20160215; Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kesavan K and Devaskar SU: Intrauterine
growth restriction: Postnatal monitoring and outcomes. Pediatr Clin
North Am. 66:403–423. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharma D, Shastri S, Farahbakhsh N and
Sharma P: Intrauterine growth restriction-part 1. J Matern Fetal
Neonatal Med. 29:3977–3987. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bernstein PS and Divon MY: Etiologies of
fetal growth restriction. Clin Obstet Gynecol. 40:723–729.
1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rosenberg A: The IUGR newborn. Semin
Perinatol. 32:219–224. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chernausek SD: Update: Consequences of
abnormal fetal growth. J Clin Endocrinol Metab. 97:689–695.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Longo S, Bollani L, Decembrino L, Di
Comite A, Angelini M and Stronati M: Short-term and long-term
sequelae in intrauterine growth retardation (IUGR). J Matern Fetal
Neonatal Med. 26:222–225. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tomi M, Zhao Y, Thamotharan S, Shin BC and
Devaskar SU: Early life nutrient restriction impairs blood-brain
metabolic profile and neurobehavior predisposing to Alzheimer's
disease with aging. Brain Res. 1495:61–75. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
An J, Wang J, Guo L, Xiao Y, Lu W, Li L,
Chen L, Wang X and Dong Z: The impact of gut microbiome on
metabolic disorders during catch-up growth in
small-for-gestational-age. Front Endocrinol (Lausanne).
12(630526)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee PA, Chernausek SD, Hokken-Koelega AC
and Czernichow P: International Small for Gestational Age Advisory
Board. International small for gestational age advisory board
consensus development conference statement: Management of short
children born small for gestational age, April 24-October 1, 2001.
Pediatrics. 111:1253–1261. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ong KK: Catch-up growth in small for
gestational age babies: Good or bad? Curr Opin Endocrinol Diabetes
Obes. 14:30–34. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eriksson JG, Forsén T, Tuomilehto J,
Winter PD, Osmond C and Barker DJ: Catch-up growth in childhood and
death from coronary heart disease: Longitudinal study. BMJ.
318:427–431. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Berends LM, Fernandez-Twinn DS,
Martin-Gronert MS, Cripps RL and Ozanne SE: Catch-up growth
following intra-uterine growth-restriction programmes an
insulin-resistant phenotype in adipose tissue. Int J Obes (Lond).
37:1051–1057. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ong KK, Ahmed ML, Emmett PM, Preece MA and
Dunger DB: Association between postnatal catch-up growth and
obesity in childhood: Prospective cohort study. BMJ. 320:967–971.
2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yan L, Wang Y, Zhang Z, Xu S, Ullah R, Luo
X, Xu X, Ma X, Chen Z, Zhang L, et al: Postnatal delayed growth
impacts cognition but rescues programmed impaired pulmonary
vascular development in an IUGR rat model. Nutr Metab Cardiovasc
Dis. 29:1418–1428. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rueda-Clausen CF, Morton JS and Davidge
ST: Effects of hypoxia-induced intrauterine growth restriction on
cardiopulmonary structure and function during adulthood. Cardiovasc
Res. 81:713–722. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kuo AH, Li C, Huber HF, Schwab M,
Nathanielsz PW and Clarke GD: Maternal nutrient restriction during
pregnancy and lactation leads to impaired right ventricular
function in young adult baboons. J Physiol. 595:4245–4260.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Mericq V, Martinez-Aguayo A, Uauy R,
Iñiguez G, Van der Steen M and Hokken-Koelega A: Long-term
metabolic risk among children born premature or small for
gestational age. Nat Rev Endocrinol. 13:50–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kelishadi R, Haghdoost AA, Jamshidi F,
Aliramezany M and Moosazadeh M: Low birthweight or rapid catch-up
growth: Which is more associated with cardiovascular disease and
its risk factors in later life? A systematic review and
cryptanalysis. Paediatr Int Child Health. 35:110–123.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z, Luo X, Lv Y, Yan L, Xu S, Wang Y,
Zhong Y, Hang C, Jyotsnav J, Lai D, et al: Intrauterine growth
restriction programs intergenerational transmission of pulmonary
arterial hypertension and endothelial dysfunction via sperm
epigenetic modifications. Hypertension. 74:1160–1171.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Luo X, Hang C, Zhang Z, Le K, Ying Y, Lv
Y, Yan L, Huang Y, Ye L, Xu X, et al: PVECs-derived exosomal
microRNAs regulate PASMCs via FoxM1 signaling in IUGR-induced
pulmonary hypertension. J Am Heart Assoc.
11(e027177)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lv Y, Tang LL, Wei JK, Xu XF, Gu W, Fu LC,
Zhang LY and Du LZ: Decreased Kv1.5 expression in intrauterine
growth retardation rats with exaggerated pulmonary hypertension. Am
J Physiol Lung Cell Mol Physiol. 305:L856–L865. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dabral S, Tian X, Kojonazarov B, Savai R,
Ghofrani HA, Weissmann N, Florio M, Sun J, Jonigk D, Maegel L, et
al: Notch1 signalling regulates endothelial proliferation and
apoptosis in pulmonary arterial hypertension. Eur Respir J.
48:1137–1149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Courboulin A, Barrier M, Perreault T,
Bonnet P, Tremblay VL, Paulin R, Tremblay E, Lambert C, Jacob MH,
Bonnet SN, et al: Plumbagin reverses proliferation and resistance
to apoptosis in experimental PAH. Eur Respir J. 40:618–629.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jimenez-Chillaron JC and Patti ME: To
catch up or not to catch up: Is this the question? Lessons from
animal models. Curr Opin Endocrinol Diabetes Obes. 14:23–29.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
National Research Council: Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press, Washington, DC, 2011.
|
|
26
|
Xu XF, Lv Y, Gu WZ, Tang LL, Wei JK, Zhang
LY and Du LZ: Epigenetics of hypoxic pulmonary arterial
hypertension following intrauterine growth retardation rat:
Epigenetics in PAH following IUGR. Respir Res.
14(20)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Barrow PC, Barbellion S and Stadler J:
Preclinical evaluation of juvenile toxicity. Methods Mol Biol.
691:17–35. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shokouhi G, Kosari-Nasab M and Salari AA:
Silymarin sex-dependently improves cognitive functions and alters
TNF-α, BDNF, and glutamate in the hippocampus of mice with mild
traumatic brain injury. Life Sci. 257(118049)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Olofinnade AT, Adeyeba A, Onaolapo AY and
Onaolapo OJ: An assessment of the effects of
azodicarbonamide-containing diet on neurobehaviour, brain
antioxidant status and membrane lipid peroxidation status in rats.
Cent Nerv Syst Agents Med Chem. 20:49–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sadiki FZ, Idrissi ME, Cioanca O, Trifan
A, Hancianu M, Hritcu L and Postu PA: Tetraclinis articulata
essential oil mitigates cognitive deficits and brain oxidative
stress in an Alzheimer's disease amyloidosis model. Phytomedicine.
56:57–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Abdulbasit A, Stephen Michael F, Shukurat
Onaopemipo A, Abdulmusawwir AO, Aminu I, Nnaemeka Tobechukwu A,
Wahab Imam A, Oluwaseun Aremu A, Folajimi O, Bilikis Aderonke A, et
al: Glucocorticoid receptor activation selectively influence
performance of Wistar rats in Y-maze. Pathophysiology. 25:41–50.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu YP, Zhu JJ, Cheng F, Jiang KW, Gu WZ,
Shen Z, Wu YD, Liang L and Du LZ: Ghrelin ameliorates
hypoxia-induced pulmonary hypertension via phospho-GSK3 β/β-catenin
signaling in neonatal rats. J Mol Endocrinol. 47:33–43.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luo F, Wang X, Luo X, Li B, Zhu D, Sun H
and Tang Y: Invasive hemodynamic assessment for the right
ventricular system and hypoxia-induced pulmonary arterial
hypertension in mice. J Vis Exp. 24:2019.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Wang Q, Shi W, Zhang Q, Feng W, Wang J,
Zhai C, Yan X and Li M: Inhibition of Siah2 ubiquitin ligase
ameliorates monocrotaline-induced pulmonary arterial remodeling
through inactivation of YAP. Life Sci. 242(117159)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jones R, Jacobson M and Steudel W:
alpha-smooth-muscle actin and microvascular precursor smooth-muscle
cells in pulmonary hypertension. Am J Respir Cell Mol Biol.
20:582–594. 1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Huang S, Yue Y, Feng K, Huang X, Li H, Hou
J, Yang S, Huang S, Liang M, Chen G and Wu Z: Conditioned medium
from M2b macrophages modulates the proliferation, migration, and
apoptosis of pulmonary artery smooth muscle cells by deregulating
the PI3K/Akt/FoxO3a pathway. PeerJ. 8(e9110)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nie X, Dai Y, Tan J, Chen Y, Qin G, Mao W,
Zou J, Chang Y, Wang Q and Chen J: α-Solanine reverses pulmonary
vascular remodeling and vascular angiogenesis in experimental
pulmonary artery hypertension. J Hypertens. 35:2419–2435.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ye L, Wang X, Cai C, Zeng S, Bai J, Guo K,
Fang M, Hu J, Liu H, Zhu L, et al: FGF21 promotes functional
recovery after hypoxic-ischemic brain injury in neonatal rats by
activating the PI3K/Akt signaling pathway via FGFR1/β-kloth. Exp
Neurol. 317:34–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Prado EL and Dewey KG: Nutrition and brain
development in early life. Nutr Rev. 72:267–284. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bond Allison M, Ming G-l and Song H: Adult
mammalian neural stem cells and neurogenesis: Five decades later.
Cell Stem Cell. 17:385–395. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wojtowicz JM and Kee N: BrdU assay for
neurogenesis in rodents. Nat Protoc. 1:1399–1405. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bateson P, Gluckman P and Hanson M: The
biology of developmental plasticity and the predictive adaptive
response hypothesis. J Physiol. 592:2357–2368. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Amruta N, Kandikattu HK and Intapad S:
Cardiovascular dysfunction in intrauterine growth restriction. Curr
Hypertens Rep. 24:693–708. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gortner L: Intrauterine growth restriction
and risk for arterial hypertension: A causal relationship? J
Perinat Med. 35:361–365. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Leunissen RWJ, Kerkhof GF, Stijnen T and
Hokken-Koelega ACS: Effect of birth size and catch-up growth on
adult blood pressure and carotid intima-media thickness. Horm Res
Paediatr. 77:394–401. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Teichert-Kuliszewska K, Tsoporis JN,
Desjardins JF, Yin J, Wang L, Kuebler WM and Parker TG: Absence of
the calcium-binding protein, S100A1, confers pulmonary hypertension
in mice associated with endothelial dysfunction and apoptosis.
Cardiovasc Res. 105:8–19. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Perrella MA, Edell ES, Krowka MJ, Cortese
DA and Burnett JC Jr: Endothelium-derived relaxing factor in
pulmonary and renal circulations during hypoxia. Am J Physiol.
263:R45–R50. 1992.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dinh-Xuan AT: Endothelial modulation of
pulmonary vascular tone. Eur Respir J. 5:757–762. 1992.PubMed/NCBI
|
|
51
|
Smith AP, Demoncheaux EA and Higenbottam
TW: Nitric oxide gas decreases endothelin-1 mRNA in cultured
pulmonary artery endothelial cells. Nitric Oxide. 6:153–159.
2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Thompson AAR and Lawrie A: Targeting
vascular remodeling to treat pulmonary arterial hypertension.
Trends Mol Med. 23:31–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ruopp NF and Cockrill BA: Diagnosis and
treatment of pulmonary arterial hypertension: A review. JAMA.
327:1379–1391. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Budhiraja R, Tuder RM and Hassoun PM:
Endothelial dysfunction in pulmonary hypertension. Circulation.
109:159–165. 2004.PubMed/NCBI View Article : Google Scholar
|