Introduction
Primary liver cancer is currently the 4th most
common malignant tumor and the 2nd leading cause of cancer-related
death in China. Hepatocellular carcinoma (HCC) accounts for 75-85%
of primary liver cancers. Programmed death-1 (PD-1), programmed
death ligand-1 (PD-L1) and other immune checkpoint inhibitors
(ICIs) are used more extensively in cancer therapy by interfering
with the immune checkpoint pathway to activate the immune system.
However, PD-1 and PD-L1 are also widely expressed in normal tissue
cells e.g. hematopoietic cells, pancreatic cells (1). Therefore, ICI therapy affects other
tissue cells and has also caused a number of immune-related adverse
events (irAEs), including a variety of endocrine disorders, such as
thyroiditis, hypopituitarism and adrenal insufficiency (2). ICI-induced type 1 diabetes mellitus
(T1DM) is an irAE with an incidence of 0.2-1.0% (3). A systematic review study demonstrated
that in 172 ICI-induced DM (ICI-DM) cases, tumor types included
melanoma (43.6%; 75/172), lung cancer (30.2%; 52/172), renal cell
carcinoma (5.8%; 10/172), breast cancer (3.5%; 6/172),
gastrointestinal cancers (3.5%; 6/172), lymphomas (2.9%; 5/172) and
hepatocellular carcinoma (1.2%; 2/172) (4). Current research also suggests that
the primary mechanism of ICI-induced T1DM is T-cell stimulation due
to the loss of interaction between PD-1 and PD-L1 in pancreatic
islets (5). A latest study
reported the first case of a patient with HCC who developed
fulminant T1DM and ketoacidosis during the therapeutic combination
of atilizumab and bevacizumab (6).
ICIs have been widely used to treat HCC for a relatively short
period of time and there is a lack of reports on the occurrence of
DM in patients with HCC treated with ICIs. Therefore, information
on such cases needs to be accumulated. The present study reported
three cases of immune-associated DM after ICI treatment for HCC.
Furthermore, the clinical attributes, epidemiology and primary
mechanism of ICI-DM were reviewed, so as to draw attention of
clinicians to this disease and, more importantly, its diagnosis and
treatment.
Case presentation
Case 1
A 27-year-old female patient was admitted to Tongji
Hospital (Wuhan, China) in January 2021 for detection of multiple
tumors in the left lobe of the liver. The patient had a history of
hepatitis B virus (HBV) infection >20 years and had never
received any antiviral treatment. The patient did not have any
complaints or discomfort and had no family or genetic history of
HCC except HBV infection of the patient's mother. There was no
positive sign such as hepatosplenomegaly or tenderness on physical
examination. Blood routine and liver-renal function laboratory
tests were normal but the biochemical examinations revealed an AFP
level of 37,966 µg/l [normal range (NR), 0-15 µg/l], elevated (↑),
and a protein induced by vitamin K absence (PIVKA-II) level of
5,834 mAU/ml (NR, 11.12-32.01 mAU/ml) ↑ (Table I). Furthermore, the MRI showed
multiple tumors in the left lobe of the liver and a tumor embolus
of the left branch of the portal vein was visible (Fig. 1). Therefore, the preliminary
diagnosis was as follows: i) Chronic hepatitis B with compensated
liver function and ii) primary HCC, China Liver Cancer (CNLC) stage
IIIa/Barcelona Clinic Liver Cancer (BCLC) stage C (7). The patient received local treatment
with drug-eluting bead transarterial chemoembolization (D-TACE) and
systemic therapy with lenvatinib 8 mg/day and tislelizumab 200 mg/3
weeks, 21 days/cycle. After one month, the MRI showed that the
patient had achieved a partial response (PR) (Fig. 1) according to the modified response
evaluation criteria in solid tumors 1.1(8) and a tumor biomarker decline. The
patient then received 4 cycles of hepatic artery infusion
chemotherapy (HAIC) and systemic therapy. The subsequent
examination showed a complete response (Fig. 1) and the tumor biomarkers had
declined to normal levels. The multidisciplinary team (MDT) refused
surgery due to inadequate residual liver volume and recommended
that the patient continued systemic therapy. However, after 6
months, contrast-enhanced ultrasonography and circulating tumor DNA
(ctDNA) analysis (9) provided
positive results, although the MRI still showed complete tumor
necrosis. Furthermore, the residual liver volume was sufficient for
operation due to left liver enlargement (Fig. S1), the liver function was
Child-Pugh grade A (10) and the
indocyanine green 15-min retention rate was 10.7% (11). Thus, right hemihepatectomy was
performed and the pathological examination showed absence of viable
cancer cells (Fig. 1). One month
after the operation, as the ctDNA analysis turned to negative and
no tumor recurrence was found in examinations including AFP,
PIVKA-II and MRI, the patient continued the systemic therapy and
received a routine examination where diabetes mellitus was
excluded. In October 2022, the patient, who had by then received 88
weeks (cycle 30) of ICI therapy, was readmitted to Tongji Hospital
(Wuhan, China) due to symptoms/complaints of nausea, vomiting,
fever and lethargy. The patient's family denied a history of
diabetes. The auxiliary examination revealed the following:
Diabetes tests: Random plasma glucose 1,082 mg/dl (NR, 70.2-199.8
mg/dl) ↑, glycated hemoglobin (HbA1c) 9.8% (NR, 4-6%) ↑, fasting
C-peptide 0.04 ng/ml (NR, 0.3-1.3 ng/ml) decreased (↓), urine
glucose (3+) and urine ketone body (3+); electrolyte examination:
Blood sodium 153.4 mmol/l (NR, 135-145 mmol/l) ↑, blood potassium
3.4 mmol/l (NR, 3.5-5.5 mmol/l) ↓, blood chlorine 107.5 mmol/l (NR,
98-106 mmol/l) ↑, effective plasma osmotic pressure 373.6 mOsm/l
(NR, 280-310 mOsm/l) ↑; which suggested that the patient had
diabetic ketoacidosis (DKA) and was in a hyperosmolar hyperglycemic
state. Islet autoantibodies were as follows: Islet cell antibody
(ICA) (+), glutamic acid decarboxylase antibody (GADA) (-), insulin
autoantibody (IAA) (-), protein tyrosine phosphatase autoantibody
(IA-2A) (-) and zinc transporter 8 autoantibody (ZnT8A) (-)
(Table II). Thyroid function
markers were as follows: Triiodothyronine (T3), 1.08 nmol/l (NR,
0.92-2.79 nmol/l); free triiodothyronine, 2.94 pmol/l (NR, 3.5-6.5
pmol/l); tetraiodothyronine (T4), 78.50 nmol/l (NR, 58.1-140.6
nmol/l); free thyroxine, 12.54 pmol/l (NR, 11.48-22.70 pmol/l); and
thyroid-stimulating hormone, 24.34 µIU/ml (NR, 2-10 µIU/ml) ↑,
which suggested that the patient had mild hypothyroidism. The
diagnosis included the following: i) DKA, ii) hyperosmolar
hyperglycemic state and iii) mild hypothyroidism. The patient was
diagnosed with ICI-DM. After ketone correction and insulin pump
therapy, the patient's ketone bodies turned negative and the
patient's treatment was switched to 4 times of intensive insulin
therapy with acceptable glycemic control. Approximately 2 months
later, the patient continued the immunological treatment when blood
glucose stability had been reached with insulin management. MRI
suggested that there was no tumor recurrence until the final
follow-up for the writing of this study in July 2023.
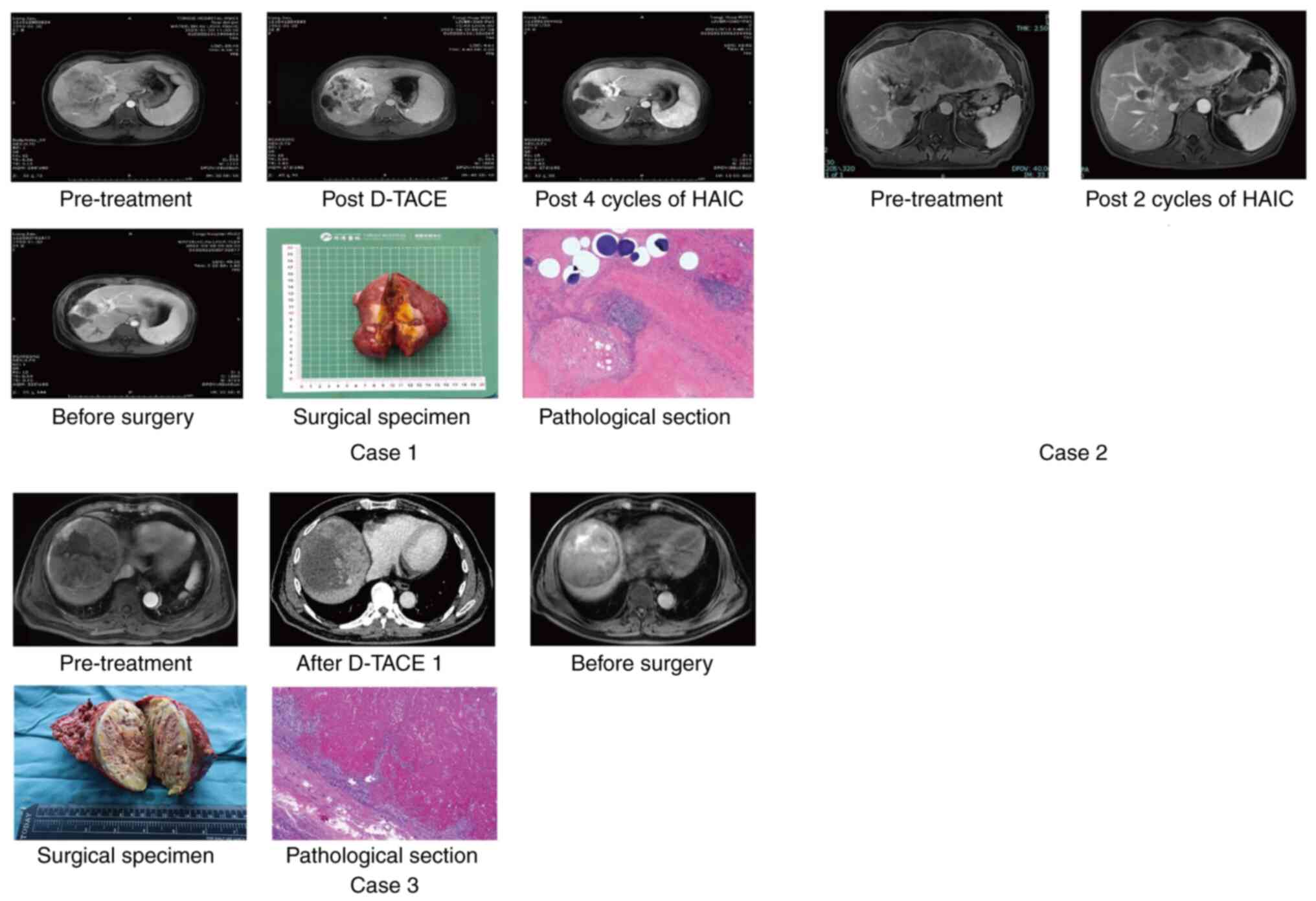 | Figure 1Treatment response of the three
cases. Case 1, female, 27 years old; HCC with CNLC stage IIIa,
multiple tumors in the left lobe of the liver and a tumor embolus
of the left branch of the portal vein was visible; received 1 cycle
of D-TACE and 4 cycles of HAIC with systemic therapy and achieved
CR by mRECIST; received surgery and got pathological response of
CR. Case 2, male, 56 years old; HCC with CNLC stage IIIa, multiple
tumors in the right lobe of the liver and a tumor embolus of the
right branch of the portal vein was visible; received 2 cycles of
HAIC with systemic therapy and achieved PR by mRECIST. Case 3,
male, 67 years old; HCC with CNLC stage IIa, two tumors in the
right lobe of the liver and the maximum tumor diameter was 8.9 cm;
received 1 cycle of D-TACE with systemic therapy and achieved PR by
mRECIST; received surgery and got pathological response of tumor
necrosis >80%. The pathological images was magnified 200 times.
HCC, hepatocellular carcinoma; CNLC, China Liver Cancer; D-TACE,
drug-eluting bead transarterial chemoembolization; HAIC, hepatic
artery infusion chemotherapy; PR, partial response; CR, complete
response; mRECIST, modified response evaluation criteria in solid
tumors. |
 | Table IClinicopathological characteristics
of the patients. |
Table I
Clinicopathological characteristics
of the patients.
| Variable | Case 1 | Case 2 | Case 3 |
|---|
| Age, years | 27 | 56 | 67 |
| Sex | Female | Male | Male |
| BMI,
kg/m2 | 20.4 | 23.3 | 25.7 |
| Family history of
DM | No | No | No |
| History of DM | No | No | No |
| Etiology of
HCC | HBV | HBV | HBV |
| Cirrhosis | No | No | No |
| Child-Pugh | A | A | A |
| AFP, µg/l (NR,
0-15) | 37966 ↑ | 5834 ↑ | 25342 ↑ |
| PIVKA, mAU/ml (NR,
11.12-32.01) | 422 ↑ | ND | 24245 ↑ |
| Tumor size, cm | 9.1 | 7.8 | 8.9 |
| Tumor number,
n | 2 | 3 | 2 |
| Portal vein tumor
thrombus | Yes | Yes | No |
| Tumor
differentiation | Moderate | Moderate | Well |
| BCLC stage | C | C | B |
| CNLC stage | IIIa | IIIa | IIa |
| Treatment
regimen |
Lenvatinib+tislelizumab |
Apatinib+camrelizumab | Tislelizumab |
| Local
treatment | D-TACE+HAIC | HAIC | D-TACE |
| Tumor response | Complete
response | Partial
response | Partial
response |
| Operation | Yes | No | Yes |
 | Table IILaboratory test results at admission
to hospital for DM. |
Table II
Laboratory test results at admission
to hospital for DM.
| Variable | Case 1 | Case 2 | Case 3 |
|---|
| Number of ICI
treatment cycles | 35 | 6 | 12 |
| Time of onset of
ICI-DM, weeks | 88 | 9 | 12 |
| Other irAEs | Hypothyroidism | RCCEP | No |
| HbA1c, % (NR,
4-6) | 9.8 ↑ | 7.7 ↑ | 7.2 ↑ |
| Casual BG, mg/dl
(NR, 70.2-199.8) | 1082 ↑ | 432 ↑ | 454 ↑ |
| C-peptide, ng/ml
(NR, 0.3-1.3) | 0.04 ↓ | 0.09 ↓ | 0.08 ↓ |
| Na+,
mmol/l (NR, 135-145) | 153.4 ↑ | 131.4↓ | 130.8↓ |
| K+,
mmol/l (NR, 3.5-5.5) | 3.4 ↓ | 4.5 | 4.8 |
| Cl+,
mmol/l (NR, 98-106) | 107.5 ↑ | 104.4 | 106.2 ↑ |
| Diabetic
ketoacidosis | Yes | No | No |
| GADA | - | + | - |
| ICA | + | - | - |
| IAA | - | - | - |
| IA-2A | - | - | - |
| ZnT8A | - | - | - |
| Continuation of
ICIs | Yes | Yes | Yes |
| Tumor response
after rechallenge | Stable disease | Partial
response | Stable disease |
Case 2
A 56-year-old male patient was admitted to Tongji
Hospital (Wuhan, China) in December 2022 for detection of multiple
tumors in the right lobe of the liver. The patient had a history of
HBV infection for 10 years and had not received any treatment. AFP
and PIVKA-II were 5,834 µg/l ↑ and 25,243 mAU/ml ↑, respectively
(Table I). The MRI showed multiple
tumors in the right lobe of the liver and a tumor embolus of the
right branch of the portal vein was visible (Fig. 1). The preliminary diagnosis was as
follows: i) Chronic hepatitis B with compensated liver function and
ii) primary HCC and CNLC IIIa/BCLC C. The patient received local
treatment with HAIC and systemic therapy with lenvatinib 8 mg/day
and tislelizumab 200 mg/3 weeks, 21 days/cycle. After 2 cycles, the
MRI indicated that the patient achieved a PR with partial tumor
necrosis (Fig. 1) and a tumor
biomarker decline. At the same time, the patient complained of mild
reactive cutaneous capillary endothelial proliferation, which was
alleviated by application of steroid hormone cream without drug
withdrawal. The patient then received the third treatment cycle.
When the patient was admitted to Tongji Hospital (Wuhan, China) for
the fourth treatment cycle, the routine examination indicated the
following: Body mass index, 23.3 kg/m2 (NR, 18.5-23.9
kg/m2); diabetes tests: HbA1c, 7.7% ↑, blood glucose,
432 mg/dl ↑, C-peptide, 0.09 ng/ml ↓, urine glucose (2+) and urine
ketone body (-), which suggested that the patient was in a
hyperglycemic state. Electrolyte examination showed as following:
Blood sodium, 131.4 mmol/l ↓; blood potassium, 4.5 mmol/l; blood
chlorine, 104.4 mmol/l; and effective plasma osmotic pressure,
295.8 mOsm/l. Islet autoantibodies were as follows: GADA (+), ICA
(-), IAA (-), IA-2A (-) and ZnT8A (-) (Table II). T3 and T4 levels were normal;
thyroid autoantibodies were negative and no other endocrine system
adverse reactions were found. The patient recovered one week after
intensive treatment with an insulin pump. The patient achieved a
stable level of blood glucose after two weeks and received low-dose
rapid-acting insulin. The patient was diagnosed ICI-DM.
Approximately 1.5 months later, the patient continued the
immunological treatment and the latest MRI in July 2023 showed a
PR.
Case 3
A 67-year-old male patient was admitted to Tongji
Hospital (Wuhan, China) in July 2022 due to detection of a
liver-occupying lesion in a routine health examination. The patient
had a history of HBV infection for 30 years and received anti-HBV
treatment for 10 years. The AFP and PIVKA-II were 25,342 µg/l ↑ and
24,245 mAU/ml ↑, respectively (Table
I). The MRI showed two tumors in the right lobe of the liver
and the maximum tumor diameter was 8.9 cm (Fig. 1). The preliminary diagnosis was as
follows: i) Chronic hepatitis B with compensated liver function and
ii) primary HCC, CNLC IIa/BCLC B. Surgery was rejected by the MDT
due to high risk of recurrence and conversion therapy with D-TACE
and tislelizumab was performed. One month later, the examination
results showed a PR (Fig. 1) and
significant tumor marker decline. The tumor situation of the
patient was reassessed and discussed by the MDT and surgery was
finally recommended. Right hemihepatectomy was successfully
performed and the pathological examination showed tumor necrosis
>80% (Fig. 1). One month after
the operation, the patient continued Tislelizumad therapy for
recurrence prevention. However, when the patient came to the
outpatient department for routine follow-up examinations 2 months
postoperatively and he had received 4 cycles of Tislelizumad
therapy, the results showed the following: Diabetes tests: Blood
glucose, 454 mg/dl ↑; HbA1c, 7.2% ↑; C-peptide, 0.08 ng/ml↓urine
glucose (2+); urine ketone body (-), which showed that the patient
was in a hyperglycemic state; electrolyte examination: Blood sodium
130.8 mmol/l ↓, blood potassium 4.8 mmol/l, blood chlorine 106.2
mmol/l ↑, effective plasma osmotic pressure 296.4 mOsm/l; and
negativity for all islet autoantibodies (Table II). T3 and T4 levels were normal;
thyroid autoantibodies were negative and no other endocrine system
adverse reactions were found. With intensive treatment using the
insulin pump, the blood glucose declined to normal levels and
remained stable. The patient then received low-dose rapid-acting
insulin. The patient was diagnosed ICI-DM. One month later, the
patient continued immunological treatment and according to the
latest MRI in July 2023, no tumor recurrence occurred.
Discussion
ICIs are significant in the history of cancer
treatment. A review by Ribas and Wolchok (12) summarized that the objective
response rate with ICI therapy in patients with Hodgkin's disease,
skin melanoma, non-small cell lung cancer, renal cell carcinoma and
HCC is 87, 35-40, 20, 25 and 20% respectively. In the tumor
microenvironment, the PD-L1 expressed on tumor-associated
macrophages, the PD-1 expressed during T and B lymphocyte
activation and the cytotoxic T lymphocyte-associated antigen-4
(CTLA-4) expressed on T-regulatory cells, are all involved in
regulating T-cell activity by T cell receptor signaling (13-15).
ICIs such as anti-PD-1 and anti-PD-L1 inhibitors, can revitalize
the anti-tumor function of immune cells by blocking the activation
of inhibitory immune checkpoints, which, however, enhances the
specific response from effector T cells to non-tumor tissues
(16). The decrease of peripheral
immune tolerance and increase of pro-inflammatory factor release
when regulatory T cells are suppressed contribute to the
development of irAEs (17). ICIs
can cause toxic damage to numerous organs and systems, including
the skin, gastrointestinal tract, musculoskeletal and oculus, the
endocrine system, the nervous system, lung, kidney and the
cardiovascular and hematologic systems (18). A systematic review showed that 14%
of patients treated with PD-(L)1 inhibitor, 34% of patients treated
with CTLA-4 inhibitors and 55% of patients on ICI combinations had
irAEs (Grade ≥3) (19). Certain
studies observed a negative impact of irAEs-related treatment
discontinuation on survival. Naqash et al (20) found that patients with permanent
ICI discontinuation due to irAEs had a 14 months shorter median
overall survival compared to those who did not have permanent ICI
discontinuation.
HCC is a typical inflammation-associated malignancy
with a complex immune microenvironment (21). Chronic HBV infection and chronic
hepatitis C virus infection create a tolerogenic immune
microenvironment through T-cell exhaustion (loss of antiviral
effector function of virus-specific CD8+ T cells) and
viral escape mutations (21,22).
ICIs, including PD-1, PD-L1 and CTLA-4, have demonstrated
significant therapeutic efficacy in the field of HCC treatment
(23). The results of one study
(CheckMate 040) showed that treatment with navulizumab
significantly reduced tumors, with objective remission rates of
15-20% (24). Pembrolizumab showed
similar results to those of navulizumab, with an overall remission
rate of 14% (25). HCC is often
combined with cirrhosis and systemic manifestations, and patients
with extrahepatic organ dysfunction may exhibit signs and symptoms
that overlap with irAEs or aggravate the severity of irAEs
(2). Furthermore, irAEs leading to
discontinuation of ICIs were also reported in 14.9% of HCC patients
receiving immune-targeted therapy (n=327/2201, 95% CI: 13.4-16.4%),
including fatigue (13.9%), diarrhea (10.2%), rash (10.0%), pruritus
(9.9%) and decreased appetite (8.5%) (26). In addition, the probability of
irAEs may be higher in patients with HCC receiving ICI combined
therapy (27). Three cases
reported had received lenvatinib and tislelizumab, apatinib and
camrelizumab, and tislelizumab, respectively.
Similar to T1DM, ICI-DM is caused by endocrine
toxicity due to ICI therapy. ICI-DM may cause lifelong persistent
insulin deficiency, increase risks associated with diabetes
complications and decrease life expectancy (28), indicating that ICI-DM should be
emphasized in clinical practice. A previous study reported on T1DM
caused by autoreactive T cell-mediated β-cell destruction (29). PD-1 and PD-L1 had inhibitory
effects on pathogenic autoreactive CD4+ T cell-mediated
tissue destruction and effector cytokine production (1,30).
PD-1 and PD-L1 deficiency accelerated the onset and frequency of
type I diabetes in non-obese diabetic mice (29,31).
Lysogenic IFN-γCD8+ T cells infiltrated pancreatic
islets in islet sections from anti-PD-1-treated patients and IFN-γ
activated the β-cell apoptotic pathway (32). In vitro experiments using
human pancreatic islets from non-diabetic patients showed that
IFN-γ promotes β-cell PD-L1 expression, which may act as a
self-defense by expressing PD-L1 in response to IFN-γ (33). Therefore, blocking the PD-1/PD-L1
pathway in ICI-treated patients may contribute to the development
of ICI-DM for aggravating the destruction of β-cells.
ICI-DM is a relatively rare but severe irAE with an
incidence of 0.86% (261/30,337 patients) (34). Furthermore, 59% of patients with
ICI-DM were complicated with DKA (35). The median age at the onset of
ICI-DM was determined to be 61 years (36). The combination therapy resulted in
an increased risk of immune-related DM compared to a single one
(37). The mean time of onset of
ICI-DM was 8.14 weeks (full range, 3.6-45 weeks) (38). One of the three patients reported
in the present study had an onset of the disease at week 88 (cycle
30 of ICI treatment) and was accompanied with DKA. The other two
patients developed ICI-DM at weeks 9 (cycle 3 of ICI treatment) and
week 12 (cycle 4 of ICI treatment), respectively, and this was not
accompanied with DKA. The clinical manifestations of ICI-DM are
atypical and vary significantly among individuals, with mild cases
showing only elevated blood glucose or severe cases showing acute
onsets, rapid progression and even DKA (39). In case 1 of the present study, the
patient presented with nausea, vomiting, fever and lethargy as
symptoms of DKA, while the other two patients were diagnosed ICI-DM
after laboratory tests during routine follow-up examinations.
According to a previous study, 44.8% of ICI-DM cases had damage to
other endocrine glands in addition to diabetes, including
hypophysitis (5.2%) and thyroiditis (30.8%) (4). In case 1 of the present study, the
pathology was accompanied by DKA and thyroiditis.
The diagnosis can be made if the patient's blood
glucose is normal before the use of ICIs and one of the following
three conditions is met after treatment: i) Typical diabetic
symptoms (thirst, increased fluid intake, urination and weight loss
caused by hyperglycemia) or acute metabolic disorders, such as
itching of the skin and blurred vision, as well as random glucose
≥11.1 mmol/l; ii) fasting plasma glucose (FPG) ≥7.0 mmol/l; iii)
2-h blood glucose after 75 g glucose load ≥11.1 mmol/l (39). Furthermore, in the cases reported
in the present study, C-peptide levels were 0.04, 0.09 and 0.08
nmol/l, respectively, which were <0.4 nmol/l in 91.6% of ICI-DM
patients according to Wu et al (28). Table
III provides certain differences and associations between
ICI-DM and T1DM and T2DM (28,40,41),
which is utilized to make differential diagnoses.
 | Table IIIDifferentiation among ICI-DM, T1DM
and T2DM. |
Table III
Differentiation among ICI-DM, T1DM
and T2DM.
| Variable | ICI-DM | T1DM | T2DM | (Refs.) |
|---|
| Age, median (IQR),
years | 63.6
(57.8-72.9) | 37.1
(27.0-51.5) | 63.8
(53.4-74.6) | (41) |
| HbA1c at first
presentation, median (IQR), % (NR, 4-6) | 10.1 (8.0-12.5)
↑ | 10.6(10.1-12.1)
↑ | 7.5 (6.3-10.1)
↑ | (41) |
| Insulin dose,
median (IQR), IU/kg/day | 0.39
(0.35-0.50) | 0.35
(0.21-0.52) | 0.31
(0.18-0.51) | (41) |
| DKA at
manifestation, % | 26.7 | 0 | 0.4 | (41) |
| Pancreatic
autoantibodies, % | 40.4(28) | 90 | NA | (28) |
| C-peptide levels,
nmol/l (NR, 0.3-1.3) | <0.3 ↓ [63.4%
(n=83)] | <0.3 ↓ | Normal or
excessive | (40) |
| Onset | Early or latent, or
after the interruption of ICIs | Acute | Slow | (28) |
| Pancreatic
enzymes | Mild increase | Lower lipase except
in fulminant phenotype | NA | (28) |
Currently, the human leukocyte antigen (HLA)
genotype and islet autoantibodies are considered useful for early
identification of patients who are more susceptible to ICI-DM.
HLA-DR4 (a HLA serotype) showed the highest association with
susceptibility to ICIs-DM (42).
In a cohort study, 76% of patients with ICI-DM expressed
HLA-DR4(35). The patients in the
present study did not undergo HLA genetic testing. Islet
autoantibodies were considered a marker of T1DM and were detected
in >90% of patients with T1DM in a previous study (43). de Filette et al (44) reported that at least one of the
islet autoantibodies was positive in 53% of patients with TIDM and
15% of them had at least two positive autoantibodies. However, the
association between islet autoantibodies and diagnosis of ICI-DM
remains unclear (45). In the case
series reported in the present study, ICA in case 1 was positive
for DKA, while GADA was positive in case 2 and the patient from
case 3 was negative for autoantibodies.
According to the Expert Consensus on Immune-related
Adverse Reactions of the Endocrine System Caused by Immune
Checkpoint Inhibitor (39), ICI-DM
can be classified into 4 grades according to the severity of
clinical symptoms and the level of FPG. According to this
consensus, Case 1 may be classified as level 4 (FPG >27.8
mmol/l) and cases 2 and 3 are classified as level 3 (FPG is from
13.9 to 27.8 mmol/l). For grade 2 (FPG is from 8.9 to 13.9 mmol/l)
and above, ICI treatment needs to be suspended until the blood
glucose is controlled. Insulin therapy should be applied promptly
for grade 3 and above, as well as for individuals with an acute
increase in blood glucose or suspected ketosis. In the present case
series, insulin therapy was used in all of the three patients when
ICI-DM was diagnosed and the level of blood glucose was rapidly
controlled under effective management. Furthermore, ICI treatment
was suspended for all these three patients, which, however, was
continued when blood glucose stability had been achieved with
insulin management 1-2 months later. No severe irAEs were noted
after the continuation of ICI treatment and no tumor recurrence or
progression occurred.
In ICI-DM, β-cell damage was irreversible, patients
required lifelong medication and steroids had no therapeutic effect
on it (4). The main focus should
be on the treatment with insulin injections and symptomatic
supportive therapy (46). Blood
glucose monitoring should be performed before each treatment cycle
and every 3-6 weeks after the end of treatment (39). Patient education on early
recognition of DM symptoms and DKA symptoms is also an important
management option for ICI-DM (40). Ultimately, ICI rechallenge is
feasible with good glycemic control (4).
In conclusion, ICI-DM is a rare but potentially
fatal irAE, as DKA is often the first manifestation. Patient
education and clinicians' awareness of adverse effects associated
with ICIs are good management options. A thorough evaluation is
needed to determine the likelihood of ICI-DM before starting ICI
therapy, including the patient's general condition, history of
previous immune disorders, laboratory tests and radiologic
examinations. Blood glucose, C-peptide levels and HbA1c are
practical screening options. At present, various therapeutic
approaches combined with ICI therapy are gradually becoming the
mainstream treatment for HCC and immunotherapy should not be easily
abandoned because of potential irAEs. Adequate clinical judgment,
close monitoring and early detection of irAEs are needed to decide
whether to continue immunotherapy or to rechallenge it according to
the combination of grading and patient condition, which aims to
achieve the maximum benefit of clinical treatment.
Supplementary Material
Remnant liver volume analysis for Case
1. The volume of the liver was calculated after three-dimensional
reconstruction.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant no. 82003403).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conception and design of the study: GW and JZ.
Acquisition of data: JW and SD. Collection of relevant articles: ZZ
and WZ. Data analysis, drafting of manuscript and critical
revision: GW. ZZ and WZ checked and confirm the authenticity of the
raw data. All authors contributed to the article and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics Review
Board of Tongji Hospital (Wuhan, China; approval no.
TJ-IRB20210935). Written informed consent for clinical research on
the data generated during therapy was obtained from all enrolled
patients.
Patient consent for publication
Written consent for the publication of potentially
identifiable patient/clinical data and/or images was obtained from
all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Keir ME, Liang SC, Guleria I, Latchman YE,
Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH and Sharpe
AH: Tissue expression of PD-L1 mediates peripheral T cell
tolerance. J Exp Med. 203:883–895. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan
X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, et al:
Treatment-related adverse events of PD-1 and PD-L1 inhibitors in
clinical trials: A systematic review and meta-analysis. JAMA Oncol.
5:1008–1019. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wright JJ, Salem JE, Johnson DB,
Lebrun-Vignes B, Stamatouli A, Thomas JW, Herold KC, Moslehi J and
Powers AC: Increased reporting of immune checkpoint
inhibitor-associated diabetes. Diabetes Care. 41:e150–e151.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu J, Shi Y, Liu X, Zhang D, Zhang H,
Chen M, Xu Y, Zhao J, Zhong W and Wang M: Clinical characteristics
and outcomes of immune checkpoint inhibitor-induced diabetes
mellitus. Transl Oncol. 24(101473)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cho YK and Jung CH: Immune-checkpoint
inhibitors-induced type 1 diabetes mellitus: From Its molecular
mechanisms to clinical practice. Diabetes Metab J. 47:757–766.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ikeda M, Tamada T, Takebayashi R, Okuno G,
Yagura I, Nakamori S, Matsumura T, Yoshioka T, Kaneko S and Kanda
N: Development of fulminant type 1 diabetes mellitus in the course
of treatment with atezolizumab for hepatocellular carcinoma. Intern
Med. 62:1775–1779. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xie DY, Ren ZG, Zhou J, Fan J and Gao Q:
2019 Chinese clinical guidelines for the management of
hepatocellular carcinoma: Updates and insights. Hepatobiliary Surg
Nutr. 9:452–463. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee JS, Choi HJ, Kim BK, Park JY, Kim DY,
Ahn SH, Han KH, Baek SE, Chung YE, Park MS, et al: The modified
response evaluation criteria in solid tumors (RECIST) yield a more
accurate prognoses than the RECIST 1.1 in hepatocellular carcinoma
treated with transarterial radioembolization. Gut Liver.
14:765–774. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Pascual J, Attard G, Bidard FC, Curigliano
G, De Mattos-Arruda L, Diehn M, Italiano A, Lindberg J, Merker JD,
Montagut C, et al: ESMO recommendations on the use of circulating
tumour DNA assays for patients with cancer: a report from the ESMO
precision medicine working group. Ann Oncol. 33:750–768.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peng Y, Qi X and Guo X: Child-Pugh versus
MELD score for the assessment of prognosis in liver cirrhosis: A
systematic review and meta-analysis of observational studies.
Medicine (Baltimore). 95(e2877)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Le Roy B, Grégoire E, Cossé C, Serji B,
Golse N, Adam R, Cherqui D, Mabrut JY, Le Treut YP and Vibert E:
Indocyanine green retention rates at 15 min predicted hepatic
decompensation in a western population. World J Surg. 42:2570–2578.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park DJ, Sung PS, Lee GW, Cho S, Kim SM,
Kang BY, Hur W, Yang H, Lee SK, Lee SH, et al: Preferential
expression of programmed death ligand 1 protein in tumor-associated
macrophages and its potential role in immunotherapy for
hepatocellular carcinoma. Int J Mol Sci. 22(4710)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rowshanravan B, Halliday N and Sansom DM:
CTLA-4: A moving target in immunotherapy. Blood. 131:58–67.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
He X and Xu C: Immune checkpoint signaling
and cancer immunotherapy. Cell Res. 30:660–669. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Passat T, Touchefeu Y, Gervois N, Jarry A,
Bossard C and Bennouna J: Physiopathological mechanisms of
immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and
anti-PD-L1 antibodies in cancer treatment. Bull Cancer.
105:1033–1041. 2018.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
18
|
Brahmer JR, Abu-Sbeih H, Ascierto PA,
Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E,
Johnson DB, et al: Society for immunotherapy of cancer (SITC)
clinical practice guideline on immune checkpoint inhibitor-related
adverse events. J Immunother Cancer. 9(e002435)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arnaud-Coffin P, Maillet D, Gan HK,
Stelmes JJ, You B, Dalle S and Péron J: A systematic review of
adverse events in randomized trials assessing immune checkpoint
inhibitors. Int J Cancer. 145:639–648. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Naqash AR, Ricciuti B, Owen DH, Florou V,
Toi Y, Cherry C, Hafiz M, De Giglio A, Muzaffar M, Patel SH, et al:
Outcomes associated with immune-related adverse events in
metastatic non-small cell lung cancer treated with nivolumab: A
pooled exploratory analysis from a global cohort. Cancer Immunol
Immunother. 69:1177–1187. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Llovet JM, Castet F, Heikenwalder M, Maini
MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS:
Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol.
19:151–172. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang CY, Liu S and Yang M: Regulatory T
cells and their associated factors in hepatocellular carcinoma
development and therapy. World J Gastroenterol. 28:3346–3358.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Callahan MK, Postow MA and Wolchok JD:
Targeting T cell co-receptors for cancer therapy. Immunity.
44:1069–1078. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ziogas IA, Evangeliou AP, Giannis D, Hayat
MH, Mylonas KS, Tohme S, Geller DA, Elias N, Goyal L and Tsoulfas
G: The role of immunotherapy in hepatocellular carcinoma: A
systematic review and pooled analysis of 2,402 patients.
Oncologist. 26:e1036–e1049. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nikoo M, Hassan ZF, Mardasi M,
Rostamnezhad E, Roozbahani F, Rahimi S and Mohammadi J:
Hepatocellular carcinoma (HCC) immunotherapy by anti-PD-1
monoclonal antibodies: A rapidly evolving strategy. Pathol Res
Pract. 247(154473)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu L, Tsang V, Menzies AM, Sasson SC,
Carlino MS, Brown DA, Clifton-Bligh R and Gunton JE: Risk factors
and characteristics of checkpoint inhibitor-associated autoimmune
diabetes mellitus (CIADM): A systematic review and delineation from
type 1 diabetes. Diabetes Care. 46:1292–1299. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fujisawa R, Haseda F, Tsutsumi C, Hiromine
Y, Noso S, Kawabata Y, Mitsui S, Terasaki J, Ikegami H, Imagawa A
and Hanafusa T: Low programmed cell death-1 (PD-1) expression in
peripheral CD4(+) T cells in Japanese patients with autoimmune type
1 diabetes. Clin Exp Immunol. 180:452–457. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang J, Yoshida T, Nakaki F, Hiai H,
Okazaki T and Honjo T: Establishment of NOD-Pdcd1-/- mice as an
efficient animal model of type I diabetes. Proc Natl Acad Sci USA.
102:11823–11828. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Perdigoto AL, Deng S, Du KC, Kuchroo M,
Burkhardt DB, Tong A, Israel G, Robert ME, Weisberg SP,
Kirkiles-Smith N, et al: Immune cells and their inflammatory
mediators modify β cells and cause checkpoint inhibitor-induced
diabetes. JCI Insight. 7(e156330)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Osum KC, Burrack AL, Martinov T, Sahli NL,
Mitchell JS, Tucker CG, Pauken KE, Papas K, Appakalai B, Spanier JA
and Fife BT: Interferon-gamma drives programmed death-ligand 1
expression on islet β cells to limit T cell function during
autoimmune diabetes. Sci Rep. 8(8295)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen X, Affinati AH, Lee Y, Turcu AF,
Henry NL, Schiopu E, Qin A, Othus M, Clauw D, Ramnath N and Zhao L:
Immune checkpoint inhibitors and risk of type 1 diabetes. Diabetes
Care. 45:1170–1176. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Stamatouli AM, Quandt Z, Perdigoto AL,
Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A,
Rushakoff R, et al: Collateral damage: Insulin-dependent diabetes
induced with checkpoint inhibitors. Diabetes. 67:1471–1480.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang Z, Sharma R, Hamad L, Riebandt G and
Attwood K: Incidence of diabetes mellitus in patients treated with
immune checkpoint inhibitors (ICI) therapy-A comprehensive cancer
center experience. Diabetes Res Clin Pract.
202(110776)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lou S, Cao Z, Chi W, Wang X, Feng M, Lin
L, Ding Y, Liu K, Qu L, Zhao G, et al: The safety concerns
regarding immune checkpoint inhibitors in liver cancer patients
rising mainly from CHB. Front Pharmacol. 14(1164309)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rodríguez de Vera-Gómez P, Piñar-Gutiérrez
A, Guerrero-Vázquez R, Bellido V, Morales-Portillo C,
Sancho-Márquez MP, Espejo-García P, Gros-Herguido N, López-Gallardo
G, Martínez-Brocca MA and Soto-Moreno A: Flash glucose monitoring
and diabetes mellitus induced by immune checkpoint inhibitors: An
approach to clinical practice. J Diabetes Res.
2022(4508633)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Immune-endocrinology Group, Chinese
society of Endocrinology, Chinese Medical Association. Chinese
expert consensus on immune checkpoint inhibitors-induced endocrine
immune-related adverse events (2020). Chin J Endocrinol Metab.
37:1–16. 2021.(In Chinese).
|
|
40
|
Lo Preiato V, Salvagni S, Ricci C,
Ardizzoni A, Pagotto U and Pelusi C: Diabetes mellitus induced by
immune checkpoint inhibitors: Type 1 diabetes variant or new
clinical entity? Review of the literature. Rev Endocr Metab Disord.
22:337–349. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tittel SR, Laubner K, Schmid SM, Kress S,
Merger S, Karges W, Wosch FJ, Altmeier M, Pavel M and Holl RW: DPV
Initiative. Immune-checkpoint inhibitor-associated diabetes
compared to other diabetes types-A prospective, matched control
study. J Diabetes. 13:1007–1014. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lin C, Li X, Qiu Y, Chen Z and Liu J: PD-1
inhibitor-associated type 1 diabetes: A case report and systematic
review. Front Public Health. 10(885001)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2020. Diabetes Care. 43 (Suppl 1):S14–S31.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
de Filette JMK, Pen JJ, Decoster L,
Vissers T, Bravenboer B, Van der Auwera BJ, Gorus FK, Roep BO,
Aspeslagh S, Neyns B, et al: Immune checkpoint inhibitors and type
1 diabetes mellitus: A case report and systematic review. Eur J
Endocrinol. 181:363–374. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Deligiorgi MV and Trafalis DT: A concerted
vision to advance the knowledge of diabetes mellitus related to
immune checkpoint inhibitors. Int J Mol Sci.
24(7630)2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Clotman K, Janssens K, Specenier P, Weets
I and De Block CEM: Programmed cell death-1 inhibitor-induced type
1 diabetes mellitus. J Clin Endocrinol Metab. 103:3144–3154.
2018.PubMed/NCBI View Article : Google Scholar
|















